Abstract
Background Not much is known about the effects of glycemic variability (GV) during the pre- and periconception period on pregnancy/perinatal complications. GV could potentially contribute to identification of high-risk pregnancies in women with type 1 diabetes.
Methods An explorative retrospective cohort study was conducted between January 2014 and May 2019. Glucose data were retrieved from electronic patient charts. Pre-/periconceptional GV and GV during all three trimesters was expressed as mean glucose, standard deviation (SD), Coefficient of Variation (CV), High Blood Glucose Index (HBGI), Low Blood Glucose Index (LBGI) and Average Daily Risk Range (ADRR). Maternal and neonatal complications were summarized using a composite total complication score. Binary logistic regression analyses were conducted to assess associations between the GV measures and a total complication score>3, a maternal complication score>1 and a neonatal complication score>1.
Results Of 63 eligible women, 29 women (38 pregnancies) were included. Women in the group with a total complication score>3 had a significantly higher ADRR at conception (OR 1.1, CI 1.0–1.2, p=0.048). No statistically significant correlations between complication score and any other GV metric besides the ADRR were found. Although not significant, in the group with a complication score>3, odds ratios>1 were found for SD in trimester 1 (OR 1.6, CI 0.6–4.5, p=0.357) and trimester 2 (OR 1.8, CI 0.5–6.2, p=0.376).
Conclusions Presence of a positive association between GV and pregnancy and perinatal complications depends on which pregnancy period is assessed and the GV metrics that are used.
Key words: Type 1 diabetes, Pregnancy, Glucose Variability, Continuous Glucose Monitoring
Introduction
Pregnant women with type 1 diabetes and their newborns have a higher risk of complications like pre-eclampsia, premature delivery, caesarean section, congenital malformations, macrosomia, neonatal hypoglycemia and perinatal mortality 1 2 3 . Higher HbA 1c values increase the risk of pregnancy complications 4 5 . Temple and co-workers have shown that pre-pregnancy care including better glycemic control is associated with fewer adverse pregnancy outcomes and fewer severe premature deliveries (<34 weeks of gestation) 6 . The risk of complications can be reduced by optimal glycemic control before and during pregnancy 7 8 . Furthermore, preconception HbA 1c levels<48 mmol/mol (<6.5%) lower the risk of congenital anomalies 9 . Women with type 1 diabetes with unplanned pregnancies have an approximately 10% risk of a serious complication (e. g. stillbirth, serious heart or birth defect), which decreases to approximately 2% when pre-conceptional care is planned together with the patient’s diabetes team 10 .
Evers et al. showed that maternal, perinatal and neonatal complications remain high despite improved glycemic control as expressed by level of HbA 1c (<53 mmol/mol [<7.0%]) in women with type 1 diabetes, 1 suggesting that HbA 1c level may not be the only factor determining the risk of these complications. Intensive insulin therapy increases the risk of maternal hypoglycemia 11 , which increases glycemic variability (GV; the cycling between high and low blood glucose levels). Kerssen et al. found that women with a ‘safe’ HbA 1c had poor glycemic control when measured by GV metrics (e. g. a substantial time below and above the targeted blood glucose range) 12 . Although the debate about a causal relationship between GV and diabetes-related complications is still ongoing, the consensus seems to be that high acute and long-term GV are at least additional risk factors for complications 13 . Indeed, GV has been associated with the risk of congenital malformations, long-term neuropsychological effects 14 and microvascular complications in a non-pregnant type 1 diabetes population 15 . GV can be assessed by monitoring glucose levels manually (self-monitoring of blood glucose [SMBG]) multiple times a day, or automatically and continuously by continuous glucose monitoring (CGM), which provides a much more detailed picture of GV than SMBG 16 . Evidence supporting CGM use in pregnancy is accumulating 17 . The CONCEPTT trial showed that compared with SMBG, using CGM resulted in lower GV 18 . Additionally, Perea et al. showed that a preconception care program for women with type 1 diabetes resulted in improved GV in the first trimester 19 . CGM was also associated with more time in targeted blood glucose range (a measure of GV), fewer occurrences of hypoglycemia and improved neonatal outcomes (which were positively associated with the increase of time in targeted blood glucose range) 18 20 .
It is known that GV contributes to the development of microvascular complications in a non-pregnant type 1 diabetes population 15 . The CONCEPTT trial found that women using CGM experienced lower GV, suggesting that CGM helps to decrease GV during pregnancy 18 . It is still unclear if the improved GV persists beyond the 1 st trimester and if improved pre- and periconceptional GV is associated with fewer pregnancy and perinatal complications. In this explorative study with real-world data we assess if GV measured in pregnant women with type 1 diabetes is associated with the occurrence of pregnancy and perinatal complications to both mother and child. We hypothesize that lower variability in pre- and periconceptional glucose levels lowers the risk of pregnancy and perinatal complications for both mother and fetus.
Methods
Study design and study population
A retrospective cohort study was performed in women with type 1 diabetes who became pregnant between January 2014 and May 2019. Participants used various blood glucose monitoring methods (i. e. SMBG, CGM or flash glucose monitoring [FGM]). The study period per pregnancy was defined as 16 weeks before conception until 7 days after delivery. Participants were recruited from Diabeter, a large multi-center clinic for focused type 1 diabetes care and research in The Netherlands. During our study period (2014–2019) the reimbursement policy for CGM and FGM for pregnant women with type 1 diabetes changed. From 2010 to 2017 CGM was reimbursed only during pregnancy. In 2018 CGM was reimbursed during the pre-pregnancy and the pregnancy period. From 2019 both CGM and FGM were reimbursed before and during pregnancy.
Inclusion and exclusion criteria
Patients were included if they became pregnant between January 2014 and May 2019, were managed by Diabeter during the preconception period, had singleton pregnancies, had≥3 blood glucose readings per day for at least 14 days per month 21 or 80% sensor time, and provided written informed consent. Patients were excluded if they were diagnosed with type 1 diabetes<1 year ago, had spontaneous abortions or were diagnosed with a disease that complicates the interpretation of GV data.
Management of diabetes in (pre-)pregnancy
All participants received standard care at Diabeter. When a patient expressed a wish to conceive, the endocrinologist referred her to a gynecologist for preconception care. The endocrinologist also initiated preconception care, e. g. prescription of folic acid, lowering target HbA 1c values, replacing potential teratogenous medication, referring to an ophthalmologist, checking urine for proteinuria, and monitoring blood pressure and thyroid function. As diabetes in pregnancy is not managed by Diabeter, patients with type 1 diabetes who became pregnant were referred to a gynecologist and endocrinologist for combined outpatient antenatal and obstetric care.
Study outcomes
Mortality and severe morbidity are uncommon in the field of obstetrics, resulting in low power to identify predictors for these parameters. For this reason composite outcomes (neonatal, maternal or combined) are commonly used in this field 22 . The primary outcome we used was a composite maternal and neonatal complication metric. Table 1 lists which maternal and neonatal complications were included. Weights were assigned and a total, maternal and neonatal score was calculated for each pregnancy. The total complication score was dichotomized in≤3 complications and>3 complications. As secondary outcomes both the maternal and the neonatal complication scores were dichotomized in 0–1 complication and>1 complications. Birth weight centiles were determined by using the Dutch Perined (Hoftiezer) reference charts 23 24 . Neonatal hypoglycemia was defined as a blood glucose<2.2 mmol/l. Severe neonatal hypoglycemia was defined as a hypoglycemia requiring glucose infusion or prolonged hospital stay. High bilirubin levels were defined as bilirubin levels requiring phototherapy. Congenital malformations were defined as malformations of any kind.
Table 1 Maternal/neonatal outcome metric and complication rates.
| Maternal complications | Score | Prevalence, n (%) |
|---|---|---|
| Pregnancy induced hypertension | 1 point | 6 (15.8%) |
| Pre-eclampsia or HELLP syndrome | 2 points | 7 (18.4%) |
| Emergency caesarean | 1 point | 11 (28.9%) |
| Forceps or vacuum extraction | 1 point | 6 (15.8%) |
| Postpartum hemorrhage (≥1000 ml blood loss) | 1 point | 3 (7.9%) |
| Shoulder dystocia | 1 point | 3 (7.9%) |
| Oxytocin stimulation for inadequate contractions | 1 point | 2 (5.3%) |
| ICU admission | 2 points | 0 (0%) |
| Hospital admission during pregnancy | 1 point | 11 (28.9%) |
| ≥2 hospital admissions during pregnancy | 2 points | 4 (10.5%) |
| Neonatal complications | ||
| Large for gestational age (LGA) | 1 point | 18 (47.4%) |
| Small for gestational age (SGA) | 1 point | 1 (2.6%) |
| Premature delivery (GA<37 weeks) | 1 point | 10 (26.3%) |
| Severe premature delivery (GA<32 weeks) | 2 points | 2 (5.3%) |
| Birth trauma | 1 point | 4 (10.5%) |
| Hypoglycaemia | 1 point | 20 (52.6%) |
| Severe hypoglycaemia | 2 points | 12 (31.6%) |
| High bilirubin levels | 1 point | 12 (31.6%) |
| Umbilical artery pH≤7.05 | 1 point | 3 (7.9%) |
| Apgar score≤7 after 5 minutes | 1 point | 5 (13.2%) |
| NICU admission | 1 point | 5 (13.2%) |
| Congenital malformation | 1 point | 4 (10.5%) |
| (Suspected) Infection | 1 point | 4 (10.5%) |
GA, gestational age; HELLP, hemolysis, elevated liver enzymes, and a low platelet count; ICU, intensive care unit; LGA, large for gestational age; NICU, neonatal intensive care unit; SGA, small for gestational age.
Glucose variability metrics
Variability metrics were calculated 16 weeks before conception (baseline), at conception and at gestational weeks 12, 24 and 34. For CGM data, seven days of data were used to calculate the mean glucose, standard deviation (SD), coefficient of variation (CV), Low Blood Glucose Index (LBGI), High Blood Glucose Index (HBGI) and Average Daily Risk Range (ADRR) 25 . Mean, SD and CV are the most commonly used metrics, allowing comparison with published literature. Their values mostly depend on hyperglycemic blood glucose levels. The LBGI was specifically developed for the hypoglycemic blood glucose range 26 while the HBGI focuses on high blood glucose excursions. Ideally a measure of glycemic variability would be equally sensitive in both extremes of the glycemic range and include both hyper- and hypoglycemia in one metric. The ADRR was developed specifically for this purpose as it combines the HGBI and LBGI 21 . In the ADRR more weight is given to fluctuations outside the target blood glucose range, as these fluctuations are assumed to contribute more to risk of complications than fluctuations within the target blood glucose range. Supplemental table 1 lists the formulas of these measures and commonly accepted reference values 21 26 27 . Increasing values imply increasing GV, i. e. increasing risk of diabetes-related complications. For the calculation of the ADRR from SMBG data, a minimum of 3 blood glucose measurements per day are needed on at least 14 days of a 30-day period 21 . Therefore, the calculation of variability metrics for SMBG data was based on a four-week period ( Fig. 1 ).
Fig. 1.
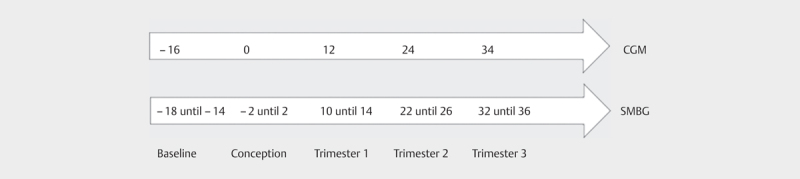
Pregnancy weeks used to calculate GV metrics for CGM and SMBG glucose data.
Data sources
Baseline data was retrieved from electronic patient charts at Diabeter. Glucose data was obtained from Diabeter’s electronic health record system Vcare to which all patients upload their glucose data (CGM and SMBG). Data is reported as a mean glucose value per hour when glucose levels are between 3.9 and 11.2 mmol/l. When a glucose value is outside this range, the most extreme value is reported, with a preference for low over high values. Clinical data on pregnancy and delivery was obtained from the medical files of both mother and baby from the hospital where the mother gave birth. If mother or baby were transferred to another hospital, these files were also requested.
Statistical analysis
Descriptive data were summarized as mean±SD for normally distributed data and n (%) for ordinal/categorical data. The unit of the analysis was the number of pregnancies, assuming that multiple pregnancies within one woman were independent. Crude and adjusted binary logistic regression analyses were conducted to estimate the odds ratios (ORs, 95% CI) between a higher continuous GV value and the dependent variable, being the composite outcome (>3 complications vs. 0–3 complications [reference]), maternal complications (>1 complication vs. 0–1 complication [reference]) and neonatal complications (>1 complication vs. 0–1 complication [reference]). Analyses were also adjusted for the following factors. The type of glucose monitoring (i. e. SMBG vs CGM vs FGM) may introduce bias 28 . Because first pregnancies are generally associated with more complications 29 we also adjusted for parity and, additionally, displayed the results for the parity 0 subgroup. Finally, adjustments were made for BMI, maternal age and duration of type 1 diabetes 30 . To avoid overfitting, adjustments were made in combinations of maximum two variables simultaneously. The significance level was set at P <0.05 (two-sided). Missing data were ignored. No formal power calculation could be performed and no adjustments were made for multiple testing, because this was an explorative pilot study. All analyses were performed with IBM SPSS Statistics 26.0 for Windows (SPSS Inc.; Chicago, IL, USA).
Results
Fig. 2 shows the patient selection. A total of 38 pregnancies in 29 women were included. Table 2 shows the baseline characteristics. Patients with a total complication score>3 had a longer diabetes duration and showed a higher incidence of hypertension, retinopathy and nephropathy. More patients with a total complication score>3 were on CSII therapy, used CGM and were primiparous.
Fig. 2.
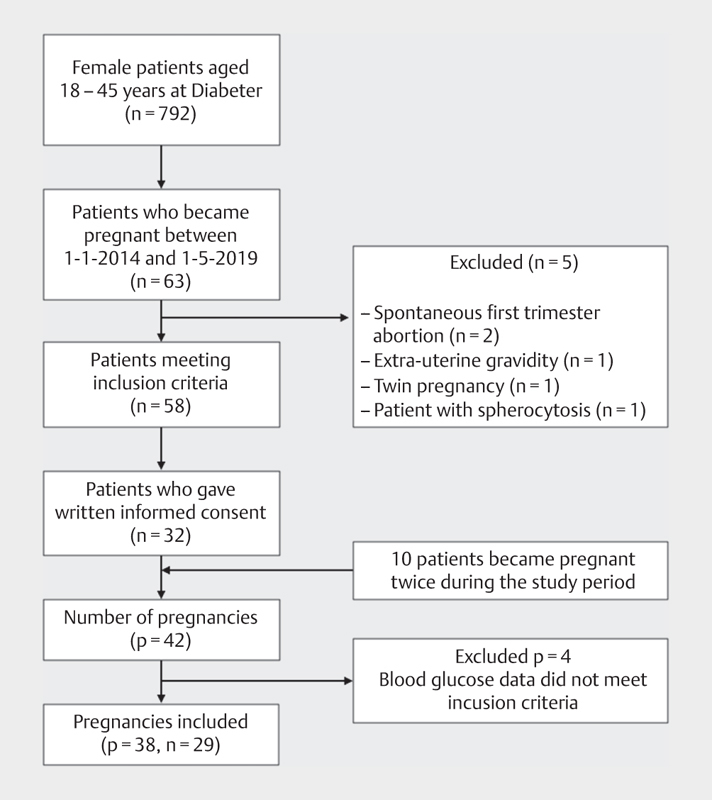
Inclusion procedure n, number of women; p, number of pregnancies.
Table 2 Baseline characteristics.
| Characteristic | All pregnancies (n=38) | Total complication score 0–3 (n=17) | Total complication score>3 (n=21) |
|---|---|---|---|
| Age at conception in years (±SD) | 27.7 (±4.5) | 27.4 (±2.9) | 28.0 (±4.5) |
| Duration of type 1 diabetes in years (±SD) | 15.1 (±7.3) | 13.8 (±6.9) | 16.2 (±7.5) |
| BMI at conception in kg/m 2 (±SD) | 25.7 (±4.3) | 25.5 (±3.7) | 25.9 (±4.7) |
| Smoking at conception | 2 (5.3%) | 1 (5.9%) | 1 (4.8%) |
| Insulin administration | |||
| MDI | 3 (7.9%) | 2 (11.8%) | 1 (4.8%) |
| CSII | 34 (89.5%) | 14 (82.4%) | 20 (95.2%) |
| Glucose monitoring | |||
| CGM | 22 (57.9%) | 8 (47.1%) | 14 (66.7%) |
| SMBG | 7 (18.4%) | 3 (17.6%) | 4 (19.0%) |
| SMBG →CGM | 8 (21.1%) | 5 (29.4%) | 3 (14.3%) |
| FGM | 1 (2.6%) | 1 (5.9%) | 0 (0%) |
| Hypertension | 4 (10.5%) | 1 (5.9%) | 3 (14.3%) |
| Retinopathy at conception | 11 (28.9%) | 4 (23.5%) | 7 (33.3%) |
| Nephropathy at conception | 3 (7.9%) | 0 (0%) | 3 (14.3%) |
| Gravida 1 | 24 (63.2%) | 9 (52.9%) | 15 (71.4%) |
| Gravida 2 | 13 (34.2%) | 8 (47.1%) | 5 (23.8%) |
| Gravida 3 | 1 (2.6%) | 0 (0%) | 1 (4.8%) |
| Para 0 | 29 (76.3%) | 12 (70.6%) | 17 (81.0%) |
| Para 1 | 9 (23.7%) | 5 (29.4%) | 4 (19.0%) |
| ART | 0 (0%) | 0 (0%) | 0 (0%) |
| HbA1c at conception | |||
| % (±SD) | 6.86 (±0.89) | 6.78 (±0.99) | 6.92 (±0.82) |
| Mmol/mol (±SD) | 51.5 (±9.7) | 50.7 (±10.9) | 52.2 (±9.0) |
| Preconception planning | 24 (63.2%) | 11 (64.7%) | 13 (61.9%) |
| Folic acid use | 30 (78.9%) | 13 (76.5%) | 17 (81.0%) |
Values are shown as mean (SD) or as number (%); ART, assisted reproductive technology; CGM, continuous glucose monitoring; CSII, continuous subcutaneous insulin infusion; FGM, flash glucose monitoring; MDI, multiple daily injections; SMBG, self-monitoring of blood glucose; SMBG →CGM, patient switched from SMBG to CGM before the end of the first trimester.
Table 1 shows the maternal and neonatal complication rates. Emergency caesarean, hospital admission during pregnancy and pre-eclampsia or HELLP-syndrome were the most frequent maternal complications. Frequent neonatal complications were hypoglycemia, LGA, hyperbilirubinemia and premature delivery.
Composite outcome, parity 0 and parity 1
Except for LBGI, the different metrics of GV seemed to decrease from the pre-conceptional baseline period to the end of the pregnancy ( Fig. 3 ). Table 3 shows results of the logistic regression between the different GV metrics and the composite outcome (i. e. combined maternal and neonatal complications) of having a total complication score>3. Our explorative analysis showed an OR>1 between SD in trimester 1 and a total complication score>3, albeit not significant (OR 1.62, p=0.357) which increased to 5.92 (p=0.051) when adjusted for glucose monitoring and parity. The same applied to SD in trimester 2 (OR 1.76, p=0.376). The ORs for SD were higher after all four adjustments were applied. An OR of similar magnitude was found between LBGI in the 2nd trimester and a total complication score>3, again not significantly so (OR 1.57, p=0.229). A higher ADRR at conception was significantly associated with a complication score>3 (OR 1.10, p=0.048). This association remained significantly different when adjusted for glucose monitoring and maternal age (OR 1.13, p=0.043). The ADRR in the 2nd trimester also showed a trend for a positive association with a complication score>3 (OR 1.14, p=0.068). This association became significant after adjustment for type of glucose monitoring and the duration of type 1 diabetes (OR 1.62, p=0.047).
Fig. 3.
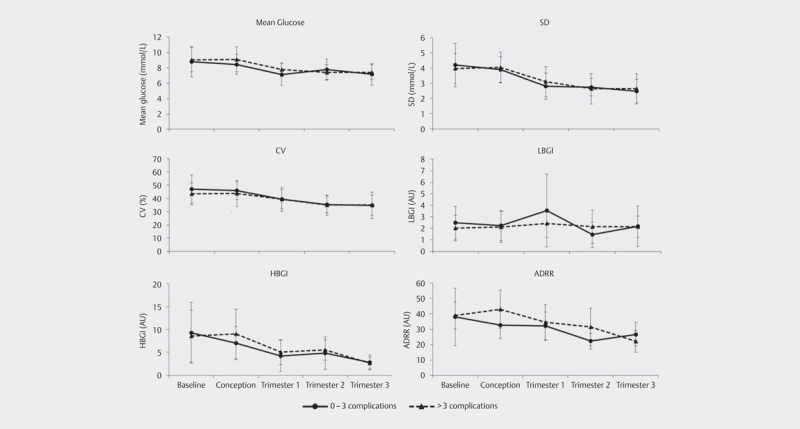
Different measures of GV before and around conception and during pregnancy Error bars: standard deviation. ADRR, average daily risk range; AU, arbitrary units; CV, coefficient of variation; HBGI, high blood glucose index; LBGI, low blood glucose index; SD, standard deviation.
Table 3 Glycemic variability in the>3 total complication score group vs. 0–3 total complication score group (reference). Results in the top line include parity 0 and parity 1 pregnancies. Results in italics only include parity 0 pregnancies.
| Overall OR (95% CI) | p | OR adjusted for type of glucose monitoring and parity (95% CI) | p | OR adjusted for type of glucose monitoring and BMI (95% CI) | p | OR adjusted for type of glucose monitoring and maternal age (95% CI) | p | OR adjusted for type of glucose monitoring and duration of type 1 diabetes (95% CI) | p | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean glucose | |||||||||||
| Baseline | 1.09 (0.71–1.67) | 0.685 | 1.05 (0.62–1.78) | 0.848 | 0.95 (0.59–1.54) | 0.840 | 0.95 (0.59–1.52) | 0.825 | 0.75 (0.41–1.36) | 0.336 | |
| 1.12 (0.65–1.93) | 0.680 | NA | NA | 0.98 (0.53–1.79) | 0.933 | 0.99 (0.54–1.81) | 0.962 | 0.80 (0.40–1.60) | 0.521 | ||
| Conception | 1.39 (0.79–2.44) | 0.253 | 1.56 (0.85–2.87) | 0.156 | 1.27 (0.70–2.29) | 0.439 | 1.45 (0.77–2.71) | 0.250 | 1.30 (0.73–2.32) | 0.374 | |
| 1.61 (0.69–3.73) | 0.271 | NA | NA | 1.70 (0.73–4.00) | 0.218 | 2.09 (0.70–6.25) | 0.187 | 1.54 (0.64–3.72) | 0.333 | ||
| Trimester 1 | 1.71 (0.79–3.75) | 0.175 | 1.97 (0.78–5.00) | 0.154 | 1.31 (0.48–3.58) | 0.605 | 1.64 (0.67–3.98) | 0.278 | 1.60 (0.64–4.02) | 0.318 | |
| 4.98 (1.01–24.48) | 0.048 | NA | NA | 6.68 (0.88–50.55) | 0.660 | 9.56 (1.02–90.21) | 0.048 | 17.60 (0.96–322.89) | 0.053 | ||
| Trimester 2 | 1.30 (0.66–2.54) | 0.451 | 1.35 (0.63–2.88) | 0.438 | 1.18 (0.56–2.45) | 0.667 | 1.16 (0.57–2.39) | 0.680 | 1.19 (0.58–2.47) | 0.637 | |
| 2.68 (0.95–7.57) | 0.063 | NA | NA | 2.55 (0.90–7.27) | 0.080 | 2.62 (0.90–7.65) | 0.078 | 2.64 (0.87–7.99) | 0.086 | ||
| Trimester 3 | 1.23 (0.61–2.47) | 0.564 | 0.93 (0.37–2.32) | 0.871 | 0.75 (0.31–1.8) | 0.525 | 0.81 (0.34–1.94) | 0.633 | 0.76 (0.32–1.80) | 0.535 | |
| 2.08 (0.73–5.90) | 0.168 | NA | NA | 1.68 (0.48–5.87) | 0.417 | 1.95 (0.48–7.86) | 0.348 | 1.45 (0.42–4.99) | 0.557 | ||
| SD | |||||||||||
| Baseline | 0.87 (0.49–1.55) | 0.626 | 0.89 (0.46–1.70) | 0.715 | 0.81 (0.43–1.55) | 0.531 | 0.86 (0.47–1.58) | 0.634 | 0.67 (0.33–1.38) | 0.280 | |
| 0.82 (0.42–1.61) | 0.572 | NA | NA | 0.83 (0.38–1.79) | 0.631 | 0.89 (0.43–1.85) | 0.752 | 0.72 (0.32–1.64) | 0.437 | ||
| Conception | 1.13 (0.59–2.16) | 0.713 | 1.63 (0.74–3.57) | 0.226 | 1.21 (0.53–2.76) | 0.654 | 1.41 (0.65–3.05) | 0.382 | 1.27 (0.57–2.83) | 0.552 | |
| 1.00 (0.40–2.49) | 0.998 | NA | NA | 1.66 (0.50–5.50) | 0.409 | 1.82 (0.51–6.53) | 0.358 | 1.38 (0.38–4.92) | 0.625 | ||
| Trimester 1 | 1.62 (0.58–4.52) | 0.357 | 5.92 (0.99–35.25) | 0.051 | 2.27 (0.49–10.47) | 0.292 | 2.78 (0.70–11.03) | 0.146 | 2.52 (0.64–9.86) | 0.186 | |
| 2.43 (0.59–9.95) | 0.218 | NA | NA | NA* | NA* | NA* | NA* | NA* | NA* | ||
| Trimester 2 | 1.76 (0.50–6.20) | 0.376 | 2.26 (0.51–10.12) | 0.286 | 2.19 (0.48–10.01) | 0.312 | 2.11 (0.47–9.44) | 0.328 | 1.80 (0.43–7.62) | 0.424 | |
| 3.04 (0.61–15.08) | 0.174 | NA | NA | 8.57 (0.89–82.77) | 0.063 | 5.79 (0.72–46.71) | 0.099 | 5.78 (0.71–46.93) | 0.100 | ||
| Trimester 3 | 1.29 (0.45–3.71) | 0.633 | 1.10 (0.30–4.03) | 0.891 | 1.29 (0.36–4.57) | 0.697 | 1.17 (0.35–3.87) | 0.796 | 1.09 (0.32–3.78) | 0.891 | |
| 1.71 (0.50–5.83) | 0.394 | NA | NA | 2.89 (0.46–18.26) | 0.260 | 1.91 (0.40–9.08) | 0.415 | 1.41 (0.30–6.60) | 0.664 | ||
| CV | |||||||||||
| Baseline | 0.96 (0.88–1.04) | 0.309 | 0.97 (0.87–1.07) | 0.500 | 0.96 (0.86–1.06) | 0.403 | 0.98 (0.89–1.08) | 0.621 | 0.95 (0.85–1.07) | 0.421 | |
| 0.95 (0.86–1.04) | 0.260 | NA | NA | 0.95 (0.85–1.07) | 0.418 | 0.98 (0.87–1.09) | 0.640 | 0.96 (0.84–1.09) | 0.488 | ||
| Conception | 0.97 (0.89–1.06) | 0.500 | 1.01 (0.90–1.14) | 0.847 | 0.99 (0.87–1.11) | 0.815 | 1.00 (0.90–1.12) | 0.912 | 0.99 (0.88–1.11) | 0.864 | |
| 0.94 (0.83–1.06) | 0.300 | NA | NA | 0.98 (0.83–1.16) | 0.809 | 1.00 (0.86–1.16) | 1.000 | 0.97 (0.84–1.13) | 0.721 | ||
| Trimester 1 | 1.00 (0.91–1.11) | 0.954 | 1.26 (0.98–1.63) | 0.073 | 1.11 (0.94–1.32) | 0.220 | 1.12 (0.95–1.32) | 0.185 | 1.10 (0.94–1.28) | 0.232 | |
| 0.99 (0.89–1.10) | 0.837 | NA | NA | 1.33 (0.94–1.87) | 0.106 | 1.51 (0.91–2.51) | 0.107 | 1.25 (0.95–1.65) | 0.117 | ||
| Trimester 2 | 1.02 (0.92–1.14) | 0.672 | 1.04 (0.91–1.19) | 0.568 | 1.06 (0.92–1.23) | 0.439 | 1.06 (0.92–1.22) | 0.460 | 1.04 (0.91–1.18) | 0.616 | |
| 1.02 (0.90–1.15) | 0.769 | NA | NA | 1.08 (0.92–1.28) | 0.360 | 1.06 (0.90–1.25) | 0.485 | 1.05 (0.89–1.23) | 0.565 | ||
| Trimester 3 | 1.00 (0.91–1.10) | 0.950 | 1.01 (0.9–1.14) | 0.811 | 1.06 (0.93–1.21) | 0.408 | 1.03 (0.92–1.15) | 0.623 | 1.03 (0.92–1.15) | 0.668 | |
| 1.00 (0.90–1.12) | 0.971 | NA | NA | 1.10 (0.92–1.32) | 0.310 | 1.03 (0.91–1.17) | 0.645 | 1.01 (0.88–1.15) | 0.923 | ||
| LBGI | |||||||||||
| Baseline | 0.73 (0.36–1.47) | 0.379 | 0.80 (0.32–1.99) | 0.633 | 0.78 (0.34–1.83) | 0.572 | 0.70 (0.29–1.70) | 0.436 | 0.64 (0.24–1.70) | 0.371 | |
| 0.91 (0.39–2.13) | 0.827 | NA | NA | 1.27 (0.42–3.86) | 0.668 | 1.04 (0.30–3.63) | 0.951 | 1.13 (0.34–3.75) | 0.844 | ||
| Conception | 0.92 (0.48–1.12) | 0.814 | 0.86 (0.38–1.94) | 0.715 | 0.93 (0.40–2.19) | 0.871 | 1.00 (0.46–2.19) | 0.997 | 1.21 (0.56–2.62) | 0.628 | |
| 0.77 (0.38–1.59) | 0.483 | NA | NA | 0.75 (0.28–1.98) | 0.558 | 0.89 (0.38–2.09) | 0.780 | 1.10 (0.45–2.65) | 0.839 | ||
| Trimester 1 | 0.78 (0.50–1.22) | 0.277 | 0.88 (0.49–1.58) | 0.669 | 0.98 (0.47–2.07) | 0.965 | 0.87 (0.49–1.56) | 0.647 | 0.83 (0.42–1.63) | 0.590 | |
| 0.51 (0.23–1.12) | 0.093 | NA | NA | 0.61 (0.22–1.71) | 0.349 | 0.56 (0.19–1.66) | 0.298 | 0.39 (0.08–1.88) | 0.240 | ||
| Trimester 2 | 1.57 (0.75–3.26) | 0.229 | 1.59 (0.69–3.67) | 0.273 | 1.61 (0.72–3.60) | 0.249 | 1.61 (0.71–3.61) | 0.253 | 1.92 (0.71–5.20) | 0.199 | |
| 1.10 (0.50–2.43) | 0.821 | NA | NA | 1.17 (0.50–2.75) | 0.723 | 1.08 (0.42–2.77) | 0.867 | 1.40 (0.50–3.94) | 0.521 | ||
| Trimester 3 | 0.99 (0.42–2.32) | 0.980 | 0.25 (0.02–2.70) | 0.255 | 1.59 (0.45–5.67) | 0.473 | 0.92 (0.28–3.07) | 0.893 | 1.20 (0.38–3.80) | 0.760 | |
| 0.71 (0.27–1.89) | 0.493 | NA | NA | NA* | NA* | 0.12 (0.01–1.93) | 0.133 | 0.25 (0.02–2.87) | 0.266 | ||
| HBGI | |||||||||||
| Baseline | 0.98 (0.85–1.13) | 0.767 | 0.79 (0.81–1.17) | 0.769 | 0.95 (0.80–1.13) | 0.586 | 0.95 (0.81–1.12) | 0.525 | 0.99 (0.74–1.09) | 0.273 | |
| 0.97 (0.80–1.17) | 0.718 | NA | NA | 0.95 (0.75–1.21) | 0.682 | 0.93 (0.74–1.17) | 0.540 | 0.92 (0.73–1.18) | 0.523 | ||
| Conception | 1.12 (0.90–1.38) | 0.317 | 1.31 (0.90–1.90) | 0.156 | 1.14 (0.91–1.42) | 0.251 | 1.22 (0.93–1.60) | 0.156 | 1.07 (0.84–1.36) | 0.600 | |
| 1.24 (0.87–1.78) | 0.242 | NA | NA | 1.37 (0.85–2.19) | 0.198 | 1.65 (0.93–2.94) | 0.090 | 1.28 (0.82–2.00) | 0.278 | ||
| Trimester 1 | 1.11 (0.82–1.50) | 0.500 | 1.21 (0.83–1.76) | 0.317 | 0.97 (0.65–1.44) | 0.879 | 1.10 (0.77–1.57) | 0.603 | 1.04 (0.70–1.52) | 0.863 | |
| 1.51 (0.86–2.67) | 0.155 | NA | NA | 2.38 (0.77–7.36) | 0.131 | 2.29 (0.81–6.46) | 0.117 | 4.96 (0.38–64.53) | 0.221 | ||
| Trimester 2 | 1.10 (0.80–1.51) | 0.565 | 1.16 (0.82–1.64) | 0.408 | 1.09 (0.79–1.52) | 0.598 | 1.08 (0.78–1.49) | 0.656 | 1.09 (0.78–1.51) | 0.612 | |
| 1.59 (0.90–2.78) | 0.108 | NA | NA | 1.65 (0.94–2.91) | 0.083 | 1.64 (0.91–2.95) | 0.102 | 1.57 (0.92–2.68) | 0.099 | ||
| Trimester 3 | 0.91 (0.41–2.05) | 0.823 | 1.97 (0.58–6.65) | 0.276 | 0.76 (0.27–2.15) | 0.602 | 1.15 (0.45–2.95) | 0.770 | 0.93 (0.38–2.26) | 0.875 | |
| 1.52 (0.54–4.29) | 0.432 | NA | NA | NA* | NA* | 2.86 (0.61–13.34) | 0.182 | 2.11 (0.57–7.88) | 0.265 | ||
| ADRR | |||||||||||
| Baseline | 1.00 (0.94–1.07) | 0.889 | 1.00 (0.93–1.09) | 0.913 | 0.98 (0.91–1.06) | 0.603 | 0.98 (0.91–1.05) | 0.562 | 0.96 (0.89–1.04) | 0.346 | |
| 1.06 (0.95–1.19) | 0.285 | NA | NA | 1.04 (0.93–1.18) | 0.475 | 1.04 (0.92–1.18) | 0.543 | 1.04 (0.92–1.18) | 0.524 | ||
| Conception | 1.10 (1.00–1.20) | 0.048 | 1.13 (0.98–1.29) | 0.084 | 1.11 (0.99–1.23) | 0.065 | 1.13 (1.00–1.26) | 0.043 | 1.09 (0.99–1.21) | 0.090 | |
| 1.12 (0.99–1.26) | 0.068 | NA | NA | 1.13 (0.98–1.30) | 0.099 | 1.20 (0.98–1.47) | 0.080 | 1.12 (0.96–1.30) | 0.155 | ||
| Trimester 1 | 1.02 (0.94–1.11) | 0.599 | 1.20 (0.98–1.50) | 0.081 | 0.98 (0.87–1.12) | 0.800 | 1.01 (0.90–1.12) | 0.93 | 0.96 (0.85–1.10) | 0.569 | |
| 1.02 (0.92–1.14) | 0.660 | NA | NA | 1.14 (0.90–1.44) | 0.289 | 1.15 (0.92–1.46) | 0.226 | 1.04 (0.84–1.28) | 0.712 | ||
| Trimester 2 | 1.14 (0.99–1.30) | 0.068 | 1.15 (0.99–1.34) | 0.077 | 1.20 (0.97–1.47) | 0.090 | 1.14 (0.98–1.31) | 0.087 | 1.62 (1.01–2.60) | 0.047 | |
| 1.10 (0.96–1.26) | 0.185 | NA | NA | 1.20 (0.96–1.50) | 0.114 | 1.11 (0.95–1.30) | 0.189 | NA* | NA* | ||
| Trimester 3 | 0.90 (0.76–1.07) | 0.251 | 0.81 (0.57–1.15) | 0.236 | 0.85 (0.65–1.11) | 0.224 | 0.75 (0.52–1.08) | 0.121 | 0.86 (0.70–1.07) | 0.181 | |
| 0.93 (0.76–1.14) | 0.472 | NA | NA | 0.83 (0.49–1.39) | 0.470 | NA* | NA* | 0.80 (0.52–1.21) | 0.285 | ||
Statistically significant Odds Ratios are displayed in bold. ; * OR cannot be estimated due to small n.
Composite outcome, parity 0 only
Table 3 also shows the results for subgroup of first pregnancies (para 0). For mean glucose in in trimester 1 a significantly increased risk of complications was now found (OR 4.98, p=0.048). For mean glucose in trimester 2 a trend for an increased risk of complications was observed (OR 2.68, p=0.063). Also, the earlier observed significant OR for ADRR at conception in the parity 0+1 group became non-significant in the parity 0 group but a trend was still observed (OR 1.10, p=0.048 vs. OR 1.12, p=0.068). In trimester 2 the trend for an increased risk of complications disappeared (OR 1.14, p=0.068 vs. OR 1.10, p=0.185).
Maternal and neonatal outcome, parity 0 and parity 1
We also performed logistic regression on the separate maternal and neonatal outcomes, comparing 0–1 complications (reference) with>1 complication (Supplemental tables 2 and 3). The OR between the SD in trimester 2 and the maternal complication score was higher compared with the composite score, but not significant (OR 2.35, p=0.206 vs. OR 1.76, p=0.376).
ORs of a similar magnitude were found between SD in trimester 1 and a neonatal complication score>1 (OR 2.11, p=0.195) and between LBGI in the trimester 2 and a neonatal complication score>1 (OR 1.91, p=0.110), although not significantly so. ADRR in trimester 2 showed a significant association with a neonatal complication score>1, when adjusted for glucose monitoring and maternal age (OR 1.20, p=0.050).
Maternal and neonatal outcome, parity 0 only
These analyses were also performed with only the first pregnancies (parity 0)(Supplemental tables 2 and 3). Mean glucose in trimester 2 showed a significant risk of maternal complications (OR 4.59, p=0.022). For HBGI in trimester 2 we observed a trend for an increased risk of maternal complications (OR 1.73, p=0.074).
For ADRR the earlier observed significant risk of neonatal complications at conception (adjustment for glucose monitoring and maternal age) became non-significant (OR 1.20, p=0.050 vs. OR 1.14, p=0.134).
Discussion
We assessed the variability of blood glucose within a defined time window from pre-conception to birth in relation to pregnancy and perinatal complications in women with type 1 diabetes and their newborns. We looked at the commonly reported GV-metrics mean glucose, SD and CV, but also at the less well-known HBGI and LBGI to assess the high end and low end of the blood glucose spectrum, respectively. To look at both ends of the spectrum simultaneously the ADRR metric was used. The results of our explorative analyses indicate that periconceptional GV and GV during the 1 st and 2 nd trimester, expressed as ADRR, is positively associated with pregnancy and perinatal complications to both mother and child. Women with a total complication score>3 had a higher ADRR at conception compared with women with a total complication score<3 (42.95 and 32.70 respectively). In both groups ADRR was relatively high, considering that ADRR<20 represents a low risk, 20–40 a moderate risk and>40 a high risk 21 . During pregnancy, ADRR decreased from high-risk values to moderate-risk values. It must be noted that these reference values are based on patients with type 1 diabetes and type 2 diabetes (males and females of all ages). In other words, our analysis suggests that higher GV is a risk factor in pregnancy complications. However, the associations for the other GV metrics were less clear. The magnitude of the ORs indicate that GV during the 1 st and 2 nd trimester may be associated with pregnancy and perinatal complications, although due to the small sample this could not be substantiated.
Our results are in accordance with previous studies: Kerssen et al. and Herranz et al. showed that LGA was related to high mean glucose levels in the second and third trimester 31 32 . Dalfrà et al. found presumptive evidence that GV is important in determining overgrowth in pregnant women with diabetes 33 . Law et al. showed that higher GV in the second trimester was associated with LGA infants 34 . However, studies concerning GV in pregnancy are difficult to compare due to use of different GV metrics and different calculation procedures. For example, in the calculation of the SD, episodes of two days in each trimester 33 35 , 5–7 days in non-specified periods 34 , 4 weeks in each trimester 36 , one week in trimester 2 and 3 18 37 , a two-week period 38 and entire trimesters 39 were used. Furthermore, only few studies used accuracy criteria for glucose data 18 38 40 . This indicates that consensus on data-management and calculation of GV metrics is urgently needed for proper comparison between studies assessing associations between GV and pregnancy outcomes 41 .
In this explorative study we found relatively high ORs between complication scores and some of the GV metrics, but due to the small sample these were not statistically significant, except for the ADRR. A majority of the GV metrics are mostly sensitive to the high end of the BG spectrum or are developed for either end of the spectrum (e. g. LBGI and HBGI). ADRR is a combination of the LBGI and the HBGI and is thus equally sensitive to the risk of hypoglycemia and hyperglycemia, because it is based on transformed glucose-values, resulting in a symmetric risk scale instead of the usual skewed scale 21 25 . This might explain that in this small study population, only the ADRR showed statistically significant associations. In pregnancy, to prepare the body for implantation and subsequent development of the embryo, a woman’s metabolic state changes in terms of the hormonal environment, adipocytes and inflammatory cytokines 14 42 43 44 45 46 47 48 49 . Studies show that extreme values on both side of the glycemic spectrum have negative effects on this fine metabolic balance. In short, it is important that any GV measure used takes into account both sides of the blood glucose spectrum.
This study has several strengths. It is a longitudinal study in which participants were monitored during the pre- and periconception period and throughout the entire pregnancy. Its novelty lies in the evaluation of associations between pre- and periconceptional GV metrics and perinatal outcomes. Data from medical records were used to calculate the perinatal outcome-metrics which resulted in more reliable outcomes compared with self-reported outcomes. Other strengths are the use of real-world data, the fact that an extensive amount of data could be included, the absence of missing values in the complication data and the use of a strict study protocol to which no concessions were made.
A limitation of this explorative, observational and retrospective pilot study is the small study size: only 29 of 63 eligible women consented to participation. Small study size and selective participation reduce power and possibly introduce bias. Some women who experienced pregnancies without any problems may not have been inclined to participate in the study. Another group of women experienced the previous pregnancy or delivery as traumatic and did not want to be reminded of that episode in their lives. Finally, some women may have thought that participating in the study would be too much hassle. This may have resulted in an underestimated complications rate in type 1 diabetes pregnancy. Also, due to heterogeneity in types of glucose monitoring, subgroups became too small to draw firm conclusions. Another limitation is that 5-minute interval glucose data was aggregated into 1-hour intervals (algorithm in Diabeter’s disease management system Vcare). The crude 5-minute interval CGM data was not available because several manufacturers could not provide us with the requested data due to storage or privacy policy issues, regardless of the patients’ informed consent to share their own data. Consequently, not all data were available and not all GV metrics could be calculated for every subject. Finally, our main results were based on the assumption that multiple pregnancies within one woman are independent, whereas they are not. The parity 0 subgroup analysis revealed that the only additional GV measure that resulted in an association with an increased risk of complications was mean glucose in trimester 2, for both composite outcome and maternal outcome. Overall this suggests that the nine secondary pregnancies did not result in major changes
Recently, Murphy et al. reported that in more than 8,000 pregnancies in women with type 1 diabetes, no improvement (possibly even a worsening) in pregnancy outcomes could be seen over a 5-year period 30 50 . Considering that these women received care in centers specializing in diabetes during pregnancy, the authors suggest that healthcare-wide changes to pregnancy care for women with diabetes are needed. Although our results are explorative and not conclusive, our study emphasizes that GV looks promising in facilitating the identification of women with type 1 diabetes with an increased risk for adverse pregnancy outcomes. If GV metrics are added to sensor output, patients and clinicians will be able to retrospectively assess periconceptional GV to identify potential risks. Also, lowering GV could become part of preconception consultation. More extensive and prospective studies are needed to confirm our results and establish GV-metric reference ranges for pregnant women with type 1 diabetes. These studies should include larger study populations, prospective and longitudinal study designs and clear agreements about access to CGM data. Further research should also assess the usability of GV metrics as markers to identify women with type 1 diabetes at increased risk of developing complications during pregnancy and/or birth. For studies concerning diabetes and pregnancy research, it would be useful to establish core outcome sets including GV metrics. Additionally, it should be elucidated in future research which GV metrics are preferable to use in type 1 diabetes and pregnancy and over which period they should be calculated.
In conclusion our data suggest that careful monitoring of GV during (pre)conception is important. However, despite the positive association between periconceptional GV as measured by ADRR and pregnancy and perinatal complications, more evidence is needed to substantiate the relation between pre- and periconceptional GV and pregnancy and perinatal complications, and to determine the optimal (combination of) GV metric(s) and cut-offs to identify women with type 1 diabetes with an increased risk for adverse pregnancy outcomes.
Ethics approval and consent to participate
The Medical Research Ethics Committee of Erasmus Medical Centre (EMC), Rotterdam, The Netherlands, declared that since participants were not subjected to any actions or restrictions and followed in regular care, this study was exempt from further approval procedures (registration number MEC-2019–0790).
Contribution statement
RH, SB and SG were responsible for the concept of the study. RH and SB were responsible for data collection. RH, SB, EB and PD researched data and performed data analysis. RH and PD drafted the initial version of the manuscript. HJD, HJV and HJA interpreted the data, reviewed the manuscript and critically revised it. All authors read and approved the final manuscript.
Acknowledgements
We thank dr. Martine de Vries, Research Program Manager at Diabeter, for reviewing the study protocol and submitting it to the medical research ethics committee.
Footnotes
Conflict of Interest SB, PD, EB, HJA and HJV are employees of Diabeter, an independent clinic which was acquired by Medtronic. The research presented here was independently performed and there are no conflicts of interest. RH, SG and HJD have nothing to disclose.
References
- 1.Evers I M, de Valk H W, Visser G H. Risk of complications of pregnancy in women with type 1 diabetes: nationwide prospective study in the Netherlands. BMJ (Clinical research ed) 2004;328:915. doi: 10.1136/bmj.38043.583160.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Persson M, Norman M, Hanson U. Obstetric and perinatal outcomes in type 1 diabetic pregnancies: A large, population-based study. Diabetes care. 2009;32:2005–2009. doi: 10.2337/dc09-0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feig D S, Hwee J, Shah B R et al. Trends in incidence of diabetes in pregnancy and serious perinatal outcomes: a large, population-based study in Ontario, Canada, 1996-2010. Diabetes care. 2014;37:1590–1596. doi: 10.2337/dc13-2717. [DOI] [PubMed] [Google Scholar]
- 4.Buschur E O, Polsky S.Type 1 Diabetes: Management in Women From Preconception to Postpartum The Journal of clinical endocrinology and metabolism 2020 10.1210/clinem/dgaa931doi:10.1210/clinem/dgaa931 [DOI] [PubMed] [Google Scholar]
- 5.Feldman A Z, Brown F M. Management of Type 1 Diabetes in Pregnancy. Current diabetes reports. 2016;16:76. doi: 10.1007/s11892-016-0765-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Temple R C, Aldridge V J, Murphy H R. Prepregnancy care and pregnancy outcomes in women with type 1 diabetes. Diabetes care. 2006;29:1744–1749. doi: 10.2337/dc05-2265. [DOI] [PubMed] [Google Scholar]
- 7.Holmes V A, Young I S, Patterson C C et al. Optimal glycemic control, pre-eclampsia, and gestational hypertension in women with type 1 diabetes in the diabetes and pre-eclampsia intervention trial. Diabetes care. 2011;34:1683–1688. doi: 10.2337/dc11-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maresh M J, Holmes V A, Patterson C C et al. Glycemic targets in the second and third trimester of pregnancy for women with type 1 diabetes. Diabetes care. 2015;38:34–42. doi: 10.2337/dc14-1755. [DOI] [PubMed] [Google Scholar]
- 9.Jensen D M, Korsholm L, Ovesen P et al. Peri-conceptional A1C and risk of serious adverse pregnancy outcome in 933 women with type 1 diabetes. Diabetes care. 2009;32:1046–1048. doi: 10.2337/dc08-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy H.Planning for pregnancy. Retrieved from URL:https://abcd.care/resource/planning-pregnancy Accessed 7-3-2022
- 11.Rosenn B M, Miodovnik M, Holcberg G et al. Hypoglycemia: the price of intensive insulin therapy for pregnant women with insulin-dependent diabetes mellitus. Obstetrics and gynecology. 1995;85:417–422. doi: 10.1016/0029-7844(94)00415-a. [DOI] [PubMed] [Google Scholar]
- 12.Kerssen A, Evers I M, de Valk H W et al. Poor glucose control in women with type 1 diabetes mellitus and ‘safe’ hemoglobin A1c values in the first trimester of pregnancy. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2003;13:309–313. doi: 10.1080/jmf.13.5.309.313. [DOI] [PubMed] [Google Scholar]
- 13.Monnier L, Colette C, Schlienger J L et al. Glucocentric risk factors for macrovascular complications in diabetes: Glucose ‘legacy’ and ‘variability’-what we see, know and try to comprehend. Diabetes & metabolism. 2019;45:401–408. doi: 10.1016/j.diabet.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 14.ter Braak E W, Evers I M, Willem Erkelens D et al. Maternal hypoglycemia during pregnancy in type 1 diabetes: maternal and fetal consequences. Diabetes/metabolism research and reviews. 2002;18:96–105. doi: 10.1002/dmrr.271. [DOI] [PubMed] [Google Scholar]
- 15.Kilpatrick E S, Rigby A S, Atkin S L. A1C variability and the risk of microvascular complications in type 1 diabetes: data from the Diabetes Control and Complications Trial. Diabetes care. 2008;31:2198–2202. doi: 10.2337/dc08-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kovatchev B. Glycemic Variability: Risk Factors, Assessment, and Control. Journal of diabetes science and technology. 2019;13:627–635. doi: 10.1177/1932296819826111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto J, Murphy H. Benefits of Real-Time Continuous Glucose Monitoring in Pregnancy. Diabetes technology & therapeutics. 2021 doi: 10.1089/dia.2020.0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feig D S, Donovan L E, Corcoy R et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet (London, England) 2017;390:2347–2359. doi: 10.1016/s0140-6736(17)32400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perea V, Giménez M, Amor A J et al. Prepregnancy care in women with type 1 diabetes improves HbA(1c) and glucose variability without worsening hypoglycaemia time and awareness: Glycaemic variability during prepregnancy care. Diabetes research and clinical practice. 2019;154:75–81. doi: 10.1016/j.diabres.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Murphy H R. Continuous glucose monitoring targets in type 1 diabetes pregnancy: every 5% time in range matters. Diabetologia. 2019;62:1123–1128. doi: 10.1007/s00125-019-4904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovatchev B P, Otto E, Cox D et al. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes care. 2006;29:2433–2438. doi: 10.2337/dc06-1085. [DOI] [PubMed] [Google Scholar]
- 22.Herman D, Lor K Y, Qadree A et al. Composite adverse outcomes in obstetric studies: a systematic review. BMC pregnancy and childbirth. 2021;21:107. doi: 10.1186/s12884-021-03588-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoftiezer L, Hukkelhoven C W, Hogeveen M et al. Defining small-for-gestational-age: prescriptive versus descriptive birthweight standards. European journal of pediatrics. 2016;175:1047–1057. doi: 10.1007/s00431-016-2740-8. [DOI] [PubMed] [Google Scholar]
- 24.Perined. Hoftiezer birth weight percentiles. Retrieved from URL:https://www.perined.nl/geboortegewichtcurven Accessed 7-3-2022
- 25.Kovatchev B P. Metrics for glycaemic control – from HbA(1c) to continuous glucose monitoring. Nature reviews Endocrinology. 2017;13:425–436. doi: 10.1038/nrendo.2017.3. [DOI] [PubMed] [Google Scholar]
- 26.Kovatchev B P, Cox D J, Gonder-Frederick L A et al. Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes care. 1998;21:1870–1875. doi: 10.2337/diacare.21.11.1870. [DOI] [PubMed] [Google Scholar]
- 27.Monnier L, Colette C, Wojtusciszyn A et al. Toward Defining the Threshold Between Low and High Glucose Variability in Diabetes. Diabetes care. 2017;40:832–838. doi: 10.2337/dc16-1769. [DOI] [PubMed] [Google Scholar]
- 28.Raman P, Shepherd E, Dowswell T et al. Different methods and settings for glucose monitoring for gestational diabetes during pregnancy. The Cochrane database of systematic reviews. 2017;10:Cd011069. doi: 10.1002/14651858.CD011069.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldman-Wohl D, Gamliel M, Mandelboim O et al. Learning from experience: cellular and molecular bases for improved outcome in subsequent pregnancies. American journal of obstetrics and gynecology. 2019;221:183–193. doi: 10.1016/j.ajog.2019.02.037. [DOI] [PubMed] [Google Scholar]
- 30.Murphy H R, Howgate C, O’Keefe J et al. Characteristics and outcomes of pregnant women with type 1 or type 2 diabetes: a 5-year national population-based cohort study. The lancet Diabetes & endocrinology. 2021 doi: 10.1016/s2213-8587(20)30406-x. [DOI] [PubMed] [Google Scholar]
- 31.Kerssen A, de Valk H W, Visser G H. Increased second trimester maternal glucose levels are related to extremely large-for-gestational-age infants in women with type 1 diabetes. Diabetes care. 2007;30:1069–1074. doi: 10.2337/dc05-1985. [DOI] [PubMed] [Google Scholar]
- 32.Herranz L, Pallardo L F, Hillman N et al. Maternal third trimester hyperglycaemic excursions predict large-for-gestational-age infants in type 1 diabetic pregnancy. Diabetes research and clinical practice. 2007;75:42–46. doi: 10.1016/j.diabres.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Dalfrà M G, Chilelli N C, Di Cianni G et al. Glucose Fluctuations during Gestation: An Additional Tool for Monitoring Pregnancy Complicated by Diabetes. International journal of endocrinology. 2013;2013:279021. doi: 10.1155/2013/279021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Law G R, Ellison G T, Secher A L et al. Analysis of Continuous Glucose Monitoring in Pregnant Women With Diabetes: Distinct Temporal Patterns of Glucose Associated With Large-for-Gestational-Age Infants. Diabetes care. 2015;38:1319–1325. doi: 10.2337/dc15-0070. [DOI] [PubMed] [Google Scholar]
- 35.Dalfrà M G, Sartore G, Di Cianni G et al. Glucose variability in diabetic pregnancy. Diabetes technology & therapeutics. 2011;13:853–859. doi: 10.1089/dia.2010.0145. [DOI] [PubMed] [Google Scholar]
- 36.McGrath R T, Glastras S J, Seeho S K et al. Association Between Glycemic Variability, HbA(1c), and Large-for-Gestational-Age Neonates in Women With Type 1 Diabetes. Diabetes care. 2017;40:e98–e100. doi: 10.2337/dc17-0626. [DOI] [PubMed] [Google Scholar]
- 37.Scott E M, Feig D S, Murphy H R et al. Continuous Glucose Monitoring in Pregnancy: Importance of Analyzing Temporal Profiles to Understand Clinical Outcomes. Diabetes care. 2020;43:1178–1184. doi: 10.2337/dc19-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kristensen K, Ögge L E, Sengpiel V et al. Continuous glucose monitoring in pregnant women with type 1 diabetes: an observational cohort study of 186 pregnancies. Diabetologia. 2019;62:1143–1153. doi: 10.1007/s00125-019-4850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta R, Khoury J, Altaye M et al. Glycemic Excursions in Type 1 Diabetes in Pregnancy: A Semiparametric Statistical Approach to Identify Sensitive Time Points during Gestation. Journal of diabetes research. 2017;2017:2.852913E6. doi: 10.1155/2017/2852913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerssen A, de Valk H W, Visser G H. Day-to-day glucose variability during pregnancy in women with Type 1 diabetes mellitus: glucose profiles measured with the Continuous Glucose Monitoring System. BJOG: an international journal of obstetrics and gynaecology. 2004;111:919–924. doi: 10.1111/j.1471-0528.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- 41.Rodbard D. Standardization versus customization of glucose reporting. Diabetes technology & therapeutics. 2013;15:439–443. doi: 10.1089/dia.2013.0116. [DOI] [PubMed] [Google Scholar]
- 42.Buschur ES B, Barbour L A.Diabetes In Pregnancy. 2018. In Endotext. Retrieved from URL:https://www.ncbi.nlm.nih.gov/books/NBK279010/Accessed 7-3-2022
- 43.Fraser R B, Waite S L, Wood K A et al. Impact of hyperglycemia on early embryo development and embryopathy: in vitro experiments using a mouse model. Human reproduction (Oxford, England) 2007;22:3059–3068. doi: 10.1093/humrep/dem318. [DOI] [PubMed] [Google Scholar]
- 44.Li R, Chase M, Jung S K et al. Hypoxic stress in diabetic pregnancy contributes to impaired embryo gene expression and defective development by inducing oxidative stress. American journal of physiology Endocrinology and metabolism. 2005;289:E591–E599. doi: 10.1152/ajpendo.00441.2004. [DOI] [PubMed] [Google Scholar]
- 45.Brown H M, Green E S, Tan TC Y et al. Periconception onset diabetes is associated with embryopathy and fetal growth retardation, reproductive tract hyperglycosylation and impaired immune adaptation to pregnancy. Scientific reports. 2018;8:2114. doi: 10.1038/s41598-018-19263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoffman R P. Sympathetic mechanisms of hypoglycemic counterregulation. Current diabetes reviews. 2007;3:185–193. doi: 10.2174/157339907781368995. [DOI] [PubMed] [Google Scholar]
- 47.Nepomnaschy P A, Welch K B, McConnell D S et al. Cortisol levels and very early pregnancy loss in humans. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3938–3942. doi: 10.1073/pnas.0511183103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu J, Kong S, Guo C et al. An exaggerated epinephrine-adrenergic receptor signaling impairs uterine decidualization in mice. Reproductive toxicology (Elmsford, NY) 2019;90:109–117. doi: 10.1016/j.reprotox.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Bartho L A, Holland O J, Moritz K M et al. Maternal corticosterone in the mouse alters oxidative stress markers, antioxidant function and mitochondrial content in placentas of female fetuses. The Journal of physiology. 2019;597:3053–3067. doi: 10.1113/jp277815. [DOI] [PubMed] [Google Scholar]
- 50.Simmons D. Adverse pregnancy outcomes in women with type 1 or type 2 diabetes. The lancet Diabetes & endocrinology. 2021 doi: 10.1016/s2213-8587(20)30427-7. [DOI] [PubMed] [Google Scholar]


