Abstract
Streptococcus pneumoniae (pneumococcus [Pn]) can be cultured from up to 50% of acute otitis media (AOM) effusions, and these bacteria are the most common cause of AOM-related complications. With the recent advent of antibiotic-resistant Pn strains, treatment of Pn infections may meet with serious difficulties. Prevention through vaccination, notably for the four most common occurring Pn serotypes in humans (i.e., Pn 6B, Pn 14, Pn 19F, and Pn 23F), is a helpful alternative. Testing of vaccine efficacy should occur in an appropriate animal AOM model, which is presented here. The four involved Pn serotypes are not pathogenic to the rat, which was chosen as the experimental animal for practical reasons. To induce a natural infection (i.e., ascending through the eustachian tube), the mucociliary clearance of the eustachian tube was impaired by infusing histamine into the tympanic cavity on 2 consecutive days before intranasal inoculation of the bacteria. With this simple protocol, high and reproducible infection rates, as determined with bacterial cultures, of Pn-induced AOM (approximately 70%) with the two major Pn serotypes 14 and 19F (Pn 14 and Pn 19F) were obtained, whereas lower infection rates (25 to 50%) with Pn 6B and Pn 23F were obtained. In this model, intranasal priming with pneumococci, as well as subcutaneous vaccination with Pn 14 tetanus toxoid-conjugated polysaccharide, induced a protective effect against the induction of otitis media with these bacteria. This shows that immunity to Pn 14 AOM can be induced by both mucosal and systemic presentations of antigen. In conclusion, we have developed an animal model for Pn-induced AOM, which is suitable for the evaluation of the protecting effect of immunization.
Acute otitis media (AOM) is characterized by acute inflammation of the middle ear and is associated with a concurrent or subsequent suppurative process in the middle ear (9). AOM is a common disease in preschool children. Review of epidemiological surveys on otitis media shows that 50 to 70% of children have experienced their first episode of otitis media before 2 or 3 years of age (11). Most cases of AOM resolve spontaneously in less than 3 or 4 weeks, but a selected population will develop recurrent and severe disease (2, 9). Possible complications include meningitis, lateral sinus thrombosis, chronic suppurative otitis media, and bacteremia (22, 23).
Streptococcus pneumoniae (pneumococcus [Pn]) can be isolated from up to 50% of AOM effusions (2) and is the most common cause of complications (23). Antibacterial treatment has shown good activity against pneumococcal AOM, but with the recent advent of antibiotic-resistant pneumococcal strains there is an increasing risk for serious and fatal infections (16, 23, 27). Preventive immunization against pneumococcal disease, especially in individuals at risk, may be preferred to treatment of existing infections. However, current licensed vaccines based on streptococcal capsular polysaccharides are poorly immunogenic in children under 2 years of age and in immunocompromised individuals (3). Therefore, new vaccines with increased immunogenicity, such as the tetanus toxoid-conjugated pneumococcal polysaccharide vaccine (24), are being constructed. There is a general need for an animal model that can test the efficacy of such vaccines and that can help to develop correlates of protection for Pn-induced diseases, particularly AOM. This animal model should meet the following conditions. (i) Infection should occur through a natural route, i.e., ascending from the nasopharynx through the eustachian tube to the middle ear, to enable determination of the effect of mucosal immunity in the eustachian tube. (ii) Minimal manipulation of the laboratory animal should facilitate induction of otitis media with the pneumococcal serotypes covering approximately 60% of Pn-induced middle ear infections in young children, i.e., Pn serotype 6B (Pn 6B), Pn 14, Pn 19F, and Pn 23F (4, 6). (iii) The laboratory animal of choice should be widely available and well characterized with respect to genetic, microbiologic, and immunologic determinants. Established clearance models for infectious otitis media employ direct instillation of bacteria into the tympanic cavity in rats or chinchillas (10, 15), thus avoiding the eustachian tube route. Other models combine infection with a respiratory virus (influenza virus A or adenovirus) and intranasal inoculation of bacteria (13, 21). These models do not suit our purpose since they were established in chinchillas, a species which doesn't meet the conditions mentioned above.
We developed a new animal model for Pn-induced otitis media in the rat. In this animal, we have employed intratympanic administration of histamine to impair ciliary activity in the tubotympanum and to induce mucosal swelling; this protocol prolongs the mucociliary clearance time from the tympanic cavity (7). The rationale for this protocol was that with malfunctioning of the mucociliary physiology of the eustachian tube there would be a decreased barrier for pneumococci to enter the tympanic cavity.
Practical employment of the protocol in evaluating protection against pneumococcal disease was tested in two different immunization schemes: intranasal priming with vital pneumococci and subcutaneous vaccination with Pn 14 tetanus toxoid-conjugated polysaccharide (PS14TT).
MATERIALS AND METHODS
Animals and experimental conditions.
Female specific pathogen-free rats of the Rivm:WU(CPB) strain, 6 weeks of age, were obtained from breeding facilities of the National Institute of Public Health and the Environment, Bilthoven, The Netherlands. During the experiments, animals were housed individually in Macolon III filter top cages, were given SSP-Tox standard diet (Hope Farms, Woerden, The Netherlands) and drinking water ad libitum, and were kept at a 12-h light–12-h dark regime. Disturbances were minimized. The appropriate number of experimental units was determined by using an analysis of statistical power (8). All procedures with the animals were performed under the supervision of the Institute's Council for Experiments on Animals, according to Dutch legislation.
Bacterial strains and culture.
Pn 19F and Pn 23F were obtained from the National Institute of Public Health and the Environment. Pn 6B (BG 7322) was obtained from A. Virolainen (University of Alabama). Pn 14 (IHU 30544) was a blood isolate from a Finnish patient. All serotypes were passed intraperitoneally through rats to increase virulence. Bacteria were isolated from peripheral blood after 18 h and stored in 20% glycerol at −70°C.
Pneumococci were serotyped by the Quellung reaction with antisera from the Statens Seruminstitut, Copenhagen, Denmark. Pneumococci were routinely grown overnight on blood agar plates (Oxoid, Haarlem, The Netherlands). Before inoculation, the bacteria were cultured at 37°C in 20 ml of Todd-Hewitt broth containing 2% inactivated horse serum and gently shaken (120 cycles/min). After 3 to 4 h, the bacteria reached log phase and were collected. After centrifugation at 3,500 rpm for 10 min, the supernatant was discarded. The pellet was resuspended in 10 ml of PBS, and the concentration of vital bacteria in the suspension was determined by culturing serial dilutions.
Experimental manipulations.
For invasive procedures, rats were anesthesized by intraperitoneal injection (0.18 ml/100 g of rat weight) with a mixture of ketamine (35 mg/ml; Kombivet, Etten-Leur, The Netherlands), xylazine (6 mg/ml; Bayer AG, Leverkusen, Germany), and atropine (0.1 mg/ml; Eurovet, Bladel, The Netherlands). Halothane anesthesia was used for short noninvasive procedures (e.g., intranasal instillation and diagnostic procedures).
Histamine (dihydrochloride; Sigma Chemical Co., St. Louis, Mo.) was administered through the tympanic membrane with a 30G1/2 needle under otoscopic surveillance. In the standard challenge procedure, 0.035 ml of a 10−4 or 10−3 M solution was given on 2 subsequent days before inoculation of bacteria. Suspensions of bacteria were prepared in a concentration of 0.7 × 109 to 3.8 × 109 CFU/ml, and 50 μl of such a suspension was inoculated intranasally with a Teflon infusion catheter. In pilot experiments, Pn 14 produced relatively high infection rates and was therefore selected for further optimization of the model. Standard evaluation was on postinfection day 6.
Protection assays.
Immunization was achieved in two ways. In the first protocol, animals were primed with a standard intranasal inoculation of homotypic bacteria 14 and 28 days before the standard challenge procedure, but without histamine. In the second protocol, animals were vaccinated subcutaneously with a vaccine preparation containing 0.1 μg of PS14TT per 0.5 ml of 0.1% (wt/vol) ALPO4 suspension (24). This vaccine was given 7 and 35 days before the standard challenge procedure.
Monitoring and evaluation.
Otomicroscopy and tympanometry were performed before experimental manipulation and at regular intervals (2 to 3 days) in the course of an experiment, to monitor the condition of the middle ear. Tympanometry was done with a GSI38 tympanometer (Lucas Grason-Stadler Inc., Milford, N.H.).
Experiments were typically terminated 6 days after inoculation of bacteria, by bleeding the animals under anesthesia. Tympanic bullae were rinsed, and bacterial culture of the lavages was performed on standard selective media for identification of intratympanic flora. These included sheep blood and chocolate agar, both also combined with clindamycin and Endo and negram agars. Suspected pneumococci isolates were serotyped, and only the confirmed presence of pneumococci in the middle ear was valued as final evidence for Pn-induced otitis media. Although otoscopy, tympanometry, and histopathology are indicative of otitis media, these parameters do not discriminate for the causative pathogenic agent. This is of importance since a potential source of bias is formed by symptoms of otitis media caused by contaminating flora. The infection rate determining the outcome of an experiment is defined as the percentage of animals in an experimental unit with a Pn-positive middle ear lavage.
Tympanic bullae were collected and processed according to standard histologic and electron microscopy (EM) procedures. Standard histologic procedures included 4% formalin fixation, decalcification in 10% EDTA solution, and paraffin embedding. Standard EM procedures included perfusion fixation with a phosphate-buffered fixative of 2% (wt/vol) paraformaldehyde and 2.5% (wt/vol) glutaraldehyde and decalcification in 5% EDTA in fixative and postfixation with 1% osmium tetroxide of selected tissue blocks, followed by dehydration and embedding in epoxy resin glycidether 100 (Merck). Ultrathin sections were prepared from selected areas, contrasted with aqueous uranyl acetate (2% [wt/vol]) and lead citrate (1% [wt/vol]), and examined in a Philips EM 201 operating at 60 kV.
Detection of immunoglobulin A (IgA)-positive cells was done on paraffin sections with an indirect immunoperoxidase procedure: a mouse anti-rat IgA monoclonal antibody (26) was detected with a peroxidase-labeled rat anti-mouse IgG (Jackson Laboratory). Peroxidase activity was visualized with 3,3′-diaminobenzidine as substrate. Cell nuclei were counterstained with hematoxilin. IgA-positive plasma cells in the mucosa of the tympanic bulla were counted with a light microscope and expressed as the number per area. Image processing and analysis were performed with IBAS (Kontron Elektronik, Munich, Germany). From each specimen, four serial sections (each 20 μm) were examined.
Anti-Pn 14-specific polysaccharide (PS14) and anti-cell wall polysaccharide (CPS) antibodies in serum were detected by enzyme-linked immunosorbent assay as described previously (24). Before analysis of anti-PS14 antibodies, sera were preincubated with 120 μg of CPSs (Statens Seruminstitut) per ml at 37°C for 30 min. All samples were serial diluted and tested in duplicate for antigen-specific IgG; the starting dilution was 1/10, and there were seven subsequent threefold serial dilutions. The spectrophotometry range was 0.050 (background) to 2.100. From the measured serial absorbances, a linear curve fit and 50% of the maximum and minimum optical density absorbances were calculated (KinetiCalc; Bio-Tek). These 50% absorbances were log10 converted to normalize distributions. From these log10 conversions, differences between values before and after immunization were calculated.
The presented results are the averages of n independent observations in immunized and sham-immunized groups. The statistical difference of ratios of otitis media between experimental groups was calculated with a Fisher's exact test. The statistical significance for differences of IgG titers between experimental groups was calculated with a one-tailed Student's t test.
RESULTS
Morphologic effects of intratympanic histamine.
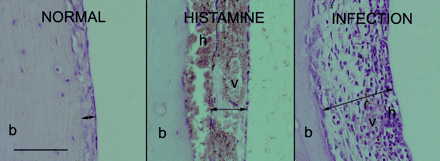
The effect of histamine on the histology of the tympanic cavity is characterized by hyperemia (vascular dilatation), intramucosal sanguineous exudation, and submucosal hemorrhage. Histamine-facilitated middle ear challenge with Pn 14 results in an inflammatory reaction, marked by an infiltration of polymorphonuclear leukocytes in the bullar mucosa (Fig. 1).
FIG. 1.
Representative illustrations of the mucosa of the tympanic cavity. Left, normal mucosa; middle, sub- and intramucosal sanguinous exudate (h) and dilated vessels (v) as a result of histamine treatment (24 and 48 h before intranasal mock infection); right, infiltrated mucosa, mainly with polymorphonucleated cells, as a result of middle ear infection (histamine 24 and 48 h before intranasal inoculation with Pn 14). Dilated vessels and hemorrhage are still visible. Tympanic bullae were prepared on day 6 after infection. Arrows, mucosa extension; b, bone of bulla wall. Bar = 0.2 mm.
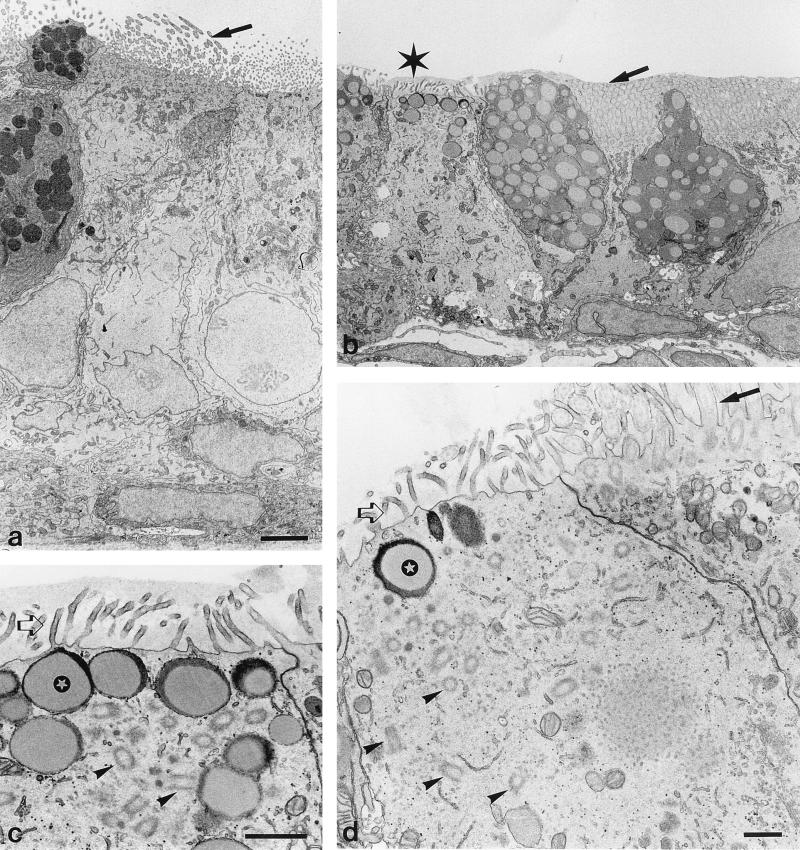
EM analysis of the epithelium of the eustachian tube shows that histamine causes epithelial cells to lose their columnar appearance (Fig. 2b), which is present in control animals (Fig. 2a). Some cells show loss of cilia, and in these cells, cilia-associated basal bodies lose their submembranous organization and are found scattered throughout the cytoplasm (Fig. 2c to d). There are mucous depositions on the apical membranes, even between cilia remnants, indicating loss of clearance function (Fig. 2b). These effects are more pronounced in the tympanic segment than in the nasopharyngeal segment of the eustachian tube.
FIG. 2.
Transmission EM of ciliated epithelium of the eustachian tube. (a) Sham-treated specimen. (b to d) Effects of histamine treatment. Histamine was administered through the tympanic membrane 48 and 24 h before sacrifice. Histamine induces loss of cilia (indicated by the asterisk in panel b; this area is magnified in panel c). Mucous deposition is present after histamine treatment over and between cilia (b) and microvilli (c); in panel b, this mucous deposition results in clumping of cilia (arrow). These depositions are not observed after sham treatment (arrow in panel a). Microvilli (open arrows) are present with (c) and without (d) mucus on epithelial cells which have lost their cilia. These cells show numerous basal bodies in a disordered manner (arrowheads). Normal apical organization of cilia-associated basal bodies is shown in a cell in panel d, which also shows normal cilia (arrow); this apparent normal cell resides adjacent to a pathologic epithelial cell. Below the apical plasma membrane of pathologic cells there are vacuoles filled with a mucous substance (indicated by the stars in panels c and d). Bars, 3 μm (a and b), 1 μm (c), and 0.5 μm (d).
Parameters determining the infection rate.
In a single dose, histamine is effective from 10−3 M (Table 1). Repeated dosage is effective from 10−4 M; lower concentrations were not tested in the repeated dosage scheme. A repeated dosage of 10−4 or 10−3 M on 2 subsequent days before intranasal administration of pneumococci proved to be adequate for a standard challenge procedure.
TABLE 1.
Parameters determining the infection rate after middle ear challenge with histamine and Pn
| Characteristic | Infection rate (%)a
|
|
|---|---|---|
| Single administration | Repeated administration | |
| Histamine concn (M) | ||
| 0 | 0.0 | 0.0 |
| 10−5 | 33.3 | |
| 10−4 | 16.6 | 73.9bc |
| 10−3 | 50.0 | 50.0 |
| 10−2 | 50.0 | 66.7 |
| Pn 14 concn (log CFU) | ||
| 4 | 0.0 | |
| 5 | 66.7 | |
| 6 | 66.7 | |
| 7 | 66.7 | |
| 8 | 73.9c | |
| No. of days after challenge | ||
| 6 | 73.9 | |
| 9 | 66.7 | |
| 12 | 16.7c | |
| 15 | 33.3 | |
| 18 | 16.7d | |
| Pn serotype | ||
| 6B | 41.7e | |
| 14 | 73.9 | |
| 19F | 50.0 | |
| 23F | 33.3 | |
All infection rates represent percentages of animals with Pn-positive middle ear lavages in separate groups with n being 6, unless otherwise indicated. Also, unless indicated otherwise, these results were obtained on day 6 after standard challenge procedure with Pn 14.
A repeated dosage of 10−4 M histamine is significantly more effective than a single dose (P < 0.05), as calculated by a Fisher's exact test.
For this group, n was 24.
The r2 for linear correlation is 0.72; the value of P is 0.0029; both were calculated over the total measurement range of 6 to 18 days.
For this group, n was 12.
With Pn 14, infection rates are not dependent on the concentration of the intranasally instilled suspension in a range of 105 to 108 CFU (Table 1); a lower concentration is ineffective. In the standard procedure, presence of bacteria in the tympanic cavity was evaluated 6 days after challenge. Thereafter, the infection rate declines. This clearance of the infection is time dependent (Table 1).
With the standard challenge procedure, middle ear infections are obtained with all four tested Pn serotypes, Pn 6B, Pn 14, Pn 19F, and Pn 23F (Table 1), although there may be differences in virulence (not tested).
In the absence of a competitive quantity of virulent bacteria, histamine treatment produces an opportunity to other (resident) flora to enter the tympanic cavity. With the standard challenge procedure, there is a high and significant inverse correlation (r2 = 0.63) between the infection rates for Pn 14 and for other flora (Table 2). This inverse correlation is also obvious between infection rates with other Pn serotypes and opportunistic flora (data not shown). Occasionally, there were concomitant infections of Pn and contaminating flora, but in these cases, middle ear lavages contained only low concentrations of vital pneumococci, as indicated by low-density colony growth in culture.
TABLE 2.
Inverse correlation between infection rates of Pn 14 and contaminating floraa
| Type of flora | Infection rate (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pn 14 | 83.3 | 66.7 | 66.7 | 66.7 | 66.7 | 50.0 | 33.3 | 33.3 | 16.7 | 0.0 |
| Contaminating flora | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 16.7 | 50.0 | 50.0 | 16.7 | 50.0 |
Infection rates are defined as in Table 1. Results were obtained on day 6 after standard challenge procedure with Pn 14. All pairs of Pn 14 and contaminating flora infection rates represent the results of a separate experiment. All groups received an identical standard histamine treatment; other conditions varied (e.g., dosage of pneumococci and intranasal priming with pneumococci). The r2 for linear correlation is 0.63; the value of P is 0.0099.
Employing the otitis media model in protection assays.
The value of this model as a read-out tool for protection after vaccination was tested in two immunization protocols. In the first protocol, the animals were intranasally primed with homotypic bacteria before the standard challenge procedure with histamine and Pn 14. This priming protocol resulted in a reproducible and significant decrease of infection rate (Table 3). Sera of these animals, drawn at the time of challenge (day 28), showed a moderate but significant increase in anti-CPS titers compared to those of sham-primed animals (Table 3), which continued to increase until the time of section (day 33). However, no anti-PS14 IgG responses were found in sera of these rats (Table 3). Immunochemical analysis of bullae of three randomly selected animals from both groups showed significantly higher numbers of IgA-positive cells in the mucosa of primed animals than in that of sham-primed animals (Table 3).
TABLE 3.
Effect of priming with homotypic pneumococci on standard middle ear challenge with histamine and Pn 14 (infection rate and immunologic parameters)
| Infection rate or parameter | PBS (sham) | Pn 14 | n (both groups) |
|---|---|---|---|
| Infection rate | 73.9% | 25.0% | 24 |
| Serum α Pn 14 IgG (days 28 and 33)a | 0.0 ± 0.0 | 0.0 ± 0.0 | 18 |
| Serum α CPS IgG (day 28)a | −0.03 ± 0.03 | 0.31 ± 0.10d | 18 |
| Serum α CPS IgG (day 33)a | −0.02 ± 0.07 | 0.58 ± 0.17d | 18 |
| IgA plasma cells/mm2 of mucosab | 5.3 ± 0.2 | 12.9 ± 1.4d | 3 |
Days 28 and 33 represent the change in value at the time of challenge and of section, respectively. IgG serum titers are the means of differences ± standard errors of the mean between the values before (day 0) and after priming (days 28 and 33); nonresponders are included. IgG titers are presented as log10 dilution at 50% maximum optical density.
For IgA immunochemistry, bullae from three randomly chosen animals from each group were prepared on day 6 after challenge.
Significantly different (P < 0.05) from values in sham-treated groups as calculated by a Fisher's exact test.
Significantly different (P < 0.05) from values in sham-treated groups as calculated by a Student's t test.
The second protocol included subcutaneous vaccination with PS14TT before standard challenge procedure with histamine and Pn 14. This protocol also results in a significant reduction of the middle ear infection rate compared to that in sham-vaccinated animals (Table 4), associated with a high and significant increase in anti-PS 14 specific IgG in the serum, measured at the moment of the booster vaccination (Table 4). Anti-CPS titers in the serum showed a small and not significant increase in the vaccinated animals (Table 4).
TABLE 4.
Effect of vaccination with PS14TT vaccine on standard middle ear challenge with histamine and Pn 14 (infection rate and Pn-specific serum IgG titers)
| Infection rate or IgG titer | PBS (sham) | PS14TT | n (both groups) |
|---|---|---|---|
| Infection rate | 72.2% | 27.8%b | 24 |
| α PS 14 IgGa | 0.0 ± 0.0 | 1.87 ± 0.17c | 18 |
| α CPS IgGa | −0.13 ± 0.07 | 0.26 ± 0.20c | 18 |
IgG serum titers are the means of differences ± standard errors of the means between the values before (day 0) and after vaccination (day 35); nonresponders are included. IgG titers are presented as log10 dilution at 50% maximum optical density.
Significantly different (P < 0.05) from values in sham-treated groups as calculated by a Fisher's exact test.
Significantly different (P < 0.05) from values in sham-treated groups as calculated by a Student's t test.
DISCUSSION
Histamine pretreatment protocol as model for human disease.
Histamine is one of the many inflammatory mediators found in middle ear effusions associated with otitis media (19). This histamine may be generated by degranulation of mast cells in local mucoperiosteum (1), e.g., as an inflammatory response triggered by viral infections (9); in patients with allergic conditions, there is an association between otitis media and histamine release from peripheral blood basophils (9). In vitro, histamine can regulate ciliary activity of eustachian tube and middle ear mucosa (7). In guinea pigs, intratympanic injection of histamine, with a similar dose as used in this study, induces the accumulation of middle ear effusion (7). Histamine also contributes to dysfunction of the eustachian tube by affecting the microcirculation and vascular permeability in its mucosa (5, 20). Dysfunction of the eustachian tube after administration of histamine is indicated by an increased mucociliary clearance time (7) and increased perfusion pressure and opening pressure (19). These findings are also relevant for our model, as indicated by the EM observation of structural (Fig. 2b to d) and functional loss of epithelial cilia in the eustachian tube and the light microscopical observation of mucosa pathology in the tympanic cavity (Fig. 1). Combined, these observations indicate that histamine can optimize conditions for bacterial passage through the eustachian tube, and this factor may therefore be a major mediator in the pathogenesis of bacterial otitis media. We used a concentration of histamine that was only 10 to 100 times higher than that detected in otitis media effusions (1), and therefore, this protocol may well mimic a major step in the natural pathogenesis of otitis media.
The histamine pretreatment protocol facilitates the ascending infection of pneumococci from the nasopharyngeal cavity to the middle ear, thus offering an appropriate model for testing acquired immunity of the eustachian tube and middle ear mucosa to these bacteria after vaccination. For this reason, this model is preferred to common clearance models employing direct inoculation of bacteria in the middle ear cavity, thus circumventing mucosal immunity in the eustachian tube (12, 14, 18, 25). Other models employ obstruction of the eustachian tube (17) or coinfection of bacteria with influenza virus A or adenovirus, the latter in chinchillas, to obtain colonization of the tympanic cavity through the eustachian tube (10, 21). Compared to most of these approaches, histamine pretreatment is a very controllable protocol, facilitating Pn-induced otitis media to a relative high rate.
Employing the model in immunization assays.
We hypothesized that a model employing entry of bacteria through the eustachian tube would enable evaluation of immunity. Indeed, both after priming of the intranasal mucosa with homotypic pneumococci and after subcutaneous immunization with a PS14TT vaccine, there were significant reductions in infection rates with pneumococci (Tables 3 and 4). These reductions were associated with mucosal and systemic immunologic responses. The low to moderate antibody responses after intranasal exposure to pneumococci point to local mucosal immunity as the effector of the reduced infection rate. This is supported by the increased number of IgA-positive plasma cells in the mucosa of the tympanic cavity. On the contrary, the high anti-PS14 titers obtained after subcutaneous vaccination indicate systemic immunity as an effector in this case.
Histamine-facilitated induction of bacterial otitis media is thus validated as a tool for the evaluation of protecting vaccines.
ACKNOWLEDGMENTS
We thank P. W. Wester for his critical support, B. J. van Middelaar, D. J. Elberts, and C. Moolenbeek for their skilled assistance in the animal facilities, L. A. Oomen for the enzyme-linked immunosorbent assays, J. Venema, H. C. W. Thuis, and R. Boot for their contributions with the bacteriological analysis, and G. Riool and S. de Waal for technical assistance with the EM.
REFERENCES
- 1.Berger G, Hawke M, Proops D W, Ranadive N S, Wong D. Histamine levels in middle ear effusions. In: Lim D, Bluestone C, Klein J, Nelson J, editors. Recent advances in otitis media with effusion. B. C. Philadelphia, Pa: Decker Inc.; 1984. pp. 195–198. [Google Scholar]
- 2.Block S L, Harrison C J, Hedrick J A, Tyler R D, Smith R A, Keegan E, Chartrand S A. Penicillin-resistant Streptococcus pneumoniae in acute otitis media: risk factors, susceptibility patterns and antimicrobial management. Pediatr Infect Dis J. 1995;14:751–759. doi: 10.1097/00006454-199509000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Bruyn G A, van Furth R. Pneumococcal polysaccharide vaccines: indications, efficacy and recommendations. Eur J Clin Microbiol Infect Dis. 1991;10:897–910. doi: 10.1007/BF02005442. [DOI] [PubMed] [Google Scholar]
- 4.Butler J C, Breiman R F, Lipman H B, Hofmann J, Facklam R R. Serotype distribution of Streptococcus pneumoniae infections among preschool children in the United States, 1978-1994: implications for development of a conjugate vaccine. J Infect Dis. 1995;171:885–889. doi: 10.1093/infdis/171.4.885. [DOI] [PubMed] [Google Scholar]
- 5.Chan K H, Swarts J D, Tan L. Middle ear mucosal inflammation: an in vivo model. Laryngoscope. 1994;104:970–980. doi: 10.1288/00005537-199408000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Douglas R M, Paton J C, Duncan S J, Hansman D J. Antibody response to pneumococcal vaccination in children younger than five years of age. J Infect Dis. 1983;148:131–137. doi: 10.1093/infdis/148.1.131. [DOI] [PubMed] [Google Scholar]
- 7.Esaki Y, Ohashi Y, Furuya H, Sugiura Y, Ohno Y, Okamoto H, Nakai Y. Histamine-induced mucociliary dysfunction and otitis media with effusion. Acta Oto-laryngol Suppl Stockh. 1991;486:116–134. doi: 10.3109/00016489109134990. [DOI] [PubMed] [Google Scholar]
- 8.Festing M F W. Experimental design. In: de Boer J, Archibald J, Downie H G, editors. An introduction to experimental surgery—a guide to experimenting with laboratory animals. Amsterdam, The Netherlands: Excerpta Medica; 1975. pp. 5–45. [Google Scholar]
- 9.Fireman P. Otitis media and eustachian tube dysfunction: connection to allergic rhinitis. J Allergy Clin Immunol. 1997;99:S787–S797. doi: 10.1016/s0091-6749(97)70130-1. [DOI] [PubMed] [Google Scholar]
- 10.Giebink G S. The pathogenesis of pneumococcal otitis media in chinchillas and the efficacy of vaccination in prophylaxis. Rev Infect Dis. 1981;3:342–353. doi: 10.1093/clinids/3.2.342. [DOI] [PubMed] [Google Scholar]
- 11.Giebink G S. Epidemiology and natural history of otitis media. In: Lim D, Bluestone C, Klein J, Nelson J, editors. Recent advances in otitis media with effusion. B.C. Philadelphia, Pa: Decker Inc.; 1984. pp. 5–9. [Google Scholar]
- 12.Giebink G S, Koskela M, Vella P P, Harris M, Le C T. Pneumococcal capsular polysaccharide-meningococcal outer membrane protein complex conjugate vaccines: immunogenicity and efficacy in experimental pneumococcal otitis media. J Infect Dis. 1993;167:347–355. doi: 10.1093/infdis/167.2.347. [DOI] [PubMed] [Google Scholar]
- 13.Giebink G S, Ripley M L, Shea D A, Wright P F, Paparella M M. Clinical-histopathological correlations in experimental otitis media: implications for silent otitis media in humans. Pediatr Res. 1985;19:389–396. doi: 10.1203/00006450-198519040-00015. [DOI] [PubMed] [Google Scholar]
- 14.Green B A, Vazquez M E, Zlotnick G W, Quigley Reape G, Swarts J D, Green I, Cowell J L, Bluestone C D, Doyle W J. Evaluation of mixtures of purified Haemophilus influenzae outer membrane proteins in protection against challenge with nontypeable H. influenzae in the chinchilla otitis media model. Infect Immun. 1993;61:1950–1957. doi: 10.1128/iai.61.5.1950-1957.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hermansson A, Emgard P, Prellner K, Hellström S. A rat model for pneumococcal otitis media. Am J Otolaryngol. 1988;9:97–101. doi: 10.1016/s0196-0709(88)80013-9. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan S L. The emergence of resistant pneumococcus as a pathogen in childhood upper respiratory tract infections. Semin Respir Infect. 1995;10:31–36. [PubMed] [Google Scholar]
- 17.Kuijpers W, van der Beek J M. The role of microorganisms in experimental eustachian tube obstruction. Acta Oto-laryngol Suppl Stockh. 1984;414:58–66. doi: 10.3109/00016488409122883. [DOI] [PubMed] [Google Scholar]
- 18.Magnuson K, Hermansson A, Melhus A, Hellström S. The tympanic membrane and middle ear mucosa during non-typeable Haemophilus influenzae and Haemophilus influenzae type b acute otitis media: a study in the rat. Acta Oto-laryngol Stockh. 1997;117:396–405. doi: 10.3109/00016489709113412. [DOI] [PubMed] [Google Scholar]
- 19.Minami T, Kubo N, Tomoda K, Kumazawa T. Effects of various inflammatory mediators on eustachian tube patency. Acta Oto-laryngol Stockh. 1992;112:680–685. doi: 10.3109/00016489209137459. [DOI] [PubMed] [Google Scholar]
- 20.Minami T, Kubo N, Tomoda K, Yamashita T, Kumazawa T. Regional blood flow volume in the eustachian tube. Acta Oto-laryngol Suppl Stockh. 1993;500:80–83. doi: 10.3109/00016489309126186. [DOI] [PubMed] [Google Scholar]
- 21.Miyamoto N, Bakaletz L O. Kinetics of the ascension of NTHi from the nasopharynx to the middle ear coincident with adenovirus-induced compromise in the chinchilla. Microb Pathog. 1997;23:119–126. doi: 10.1006/mpat.1997.0140. [DOI] [PubMed] [Google Scholar]
- 22.Ottolini M G, Ascher D P, Cieslak T J, Modica Lucero S. Pneumococcal bacteremia during oral treatment with cefixime for otitis media. Pediatr Infect Dis J. 1991;10:467–468. doi: 10.1097/00006454-199106000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Poole M D. Otitis media complications and treatment failures: implications of pneumococcal resistance. Pediatr Infect Dis J. 1995;14:S23–S26. doi: 10.1097/00006454-199504001-00005. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez M E, van den Dobbelsteen G P J M, Oomen L A, de Weers O, van Buren L, Beurret M, Poolman J, Hoogerhout P. Immunogenicity of Streptococcus pneumoniae type 6B and 14 polysaccharide-tetanus toxoid conjugates and the effect of uncoupled polysaccharide on the antigen-specific immune response. Vaccine. 1998;16:1941–1949. doi: 10.1016/s0264-410x(98)00129-7. [DOI] [PubMed] [Google Scholar]
- 25.Svinhufvud M, Hermansson A, Prellner K. Active immunisation and resistance to experimental acute pneumococcal otitis media. Int J Pediatr Otorhinolaryngol. 1993;25:91–103. doi: 10.1016/0165-5876(93)90013-s. [DOI] [PubMed] [Google Scholar]
- 26.van Loveren H, Osterhaus A D, Nagel J, Schuurman H J, Vos J G. Detection of IgA antibodies and quantification of IgA antibody-producing cells specific for ovalbumin or Trichinella spiralis in the rat. Scand J Immunol. 1988;28:377–381. doi: 10.1111/j.1365-3083.1988.tb01463.x. [DOI] [PubMed] [Google Scholar]
- 27.Zenni M K, Cheatham S H, Thompson J M, Reed G W, Batson A B, Palmer P S, Holland K L, Edwards K M. Streptococcus pneumoniae colonization in the young child: association with otitis media and resistance to penicillin. J Pediatr. 1995;127:533–537. doi: 10.1016/s0022-3476(95)70108-7. [DOI] [PubMed] [Google Scholar]