Dear Editor,
We read with interest the article of Marra et al,1 who evaluated the short-term effectiveness of the COVID-19 mRNA vaccine in immunocompromised patients. They found that a reduced immune response to the COVID-19 mRNA vaccine was present in patients with solid organ transplant and malignant diseases compared to controls. Patients with solid malignancies are often immunocompromised, so we will further explore serological response to the COVID-19 vaccine in patients with solid malignancies.
At present, the latest data show that the safety and effectiveness of COVID-19 vaccine have been fully verified in the general population.2 but evidence regarding its performance is insufficient in cancer patients, as cancer patients are mostly excluded in vaccine trials. Patients with haematological malignancies are less immunogenic to the COVID-19 vaccine than patients with solid malignancies.3 However, relevant studies on the effectiveness of the COVID-19 vaccine in patients with solid malignancies are still limited, and most of them are small samples. Despite the growing immunogenicity data on patients with solid malignancies, the assessment of serological response to COVID-19 vaccine in patients with solid malignancies receiving active treatment is not completely conclusive.
To gain a more definitive understanding of the effect of COVID-19 vaccine in patients with solid malignancies. Here, we aimed to conduct this meta-analysis to integrate findings to determine the serological response to COVID-19 vaccine in patients with solid malignancies and the predictors of poor seroconversion.
The study was reported according to PRISMA and MOOSE guidelines and has been registered with PROSPERO (CRD42022359242). PubMed, Cochrane Library and EMBASE were searched from inception to 13 September 2022 using medical subject headings terms or keywords related to “Cancer”, “COVID-19 vaccine”, “Cohort study”, “Prospective study”. The inclusion criteria were as follows: (1) The study was a prospective or retrospective cohort study; (2) Studies have evaluated the serological response of patients with solid malignancies to COVID-19 vaccine and the predictors of poor seroconversion; (3) Corresponding RRs and 95% confidence intervals (CIs) are available.
Literature quality was assessed according to the Newcastle Ottawa scale (Supplementary Material 1). The main outcome was the serological response rate of patients with solid malignancies to COVID-19 vaccine (the first dose and the second dose). The secondary outcome was the effect of age, sex, metastasis, chemotherapy, immunotherapy, targeted therapy and endocrine therapy on the serological response rate after COVID-19 vaccine. Revman (version 5.3.3; the Cochrane Collaboration) were selected for analysis.
A total of 16 prospective cohort studies with 4274 patients with solid malignancies were included (Table 1 ; Supplementary Material 2). Cohorts were of European (including Italy, Britain, France) and Asian (including Turkey, Israel, Iran) origin.
Table 1.
Baseline characteristics and outcomes of the 16 cohorts included in the meta-analysis.
| First author | Year | Country | Patient numbers and description | Vaccine dose | Vaccine type | Age (years) |
Sex (Male%) |
Risk factors |
|---|---|---|---|---|---|---|---|---|
| Amatu | 2022 | Italy | 171 solid malignancies (Colorectal 24.6%, Breast 21.1%, Non-small cell lung 15.8%, Ovarian 7.6%, Pancreatic 7.0%, Stomach 7.0%, Others 16.9%) | Second | BNT162b2/ mRNA-1273 | 68 (58–73) | 41% | 1,2,3,5,6,7,8 |
| Cavanna | 2021 | Italy | 257 solid malignancies (Gastrointestinal 26.1%, Breast 27.2%, Lung 13.2%, Gynaecological 9.7%, Other 23.7%) | Second | BNT162b2/ mRNA-1273 | 65 (57–72) | 44% | 1,2,3,5,6,7 |
| Di Noia | 2021 | Italy | 816 solid malignancies (Breast 30.6%, Lung 20.6%, Melanoma 14.7%, Gastrointestinal 8.6%, Gynecologic 5.6%, Genitourinary 10.9%, Sarcoma 6.6%, Head-neck 1.1%, Cerebral 0.4%, NE tumor 0.9%) | Second | BNT162b2 | 62 (21–97) | 41% | 1,6,7 |
| Erdoğan | 2022 | Turkey | 218 solid malignancies (Head/neck 2.8%, Gastrointestinal 21.1%, Genitourinary 16.5%, Breast 46.8%, Thoracic cavity 6.0%, Rare tumors and others 6.9%) | Third | CoronaVac/ BNT162b2 |
57.6±11.5 | 32% | 1,2,3 |
| Fendler | 2021 | Britain | 446 solid malignancies (Genitourinary 21%, Skin 20%, Gastrointestinal 19%, Thoracic 14%, Breast 12%, Gynaecological 6%, Head and Neck 3%, Other 5%) | Second | AZD1222/ BNT162b2 | 60 (52–68) | 60% | 1 |
| Fenioux | 2022 | France | 163 solid malignancies (Digestive 41%, Urologic 25%, Breast 31%, Others 3%) | Third | BNT162b2 | 66 (27–89) | 53% | 1,2,3,5 |
| Goshen-Lago | 2021 | Israel | 232 solid malignancies (Gastrointestinal 27%, Breast 18%, Genitourinary 21%, Gynecologic 5%, Head and neck 5%, Lung 19%, Melanoma 2%, Neurologic 2%, Sarcoma 1%) | Second | BNT162b2 | 68 (25-88) | 57% | 1,3,5,6,7 |
| Gounant | 2022 | France | 306 solid malignancies (Lung Non-SCC 68.9%, Lung SCC 16%, Lung NSCLC 84.9%, Lung SCLC 7.2%, Pleural mesothelioma 4.2%, Others 3.5%) | Second | BNT162b2/ mRNA-1273/ AZD1222 |
67 (27–92) | 59% | 1,2,3,45 |
| Margalit | 2022 | Iran | 93 solid malignancies (Gastrointestinal 43.0%, Breast 24.7%, Lung 9.7%, Melanoma 10.8%, Genitourinary 7.5%, Others 4.3%) | Second | BNT162b2 | 60.8±12.5 | 40% | 1 |
| Massarweh | 2022 | Israel | 17 solid malignancies (Glioblastoma 76%, Anaplastic astrocytoma 12%, Oligodendroglioma 6%, Atypical meningioma 6%) | Second | BNT162b2 | 65 (58–71) | 65% | 1 |
| Monin | 2021 | Britain | 95 solid malignancies (gynaecological, breast 35%, Urological cancers 16%, Skin cancers 13%, Thoracic malignancies 22%, Gastrointestinal cancers 13%, Head and neck cancer 1%, Glioblastoma 1%) | Second | BNT162b2 | 73.0(64.5–79.5) | 52% | 1 |
| Palich | 2021 | France | 110 solid malignancies (Breast 34%, Lung 14%, Gynecological 14%, Prostate 10%, Digestive 7.3%, Kidney 6.4%, Bladder 4.5%, Upper aero-digestive tract 5.5%, Thyroid 4.5%, Others 2.7%) | One | BNT162b2 | 66 (54-74) | 48% | 1,2,5 |
| Shmueli | 2021 | Israel | 129 solid malignancies (Gastrointestinal 42.6%, Breast 20.2%, Lung 14.7% Melanoma 10.9%, Genitourinary 7.8%, Others 3.9%) | Second | BNT162b2 | 62.4±12.8 | 48% | 1 |
| Waldhorn | 2021 | Israel | 154 solid malignancies (Gastrointestinal 36%, Lung 23%, Breast 17%, Genitourinary 12%, Head and neck 3%, Gynecologic 3%, Neurologic 2%, Melanoma 1%, Sarcoma 1%, Unknown primary 1%) | Second | BNT162b2 | 67 (32–87) | 55% | 1,3,4,5,6,7 |
| Webber | 2021 | Italy | 291 solid malignancies (Digestive 34.0%, Lung 10.3%, Breast 24.8%, Genitourinary and gynaecologic 27.1%, Others 3.8%) | Second | BNT162b2 | 68.2 (59.7–75.0) | 41% | 1,4,5,6,7,8 |
| Yasin | 2022 | Turkey | 776 solid malignancies (Breast 32.3%, Gastrointestinal 22.4%, Genitourinary 13.8%, Lung 23.6%, Others 7.9%) | Second | CoronaVac | 64 (20–88) | 44% | 1,2,3,4,5,7 |
1. Rate of serologic response; 2. Age; 3. Sex; 4. Metastasis; 5. Chemotherapy; 6. Immunotherapy; 7. Targeted therapy; 8. Endocrine therapy.
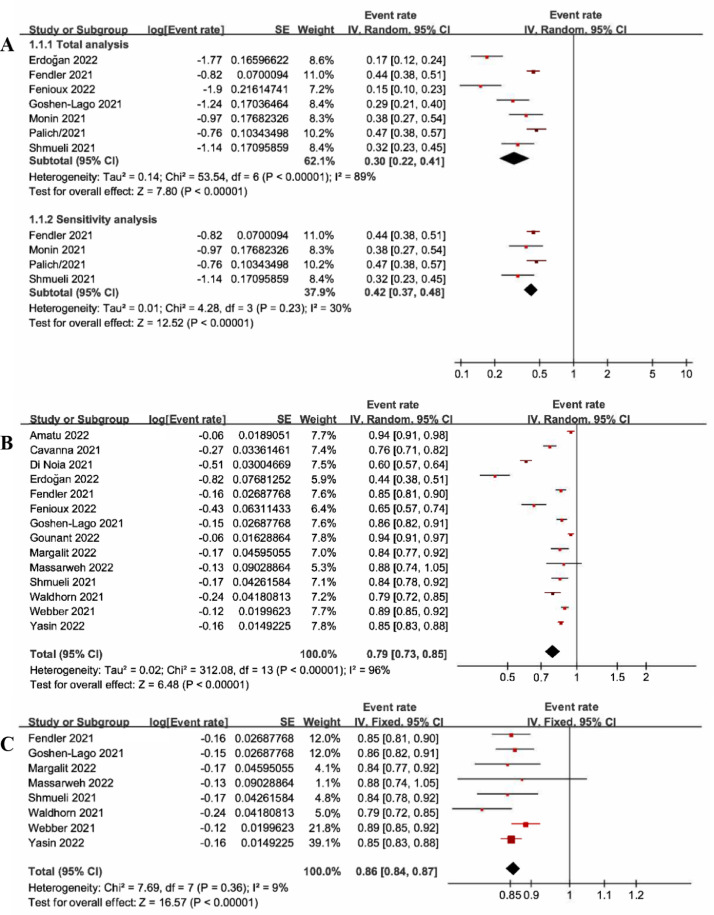
The meta-analysis indicated that the pooled proportion of patients with solid malignancies achieving a serological response after one dose of COVID-19 vaccine was (30%, 95%CI: 22%-41%, P<0.01) (Fig. 1 A). sensitivity analysis was performed to exclude the heterogeneity and the pooled results were (42%, 95%CI: 37%-48%, P<0.01). According to the subgroup analysis of vaccine type, the serological response rates were CoronaVac combined with BNT162b2 (17.0%), BNT162b2 (31.0%), and AZD1222 combined with BNT162b2 (44.0%), respectively. (Supplementary Material 3A).
Fig. 1.
A. Forest plot of serological response after one dose of vaccine; B. Forest plot of serological response after two doses of vaccine; C. Sensitivity analysis of serological response after two doses of vaccine.
The meta-analysis indicated that the pooled proportion of patients with solid malignancies after two doses of COVID-19 vaccine achieving a serological response was (79%, 95%CI:73%-85%, P<0.01) (Fig. 1B-1C). sensitivity analysis was performed to exclude heterogeneity and the pooled results were (86%, 95%CI:84%-87%, P<0.01). According to the subgroup analysis of vaccine type, the serological response rates were CoronaVac combined with BNT162b2 (44.0%), BNT162b2 combined with mRNA-1273 (85.0%), BNT162b2 (79.0%), AZD1222 combined with BNT162b2 (85.0%), CoronaVac (85.0%), and BNT162b2 as well as mRNA-1273 combined with AZD1222 (94.0%), respectively. (Supplementary Material 3B).
The meta-analysis indicated that age (RR=0.96, 95%CI: 0.94-0.98, P<0.01), chemotherapy (RR=0.34, 95%CI: 0.24-0.50, P<0.01), endocrine therapy (RR=0.15, 95%CI: 0.03-0.86, P=0.03) reduced the serological response rate to COVID-19 vaccine, while no significant association was observed between sex, immunotherapy, targeted therapy and metastatic disease. (Supplementary Material 4).
In this study, we found that only 42% of patients with solid malignancies achieved serological response to one dose of COVID-19 vaccine, which rose to 86% after two doses. In terms of cancer treatment strategies, we also found that chemotherapy and endocrine therapy were negatively correlated with the seroconversion to the COVID-19 vaccine.
A previous study4 found that the serological response rate of cancer patients under 60 years old after receiving two doses of COVID-19 vaccine was 84.1%, while that of patients over 60 years old was 59.3%, which was similar to our results. Our study found that with the increase of age (RR=0.96, 95%CI: 0.94-0.98, P<0.01), the serological response rate of patients with solid malignancies gradually decreased. This may be related to the poor general condition of elderly cancer patients, because elderly cancer patients are usually accompanied by complications. In addition, the unresponsiveness of the immune system in cancer patients of advanced age has also contributed to their greater risk of death after COVID-19 infection.5
Due to the influence of the immunosuppressive characteristics of cancer, cancer patients usually have low immune function.6 Therefore, cancer patients are a highly susceptible population and a vulnerable group for priority vaccination. It is unclear whether it is better for cancer patients to continue or start active treatment when receiving COVID-19 vaccine. We sought to identify predictors of poor seroconversion in patients with solid malignancies. Our results show that chemotherapy and endocrine therapy can affect the seroconversion rate of COVID-19 vaccine. Chemotherapy may lead to short-term depletion of circulating lymphocytes of major subtypes and long-term depletion of B and CD4+T cells, thus increasing the risk of infection and reducing the serological response to vaccine.7 But the results of endocrine therapy were contrary to recent findings,8 which may be related to the immunosuppressive mechanism of hormone deficiency, as CDK4/6 inhibitors did not have an additive effect. Since estrogen enhances the immune system and steroids have been associated with differences in the immune response to COVID-19, some endocrine therapies may induce immunosenescence in this population.9 , 10
There are still some limitations in our study. The type of vaccine used by most articles was BNT162b2, and other types of vaccines still need further in-depth study. The number of included articles in part of the studies was small, which may cause some differences in the results. Finally, due to the limited included studies, the serological response rate to the COVID-19 vaccine may differ among different types of solid malignancies.
In summary, patients with solid malignancies have a reduced serological response to COVID-19 vaccine. Chemotherapy and endocrine therapy may affect serological response to COVID-19 vaccine in patients with solid malignancies. Therefore, safety measures still need to be followed after vaccination in patients with solid malignancies, and actively treated patients require an adapted vaccination strategy.
Authors’ contributions
D.C. designed and wrote the manuscript, Y.C.P., Y.W., X.C. collected and analyzed data, J.H.Z., T.J.D., Z.Z.Y., T.C.L., Y.Y.Z., J.Y.W. performed article selection, and manuscript review and revision, W.J.K., Y.T.Z., H.M.L., X.H.Y, G.W.Y. participated in the interpretation of the results, D.C., G.L.Z., X.M.W conceived of the study, assisted with analysis, and revised manuscript. D.C., G.L.Z., X.M.W is the guarantor of this work and, as such, takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This work was supported by the National Natural Science Foundation of China (82074182, 82174454, 82274599).
Declaration of Competing Interests
The authors declare that they have no competing interests.
Acknowledgements
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2022.11.012.
Appendix. Supplementary materials
References
- 1.Marra A.R., Kobayashi T., Suzuki H., Alsuhaibani M., Tofaneto B.M., Bariani L.M., et al. Short-term effectiveness of COVID-19 vaccines in immunocompromised patients: A systematic literature review and meta-analysis. J Infect. 2022;84(3):297–310. doi: 10.1016/j.jinf.2021.12.035. PubMed PMID: 34982962. Pubmed Central PMCID: PMC8720049. Epub 2022/01/05.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. PubMed PMID: 33301246. Pubmed Central PMCID: PMC7745181. Epub 2020/12/11.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghione P., Gu J.J., Attwood K., Torka P., Goel S., Sundaram S., et al. Impaired humoral responses to COVID-19 vaccination in patients with lymphoma receiving B-cell-directed therapies. Blood. 2021;138(9):811–814. doi: 10.1182/blood.2021012443. PubMed PMID: 34189565. Pubmed Central PMCID: PMC8245303. Epub 2021/07/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ariamanesh M., Porouhan P., PeyroShabany B., Fazilat-Panah D., Dehghani M., Nabavifard M., et al. Immunogenicity and Safety of the Inactivated SARS-CoV-2 Vaccine (BBIBP-CorV) in Patients with Malignancy. Cancer Invest. 2022;40(1):26–34. doi: 10.1080/07357907.2021.1992420. PubMed PMID: 34634986. Pubmed Central PMCID: PMC8567287. Epub 2021/10/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyashita H., Mikami T., Chopra N., Yamada T., Chernyavsky S., Rizk D., et al. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol. 2020;31(8):1088–1089. doi: 10.1016/j.annonc.2020.04.006. PubMed PMID: 32330541. Pubmed Central PMCID: PMC7172785. Epub 2020/04/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill J.A., Seo SK. How I prevent infections in patients receiving CD19-targeted chimeric antigen receptor T cells for B-cell malignancies. Blood. 2020;136(8):925–935. doi: 10.1182/blood.2019004000. PubMed PMID: 32582924. Pubmed Central PMCID: PMC7441168 Therapeutics, Inc, Amplyx, and Gilead Sciences, and has received research support from Nohla Therapeutics, Karius, and Takeda (formerly Shire). S.K.S. declares no competing financial interests. Epub 2020/06/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verma R., Foster R.E., Horgan K., Mounsey K., Nixon H., Smalle N., et al. Lymphocyte depletion and repopulation after chemotherapy for primary breast cancer. Breast Cancer Res: BCR. 2016;18(1):10. doi: 10.1186/s13058-015-0669-x. PubMed PMID: 26810608. Pubmed Central PMCID: PMC4727393. Epub 2016/01/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thakkar A., Gonzalez-Lugo J.D., Goradia N., Gali R., Shapiro L.C., Pradhan K., et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell. 2021;39(8):1081–1090. doi: 10.1016/j.ccell.2021.06.002. e2PubMed PMID: 34133951. Pubmed Central PMCID: PMC8179248. Epub 2021/06/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gadi N., Wu S.C., Spihlman A.P., Moulton VR. What's Sex Got to Do With COVID-19? Gender-Based Differences in the Host Immune Response to Coronaviruses. Front Immunol. 2020;11:2147. doi: 10.3389/fimmu.2020.02147. PubMed PMID: 32983176. Pubmed Central PMCID: PMC7485092. Epub 2020/09/29.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Straub R.H., Miller L.E., Schölmerich J., Zietz B. Cytokines and hormones as possible links between endocrinosenescence and immunosenescence. J Neuroimmunol. 2000;109(1):10–15. doi: 10.1016/s0165-5728(00)00296-4. PubMed PMID: 10969175.Epub 2000/09/02.eng. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.