Abstract
Background:
In the WHO 2016 classification of central nervous system tumors, solitary fibrous tumors (SFT) and hemangiopericytomas (HPC) were considered part of the same category given a shared mutation. Nevertheless, since the new 2021 WHO classification, the term “hemangiopericytoma” has been retired, and SFT is considered an independent pathological entity.
Methods:
We reviewed the literature following preferred reporting items for systematic reviews and meta-analyses guidelines focusing on the treatment options and prognosis of patients with cervical SFT. We also present a 68-year-old female with spinal intradural extramedullary SFT complicated by diffuse extension into paravertebral tissues and muscles.
Results:
We found 38 cervical SFT in the literature. Patients averaged 47.3 years of age and 47.4% were female. Typically, these lesions spanned two spinal levels resulting in cord compression and most frequently exhibited benign features (i.e., diagnosed as Grade I SFTs). Interestingly, two patients exhibited distant metastases and had initial pathology consistent with grade II SFT.
Conclusion:
SFT of the cervical spine is rare and its management varies according to the histological grade and the clinical behavior, generally warranting surgical excision and adjuvant radiation therapy and/or systemic chemotherapy.
Keywords: Adjuvant chemotherapy, Cervical spine, Hemangiopericytoma, Solitary fibrous tumor, Spinal tumor

INTRODUCTION
Solitary fibrous tumor/hemangiopericytoma (SFT/HPC) are since the new 2021 WHO classification of central nervous system tumors the same entity and account for 1.9–4% of intracranial neoplasms, with few being found in the spine.[6] Despite gross-total surgical excision of spinal lesions (i.e., preferred treatment), higher grade lesions exhibit an increased survival rate but higher recurrence rate (i.e., especially for the WHO-2021 Grade III tumors) despite the utilization of adjuvant radiation or chemotherapy.[8] Given the recent finding of STAT6 expression in more than 55% of the cases, it is now agreed on most authors that the tumor has a mesenchymal origin.[3] Here, we present a patient with an intra-extradural cervical WHO-2021 Grade I SFT with diffuse paraspinal extension. As gross-total surgical resection was not feasible due to the high risk to adjacent neurological structures, sub-total tumor resection plus radiation therapy and chemotherapy increased the patient’s quality of life and survival (i.e., durable disease control).
MATERIALS AND METHODS
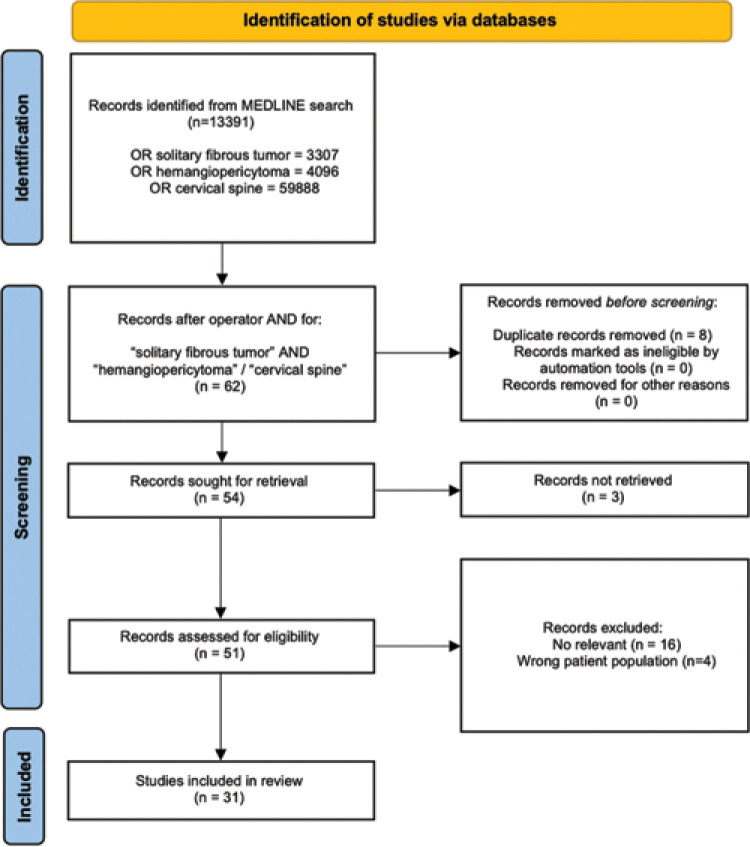
A qualitative review of the literature was performed in compliance with the updated preferred reporting items for systematic reviews and meta-analyses guidelines using the electronic database MEDLINE/PubMed.[7] The search used “solitary fibrous tumor,” “hemangiopericytoma,” and “cervical spine.” Full-text screening for inclusion was operated by two independent reviewers (F.C. and N.P.F.). Finally, 31 papers were included in the qualitative analysis [Figure 1].
Figure 1:
The preferred reporting items for systematic reviews and meta-analyses protocol used for the qualitative review.
CASE DESCRIPTION
Clinical and radiological presentation
A 68-year-old female presented with a 6-month history of poorly localized cervicalgia, bilateral brachialgia, and hyposthenia of the upper extremities accompanied by progressive 2/5 paresis of the upper extremities with decreased sensation to touch and burning paresthesia in both arms (i.e., increased in the left hand). Proprioception and vibration were intact.
Radiological assessment
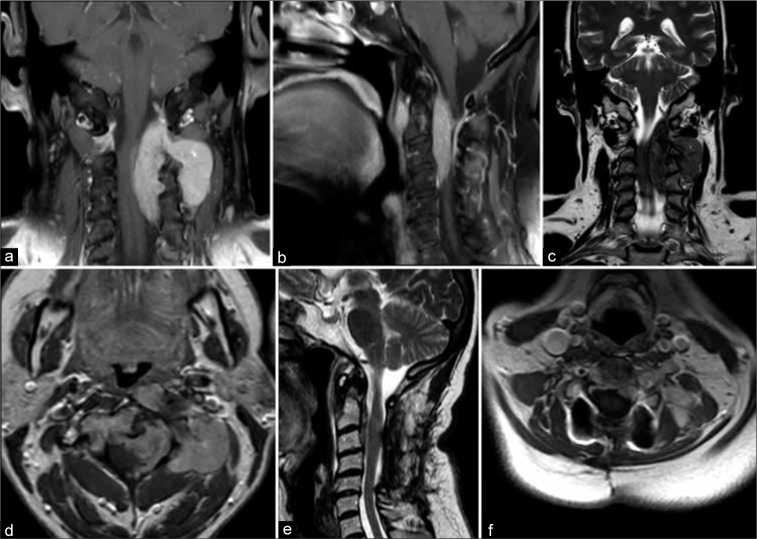
The entire spine was evaluated with magnetic resonance imaging (MRI). It revealed a cervical 8 cm, poorly circumscribed, intra-extradural tumor located between C0 and C5 level with epidural extension, and cord compression [Figure 2]. Posterior extension through the left neural foramina of C2–C3 and C3–C4 was also noted (i.e., 4 × 2.5 cm) with involvement of the paraspinal muscles. Anteriorly, it involved C2 and C3 vertebrae and the MRA showed a compromise of the left vertebral artery.
Figure 2:
(a) Coronal and (b) sagittal T1-weighted images demonstrating the diffusely infiltrating hyperintense tumor causing marked compression on the left and anterior spinal cord surfaces. Of notice the extraspinal extension through the left third and fourth foramina and the anterior component of the lesion located anteriorly to the third and fourth vertebral bodies. (c) Sagittal T2-weighted scan showing the inhomogeneous hypointensity of the lesion. (d) The paraspinal and the intra-extradural components of the tumor exhibited homogeneous gadolinium enhancement. (e) Postoperative T2-weighted image documented successful decompression of the spinal cord and debulking of the intraspinal component of the tumor. (f) Axial T1-weighted image after the administration of contrast showing considerable reduction of the paraspinal component of the solitary fibrous tumors/hemangiopericytomas following the four cycles of chemotherapy.
Surgical procedure
The patient agreed to a C2–C5 laminectomy with bilateral lateral mass resection and instrumented fusion to achieve a subtotal tumor resection (STR) through a posterior-only approach. The paramedian dural incision revealed a white-gray-colored mass compressing the left anterolateral surface of the spinal cord shifting tumor to the right. Tumor was debulked/decompressed, as confirmed by the postoperative MRI [Figure 2e].
Postoperative course and pathology
Postoperatively, the patient improved demonstrating reduced muscular weakness and brachialgia; she was discharged on the 4th postoperative day and referred to a rehabilitation center. She refused a potential secondary anterior tumor resection due to the risk of increased morbidity.
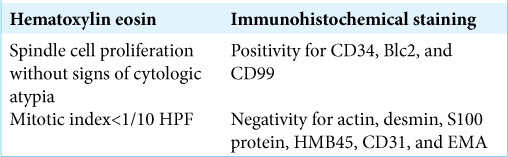
The permanent histological sections documented a pattern consistent with the diagnosis of SFT [Figure 3 and Table 1].
Figure 3:
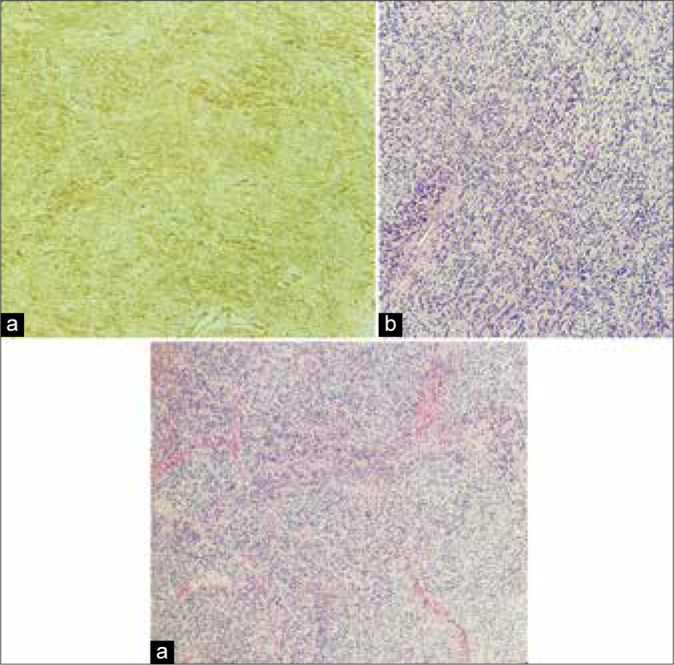
Histological images showing (a) a moderately cellular tumor exhibiting ovoidal and spindle cells arranged in small fascicles and embedded in a collagenous stroma (Hematoxylin eosin). (b) Malignant cells did not show neither signs of cytologic atypia nor necrosis and present a low mitotic count (Hematoxylin eosin). (c) Immunohistochemistry shows strong and diffuse positivity for CD34, a pattern consistent with the diagnosis of solitary fibrous tumors.
Table 1:
Clinical data and histology with immunohistochemical summary.
Adjuvant therapy and follow-up
A month after surgical debulking, the patient received radiation therapy (24 Gray) but no further surgery. She also agreed to undergo adjuvant chemotherapy with four cycles of Epirubicin. An MRI following the completion of chemotherapy revealed a sensible reduction of the residual extraspinal component of tumor-infiltrating the paraspinal muscles [Figure 2f]. At 2-year clinical follow-up, she had no symptoms/signs of SFT recurrence.
REVIEW OF THE LITERATURE
Patient characteristics
We evaluated 31 articles concerning 38 cases (including this one) of cervical SFT/HPC: 29 case reports and two case series (i.e., largest cohort of five patients). Patients averaged 48.2 years of age for Grade I, 45.7 years old with Grade II SFT, and 40.5 for Grade III HPC lesions. Seven tumors (18.4%) involved one level, while 21 (55.3%) occupied two levels of the spinal cord, three tumors (7.9%) extended to three levels, 5 (10.5%) involved four levels, and two tumors (5.3%) extended for >4 levels [Table 2].
Table 2:
Demographic and pathological characteristics of previously reported cases with associated treatment strategies.

Tumor characteristics and management
In 25 cases (65%), gross-total resection (GTR) of the tumor was performed as the sole treatment, whereas in three cases (7.9%), surgical treatment was followed by radiation therapy and in four patients (10.5%), adjuvant chemotherapy was also deployed.
DISCUSSION
Since 2021, the WHO classified SFT into three grades: a Grade I that corresponds to the highly collagenous, relatively low cellularity with typical spindle cells previously diagnosed as SFT; a Grade II, frequently more cellular and exhibiting less collagenous component with “staghorn” vasculature that was previously designated as HPC; and a Grade III that most often corresponds to what was termed anaplastic HPC.[6]
In their systematic review, Giordan et al.[2] analyzed a sample of 368 patients, histologically diagnosed with SFT/HPC of the CNS, and found that 70.4% of tumors were primarily intracranial with only 109 cases of spinal involvement. The majority of spinal SFT/HPCs were identified at the thoracic level (45.3%) followed by cervical and lumbar segments with the primary intradural localization reported in 27.5% of patients.
Microscopic examination of this neoplasm demonstrates a patternless architecture composed of spindle-shaped cells with different grades of atypia disposed between thick bands of collagen. Confirmation of the diagnosis is made with immunohistochemical staining, with abnormal cells exhibiting positivity for CD34, CD99, Bcl2, vimentine, and no signs of reaction for S100 protein and EMA.[1] Morphological features range from more ill-circumscribed lesions associated with bony erosions, preferably seen in Grade III SFT, to well-defined ovoid or dumbbell-shaped tumors exhibiting less aggressive behavior [Table 1].[4]
RADIOLOGICAL DIAGNOSIS
Frequently encountered radiological features of SFT include hypo/isointensity on T1-weighted images with a heterogeneous enhancement after the administration of contrast.[5]
Prognosis
A recent retrospective study Sung et al.[8] comparing treatment results based on the 2016 WHO classification of SFT/HPC revealed relatively benign and indolent courses for grade I lesions, with a 25% of recurrence rate, which was strongly dependent on the volume of the residual tumor after resection. However, more aggressive lesions exhibited a significantly shorter overall survival regardless of the extent of surgical resection or the adjuvant therapy performed.
CONCLUSION
SFT of the cervical spine is rare and relatively benign tumors, whose preferable treatment lays within a combination of surgical tumor resection, radiation therapy, and adjuvant chemotherapy.
Footnotes
How to cite this article: Colamaria A, Carbone F, Sacco M, Corsi F, Leone A, Parbonetti G, et al. Solitary fibrous tumor/hemangiopericytoma of the cervical spine: A systematic review of the literature with an illustrative case. Surg Neurol Int 2022;13:532.
Contributor Information
Antonio Colamaria, Email: colamariaa@gmail.com.
Francesco Carbone, Email: francesco.carbone615@gmail.com.
Matteo Sacco, Email: matteosacco88@gmail.com.
Fabrizio Corsi, Email: fabcorsi@yahoo.it.
Augusto Leone, Email: augustoleone96@gmail.com.
Giovanni Parbonetti, Email: parbonetti.giovanni@gmail.com.
Matteo de Notaris, Email: matteodenotaris@gmail.com.
Nicola Pio Fochi, Email: nicola_fochi.556052@unifg.it.
Matteo Landriscina, Email: matteo.landriscina@unifg.it.
Giulia Coppola, Email: coppola1537902@studenti.uniroma1.it.
Elena de Santis, Email: elenadesantis@uniroma1.it.
Guido Giordano, Email: guido.giordano@unifg.it.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
REFERENCES
- 1.Carneiro SS, Scheithauer BW, Nascimento AG, Hirose T, Davis DH. Solitary fibrous tumor of the meninges: A lesion distinct from fibrous meningioma. A clinicopathologic and immunohistochemical study. Am J Clin Pathol. 1996;106:217–24. doi: 10.1093/ajcp/106.2.217. [DOI] [PubMed] [Google Scholar]
- 2.Giordan E, Marton E, Wennberg AM, Guerriero A, Canova G. A review of solitary fibrous tumor/hemangiopericytoma tumor and a comparison of risk factors for recurrence, metastases, and death among patients with spinal and intracranial tumors. Neurosurg Rev. 2021;44:1299–312. doi: 10.1007/s10143-020-01335-x. [DOI] [PubMed] [Google Scholar]
- 3.Gubian A, Ganau M, Cebula H, Todeschi J, Scibilia A, Noel G, et al. Intracranial solitary fibrous tumors: A heterogeneous entity with an uncertain clinical behavior. World Neurosurg. 2019;126:e48–56. doi: 10.1016/j.wneu.2019.01.142. [DOI] [PubMed] [Google Scholar]
- 4.Jia Q, Zhou Z, Zhang D, Yang J, Liu C, Wang T, et al. 2018 Surgical management of spinal solitary fibrous tumor/ hemangiopericytoma: A case series of 20 patients. Eur Spine J. 2018;27:891–901. doi: 10.1007/s00586-017-5376-0. [DOI] [PubMed] [Google Scholar]
- 5.Kurtkaya O, Elmaci I, Sav A, Pamir MN. Spinal solitary fibrous tumor: Seventh reported case and review of the literature. Spinal Cord. 2001;39:57–60. doi: 10.1038/sj.sc.3101104. [DOI] [PubMed] [Google Scholar]
- 6.Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021;23:1231–51. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sung KS, Moon JH, Kim EH, Kang SG, Kim SH, Suh CO, et al. Solitary fibrous tumor/hemangiopericytoma: Treatment results based on the 2016 WHO classification. J Neurosurg. 2018:1–8. doi: 10.3171/2017.9.JNS171057. [DOI] [PubMed] [Google Scholar]