Abstract
Thoracic endovascular stent grafting has been increasingly used in patients with type B aortic dissection (TBAD). We describe a patient with worsening abdominal pain and a rapidly enlarging common iliac artery aneurysm associated with TBAD. The patient underwent open aortoiliac replacement followed by thoracic stent grafting of the TBAD. Computed tomography imaging indicated positive remodeling of the aortic dissection at 3 years. Open abdominal aortic replacement before thoracic endovascular aortic repair may be a useful strategy in patients with TBAD with negative predictors of aneurysmal degeneration.
Keywords: Aortic dissection, Thoracic, Endovascular
Thoracic endovascular aortic repair (TEVAR) is now considered first-line intervention for patients with acute type B aortic dissection (TBAD) complicated by malperfusion or rupture.1 TBAD may also be considered complicated if patients have refractory hypertension, pain, or rapid aortic expansion. The role of TEVAR in uncomplicated TBAD is controversial. Traditionally, uncomplicated TBAD has been managed medically with anti-impulse therapy. However, the paradigm has been evolving. Of uncomplicated TBAD, 20% to 50% will become complicated with continued aortic degeneration.2, 3, 4 Prognostic factors for aneurysmal degeneration include an aortic diameter of more than 40 mm, a false lumen diameter of more than 22 mm, a proximal intimal tear of more than 10 mm, and a patent or partially thrombosed false lumen.5 Uncomplicated TBADs with unfavorable anatomical factors may be considered for pre-emptive TEVAR. By covering the primary tear and directing blood flow into the true lumen, TEVAR may promote false lumen thrombosis and enhance aortic remodeling. We describe a patient with TBAD and rapid symptomatic iliac artery expansion treated with a strategy of open aortoiliac replacement first. TEVAR was then used to treat the residual TBAD with high-risk features of aneurysmal degeneration.
Case report
Informed consent was obtained for the publication of this case report and associated images. A 63-year-old man with a history of hypertension presented to an outside facility with sudden onset of chest pain and right lower extremity pain. He had no right lower extremity pulses nor signals. Computed tomographic angiography (CTA) indicated TBAD extending from the left subclavian artery into the iliac arteries (Society for Vascular Surgery/Society of Thoracic Surgeons B3,10). The true lumen supplied the celiac, superior mesenteric, and bilateral renal arteries. Complete occlusion of the right iliac artery was caused by the dissection flap at the aortic bifurcation (Fig 1). Upon review of the outside records, he was treated with analgesics and anti-impulse therapy with resolution of chest pain and restoration of right foot pulses after several hours. He was discharged with no plans for further treatment. Two months later, he presented to our facility with progressively worsening right lower quadrant abdominal pain and tenderness. CTA indicated similar TBAD but with a widely patent right iliac artery and an increase in the right common iliac artery diameter from 30 to 40 mm (Fig 2). He had unfavorable predictors of aneurysmal degeneration (Fig 1). Because of the rapid expansion of the right common iliac artery and escalating symptomatology, urgent repair was deemed necessary. Our institution protocol is to offer TEVAR (coverage up to 20 cm) to anatomically suitable patients with complicated TBAD or with uncomplicated TBAD exhibiting adverse morphological features within 90 days.
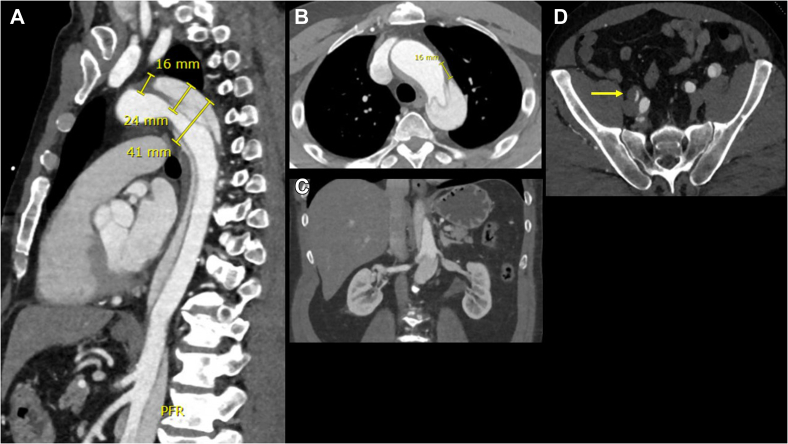
Fig 1.
A, The patient presenting with type B aortic dissection (TBAD) with negative anatomical predictors for aneurysmal degeneration (aortic diameter of >40 mm, false lumen diameter of >22 mm, entry tear of >10 mm). True lumen supplies the celiac and superior mesenteric arteries. B, The intimal tear is at the convexity of the descending thoracic aorta with diameter of greater than 10 mm. C, The true lumen supplies the bilateral renal arteries. D, TBAD with occlusion of the right external iliac artery (yellow arrow).
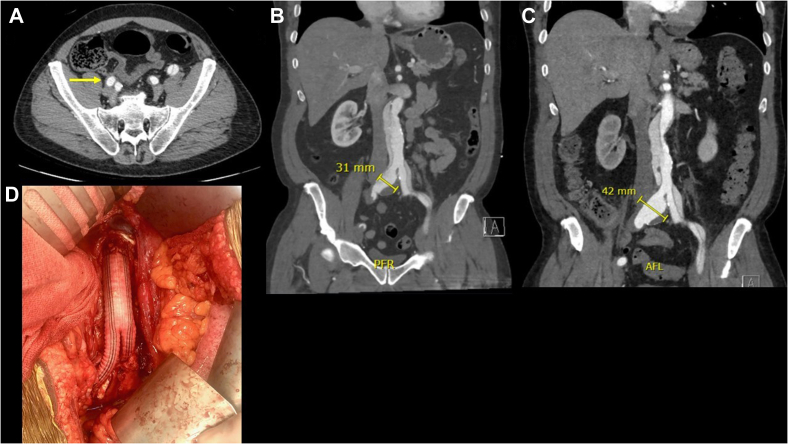
Fig 2.
A, Widely patent right external iliac artery 2 months after the onset of acute type B aortic dissection (TBAD) (yellow arrow). B and C, Increase in the right common iliac artery aneurysm from 31 to 42 mm after 2 months. D, Aortoiliac bypass with proximal anastomosis at the infrarenal aorta and distal anastomoses to distal bilateral common iliac arteries with reapproximation of true and false lumens.
In this case, a TEVAR-first strategy would not have provided definitive immediate treatment of the iliac artery aneurysm. Therefore, he underwent urgent open aortoiliac reconstruction using a 20 × 10-mm bifurcated Dacron graft. Graft sizing was based on intraoperative estimates of aortic and iliac diameters and capability to accommodate a 24F device for delayed TEVAR. The main body of the bifurcated graft was left long (9 cm length) to allow for future branched or fenestrated devices. An infrarenal clamp was placed and the proximal anastomosis was performed after the pliable septum was excised and a single channel was created for anastomosis. The distal anastomoses were performed to both common iliac arteries after the two lumens were reapproximated. Pledgeted mattress sutures were used to create secure anastomoses. Clamp time was 73 minutes. Blood loss was 1500 mL. Postoperative recovery was uneventful. Our plan was to perform pre-emptive TEVAR within the 90-day subacute period. However, the patient declined intervention during this therapeutic window because of postoperative fatigue. Five months later, he agreed to undergo TEVAR. He underwent left carotid-subclavian artery bypass graft followed by thoracic stent graft coverage of the proximal intimal tear with distal bare-metal stent extension into the body of the bifurcated aortic graft (Provisional ExTension To Induce COmplete ATtachment [PETTICOAT]). General anesthesia was used. Right common femoral artery was exposed through an oblique incision. The artery was normal with no evidence of dissection. An intravascular ultrasound (IVUS) catheter (Philips Visions PV 0.035 Catheter System, San Diego, CA) was used to confirm true lumen access from the femoral artery to the ascending aorta and high intimal flap mobility (amplitude of >3 mm). Left brachial artery exposure was performed through a longitudinal incision above the antecubital fossa. Pigtail catheter was placed into the ascending aorta via the brachial access. Thoracic aortogram demonstrated a large fenestration 1 cm distal to the left subclavian artery (Fig 3). A 40 × 36 × 160-mm tapered Dissection Endovascular Graft TX2 with Pro-Form (Cook Medical, Bloomington, IN) was deployed from zone 2 into the mid descending thoracic aorta over a double-curve Extra-Stiff Lunderquist wire. Given persistent true lumen collapse, two overlapping 36 × 180-mm bare-metal dissection stents (Zenith Dissection Endovascular Stent, Cook Medical) were implanted from the mid descending thoracic aorta into the main body of the bifurcated graft. Postdeployment compliant balloon molding was not used. Embolization of the proximal left subclavian artery was performed using an 18-mm Amplatzer Plug II (Abbott Laboratories, Chicago, IL) via the brachial artery access. IVUS confirmed significant expansion of the true lumen. A postdeployment angiogram demonstrated coverage of the dissection entry tear, nonopacification of the false lumen proximally, and brisk antegrade opacification of the visceral and iliac arteries (Fig 3). His postoperative course was uncomplicated and he was discharged 4 days later. Postoperative CTA at 3 years indicated complete thrombosis of the false lumen in the descending thoracic aorta and positive remodeling in the abdominal aorta (Figs 4 and 5).
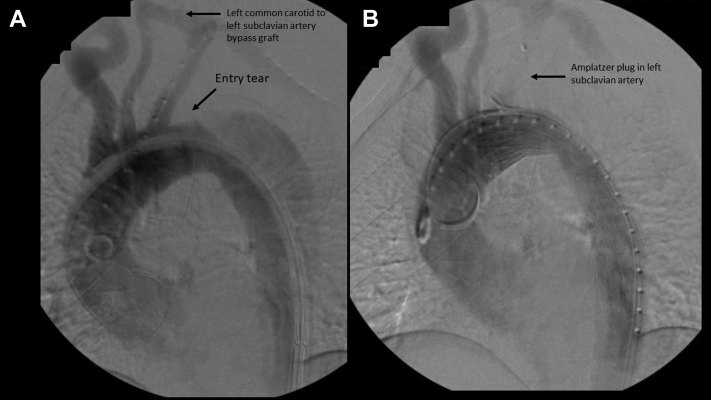
Fig 3.
A, Thoracic angiogram 5 months after an open aortoiliac bypass indicating patent left common carotid to left subclavian bypass and entry tear approximately 1 cm distal to the origin of the left subclavian artery on the outer convexity. B, Placement of thoracic stent graft just beyond the origin of the left common carotid artery and embolization of the proximal left subclavian artery. There is no filling of the false lumen in the proximal descending thoracic aorta.
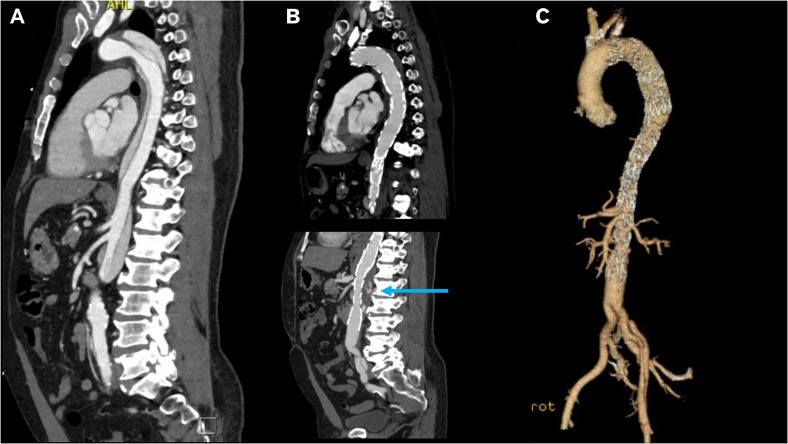
Fig 4.
A, Computed tomography angiography (CTA) indicating type B aortic dissection (TBAD) at initial presentation. B, Postoperative CTA at 3 years indicating complete thrombosis of the descending thoracic aorta and partial thrombosis of the false lumen in the visceral aorta (blue arrow). C, Three-dimensional reconstruction indicating patent aortoiliac bypass graft and thoracic endovascular aortic repair (TEVAR) with Provisional ExTension To Induce COmplete ATtachment (PETTICOAT) extending into the main body of the bifurcated Dacron graft.
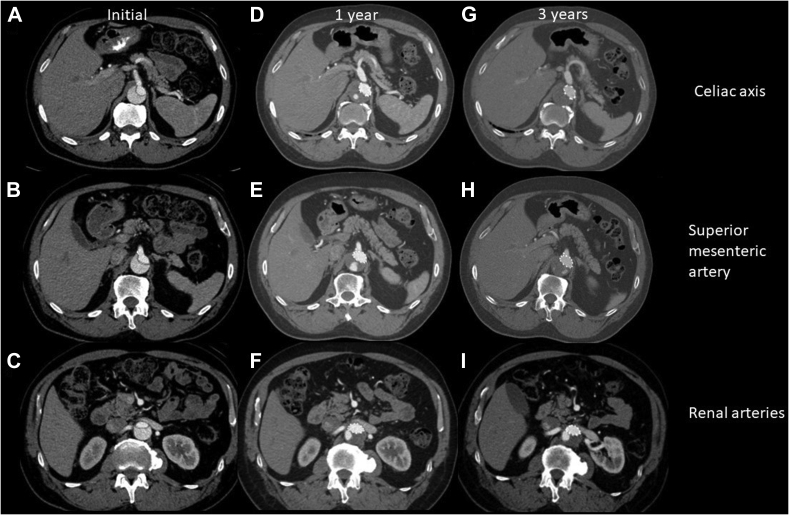
Fig 5.
Computed tomography angiography (CTA) indicating the celiac axis (A), superior mesenteric artery (B), and renal arteries (C) at the time of initial presentation, 1 year after thoracic endovascular aortic repair (TEVAR) (D–F), and 3 years after TEVAR (G–I). Note the progression of thrombosis in the false lumen at the celiac and superior mesenteric artery levels. The false lumen thrombosis status at the renal arteries remains unchanged.
Discussion
Rapid aortic expansion falls under the rubric of complicated TBAD. In this case, the patient initially presented to an outside facility with lower extremity malperfusion associated with acute TBAD with unfavorable morphological predictors. His malperfusion resolved with optimal medical therapy within hours. TEVAR was not offered to the patient by the outside facility. Two months later, the patient presented to our institution with worsening right lower quadrant pain and tenderness and a 10-mm increase in the right common iliac artery diameter. If TEVAR had been performed upon initial presentation, it is unclear whether progression of iliac aneurysmal disease would have been stymied or slowed by coverage of the initial primary tear. The benefit of TEVAR in aortic remodeling has mostly occurred in the descending thoracic aorta; its role in abdominal aorta remodeling is unclear. The Investigation of Stent Grafts in Patients with Type B Aortic Dissection (INSTEAD) trial compared best medical treatment with stent grafting in 140 patients with uncomplicated subacute and early chronic type B dissection (2-52 weeks). False lumen thrombosis in the descending thoracic aorta was enhanced with TEVAR (91.3% vs 19.4%) at 2 years. Despite this favorable aortic remodeling, all-cause mortality and aorta-related death did not improve.6 However, an extended follow-up study at 5 years (INSTEAD-XL) showed that TEVAR reduced all-cause mortality (11.1% vs 19.3%), aorta-specific mortality (6.9% vs 19.3%), and progression of dissection (27% vs 46.1%).7 Thus, the clinical benefit of TEVAR may not become evident until 2 to 5 years after TEVAR. Unfortunately, there are no data on the prevention of long-term aneurysmal degeneration in the abdominal aorta.8, 9, 10
A purely endovascular management strategy at the outset in our case would entail thoracic stent graft coverage of the proximal intimal tear with laser fenestration of the left subclavian artery or a branched device to ensure a proximal landing zone in healthy aorta. The thoracic stent graft would extend to the celiac axis and then be further lengthened to the infrarenal aorta with a branched or fenestrated endograft. It would be necessary to secure a distal landing zone in the right external iliac artery with embolization of the right hypogastric artery or use an iliac branch device to exclude the right common iliac artery aneurysm. A one-stage strategy would incur an excessive risk of paraplegia, whereas a staged approach would delay treatment of the symptomatic common iliac artery aneurysm. Another endovascular option would be cheese-wire or laser-assisted fenestration of the infrarenal aorta before the placement of a standard infrarenal endograft.11,12 The septum is torn to create a proximal infrarenal landing zone.
We elected to perform urgent open replacement of the dissected infrarenal aorta and iliac arteries to treat the rapid iliac artery expansion. An infrarenal clamp was well-tolerated. The septum was pliable and was resected easily. The true and false lumens were readily differentiated and reapproximated into a single channel. Sultan et al13 used left medial visceral rotation, supraceliac clamping, aorto-visceral-iliac endarterectomy followed by TEVAR 12 weeks later. We posit that supraceliac clamping and septectomy of the visceral aorta would add increased complexity and morbidity to the procedure. We performed TEVAR with PETTICOAT 7 months after the initial presentation to treat the residual type B dissection, which was well outside the subacute (2 weeks to 3 months) period of optimal septal plasticity.14 Fortunately, the intimal flap was sufficiently mobile on IVUS to allow for successful TEVAR. Lee et al15 suggested that 1 year is the upper time threshold for achieving optimal outcomes with TEVAR. In our patient, there was complete false lumen thrombosis in the descending thoracic aorta; however, there was residual flow in the false lumen within the visceral aorta at 3 years. We acknowledge that visceral septectomy would have obviated the need for the PETTICOAT and expedite false lumen thrombosis. We eliminated the most distal reentry tears by open aortoiliac replacement. There were no visceral branches arising from the false lumen. These factors favor eventual thrombosis of the false lumen.16 If visceral vessels had arisen from the false lumen, pre-emptive TEVAR may risk end-organ ischemia.
The use of the PETTICOAT technique is contentious. We elected to use the PETTICOAT technique because of continued compromise of the true lumen after proximal stent graft deployment. Moreover, the incidence of distal stent graft-induced new entry tear may be reduced with the PETTICOAT technique, particularly with more chronic (>3 months) dissections.17,18 In the Staged Thoraco-Abdominal and Branch vessel Endoluminal repair (STABLE) I prospective multicenter trial, the use of the Zenith dissection endovascular system (proximal stent graft across the primary entry tear and distal bare metal stent across the visceral segment) was used in 86 patients with acute and subacute TBAD presenting with malperfusion, rupture, refractory pain, hypertension, rapid aortic growth, or large transaortic diameter. Complete false lumen thrombosis within the descending thoracic aorta occurred in 58.8% of patients with subacute disease at 5 years. However, no clear advantage in preventing aneurysmal degeneration in the abdominal aorta could be demonstrated.19 Moreover, recently published 5-year results of the STABLE II trial of 73 patients with acute type B dissection complicated by malperfusion and/or rupture indicate that 62% of patients with a dissection stent had an increase in the transaortic diameter at 5 years.20 Conversely, others have reported that bare-metal stenting may offer positive remodeling in the distal aorta.21, 22, 23, 24, 25 Concerns have also been raised that the PETTICOAT technique may jeopardize future adjunctive endovascular procedures because of stent strut interference. Other investigators have countered that a more expanded true lumen facilitates adjunctive procedures by virtue of the increase in workspace. The use of the baremetal stent did not hinder the performance of secondary interventions in the STABLE II trial.20
Conclusions
Infrarenal aortoiliac replacement for postdissection aneurysm before TEVAR with PETTICOAT can lead to favorable aortic remodeling.
From the Society for Clinical Vascular Surgery
Footnotes
Author conflict of interest: none.
References
- 1.Harky A., Chan J.S.K., Wong C.H.M., Francis N., Grafton-Clarke C., Bashir M. Systemic review and meta-analysis of acute type B thoracic aortic dissection, open, or endovascular repair. J Vasc Surg. 2019;69:1599–1609. doi: 10.1016/j.jvs.2018.08.187. [DOI] [PubMed] [Google Scholar]
- 2.DeBakey M.E., McCollum C.H., Crawford E.S., Morris G.C., Howell J., Noon G.P., et al. Dissection and dissecting aneurysms of the aorta: twenty-year follow-up of five hundred twenty-seven patients treated surgically. Surgery. 1982;92:1118–1134. [PubMed] [Google Scholar]
- 3.Reutersberg B., Trenner M., Haller B., Geisbusch S., Reeps C., Eckstein H. The incidence of delayed complications in acute type B aortic dissections is underestimated. J Vasc Surg. 2018;68:356–363. doi: 10.1016/j.jvs.2017.11.089. [DOI] [PubMed] [Google Scholar]
- 4.Fattori R., Montgomery D., Lovato L., Kische S., Di Eusanio M., Ince H., et al. Survival after endovascular therapy in patients with type B aortic dissection: a report from the International Registry of Acute Aortic Dissection (IRAD) JACC Cardiovasc Interv. 2013;6:876–882. doi: 10.1016/j.jcin.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Evangelista A., Salas A., Ribera A., Ferreira-Gonzalez I., Cuellar H., Pineda V., et al. Long-term outcome of aortic dissection with patent false lumen: predictive role of entry tear size and location. Circulation. 2012;125:3133–3141. doi: 10.1161/CIRCULATIONAHA.111.090266. [DOI] [PubMed] [Google Scholar]
- 6.Nienaber C.A., Rousseau H., Eggebrecht H., Kische S., Fattori R., Rehders T.C., et al. Randomized comparison of strategies for type B aortic dissection: the Investigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial. Circulation. 2009;120:2519–2528. doi: 10.1161/CIRCULATIONAHA.109.886408. [DOI] [PubMed] [Google Scholar]
- 7.Nienaber C.A., Kische S., Rousseau H., Eggebrecht H., Rehders T.C., Kundt G., et al. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv. 2013;6:407–416. doi: 10.1161/CIRCINTERVENTIONS.113.000463. [DOI] [PubMed] [Google Scholar]
- 8.Famularo M., Meyermann K., Lombardi J.V. Aneurysmal degeneration of type B aortic dissections after thoracic endovascular aortic repair: a systematic review. J Vasc Surg. 2017;66:924–930. doi: 10.1016/j.jvs.2017.06.067. [DOI] [PubMed] [Google Scholar]
- 9.Conrad M.F., Carvalho S., Ergul E., Kwolek C.J., Lancaster R.T., Patel V.I., et al. Late aortic remodeling persists in the stented segment after endovascular repair of acute complicated type B aortic dissection. J Vasc Surg. 2015;62:600–605. doi: 10.1016/j.jvs.2015.03.064. [DOI] [PubMed] [Google Scholar]
- 10.Canaud L., Faure E.M., Ozdemir B.A., Alric P., Thompson M. Systematic review of outcomes of combined proximal stent-grafting with distal bare stenting for management of aortic dissection. Ann Cardiothorac Surg. 2014;3:223–233. doi: 10.3978/j.issn.2225-319X.2014.05.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ullery B.W., Chandra V., Dake M., Lee J.T. Cheese wire fenestration of a chronic juxtarenal dissection flap to facilitate proximal neck fixation during EVAR. Ann Vasc Surg. 2015;29:124.e1–124.e5. doi: 10.1016/j.avsg.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 12.Qato K., Conway A., Northfield E., Giangola G., Brinster D., Carroccio A. Laser-assisted “scissor” technique to facilitate thoracic endovascular aortic repair for chronic type B aortic dissection. Ann Vasc Surg. 2021;77:347.e7–347.e11. doi: 10.1016/j.avsg.2021.04.048. [DOI] [PubMed] [Google Scholar]
- 13.Sultan S., Barrett N., Kamal M.H., Tawfick W., Atteia E.M., Clarkson K., et al. Staged hybrid single lumen reconstruction (TIGER) in management of chronic symptomatic complex type B aortic dissection, techniques, and literature review. Ann Vasc Surg. 2020;65:261–270. doi: 10.1016/j.avsg.2019.12.028. [DOI] [PubMed] [Google Scholar]
- 14.VIRTUE Registry Investigators Mid-term outcomes and aortic remodelling after thoracic endovascular repair for acute, subacute, and chronic aortic dissection: the VIRTUE Registry. Eur J Vasc Endovasc Surg. 2014;48:363–371. doi: 10.1016/j.ejvs.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Lee S.J., Kang W.C., Ko Y.G., Woo Y., Ahn C.M., Won J.Y., et al. Aortic remodeling and clinical outcomes in Type B aortic dissection according to the timing of thoracic endovascular aortic repair. Ann Vasc Surg. 2020;67:322–331. doi: 10.1016/j.avsg.2020.03.022. [DOI] [PubMed] [Google Scholar]
- 16.Kamman A.V., Brunkwall J., Verhoeven E.L., Heijmen R.H., Trimarchi S., ADSORB trialists Predictors of aortic growth in uncomplicated type B aortic dissection from the Acute Dissection Stent Grafting or Best Medical Treatment (ADSORB) database. J Vasc Surg. 2017;65:964–997. doi: 10.1016/j.jvs.2016.09.033. [DOI] [PubMed] [Google Scholar]
- 17.Civilini E. PETTICOAT technique to prevent distal stent graft-induced new entry tears. Ann Thorac Surg. 2017;103:2023. doi: 10.1016/j.athoracsur.2016.09.076. [DOI] [PubMed] [Google Scholar]
- 18.Ouchi T., Kato N., Kato H., Higashigawa T., Ito H., Nakajima K., et al. Relevance of aortic dissection chronicity to the development of stent graft-induced new entry. Ann Thorac Surg. 2020;110:1983–1989. doi: 10.1016/j.athoracsur.2020.04.032. [DOI] [PubMed] [Google Scholar]
- 19.Lombardi J.V., Cambria R.P., Nienaber C.A., Chiesa R., Mossop P., Haulon S., et al. Five-year results from the Study of Thoracic Aortic Type B Dissection Using Endoluminal Repair (STABLE I) study of endovascular treatment of complicated type B aortic dissection using a composite device design. J Vasc Surg. 2019;70:1072–1081. doi: 10.1016/j.jvs.2019.01.089. [DOI] [PubMed] [Google Scholar]
- 20.Lombardi J.V., Gleason T.G., Panneton J.M., Starnes B.W., Dake M.D., Haulon S., et al. Five-year results of the STABLE II study for the endovascular treatment of complicated, acute type B aortic dissection with a composite device design. J Vasc Surg. 2022;76:1189–1197.e3. doi: 10.1016/j.jvs.2022.06.092. [DOI] [PubMed] [Google Scholar]
- 21.Sultan I., Dufendach K., Kilic A., Bianco V., Trivedi D., Althouse A.D., et al. Bare metal stent use in Type B aortic dissection may offer positive remodeling for the distal aorta. Ann Thorac Surg. 2018;106:1364–1370. doi: 10.1016/j.athoracsur.2018.06.042. [DOI] [PubMed] [Google Scholar]
- 22.Hashizume K., Honda M., Mori M., Yagami T., Takaki H., Matsuoka T., et al. Full PETTICOAT in acute type B aortic dissection with patent false lumen may offer positive remodeling for the distal aorta. Gen Thorac Cardiovasc Surg. 2021;69:926–933. doi: 10.1007/s11748-020-01548-3. [DOI] [PubMed] [Google Scholar]
- 23.Nienaber C.A., Yuan X., Aboukoura M., Blanke P., Jakob R., Janosi R.A., et al. ASSIST study group Improved remodeling with TEVAR and distal bare-metal stent in acute complicated Type B dissection. Ann Thorac Surg. 2020;110:1572–1579. doi: 10.1016/j.athoracsur.2020.02.029. [DOI] [PubMed] [Google Scholar]
- 24.Matsuoka T., Hashizume K., Honda M., Harada D., Ohno M., Ikebata K., et al. The provisional extension to induce complete attachment technique is associated with abdominal aortic remodeling and reduces aorta-related adverse events after aortic dissection. J Vasc Surg. 2021;74:45–52. doi: 10.1016/j.jvs.2020.11.038. [DOI] [PubMed] [Google Scholar]
- 25.Rong D., Ge Y., Liu J., Liu X., Guo W. Combined proximal descending aortic endografting plus distal bare metal stenting (PETTICOAT technique) versus conventional proximal descending aortic stent graft repair for complicated type B aortic dissections. Cochrane Database Syst Rev. 2019;10:CD013149. doi: 10.1002/14651858.CD013149.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]