Abstract
Coreopsis tinctoria (CT) flower is widely consumed as flower tea with many good healthcare benefits in China, and room drying is the most common drying method of CT, while many rapid and controllable modern instrument drying methods have been used for the drying of flowers teas. In the present study, effects of drying methods on chemical components, antioxidant activity and anti-α-glucosidase activity of CT have been compared to search for proper drying methods. In the results, drying methods have significant effects on the chemical composition, antioxidant activity and anti-α-glucosidase activity of CT. Vacuum drying at 50 °C, hot air drying at 30 °C and hot air drying at 50 °C are the top three drying methods to produce dried CT of high quality, and hot air drying at 30 °C is more energy-efficient and time-saving. Our results provide some new choices for CT in the future.
Keywords: Coreopsis tinctoria, Drying method, Hot air drying, Vacuum drying
Coreopsis tinctoria; Drying method; Hot air drying; Vacuum drying.
1. Introduction
Coreopsis tinctoria Nutt. (CT), also named Xueju in China, is the dried capitula of the plant CT, which is an annual herb of the Compositae family and widely planted in Xinjiang province of China. Usually, dried CT always is consumed as flower tea with many health benefits, such as antihyperglycemic effect (Dias et al., 2010; Li et al., 2020), antihypertensive effect (Yang et al., 2014), antioxidant (Ma et al., 2016), anti-inflammatory (Zhang et al., 2013), and so on. There are more than 100 chemical compounds that have been isolated or identified from CT, mainly including flavonoids, polyacetylenes, polysaccharides, phenylpropanoids, and volatile oil (Shen et al., 2021). Especially, flavonoids (such as marein, flavonemarein, okanin, iso-okanin, rutin and quercetin) and phenolics (for example, gallic acid and chlorogenic acid) are the main active components in CT (Bai et al., 2018; Chen, 2014).
In May 2021, the World Health Organization (WHO) released the 2021 World Health Statistics Report, and which showed that diabetes and its complications have become a major public health problem in the world in the 21st Century, and it has been the third most common chronic noncommunicable disease. Due to the toxic and side effects of synthetic drugs, natural plant resources have become interesting good origin for the candidates of diabetes drugs. CT has caused the interests of researchers, because it has been applied to control blood glucose, blood lipid and blood pressure by nomadic people in Xinjiang province of China for many years. Drying method is the most important process for flower teas and many other traditional herbal medicine, and proper drying method would help to improve active compounds in flower teas and herbs, which always would contribute to the promotion of qualities and healthcare benefits of flower teas and herbs (Wang et al., 2016). Furthermore, drying process is the most important link in the production chain of flower teas and herbs, due to fresh materials are difficult to preserve. Regrettably, CT still is dried by room drying for 10 to more than 20 days after being recovered from August to October every year in the main plant area, while there are many kinds of modern drying technologies applied in the drying process of flower teas and foods, such as hot air drying, freeze drying, vacuum drying, microwave drying, and so on (Zheng et al., 2015; Qin et al., 2020; Shi et al., 2020).
Sun drying, room drying and hot air drying are the most widely used methods for their lower costs (Soysal, 2004), while hot air drying is a commonly used method as it can produce dried material in shorter time when compared to sun drying and room drying. However, antioxidants and active compounds in CT, particularly phenolics, flavonoids, and anthocyanins, are sensitive to heat, oxygen and light in the drying process. In this study, we compared the antioxidant and hypoglycemic activities of CT dried by different drying methods, as well as their active chemical components, in order to filter out the most suitable methods for the production of dry CT in the plant area. By doing all these work, quality of dried CT also would be improved.
2. Materials and methods
2.1. Materials and chemical reagents
Fresh capitula of CT were collected from Xigou Township, Dabancheng District, Urumqi city in Xinjiang province of China, at their blooming stage in September 2020, and stored in a refrigerator at 4 °C immediately.
DPPH· (2, 2-diphenyl-1-picrylhydrazyl, purity ≥98.0%) and p-nitrophenyl-α-D- glucopyranoside (pNPG, N7006) were purchased from Sigma-Aldrich Co., LLC., while acetonitrile (HPLC grade) was obtained from Merck Chemical Technology (Shanghai) Co., Ltd. (China). Folin-Ciocalteu reagent (FC reagent, puriss, 1 N) and ABTS (2,2’-azino-bis(3-ethyl-benzothiazoline-6-sulfonic acid) diammonium salt) were provided by Beijing Solarbio Technology Co., Ltd. (Beijing, China), and α-glucosidase (E0035, 50 u/mg) was bought from Nanjing Dulai Biotechnology Co., Ltd. (Nanjing, China). Glacial acetic acid was of analytical grade (purity>90%) and purchased from Tianjin Kunhua Chemical Technology Co., Ltd. (Tianjin, China). All other chemical reagents were of analytical grade and supplied by Tianjin Jiangtian Chemical Technology Co., Ltd. (Tianjin, China).
Standards of gallic acid (NO. MUST-20032802, purity is 99.04%), chlorogenic acid (NO. MUST-20032310, purity is 99.73%), catechin (NO. MUST-19080110, purity is 99.52%), quercetin (NO. MUST-19101104, purity is 99.35%), marein (NO. MUST-20042013, purity is 99.94%), flavanomarein (NO. MUST-20062815, purity is 95.33%), and procyanidin B2 (NO. MUST-29106498, purity is more than 98%) were collected from Chengdu Manster Biotechnology Co., Ltd. (Sichuan province, China), while okanin (NO. 20052602), isookanin (NO. 20052697) and rutin (NO. 20030203) with the purity of more than 98% were purchased from Tianjin Zhonghe Biotechnology Co., Ltd. (Tianjin city, China).
2.2. Drying methods
Fresh capitulas of CT were selected, and the slender stalks were deleted before drying process. Flowers were evenly divided into 11 parts for the following drying process. Four parts were dried at 30, 40, 50 and 60 °C at a vacuum value of 0.02 MPa in a vacuum drying oven for 36, 24, 12 and 6 h, respectively. Another 4 parts were dried at 30, 40, 50 and 60 °C in a hot air drying oven for 12, 8, 4 and 2 h, respectively. The remaining three parts were dried by room drying (dried in the room with no sunshine for about 10 days, and room temperature ranged from 12 to 26 °C), sun drying (dried in the sunshine for about 3 days, and room temperature ranged from 12 to 26 °C), and freeze drying (dried at −45 °C with a vacuum value of 0.01 MPa for 48 h), respectively.
2.3. Sample preparation
CT samples dried by different methods previously mentioned were crushed until passed 40 mesh sieve with a grinder, and powder of all samples were stored in plastic bags in a 4 °C refrigerator, respectively.
Powder of each sample (1 g, weighed exactly) was transferred into a flask with stopper, and then 50 mL 60% ethanol/water (V/V = 60/40) was added before they were put into an ultrasonic bath to extract for 30 min. The filtrate was collected separately after filtering, and the residual was re-extracted for another twice in the same procedure. All three filtrates were collected together and concentrated on a rotary evaporator at 50 °C under vacuum until ethanol was removed completely. The concentrated solution was moved into a volumetric flask, and then distilled water was added to adjust the volume to 10 mL. All these extract solutions were stored at 4 °C until the following tests.
2.4. Determination of total phenolics content (TPc)
TPc values of all extracts were determined using Folin-Ciocalteu's reagent as described by Miao et al. (2018).
2.5. Determination of total flavonoids content (TFc)
TFc values were measured by aluminum chloride colorimetric method described by Miao et al. (2018).
2.6. Determination of total anthocyanins content (TAc)
The content of total anthocyanins was established by iron salt catalytic colorimetry method described by He et al. (2013). Chromogenic solution was developed with n-butanol, concentrated hydrochloric acid, and 10% ammonium ferric sulfate aqueous solution with the proportion of 83:6:1. Briefly, 0.1 mL sample solution was mixed together with 0.9 mL chromogenic solution in a test tube with plug, and the test tube was boiled in the boiling water for 40 min after shook well. After that, the test tube was cooled to room temperature with cooling water immediately, and the absorbance was determined at 550 nm with a full wavelength microplate reader. Procyanidin B1 was used as a standard, and TAc values of samples were expressed as mg procyanidin B1 equivalents per g dry weight.
2.7. Antioxidant activity
2.7.1. Total ferric reducing power (TFRP)
Total ferric reducing power was evaluated according to the method of Miao et al. (2018).
2.7.2. DPPH assay
DPPH free radical scavenge capacity was assessed by the method described by Miao et al. (2018), and EC50 value of each sample was counted.
2.7.3. ABTS assay
ABTS cation radical scavenging activity was performed according to the method reported by Miao et al. (2018). The results were presented as the EC50 values.
2.8. Anti-α-glucosidase activity
The α-glucosidase inhibitory activity was evaluated by the method described by Miao et al. (2018). In the experiment, solution of each sample was diluted to different concentrations, including 20, 10, 2, 1, 0.5, 0.25 and 0.05 mg dry weight/mL. The α-glucosidase inhibitory ratio was calculated, and IC50 value was worked out and expressed as mg dry weight per milliliter.
2.9. Qualitative analysis of nine monomer compounds by UPLC
Contents of nine monomer compounds in CT were determined by a Waters ACQUITY UPLC system, which combined with a reversed phase C18 column (2.1 mm × 100 mm, 2.7 μm, HALO 90 Å C18) at a flow rate of 0.2 mL/min with the injection volume of 5 μL. A binary solvent system was employed consisting of acetic acid/water (0.1/99.9, v/v) as solvent A and acetonitrile as solvent B. The diode array UV detector (DAD) was set at 280 nm to record the peak intensity of gallic acid, catechin, flavanomarein and isookanin, while 330 nm was set to record the signals of chlorogenic acid, quercetin, marein, okanin and rutin. The gradient program was 0–3 min with 98–80% solvent A, 3–7.8 min with 80% solvent A, 7.8–9 min with 80–52% solvent A, 9–14 min with 52% solvent A, and 14–15 min with 52–0% solvent A.
All the nine compounds in their UPLC chromatograms were identified by retention time, respectively, and their concentrations were reported as mg per g dry weight.
2.10. Statistical analysis
All data were calculated by three replicates and expressed as mean ± standard error of mean. The SPSS version 17.0 statistical software package was employed for all statistical analysis. The significant differences were detected by LSD and S–N–K tests, correlation matrix was analyzed by Pearson correlation coefficient, and cluster analysis was finished by hierarchical cluster procedure.
3. Results and discussion
3.1. Chemical composition
3.1.1. TPc, TFc and TAc in CT
Total phenolics are one kind of important component in CT contribute to antioxidant activity (Bai et al., 2018) and protective effect against acute liver damage (Zhi et al., 2018). Table 1 indicates CT dried by hot air drying at 50 °C has the highest TPc, and then followed by CT dried by sun drying and hot air drying at 30 °C.
Table 1.
Contents of total phenolics, flavonoids and anthocyanins in CT dried by different drying methods.
| Drying method | TPc (mg gallic acid/g dry weight) | TFc (mg rutin/g dry weight) | TAc (mg procyanidin B1/g dry weight) |
|---|---|---|---|
| vacuum drying at 30 °C | 42.67 ± 1.10bcd | 447.78 ± 2.60f | 1.26 ± 0.03b |
| vacuum drying at 40 °C | 40.20 ± 0.51a | 403.05 ± 12.91cd | 2.18 ± 0.05d |
| vacuum drying at 50 °C | 44.24 ± 1.90cde | 413.05 ± 4.71d | 2.46 ± 0.14e |
| vacuum drying at 60 °C | 44.53 ± 0.56def | 394.14 ± 4.96c | 2.02 ± 0.19d |
| hot air drying at 30 °C | 45.42 ± 0.47ef | 409.15 ± 4.88 cd | 0.91 ± 0.05a |
| hot air drying at 40 °C | 43.95 ± 0.35cdef | 365.34 ± 1.71b | 1.57 ± 0.12c |
| hot air drying at 50 °C | 46.45 ± 0.46f | 399.46 ± 7.91 cd | 1.59 ± 0.04c |
| hot air drying at 60 °C | 41.86 ± 0.92b | 396.48 ± 4.87c | 1.39 ± 0.14b |
| room drying | 43.73 ± 1.57 cd | 433.79 ± 2.48e | 1.52 ± 0.07c |
| sun drying | 46.00 ± 1.00 ef | 468.67 ± 6.38g | 0.98 ± 0.07a |
| freeze drying | 41.14 ± 1.13 ab | 331.29 ± 3.28a | 1.62 ± 0.03c |
Note: Different letters indicate that there are significant differences between the data at P < 0.05 level.
CT dried by hot air drying at 30 °C, 40 °C and 50 °C have higher TPc than samples dried by vacuum drying at 30 °C, 40 °C and 50 °C. CT dried by vacuum drying at 40 °C, freeze drying and hot air drying at 60 °C have lower TPc than other samples. In the vacuum drying samples, TPc decreases when temperature increases from 30 °C to 40 °C, and then increases when temperature increases from 40 °C to 60 °C. In the hot air drying samples, TPc changes in the similar trend with vacuum drying samples. Although samples dried by hot air drying at 30 °C, 40 °C and 50 °C show higher TPc values than samples dried by vacuum drying at 30 °C, 40 °C and 50 °C, while sample dried by hot air drying at 60 °C shows lower TPc values than samples dried by vacuum drying at 60 °C.
Total flavonoids is an well-known active component with antioxidant (Qiu, 2015), antidiabetic (Dias et al., 2010) and antihypertensive (Yang et al., 2014) activities, and TFc values of all dried CT are listed in Table 1. TFc values of CT dried by different methods vary significantly, and the highest TFc value appears in sun drying CT, followed by vacuum drying at 30 °C and room drying CT, while the lowest TFc value exists in freeze drying CT. Among vacuum drying group, CT dried by vacuum drying at 30 °C shows the highest TFc value, followed by CT dried by vacuum drying at 50 °C, 40 °C, and 60 °C, while CT dried by hot air drying at 30 °C shows the highest TFc value in hot air drying group, and then followed by CT dried by hot air drying at 50 °C, 60 °C, and 40 °C. Furthermore, hot air drying samples show lower TFc values than vacuum drying samples, this reduction was caused by the reaction of flavonoids with oxidase that occurred at elevated temperatures (Wang et al., 2016). Sun drying CT has the highest TFc, and in Scutellaria baicalensis flower sun drying sample also show higher TFc value than vacuum drying and room drying samples (Xiao et al., 2013), but the mechanism has not been reported.
Total anthocyanins also is another important active component with good antioxidant activity in CT (Xu et al., 2017). As list in Table 1, different dry CT have different TAc values, because the stability of anthocyanins could be affected by many factors, such as temperature, time, pH, oxygen, and so on (Ekici et al., 2014). The lowest TAc values present in CT dried by hot air drying at 30 °C and sun drying, while the highest TAc value appears in CT dried by vacuum drying at 50 °C. These results demonstrate that vacuum drying at 50 °C is helpful for the preservation of total anthocyanins in CT. In the vacuum drying samples, TAc increases constantly when temperature increases from 30 °C to 50 °C, and then decreases slightly when temperature increases from 50 °C to 60 °C, while hot air drying samples exhibit a similar tendency as vacuum drying samples.
However, different drying methods have different effects on the chemical compositions in CT. As we all know, a vacuum environment can reduce the exposition of the sample to oxygen, and hence protect some compounds before oxidation occurs. Therefore, TPc, TFc, and TAc in CT dried by vacuum drying methods always are higher than those contents in CT dried by hot air drying methods at the same temperature. Furthermore, anthocyanin is unstable to some drying factors, including high temperature, light, oxygen, and long drying duration (Lu et al., 2021). In this study, sun drying CT shows lower TAc value than room drying CT. It is similar to the production experience that sun drying CT would lose its bright yellow color. And TPc, TFc, and TAc all change in the same trends when drying temperature increases during the hot air drying and vacuum drying processes of CT. The decrease of TPc and TFc may mainly due to the effects of high drying temperature and enzymatic degradation which degraded flavonoids and phenolics (Kolla et al., 2021). Briefly, TPc, TFc, and TAc are mainly affected by enzymatic degradation and oxygen when CT is dried at 30 °C and 40 °C, while these three values are mainly affected by high drying temperature and oxygen when CT is dried at 50 °C and 60 °C at different extends.
Generally, freeze drying always protect active components from degradation in plants, flowers and fruits, but in the present study freeze drying CT provides the lowest TFc value. This may be due to the low enzymatic activity, which can promote the accumulation of total flavonoids and total phenolics, at −55 °C in the freeze drying environment. Moreover, many key enzymes would continuously influence the level of metabolisms of grape in response to dehydration treatments (Conde et al., 2018). Also, proper dehydration temperature can promote the accumulation of specific components. For instance, 30 °C and 40 °C could raise the content of chalcones, dihydroflavones, dihydroflavonols, flavanols, flavonoid carbonosides, proanthocy-anidins, and other phenols (Chen et al., 2022).
3.1.2. Antioxidant activity and anti-α-glucosidase activity of CT
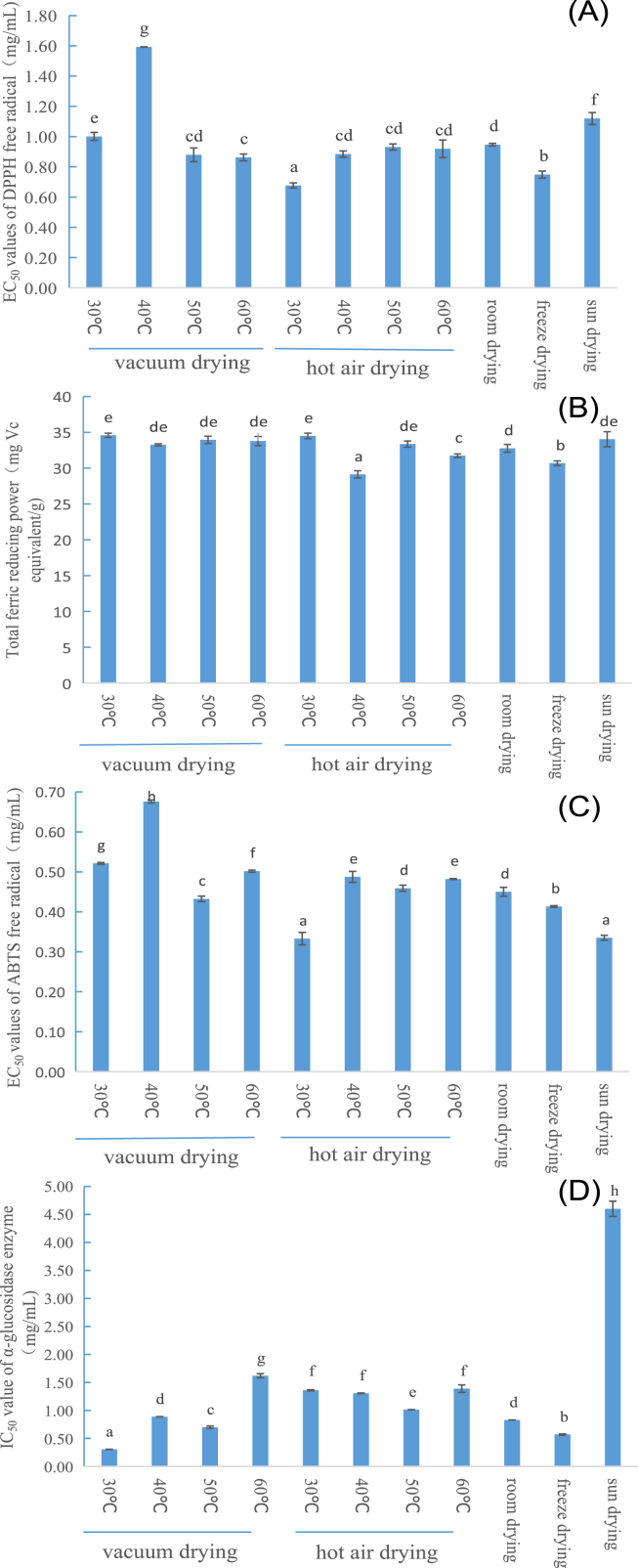
Due to the differences in chemical components in different CT, their antioxidant activity and anti-α-glucosidase activity both have significant differences. As we all know, different drying methods and drying temperatures both can affect the chemical composition, and they also can affect bioactivities of plants (Lou et al., 2015; Samoticha et al., 2016). In Figure 1(A), CT dried by hot air drying at 30 °C has the strongest scavenging activity on DPPH free radical, and then followed by samples dried by freeze drying and vacuum drying at 60 °C, while sample dried by vacuum drying at 40 °C has the weakest scavenging activity on DPPH free radical. As shown in Figure 1(B), TFRP of all samples ranged from 29.13 to 34.59 mg Vc equivalent/g dry weight, and the lowest TFRP exists in CT dried by hot air drying at 40 °C, while CT dried by hot air drying at 30 °C and vacuum drying at 30 °C both have the highest TFRP. Figure 1(C) also indicates that sun drying sample and 30 °C hot air drying sample both have the highest scavenging activity among all dry CT samples, and then followed by samples dried with freeze drying, vacuum drying at 50 °C, room drying and hot air drying at 50 °C.
Figure 1.
Antioxidant activity and anti-α-glucosidase activity of CT dried by different drying methods. (A) DPPH scavenging capacity, (B) FRAP, (C) ABTS scavenging capacity, and (D) anti-α-glucosidase activity.
Anti-α-glucosidase activities of all dried CT are listed in Figure 1(D), sun drying sample has the lowest anti-α-glucosidase activity, while sample dried by vacuum drying at 30 °C shows the highest anti-α-glucosidase activity, which is 14.8 times higher than sun drying sample, and followed by freeze drying, vacuum drying at 50 °C, room drying and vacuum drying at 50 °C. CT dried by hot air drying at 30 °C, 40 °C, 50 °C and 60 °C always have lower anti-α-glucosidase activity than freeze drying and room drying samples.
In fact, antioxidant activity and anti-α-glucosidase activity of CT already have been reported. Furthermore, CT extract shows good antioxidant activity both in vitro (Ma et al., 2016) and in vivo (Tian et al., 2019). Water extract and ethanol extract of CT both show good inhibitory effect on α-glucosidase enzyme, which is stronger than the inhibitory effect on α-glucosidase enzyme of acarbose (Zhang, 2011). However, in our study CT extracts didn't show higher inhibitory effect on α-glucosidase than acarbose. This difference may be ascribed to the different plant area of CT.
3.1.3. Contents of the nine selected active compounds in CT
Each active chemical component contains many active chemical compounds, and nine active chemical compounds have been analyzed in order to reveal the effects of drying methods on active chemical compounds. As listed in Table 2, contents of nine selected compounds in different CT samples varied significantly, and contents of gallic acid and quercetin both are lower than other compounds determined. CT dried by vacuum drying at 30 °C has the highest contents of flavanomarein and marein, CT dried by vacuum drying at 50 °C shows the highest contents of chlorogenic acid, okanin and quercetin, and sample dried by hot air drying at 30 °C shows the highest content of isookanin. At the same time, samples dried by room drying and freeze drying demonstrate the highest contents of rutin and catechin, respectively. Among these compounds, flavanomarein, isookanin, marein and okanin have been determined to evaluate the quality of CT (Gao et al., 2016), and marein and okanin both are the main chalcone in CT, which can affect the color and proportion of reddish brown part of the flower (Qi et al., 2017).
Table 2.
Contents of nine selected active compounds in CT dried by different drying methods.
| Drying method | Gallic acid | Chlorogenic acid | Catechin | Flavanomarein | Rutin | Isookanin | Marein | Okanin | Quercetin |
|---|---|---|---|---|---|---|---|---|---|
| vacuum drying at 30 °C | 0.21 ± 0.01b | 7.65 ± 0.07ab | 5.87 ± 0.01d | 11.97 ± 0.03i | 1.48 ± 0.00c | 4.54 ± 0.00h | 35.42 ± 0.02h | 1.18 ± 0.05c | 0.15 ± 0.00b |
| vacuum drying at 40 °C | 0.45 ± 0.00e | 12.89 ± 0.46g | 4.09 ± 0.03b | 10.13 ± 0.02d | 2.99 ± 0.06f | 3.74 ± 0.00e | 14.97 ± 0.07b | 0.95 ± 0.00b | 0.30 ± 0.01e |
| vacuum drying at 50 °C | 0.24 ± 0.01b | 14.01 ± 0.13h | 35.42 ± 0.02h | 11.07 ± 0.03f | 3.35 ± 0.01g | 5.89 ± 0.00j | 27.77 ± 0.04e | 2.60 ± 0.00i | 0.30 ± 0.00e |
| vacuum drying at 60 °C | 0.59 ± 0.01f | 7.12 ± 0.08a | 4.25 ± 0.01b | 11.08 ± 0.27f | 1.73 ± 0.01d | 4.79 ± 0.00i | 27.18 ± 0.01d | 1.90 ± 0.00f | 0.30 ± 0.00e |
| hot air drying at 30 °C | 0.58 ± 0.01f | 10.91 ± 0.43e | 5.62 ± 0.02cd | 10.98 ± 0.03f | 1.70 ± 0.01d | 6.16 ± 0.03k | 28.74 ± 0.05f | 2.05 ± 0.00g | 0.16 ± 0.00b |
| hot air drying at 40 °C | 0.34 ± 0.01c | 8.16 ± 0.03bc | 3.54 ± 0.59a | 8.99 ± 0.01c | 1.33 ± 0.00b | 3.68 ± 0.00d | 23.12 ± 0.04c | 1.27 ± 0.00d | 0.08 ± 0.00a |
| hot air drying at 50 °C | 0.18 ± 0.00a | 7.17 ± 0.01a | 3.48 ± 0.01a | 8.45 ± 0.06b | 1.11 ± 0.00a | 4.22 ± 0.01g | 28.15 ± 0.04e | 1.88 ± 0.06f | 0.18 ± 0.00c |
| hot air drying at 60 °C | 0.41 ± 0.01d | 12.00 ± 0.21f | 3.85 ± 0.10ab | 10.69 ± 0.05e | 1.87 ± 0.17e | 3.97 ± 0.01f | 30.90 ± 0.89g | 1.19 ± 0.01c | 0.19 ± 0.04c |
| room drying | 0.48 ± 0.06e | 7.77 ± 0.08bc | 3.84 ± 0.01ab | 11.31 ± 0.23g | 22.64 ± 0.00j | 3.01 ± 0.01b | 10.05 ± 0.01a | 0.29 ± 0.00a | 0.08 ± 0.00a |
| sun drying | 0.58 ± 0.01f | 9.05 ± 0.45d | 3.59 ± 0.10a | 11.77 ± 0.05h | 17.74 ± 0.09i | 3.33 ± 0.03c | 10.05 ± 0.00a | 1.65 ± 0.02e | 0.21 ± 0.00d |
| freeze drying | 0.58 ± 0.02f | 8.28 ± 0.36c | 9.06 ± 0.02e | 6.27 ± 0.04a | 13.50 ± 0.04h | 1.73 ± 0.01a | 10.38 ± 0.02a | 2.36 ± 0.03h | 0.15 ± 0.01b |
Note 1: Different letters indicate that there are significant differences between the data at P < 0.05 level.
Note 2: Units of all numbers are mg/g dry weight.
3.1.4. Antioxidant activity and anti-α-glucosidase activity of nine selected active compounds in CT
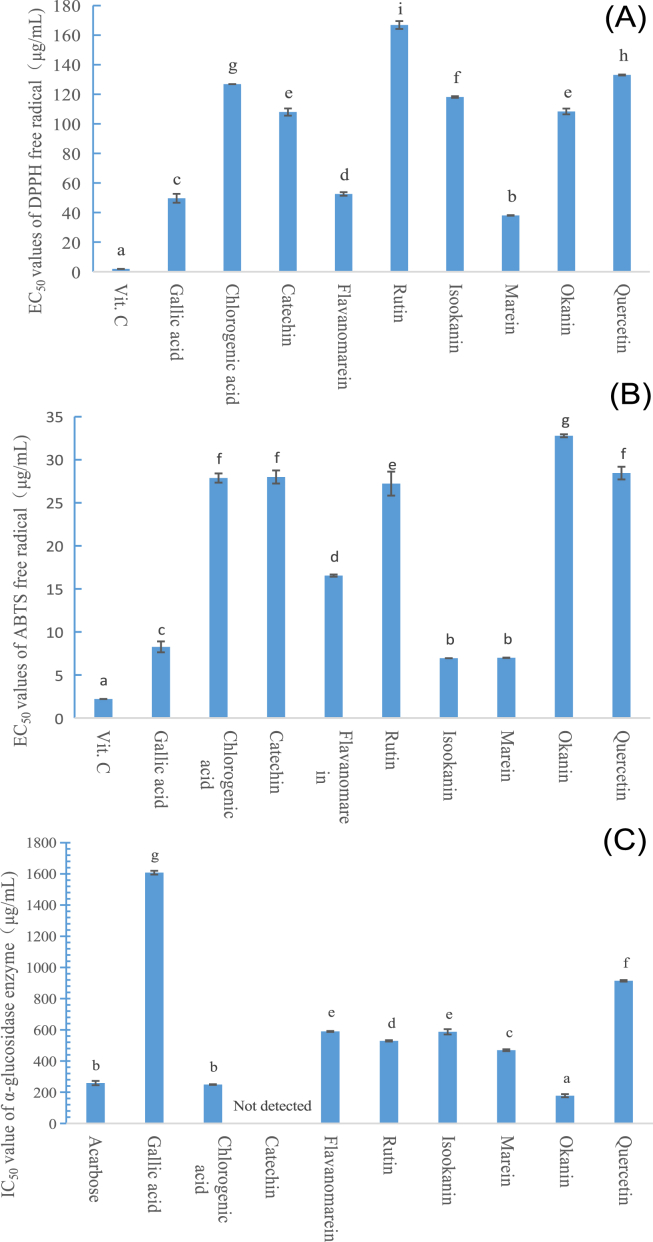
Many chemical components in CT have been identified as active components, such as flavone and phenol (Ma et al., 2016). Therefore, antioxidant activity and anti-α-glucosidase activity of nine selected active chemical compounds in CT were evaluated, and in this part antioxidant activity was evaluated by DPPH assay and ABTS assay.
Flavonoids and phenolics acid are resources of valuable antioxidants in CT, and nine compounds determined all show good DPPH and ABTS free radical scavenging activity. In Figure 2(A), nine active compounds all show weaker scavenging capacity on DPPH free radical than vitamin C, and marein shows the highest DPPH free radical scavenging capacity, and then followed by gallic acid, flavanomarein, catechin and okanin. Okanin and isookanin have been considered as the strongest antioxidants in CT ethanol extract when evaluated by DPPH assay (Ma et al., 2016), and in the present study marein and gallic acid both show better DPPH scavenging capacity than okanin and isookanin. As listed in Figure 2(B), marein and isookanin both show the best ABTS free radical elimination capacity than other seven compounds, and then followed by gallic acid and flavanomarein. Marein has been reported as the most important antioxidant in the total flavonoids of CT (Chen, 2014), okanin and isookanin both are good antioxidants in CT (Ma et al., 2016), and these reports are similar to the results of this study.
Figure 2.
Antioxidant activity and anti-α-glucosidase activity of nine active compounds in dried CT. (A) DPPH scavenging capacity, (B) ABTS scavenging capacity, and (C) anti-α-glucosidase activity.
According to Figure 2(C), okanin shows the highest anti-α-glucosidase activity, and then followed by chlorogenic acid, marein, rutin, isookanin, flavanomarein, quercetin and gallic acid, while catechin shows the lowest anti-α-glucosidase activity. Furthermore, okanin shows higher anti-α-glucosidase activity than acarbose, and chlorogenic acid shows similar anti-α-glucosidase activity to that of acarbose. Total flavonoids of CT can promote glucose tolerance regain through pancreatic function recovery in streptozotocin-induced glucose-intolerant rats (Dias et al., 2010), and also can help to protect diabetic endothelial (Li et al., 2020). As the main compounds in the total flavonoids of CT, marein can ameliorates diabetic nephropathy by inhibiting renal sodium glucose transporter 2 and activating the AMPK signaling pathway in db/db mice and high glucose-treated HK-2 cells (Guo et al., 2020), marein and flavanomarein both show cytoprotective effect on tBHP and cytokine-induced cell injury in pancreatic MIN6 cells (Dias et al., 2010). Moreover, CT extract could attenuate the cognitive damage and improve parameters related to brain senescence, partly by reducing oxidative stress and hippocampal damage, in which three phenolics acid and nine flavones have been detected (He et al., 2020).
By now, more and more people have realized the nutritional and health care function of CT. According to the reports, chlorogenic acid, flavanomarein and marein in CT could be absorbed in the intestine of rats, and mainly in the ileum (Wang et al., 2016), and concentration of okanin and isookanin in plasma would reach the peak after 20 min of intake (Ma et al., 2016).
Actually, CT dried by sun drying shows higher TPc and TFc values, as well as higher contents of gallic acid, chlorogenic acid, rutin and quercetin, than CT dried by vacuum drying at 30 °C, although sun drying sample owns the lowest anti-α-glucosidase activity. In all, more than 57 flavonoids and 24 phenolics have been reported by 2021 (Shen et al., 2021). Therefore, we have reasons to ascribe this unusual result to active compounds else with good anti-α-glucosidase activity other than the nine determined compounds.
3.1.5. Correlation analysis
Correlation analysis is finished by Pearson correlation coefficient with a two-tailed test, and the correlation coefficients are exhibited in Table 3. Contents of total flavonoids and flavanomarein both correlate with TFRP significantly at P < 0.05 level with the correlation coefficients of 0.725 and 0.663, respectively, and content of total phenolics correlates with ABTS elimination capacity significantly at P < 0.05 level with the correlation coefficients of 0.614. Total flavonoids and phenolics are well-known antioxidants in CT (Bai et al., 2018; Chen, 2014), and flavanomarein shows good antioxidant activity in this study. Thus, these results of correlation analysis are consistent with our understanding.
Table 3.
Correlation analysis between chemical composition and antioxidant and anti-α-glucosidase activities of CT.
| 1/EC50 of DPPH | 1/EC50 of ABTS | TFRP | 1/EC50 of α-Glucosidase enzyme | |
|---|---|---|---|---|
| TFc | −0.424 | 0.222 | 0.725∗ | 0.049 |
| TPc | 0.307 | 0.614∗ | 0.344 | −0.401 |
| TAc | −0.294 | −0.621∗ | −0.072 | 0.066 |
| Gallic acid | 0.218 | 0.415 | −0.024 | −0.479 |
| Chlorogenic acid | −0.180 | −0.048 | 0.153 | −0.133 |
| Catechin | 0.500 | 0.184 | −0.081 | 0.528 |
| Flavanomarein | −0.281 | 0.082 | 0.663∗ | −0.001 |
| Rutin | −0.083 | 0.373 | −0.056 | −0.117 |
| Isookanin | 0.233 | 0.141 | 0.590 | −0.034 |
| Marein | 0.172 | −0.268 | 0.242 | 0.242 |
| Okanin | 0.529 | 0.426 | 0.130 | −0.061 |
| Quercetin | −0.360 | −0.234 | 0.491 | −0.171 |
means significantly correlated with two-tailed test at the level of P < 0.05.
3.1.6. Cluster analysis and principal components analysis (PCA)
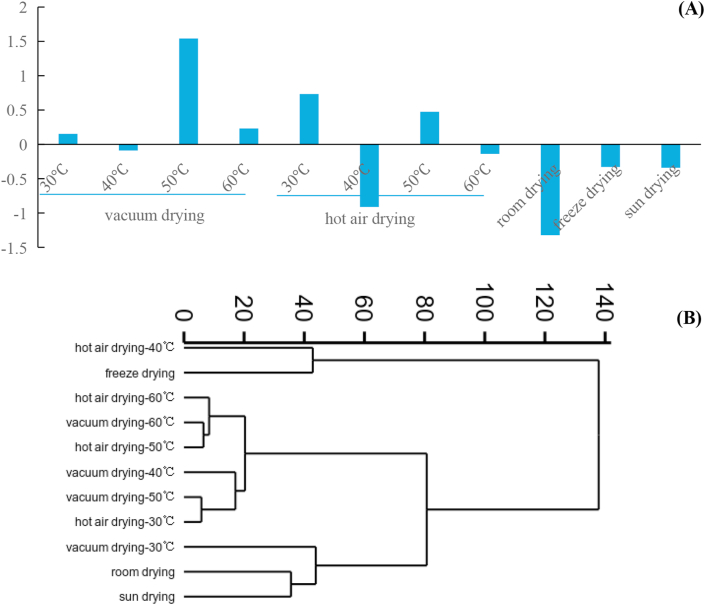
It is obvious that drying methods have significant effect on chemical composition and bioactivities of CT, which also exist in other flowers, such as Chrysanthemum morifolium Ramat (Wang et al., 2016). However, results which subjected to PCA analysis and cluster analysis are shown in Figure 3(A) & (B), respectively.
Figure 3.
Scores histogram of PCA (A) and cluster analysis of CT dried by different methods (B).
Based on the reduction of variables from a large number to a small amount of principal components, which can explain the analyzed data to the greatest extent, and the standardized data, factor analysis of PCA was performed. In this work, data of different CT were simplified and reduced to six principal components, and score scatter plot of PCA is shown in Figure 3 (A). In the process of PCA, all variables were reduced to six principal components, and they explained 92.56% of total variance. The first principal component explained 24.74% of the total variance, and contained TFRP, isookanin, flavanomarein and marein. The second principal component explained 20.73% of the total variance, and contained total flavonoids and rutin, while the third principal component explained 17.60% of the total variance containing DPPH elimination capacity, total phenolics, ABTS scavenging activity and okanin. The forth principal component consists quercetin and chlorogenic acid to explain 11.62% of the total variance, while the fifth principal component consists catechin and anti-α-glucsidase activity, and can explain 9.91% of the total variance, although the sixth principal component only consists of total anthocyanins and gallic acid, and explain 7.95% of the total variance. All CT samples, dried by eleven different drying methods, are scored in a comprehensive level (Zhang, 2005), and higher score means better quality in a comprehensive level based on chemical component and bioactivities. According to the results, sample dried by vacuum drying at 50 °C gets the highest score, and then closely followed by samples dried by hot air drying at 30 °C and hot air drying at 50 °C, while samples dried by freeze drying, sun drying, room drying, hot air drying at 40 °C and 60 °C, and vacuum drying at 40 °C all get negative scores. Furthermore, CT dried by room drying gets the lowest score. Thus, room drying is not the most ideal drying method for CT, and quality of CT could be greatly promoted by vacuum drying at 50 °C and hot air drying at 30 °C.
Cluster analysis was finished by within groups method and measured by euclidean distance method. In Figure 3 (B), CT samples dried by room drying and sun drying are divided into one subclass, which is divided into one larger subclass with sample dried by vacuum drying at 40 °C, while CT dried by freeze drying and hot air drying at 40 °C are separately divided into a single subclass. CT samples dried by hot air drying at 30 °C and vacuum drying at 50 °C are divided into one subclass with CT dried by vacuum drying at 40 °C. Meanwhile, samples dried by hot air drying at 50 °C and vacuum drying at 60 °C are differentiated into one subclass with CT dried by hot air drying at 60 °C.
Obviously, chemical components and bioactivities of CT can be affected by drying method, which always affects the quality of CT flower tea. Room drying is a low-cost and convenient method but with some shortcomings, such as needing large square, long-term, low speed and uncontrolled quality. All the results show hot air drying at 30 °C and vacuum drying at 50 °C are good choices for CT instead of room drying, and CT dried by hot air drying at 30 °C and vacuum drying at 50 °C both have similar quality in particular. In fact, freeze drying always can be seen as the most ideal drying method for many plants and flowers, while freeze drying can lower the quality of CT flower tea. Furthermore, hot air drying at 30 °C is a more energy-efficient and time-saving drying method than vacuum drying at 50 °C, because vacuum drying at 50 °C would take longer time and higher energy. Therefore, hot air drying at 30 °C is more useful and practiced for CT drying in the plant area.
According to Table 2, CT dried by vacuum drying at 50 °C shows the highest content of chlorogenic acid, and followed by CT dried by hot air drying at 30 °C and sun drying, while CT dried by hot air drying at 30 °C shows the highest content of okanin, and followed by CT dried by sun drying and vacuum drying at 50 °C. Chlorogenic acid and okanin both are active compounds with anti-α-glucosidase activity in CT, so drying method which can increase contents of chlorogenic acid and okanin would be a great help for the anti-α-glucosidase activity of CT. And CT dried by vacuum drying at 50 °C has the highest contents of chlorogenic acid and okanin. Generally speaking, contents of flavanomarein and marein have been used to evaluate quality of CT (Chen, 2014), and in the present study contents of flavanomarein and marein both increased when dried by hot air drying and vacuum drying, and CT dried by vacuum drying at 30 °C has the highest contents of flavanomarein and marein, but the change mechanism have not been studied yet. It is very clear that something is happening during the drying process of CT, and enzyme reaction in CT dried by freeze drying hardly occurs due to the low drying temperature. Consequently, the increased active compounds and bioactivities of CT are mainly caused by drying method, including enzyme reaction and oxidation reaction, while drying mechanism still is unexplored.
Above all, hot air drying and vacuum drying both can help to improve the quality of CT when compared with room drying and sun drying. However, only single drying method of CT is studied in the present study, so the combination of two or three kinds of drying methods would become the future research interest for the quality promotion of CT.
4. Conclusions
Drying methods would affect chemical compounds and bioactivities of CT, and different drying methods and drying temperatures have different effects. In the present study, effects of eleven drying methods on chemical composition and bioactivities of CT flower tea are evaluated in order to seek a more proper drying method for CT than room drying. As a result, vacuum drying at 50 °C, hot air drying at 30 °C and hot air drying at 50 °C are the top three drying methods for CT. And hot air drying at 30 °C would be better choice than vacuum drying at 50 °C, because hot air drying at 30 °C is more energy-efficient and time-saving than vacuum drying at 50 °C and hot air drying at 50 °C. Our results provide some new choices for the future drying process of CT.
Declarations
Author contribution statement
Jing Miao: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Jingjing Liu; Xiaoxia Gao; Fangyi Lu; Xue Yang: Performed the experiments; Analyzed and interpreted the data.
Funding statement
Dr. Jing Miao was supported by Tianchi Doctoral program in 2018 from the Xinjiang Uygur Autonomous Region (tcbs201815) and the Science and Technology Program from Xinjiang University (BS180227).
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
Supplementary content related to this article has been published online at [URL].
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Bai Y., Wang G., Wen H., Zhang L., Ni Y.Y., Li J.M. Effects of polyphenols from Kunlun chrysanthemum (Coreopsis tinctoria) on the oxidative stability of peanut oil. Food Sci. (N. Y.) 2018;39(10):52–58. [Google Scholar]
- Chen F. Suzhou University; 2014. Extraction Process of Total Flavonoid from Coreopsis tinctoria. [Google Scholar]
- Chen K.Q., Hu Y.J., Chen L., Zhang J.X., Qiao H.R., Li W.P., Zhang K.K., Fang Y.L. Role of dehydration temperature on flavonoids composition and free-form volatile profile of raisins during the drying process. Food Chem. 2022;374 doi: 10.1016/j.foodchem.2021.131747. [DOI] [PubMed] [Google Scholar]
- Conde A., Soares F., Breia R., Ger´os H. Postharvest dehydration induces variable changes in the primary metabolism of grape berries. Food Res. Int. 2018;105:261–270. doi: 10.1016/j.foodres.2017.11.052. [DOI] [PubMed] [Google Scholar]
- Dias T., Bronze M.R., Houghton P.J., Mota-Filipe H., Paulo A. The flavonoid-rich fraction of Coreopsis tinctoria promotes glucose tolerance regain through pancreatic function recovery in streptozotocin-induced glucose-intolerant rats. J. Ethnopharmacol. 2010;132:483–490. doi: 10.1016/j.jep.2010.08.048. [DOI] [PubMed] [Google Scholar]
- Ekici L., Simsek Z., Ozturk I., Sagdic O., Yetim H. Effects of temperature, time, and pH on the stability of anthocyanin extracts: prediction of total anthocyanin content using nonlinear models. Food Anal. Methods. 2014;7(6):1328–1336. [Google Scholar]
- Gao F., Chen F., Ma Q.L., Hao L.L. Simultaneous Determination of Four Flavonoids Components in Coreopsis tinctoria by HPLC. Chinese J. Exp. Tradit. Medical Formulae. 2016;22(20):49–52. [Google Scholar]
- Guo Y.L., Zheng R., Zhang Y.W., Song Z.P., Wang L.F., Yao L., Zhang M.F., Xin J.L., Mao X.M. Marein ameliorates diabetic nephropathy by inhibiting renal sodium glucose transporter 2 and activating the AMPK signaling pathway in db/db mice and high glucose-treated HK-2 cells. Biomed. Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110684. [DOI] [PubMed] [Google Scholar]
- He C., Wang Y.F., Ma C., Lu X.J., Wu Y. Optimization of ultrasonic-assisted extraction of proanthocyanidins from Kunlun chrysanthemum by response surface methodology. China Brew. 2013;8:107–111. [Google Scholar]
- He X.Q., Tian Y., Lei L., Zhi Q., Zhao J.C., Ming J. Protective effects of Coreopsis tinctoria buds extract against cognitive impairment and brain aging induced by D-galactose. J. Funct.Foods. 2020;73 [Google Scholar]
- Kolla M.C., Laya A., Bayang J.,P., Koubala B.B. Effect of different drying methods and storage conditions on physical, nutritional, bioactive compounds and antioxidant properties of doum (hyphaene thebaica) fruits. Heliyon. 2021 doi: 10.1016/j.heliyon.2021.e06678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.J., Huang C.R., Fu W.W., Zhang H. Screening of the active fractions from the Coreopsis tinctoria Nutt. Flower on diabetic endothelial protection and determination of the underlying mechanism. J. Ethnopharmacol. 2020;253 doi: 10.1016/j.jep.2020.112645. [DOI] [PubMed] [Google Scholar]
- Lou S.N., Lai Y.C., Huang J.D., Ho C.T., Ferng L.H.A., Chang Y.C. Drying effect on flavonoid composition and antioxidant activity of immature kumquat. Food Chem. 2015;171:356–363. doi: 10.1016/j.foodchem.2014.08.119. [DOI] [PubMed] [Google Scholar]
- Lu J.D., Wang Z., Qin L.H., Shen J.Y., He Z., Shao Q.S., Lin J. Drying methods affect bioactive compound contents and antioxidant capacity of Bletilla striata (Thunb.) Reichb.f. flower. Ind. Crop. Prod. 2021;164 [Google Scholar]
- Ma Z.Y., Zeng S.R., Han H.X., Meng J., Yang X., Zeng S., Zhou H., Liang H.D. The bioactive components of Coreopsis tinctoria (Asteraceae) capitula: antioxidant activity in vitro and profile in rat plasma. J. Funct.Foods. 2016;20:575–586. [Google Scholar]
- Miao J., Li X., Zhao C.C., Gao X.X., Wang Y., Gao W.Y. Active compounds, antioxidant activity and α-glucosidase inhibitory activity of different varieties of Chaenomeles fruits. Food Chem. 2018;248:330–339. doi: 10.1016/j.foodchem.2017.12.018. [DOI] [PubMed] [Google Scholar]
- Qi L., Luo Q.Z., Dai D., Zhang Y. Study on correlation between macroscopic properties and qualities of head inflorescences of Coreopsis tinctoria. Mod. Chin. Med. 2017;19(8):1126–1130. [Google Scholar]
- Qin L.W., Wang H., Zhang W., Pan M.Q., Xie H., Guo X.B. Effects of different drying methods on phenolic substances and antioxidant activities of seedless raisins [J] LWT–Food Sci. Technol. 2020;131 [Google Scholar]
- Qiu J.J. Suzhou University; 2015. Preparation and Antioxidant Capacities of Total Flavonoid from Coreopsis Tinctoria. [Google Scholar]
- Samoticha J., Wojdyło A., Lech K. The influence of different the drying methods on chemical composition and antioxidant activity in chokeberries. LWT--Food Sci. Technol. 2016;66:484–489. [Google Scholar]
- Shen J., Hu M.Y., Tan W., Ding J.W., Jiang B.P., Xu L., Hamulati H., He C.N., Sun Y.H., Xiao P.G. Traditional uses, phytochemistry, pharmacology, and toxicology of Coreopsis tinctoria Nutt.: a review. J. Ethnopharmacol. 2021;269 doi: 10.1016/j.jep.2020.113690. [DOI] [PubMed] [Google Scholar]
- Shi Y., Chen G.J., Chen K.W., Chen X.H., Hong Q.Y., Kan J.Q. Assessment of fresh star anise (Illicium verum Hook.f.) drying methods for influencing drying characteristics, color, flavor, volatile oil and shikimic acid. Food Chem. 2020;342(1) doi: 10.1016/j.foodchem.2020.128359. [DOI] [PubMed] [Google Scholar]
- Soysal Y. Microwave drying characteristics of parsley. Biosyst. Eng. 2004;89(2):167–173. [Google Scholar]
- Tian Y., Wen Z., Li L., Li F., Ming J. Coreopsis tinctoria flowers extract ameliorates D-galactose induced aging in mice via regulation of Sirt1-Nrf2 signaling pathway[J] J. Funct.Foods. 2019;60 [Google Scholar]
- Wang J., Aierken G., Li X.X., Li L.L., Mao X.M. Testing the absorption of the extracts of Coreopsis tinctoria Nutt. in the intestinal canal in rats using an Ussing chamber. J. Ethnopharmacol. 2016;186:73–83. doi: 10.1016/j.jep.2016.03.061. [DOI] [PubMed] [Google Scholar]
- Xiao S.P., He C.N., Zeng Y., Tian Z., Zhao R.H., Wang J.Y., Song G.H., Hang Z.B., Du Y.J., Tian D.F. Effects of different drying methods and harvesting time on content of flavonoids from flowers of Scutellaria baicalensis. Modern Chin. Med. 2013;15(11):975–980. [Google Scholar]
- Xu D.D., Chen A.X., Ding H.L. Optimization of ultrasonic-microwave synergistic extraction of pigment from Kunlun Coreopsis tinctoria and its antioxidant activity. China Food Addit. 2017;2:91–98. [Google Scholar]
- Yang Q., Sun Y.H., Zhang L., Xu L., Hu M.Y., Liu X.Y., Shi F.Y., Gu Z.Y. Antihypertensive effects of extract from flower buds of Coreopsis tinctoria on spontaneously hypertensive rats. Chin. Herbal Med. 2014;6(2):103–109. [Google Scholar]
- Zhang W.L. Operation and application of principal component analysis in SPSS. Market. Res. 2005;12:31–34. [Google Scholar]
- Zhang S.P. Xinjiang Mecical University; 2011. The Research of Coreopsis tinctoria Flowers Extracts on Glucose Metabolism in Vitro. [Google Scholar]
- Zhang Y., Shi S., Zhao M., Chai X., Tu P. Coreosides A-D, C14-polyacetylene glycosides from the capitula of Coreopsis tinctoria and its anti-inflammatory activity against Cox-2. Fitoterapia. 2013;87:93–97. doi: 10.1016/j.fitote.2013.03.024. [DOI] [PubMed] [Google Scholar]
- Zheng M.Y., Xia Q.L., Lu S.M. Study on drying methods and their influences on effective components of loquat flower tea. LWT--Food Sci. Technol. 2015;63(1):14–20. [Google Scholar]
- Zhi Q., Li Y., Li F., Tian Y., Li F., Tang Y., Yang Y., Yin R., Ming J. Polyphenols extracted from Coreopsis tinctoria buds exhibited a protective effect against acute liver damage. J. Funct.Foods. 2018;44:201–208. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.