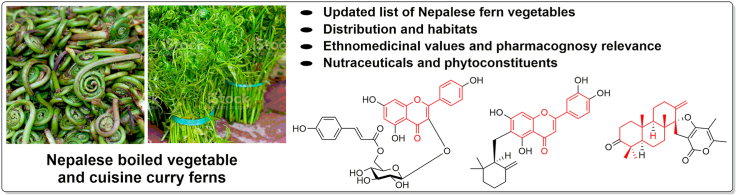
Abstract
Ferns are used as traditional and fascinating foods in many countries. They are also considered to possess important ethnomedicinal values; however, ferns are one of the underutilized plant resources by both scientific and local communities. Pharmagonostical studies reveal that ferns and fern-allies possess several biological activities including antibacterial, antiviral, antifungal, antimalarial, antidiarrheal, anthelmintic, analgesic, anti-inflammatory, antidiabetic, anticancer, neuroprotective, nephroprotective, hepatoprotective, antifertility, etc. Flavonoids and terpenoids are major secondary metabolites present in ferns. Ugonins, particularly isolated from Helminthostachys zeylanica, have found diverse bioactivities. Ptaquiloside, a norsesquiterpene glucoside, found in Pteridium revolutum, Dryopteris cochleata and Polystichum squarrosum, is one of the hazardous metabolites of ferns which is responsible for the toxic effect. Alkaloids are reported to be present in the ferns; however, the qualitative data are uncertain. Some fern metabolites, such as cyanogenic glycosides and terpenoids, are considered to possess defensive activity against animal attacks. Some ferns are also used for manuring as biological alternative to pesticides. Nepalese have consumed at least 33 species of ferns and fern-allies belonging to 13 families, 20 genera as cooked vegetable foods. The aim of this review is compilation of information available on their distribution, ethnomecinal values, pharmcognosy, pharmacology and phytochemistry.
Keywords: Bioactivity, Biodiversity, Food, Pteridophytes, Secondary metabolite, Traditional knowledge
Graphical abstract
Highlights
-
•
Ferns and fern-allies are consumed as a vegetable in many countries.
-
•
Ferns possess several ethnomedicinal values, but are less explored scientifically.
-
•
Flavonoids, terpenoids and steroids are major secondary metabolites found in ferns.
-
•
This review compiles pharmcognosy and phytochemistry of 33 wild ferns eaten by Nepalese as cooked vegetable foods.
Bioactivity; Biodiversity; Food; Pteridophytes; Secondary metabolite; Traditional knowledge.
1. Introduction
Nepal is a small land-locked country resides on the lap of the great Himalaya, in the north. It is sandwitched between China and India. Despite occupying only 0.1% of the global area, the country is naturally blessed and enriched with cultural, geographical and biological diversities [1]. Its cultural diversities are interlinked with more than 60 ethnic groups speaking more than 100 languages. Geographically, it has a large altitudinal variations ranging from a low land (altitude nearly 60 m in south) to the higest peak of the earth (Mt. Everest, 8,848.86 m in north) within a span of just about 200 km of aerial expansion from south to north. Its physiographic regions comprises (1) Tarai, (2) Siwaliks and Chure, (3) Mahabharat, (4) Midlands or central hills, (5) Himalayas, and (6) Inner Himalayas and Tibetan marginal mountains [2]. The country has 118 different types of ecosystems harboring 3.2% of the world's flora including 5.1% of gymnosperms, 3.2% of angiosperms, 5.1% of pteridophytes, 8.2% of bryophytes, 2.5% of algae, 2.6% of fungi and 2.3% of lichens [1].
For food and livelihood securities, Nepalese significantly depend on cultivated and wild vegetables [3]. In Nepal, it has been estimated that >650 species are used as food materials, and >1,000 species possess medicinal values [4]. Recently, we have listed 318 wild plant species (excluding mushrooms, fruits, spices and condiments, seeds and pulses) that are consumed by Nepalese as vegetables [5]. Wild vegetables are indispensable constituent of the human diet and are the nature's gift to mankind. They are not only cheap but also supply minerals, vitamins and certain hormone precursors. Among them, wild edible ferns play an important role in diet, and they are also a measure of income source with no investment.
Wild plants are globally used by human societies for food, medicine, ornament, defence and industrial purposes. Ferns and fern-allies have unique features in their appearance, natural habitat, food value and ethnomedicinal properties. Ferns possess a wide range of medicinal activities; unfortunately, they are poorly investigated. Comprehensive review on phytochemicals and bioactivities of ferns and fern-allies is rare. It is commonly considered that plants grown in harsh environmental conditions usually possess incredible bioactivity. Therefore, writing of this review article is envisioned to increase scientific attention on the fern species, particularly those grown in high altitudes of Nepal. Consequently, an extensive literature search was conducted. Dissertations, books, journal articles and conference proceedings on ethnobotonical surveys in Nepal were accessed in the libraries to generate a list of wild vegetable food ferns of Nepal. Taxonomic names mentioned in the original articles were verified using several efloral databases. Online literature search was conducted in Google Scholar and Pub Med by using the terms ferns, pteridophytes and individual name of the fern species. Cited references in the published research articles were also traced to obtain information on pharmacognosy, phytochemicals and bioactivites of the fern species grown across the globe.
2. Ferns and fern-allies (pteridophytes)
Fern and fern-allies (pteridophytes) are considered as a very ancient family. They are the first vascular land plants that evoluted during the Devonian and Carboniferous periods of the Paleozoic era [6]. Pteridophytes dominated the earth's vegetation in the beginning of the Mesozoic era, about 280–230 million years ago [7, 8]. Ferns are consumed as vegetables in many countries across the globe and they are also used in the traditional Chinese medicines [9, 10]. However, surprisingly, compared to other vascular plants, pteridophytes are remain under-explored both in ethnobotanical and pharmacological aspects. In the last decade, some natural compounds have been isolated from different fern species and some phramacognostical studies are begun [11]. Initially, traditional knowledge based ferns were ethnomedicinally used for some ailments, and the major studies on pteridophytes were primarily focused on taxonomic identification.
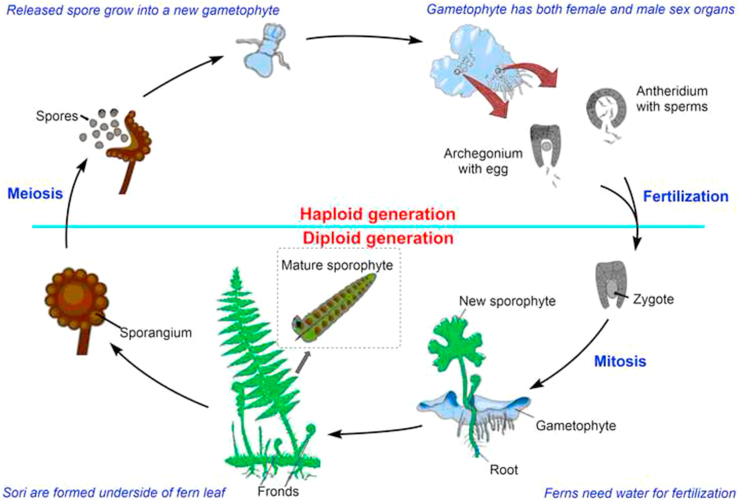
Ferns are seedless vascular plants and they reproduce by sporulation. They have many similar features of mosses and algae, but are usually differentiated from them by having xylem and phloem to transport water and nutrient materials. Like other vascular plants (gymnosperms and angiosperms), they possess stems, fronds, pinnae and roots. The life-cycle of fern is referred to as alternation of generations with two different stages‒gametophyte phase (sexual) and sporophyte phase (asexual) (Figure 1). Motile male gametes (antherozoids) are produced from antheridia and non-motile female gametes (egg cells) are bornt singly in archegonia. Fusion of both the gametes results in the formation of a zygote, which develops into the sporophyte (diploid) after mitotic divisions. Fern sporophytes are free-living, independent on the gametophyte (prothallus), dominant and grow to a much larger size. Mature sporophyte bears sori on the underside of the blade. Sporangia release a number of non-motile spores that germinate and grow by mitotic divisions into haploid gametophytes.
Figure 1.
Fern lifecycle.
It has been estimated that there are around 4,03,000 plant species in the earth including phanerogams and cryptogams [12]. About 13,271 species of pteridophytes are distributed which forms nearly 3% of the world flora [13]. The Plant List has listed 35 plant families and 568 genera of pteridophytes [14]. About 63 families, 230 genera and 2,600 species of pteridophytes have been reported in China [15]. From India, 33 families, 130 genera and 1,267 species of ferns and fern-allies are reported [16]. About 37 families, 687 species in Japan [17]; 801 species in Taiwan [18]; 39 families, 144 genera, 1,100 species in the Philippines [19]; 1,165 species in Malaysia [20], and 670 species of pteridophytes in Thailand [21] have been reported.
In Nepal, ferns are called as “Unyu/Oony” and they are distributed between 60 to 4,800 m above sea level (asl) [22, 23]. Thapa et al. [24] have listed 535 species of pteridophytes belonging to 35 families and 102 genera from Nepal. Recently, a book Ferns and Fern-allies of Nepal [25] has been published including annotated checklist and critical account of 550 species and additional 32 sub-species, altogether 582 taxa of fern and fern-allies of Nepal belonging to 32 families and 99 genera. In Nepal, Deparia boryana, Diplazium esculentum, Dryopteris cochleata, Ophioglossum vulgatum, Pteridium revolutum, Diplazium maximum, Diplazium spectabile, Diplazium stoliczkae, Polystichum squarrosum, Pteris biaurita, Tectaria gemmifera, etc. are popularly consumed as vegetables, and they are also sold in the markets [5, 26]. Popularity gained in consumption of ferns in Nepalese communities is not only due to their unique taste but also due to beliefs of their high nutritional contents such as vitamin C and iron [27]. Obviously, it is considered that the nutritional value and mineral content in wild edible plants are richer than that of commercial vegetables [28]; therefore, their consumption for the nutritional purpose should be encouraged. At the same time, their poor availability and prone vulnerability are the serious issues.
3. History, expeditions and literature on Nepalese pteridophytes
Species Plantarum, originally published in 1753 by Carl Linnaeus, is the first botanical work that applied the binomial nomenclature sytem for the taxonomic description of 5,940 plant species, and recognized 15 fern genera [29]. The pioneering plant collection and taxonomic study of Nepalese pteridophytes were first begun in the early 19th century when Scottish Franchis Buchannan (later known as Franchis Hamliton) collected 433 plant specimens including 34 species of Nepalese pteridophytes in 1802–1803 and published An Account of Kingdom of Nepal [30, 31, 32]. In subsequent historical book publications, namely, Tentamen Florae Nepalensis Illustratae by Wallich [33], Prodromus Florae Nepalensis by Don [34], The Flora of British India by Hooker [35], Index Filicum by Moore [36], A Priced Catalogue of Hardy Exotic and British Ferns by Sim [37], Catalogue of the Plants of Kumaon by Duthie [38], Notes from a Journey to Nepal by Burkill [39] and A Plantsman in Nepal by Lancaster [40] have documented many Nepalese ferns. Some foreign authors have also contributed in the taxonomic identification of pteridophytes of Nepal [41, 42, 43, 44].
After establishement of Botanical Survey and Herbarium Office by Nepal Government in 1961 (later renamed as National Herbarium and Plant Laboratories), Nepalese botanists have started collection of plants and preservation of herbariums, and then they could publish several articles and books [45]. Earlier contributions are made by Gurung [46, 47, 48, 49] and Manandhar [50, 51, 52, 53] followed by others [24, 54, 55, 56, 57].
A major contribution of plant collection and taxonomic studies on Nepalese pteridophytes including Sikkim Himalayan ferns has been made by Christopher Fraser-Jenkins, a Welsh pteridologist, who resides for 40 years in Kathmandu, Nepal. Formerly, he was a research fellow at the Natural History Museum, London, and at the Royal Botanic Garden, Edinburgh. Lately, he worked in conjunction with the National Herbarium and Plant Laboratories Godawari, Nepal. He has spent more than five and half decades in studying Himalayan pteridophytes and published Ferns and Fern-allies of Nepal, Vols. 1–3 [58, 59, 25]; Annotated Checklist of Indian Pteridophytes, Parts 1–3 [60, 61, 62]; including a series of other publications [63, 64, 65].
A number of works on exploration, taxonomic identification and documentation along with rare molecular study on Nepalese pteridophytes have been made; however, not only Nepalese but also global edible peridophytes are poorly investigated for their phytochemical constituents and pharmacological effects. Therefore, exploitation of both edible and non-edible fern flora deserves serious attention by scientific communities [66].
4. A list of wild edible ferns of Nepal
From the literature survey of the publications on Nepalese ferns by various authors, we have generated a list of 33 edible fern species (Table 1). The taxonomic identification of the species was followed according to Kramer and Green [67], modified by Fraser-Jenkins [65], and was cross-verified in the e-databases of The Plant List [68] and Flora of China [69]. Cornopteris decurrenti-alata and Matteuccia intermedia are rarely occurred in Nepal, while Blechnum orientale, Osmunda japonica, Coniogramme intermedia and Pteris wallichiana are common species [31]. These species are used as fern food in China [70]. We could not find proper documentation for these pteridophytes as wild edible ferns of Nepal; therefore, they are omitted in the list. The pteridophytes, which consumed for the medicinal values, but not eaten as salad or cooked vegetables, are also not included in the table [71].
Table 1.
List of edible ferns and fern-allies of Nepal that eaten as vegetables.
| SN | Botanical name [synonym] | Local name (in different community) | Habit | Vegetative parts | Altitude | Season | Availability | References |
|---|---|---|---|---|---|---|---|---|
| Athyriaceae | ||||||||
| 1 | Athyrium atkinsonii Bedd. | Niuro | Herb | Young leaf | 1,200‒4,000 | - | Common | [25, 31, 72] |
| 2 | Athyrium strigillosum (T. Moore ex E. J. Lowe) T. Moore ex Salomon | Niguro | Herb | Young frond | 600‒2,600 | - | Common | [25, 31] |
| 3 | Deparia boryana (Willd.) M. Kato [Dryoathyrium boryanum (Willd.) Ching] | Kalo niuro, Dhengan (Tamang) | Herb | Leaf, tender shoot | 400‒3,300 | May–Jul | Common | [26, 31, 73, 74, 75, 76] |
| 4 | Diplazium esculentum (Retz.) Sw. | Masino niuro, Chipley niuro, Kuturke, Pani nyuro, Piraunli | Herb | Young leaf | 100‒1,300 | Apr–Oct | Common | [23, 26, 31, 73, 74, 77, 78] |
| 5 | Diplazium kawakamii Hayata | Niuro, Jire ningro (Darjiling) | Herb | Tender shoot | 1,300‒2,400 | - | Common | [31, 59, 72] |
| 6 | Diplazium maximum (D. Don) C. Chr. [Allantodia maxima (D. Don) Ching] | Neuro | Herb | Leaf | 9,00–1,800 | May–Jul | Common | [26, 31, 79] |
| 7 | Diplazium spectabile (Wall. ex. Mett.) Ching [Allantodia spectabilis (Wall. ex Mett.) Ching] | Saune niuro | Herb | Leaf | 1,500‒2,700 | May–Jul | Common | [26, 31] |
| 8 | Diplazium stoliczkae Bedd. [Allantodia stoliczkae (Bedd.) Ching] | Kalo niuro, Lekh chipley niuro, Bhandhengan (Tamang) | Shrub | Tender shoot, leaf | 1,300‒4,000 | May–Jun | Common | [26, 31, 76, 80] |
| Blechnaceae | ||||||||
| 9 | Stenochlaena palustris (Burm. f.) Bedd. | - | Climber | Young shoot | 60–900 | - | Rare | [25, 31, 72] |
| 10 | Woodwardia unigemmata (Makino) Nakai | - | Shrub | Stem | 400‒3,000 | Jun–Jul | Common | [26, 31, 73, 74] |
| Cyatheaceae | ||||||||
| 11 | Cyathea spinulosa Wall. ex Hook. [Alsophila spinulosa (Wall. ex Hook.) R.M. Tryon] | Chattre, Rukh uniyu, Thulo uniyu, Jatashankari (Indian) | Shrub | Young shoot | 300‒1,600 | Jul–Aug | Scattered to common | [23, 31, 81] |
| Dennstaedtiaceae | ||||||||
| 12 | Pteridium revolutum (Blume) Nakai [Pteridium aquilinum subsp. wightianum (J. Agardh) W.C. Shieh] | Saune niuro, Ainu | Herb | Shoot | 600‒3,000 | Jul–Jul | Common | [8, 26, 31, 73, 74] |
| Dryopteridaceae | ||||||||
| 13 | Dryopteris cochleata (D. Don) C. Chr. | Pani niuro, Ghee niuro, Danthe niuro, Lotah niuro | Herb | Tender leaf | 1,200‒1,600 | Mar–Oct | Common | [23, 26, 31, 72, 78, 80, 82] |
| 14 | Dryopteris splendens (Hook. ex Bedd.) Kuntze | Danthe neuro | Herb | <1,000 | Jul–Sep | Scattered | [31, 83] | |
| 15 | Polystichum squarrosum (D. Don) Fée [Polystichum apicisterile Ching & S.K. Wu] | Phusre niuro, Rato unyu | Herb | Shoot | 1,900‒2,400 | May–Jun | Common | [26, 31, 73, 74, 80] |
| Lygodiaceae | ||||||||
| 16 | Lygodium flexuosum (L.) Sw. | Parandi sag, Lahare unyu | Climber | Young shoot, leaf | 60‒1,000 | Whole year | Common | [23, 31, 74] |
| 17 | Lygodium japonicum (Thunb.) Sw. | Janai lahara, Nagbeli, Lute jhar, Ukuse jhar (Magar), Pinse (Tamang) | Climber | Leaf | 60‒3,900 | May–Jun | Common | [23, 26, 31, 74, 84] |
| Marsileaceae | ||||||||
| 18 | Marsilea quadrifolia L. | Charpate behuli | Herb | Tender shoot | 900‒1,700 | [85] | ||
| Nephrolepidaceae | ||||||||
| 19 | Nephrolepis cordifolia (L.) C. Prest | Pani amala, Bhui amala, Ras amala, Ambeli (Tamang), Pani saro (Majhi) | Herb | Tuber, shoot | 60‒2,400 | Jul–Sep | Common | [23, 31, 74, 75, 77, 81] |
| Ophioglossaceae | ||||||||
| 20 | Botrychium lanuginosum Wall. ex Hook. & Grev. | Jaluko, Bayakhra | Herb | Shoot | 1,000–3,000 | May–Jun | Common | [26, 31, 74] |
| 21 | Helminthostachys zeylanica (L.) Hook | Majurgoda (Chepang), Kamsaj (Tharu) | Herb | Tender shoot | <1,000 | Scattered | [31, 75] | |
| 22 | Ophioglossum nudicaule L.f. | Jibre sag | Herb | Leaf | 1,800‒4,300 | Mar–Apr | Common | [26, 73, 74] |
| 23 | Ophioglossum petiolatum Hook. | Jibre sag | Herb | Leaf | 200‒3,300 | Feb–Apr | Scattered | [31, 84] |
| 24 | Ophioglossum reticulatum L. | Jibre sag, Ek patiya (Tharu), Jibre dhap (Tamang) | Herb | Young leaf, tender shoot | 1,100‒4,000 | Mar–Apr | Common | [26, 31, 73, 74, 77, 80] |
| 25 | Ophioglossum vulgatum L. | Jibre sag | Herb | Whole plant | <3,000 | Mar–Apr | Common | [74] |
| Osmundaceae | ||||||||
| 26 | Osmunda claytoniana L. [Osmunda claytoniana subsp. vestita (Milde) Á. Löve & D. Löve] | Kuthurke | Herb | Leaf | 1,600‒3,400 | May–Jun | Common | [26, 31, 73, 74, 77] |
| Pteridaceae | ||||||||
| 27 | Ceratopteris thalictroides (L.) Brongn. | Pani dhaniya, Dhaniya jhar | Herb | Whole plant | <1,000 | Jun–Aug | Scattered | [23, 31, 74, 85, 86, 87] |
| 28 | Pteris biaurita L. [Pteris flavicaulis Hayata] | Haade unyu, Kuthurke niuro (Raji) | Herb | Young leaf, shoot | 200‒2,500 | Aug–Oct | Common | [23, 31, 78, 88] |
| 29 | Pteris vittata L. | Unigar, Urakthewn | Herb | Shoot, rhizome | 400‒3,000 | Jul–Sep | Common | [23, 31, 74] |
| Tectariaceae | ||||||||
| 30 | Tectaria gemmifera (Fée) Alston [Tectaria coadunata C. Chr.] | Danthe niuro, Kuthurke, Toplign degni (Tamang) | Herb | Young leaf, shoot | 500‒2,500 | Jun–Oct | Common | [23, 26, 31, 73, 74, 78, 82, 86, 89, 90] |
| 31 | Tectaria zeylanica (Houtt.) Sledge [Osmunda zeylanica L.] | Mayur kutea, Dhagrajawa (Tharu) | Herb | Leaf | 100‒1,000 | Mar–Apr | [74, 77] | |
| Thelypteridaceae | ||||||||
| 32 | Thelypteris auriculata (J. Sm.) K. Iwats [Cyclosorus auriculata (J. Sm.) C.M. Kuo] | Kochaya | Herb | Shoot | 1,600‒1,800 | Mar–May | Common | [31, 74] |
| 33 | Thelypteris nudata (Roxb.) C.V. Morton [Thelypteris multilineata (Wall. ex Hook.) C.V. Morton; Pronephrium nudatum (Roxb.) Holttum] | Koche (Tharu) | Herb | Shoot | 2,100 | Jun–Jul | Common | [26, 31, 73, 74] |
5. Distribution, ethnomedicine, pharmacognosy and phytochemistry of the wild edible ferns
It has been recently reported that there are 582 taxa of pteridophytes in Nepal [72]; however, Nepalese researchers have not been focused in the ethnopteridological studies in traditional medicinal knowledge, food safety and phytoconstituents present in the pteridophytes. At the same time, surprisingly, very few scientific studies have been carried out globally in the areas of the chemical constituents and pharmacological activites of pteridophytes [71]. In this section, we accumulate available information on the distribution, ethnomedicinal uses, pharmacognosy and phytochemistry of the wild edible ferns listed in Table 1.
C (central), N (north), E (east), W (west) and S (south) abbreviations are used to indicate distribution of the fern species. The edible fern distribution in China and India is specified as available in different provinces.
5.1. Athyriaceae
5.1.1. Athyrium atkinsonii
5.1.1.1. Distribution
W, C and E Nepal; Chongqing, Fujian, Gansu, Guizhou, Henan, Hubei, Hunan, Jiangxi, Shaanxi, Shanxi, Sichuan, Xizang and Yunnan; Arunachal, Sikkim, Darjeeling, Uttarakhand, Himachal, Jammu and Kashmir; Pakistan; Bhutan; Myanmar; Taiwan; S Korea; Japan; etc. [31, 69].
5.1.1.2. Habitat
Damp forest at high altitude.
5.1.1.3. Phytochemistry
The plant material contains carbohydrates, proteins, alkaloids, phenolics, flavonoids, etc. [91].
5.1.2. Athyrium strigillosum
5.1.2.1. Distribution
W, C and E Nepal; Guangdong, Guangxi, Guizhou, Hunan, Jiangxi, Sichuan, Xizang and Yunnan; Sikkim, Darjeeling, Uttarakhand, Himachal, Jammu and Kashmir; Pakistan; Bhutan; Myanmar; Taiwan; Japan; etc. [31, 69].
5.1.2.2. Habitat
Wet forest and partially exposed area or along banks and beds of well-shaded ravines.
5.1.3. Deparia boryana [Dryoathyrium boryanum]
5.1.3.1. Distribution
W, C and E Nepal; Fujian, Guangdong, Guangxi, Guizhou, Hainan, Hunan, Shaanxi, Sichuan, SE Xizang, Yunnan and Zhejiang; Arunachal, Sikkim, Darjeeling, Uttarakhand, Himachal, Jammu and Kashmir; Bhutan; Sri Lanka; Myanmar; Thailand; Vietnam; Malaysia; Indonesia; Philippines; Taiwan; Africa; etc. [31, 69].
5.1.3.2. Habitat
Densely forested ravine with waterfalls, moist and shade places.
5.1.3.3. Ethnomedicine
The tender shoot is used as demulcent, stomachic and laxative [92]. The powder or paste is used to treat dysentery [93]. Rhizome is used in abdominal spasm [75]. Root paste is applied externally to treat burns, injury and wounds [76].
5.1.3.4. Pharmacognosy
The ethyl acetate extract inhibits the lipid formation with 35% at 100 μg/mL in 3T3-L1 cell model [92]. Antioxidant as well as anticancer activities are explored [92, 94].
5.1.3.5. Phytochemistry
The polyphenolic content in the aerial parts is 266 μg gallic acid equivalent/mg [92]. The total flavonoids content is estimated about 145.8 mg/g [94]. By using HPLC–DAD–ESI–MS, 3-hydroxyphloretin 6′-O-hexoside, quercetin-7-hexoside, apigenin7-O-glucoside, luteolin 7-O-glucoside, apigenin 7-O-galactoside, acacetin 7-O-(α-D-apio-furanosyl) (1→6)-β-D-glucoside, 3-hydroxy phloretin 6-O-hexoside, luteolin-6-C-glucoside, kaempferol 3-O-glucoside, etc. are tentatively identified in the plant extract [94].
5.1.4. Diplazium esculentum
5.1.4.1. Distribution
W, C and E Nepal; Anhui, Fujian, Guangdong, Guangxi, Guizhou, Hainan, Hunan, Jiangxi, Sichuan, Xizang, Yunnan and Zhejiang; Arunachal; Sikkim, Darjeeling, Uttarakhand, Himachal, Jammu and Kashmir; Pakistan; Bhutan; Taiwan; etc. [31, 69].
5.1.4.2. Habitat
Moist, shade forest and rocks.
5.1.4.3. Ethnomedicine
Young fronds are used as tonic and eaten for laxative effect [95]. Aerial parts are used to treat fever, pain, glandular swelling, diarrhea, dermatitis, wound and measles. The rhizome is considered as anthelmintic, antidysenteric, antidiarrheal and pest repellent [96]. It is also used in cough, asthma, fever, dyspepsia and stomachache. Decoction of rhizome and young leaves are useful in haemoptysis and constipation. Rhizomes are also used in scabies and boils [53]. A paste of leaf and stem is applied externally to treat cuts and wounds [97].
5.1.4.4. Pharmacognosy
Biological activities of the leaf extracts such as antimicrobial [98, 99, 100], antioxidant [101, 102, 103, 104], antidiabetic [103], anticaner [105], anti-inflammatory [99, 106], hepatoprotective [103], anti-Alzheimer's [104], etc. have been reported. Antibacterial [96] and anthelmintic activities of the rhizomes are reported [107].
5.1.4.5. Phytochemistry
Estimation of quercetin in the plant is performed [108]. Proximate analysis shows the presence of fat (3.4%), protein (10.7%), fiber (9.1%), total phenolics (125.6 mg gallic acid equivalent/100 g) and total flavonoids (110.8 mg quercetin equivalent/100 g) [109]. Total phenolics (167.7 mg gallic acid equivalent/100 g) and total flavonoids (70.8 mg quercetin equivalent/100 g) in the hydroalcoholic extract are separately reported by Junejo et al. [103]. Qualitative phytochemical screening shows the presence of carbohydrates, fats, alkaloids, anthraquinones, phenolics, saponins, cyanidins, cardiac glycosides, steroids, flavonoids, terpenoids, etc. [110]. Evaluation of vitamin C (22.2 mg/100 g), mineral contents (Ca, Cu, Fe, Mg, Mn, Na and Zn), heavy metals (Al, As, Cd, Hg, Li, Ni and Pb), etc. are performed in the leaves [27]. β-Pinene (17.2%), α-pinene (10.5%), caryophyllene oxide (7.5%), sabinene (6.1%), 1,8-cineole (5.8%), α-cardinol (4.8), β-caryophyllene (4.3%) are identified as the major volatile constituents in the essential oil obtained from the leaves [111]. Pentadecanoic acid, α-linolenic acid, phytol, β-sitosterol, stigmasta-5,22-dien-3-ol, methyl palmitate, diisobutyl phthalate, neophytadiene, 10,12-hexadecadien-1-ol, etc. are identified in the extracts of young fronds from Indian Himalaya [112].
Ascorbic acid [113], eriodictyol 5-O-methyl ether 7-O-β-D-xylosylgalactoside [114], α-tocopherol [115], quercetin [108, 116], ptaquiloside [117], pterosin [108], hopan-triterpene lactone [118], lutein [119], etc. are isolated from the edible parts of D. esculentum. Phenolics ((2R)-3-(4′-hydroxyphenyl)lactic acid, trans-cinnamic acid, protocatechuic acid and rutin) and ecdysteroids (amarasterone A1, makisterone C and ponasterone A) are recently isolated from the young fronds collected in Japan [120].
5.1.5. Diplazium kawakamii
5.1.5.1. Distribution
E Nepal; Yunnan; Arunachal, Sikkim and Darjeeling; Bhutan; Taiwan; Japan; etc. [31, 69].
5.1.5.2. Habitat
Wet, densely forested ravine and besides streamlets.
5.1.6. Diplazium maximum [Allantodia maxima]
5.1.6.1. Distribution
W, C and E Nepal; Fujian, Guangxi, Guizhou, Hainan, Jiangxi, Xizang and Yunnan; Arunachal, Sikkim, Darjeeling, Uttarakhand, Himachal, Jammu and Kashmir; Pakistan; Afghanistan; Bhutan; Myanmar; etc. [31, 69].
5.1.6.2. Habitat
Valley, evergreen forest, besides streamlets and north-west facing slopes.
5.1.6.3. Ethnomedicine
Young shoots are used in stomach problem [79].
5.1.6.4. Pharmacognosy
Fronds exhibit antioxidant potentiality [121].
5.1.6.5. Phytochemistry
The plant material contains alkaloids, phenolics, tannins, saponins, flavonoids, terpenoids, etc. [91]. Chettri et al. have estimated total crude fat (2.5%), fiber (11.3%), protein (13.4%), vitamin C (25.4 mg/100 g) and several metal contents [27]. Fronds have high contents of dietary fibre (38.3%) and crude protein (25.4%) [121]. It constitutes several fatty acids and phenolics like catechin, epicatechin, procatechuric acid, myricetin, etc.
5.1.7. Diplazium spectabile [Allantodia spectabilis]
5.1.7.1. Distribution
W, C and E Nepal; Yunnan and Xizang; Arunachal, Sikkim, Darjeeling, Uttarakhand, Himachal, Jammu and Kashmir; Bhutan; etc. [31, 69].
5.1.7.2. Habitat
South facing forest and valley, evergreen broad-leaved forests and besides streamlets.
5.1.7.3. Phytochemistry
Crude fat (0.2%), fiber (3.4%), protein (3.6%), vitamin C (21.6 mg/100 g), mineral contents (Ca, Cu, Fe, Mg, Mn, Na and Zn), heavy metals (Al, As, Cd, Hg, Li, Ni and Pb), etc. are determined in the leaves [27].
5.1.8. Diplazium stoliczkae [Allantodia stoliczkae]
5.1.8.1. Distribution
C and E Nepal; Xizang; Arunachal; Sikkim and Darjeeling; Bhutan; etc. [31].
5.1.8.2. Habitat
Dense forest.
5.1.8.3. Ethnomedicine
Root paste is applied externally to cure burn, injury and wounds [53]. Leaf juice is given to treat diarrhea and dysentery [122].
5.1.8.4. Phytochemistry
Total phenolic content (440.6 mg gallic acid equivalent/g) and flavonoid content (625.5 quercetin equivalent/g) together with antioxidant activity in the rhizomes have been investigated [123].
5.2. Blechnaceae
5.2.1. Stenochlaena palustris
5.2.1.1. Distribution
E Nepal; Guangdong, Guangxi, Hainan and Yunnan; Arunachal, Assam, Sikkim and Darjeeling; Thailand; Laos; Vietnam; Cambodia; Malaysia; Indonesia; Australia; Pacific islands; etc. [31, 69].
5.2.1.2. Habitat
Secondary forest, open forests and interior on the hills.
5.2.1.3. Ethnomedicine
Leaves decoction cures fever [124]. Fronds are used traditionally to treat fever, diarrhea, skin diseases, cutaneous disorders and gastric ulcers [125, 126].
5.2.1.4. Pharmacognosy
Fronds extracts show antifungal [127], antibacterial [128], antioxidant [[129], [130], [131]], and butyrylcholinesterase inhibitory [126] activities. Antimalarial activity of the leaf extract is recently reported [132].
5.2.1.5. Phytochemistry
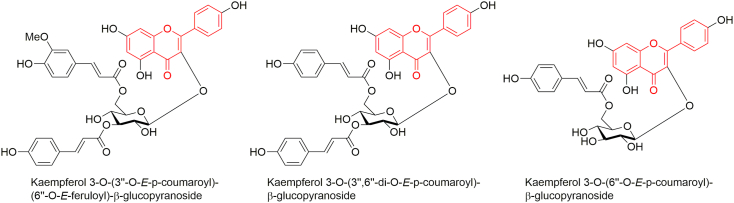
Leaves constitute stenopalustrosides A−E, kaem-pferol 3-O-(3″-O-E-p-coumaroyl)-(6″-O-E-feruloyl)-β-D-glucopyranoside, kaempferol 3-O-(3″,6″-di-O-E-p-coumaroyl)-β-D-glucopyranoside, kaempferol 3-O-(3″-O-E-p-coumaroyl)-β-D-glucopyranoside, kaempferol 3-O-(6″-O-E-p-coumaroyl)-β-D-glucopyranoside, kaempferol 3-O-β-D-glucopyranoside, (4S∗,5R∗)-4-[(9Z)-2,13-di-(O-β-D-glucopyranosyl)-5,9,10-trimethyl-8-oxo-9-tetradecene-5-yl]-3,3,5-trimethylcyclohexanone, 1-O-β-D-glucopyranosyl-(2S∗,3R∗,4E,8Z)-2-N-[(2R)-hydroxytetracosanoyl]octadecasphinga-4,8-dienine, 3-oxo-4,5-dihydro-α-ionyl β-D-glucopyranoside, 3-formylindole, lutein and β-sitosterol-3-O-β-D-glucopyranoside [128, 133].
Fronds contain kaempferol 3-O-β-glucopyranoside, kaempferol 3-O-(3″-O-E-p-coumaroyl)-(6″-O-E-feruloyl)-β-glucopyranoside, kaempferol 3-O-(3″,6″-di-O-E-p-coumaroyl)-β-glucopyranoside, kaempferol 3-O-(6″-O-E-p-coumaroyl)-β-glucopyranoside, kaempferol 3-O-(3″-O-E-p-coumaroyl)-β-glucopyranoside, kaempferol 3-O-α-rhamnopyranoside and kaempferol 3-O-(6″-O-α-rhamnopyranoside)-β-glucopyranoside [126].
5.2.2. Woodwardia unigemmata [Woodwardia biserrata]
5.2.2.1. Distribution
W, C and E Nepal; Fujian, Gansu, Guangdong, Guangxi, Guizhou, Hubei, Hunan, Jiangxi, Shanxi, Sichuan, Xizang and Yunnan; Arunachal, Sikkim, Darjeeling, Uttarakhand, Himachal, Jammu and Kashmir; Pakistan; Bhutan; Myanmar; Vietnam; Philippines; Taiwan; Japan; etc. [31, 69].
5.2.2.2. Habitat
Densely forested gully and slopes below trees, exposed and shaded places or wet localities along roadsides.
5.2.2.3. Ethnomedicine
Fronds are used in dysentery, skin diseases and infirtility [134, 135, 136]. Leaf is used for abdominal pain, constipation and sore throat [135]. Rhizome is used as purgative.
5.2.2.4. Pharmacognosy
Antioxidant [137, 138] and antibacterial [138] activitities of the aerial parts are reported. Anti-HIV activity has been shown by the rhizome extracts [139, 140].
5.2.2.5. Phytochemistry
Total phenolic and flavonoid contents in the aerial parts are reported [137, 138]. GC-MS analysis of the aerial parts shows the presence of catechol (22%), glycerol (20.2%), n-pentadecanoic acid (6.9%), glyceryl monoacetate (6.3%), ethyl acetimidate (5.4%) and 3-hydroxy-2,3-dihydromaltol (5.4%) in the aqueous extract; β-sitosterol (17.4%), pentadecanoic acid (9.8%), vitamin E (7.8%) and glycerol (7.0%) in the methanolic extract; and γ-sitosterol (33.4%), vitamin E (10.0%) and campesterol (7.3%) in the hexane extract [138]. From the roots, crassirhizomosides B–C, sutchuenoside A, kaempferol 3-O-α-L-(3-O-acetyl) rhamopyranoside-7-O-α-L-rhanopyranoside, kaempferol 3-O-α-L-(2-O-acetyl) rhamopyranoside-7-O-α-L-rhanopyranoside, kaempferol 3-O-α-L-rhamopyranoside-7-O-α-L-rhanopyranoside and kaempferol 3-O-α-L-(3,4-O-di-acetyl) rhamopyranoside-7-O-α-L-(4-O-acetyl)rhanopyranoside are isolated [11].
5.3. Cyatheaceae
5.3.1. Cyathea spinulosa [Alsophila spinulosa]
5.3.1.1. Distribution
W, C and E Nepal; Chongqing, Fujian, Guangdong, Guangxi, Guizhou, Hainan, Jiangxi, Sichuan, Xizang and Yunnan; Arunachal, Sikkim, Darjeeling and Uttarakhand; Bhutan; Bangladesh; Sri Lanka; Myanmar; N Thailand; Taiwan; S Japan; etc. [31, 69].
5.3.1.2. Habitat
Densely forested ravine with waterfalls and shade places.
5.3.1.3. Ethnomedicine
It is useful as hair tonic, sudorific and aphrodisiac [141]. Young leaf is taken orally in excessive bleeding during menstrual period [97]. Pith decoction is given in pains and bodyaches [142]. Rhizomes are used againts snake bites [143].
5.3.1.4. Pharmacognosy
Leaves possess antioxidant and anti-Alzheimer's potentiality [144].
5.3.1.5. Phytochemistry
From the fronds, hegoflavones A−B are isolated [145]. From the leaves, (2S,3S,4R)-2-[(2′R)-2′-hydroxytetracosanoylamino]-1,3,4-octadecanetriol, 1-O-β-D-glucopyranosyl-(2S,3R,4E,8Z)-2-[(2-hydroxyoctadecanoyl) amido]-4,8-octadecadiene-1,3-diol, 3α-hydroxyfilic-4(23)-ene, 2-oxofilic-3-ene, hop-22(29)-ene fern-7-ene, cycloaudenyl palmitate, daucosterol, dryocrasol, ergosterol, fern-9(11)-ene, filic-3-ene, hopan-29,17α-olide, hopan-17α,29-epoxide, hydroxyhopane, protocatechuic aldehyde, sitostanol, sitosterol, sitosteryl palmitate, stigmast-3,6-dione, stigmast-4-ene-3,6-dione and tetrahymanol are isolated [146, 147]. From the stalks, (E)-4-O-β-D-glucopyranosyl caffeic acid, 1-O-hexadecanolenin, 3,4,6-trihydroxy-1-ring vinyl carboxylic acid, 30-O-β-D-xylopyranosyl-dryocrassol, 4-O-β-D-glucopyranosyl-p-coumaric acid, 6β-hydroxy-24-ethyl-cholest-4-en-3-one, 9α-hydroxy-1β-methoxycaryolanol, clovandiol, cyathenosin A, daucosterol, decumbic acid, isorientin, n-tetracosane, pimaric acid, protocatechualdehyde, protocatechuic acid, stigmastane-3,6-dione, vitexin and β-sitosterol are isolated [148, 149].
5.4. Dennstaedtiaceae
5.4.1. Pteridium revolutum [Pteridium aquilinum subsp. Wightianum]
5.4.1.1. Distribution
W, C and E Nepal; Gansu, Guangdong, Guangxi, Guizhou, Henan, Hubei, Hunan, Jiangxi, Shaanxi, Sichuan, Xizang, Yunnan and Zhejiang; Arunachal, Sikkim, Darjeeling, Uttarakhand, Himachal, Jammu and Kashmir; Pakistan; Bhutan; Taiwan; N Australia; etc. [31, 69]. P. aquilinum, known as bracken fern, is one of the most commonly distributed weed species that grows in many types of soil with extreme high rate of reproduction [150].
5.4.1.2. Habitat
Exposed and shaded places, and open humus-rich forests.
5.4.1.3. Ethnomedicine
It is an ornamental fern that planted for indoor decoration and borders [151]. It is traditionally used for animal bedding [52]. The rhizomes are astringent and useful in diarrhea, intestinal inflammation and wounds. The rhizomes are also used for making breads and brews. The plant juice is considered antibacterial.
5.4.1.4. Pharmacognosy
It contains poisonous cyanogen glycosides which are antithiamine and carcinogenic. It has been demonstrated that feeding of the plant induces urinary bladder tumors, illeum sarcomas, pulmonary adenomas and leukemia [152, 153]. However, Yoshihira et al. performed toxicity tests and found that none of the plant extracts and the fractions could induce tumors under the conditions studied [154].
5.4.1.5. Phytochemistry
The rhizome constitutes the bitter starch [151]. It contains carbohydrate (51%), protein (9.5%), fat (1.2%), fibre (20%) and ash (8.3%). The fronds contain protein (1%), fat (0.1%), fibre (1.4%), minerals (0.6%), β-carotene (1 mg/100 g) and iodine (900 μg/kg). Phytochemical screening of the fronds grown in the Philippines reveals the presence of alkaloids, flavonoids and tannins [99]. Prunasin, a main cyanogen component in the fern that can release HCN to induce cyanogenesis, has been estimated about 10.4–61.3 mg/g in fresh plant tissue [155]. Ptaquiloside and quercetin are estimated in the plant material [108].
Several sesquiterpens have been isolated from the fronds that include pterosins A‒G, I‒J, K‒L, N, O, Z, etc. [156, 157, 158, 159]. Thiaminases 1 and 2 are isolated, which cause vitamin B1 deficiency in animals [160]. Steroids α- and β-ecdysones are identified in the bracken fern [161].
5.5. Dryopteridaceae
5.5.1. Dryopteris cochleata
5.5.1.1. Distribution
W, C and E Nepal; Guizhou, Sichuan and Yunnan; Arunachal, Sikkim, Darjeeling, Uttarakhand, Himachal, Jammu and Kashmir; Pakistan; Bhutan; Bangladesh; Myanmar; Thailand; Indonesia; Philippines; etc. [31, 69].
5.5.1.2. Habitat
Moist and shady slopes, and roadsides.
5.5.1.3. Ethnomedicine
Decoction of rhizome, stem and stripe is given for blood purification [162]. Dried rhizome powder is internally taken to cure diarrhea [134]. Root decoction is used in cuts and throat problems. It is antibacterial, antiepileptic, and effective in rheumatism, amoebic dysentery and leprosy [163]. The juice of fronds is used to treat muscular and rheumatic pain [164].
5.5.1.4. Pharmacognosy
Antibacterial [165] and antioxidant [166] activities of the leaf extracts, and antimicrobial [167] activity of the rhizome extracts have been reported.
5.5.1.5. Phytochemistry
Leaves contain fat (3.6%), fiber (11.2%), protein (20.1%), vitamin C (23.1 mg/100 g) and minerals (Ca, Cu, Fe, Mg, Mn, Mo, Na, Zn, Al, As, Cd, Hg, Li, Ni and Pb) [27]. Preliminary phytochemical screenings and GC-MS analyses of the rhizomes and leaves extracts are reported [166, 167, 168]. The amounts of ptaquiloside and quercetin are estimated in the plant material [108].
5.5.2. Dryopteris splendens
5.5.2.1. Distribution
E. Nepal; Yunnan; Arunachal, Sikkim and Darjeeling; Bhutan; etc. [31, 69].
5.5.2.2. Habitat
Densely forested gully and slopes below trees, and evergreen and deciduous braod-leaved forests.
5.5.3. Polystichum squarrosum [Polystichum apicisterile]
5.5.3.1. Distribution
W, C and E Nepal; S Xizang; Arunachal, Sikkim, Darjeeling, Uttarakhand, Himachal, Jammu and Kashmir; Pakistan; Bhutan; etc. [31, 69].
5.5.3.2. Habitat
Near streams and tree shades.
5.5.3.3. Pharmacognosy
Leaf extracts show antifungal [169] and antioxidant [165] activities. P. squarrosum fed rats show moderate mortality, splenomegaly and develop neoplastic lesions awaring its toxic effects [170].
5.5.3.4. Phytochemistry
The amounts of ptaquiloside and quercetin are estimated in the leaves [108].
5.6. Lygodiaceae
5.6.1. Lygodium flexuosum
5.6.1.1. Distribution
W, C and E Nepal; Fujian, Guangdong, Guangxi, Guizhou, Hainan, Hunan and Yunnan; Arunachal, Sikkim, Darjeeling, Uttarakhand and Himachal; Bhutan; Sri Lanka; Thailand; Vietnam; Malaysia; Philippines; S Japan; E Australia; etc. [31, 69].
5.6.1.2. Habitat
Interior exposed area.
5.6.1.3. Ethnomedicine
The plant is used as an expectorant [171]. Root is smoked as cigarette [172]. Fresh root is boiled with mustard oil and then used for massage to treat rheumatism and sprains. It is also used for the treatment of jaundice, dysmenorrhea, wounds and eczema. Plant ash is used for treating gonorrhea, herpes, and other foot and mouth diseases. The whole plant is used in sprain, fracture, cuts and wounds [173].
5.6.1.4. Pharmacognosy
Antifertility [174, 175], antiproliferative, apoptotic, hepatoprotective [176, 177], antibacterial properties [178, 179] are reported.
5.6.1.5. Phytochemistry
It contains lygodinolide, coumaryl dryocrassol, tectoquinone, kaempferol, kaempferol 3-β-D-glucoside, β-sitosterol and stigmasterol [180, 181, 182]. Several anhidiogens are identified from the spores [183].
5.6.2. Lygodium japonicum
5.6.2.1. Distribution
W, C and E Nepal; Anhui, Chongqing, Fujian, Gansu, Guangdong, Guangxi, Guizhou, Hainan, Henan, Hubei, Hunan, Jiangsu, Jiangxi, Shaanxi, Shanghai, Sichuan, Xizang, Yunnan and Zhejiang; Arunachal, Sikkim, Darjeeling, Uttarakhand, Himachal, Jammu and Kashmir; Pakistan; Bhutan; Sri Lanka; Indonesia (Java); Philippines; Taiwan; Korea; Japan; Australia, N America; etc. [31, 69].
5.6.2.2. Habitat
Moist and exposed area, and roadsides.
5.6.2.3. Ethnomedicine
It has been used in China for the treatment of calculi, edema, dysentery, hepatitis, pneumonia, gonorrhea, etc. [184]. Vegetative parts and spores decoction is used for diuretic or cathartic effects [171]. The plant is also used as an expectorant. The plant juice is applied in scabies, boils and wounds, and the paste is used in joint pains [51, 185]. The whole plant is applied to treat whitlow [52].
5.6.2.4. Pharmacognosy
The methanolic extract of the spores shows neuroprotective effect [186].
5.6.2.5. Phytochemistry
The amount of quercetin present in L. japonicum is estimated [108]. GC-MS analysis of the essential oil from the whole herb leads to identify 52 compounds including 3-methyl-1-pentanol (4%), 2-(methylacetyl)-3-carene (4.2%), cyclooctenone (7.6%), (E)-2-hexenoic acid (12.8%) and 1-undecyne (28.6%) [187].
Roots contain 1-hentriacontanol, hexadecanoic acid 2,3-dihydroxy-propyl ester, kaempferol, kaempferol-7-O-α-L-rhamnopyranoside, lygodiumsteroside B, p-coumaric acid, tilianin, daucosterol and β-sitosterol [188, 189]. From the aerial parts, 2-O-caffeoyl-D-glucopyranose, 3-O-caffeoyl-D-glucopyranose, 4-O-caffeoyl-D-glucopyranose, 4-O-pcoumaroyl-D-glucopyranose, 6-O-caffeoyl-D-glucopyranose, 6-O-p-coumaroyl-D-glucopyranose, caffeic acid and p-coumaric acid are isolated [190]. Antheridiogens are identified in the spores [191, 192].
Interestingly, L. japonicum grown in the metal-enriched conditions can accumulates copper in the cell wall pectin [193].
5.7. Marsileaceae
5.7.1. Marsilea quadrifolia
5.7.1.1. Distribution
C and E Nepal; Gansu, Hebei, Heilongjiang, Henan, Jilin, Liaoning, Nei Mongol, Ningxia, Qinghai, Shaanxi, Shandong and Shanxi; Arunachal, Sikkim and Darjeeling; Bhutan; Korea; Japan; America; Europe; etc. [69, 85].
5.7.1.2. Habitat
Ponds and paddy fields.
5.7.1.3. Ethnomedicine
It produces aphordisiac effect [124]. Leaf juice is anti-inflammatory, diuretic, depurative, febrifuge and refrigerant [194]. The plant is useful to relieve hypertension, sleep disorder, headache, cough, convulaion, repiratory troubles, nervous problems, constipation, dyspepsia, etc. [195, 196]. It is also used in the treatment of leprosy, haemorrhoids, fever and insomnia [197]. It is a famine food and only used during food scarcity.
5.7.1.4. Pharmacognosy
The plant extracts exhibit antibacterial [196, 198, 199, 200], antidiarrheal [194], antifungal [201], analgesic [194], anti-inflammatory [202], antioxidant [203, 204], antidiabetic [205], anti-Alzheimer's [206, 207], antiepileptic [208], anticonvulsive [209], nephroprotective [210] and antitumor [211] activities.
5.7.1.5. Phytochemistry
The plant material contains carbohydrate (19.5% g), protein (4.9%), fat (0.2%), amino acid (3%), flavonoids (0.3%) and saponins (0.3%) [212]. Seasonal variations in the compositions of sodium, potassium, calcium, phosphorus and β-carotene contents in the whole plant have been reported [213]. Total phenolics, flavonoids, alkaloids, tannins and saponins in both leaves and stems have been separately evaluated [214].
Leaves possess alkaloids, steroids, tannins, terpenoids, flavonoids, etc. [200, 202, 208, 214]. 2,3,7,8-Tetracholorodibenzofuran, 3-hydoxy-triacontan-11-one, chlorogenic acid, hentriacontan-6-ol, marsileagenin A, marsilin (1-triacontanol-cerotate), methylamine, pentachlorophenylacetate, quercetin, triacontyl hexacosanoate, tridecyliodide, β-sitosterol, etc. are identified in M. quadrifolia [198, 202, 204].
Zhang et al. have isolated (±)-(E)-4b-methoxy-3b,5b-dihydroxyscirpusin A, (±)-(E)-cyperusphenol A, (±)-(E)-scirpusin A, (E)-caffeic acid 4-O-β-D-glucopyranoside, (E)-ethyl caffeate, 4-methy-3′-hydroxypsilotinin, hegoflavone A, kaempferol 3-O-(2″-O-E-caffeoyl)-β-D-glucopyranoside, kaempferol 3-O-(3″-O-E-caffeoyl)-α-L-arabinopyranoside, kaempferol 3-O-(4″-O-E-caffeoyl)-α-L-arabinopyranoside, kaempferol 3-O-α-L-arabinopyranoside, kaempferol 3-O-β-D-glucopyranoside, kaempferol, mesocyperusphenol A, quercetin, quercetin 3-O-(6″-O-E-caffeoyl)-β-D-glucopyranoside, quercetin 3-O-α-L-arabinopyranoside and quercetin 3-O-β-D-glucopyranoside from the ethanolic extract of M. quadrifolia, and their antioxidant capacity is evaluated [215].
5.8. Nephrolepidaceae
5.8.1. Nephrolepis cordifolia [Nephrolepis auriculata]
5.8.1.1. Distribution
W, C and E Nepal; Fujian, Guangdong, Guangxi, Guizhou, Hainan, Hunan, Taiwan, Xizang, Yunnan and Zhejiang; Arunachal, Sikkim, Darjeeling and Uttarakhand; Pakistan; Bhutan; Bangladesh; Sri Lanka; Myanmar; Thailand; Laos; Vietnam; Cambodia; Malaysia; Singapore; Indonesia; Philippines; Korea; Japan; Australia; N and S America; Africa; etc. [31, 69].
5.8.1.2. Habitat
Moist place and roadsides.
5.8.1.3. Ethnomedicine
Tuberous root is used in indigestion, cold, cough, fever, jaundice and burning urination [52, [216]]. It is eaten to cure hypertension and inflammation [185]. Root paste is used in boils and skin problems [217]. Tuber juice is useful in dehydration [218]. Whole plant is used to cure renal, liver and skin disorders [219]. It is useful in chest congestion, rheumatism, jaundice and wounds [220]. Cough is cured by a decoction of the fresh fronds [221].
5.8.1.4. Pharmacognosy
Frond possesses antibacterial and antifungal potentials [99, 222]. Fruit and leaf are evaluated for the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging, α-amylase inhibitory and glucose diffusion inhibition activities [223]. Anthelmintic activity of the aqueous extract of leaves is claimed as it paralyzes earthworms (Pheretima posthuma) at the concentration of 10 mg/mL [224]. Diuretic potential of the rhizome juice is shown [225].
5.8.1.5. Phytochemistry
Proximate analysis of different parts of N. cordifolia grown in Nepal and Nigeria has been reported [226, 227]. Tannins (11.5 mg/100 g), alkaloids (9.1 mg/100 g), flavonoids (16.5 mg/100 g), phenols (8.3 mg/100 g), saponin (1.2 mg/100 g) and glycosides (5.4 mg/100 g) are estimated in the leaflets [227]. Essential oil obtained from the aerial parts constitutes nonanal (10.6%), β-ionone (8%), eugenol (7.2%) and anethol (4.6%) [228]. Sequoyitol, β-sitosterol, fern-9(11)-ene, oleanolic acid, myristic acid octadecylester, hentriacontanoic acid and triacontanol are isolated from the plant material [229].
5.9. Ophioglossaceae
5.9.1. Botrychium lanuginosum
5.9.1.1. Distribution
W, C and E Nepal; Guangxi, Guizhou, Hunan, Sichuan, Xizang and Yunnan; Arunachal, Sikkim, Darjeeling, Uttarakhand and Himachal; Bhutan; Sri Lanka; Myanmar; Thailand; Vietnam; Malaysia; Indonesia; Philippines; Taiwan; New Guinea; etc. [31, 69].
5.9.1.2. Habitat
Moist and shady forest floor.
5.9.1.3. Ethnomedicine
It is used in dysentery and wounds [134, 163, 220, 230]. Rhizomes are used in pneumonia, catarrh and cough [124]. The paste is used as a facial mask, and it is applied in boils on the tongue and on the forehead to relieve headache [50]. Shoots are used in body ache [84].
5.9.1.4. Phytochemistry
Apigenin, luteolin, luteolin-7-O-glucoside, daucosterol, β-sitosterol, (6′-O-palmitoyl)-sitosterol-3-O-β-D-glucoside, thunberginol A, 30-nor-21β-hopan-22-one, 1-O-β-D-glucopyranosyl-(2S,3R,4E,8Z)-2-[(2R-hydroxy hexadecanoyl) amino]-4,8-octadecadiene-1,3-diol and sucrose are isolated from B. lanuginosum [231].
5.9.2. Helminthostachys zeylanica
5.9.2.1. Distribution
W, C and E Nepal; Guangdong, Hainan and Yunnan; Arunachal, Assam, Darjeeling, Bihar and Uttarakhand; Sri Lanka; Thailand; Laos; Vietnam; Cambodia; Philippines; Taiwan; Japan; Australia; Pacific islands; etc. [31, 69].
5.9.2.2. Habitat
Forest, edges of marshes and swampy places.
5.9.2.3. Ethnomedicine
Fronds are used as aphrodisiac [232]. The plant is considered as intoxicant and anodyne. It is useful in impotency, sciatica, dysentery, malaria and to relieve blisters on the tongue [124]. The rhizome is regarded as a tonic [233]. It is also used in dysentery, catarrh and phthisis. The rhizomes are popularly used as an antipyretic and anti-inflammatory Chinese herbal medicine, Daodi-Ugon [234, 235].
5.9.2.4. Pharmacognosy
Rhizomes show hepatoprotective [236, 237], anti-inflammatory [[238], [239], [240]], antihyperuricemia [241], cytotoxic [242], anticancer [243], antidiabetic [244] and antibacterial [245] activities.
5.9.2.5. Phytochemistry
The plant material contains fat (0.6%), crude fibre (1%), calcium (47.9 mg/100 g), phosphorus (91.5 mg/100 g), iron (1.8 mg/100 g), carotene (2.1 mg/100 g), riboflavin (0.1 mg/100 g), niacin (0.9 mg/100 g) and ascorbic acid (45.9 mg/100 g) [233].
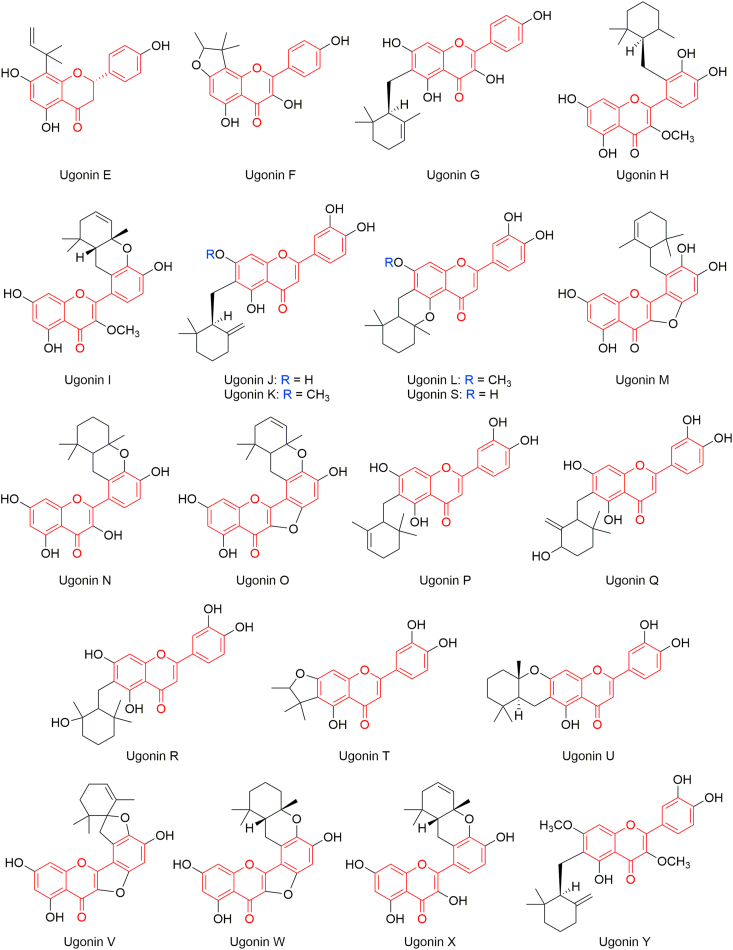
From the rhizomes, flavonoids viz. ugonins E‒Y, quercetin, quercetin-4′-O-β-D-glucopyranosyl-(1→2)-β-D-glucopyranoside, quercetin-3-O-β-D-glucopyranosyl-4′-O-β-D-glucopyranosyl-(1→2)-β-D-glucopyranoside, 2-(3,4-dihydroxyphenyl)-6-((2,2-dimethyl-6-methylenecyclohexyl)methyl)-5,7-dihydroxy-chroman-4-one, 2-(3,4-dihydroxy-2-[(2,6,6-trimethylcyclohex-2-enyl)methyl]phenyl)-3,5,7-trihydroxy-4 H-chromen-4-one and 5,4′-dihydroxy-4″,4″-dimethyl-5″-methyl-5″H-dihydrofurano[2″,3′′:6,7]flavanone are isolated by Huang et al. [[235], [246], [247], [248]]. Quality of the root and rhizome of H. zeylanica can be determined by using ugonins as markers [249]. Geranyl stilbenes ugonstilbenes A–C and 3-hydroxyacetophenone are isolated from the rhizomes by Chen et al. [234]. Ugonstilbenes A‒C exhibit moderate antioxidant activity. 4′-O-β-D-Glucopyranosyl-quercetin-3-O-β-D-glucopyranosyl-(1→4)-β-D-glucopyranoside and 4′-O-β-D-glucopyranosyl-(1→2)-β-D-glucopyranosyl-quercetin-3-O-β-D-glucopyranosyl-(1→4)-β-D-glucopyranoside are isolated by Yamauchi et al. [250]. These glycosides display melanogenic acceleratory effect.
From the whole plant, neougonins A‒B, ugonins D‒E, ugonin J, ugonin L, ugonin N, ugonin S, 2-(3,4-dihydroxyphenyl)-6-((2,2-dimethyl-6-methylenecyclohexyl)methyl)-5,7-dihydroxy-chroman-4-one, etc. are isolated [251].
5.9.3. Ophioglossum nudicaule
5.9.3.1. Distribution
Nepal; SW Sichuan, Xizang, C and NW Yunnan; Darjiling, Sikim, Himanchal, Jharkand, Karnataka, Kerala, Tamilnadu, Jammu and Kashmir; Indonesia; Malaysia; Thailand; etc. [69, 252].
5.9.3.2. Habitat
Rainforest, impermeable soil in open places and by culverts on roadsides in the hills.
5.9.3.3. Ethnomedicine
Fronds are used as a tonic and styptic [253]. It is used in wounds and old skin diseases [52, 254].
5.9.4. Ophioglossum petiolatum
5.9.4.1. Distribution
C and E Nepal; Fujian, Guizhou, Hainan, Hubei, Sichuan, Yunnan and Xizang; Arunachal, Sikkim, Darjeeling, Uttarakhand, Himachal, Jammu and Kashmir; Pakistan; Uzbekistan; Bhutan; Sri Lanka; Thailand; Indonesia; Philippines; Taiwan; Japan; Australia; New Zealand; N America; England; Fiji; etc. [31, 69, 252].
5.9.4.2. Habitat
Moist exposed grassy areas.
5.9.4.3. Ethnomedicine
It is used for checking of nose bleeding [84]. It has been used as a detoxifying agent and found effective against hepatitis [255, 256].
5.9.4.4. Phytochemistry
Ophioglonin, ophioglonin 7-O-β-D-glucopyranoside, ophioglonol, ophioglonol prenyl ether, ophioglonol 4′-O-β-D-glucopyranoside, isoophioglonin 7-O-β-D-glucopyranoside, quercetin, luteolin, kaempferol, 3,5,7,3′,4′-pentahydroxy-8-prenylflavone, and quercetin 3-O-methyl ether are isolated from the whole plant [256].
5.9.5. Ophioglossum reticulatum
5.9.5.1. Distribution
W, C and E Nepal; Fujian, Gansu, Guizhou, Henan, Hubei, Jiangxi, Shaanxi, Sichuan, Xizang and Yunnan; Arunachal, Sikkim, Darjeeling, Uttarakhand, Himachal, Jammu and Kashmir; Pakistan; Bhutan; Sri Lanka; Philippines; Korea; S America; Madagascar; Africa; etc. [31, 69, 252].
5.9.5.2. Habitat
Roadsides, under beneath of tree in the forests and grassy plateaus.
5.9.5.3. Ethnomedicine
It is used as cooling agent in burns, tonic and wounds remedy [230]. Fronds are used as styptic in contusions and haemorrages [163]. Leaf extract is vulnerary [124]. Leaf juice is useful against heart spasms [257]. Rhizome decoction is used in boils and the extract is used as an antidote in snake bite.
5.9.6. Ophioglossum vulgatum
5.9.6.1. Distribution
Nepal; Anhui, Fujian, Guangdong, Guizhou, Henan, Hubei, Hunan, Jiangxi, S Shaanxi, Sichuan, Xizang, Yunnan and Zhejiang; India; Sri Lanka; Korea; Japan; Australia, N America; Europe; etc. [69].
5.9.6.2. Habitat
Naturally grow in damp grasslands and semi-evergreen forests, particularly after fire [258].
5.9.6.3. Ethnomedicine
It possesses detergent, antiseptic, styptic and vulnerary properties [259]. Rhizome is used to treat boils. Pulverized rhizome is externally applied to sores, burns and wounds [135]. Decoction is useful in heart diseases [134]. It is useful in clearing heat, detoxifying toxins, activating blood circulation, treating hepatitis and pneumonia, etc. [260].
5.9.6.4. Pharmacognosy
O. vulgatum exhibits wound healing [261, 262], antidiarrheal [263] and antiviral [264] properties.
5.9.6.5. Phytochemistry
The whole plant material constitutes total flavonoid and total polyphenols in 2.7 and 1.5%, respectively [263]. Flavonoids, glycerides and amino acids are the constituents in O. vulgatum [265]. From the aerial parts, 3-O-methylquercetin 7-O-diglycoside 4′-O-glycoside, quercetin-3-O-[(6-caffeoyl)-β-glucopyranosyl(1→3) α-rhamnopyranoside]-7-O-α-rhamnopyranoside, kaempferol-3-O-[(6-caffeoyl)-β-glucopyranosyl (1→3) α-rhamnopyranoside]-7-O-α-rhamnopyranoside and quercetin-3-O-methyl ether have been isolated [261, 266].
5.10. Osmundaceae
5.10.1. Osmunda claytoniana [Osmunda claytoniana subsp. Vestita]
5.10.1.1. Distribution
W, C and E Nepal; Chongqing, Guizhou, Hubei, Hunan, Liaoning, Sichuan, Taiwan, Xizang and Yunnan; Arunachal, Sikkim, Darjeeling, Uttarakhand, Himachal, Jammu and Kashmir; Pakistan; Bhutan; Korea; Japan; E Russia; N America; etc. [31, 69].
5.10.1.2. Habitat
At high altitude above in meadows, moist and shady forest area.
5.10.1.3. Ethnomedicine
Plant paste is applied on wounds externally [134]. The rhizome and stipe bases are used for adulteration as a substitute of Filix-mas for vermifuge effect and flavor [267].
5.10.1.4. Pharmacognosy
α-Glucosidase inhibitory activity [268] and antitrypanosomal activity [269] of the plant extracts are evaluated.
5.10.1.5. Phytochemistry
The plant material consists of phenolics, tannins, flavonoids, saponins, terpoenoids, etc. [91].
5.11. Pteridaceae
5.11.1. Ceratopteris thalictroides
5.11.1.1. Distribution
W and C Nepal; Anhui, Fujian, Guangdong, Guangxi, Guizhou (Liping), Hainan, Hubei, Jiangsu, Jiangxi, Shandong, Sichuan, Yunnan and Zhejiang; Assam, W Bengal, Bihar, Uttarakhand, Himachal and C India; Sri Lanka; Myanmar; Thailand; Vietnam; Malaysia; Indonesia; Philippines; Taiwan; Japan; New Guinea; Australia; C, N and S America; Madagascar; Pacific islands; West Indies; Africa; etc. [31, 69].
5.11.1.2. Habitat
Marshy areas.
5.11.1.3. Ethnomedicine
The fronds are used as a poultice for skin problems [163, 270, 271]. It's paste with turmeric is applied in cuts and wounds. It is also used as a tonic and styptic [272], and it is also reported to be toxic [163].
5.11.1.4. Pharmacognosy
The plant extracts possess antibacterial [273], anti-inflammatory [274], antioxidative [275] and antidiabetic activities [276].
5.11.1.5. Phytochemistry
The leaves contain protein (13.4%), carbohydrate (54.3%), fat (3.2%), vitamin C (6.1 mg/100 g) and minerals (eg. Na, K, P, Mg, Ca, Mn, Fe, Zn and Cu) [277]. The plant materials showed the presence of alkaloids, steroids, coumarins, tannins, saponins, flavonoids, quinones, anthroquinones, phenols, proteins, xanthoproteins, carbohydrates, glycosides, catachins, sugars and terpenoids [270, 277]. 2-Nitroethyl benzene, γ-sitosterol, n-hexadecanoic acid and linalool are the major phytochemicals present in the leaves.
5.11.2. Pteris biaurita [Pteris flavicaulis]
5.11.2.1. Distribution
W, C and E Nepal; Guangdong, Guangxi (Baise), Guizhou, Hainan, Xizang and Yunnan; Arunachal, Sikkim, Darjeeling and Uttarakhand; Bhutan; Bangladesh; Sri Lanka; Thailand; Laos; Malaysia; Indonesia; Philippines; C and S Taiwan; etc. [31, 69].
5.11.2.2. Habitat
Moist, shady and exposed areas, and roadsides.
5.11.2.3. Ethnomedicine
The rhizome and frond decoction is used to treat chronic disorders [278]. To relieve body pain, the rhizome paste is applied [279]. Frond juice and paste are applied on cuts and bruises [53, 280].
5.11.2.4. Pharmacognosy
The leaves exhibit antimicrobial activity [281]. The fronds are evaluated for the antioxidant activity [278].
5.11.2.5. Phytochemistry
Fronds constitute alkaloids (16.4 mg/g), phenolics (13.2 mg/g), saponins (11.1 mg/g), tannins (6.2 mg/g), flavonoids (17.5 mg/g) as well as steroids, triterpenoids, anthraquinones, amino acids and sugars [282]. Leaves contain flavonoids (14.5 mg/g), total phenol (10.4 mg/g), ascorbate (1.6 mg/g), α-tocophenol (0.9 mg/g) and total sugars (125 mg/g) [278]. Total protein (110.5 mg/g), total chlorophyll (0.9 mg/g) and carotenoids (62.3 μg/g) in the fresh material are also estimated. Eicosenes and heptadecanes present in the leaves are attributed for the antimicrobial activity [281].
5.11.3. Pteris vittata
5.11.3.1. Distribution
W, C and E Nepal; Anhui, Fujian, SE Gansu (Kangxian), Guangdong, Guangxi, Guizhou, SW Henan, Hubei, Hunan, Jiangxi, Shaanxi, Sichuan, Xizang, Yunnan and Zhejiang; Arunachal, Sikkim, Darjeeling and Uttarakhand; Bhutan; Taiwan; etc. [31, 69]. It is commonly known as brake fern.
5.11.3.2. Habitat
Moist and shady areas, roadsides and tropical semi-evergreen forests.
5.11.3.3. Ethnomedicine
It is a poisonous plant and therefore it is planted on village borders to keep enemies away [283]. It is used in tongue sores, burns and glandular swellings and for population control [136, 220, 279]. Herb juice is used for treating dysentery and diarrhea [284]. Young fronds are used in manuring [134]. Plants are used as fodder and thatching [52].
5.11.3.4. Pharmacognosy
It's antibacterial property is explored [285, 286]. It has been reported that P. vittata hyperaccumulates arsenic [287, 288].
5.11.3.5. Phytochemistry
Leaves contain leucocyanidin, leucodelphinidin, apigenin 7-O-p-hydroxybenzoate, leutolin, kaempherol, quercetin, rutin, etc. [285, 289, 290].
5.12. Tectariaceae
5.12.1. Tectaria gemmifera [Tectaria coadunata; Tectaria macrodonta]
5.12.1.1. Distribution
W, C and E Nepal; Guangdong, Guangxi, Guizhou, Sichuan, Xizang and Yunnan; Arunachal, Sikkim, Darjeeling, Uttarakhand, Himachal, Jammu and Kashmir; Bhutan; Sri Lanka; Myanmar; Thailand; Laos; Vietnam; Malaysia; Taiwan; Madagascar; Africa; etc. [31, 69].
5.12.1.2. Habitat
North-west facing moist and rocky slopes, exposed areas and roadsides or in rock crevices near water channels.
5.12.1.3. Ethnomedicine
Rhizome decoction is given to treat stomachache and gastrointestinal disorders [291]. Root juice is used to treat diarrhea and dysentery [142]. Leaf decoction is given to treat asthma and bronchitis [253]. Leaf paste is applied in stings of honeybee, centipeds, etc. Whole plant is used in eczema, scabies and jaundice [254, 292]. Leaves are used as fodder [134].
5.12.1.4. Pharmacognosy
Antibacterial [293, 294, 295, 296], antioxidant [295, 297, 298] and antidiabetic [299] activities are reported. Cholinesterases and tyrosinase inhibitory activities together with cytotoxic effect on the 2008 and BxPC3 cell lines of the plant extract from twigs and rhizomes are reported [297].
5.12.1.5. Phytochemistry
Crude fat (0.2%), fiber (2.3%), protein (12.1%), vitamin C (22.2 mg/100 g), mineral contents (Ca, Cu, Fe, Mg, Mn, Na and Zn), heavy metals (Al, As, Cd, Hg, Li, Ni and Pb), etc. are determined in T. gemmifera [27]. The fronds constituted of anthraquinones, coumarins, flavonoids, steroids, alkaloids, tannins, phenolics, etc. [296, 300], and the rhizomes constitutes alkaloids, coumarins, phenolics, flavonoids, quinones, etc. [294, 296, 301]. GC-MS analysis of the methanolic extract of leaves show the presence of palmitic acid, methyl palmitate, phytol, etc. as the major constituents [295], while the rhizome constitutes fatty acids [295, 302]. The qualitative and quantitative analyses reveal that the twig and rhizome contain a large amount of procyanidins as well as eriodictyol-7-O-glucuronide and luteolin-7-O-glucoronide [297].
5.12.2. Tectaria zeylanica [Osmunda zeylanica]
5.12.2.1. Distribution
W, C and E Nepal; Fujian, Guangdong, Guangxi, Guizhou, Hainan and Yunnan; S India; Sri Lanka; Thailand; Laos; Vietnam; Malaysia; Indonesia; Philippines; Taiwan; Indian Ocean islands (Mauritius); Pacific islands (Polynesia); etc. [69].
5.12.2.2. Habitat
Muddy rocks in forests, near streams and on steep banks.
5.12.2.3. Pharmacognosy
Ethanolic extract of the leaves shows antioxidant activity by scavenging DPPH free radical with the IC50 value of 0.78 mg/mL [303].
5.12.2.4. Phytochemistry
Leaf constitutes alkaloids, terpenoids, flavonoids, tannins, etc. [303]. Fronds constitute phenolics, saponins, steroids, tannins, coumarins, etc. [304].
5.13. Thelypteridaceae
5.13.1. Thelypteris auriculata [Cyclosorus auriculata]
5.13.1.1. Distribution
W, C and E Nepal; Guangxi, Guizhou, Xizang and Yunnan; Arunachal, Sikkim, Darjeeling, Uttarakhand and Himachal; Bhutan; Sri Lanka; Thailand; Vietnam; etc. [31, 69].
5.13.1.2. Habitat
Along river banks and forest margins.
5.13.2. Thelypteris nudata [Thelypteris multilineata; Pronephrium nudatum]
5.13.2.1. Distribution
W, C and E Nepal; Guizhou, Xizang and Yunnan; Arunachal, Sikkim, Darjeeling, Uttarakhand, Himachal, Jammu and Kashmir; Bhutan; Indonesia; Vietnam; etc. [31, 69].
5.13.2.2. Habitat
Shaded sparse forests on slopes.
5.13.2.3. Pharmacognosy
The frond extract exhibits synergistic antibacterial effect against methicillin resistant Staphylococcus aureus (MRSA) [305].
6. Altitudinal zones-based categorization of Nepalese wild edible ferns
The distribution of ferns is varied with different altitudinal zones. Nepal is particularly considered to be divisible into three altitudinal zones, namely, (a) Tarai (plain area), (b) Pahad (hilly area) and (c) Himal (mountainous area). Accordingly, based on the species found at different altitudes, the Nepalese wild edible vegetable ferns can be categorized into three:
6.1. Lower altitude species (60−1,200 m asl)
The species grown in low-lands, i.e. Tarai, Siwalik and Chure areas of Nepal, include: A. strigillosum, D. boryana, D. esculentum, S. palustris, W. unigemmata, C. spinulosa, P. revolutum, D. maximum, D. splendens, D. cochleata, L. flexuosum, L. japonicum, N. cordifolia, B. lanuginosum, H. zeylanica, O. petiolatum, O. reticulatum, C. thalictroides, P. biaurita, P. vittata, T. gemmifera and T. zeylanica.
6.2. Mid-hills species (1,200−2,500 m asl)
The species grown in mid-lands, i.e. Chure and Mahabharat areas of Nepal, include: A. atkinsonii, A. strigillosum, D. boryana, D. esculentum, D. kawakamii, D. spectabile, D. stoliczkae, W. unigemmata, C. spinulosa, P. revolutum, D. maximum, D. cochleata, P. squarrosum, M. quadrifolia, L. japonicum, N. cordifolia, B. lanuginosum, B. lanuginosum, H. zeylanica, O. nudicaule, O. petiolatum, O. reticulatum, O. vulgatum, O. claytoniana, P. biaurita, P. vittata, T. gemmifera, T. auriculata and T. multilineata.
6.3. High altitude species (2,500−4,000 m asl)
The species grown in upper-lands, i.e. Himalayas or mountainous areas of Nepal, include: D. boryana, D. spectabile, D. stoliczkae, P. squarrosum, L. japonicum, W. unigemmata, P. revolutum, B. lanuginosum, O. nudicaule, O. petiolatum, O. reticulatum, O. vulgatum and O. claytoniana.
7. Inhabitations-based distribution of Nepalese wild edible ferns
Virtually, the ferns are inhabitated where flowering plants are found [32]. Humid, moist and shady forests are more suitable for their growth. Based on the species found at different ecological habitats, the edible vegetable ferns of Nepal are categorized into epiphytes, lithohytes, terrestrials, climbers and hydrophytes as given below. Noteworthly, some species could be found in more than one habitat.
7.1. Epiphytes
These species grow on bases, trunks and branches of trees, and are covered with mosses and liverworts. Rainy season, hill-side evergreen forest and moist forest are more suitable for their growth. In middle elevation, altitude up to 2,000 m, the distribution of ephiphytes is very few. Examples of epiphytes are: N. cordifolia, B. lanuginosum and P. vittata.
7.2. Lithophytes
These species grow in rock crevices; humus rich rocks in shady areas; moist, shaded and exposed forests; crevices of brick walls; and muddy rocks in streamlets. Examples of lithophytes are: W. unigemmata, D. cochleata, N. cordifolia, B. lanuginosum, P. biaurita, P. vittata and T. gemmifera.
7.3. Terrestrials
They grow in evergreen and semi-evergreen forests that enriched with humus and organic nutrients. They also grow in shady areas, stream banks, moist hill slopes and shaded roadsides. Examples of terrestrial species are: A. strigillosum, D. boryana, D. esculentum, D. maximum, D. stoliczkae, W. unigemmata, C. spinulosa, P. revolutum, D. cochleata, D. splendens, P. squarrosum, N. cordifolia, B. lanuginosum, H. zeylanica, O. nudicaule, O. petiolatum, O. reticulatum, O. claytoniana, P. biaurita, P. vittata, T. gemmifera, T. zeylanica and T. nudata.
7.4. Climbers
They grow on rich humus soil. They climb other trees growing inside shaded forests, forerst edges and tropical areas. Examples of climber species include: S. palustris, L. flexuosum and L. japonicum.
7.5. Hydrophytes
They are water loving species. They occur on wet or marshy border of ponds, lakes and waterfalls. They also occur in paddy fields. Examples of hydrophytes include: M. quadrifolia and C. thalictroides.
8. Medicinal ailments categorization of Nepalese wild edible ferns
Based on pharmacognosy of the plant extracts of ferns and fern-allies reported by several authors, the medicinal ailments categorization of Nepalese wild edible vegetable food ferns is summarized in Table 2.
Table 2.
Summary of the medicinal ailments of the vegetable food ferns.
| SN | Medicinal ailments | Ferns | Parts used | References |
|---|---|---|---|---|
| 1 | Antibacterial | D. esculentum | Rhizome, leaf | [96, 98, 99, 100] |
| S. palustris | Frond | [128] | ||
| W. unigemmata | Aerial part | [138] | ||
| D. cochleata | Leaf, rhizome | [165, 167] | ||
| L. flexuosum | Frond, whole plant | [178, 179] | ||
| M. quadrifolia | Stem, leaf, aerial part | [196, 198, 199, 200] | ||
| N. cordifolia | Frond | [99, 222] | ||
| H. zeylanica | Rhizome | [245] | ||
| O. vulgatum | Frond, leaf and stem | [261, 262] | ||
| C. thalictroides | Leaf | [273] | ||
| P. vittata | Frond | [285, 286] | ||
| T. gemmifera | Rhizome, leaf, frond | [293, 294, 295, 296] | ||
| 2 | Antiviral | W. unigemmata | Rhizome | [139, 140] |
| O. vulgatum | Not specified | [264] | ||
| 3 | Antifungal | S. palustris | Frond | [127] |
| P. squarrosum | Leaf | [169] | ||
| M. quadrifolia | Whole part | [201] | ||
| N. cordifolia | Frond | [222] | ||
| P. biaurita | Leaf | [281] | ||
| 4 | Antimalarial | S. palustris | Leaf | [132] |
| 5 | Antidiarrheal | M. quadrifolia | Aerial part | [194] |
| O. vulgatum | Whole part | [263] | ||
| 6 | Anthelmintic | D. esculentum | Rhizome | [107] |
| N. cordifolia | Leaf | [224] | ||
| 7 | Analgesic and anti-inflammatory | D. esculentum | Leaf | [99, 106] |
| M. quadrifolia | Aerial part, leaf | [194, 202] | ||
| H. zeylanica | Rhizome | [238, 239, 240] | ||
| C. thalictroides | Spore | [274] | ||
| 8 | Antidiabetic | D. esculentum | Leaf | [103] |
| M. quadrifolia | Aerial part | [205] | ||
| H. zeylanica | Rhizome | [244] | ||
| O. claytoniana | Frond, rhizome | [268] | ||
| C. thalictroides | Whole part | [276] | ||
| T. gemmifera | Frond, rhizome | [299, 301] | ||
| 9 | Antioxidant and anticancer | D. boryana | Aerial part | [92, 94] |
| D. esculentum | Leaf | [101, 102, 103, 104, 105] | ||
| D. maximum | Frond | [121] | ||
| S. palustris | Frond | [129, 130, 131] | ||
| W. unigemmata | Aerial part | [137, 138] | ||
| C. spinulosa | Leaf | [144] | ||
| D. cochleata | Leaf | [166] | ||
| P. squarrosum | Leaf | [165] | ||
| M. quadrifolia | Aerial part, leaf | [203, 204, 211] | ||
| N. cordifolia | Fruit, leaf | [223] | ||
| H. zeylanica | Rhizome | [242, 243] | ||
| C. thalictroides | Frond | [275] | ||
| P. biaurita | Frond | [278] | ||
| T. gemmifera | Rhizome, leaf, twig | [295, 297, 298] | ||
| T. zeylanica | Leaf | [303] | ||
| 10 | Neuroprotective | D. esculentum | Leaf | [104] |
| C. spinulosa | Leaf | [144] | ||
| L. japonicum | Spore | [186] | ||
| M. quadrifolia | Whole plant, leaf | [206, 207, 208, 209] | ||
| 11 | Nephroprotective | M. quadrifolia | Leaf | [210] |
| N. cordifolia | Rhizome | [225] | ||
| 12 | Hepatoprotective | D. esculentum | Leaf | [103] |
| L. flexuosum | Whole plant | [176, 177] | ||
| H. zeylanica | Rhizome | [236, 237] | ||
| 13 | Antifertility | L. flexuosum | Not specified | [174, 175] |
9. Important phytochemicals present in the wild edible ferns
The major bioactive compounds present in the ferns and fern-allies are flavonoids, terpenoids, steroids and alkaloids.
9.1. Flavonoids
The total flavonoid content has been reported in the edible parts of some ferns, for examples, 145.8 mg/g in D. boryana (arial parts) [94], 110.8 mg quercetin equivalent/100 g in D. esculentum (arial parts) [109], 151 mg quercetin equivalent/g in W. unigemmata (arial parts) [138], 3.5 mg/g in M. quadrifolia (arial parts) [212], 7.5 mg/g in M. quadrifolia (leaves) [214], 6.4 mg/g M. quadrifolia (stems) [214], 27.4 mg/g in O. vulgatum (whole plant) [263], 17.5 mg/g in P. biaurita (fronds) [282], 14.5 mg/g in P. biaurita (leaves) [278], etc. A higher total flavonoid content in the plant material is usually correlated with a more antioxidant capacity and thus potentially anticarcinogenic [306, 307].
Several flavonoid glycosides have been reported from the leaves [128] and fronds of S. palustris [126] (Figure 2). The isolated flavonoids viz. kaempferol 3-O-(3″-O-E-p-coumaroyl)-(6″-O-E-feruloyl)-β-glucopyranoside and kaempferol 3-O-(3″,6″-di-O-E-p-coumaroyl)-β-glucopyranoside moderately inhibit butyrylcholieserase (BChE) with IC50 values of 113.66 and 85.37 μM, respectively [126]; and kaempferol 3-O-(6″-O-E-p-coumaroyl)-β-glucopyranoside strongly inhibits acetylcholinesterase (AChE) with IC50 value of 23.5 μM [308] exhibiting potentiality against Alzheimer's disease.
Figure 2.
Some flavonoids isolated from S. palustris.
Huang et al. reported isolation of several flavonoids (ugonins E–Y) from the rhizomes of H. zeylanica [235, 246, 247, 248] (Figure 3). Ugonins J‒L are found more active than trolox, with IC20 values of 5.29 ± 0.32, 7.23 ± 0.22 and 7.93 ± 0.31 μM, respectively, in antioxidative activity using the DPPH assay; ugonins L,M,O,Q,S and T are found anti-inflammatory; and ugonin K is found antiosteoporosis. Ugonins J,K,L,M,S and U possess antidiabetic activity by inhibiting α-glucosidase [309, 310]. Ugonin J is found effective in inhibition of neointima formation [311]. Being a novel viral protease inhibitor, ugonin J inhibits severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) 3CLpro activity and hence is therapeutically potential for SARS-CoV-2 infection [312]. Ugonin K induces apoptosis [313]. Neuroprotective [314] and osteogenesis [315] effects of ugonin K are reported. Ugonin M is considerably potential to develop hepatoprotective and anti-inflammatory natural agents [316]. Ugonin U shows immunomodulatory effect in human neutrophils via stimulation of phospholipase C [317], and induces Ca2+ mobilization and eventually activates the NLRP3 inflammasome [318].
Figure 3.
Ugonins isolated from H. zeylanica rhizomes.
From the whole plant of H. zeylanica, Su et al. have isolated several prenylated flavonoids [251]. Among them, neougonin A (Figure 4) inhibited NO production in lipopolysaccharide-treated RAW264.7 cells with an IC50 value of 3.32 μM, and it displayed a moderate cytotoxicity with IC50 value of 16.13 μM in the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.
Figure 4.
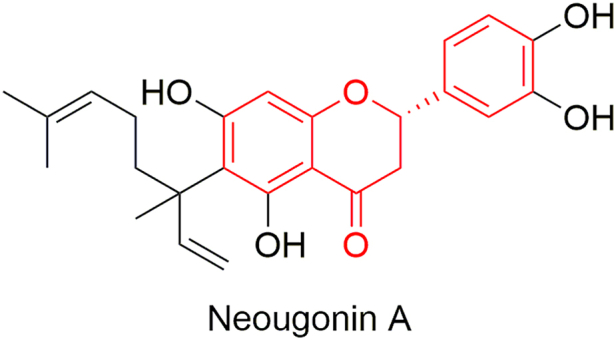
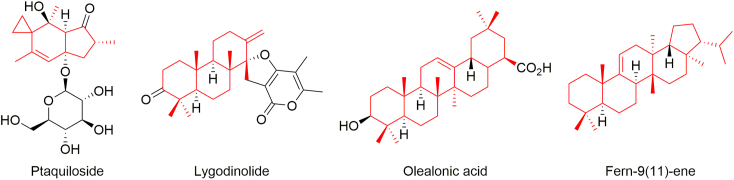
Neougonin A isolated from H. zeylanica.
9.2. Terpenoids
Ptaquiloside (Figure 5) is one of the main hazardous metabolites found in the fern that is considered to be responsible for the toxicological problems to ruminant and non-ruminant animals, and then to human alike through the milk and meat [150]. The amounts of ptaquiloside have been estimated in P. revolutum, D. cochleata and P. squarrosum (leaves) that collected from different parts of India [108]. P. revolutum is considered as carcinogenic [152]. It has been demonstrated that feeding of bracken fern induces urinary bladder tumors, illeum sarcomas, pulmonary adenomas and leukemia [153]. On the other hand, Yoshihira et al. have isolated several sesquiterpens, namely, pterosins A‒G, J‒L, N, O, Z, pterosides A‒C, etc., from the fronds of Japanese bracken fern and performed cytotoxicity test (Table 3) [154]. They have mentioned that although these indanonone derivatives showed some cytotoxicity to HeLa cells; however, none of them could induce tumors under the conditions studied.
Figure 5.
Some bioactive terpenoids obtained from food ferns.
Table 3.
Cytotoxicity (HeLa Cell) of pterosins and pterosides isolated from Pteridium aquilinum var. latiusculum.
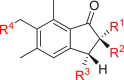 | |||||
|---|---|---|---|---|---|
| Compound | R1 | R2 | R3 | R4 | Cytotoxicity IC50 (μg/mL) |
| Pterosin A | ‒CH2OH | ‒CH3 | H | ‒CH2OH | 320 |
| Pterosin B | ‒CH3 | H | H | ‒CH2OH | 100 |
| Pterosin C | H | ‒CH3 | ‒OH | ‒CH2OH | 320 |
| Pterosin D | ‒CH3 | ‒CH3 | ‒OH | ‒CH2OH | >320 |
| Pterosin E | ‒CH3 | H | H | ‒CO2H | 30 |
| Pterosin F | ‒CH3 | H | H | ‒CH2Cl | 65 |
| Pterosin G | ‒CH2OH | H | H | ‒CH2OH | 180 |
| Pterosin J | H | ‒CH3 | ‒OH | ‒CH2Cl | >100 |
| Pterosin K | ‒CH2OH | ‒CH3 | H | ‒CH2Cl | >100 |
| Pterosin L | ‒CH2OH | ‒CH3 | ‒OH | ‒CH2OH | >100 |
| Pterosin N | ‒CH3 | ‒OH | H | ‒CH2OH | 180 |
| Pterosin O | ‒CH3 | H | H | ‒CH2OCH3 | 30 |
| Pterosin Z | ‒CH3 | ‒CH3 | H | ‒CH2OH | 10 |
| Pteroside A | ‒CH2OH | ‒CH3 | H | ‒CH2O glucose | >320 |
| Pteroside B | ‒CH3 | H | H | ‒CH2O glucose | >100 |
| Pteroside C | H | ‒CH3 | ‒OH | ‒CH2O glucose | >320 |
Lygodinolide (Figure 5), present as major constituent in L. flexuosum, is attributed for the wound healing property [[181], [182]].
From N. cordifolia, oleanolic acid and fern-9(11)-ene have been isolated (Figure 5) [229]. Fern-9(11)-ene has also been isolated from C. spinulosa leaves [146]. Oleanolic acid shows antimicrobial activity against vancomycin resistant enterococci, Streptococcus pneumoniae and MRSA [319]. It is also found to exhibit anticancer activity [320]. Fern-9(11)-ene is found to be susceptible against Salmonella typhi and Pseudomona aeruginosa [321].
9.3. Steroids
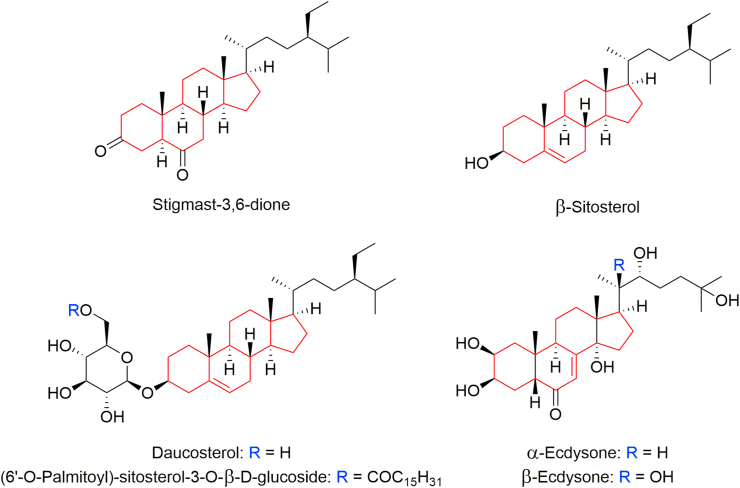
Stigmast-3,6-dione, β-sitosterol and daucosterol in the leaves and stalks of C. spinulosa [147, 148, 149]; α- and β-ecdysones in the arial parts of P. revolutum [161]; and daucosterol, β-sitosterol, (6′-O-palmitoyl)-sitosterol-3-O-β-D-glucoside in B. lanuginosum [231] are some examples of alkaloids present in different edible parts of the ferns and fern-allies (Figure 6).
Figure 6.
Steroids found in food ferns.
9.4. Alkaloids
Total alkaloids of 6.1 mg/g in M. quadrifolia (leaves) [214], 5.9 mg/g M. quadrifolia (stems) [214], 0.1 mg/g in N. cordifolia (leaves) [227] and 16.4 mg/g in P. biaurita (fronds) [282] are reported.
10. Conclusion
Taxonomic and ethnobotanical studies on the ferns of Nepal are exploited; however, chemical and biological investigations on Nepalese ferns and fern-allies are limited. This review primarily briefs historical background on expeditious journey on Nepalese pteridophytes, updates number of ferns consumed as vegetables by Nepalese, outlines their ethnomedicinal uses, and compiles information on their pharmacognosy, pharmacology and phytochemistry that reported from elsewhere. In Nepal, some species of ferns that primarily collected from nearby forests are sold in the markets. Some species are consumed by rural Nepalese as the last option of food and there is no proper information transformation to the next generation about ferns as food materials and their potential medicinal values. C. decurrenti-alata, M. intermedia, B. orientale, O. japonica, C. intermedia and P. wallichiana are available in Nepal, but there is no precedented report for their consumption by Nepalese; however, they are consumed in China. The traditional ethnopharmacological knowledge on ferns and fern-allies is neglected both by locals and scientific community.
11. Future recommendation
Most of the ferns and fern-allies, collected for vegetable food by Nepalese, grow in their natural habitat. Lack of sharing of traditional ethnomedicinal knowledge to new generation and unsustainable collection practice making them vulnerable. Only in the recent past, a very few species bearing economical value are agricultured in local farms. From the literature evidences, we believe that investigations on unfocused ferns can provide new research opportunities and potent bioactive phytochemicals.
Parts of some species such as P. revolutum, P. squarrosum, C. thalictroides, P. vittata, etc. are considered to be toxic, thus they warrant immediate further investigations. P. revolutum constitutes cyanogen glycosides. P. squarrosum constitutes prunasin that can release HCN. P. vittata hyperaccumulates arsenic. P. revolutum and P. squarrosum can induce carcinogenesis. On the other hand, the antioxidant capacity of the extracts of D. boryana, D. esculentum, D. maximum, S. palustris, W. unigemmata, D. cochleata, P. squarrosum, M. quadrifolia, N. cordifolia, P. biaurita, T. gemmifera, T. zeylanica, etc. has been reported indicating their role in cancer chemoprevention. Further investigations of these plant materials to isolate and identify effective anticancer agents are highly desirable.
Rhizomes of C. spinulosa and O. reticulatum have found their traditional uses against snake bites. Ugonins, particularly isolated from the rhizomes of H. zeylanica, have found diverse bioactivities. Recently, ugonin J has reported to be effective against coronavirus disease 2019 (COVID-19). Hence, not only the vegetative parts of the ferns but also other parts should be investigated in order to search for potential drug candidates.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Nepal Academy of Science and Technology (NAST), Khumaltar, Lalitpur, Nepal for providing a platform to write this article. Tribhuvan University Central Library, Kirtipur, Kathmandu, Nepal is acknowledged for providing literature access.
References
- 1.GoN/MoFSC, Nepal National Biodiversity Strategy and Action Plan: 2014-2020. Ministry of Forests and Soil Conservation, Government of Nepal; Kathmandu: 2014. http://mofe.gov.np/downloadfile/29_Strategy%20and%20action%20plan_1526382258.pdf [Google Scholar]
- 2.Paudel P.K., Bhattarai B.P., Kindlmann P. In: Himalayan Biodiversity in the Changing World. Kindlmann P., editor. Springer; Dordrecht: 2012. An overview of the biodiversity in Nepal. [Google Scholar]
- 3.Shyaula S.L., Bajracharya G.B., Shakya S.M., Subba D. Khumaltar, Lalitpur; Nepal: 2021. Comprehensive Insights in Vegetables of Nepal, Nepal Academy of Science and Technology.https://nast.gov.np/documentfile/Comprehensive-Insights-in-Vegetables-Book.pdf [Google Scholar]
- 4.Background Information about Nepal. http://www.floraofnepal.org/countryinformation
- 5.Bajracharya G.B., Bajracharya B. In: Comprehensive Insights in Vegetables of Nepal, Nepal Academy of Science and Technology. Shyaula S.L., Bajracharya G.B., Shakya S.M., Subba D., editors. Khumaltar, Lalitpur; Nepal: 2021. Diversity, availability, using status, nutritional and pharmacological aspects of wild vegetables of Nepal; pp. 157–200.https://nast.gov.np/documentfile/Comprehensive-Insights-in-Vegetables-Book.pdf [Google Scholar]
- 6.Pandey S.N., Trivedi P.S., Mishra S.P. II. Vikas Publishing House Pvt. Ltd.; New Delhi, India: 1977. A Textbook of Botany. [Google Scholar]
- 7.Khare P.B. Ferns and fern allies - their significance and fantasies. Appl. Bot. Abstr. 1996;16(1):50–61. [Google Scholar]
- 8.Thakur C., Rajbhandary S. Fern and fern allies of panchase protected forest, central Nepal. J. Plant Res. 2018;16(1):39–45. [Google Scholar]
- 9.Gao Z.P., Li R.F., Wang B.H., Lu Y.R. Progress in chemical constituents of genus Dryopteris, Chin. J. Exp. Tradit. Med. Formulae. 2003;9(3):50–55. [Google Scholar]
- 10.Wang L., He Z.R. Advances in medical components of pteridophytes. Chin. Wild Plant Resour. 2006;25:1–4. [Google Scholar]
- 11.Ma R., Pan H., Shen T., Li P., Chen Y., Li Z., Di X., Wang S. Interaction of flavonoids from Woodwardia unigemmata with bovine serum albumin (BSA): application of spectroscopic techniques and molecular modeling methods. Molecules. 2017;22:1317. doi: 10.3390/molecules22081317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaudhary R.P. Tec Press Books; Bangkok: 1998. Biodiversity in Nepal: Status and Conservation. [Google Scholar]
- 13.Hassler M. In: Species 2000 and ITIS Catalogue of Life, 2018 Annual Checklist, Species 2000. Roskov Y., Abucay L., Orrell T., Nicolson D., Bailly N., Kirk P.M., Bourgoin T., De Walt R.E., Decock W., De Wever A., van Nieukerken E., Zarucchi J., Penev L., editors. Naturalis; Leiden, the Netherlands: 2018. World ferns: checklist of ferns and lycophytes of the world (version Apr. 2018)http://www.catalogueoflife.org/annual-checklist/2018 [Google Scholar]
- 14.The Pteridophytes (Fens and Fern Allies) http://www.theplantlist.org/browse/P/
- 15.Lu S.G. Higher Education Press; Beijing: 2007. Pteridology. [Google Scholar]
- 16.Pteridophytes. https://bsi.gov.in/page/en/pteridophytes
- 17.Ebihara A., Nitta J.H. An update and reassessment of fern and lycophyte diversity data in the Japanese Archipelago. J. Plant Res. 2019;132:723–738. doi: 10.1007/s10265-019-01137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knapp R., Hsu T.C. Second supplement. KBCC Press; Taiwan: 2017. Ferns and Fern Allies of Taiwan. [Google Scholar]
- 19.delos Angeles M.D.A., Buot I.E., Jr. Orders and families of Philippine pteridophytes. J. Nat. Stud. 2012;11(1-2):19–33. https://www.journalofnaturestudies.org/files/19-33-delos-angeles-full.pdf [Google Scholar]
- 20.Yusuf U.K. Universiti Putra Malaysia Press; Serdang: 2010. Ferns of Malaysian Rain Forest. A Journey through the Fern World. [Google Scholar]
- 21.Lindsay S., Middleton D.J., Boonkerd T., Suddee S. Towards a stable nomenclature for Thai ferns. Thai For. Bull. (Bot.) 2009;37:64–106. https://li01.tci-thaijo.org/index.php/ThaiForestBulletin/article/view/24338 [Google Scholar]
- 22.Bhattarai K.R. Ethnobotanical survey on plants used in Mai Municipality of Ilam district, eastern Nepal. Banko Janakari. 2020;30(2):11–35. http://bankojanakari.gov.np/articles/2-BJ_Ethnobotanical_Bhattarai_final_1606748171.pdf [Google Scholar]
- 23.Bhagat I.M., Shrestha S. Fern and fern-allies of eastern terai, Nepal. Our Nat. 2010;8:359–362. [Google Scholar]
- 24.Thapa N. In: Bulletin of Department of Plant Resources No. 19, His Majesty’s Government of Nepal, Ministry of Forests and Soil Conservation. Bista M.S., Adhikari M.K., Rajbhandari K.R., editors. Department of Plant Resources, National Herbarium and Plant Laboratories; Lalitpur: 2002. Pteridophytes of Nepal; pp. 1–175. [Google Scholar]
- 25.Kandel D.R., Fraser-Jenkins C.R. Vol. 3. Ministry of Forests and Environment, Department of Plant Resources, National Herbarium and Plant Laboratories; Godawari, Lalitpur, Nepal: 2020. Ferns and Fern-Allies of Nepal. [Google Scholar]
- 26.Joshi N., Kehlenbeck K., Maass B.L. Conference on International Agricultural Research for Development, Tropentag 2007. University of Kassel-Witzenhusen and University of Göttingen, October 9-11; 2007. Traditional, neglected vegetables of Nepal: their sustainable utilization for meeting human needs.https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.624.5143&rep=rep1&type=pdf [Google Scholar]
- 27.Chettri S., Manivannan S., Muddarsu V.R. Nutrient and elemental composition of wild edible ferns of the Himalaya. Am. Fern J. 2018;108(3):95–106. [Google Scholar]
- 28.Seal T. Evaluation of nutritional potential of wild edible plants, traditionally used by the tribal people of Meghalaya State in India. Am. J. Plant Nutr. Fertilization Technol. 2012;2:19–26. [Google Scholar]
- 29.Aulakh M.K., Kaur N., Saggoo M.I.S. Bioactive phytoconstituents of Pteridophytes - a review. Indian Fern J. 2019;36:37–79. [Google Scholar]
- 30.Buchanan-Hamilton F. Asian Educational Service; New Delhi: 1986. An Account of the Kingdom of Nepal. [Google Scholar]
- 31.Fraser-Jenkinns C.R. Nepal’s Little Known Pteridophytes, the Hidden Works of David Don, and the Geography and Distribution of Indo-Himalayan Ferns, with State Lists, Website Version. 2010. www.groups.yahoo.com/group/Indian-Ferns updated 31 Dec. 2010, 2011.
- 32.Rajbhandary S. In: Frontiers of Botany. Jha P.K., Siwakoti M., Rajbhandary S., editors. Central Department of Botany, Tribhuvan University; Kirtipur, Kathmandu: 2016. Fern and fern allies of Nepal; pp. 124–150. [Google Scholar]
- 33.Wallich N. Asiatic Lithographical Press; Calcutta, Serampore: 1826. Tentamen Florae Nepalensis Illustratae Consisting of Botanical Descriptions and Lithographic Figures of Select Nipal Plants.https://www.biodiversitylibrary.org/page/58005015#page/7/mode/1up [Google Scholar]
- 34.Don D. J. Gale; London, England: 1825. Prodromus Florae Nepalensis. [Google Scholar]
- 35.Hooker J.D. 1–7. L. Reeve and Co.; London: 1872. The Flora of British India. [Google Scholar]
- 36.Moore T. William Pamplin; London: 1857. Index Filicum. [Google Scholar]
- 37.Sim R. Grown for Sale by Robert Sim, Nurseyman, Foot’s Cray; Kent, London: 1859. A Priced Catalogue with Brief Descriptive and Cultural Remarks on the Extensive Collection of Stove, Greenhouse and Hardy Exotic and British Ferns.https://books.google.com.np/books?id=VNRbAAAAQAAJ&pg=PA1&lpg=PA1&dq#v=onepage&q&f=false [Google Scholar]
- 38.Strachey Sir R., Duthie J.F. Lovell Reeve and Co. Ltd.; London: 1906. Catalogue of the Plants of Kumaon and of the Adjacent Portions of Garhwal and Tibet, Based on the Collections Made by Strachey and Winterbottom during the Years 1846 to 1849 and on the Catalogue Originally Prepared in 1852 by Sir Richard Strachey. [Google Scholar]
- 39.Burkill I.H. Superintendent Government Printing; Calcutta, India: 1910. Notes from a Journey to Nepal. [Google Scholar]
- 40.Lancaster C.R. 1981. A Plantsman in Nepal. London. [Google Scholar]
- 41.Raizada M.B., Vaid K.M. Fern of Nepal. India Forester. 1952;78:576–581. [Google Scholar]
- 42.Panday B.D. Some aspects of the vegetation of Nepal. Bull. Bot. Survey India. 1962;4(4):135–140. [Google Scholar]
- 43.Tagawa M. Contribution to the ferns of Annapurna-Dhaulagiri range, central Nepal. J. Bombay Nat. Hist. Soc. 1975;72(3):728–731. [Google Scholar]
- 44.Iwatsuki K. In: The Himalayan Plants. Ohba H., Malla S.B., editors. University of Tokyo Press; Tokyo: 1988. An enumeration of the pteridophytes of Nepal; pp. 231–339.http://umdb.um.u-tokyo.ac.jp/DKankoub/Bulletin/no31/no31018.html [Google Scholar]
- 45.Rajbhandari K.R. History of botanical explorations in Nepal. J. Bombay Nat. Hist. Soc. 1976;73:468–481. [Google Scholar]
- 46.Gurung V.L. In: Catalogue of Nepalese Vascular Plants, Bulletin of the Department of Medicinal Plant Nepal No. 7, His Majesty’s Government of Nepal. Malla S.B., Shrestha A.B., Rajbhandari S.B., Shrestha T.B., Adhikari P.M., Adhikari S.R., editors. Ministry of Forests, Department of Medicinal Plants; Thapathali, Kathmandu, Nepal: 1976. Pteridophyta; pp. 1–27. [Google Scholar]
- 47.Gurung V.L. In: Nepal - Nature’s Paradise. Majapuria T.C., editor. White Lotus Co. Ltd.; Bangkok, Thailand: 1984. Ferns; pp. 194–211. [Google Scholar]
- 48.Shrestha R., Gurung V.L. Proceedings of National Conference on Science and Technology, April 24-29, 1988. Royal Nepal Acadamy of Science and Technology; Kathmandu, Nepal: 1989. A study on ecology of eusporangiate ferns of Nepal Himalaya; pp. 359–364. [Google Scholar]
- 49.Gurung V.L. Sahayogi Press Pvt. Ltd.; Kathmandu: 1991. Ferns: the Beauty of Nepalese Flora. [Google Scholar]
- 50.Manandhar N.P. A survey of medicinal plants of Jajarkot district, Nepal. J. Ethnopharmacol. 1995;48:1–6. doi: 10.1016/0378-8741(95)01269-j. [DOI] [PubMed] [Google Scholar]
- 51.Manandhar N.P. An inventory of some herbal drugs of Myagdi district, Nepal, Econ. Bot. 1995;49(4):371–379. [Google Scholar]
- 52.Manadhar N.P. In: Ethnobotany in South Asia. Maheshwari J.K., editor. Scientific Publishers; Jodhpur, India: 1996. Ethnobotanical observation on ferns and fern allies of Nepal; pp. 414–422. [Google Scholar]
- 53.Manandhar N.P. Timber Press; Portland, Oregon: 2002. Plants and People of Nepal. [Google Scholar]
- 54.Subba D.K., Rai B.K., Dhakal M.R. Food value of some edible ferns from Dharan, south eastern Nepal. J. Bombay Nat. Hist. Soc. 2001;98(3):499–502. [Google Scholar]
- 55.Baral S.R., Kurmi P.P. Rachana Sharma, Maijubahal; Kathmandu, Nepal: 2006. A Compendium of Medicinal Plants in Nepal. [Google Scholar]
- 56.Phuyal N., Bhatta G.D., Maharjan S., Pokharel K.K. A contribution to the pteridophytic flora of Makwanpur district, central Nepal. Bull. Dept. Plant Resour. 2011;33:45–59. [Google Scholar]
- 57.Pathak M., Phuyal N., Tharu L.R. Inventory of the pteridophytic flora of Sankhuwasabha district, eastern Nepal with notes on medicinal values. Bull. Dept. Plant Resour. 2012;34:47–55. [Google Scholar]
- 58.Fraser-Jenkins C.R., Kandel D.R., Pariyar S. Vol. 1. Ministry of Forests and Soil Conservation, Department of Plant Resources, National Herbarium and Plant Laboratories; Nepal: 2015. Ferns and Fern-Allies of Nepal. [Google Scholar]
- 59.Fraser-Jenkins C.R., Kandel D.R. Vol. 2. Ministry of Forests and Soil Conservation, Department of Plant Resources, National Herbarium and Plant Laboratories; Nepal: 2019. Ferns and Fern-Allies of Nepal. [Google Scholar]
- 60.Fraser-Jenkins C.R., Gandhi K.N., Kholia B.S. Bishen Singh Mahendra Pal Singh; Dehra Dun, India: 2016. An Annotated Checklist of Indian Pteridophytes Part-1 (Lycopodiaceae to Thelypteridaceae) [Google Scholar]
- 61.Fraser-Jenkins C.R., Gandhi K.N., Kholia B.S. Bishen Singh Mahendra Pal Singh; Dehra Dun, India: 2018. An Annotated Checklist of Indian Pteridophytes Part-2 (Woodsiaceae to Dryopteridaceae) [Google Scholar]
- 62.R Fraser-Jenkins C., Gandhi K.N., Kholia B.S., Kandel D.R. Bishen Singh Mahendra Pal Singh; Dehra Dun, India: 2020. An Annotated Checklist of Indian Pteridophytes Part-3 (Lomariopsidaceae to Salviniaceae) [Google Scholar]
- 63.Fraser-Jenkins C.R. International Book Distributors; Dehra Dun, India: 1997. New Species Syndrome in Indian Pteridology and the Ferns of Nepal. [Google Scholar]
- 64.Fraser-Jenkins C.R. Bishen Singh Mahendra Pal Singh; Dehra Dun, India: 2008. Taxonomic Revision of Three Hundred Indian Subcontinental Pteridophytes with a Revised Census-List. [Google Scholar]
- 65.Fraser-Jenkins C.R. A brief comparison of modern pteridophyte classifications (families and genera in India) Indian Fern J. 2010;26:107–131. [Google Scholar]
- 66.Shrestha T.B., Bhandari B., Belbase N. IUCN Nepal; Kathmandu: 1999. Nepal Country Report on Biological Diversity. [Google Scholar]
- 67.Kramer K.U., Green P.S. In: The Families and Genera of Vascular Plants. Kubitzki K., editor. Vol. 1. Pteridophytes and Gymnosperms, Springer-Verlag; 1990. Pteridophytes; pp. 12–279. [Google Scholar]
- 68.The Plant List. http://www.theplantlist.org/
- 69.eFloras. http://www.efloras.org/
- 70.Liu Y., Wujisguleng W., Long C. Food uses of ferns in China: a review. Acta Soc. Bot. Pol. 2012;81(4):263–270. [Google Scholar]
- 71.Ojha R., Devkota H.P. Edible and medicinal pteridophytes of Nepal: a review. Ethnobot. Res. Appl. 2021;22:16. [Google Scholar]
- 72.Kandel D.R. In: Plant Diversity of Nepal. Siwakoti M., Jha P.K., Rajbhandary S., Rai S.K., editors. Botanical Society of Nepal; Kathamandu: 2020. Pteridophytes of Nepal; pp. 10–22. [Google Scholar]
- 73.Shrestha D. Indigenous vegetables of Nepal for biodiversity and food security. Int. J. Biodivers. Conserv. 2013;5(3):98–108. [Google Scholar]
- 74.Dangol D.R., Maharjan K.L., Maharjan S.K., Acharya A.K. In: Conservation and Utilization of Agricultural Plant Genetic Resources in Nepal, Proceedings of 2nd National Workshop, 22-23 May 2017, Dhulikhel, National Agriculture Genetic Resources Center. Joshi B.K., KC H.B., Acharya A.K., editors. Fod Development Directorate, Department of Agriculture, Ministry of Agricultural Development; Kathmandu, Nepal: 2017. Wild edible plants in Nepal; pp. 390–407.https://nepalindata.com/media/resources/bulkuploaded/Plant_Genetic_Resources_CUAPGR_Nepal_OCT17.pdf [Google Scholar]
- 75.Rijal A. Surviving on knowledge: ethnobotany of Chepang community from mid-hills of Nepal. Ethnobot. Res. Appl. 2011;9:181–215. https://ethnobotanyjournal.org/index.php/era/article/view/249 [Google Scholar]
- 76.Rokaya M.B., Münzbergová Z., Timsina B. Ethnobotanical study of medicinal plants from the Humla district of western Nepal. J. Ethnopharmacol. 2010;130:485–504. doi: 10.1016/j.jep.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 77.Uprety Y., Poudel R.C., Shrestha K.K., Rajbhandary S., Tiwari N.N., Shrestha U.B., Asselin H. Diversity of use and local knowledge of wild edible plant resources in Nepal. J. Ethnobiol. Ethnomed. 2012;8:16. doi: 10.1186/1746-4269-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Joshi N., Siwakoti M. Wild vegetables used by local community of Makawanpur district and their contribution to food security and income generation. Nepal J. Sci. Technol. 2012;13(1):59–66. [Google Scholar]
- 79.Rana S.K., Oli P.S., Rana H.K. Traditional botanical knowledge (TBK) on the use of medicinal plants in Sikles area, Nepal. Asian J. Plant Sci. Res. 2015;5(11):8–15. [Google Scholar]
- 80.Shrestha P.M., Dhillion S.S. Diversity and traditional knowledge concerning wild food species in a locally managed forest in Nepal. Agrofor. Syst. 2006;66:55–63. [Google Scholar]
- 81.Bhattarai S., Rajbhandary S. Pteridophyte flora of manaslu conservation area, central Nepal. Am. J. Plant Sci. 2017;8(4):680–687. [Google Scholar]
- 82.Kandel D.R., Pathak M. Documentation of ferns from subtropical forests of Pyuthan district, western Nepal. J. Dept. Plant Resour. 2013;35:46–49. [Google Scholar]
- 83.Shrestha S., Rai S.K. Survey of marketable vegetables and edible fruits in Dharan, eastern Nepal. Nepalese J. Biosci. 2012;2:134–147. https://www.nepjol.info/index.php/NJBS/article/view/7501 [Google Scholar]
- 84.Singh A.G. Survey of some medicinally important leafy vegetables in Rupandehi district of western Nepal. Int. J. Appl. Sci. Biotechnol. 2015;3:111–118. [Google Scholar]
- 85.Joshi A.R., Joshi K. Indigenous uses of wetland plant diversity of two valleys (Kathmandu and Pokhara) in Nepal. Ethnobotany. 2009;21:11–17. [Google Scholar]
- 86.Dangol D.R. Traditional uses of plants of commonland habitats in western Chitwan, Nepal. J. Inst. Agric. Anim. Sci. 2008;29:71–78. [PMC free article] [PubMed] [Google Scholar]
- 87.Bhatt M.D., Tewari A., Singh S.P. Floristic composition of weeds in paddy fields in Mahendranagar, Nepal. Ecoprint. 2009;16:15–19. [Google Scholar]
- 88.Thapa L.B., Dhakal T.M., Chaudhary R. Wild edible plants used by endangered and indigenous Raji tribes in western Nepal. Int. J. Appl. Sci. Biotechnol. 2014;2(3):243–252. https://www.nepjol.info/index.php/IJASBT/article/view/10969/9012 [Google Scholar]
- 89.Aryal K.P., Berg Å., Ogle B. Uncultivated plants and livelihood support - a case study from the Chepang people of Nepal. Ethnobot. Res. Appl. 2009;7:409–422. https://ethnobotanyjournal.org/index.php/era/article/view/255 [Google Scholar]
- 90.Luni P., Maharjan K., Joshi N. Forest and food security of indigenous people: a case of Chepangs in Nepal. J. Int. Dev. Cooperation. 2011;17(1):113–135. [Google Scholar]
- 91.Mir S.A., Mishra A.K., Reshi Z.A., Sharma M.P. Preliminary phytochemical screening of some pteridophytes from district Shopian (J & K) Int. J. Pharm. Pharmaceut. Sci. 2013;5(S4):632–637. [Google Scholar]
- 92.Poudel A., Kim S.G., Kim D.K., Kim Y.K., Lee Y.S., Lee G.W., Min B.S., Jung H.J. Antioxidative and antiobesity activity of Nepalese wild herbs. Nat. Prod. Sci. 2011;17:123–129. https://www.koreascience.or.kr/article/JAKO201121055567508.page [Google Scholar]
- 93.Acharya K.P., Rokaya M.B. Ethnobotanical survey of medicinal plants traded in the streets of Kathmandu valley. Sci. World. 2005;3(3):44–48. [Google Scholar]
- 94.Cao J., Xia X., Dai X., Xiao J., Wang Q., Andrae-Marobela K., Okatch H. Flavonoids profiles, antioxidant, acetylcholinesterase inhibition activities of extract from Dryoathyrium boryanum (Willd.) Ching. Food Chem. Toxicol. 2013;55:121–128. doi: 10.1016/j.fct.2012.12.051. [DOI] [PubMed] [Google Scholar]
- 95.Dash G.K., Khadidi S.K.J., Shamsuddin A.F. Pharmacognostic studies on Diplazium esculentum (retz.) sw. Der Pharm. Lett. 2017;9:113–120. https://www.scholarsresearchlibrary.com/articles/pharmacognostic-studies-on-diplazium-esculentum-retz-sw.pdf [Google Scholar]
- 96.Semwal A., Kaushik S., Bhatt S.P., Negi A. Antibacterial activity of Diplazium esculentum (retz.) sw. Pharmacogn. J. 2011;3:77–79. [Google Scholar]
- 97.Bhattarai K.R., Khadka M.K. Ethnobotanical survey of medicinal plants from Ilam district, east Nepal. Our Nat. 2016;14(1):78–91. [Google Scholar]
- 98.Akter S., Hossain Md M., Ara I., Akhtar P. Investigation of in vitro antioxidant, antimicrobial and cytotoxic activity of Diplazium esculentum (RETZ). SW. Int. J. Adv. Pharm. Biol. Chem. 2014;3(3):723–733. http://www.ijapbc.com/files/32-3369.pdf [Google Scholar]
- 99.Amoroso V.B., Antesa D.A., Buenavista D.P., Coritico F.P. Antimicrobial, antipyretic, and anti-inflammatory activities of selected Philippine medicinal pteridophytes. Asian J. Biodivers. 2014;5:18–40. http://www.asianscientificjournals.com/new/publication/index.php/ajob/article/viewFile/479 [Google Scholar]
- 100.Ullah M.O., Haque M., Urmi K.F., Md Zulfiker A.H., Anita E.S., Begum M., Hamid K. Antibacterial activity and brine shrimp lethality bioassay of methanolic extracts of fourteen different edible vegetables from Bangladesh. Asian Pac. J. Trop. Biomed. 2013;3:1–7. doi: 10.1016/S2221-1691(13)60015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Roy S., Hazra B., Mandal N., Chaudhuri T.K. Assessment of the antioxidant and free radical scavenging activities of methanolic extract of Diplazium esculentum. Int. J. Food Prop. 2013;16:1351–1370. [Google Scholar]
- 102.Kaushik A., Jijta C., Kaushik J.J., Zeray R., Ambesajir A., Beyene L. FRAP (Ferric reducing ability of plasma) assay and effect of Diplazium esculentum (Retz) Sw. (a green vegetable of north India) on central nervous system. Indian J. Nat. Prod. Resour. 2012;3:228–231. http://nopr.niscair.res.in/bitstream/123456789/14404/1/IJNPR%203%282%29%20228-231.pdf [Google Scholar]
- 103.Junejo J.A., Gogoi G., Islam J., Rudrapal M., Mondal P., Hazarika H., Zaman K. Exploration of antioxidant, antidiabetic and hepatoprotective activity of Diplazium esculentum - a wild edible plant from north eastern India. Future J. Pharm. Sci. 2018;4:93‒101. [Google Scholar]
- 104.Kunkeaw T., Suttisansanee U., Trachootham D., Karinchai J., Chantong B., Potikanond S., Inthachat W., Pitchakarn P., Temviriyanukul P. Diplazium esculentum (Retz.) Sw. reduces BACE-1 activities and amyloid peptides accumulation in Drosophila models of Alzheimer’s disease. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-03142-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rahmat A. Determination of total antioxidant activity in three types of local vegetables shoots and the cytotoxic effect of their ethanolic extracts against different cancer cell lines, Asia Pac. J. Clin. Nutr. 2003;12(3):308–311. [PubMed] [Google Scholar]
- 106.Kaushik A., Kaushik J.J., Das A., Gemal S., Gaim D. Preliminary studies on anti-inflammatory activities of Diplazium esculentum in experimental animal models. Int. J. Pharm. Sci. Res. 2011;2:1251–1253. [Google Scholar]
- 107.Semwal A., Farswan M.S. In-vitro anthelmintic activity of Diplazium esculentum (Retz.) Swiss rhizome extract. J. Pharmacogn. Phytochem. 2012;1:84–87. https://www.phytojournal.com/archives/2012/vol1issue4/PartA/11.1.pdf [Google Scholar]
- 108.Pathania S., Kumar P., Singh S., Khatoon S., Rawat A.K.S., Punetha N., Jensen D.J., Lauren D.R., Somvanshi R. Detection of ptaquiloside and quercetin in certain Indian ferns. Curr. Sci. 2012;102:1683–1691. https://www.currentscience.ac.in/Volumes/102/12/1683.pdf [Google Scholar]
- 109.Tongco J.V.V., Villaber R.A.P., Aguda R.M., Razal R.A. Nutritional and phytochemical screening, and total phenolic and flavonoid content of Diplazium esculentum (Retz.) Sw. from Philippines. J. Chem. Pharmaceut. Res. 2014;6(8):238–242. https://www.jocpr.com/articles/nutritional-and-phytochemical-screening-and-total-phenolic-and-flavonoid-content-of-diplazium-esculentum-retz-sw-from-ph.pdf [Google Scholar]
- 110.Semwal P., Painuli S., Painuli K.M., Antika G., Tumer T.B., Thapliyal A., Setzer W.N., Martorell M., Alshehri Md M., Taheri Y., Daştan S.D., Ayatollahi S.A., Petkoska A.T., Sharifi-Rad J., Cho W.C. Diplazium esculentum (Retz.) Sw.: ethnomedicinal, phytochemical, and pharmacological overview of the Himalayan ferns. Oxid. Med. Cell. Longev. 2021 doi: 10.1155/2021/1917890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Essien E.E., Ascrizzi R., Flamini G. Characterization of volatile compounds of Diplazium esculentum. Chem. Nat. Compd. 2019;55:958–959. [Google Scholar]
- 112.Naik B., Maurya V.K., Kumar V., Kumar V., Upadhyay S., Gupta S. Phytochemical analysis of Diplazium esculentum reveals the presence of medically important components. Curr. Nutr. Food Sci. 2021;17:210–215. [Google Scholar]
- 113.Caldwell M.J. Ascorbic acid content of Malaysian leaf vegetables. Ecol. Food Nutr. 1972;1:313–317. [Google Scholar]
- 114.Srivastava S.K., Srivastava S.D., Saksena V.K., Nigam S.S. A flavanone glycoside from Diplazium esculentum. Phytochemistry (Oxf.) 1981;20:862. [Google Scholar]
- 115.Ching L.S., Mohamed S. Alpha-tocopherol content in 62 edible tropical plants. J. Agric. Food Chem. 2001;49:3101–3105. doi: 10.1021/jf000891u. [DOI] [PubMed] [Google Scholar]
- 116.Miean K.H., Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001;49:3106–3112. doi: 10.1021/jf000892m. [DOI] [PubMed] [Google Scholar]
- 117.Somvanshi R., Lauren D.R., Smith B.L., Dawra R.K., Sharma O.P., Sharma V.K., Singh A.K., Gangwar N.K. Estimation of the fern toxin, ptaquiloside, in certain Indian ferns other than bracken. Curr. Sci. 2006;91:1547–1552. https://www.currentscience.ac.in/Volumes/91/11/1547.pdf [Google Scholar]
- 118.Astuti M.D., Kuntorini E.M., Wisuda F.E.P. Isolasi dan identifikasi terpenoid Dari fraksi n-butanol herba lampasau (Diplazium esculentum Swartz) Jurnal Kimia Valensi. 2014;4:20–24. [Google Scholar]
- 119.Wali A., Sharma S., Walia M., Kumar P., Thakur S., Kumari A., Lal B., Agnihotri V.K. Two edible ferns of western Himalaya: a comparative in vitro nutritional assessment, antioxidant capacity and quantification of lutein by UPLC-DAD. Int. J. Food Nutr. Sci. 2016;5(9):9‒18. [Google Scholar]
- 120.Watanabe M., Miyashita T., Devkota H.P. Phenolic compounds and ecdysteroids of Diplazium esculentum (Retz.) Sw. (Athyriaceae) from Japan and their chemotaxonomic significance. Biochem. Systemat. Ecol. 2021;94 [Google Scholar]
- 121.Sareen B., Bhattacharya A., Srivatsan V. Nutritional characterization and chemical composition of Diplazium maximum (D. Don) C. Chr. J. Food Sci. Technol. 2021;58:844–854. doi: 10.1007/s13197-020-04598-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Malla B., Chhetri R.B. Indigenous knowledge on ethnobotanical plants of Kavrepalanchowk district. Kathmandu Univ. J. Sci. Eng. Technol. 2009;5(2):96–109. [Google Scholar]
- 123.Parajuli S., Pun N.T., Parajuli S., Jamarkattel-Pandit N. Antioxidant activity, total phenol and flavonoid contents in some selected medicinal plants of Nepal. J. Health Allied Sci. 2012;2:27–31. [Google Scholar]
- 124.Goswami H.K., Sen K., Mukhopadhyay R. Pteridophytes: evolutionary boon as medicinal plants. Plant Genetic Resour. 2016;14(4):328–355. [Google Scholar]
- 125.Adenan, Suhartono E. Stenochlaena palustris aqueous extract reduces hepatic peroxidative stress in Marmota caligata with induced fever. Univ. Medicina. 2010;29(3):123–128. [Google Scholar]
- 126.Chear N.J.Y., Khaw K.Y., Murugaiyah V., Lai C.S. Cholinesterase inhibitory activity and chemical constituents of Stenochlaena palustris fronds at two different stages of maturity. J. Food Drug Anal. 2016;24:358–366. doi: 10.1016/j.jfda.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sumathy V., Lachumy S.J., Zuraini Z., Sasidharan S. Effects of Stenochlaena palustris leaf extract on growth and morphogenesis of food borne pathogen Aspergillus niger. Malays J. Nutr. 2010;16:439–446. [PubMed] [Google Scholar]
- 128.Liu H., Orjala J., Sticher O., Rali T. Acylated flavonol glycosides from leaves of Stenochlaena palustris. J. Nat. Prod. 1999;62:70–75. doi: 10.1021/np980179f. [DOI] [PubMed] [Google Scholar]
- 129.Chai T.T., Panirchellvum E., Ong H.C., Wong F.C. Phenolic contents and antioxidant properties of Stenochlaena palustris, an edible medicinal fern. Bot. Stud. 2012;53:439–446. [Google Scholar]
- 130.Ponnusamy Y., Chear N.J.Y., Ramanathan S., Murugaiyah V., Lai C.S. Antioxidant and antibacterial properties of Malaysian ferns used traditionally against infection. J. Nat. Prod. Plant Resour. 2013;3(6):14–18. https://www.scholarsresearchlibrary.com/articles/antioxidant-and-antibacterial-properties-of-malaysian-ferns-used-traditionallyagainst-infection.pdf [Google Scholar]
- 131.Chai T.T., Kwek M.T., Ong H.C., Wong F.C. Water fraction of edible medicinal fern Stenochlaena palustris is a potent α-glucosidase inhibitor with concurrent antioxidant activity. Food Chem. 2015;186:26–31. doi: 10.1016/j.foodchem.2014.12.099. [DOI] [PubMed] [Google Scholar]
- 132.Azizah L.N., Wardhani P., Arwati H. Antimalarial activity of ethanol extract of kelakai leaves (Stenochlaena palustris) to parasitemia and splenomegaly in BALB/c mice infected with Plasmodium berghei ANKA. J. Ilmiah Mahasiswa Kedokteran Univ. Airlangga. 2022;13(1) [Google Scholar]
- 133.Liu H., Orjala J., Rali T., Sticher O. Glycosides from Stenochlaena palustris. Phytochemistry (Oxf.) 1998;49:2403–2408. [Google Scholar]
- 134.Gaur R.D., Bhatt B.P. Folk utilization of some pteridophytes of deoprayag area in garhwal Himalaya: India, econ. Bot. 1994;48:146–151. [Google Scholar]
- 135.Nwosu M.O. Ethnobotanical studies on some pteridophytes of southern Nigeria. Econ. Bot. 2002;56:255–259. [Google Scholar]
- 136.Verma S.K., Kanwar S. Medicinal pteridophytes used in the treatment of various diseases by the inhabitants of Sarkaghat Tehsil, Mandi district, Himachal Pradesh. J. Pharmaceut. Sci. Res. 2020;12(3):360–364. https://www.jpsr.pharmainfo.in/Documents/Volumes/vol12issue03/jpsr12032004.pdf [Google Scholar]
- 137.Ding Z.T., Fang Y.S., Tai Z.G., Yang M.H., Xu Y.Q., Li F., Cao Q.E. Phenolic content and radical scavenging capacity of 31 species of ferns. Fitoterapia. 2008;79:581–583. doi: 10.1016/j.fitote.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 138.Takuli P., Khulbe K., Kumar P., Parki A., Syed A., Elgorban A.M. Phytochemical profiling, antioxidant and antibacterial efficacy of a native Himalayan fern: Woodwardia unigemmata (Makino) Nakai. Saudi J. Biol. Sci. 2020;27:1961–1967. doi: 10.1016/j.sjbs.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chang R.S., Yeung H.W. Inhibition of growth of human immunodeficiency virus in vitro by crude extracts of Chinese medicinal herbs. Antivir. Res. 1988;8:163–176. doi: 10.1016/0166-3542(88)90001-0. [DOI] [PubMed] [Google Scholar]
- 140.Collins R.A., Ng T.B., Fong W.P., Wan C.C., Yeung H.W. A comparison of human immunodeficiency virus type 1 inhibition by partially purified aqueous extracts of Chinese medicinal herbs. Life Sci. 1997;60:345–351. doi: 10.1016/s0024-3205(97)00227-0. [DOI] [PubMed] [Google Scholar]
- 141.Singh A.V. Edible and medicinal ferns of Lakhimpur, Assam. Int. J. Eng. Sci. Math. 2017;6(3):1–16. https://www.ijesm.co.in/abstract.php?article_id=3101&title=EDIBLE%20AND%20MEDICINAL%20FERNS%20OF%20LAKHIMPUR,%20ASSAM [Google Scholar]
- 142.Adhikari M., Thapa R., Kunwar R.M., Devkota H.P., Poudel P. Ethnomedicinal uses of plant resources in the machhapuchchhre rural municipality of kaski district, Nepal. Medicines. 2019;6(2):69. doi: 10.3390/medicines6020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Barukial J., Sarmah J.N. Ethnomedicinal plants used by the people of Golaghat district, Assam, India. Int. J. Med. Aromatic Plants. 2011;1(3):203‒211. [Google Scholar]
- 144.Yan N., Zhang H., Zhang Z., Zhang H., Zhou L., Chen T., Feng S., Ding C., Yuan M. The extraction, antioxidant and against β-amyloid induced toxicity of polyphenols from Alsophila spinulosa leaves. Arab. J. Chem. 2022;15 [Google Scholar]
- 145.Wada H., Satake T., Murakami T., Kojima T., Saiki Y., Chen C.M. Chemische und chemotoxonomische untersuchungen der pterophyten. LIX. Chemische untersuchungen der inhaltsstoffe von Alsophila spinulosa Tryon. Chem. Pharm. Bull. 1985;33:4182–4187. [Google Scholar]
- 146.Arai Y., Koide N., Ohki F., Ageta H., Yang L.L., Yen K.Y. Fern constituents: triterpenoids isolated from leaflets of Cyathea spinulosa. Chem. Pharm. Bull. 1994;42:228–231. [Google Scholar]
- 147.Jiang J.S., Zhan Z.L., Feng Z.M., Yang Y.N., Zhang P.C. Study on the chemical constituents from Cyathea spinulosa. J. Chin. Med. Mater. 2012;35(4):568–570. [PubMed] [Google Scholar]
- 148.Cheng Y., Chen F.Z., He X.J. Isolation of three chemical constituents from Alsophila spinulosa stalks for the first time. Med. Plants. 2011;2(11):5–7. [Google Scholar]
- 149.M Lu R., Cao M., Liao P.Y., Wei J.H. Chemical constituents in stems of Zhuang medicine Alsophila spinulosa. Chin. Tradit. Herb. Drugs. 2013;44(16):2195. –2199. [Google Scholar]
- 150.Vetter J. A biological hazard of our age: bracken fern [Pteridium aquilinum (L.) Kuhn] – a review. Acta Vet. Hung. 2009;57(1):183–196. doi: 10.1556/AVet.57.2009.1.18. [DOI] [PubMed] [Google Scholar]
- 151.CSIR, The Wealth of India . VIII. Ph-Re, Publications and Information Directorate, Council of Scientific and Industrial Research; New Delhi: 1969. A dictionary of Indian raw materials and industrial products. Raw Materials; pp. 299–300. [Google Scholar]
- 152.Evans I.A., Mason J. Carcinogenic activity of bracken. Nature. 1965;208:913–914. doi: 10.1038/208913a0. [DOI] [PubMed] [Google Scholar]
- 153.Pamukcu A.M., Ertük E., Price J.M., Bryan G.T. Lymphatic leukemia and pulmoinary tumors in female Swiss mice fed bracken fern (Pteris aquilina) Cancer Res. 1972;32:1442–1445. https://aacrjournals.org/cancerres/article/32/7/1442/478915 [PubMed] [Google Scholar]
- 154.Yoshihira K., Fukuoka M., Kuroyanagi M., Natori S., Umeda M., Morohoshi T., Enomoto M., Saito M. Chemical and toxicological studies on bracken fern, Pteridium aquilinum var. latiusculum. I. Introduction, extraction and fractionation of constituents, and toxicological studies including carcinogenicity tests. Chem. Pharm. Bull. 1978;26:2346–2364. doi: 10.1248/cpb.26.2346. [DOI] [PubMed] [Google Scholar]
- 155.Alonso-Amelot M.E., Oliveros A. A method for the practical quantification and kinetic evaluation of cyanogenesis in plant material. Application to Pteridium aquilinum and Passiflora capsularis. Phytochem. Anal. 2000;11:309–316. [Google Scholar]
- 156.Hikino H., Takahashi T., Arihara S., Takemoto T. Structure of pteroside B, glycoside of Pteridium aquilinum var. latiusculum, Chem. Pharm. Bull. 1970;18:1488–1489. [Google Scholar]
- 157.Hikino H., Takahashi T., Takemoto T. Structure of pterosides Z and D, glycosides of Pteridium aquilinum var. latiusculum, Chem. Pharm. Bull. 1971;19:2424–2425. [Google Scholar]
- 158.Hikino H., Takahashi T., Takemoto T. Structure of pteroside A and C, glycosides of Pteridium aquilinum var latiusculum. Chem. Pharm. Bull. 1972;20:210–212. [Google Scholar]
- 159.Fukuoka M., Kuroyanagi M., Yoshihira K., Natori S. Chemical and toxicological studies on bracken fern, Pteridium aquilinum var. latiusculum. II. Structures of pterosins, sesquiterpens having 1-indanone skeleton. Chem. Pharm. Bull. 1978;26(8):2365–2385. doi: 10.1248/cpb.26.2365. [DOI] [PubMed] [Google Scholar]
- 160.Meyer P. Thiaminase activities and thiamine content of Pteridium aquilinum, Equisetum ramossisimum, Malva parviflora, Pennisetum clandestinum and Medicago sativa. Onderstepoort J. Vet. Res. 1989;56:145–146. [PubMed] [Google Scholar]
- 161.Selvaraj P., de Britto A.J., Sahayaraj K. Phytoecdysone of Pteridium aquilinum (L) Kuhn (Dennstadtiaceae) and its pesticidal property on two major pests. Arch. Phytopathol. Plant Protect. 2005;38:99–105. [Google Scholar]
- 162.Shweta S., Dixit R.D., Sahu T.R. Ethnomedical uses of pteridophytes of Amarkatak, Madhya Pradesh. Int. J. Tradit. Knowl. 2005;4(4):392–395. http://nopr.niscair.res.in/handle/123456789/8542 [Google Scholar]
- 163.Banjamin A., Manickam V.S. Medicinal pteridophytes from the western ghats. Indian J. Tradit. Knowl. 2007;6(4):611–618. http://nopr.niscair.res.in/handle/123456789/1013 [Google Scholar]
- 164.Kunjani J., Joshi A.R. Ethnobotanical studies on some lower plants of the central development region, Nepal. Ethanobot. Leafl. 2008;12(1):832–840. https://opensiuc.lib.siu.edu/ebl/vol2008/iss1/113 [Google Scholar]
- 165.Parihar P., Parihar L., Bohra A. In vitro antibacterial activity of fronds (leaves) of some important pteridophytes. J. Microbiol. Antimicrob. 2010;2(2):19–22. https://academicjournals.org/journal/JMA/article-full-text-pdf/A1C09D59652 [Google Scholar]
- 166.Kathirvel A., Sujatha V. Phytochemical studies, antioxidant activities and identification of active compounds using GC-MS of Dryopteris cochleata leaves. Arab. J. Chem. 2016;9(S2):S1435–S1442. [Google Scholar]
- 167.Kathirvel A., Rai A.K., Maurya G.S., Sujatha V. Dryopteris cochleata rhizome: a nutritional source of essential elements, phytochemicals, antioxidants and antimicrobials. Int. J. Pharm. Pharmaceut. Sci. 2014;6(S2):179–188. [Google Scholar]
- 168.Dubal K., Patil S., Dongare M., Kale M. Investigation of chemical composition from Dryopteris chochleata (D. Don) C. Chr. (Dryopteridaceae) Asian J. Pharmaceut. Clin. Res. 2015;8:311–314. https://innovareacademics.in/journals/index.php/ajpcr/article/view/6821 [Google Scholar]
- 169.Tapwal A., Nisha, Garg S., Gautam N., Kumar R. In vitro antifungal potency of plant extracts against five phytopathogens. Braz. Arch. Biol. Technol. 2011;54(6):1093–1098. [Google Scholar]
- 170.Sivasankar S., Somavanshi R. Pathological evaluation of Polystichum squarrosum (D. Don) fern in laboratory rats. Indian J. Exp. Biol. 2001;39:772–776. http://hdl.handle.net/123456789/23855 [PubMed] [Google Scholar]
- 171.CSIR, The Wealth of India . VI. L-M, Council of Scientific and Industrial Research; New Delhi: 1962. A dictionary of Indian raw materials and industrial products. Raw Materials; pp. 199–200. [Google Scholar]
- 172.Yadav E., Mani M., Chandra P., Sachan N., Ghosh A.K. A review on therapeutic potential of Lygodium flexuosum Linn. Phcog. Rev. 2012;6:107–114. doi: 10.4103/0973-7847.99944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bhattarai K.R., Vetaas O.L., Grytness J.A. Fern species richness along a central Himalayan elevation gradient, Nepal. J. Biogeogr. 2004;31:389–400. [Google Scholar]
- 174.Gaitonde B.B., Mahajan R.T. Antifertility activity of Lygodium flexuosum. Indian J. Med. Res. 1980;12:597–604. [PubMed] [Google Scholar]
- 175.Nangare V.G., Jadhav R.L. Evaluation of contraceptive efficacy of ethanolic extract of Lygodium flexuosum in male Albino rat: a histological approach. J. Glob. Biosci. 2021;10:8968–8979. https://www.mutagens.co.in/jgb/vol.10/09/100901.pdf [Google Scholar]
- 176.Wills P.J., Asha V.V. Protective effect of Lygodium flexuosum (L.) Sw. extract against carbon tetrachloride-induced acute liver injury in rats. J. Ethnopharmacol. 2006;108:320–326. doi: 10.1016/j.jep.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 177.Wills P.J., Asha V.V. Chemopreventive action of Lygodium flexuosum extract in human hepatoma PLC/PRF/5 and Hep 3B cells. J. Ethnopharmacol. 2009;122:294–303. doi: 10.1016/j.jep.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 178.Nayak N., Rath S., Mishra M.P., Ghosh G., Padhy R.N. Antibacterial activity of the terrestrial fern Lygodium flexuosum (L.) Sw. against multidrug resistant enteric- and uro-pathogenic bacteria. J. Acute Dis. 2013;2(4):270–276. [Google Scholar]
- 179.Jeeshna M.V. Antimicrobial activities and phytochemical investigation of fern, Lygodium flexuosum (Linn) Sw. Kongunadu Res. J. 2017;4:87–91. [Google Scholar]
- 180.Achari B., Basu K., Saha C.R., Prakashi S.C. A new triterpene ester, an anthraquinone and other constituents of the fern Lygodium flexuosum. Planta Med. 1986;52:329–330. doi: 10.1055/s-2007-969169. [DOI] [PubMed] [Google Scholar]
- 181.Basudev A., Chaudhuri C., Saha C.R., Prakashi S.C., McPhail D.R., McPhail A.T. X-ray crystal structure of lygodinolide: a novel spiro furopyran-perhydrophenanthrene derivatives from Lygodium flexuosum. J. Org. Chem. 1990;55:4977–4978. [Google Scholar]
- 182.Rao S. Pharmacognostical studies on Lygodium flexuosum. J. Pharm. Res. 2010;3:1976–1978. [Google Scholar]
- 183.Yamauchi T., Oyama N., Yamane H., Murofushi N., Schraudolf H., Pour M., Furber M., Mander L.N. Identification of antheridiogens in Lygodium circinnatum and Lygodium flexuosum. Plant Physiol. 1996;111:741–745. doi: 10.1104/pp.111.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang K.R., Nan C., Ding R., Lin J., Hu D.J., Zhong S.H., Gu R. A cross-national comparaison of medicinal plants used by the Miao, Yi and Lisu ethnic groups in Yanbian, China. Res. Square. 2020 [Google Scholar]
- 185.Malla B., Gauchan D.P., Chhetri R.B. An ethnobotanical study of medicinal plants used by ethnic people in Parbat district of western Nepal. J. Ethnopharmacol. 2015;165:103–107. doi: 10.1016/j.jep.2014.12.057. [DOI] [PubMed] [Google Scholar]
- 186.Won J.B., Ma C.J. Neuroprotective activities of some medicinal plants against glutamate-induced neurotoxicity in primary cultures of rat cortical cells. Nat. Prod. Sci. 2009;15:125–129. https://www.koreascience.or.kr/article/JAKO200929349389228.pdf [Google Scholar]
- 187.Ni S.F., Pan Y.J., Wu P., Chen Y.C., Fu C.X., Xu H. Analysis of volatile oil from Lygodium japonicum by GC-MS. Chin. Pharmaceut. J. 2004;39(2):99–100. http://hdl.handle.net/10397/62568 [Google Scholar]
- 188.Zhang L.H., Yin Z.Q., Ye W.C., Zhao S.X., Wang L., Hu F. Studies on the chemical constituents in herb of Lygodium japonicum, China. J. Chin. Mater. Med. 2005;30(19):1522–1524. [PubMed] [Google Scholar]
- 189.Zhu L., Zhang G., Wang S., Li L., Li P., Wu S., Wang X., Chen L., Pan C., Mi W. A new compound from Lygodium japonicum (Thunb.) Sw. Nat. Prod. Res. 2009;23:1284–1288. doi: 10.1080/14786410802420820. [DOI] [PubMed] [Google Scholar]
- 190.Duan Y.H., Dai Y., He R.R., Kurihara H., Li Y.L., Yao X.S. A new phenylpropanoid glucoside from the aerial parts of Lygodium japonicum. J. Asian Nat. Prod. Res. 2012;14:286–292. doi: 10.1080/10286020.2011.650690. [DOI] [PubMed] [Google Scholar]
- 191.Yamane H., Takahashi N., Takeno K., Furuya M. Identification of gibberellin A9 methyl ester as a natural substance regulatingformation of reproductive organs in Lygodium japonicum. Planta. 1979;147:251–256. doi: 10.1007/BF00388747. [DOI] [PubMed] [Google Scholar]
- 192.Yamane H., Satoh Y., Nohara K., Nakayama M., Murofushi N., Takahashi N., Takeno K., Furuya M., Furber M., Mander L.N. The methyl ester of a new gibberellin, GA73: the principal antheridiogen in Lygodium japonicum. Tetrahedron Lett. 1988;29:3959–3962. [Google Scholar]
- 193.Konno H., Nakato T., Nakashima S., Katoh K. Lygodium japonicum fern accumulates copper in the cell wall pectin. J. Exp. Bot. 2005;56:1923–1931. doi: 10.1093/jxb/eri187. [DOI] [PubMed] [Google Scholar]
- 194.Nahar L., Ripa F.A., Zahan R., Bulbul I.J., Parvej I. Evaluation of analgesic and antidiarrhoeal effects of methanolic extract of Marsilea quadrifolia in rat. J. Pharm. Res. 2011;4(12):4374–4376. [Google Scholar]
- 195.Soni P., Singh L. Marsilea quadrifolia Linn. - a valuable culinary and remedial fern in Jaduguda, Jharkhand, India. Int. J. Life Sci. Pharm. Res. 2012;2(3):L99–L104. https://www.ijlpr.com/admin/php/uploads/106_pdf.pdf [Google Scholar]
- 196.Gopalakrishnan K., Udayakumar R. Antimicrobial activity of Marsilea quadrifolia (L.) against some selected pathogenic microorganisms. Microbiol. Res. J. Int. 2014;4(9):1046–1056. https://www.journalmrji.com/index.php/MRJI/article/view/6788 [Google Scholar]
- 197.Reddy K.S., Reddy C.S., Ganapaty S. Psychopharmacological studies of hydro alcoholic extract of whole plant of Marsilea quadrifolia. J. Sci. Res. 2012;4:279–285. [Google Scholar]
- 198.Ripa F.A., Nahar L., Haque M., Islam Md M. Antibacterial, cytotoxic and antioxidant activity of crude extract of Marsilea quadrifolia. Eur. J. Sci. Res. 2009;33(1):123–129. [Google Scholar]
- 199.Sethi P. Antimicrobial activities of Turbinaria conoides (J. Agardh) kutzing and Marsilea quadrifolia linn. Asian J. Plant Sci. Res. 2014;4(6):36–40. https://www.imedpub.com/articles/antimicrobial-activities-of-turbinaria-conoides-j-agardh-kutzing-andmarsilea-quadrifolia-linn.pdf [Google Scholar]
- 200.Nivas R.K., Boominathan M. Antimicrobial evaluation of selected south Indian medicinal plants against Streptococcus pneumoniae. Int. J. Curr. Microbiol. Appl. Sci. 2015;4:835–840. https://www.ijcmas.com/vol-4-3/R.%20Kadhar%20Nivas%20and%20M.%20Boominathan.pdf [Google Scholar]
- 201.Gini T.G., Jothi G.J. In vitro screening of antibacterial and antifungal activity of Marsilea quadrifolia (Marsileaceae) Linn. extract. Am. J. Phytomed. Clin. Ther. 2015;3(4):313–329. [Google Scholar]
- 202.Vijayalakshmi M., Kiruthika R., Bharathi K., Ruckmani K. Phytochemical screening by LC-MS analysis and invitro anti-inflammatory activity of Marselia quadrifolia plant extract. Int. J. Pharm. Tech. Res. 2015;8:148–157. https://sphinxsai.com/2015/ph_vol8_no9/1/(148-157)V8N9PT.pdf [Google Scholar]
- 203.Mathangi T., Prabhakaran P. DPPH free radical scavenging activity of the extracts of the aquatic fern Marsilea quadrifolia Linn. Int. J. Pharm. Pharmaceut. Sci. 2013;2:534–536. https://www.ijcmas.com/vol-2-10/T.%20Mathangi%20and%20P.Prabhakaran.pdf [Google Scholar]
- 204.Chowdhury A., Panneerslevam T., Suthendran K., Bhattachejee C., Balasubramanian S., Murugesan S., Suraj B., Selvaraj K. Optimization of microwave-assisted extraction of bioactive polyphenolic compounds from Marsilea quadrifolia L. using RSM and ANFIS modelling. Indian J. Nat. Prod. Resour. 2018;9(3):204–221. http://nopr.niscair.res.in/bitstream/123456789/45556/1/IJNPR%209%283%29%20204-221.pdf [Google Scholar]
- 205.Dongare S.S., Maske A.P., Patil S.M., Umbare R.P., Mate G.S. Antidiabetic activity of Marsilea quadrifolia Linn in alloxan-diabetic rats. Res. J. Pharmacogn. Phytochem. 2009;1:15–17. https://rjpponline.org/AbstractView.aspx?PID=2010-2-6-14 [Google Scholar]
- 206.Ashwini G., Pranay P., Thrinath G., Karnaker Reddy T., Giri Prasad V.S. Pharmacological evalution of Marsilea qudrifolia plant extracts against Alzheimer’s disease. Int. J. Drug Dev. Res. 2012;4(2):153–158. https://www.ijddr.in/drug-development/pharmacological-evalution-of-marsilea-qudrifolia-plant-extractsagainst-alzheimers-disease.pdf [Google Scholar]
- 207.Bhadra S., Mukherjee P.K., Bandyopadhyay A. Cholinesterase inhibition activity of Marsilea quadrifolia Linn. an edible leafy vegetable form West Bengal, India. Nat. Prod. Res. 2012;26(16):1519–1522. doi: 10.1080/14786419.2011.565006. [DOI] [PubMed] [Google Scholar]
- 208.Snehunsu A., Mukunda N., Kumar M.C.S., Sadhana N., Narayanan S.N., Kapgal K.V., Avinash H., Chandrashekar B.R., Rao K.R., Nayak B.S. Evaluation of anti-epileptic property of Marsilea quadrifolia Linn. in maximal electroshock and pentylenetetrazole-induced rat models of epilepsy. Brain Inj. 2013;27:1707–1714. doi: 10.3109/02699052.2013.831121. [DOI] [PubMed] [Google Scholar]
- 209.Sahu S., Dutta G., Mandal N., Goswami A.R., Ghosh T. Anticonvulsant effect of Marsilea quadrifolia Linn. on pentylenetetrazole induced seizure: a behavioral and EEG study in rats. J. Enthnopharmacol. 2012;141:537–541. doi: 10.1016/j.jep.2012.02.039. [DOI] [PubMed] [Google Scholar]
- 210.Vallabi D.E., Elango V. Antioxidant activity of Cyathula prostrate, Ipomea obscura and Marsilea quadrifolia in nephrotoxicity induced male Albino rats. World J. Pharmaceut. Res. 2016;5(8):1000–1006. [Google Scholar]
- 211.Uma R., Pravin B. Invitro cytotoxic activity of Marsilea quadrifolia Linn of MCF-7 cells of human breast cancer. Int. Res. J. Med. Sci. 2013;1:10–13. http://www.isca.in/MEDI_SCI/Archive/v1/i1/2.ISCA-IRJMedS-2013-006.pdf [Google Scholar]
- 212.Agarwal S.K., Roy S., Pramanick P., Mitra P., Gobato R., Mitra A. Marsilea quadrifolia: a floral species with unique medicinal properties. Parana J. Sci. Edu. 2018;4(5):15–20. [Google Scholar]
- 213.Dewanji A., Matai S., Si L., Barik S., Nag A. Chemical composition of two semi-aquatic plants for food use. Plant Foods Hum. Nutr. 1993;44:11–16. doi: 10.1007/BF01088478. [DOI] [PubMed] [Google Scholar]
- 214.Gopalakrishnan K., Udayakumar R. Phytochemical content of leaf and stem of Marsilea quadrifolia (L.) J. Plant Sci. Phytopathol. 2017;1:26–37. [Google Scholar]
- 215.Zhang Y., Tian H.Y., Tan Y.F., Wong Y.L., Wu H.Y., Jia J.F., Wang G.E., Gao J.J., Li Y.F., Kurihara H., Shaw P.C., Jiang R.W. Isolation and identification of polyphenols from Marsilea quadrifolia with antioxidant properties in vitro and in vivo. Nat. Prod. Res. 2016;30:1404–1410. doi: 10.1080/14786419.2015.1062377. [DOI] [PubMed] [Google Scholar]
- 216.Singh A.G., Kumar A., Tewari D.D., Bharati K.A. New ethnomedicinal claims from Magar community of Palpa district, Nepal. Indian J. Tradit. Knowl. 2018;17(3):499–511. http://nopr.niscair.res.in/handle/123456789/44583. [Google Scholar]
- 217.Pradhan S.P., Chaudhary R.P., Sigdel S., Pandey B.P. Ethnobotanical knowledge of Khandadevi and Gokulganga rural municipality of Ramechhap district of Nepal. Ethnobot. Res. Appl. 2020;20:1–32. https://ethnobotanyjournal.org/index.php/era/article/view/2027 [Google Scholar]
- 218.Silwal G.R. Medicinal plants and their traditional uses in Ramkot village, Kathmandu Nepal. Patan Pragya. 2020;6(1):180–190. https://www.nepjol.info/index.php/pragya/article/view/34431 [Google Scholar]
- 219.Dhiman A.K. Ethnomedicinal uses of some pteridophytic species in India. Indian Fern J. 1998;15(1-2):61–64. [Google Scholar]
- 220.Singh H.B. Potential medicinal pteridophytes of India and their chemical constituents. J. Econ. Taxon. Bot. 1999;23(1):63–78. [Google Scholar]
- 221.CSIR, The Wealth of India . VII. N-Pe, Publications and Information Directorate, Council of Scientific and Industrial Research; New Delhi: 1966. A dictionary of Indian raw materials and industrial products. Raw Materials; pp. 14–15. [Google Scholar]
- 222.Rani D., Khare P.B., Dantu P.K. In vitro antibactarial and antifungal properties of aqueous and non-aqueous frond extracts of Psilotum nudum, Nephrolepsis biserrata and Nephrolepsis cordifolia. Indian J. Pharmaceut. Sci. 2010;72(6):818–822. doi: 10.4103/0250-474X.84606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Baral R., Subedi L., Gurung M., Ojha S., Shrestha B., Chaudhary D., Jammarkattel N. Phytochemical screening, free radical scavenging activity, in-vitro alpha-amylase enzyme and glucose diffusion inhibition activity of ethyl acetate and water extracts of selected medicinal plants of Nepal. Int. J. Herb. Med. 2021;9(3):18–27. https://www.florajournal.com/archives/2021/vol9issue3/PartA/8-5-64-832.pdf [Google Scholar]
- 224.Pal R. In-vitro evaluation of anthelmintic activity of Nephrolepis cordifolia leaves. J. Pharm. Exp. Med. 2021;1:176. [Google Scholar]
- 225.Rajasekaran A., Sivakumar V. Evaluation of diuretic potential of Nephrolepis cordifolia rhizome juice in Wistar rats. Sains Malayysiana. 2009;38(1):57–59. [Google Scholar]
- 226.Gauchan D.P., Manandhar D., Shrestha N., Suwal S.K. Nutrient analysis of Nephrolepis cordifolia (L.) C. Presl, Kathmandu univ . J. Sci. Eng. Technol. 2008;4(1):68–72. [Google Scholar]
- 227.Adebiyi A.O. Phytochemical constituents and proximate composition of Nephrolepis cordifolia (L) C. Presl grown in Nigeria. New York Sci. J. 2016;9(2):79–82. http://www.sciencepub.net/newyork/ny090216/013_30082nys090216_79_82.pdf [Google Scholar]
- 228.El-Tantawya M.E., Shams M.M., Afifi S. Chemical composition and biological evaluation of the volatile constituents from the aerial parts of Nephrolepis exaltata (L.) and Nephrolepis cordifolia (L.) C. Presl grown in Egypt. Nat. Prod. Res. 2016;30:1197–1201. doi: 10.1080/14786419.2015.1046070. [DOI] [PubMed] [Google Scholar]
- 229.Liang Z., Yang X., Zhu H., Hao X. Chemical constituents of Nephrolepis cordifolia. Guang Xi Zhi Wu. 2008;28:420–421. [Google Scholar]
- 230.Singh B.P., Upadhyay R. Medicinal pteridophytes of Madhya Pradesh. J. Pharmacogn. Phytochem. 2014;3:173–176. https://www.phytojournal.com/vol3Issue3/Issue_sep_2014/19.1.pdf [Google Scholar]
- 231.Wang D., Liu X.Q., Yao C.S., Fang W.S. Studies on chemical constituents from herbs of Botrychium lanuginosum, China. J. Chin. Mater. Med. 2008;33(22):2627–2629. [PubMed] [Google Scholar]
- 232.Upreti K., Jalal J.S., Tewari L.M., Joshi G.C., Pangtey Y.P.S., Tewari G. Ethnomedicinal uses of pteridophytes of kumaun Himalaya, Uttarakhand, India. J. Am. Sci. 2009;5:167–170. http://www.jofamericanscience.org/journals/am-sci/0504/24_0797_pteridophyte_am0504.pdf [Google Scholar]
- 233.CSIR, The Wealth of India . V. H-K, Council of Scientific and Industrial Research; New Delhi: 1959. A dictionary of Indian raw materials and industrial products. Raw Materials; p. 31. [Google Scholar]
- 234.Chen C.C., Huang Y.L., Yeh P.Y., Ou J.C. Cyclized geranyl stilbenes from the rhizomes of Helminthostachys zeylanica. Planta Med. 2003;69:964–967. doi: 10.1055/s-2003-45112. [DOI] [PubMed] [Google Scholar]
- 235.Huang Y.L., Yeh P.Y., Shen C.C., Chen C.C. Antioxidant flavonoids from the rhizomes of Helminthostachys zeylanica. Phytochemistry (Oxf.) 2003;64:1277–1283. doi: 10.1016/j.phytochem.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 236.Chang T.C., Chiang H., Lai Y.H., Huang Y.L., Huang H.C., Liang Y.C., Liu H.K., Huang C. Helminthostachys zeylanica alleviates hepatic steatosis and insulin resistance in diet-induced obese mice. BMC Compl. Alternative Med. 2019;19:368. doi: 10.1186/s12906-019-2782-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Suja S.R., Latha P.G., Pushpangadan P., Rajasekharan S. Evaluation of hepatoprotective effects of Helminthostachys zeylanica (L.) Hook against carbon tetrachloride-induced liver damage in Wistar rats. J. Ethnopharmacol. 2004;92:61–66. doi: 10.1016/j.jep.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 238.Hsieh H.L., Yang S.H., Lee T.H., Fang J.Y., Lin C.F. Evaluation of anti-inflammatory effects of Helminthostachys zeylanica extracts via inhibiting bradykinin-induced MMP-9 expression in brain astrocytes. Mol. Neurobiol. 2016;53:5995–6005. doi: 10.1007/s12035-015-9511-9. [DOI] [PubMed] [Google Scholar]
- 239.Liou C.J., Huang Y.L., Huang W.C., Yeh K.W., Huang T.Y., Lin C.F. Water extract of Helminthostachys zeylanica attenuates LPS-induced acute lung injury in mice by modulating NF-κB and MAPK pathways. J. Ethnopharmacol. 2017;199:30–38. doi: 10.1016/j.jep.2017.01.043. [DOI] [PubMed] [Google Scholar]
- 240.Huang W.C., Ting N.C., Huang Y.L., Chen L.C., Lin C.F., Liou C.J. Helminthostachys zeylanica water extract ameliorates airway hyperresponsiveness and eosinophil infiltration by reducing oxidative stress and Th2 cytokine production in a mouse asthma model. Mediat. Inflamm. 2020 doi: 10.1155/2020/1702935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Fitrya, Muharni An antihyperuricemia effect of ethanol extract of Tunjuk Langit rhizome (Helmynthostachys Zeylanica Linn Hook) on Swiss male mice. Tradit. Med. J. 2014;19(1):14–18. [Google Scholar]
- 242.Anwar F.D.L. Uji aktivitas antikanker secara in vitro dengan sel murine P-388 senyawa flavonoid Dari fraksi etilasetat akar tumbuhan tunjuk langit (Helminthostchys zeylanica (Linn) Hook) J. Penelitian Sains. 2009;12(1) [Google Scholar]
- 243.Tsai M.M., Lin H.C., Yu M.C., Lin W.J., Chu M.Y., Tsai C.C., Cheng C.Y. Anticancer effects of Helminthostachys zeylanica ethyl acetate extracts on human gastric cancer cells through downregulation of the TNF-α-activated COX-2-cPLA2-PGE2 Pathway. J. Cancer. 2021;12:7052–7068. doi: 10.7150/jca.64638. https://www.jcancer.org/v12p7052.htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ridhasya F.E., Teruna H.Y., Hendra R., Almurdani Md. Natural antidiabetic of Tunjuk Langit (Helminthostachys zeylanica) rhizome extracts. Pharmacol. Clin. Pharm. Res. 2019;4(3):67–70. [Google Scholar]
- 245.Yenn T.W., Ring L.C., Zahan K.A., Rahman Md S.A., Tan W.N., Alaudin B.J.S. Chemical composition and antimicrobial efficacy of Helminthostachys zeylanica against foodborne Bacillus cereus. Nat. Prod. Sci. 2018;24(1):66–70. [Google Scholar]
- 246.Huang Y.C., Hwang T.L., Chang C.S., Yang Y.L., Shen C.N., Liao W.Y., Chen S.C., Liaw C.C. Anti-inflammatory flavonoids from the rhizomes of Helminthostachys zeylanica. J. Nat. Prod. 2009;72:1273–1278. doi: 10.1021/np900148a. [DOI] [PubMed] [Google Scholar]
- 247.Huang Y.C., Hwang T.L., Yang Y.L., Wu S.H., Hsu M.H., Wang J.P., Chen S.C., Huang L.J., Liaw C.C. Acetogenin and prenylated flavonoids from Helminthostachys zeylanica with inhibitory activity on superoxide generation and elastase release by neutrophils. Planta Med. 2010;76:447–453. doi: 10.1055/s-0029-1186221. [DOI] [PubMed] [Google Scholar]
- 248.Huang Y.L., Shen C.C., Shen Y.C., Chiou W.F., Chen C.C. Anti-inflammatory and antiosteoporosis flavonoids from the rhizomes of Helminthostachys zeylanica. J. Nat. Prod. 2017;80:246–253. doi: 10.1021/acs.jnatprod.5b01164. [DOI] [PubMed] [Google Scholar]
- 249.Wu K.C., Kao C.P., Ho Y.L., Chang Y.S. Quality control of the root and rhizome of Helminthostachys zeylanica (Daodi-Ugon) by HPLC using quercetin and ugonins as markers. Molecules. 2017;22:1115. doi: 10.3390/molecules22071115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Yamauchi K., Mitsunaga T., Batubara I. Novel quercetin glucosides from Helminthostachys zeylanica root and acceleratory activity of melanin biosynthesis. J. Nat. Med. 2013;67:369–374. doi: 10.1007/s11418-012-0672-9. [DOI] [PubMed] [Google Scholar]
- 251.Su L.H., Li Y.P., Li H.M., Dai W.F., Liu D., Cao L., Li R.T. Anti-inflammatory prenylated flavonoids from Helminthostachys zeylanica. Chem. Pharm. Bull. 2016;64:497–501. doi: 10.1248/cpb.c15-00661. [DOI] [PubMed] [Google Scholar]
- 252.Patil S., Dongare M. The genus Ophioglossum from western ghats of India. Indian Fern J. 2014;31:17–24. [Google Scholar]
- 253.Parihar P., Parihar L. Some pteridophytes of medicinal importance from Rajasthan. Nat. Product. Radiance. 2006;5(4):297–301. [Google Scholar]
- 254.Singh B.P., Upadhyay R. Pteridophytes diversity of Satpura hills. Int. J. Curr. Microbiol. Appl. Sci. 2014;3:162–168. https://www.ijcmas.com/vol-3-9/Balendra%20Pratap%20Singh%20and%20Ravi%20Upadhyay.pdf [Google Scholar]
- 255.Editorial Committee of the Flora of Taiwan . second ed. Vol. 1. 1994. Flora of Taiwan; p. 71. Taipei, Taiwan. [Google Scholar]
- 256.Lin Y.L., Shen C.C., Huang Y.J., Chang Y.Y. Homoflavonoids from Ophioglossum petiolatum. J. Nat. Prod. 2005;68:381–384. doi: 10.1021/np0401819. [DOI] [PubMed] [Google Scholar]
- 257.van der Burg W.J. In: Plant Resources of Tropical Africa 2: Vegetables. Grubben G.J.H., Denton O.A., editors. 2004. Ophioglossum reticulatum L; pp. 173–175. Prota Foundation, Wageningen, Netherlands. [Google Scholar]
- 258.Benedict R.C. What is the habitat of Ophioglossum vulgatum. Am. Fern J. 1914;4:121–122. [Google Scholar]
- 259.CSIR, The Wealth of India . VII. N-Pe, Publications and Information Directorate, Council of Scientific and Industrial Research, New Delhi; 1966. A dictionary of Indian raw materials and industrial products; pp. 97–98. [Google Scholar]
- 260.Zhao J.Z. Treatment of snake bite with Ophioglossum vulgatum L. of traditional Chinese medicine. Gansu Chin. Med. 2001;14:51–52. [Google Scholar]
- 261.Clericuzio M., Tinello S., Burlando B., Ranzato E., Martinotti S., Cornara L., La Rocca A. Flavonoid oligoglycosides from Ophioglossum vulgatum L. having wound healing properties. Planta Med. 2012;78:1639–1644. doi: 10.1055/s-0032-1315149. [DOI] [PubMed] [Google Scholar]
- 262.Clericuzio M., Burlando B., Gandini G., Tinello S., Ranzato E., Martinotti S., Cornara L. Keratinocyte wound healing activity of galactoglycerolipids from the fern Ophioglossum vulgatum L. J. Nat. Med. 2014;68:31–37. doi: 10.1007/s11418-013-0759-y. [DOI] [PubMed] [Google Scholar]
- 263.Zhang J., Yang L., Fang Y., Li J., Gu C., Zhou Q., Zheng Q., Chen W., Zhou Y., Zhuang M., Liu Q., Li C., Zhang Y. Antidiarrheal activity of ethanol extract of Ophioglossum vulgatum in mice and spasmolytic effect on smooth muscle contraction of isolated jejunum in rabbits. Res. Square. 2020 [Google Scholar]
- 264.Herrmann F., Romero M.R., Blazquez A.G., Kaufmann D., Ashour Md L., Kahl S., Marin J.J.G., Efferth T., Wink M. Diversity of pharmacological properties in Chinese and European medicinal plants: cytotoxicity, antiviral and antitrypanosomal screening of 82 herbal drugs. Diversity. 2011;3:547–580. [Google Scholar]
- 265.Hu W.C., Meng Q.Y., Wan C.X. Fingerprint analysis of Ophioglossum HPLC. Chin. J. Exp. Tradit. Med. Form. 2016;22(6):61–65. [Google Scholar]
- 266.Markham K.R., Mabry T.J., Voirin B. 3-O-methylquercetin 7-O-diglucoside 4′-O-glucoside from the fern. Ophioglossum vulgatum, Phytochem. 1969;8:469–472. [Google Scholar]
- 267.CSIR, The Wealth of India . VII. N-Pe, Publications and Information Directorate, Council of Scientific and Industrial Research; New Delhi: 1966. A dictionary of Indian raw materials and industrial products. Raw Materials; pp. 193–194. [Google Scholar]
- 268.Kim N.R., Chi L.W., Lee C.H. Alpha-glucosidase inhibition activity of methanol extracts obtained from nine pteridophyte species native to Korea. Korean J. Polar Res. 2013;26(4):411–416. [Google Scholar]
- 269.Jain S., Jacob M., Walker L., Tekwani B. Screening North American plant extracts in vitro against Trypanosoma brucei for discovery of new antitrypanosomal drug leads. BMC Compl. Alternative Med. 2016;16:131. doi: 10.1186/s12906-016-1122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Smitha V., Vadivel V. Phytochemical screening for active compounds in Ceratopteris thalictroides (L.) Brogn. J. Pharmacogn. Phytochem. 2019;8:3556–3559. https://www.phytojournal.com/archives/2019/vol8issue3/PartAZ/8-3-382-884.pdf [Google Scholar]
- 271.Bhatt M.D., Adhikari Y.P., Kunwar R.M. Ethnomedicinal values of weeds in Kanchanpur district, far-western Nepal. Ethnobot. Res. Appl. 2021;21:1–19. https://ethnobotanyjournal.org/index.php/era/article/view/2613 [Google Scholar]
- 272.CSIR The wealth of India: a dictionary of Indian raw materials and industrial products. Raw Materials. 1950;II:122. Publications and Information Directorate, Council of Scientific and Industrial Research, New Delhi. [Google Scholar]
- 273.Anto P.V., Greeshma K.V., Neenu A.S. Antibacterial activity of five selected species of pteridophytes. Int. J. Innov. Res. Dev. 2015;4 http://www.internationaljournalcorner.com/index.php/ijird_ojs/article/view/135914 [Google Scholar]
- 274.Antonysamy J.M.A., Ramakrishnan P., Perumal S., Shibila T. Anti inflammatory activity of selected pteridophytes from western ghats. Int. J. Complement. Alt. Med. 2017;9:307. [Google Scholar]
- 275.Amoroso V.B., Mendez R.A., Villalobos A.P. Bringing back the lost values of Phillipine edible ferns: their antioxidant, proteins and utilization. Int. J. Adv. Res. 2017;5:757–770. [Google Scholar]
- 276.Chen C.Y., Chiu F.U., Lin Y., Haung W.J., Hsieh P.S., Hsu F.L. Chemical constituents analysis and antidiabetic activity validation of four fern species from Taiwan. Int. J. Mol. Sci. 2015;16:2497–2516. doi: 10.3390/ijms16022497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Adebiyi A.O., Oyeyemi S.D., Tedela P.O., Ojo V.I. Chemical composition of the leaves of Ceratopteris thalictroides (L.) Brongn. Scholars Acad. J. Biosci. 2019;7:377–386. [Google Scholar]
- 278.Jaishee N., Chakraborty U. Evaluation of in-vitro antioxidant activities of Pteris biaurita L. Int. J. Pharm. Pharmaceut. Sci. 2014;6(S2):413–421. [Google Scholar]
- 279.Rout S.D., Panda T., Mishra N. Ethnomedicinal studies on some pteridophytes of similipal biosphere reserve, Orissa, India. Int. J. Med. Med. Sci. 2009;1:192–197. [Google Scholar]
- 280.Ale R., Raskoti B.B., Shrestha K. Ethnobotanical knowledge of Magar community in Siluwa VDC, Palpa district, Nepal. J. Nat. Hist. Mus. 2009;24:58–71. [Google Scholar]
- 281.Dalli A.K., Saha G., Chakraborty U. Characterization of antimicrobial compounds from a common fern, Pteris biaurita. Indian J. Exp. Biol. 2007;45:285–290. http://hdl.handle.net/123456789/5249 [PubMed] [Google Scholar]
- 282.Gracelin D.H.S., de Britto A.J., Kumar P.B.J.R. Qualitative and quantitative analysis of phytochemicals in five Pteris species. Int. J. Pharm. Pharmaceut. Sci. 2013;5(S1):105–107. [Google Scholar]
- 283.Lans C.A. Ethnomedicines used in Trinidad and Tobago for urinary problems and diabetes mellitus. J. Ethnobiol. Ethnomed. 2006;2:45. doi: 10.1186/1746-4269-2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Thulsi Rao K., Reddy R.N., Pattanaik C., Reddy C.S. Ethnomedicinal importance of pteridophytes used by Chenchus of Nallamalais, Andhra Pradesh, India. Ethnobot. Leafl. 2007;11:6–10. https://opensiuc.lib.siu.edu/ebl/vol2007/iss1/2/ [Google Scholar]
- 285.Singh M., Govindarajan R., Rawat A.K.S., Khare P.B. Antimicrobial flavonoid rutin from Pteris vittata L. against pathogenic gastrointestinal microflora. Am. Fern J. 2008;98:98–103. [Google Scholar]
- 286.Nath K., Bhattacharya M.K., Ka S. Antimicrobial potential of ethnomedicinal ferns of southern Assam, India. Indian J. Pharmaceut. Sci. 2018;80(3):556–560. [Google Scholar]
- 287.Ma L.Q., Komar K.M., Tu C., Zhang W., Cai Y., Kennelly E.D. A fern that hyperaccumulated arsenic. Nature. 2001;409:579–580. doi: 10.1038/35054664. [DOI] [PubMed] [Google Scholar]
- 288.Danh L.T., Truong P., Mammucari R., Foster N. A critical review of the arsenic uptake mechanisms and phytoremediation potential of Pteris vittata. Int. J. Phytoremediation. 2014;16:429–453. doi: 10.1080/15226514.2013.798613. [DOI] [PubMed] [Google Scholar]
- 289.Salatino M.L.F., Prado J. Flavonoid glycosides of pteridaceae from Brazil. Biochem. Systemat. Ecol. 1998;26:761–769. [Google Scholar]
- 290.Imperato F. The new flavone ester apigenin-7-O-p-hydroxybenzoate and three 3-di-C-glycosylflavones from Pteris vittata. Am. Fern J. 2006;96:62–65. [Google Scholar]
- 291.Joshi K., Joshi R., Joshi A.R. Indigenous knowledge and uses of medicinal plants in Macchegaun, Nepal. Indian J. Tradit. Knowl. 2011;10(2):281–286. http://nopr.niscair.res.in/handle/123456789/11504 [Google Scholar]
- 292.Subba B., Srivastav C., Kandel R.C. Scientific validation of medicinal plants used by Yakkha community of Chanuwa VDC, Dhankuta, Nepal. SpringerPlus. 2016;5:155. doi: 10.1186/s40064-016-1821-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Masal V.P., Dongare M.M. Studies on ethnomedicinal uses of pteridophytes of Ratnagiri district (Maharashtra, India) Indian Fern J. 2010;27(12):88–93. [Google Scholar]
- 294.Neel R.S., Jadhao A.S., Bhuktar A.S. Antibacterial activity of rhizomes and leaf of Tectaria gemmifera (Fee.) Alston. Int. J. Bot. Stud. 2017;2(5):89–92. [Google Scholar]
- 295.Marahatta A.B., Poudel B., Basnyat R.C. The phytochemical and nutritional analysis and biological activity of Tectaria coadunate Linn. Int. J. Herb. Med. 2019;7:42–50. https://www.florajournal.com/archives/?year=2019&vol=7&issue=2&part=A&ArticleId=567 [Google Scholar]
- 296.Thomas A.P., Sindhu N., Dileesh S. Comparative phytochemical and antibacterial efficacy of Tectaria coadunata and Tectaria wightii. Int. J. Res. Anal. Rev. 2021;8:152–158. https://ijrar.org/papers/IJRAR1CEP024.pdf [Google Scholar]
- 297.Shrestha S.S., Sut S., Di Marco S.B., Zengin G., Gandin V., De Franco M., Pant D.R., Mahomoodally Md F., Dall’Acqua S., Rajbhandary S. Phytochemical fingerprinting and in vitro bioassays of the ethnomedicinal fern Tectaria coadunata (J. Smith) C. Christensen from central Nepal. Molecules. 2019;24:4457. doi: 10.3390/molecules24244457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Reddy M.N., Adnan Md, Alreshidi M.M., Saeed Md, Patel M. Evaluation of anticancer, antibacterial and antioxidant properties of a medicinally treasured fern Tectaria coadunata with its phytoconstituents analysis by HR-LCMS. Anti Cancer Agents Med. Chem. 2020;20:1845–1856. doi: 10.2174/1871520620666200318101938. [DOI] [PubMed] [Google Scholar]
- 299.Pant D.R., Aryal B., Pun D., Sharma S., Joshi G.P. Inhibition of α-amylase and α-glucosidase activities in vitro by extracts of selected medicinal plants. Biodiversitas. 2021;22:1187–1193. [Google Scholar]
- 300.Pawar S.G., Kamble S.Y., Patil S.R., Sawant P.S., Singh E.A. Preliminary phytochemical investigations of three species of traditional medicinal plants of tribal regions of Maharashtra (India) Int. J. Pharmacogn. Phytochem. Res. 2016;8(5):742–745. http://impactfactor.org/PDF/IJPPR/8/IJPPR,Vol8,Issue5,Article6.pdf [Google Scholar]
- 301.Rai J., Sharma K.R., Pokharel Y.R. Antioxidant and alpha amylase inhibitory activity of Nepalese medicinal plants from Gorkha district. J. Pharmacogn. Phytotherapy. 2020;12(2):28–35. [Google Scholar]
- 302.Dubal K.N., Ghorpade P.N., Kale M.V. Studies on bioactive compounds of Tectaria coadunata (wall. Ex hook. & grev.) C. Chr. Asian J. Pharmaceut. Clin. Res. 2013;6(S2):186–187. https://innovareacademics.in/journal/ajpcr/Vol6Suppl2/1723.pdf [Google Scholar]
- 303.Maridass M., Ravichandran B. The screening of phytochemical and antioxidant activity of two endemic ferns, Mycodium excertum (Wall. Ex Hook) Copel and Tectaria Zeilanica (Holtt.) Sledge. Pharmacologyonline. 2009;1:56–63. https://pharmacologyonline.silae.it/files/newsletter/2009/vol1/9.Maridass.pdf [Google Scholar]
- 304.Sukumaran S., Mahesh M., Jeeva S. Bioactive constituents of oak leaf fern - Tectaria zeylanica (Houtt.) Sledge from southern western ghats. Asian Pac. J. Trop. Biomed. 2012;2(S1):S64–S66. [Google Scholar]
- 305.Nath K., Talukdar A.D., Bhattacharya M.K., Bhowmik D., Chetri S., Choudhury D., Mitra A., Bhattacharjee A. Antibacterial activity of certain ferns against multi drug resistant organisms. J. Nat. Remedies. 2017;17 [Google Scholar]
- 306.Cao J., Xia X., Chen X., Xiao J., Wang Q. Flavonoids contents, antioxidant and anticancer activities of 72 species of ferns from China. Biomed. Pap. 2012;156(S1):S33. https://biomed.papers.upol.cz/incpdfs/pri-990000-0200_10_026.pdf [Google Scholar]
- 307.Cao J., Xia X., Chen X., Xiao J., Wang Q. Characterization of flavonoids from Dryopteris erythrosora and evaluation of their antioxidant, anticancer and acetylcholinesterase inhibition activities. Food Chem. Toxicol. 2013;51:242–250. doi: 10.1016/j.fct.2012.09.039. [DOI] [PubMed] [Google Scholar]
- 308.Jung M., Park M. Acetylcholinesterase inhibition by flavonoids from Agrimonia pilosa. Molecules. 2007;12:2130–2139. doi: 10.3390/12092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Ridhasya F.E., Rahim N., Almurdani Md, Hendra R., Teruna H.Y. Antidiabetic constituents from Helminthostachys zeylanica (L) hook (ophioglossaceae), pharmacogn. J. 2020;12(2):223–226. [Google Scholar]
- 310.Shah A.B., Yoon S., Kim J.H., Zhumanova K., Ban Y.J., Lee K.W., Park K.H. Effectiveness of cyclohexyl functionality in ugonins from Helminthostachys zeylanica to PTP1B and α-glucosidase inhibitions. Int. J. Biol. Macromol. 2020;165:1822–1831. doi: 10.1016/j.ijbiomac.2020.10.061. [DOI] [PubMed] [Google Scholar]
- 311.Wu C.H., Yeh W.T., Li P.C. Ugonin J, a bioactive compound isolated from Helminthostachys zeylanica (L) Hook. (Helminthostachyaceae), demonstrates inhibitory effects on neointima formation in the rat carotid artery. Exp. Biol. 2013;27(S1):922. [Google Scholar]
- 312.Chiou W.C., Lu H.F., Hsu N.Y., Chang T.Y., Chin Y.F., Liu P.C., Lo J.M., Wu Y.B., Yang J.M., Huang C. Ugonin J acts as a SARS-CoV-2 3C-like protease inhibitor and exhibits anti-inflammatory properties. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.720018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Chan L.P., Chou T.H., Wang G.H., Tseng Y.P., Chen P.J., Cheng Da L., Liang C.H. Ugonin K induces cell cycle arrest and apoptosis through reactive oxygen species/apoptosis signal pathway in human skin cancer cells. Liu X., Zhang K., Li M., editors. Adv. Mater. Res. 2013;690–693:1422–1425. Trans Tech Publications Ltd. [Google Scholar]
- 314.Lin Y.C., Huang Y.C., Chen S.C., Liaw C.C., Kuo S.C., Huang L.J., Gean P.W. Neuroprotective effects of ugonin K on hydrogen peroxide-induced cell death in human neuroblastoma SH-SY5Y cells. Neurochem. Res. 2009;34:9223–9930. doi: 10.1007/s11064-008-9860-0. [DOI] [PubMed] [Google Scholar]
- 315.Lee C.H., Huang Y.L., Liao J.F., Chiou W.F. Ugonin K-stimulated osteogenesis involves estrogen receptor-dependent activation of non-classical Src signaling pathway and classical pathway. Eur. J. Pharmacol. 2012;676:26–33. doi: 10.1016/j.ejphar.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 316.Wu K.C., Ho Y.L., Kuo Y.H., Huang S.S., Huang G.J., Chang Y.S. Hepatoprotective effect of ugonin M, a Helminthostachys zeylanica constituent, on acetaminophen-induced acute liver injury in mice. Molecules. 2018;23:2420. doi: 10.3390/molecules23102420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Chen C.Y., Liaw C.C., Chen Y.H., Chang W.Y., Chung P.J., Hwang T.L. A novel immunomodulatory effect of ugonin U in human neutrophils via stimulation of phospholipase C. Free Radic. Biol. Med. 2014;72:222–231. doi: 10.1016/j.freeradbiomed.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 318.Chen C.Y., Yang C.H., Tsai Y.F., Liaw C.C., Chang W.Y., Hwang T.L. Ugonin U stimulates NLRP3 inflammasome activation and enhances inflammasome-mediated pathogen clearance. Redox Biol. 2017;11:263–274. doi: 10.1016/j.redox.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Kumiko H., Shiota S., Hatano T., Yoshida T., Kuroda T., Tsuchiya T. Antimicrobial activity of oleanolic acid from Salvia officinalis and related compounds on vancomycin-resistant Enterococci (VRE) Biol. Pharm. Bull. 2007;30(6):1147–1149. doi: 10.1248/bpb.30.1147. [DOI] [PubMed] [Google Scholar]
- 320.Wang X., Bai H., Zhang X., Liu J., Cao P., Liao N., Zhang W., Wang Z., Hai C. Inhibitory effect of oleanolic acid on hepatocellular carcinoma via ERK–p53-mediated cell cycle arrest and mitochondrial-dependent apoptosis. Carcinogenesis. 2013;34:1323–1330. doi: 10.1093/carcin/bgt058. [DOI] [PubMed] [Google Scholar]
- 321.N Reddy V.L., Ravikanth V., Rao T.P., Diwan P.V., Venkateswarlu Y. A new triterpenoid from the fern Adiantum lunulatum and evaluation of antibacterial activity. Phytochemistry (Oxf.) 2001;56:173–175. doi: 10.1016/s0031-9422(00)00334-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.