Abstract
We describe here a side-by-side comparison of murine respiratory infection by Bordetella pertussis and Bordetella bronchiseptica strains whose genomes are currently being sequenced (Tohama I and RB50, respectively). B. pertussis and B. bronchiseptica are most appropriately classified as subspecies. Their high degree of genotypic and phenotypic relatedness facilitates comparative studies of pathogenesis. RB50 and Tohama I differ in their abilities to grow in the nose, trachea, and lungs of BALB/c mice and to induce apoptosis, lung pathology, and an antibody response. To focus on the interactions between the bacteria and particular aspects of the host immune response, we used mice with specific immune defects. Mice lacking B cells and T cells were highly susceptible to B. bronchiseptica and were killed by intranasal inoculation with doses as low as 500 CFU. These mice were not killed by B. pertussis, even when doses as high as 105 CFU were delivered to the lungs. B. bronchiseptica, which was highly resistant to naive serum in vitro, caused bacteremia in these immunodeficient mice, while B. pertussis, which was highly sensitive to naive serum, did not cause bacteremia. B. bronchiseptica was, however, killed by immune serum in vitro, and adoptive transfer of anti-Bordetella antibodies protected SCID-beige mice from B. bronchiseptica lethal infection. Neutropenic mice were similarly killed by B. bronchiseptica but not B. pertussis infection, suggesting neutrophils are critical to the early inflammatory response to the former but not the latter. B. bronchiseptica was dramatically more active than B. pertussis in mediating the lysis of J774 cells in vitro and in inducing apoptosis of inflammatory cells in mouse lungs. This side-by-side comparison describes phenotypic differences that may be correlated with genetic differences in the comparative analysis of the genomes of these two highly related organisms.
Bordetella pertussis and Bordetella bronchiseptica are closely related gram-negative bacteria that cause respiratory tract infections in their respective hosts. Although extensively studied in vitro, limitations of the B. pertussis mouse model have hindered a full exploration of the infectious process in vivo. While B. pertussis is highly infectious in humans, mouse infection models require inoculation with high numbers of bacteria delivered in a large liquid volume or in aerosolized form directly to the lungs. The bacteria grow transiently and are cleared from the animals over a period of weeks. These models are likely to bypass many of the mechanisms that normally allow small numbers of bacteria to efficiently adhere, grow, and spread throughout the respiratory tract during a natural infection. The mouse model is therefore unlikely to accurately reflect the roles of adhesins and colonization factors that are believed to contribute to the highly infectious nature of B. pertussis in humans.
B. pertussis and B. bronchiseptica are so closely related as to be considered subspecies (20). They express a similar set of virulence factors and use a conserved and functionally interchangeable two-component signal transduction system, BvgAS, to regulate the expression of virulence genes (4, 17). They differ, however, in their propensity to cause disease and in their nonoverlapping host ranges. While B. pertussis causes a severe, acute disease that can be life-threatening in unvaccinated patients, B. bronchiseptica typically establishes asymptomatic infections that persist indefinitely in the upper respiratory tract of infected animals. B. pertussis has a highly restricted host range which is exclusively confined to humans. In contrast, B. bronchiseptica has a broad host range which includes rodents, pigs, dogs, and cats but only rarely humans (11, 29). This broad host range has allowed the development of natural host animal models that use B. bronchiseptica and rabbits, rats, and mice (1, 5, 12). The remarkable efficiency with which B. bronchiseptica establishes persistent colonization in rodents suggests that relevant virulence factors are functional in these models. In contrast to B. pertussis, B. bronchiseptica requires fewer than 10 organisms delivered in a 5-μl droplet to the external naris to establish murine infections that last for the life of the animal (12). In the context of this infection model, interactions between bacteria and host may be dissected at the molecular level with the confidence that findings are likely to be relevant to natural infections.
Here we describe a side-by-side comparison of the B. pertussis and B. bronchiseptica strains whose genomes are currently being sequenced, Tohama I and RB50, respectively (20a). Sequence data will allow future comparative studies to use powerful genome-based techniques to investigate the genetic basis for differences between these closely related organisms. The prerequisite for such studies is a careful comparative analysis of their phenotypic differences. Mice are the most advanced system available to study the role of host immune functions and have been used extensively in the study of B. pertussis. We have recently shown that the manipulation of mouse immunity can increase the sensitivity of infection models to the effects of specific bacterial virulence factors (12). This approach is now applied to the comparative analysis of B. bronchiseptica and B. pertussis.
Strains Tohama I and RB50 differ in their ability to grow in various respiratory organs and to induce lung pathology and antibody responses in BALB/c mice. We used immunocompromised mice to compare the role of particular aspects of the host immune response to each subspecies. Mice deficient in either acquired immunity or neutrophils were highly susceptible to lethal infections by low doses of B. bronchiseptica, but were not killed by B. pertussis, even when doses as high as 105 CFU were delivered to the lungs. B. bronchiseptica was resistant to serum killing, was highly cytotoxic for J774 cells in vitro, and induced apoptosis of inflammatory cells in the lungs of infected mice. B. pertussis was deficient in all of these virulence characteristics, indicating that this subspecies has a dramatically different virulence strategy or that the B. pertussis mouse model does not accurately reflect natural infection of its human host.
MATERIALS AND METHODS
Bacteria.
Bacteria were maintained on Bordet-Gengou (BG) agar (Difco) and were grown to mid-log phase in Stainer-Scholte broth for assays and inoculations. Tohama I was originally isolated from a human patient with whooping cough and has been extensively studied for 4 decades (13). RB50 was isolated from a naturally infected rabbit colony (5). Bvg+ and Bvg− derivatives of RB50 (RB53 and RB54, respectively) and Tohama I (370 and 369, respectively) have been described (5, 18, 25, 26).
Animals.
Bordetella-free rabbits were obtained from Charles River Laboratories and inoculated as described earlier (5) to obtain immune serum. BALB/c mice were obtained from Charles River Laboratories. G-CSF−/− and beige mice were obtained from Jackson Laboratories. SCID-beige and SCID mice were obtained from facilities at the University of California, Los Angeles. Four- to six-week-old, female BALB/c or SCID-beige mice or four- to eight-week-old G-CSF−/− mice were used. Mice lightly sedated with halothane were inoculated with the indicated dose by pipetting the inoculum into the tip of the external nares. For the time course experiment, groups of four animals were sacrificed at days 0, 3, 5, 7, 14, 21, 28, and 50 postinoculation. Colonization of the nasal cavity, a 0.5-cm portion of the trachea, and the entire lungs was quantified by homogenizing each tissue in phosphate-buffered saline (PBS), plating aliquots onto BG blood agar, and counting the colonies after 2 (B. bronchiseptica) or 4 (B. pertussis) days of incubation at 37°C. BALB/c mice were made neutropenic by intraperitoneal injection of 0.25 g of cyclophosphamide per kg of body weight 4 days prior to inoculation. Blood leukocytes were counted by using a hemocytometer, and blood smears were stained with Diff-Quik (Baxter Scientific Products) and observed microscopically to confirm that neutrophils were reduced by more than 90%. For survival curves, after the progression of the disease became clear, moribund animals were euthanized to prevent unnecessary suffering. Antibody treatment involved intraperitoneal injection of 0.2 ml of immune rabbit serum once every 45 days starting 1 day postinoculation. Animals were handled in accordance with institutional guidelines. Statistical significance was determined by using an unpaired t test.
Histology and apoptosis assays.
For histological examination, lungs and trachea were excised as a unit. The trachea was cannulated with a blunt-ended 18-gauge needle attached to a syringe. Lungs were carefully inflated with 10% formalin and immersed in 10% formalin for 24 h before they were embedded in paraffin and sectioned. Sections were hematoxylin and eosin stained and graded by observers blinded as to the treatment of the samples, and the results were confirmed by an independent assessment by a pathologist consultant. For apoptosis assays, paraffin was removed from sections by xylene-ethanol washes, and sections were rehydrated in water before the fluorescent TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling)-based In Situ Cell Death Detection Kit was used according to manufacturer's protocol (Boehringer Mannheim).
Cytotoxicity.
J774 cells were cultured in Dulbecco modified Eagle medium with 10% fetal bovine serum. Cells were grown to approximately 80% confluency, and bacteria in 10 μl of PBS were added at a multiplicity of infection (MOI) of 10. After a 5-min centrifugation at 500 × g, the mixture was incubated at 37°C for 4 h. The cytotoxicity was determined by using the Cytotox96 Kit (Promega) according to the manufacturer's protocol.
Analysis of antibody response and serum resistance.
Blood was collected from rabbits that were Bordetella-free (naive) or B. bronchiseptica infected for 6 months (immune). Serum aliquots were frozen at −80°C. Bacteria were grown in Stainer-Scholte broth to mid-log phase and diluted in PBS to 100 CFU/μl. Serum was thawed on ice, and 90 μl of serum or PBS was mixed with 10 μl of PBS containing 1,000 CFU of bacteria. The mixture was incubated at 37°C for 1 h. Dilutions from samples were spread on BG plates and incubated for 2 to 4 days to determine the bacterial numbers. The classical pathway of complement activation was inhibited with 10 mM EGTA and 5 mM MgCl2. For immunoblots, total bacterial cell proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene difluoride membranes, and probed with sera from mice infected for 28 days with B. bronchiseptica (RB50) or B. pertussis (Tohama I). Enzyme-linked immunosorbent assays (ELISAs) were performed as previously described (5). Immunoelectron microscopy was performed by using immune serum as previously described (1).
RESULTS
Respiratory tract colonization of BALB/c mice by B. pertussis and B. bronchiseptica.
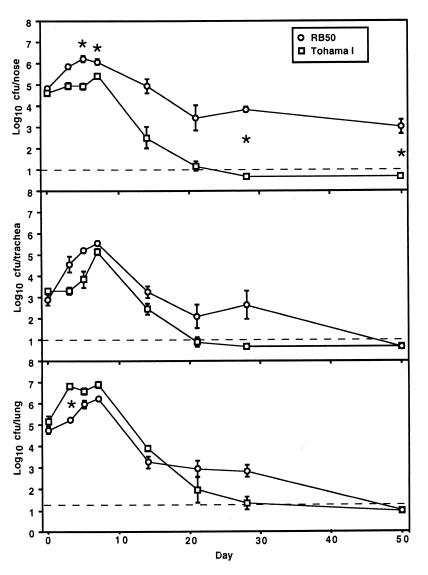
BALB/c mice were inoculated intranasally with 5 × 105 CFU of B. bronchiseptica (RB50) or B. pertussis (Tohama I) in 50 μl of PBS to compare their relative abilities to colonize the respiratory tract. This inoculation regimen consistently delivers bacteria to the nasal cavity, trachea, and lungs (Fig. 1, day 0). Both B. pertussis and B. bronchiseptica were subsequently recovered from all three sites at higher numbers, indicating that both subspecies were able to colonize and multiply throughout the respiratory tract. B. bronchiseptica grew to higher numbers than B. pertussis in the nasal cavity and the trachea. This situation was reversed in the lungs, however, where B. pertussis colony counts were higher than that of B. bronchiseptica on day 3 postinoculation. By later time points, both subspecies were cleared from the lower respiratory tract. B. bronchiseptica, but not B. pertussis, persisted in the nasal cavity for at least 270 days. These data demonstrate several differences in the abilities of B. bronchiseptica and B. pertussis to infect the respiratory tract of mice. B. bronchiseptica more efficiently infects the nasal cavity and trachea and persists in the nose. B. pertussis, however, grows more rapidly in the lungs early after inoculation. We also intranasally inoculated BALB/c mice with low numbers of bacteria in 5 μl of PBS and recovered bacteria 7 days later to determine the 50% infective dose (ID50). While mice inoculated with doses as low as 20 CFU of B. bronchiseptica were consistently infected with ca. 106 bacteria in the nasal cavity, B. pertussis required 500 CFU to consistently infect these mice, and roughly 104 bacteria were recovered from the nasal cavity of these animals.
FIG. 1.
Kinetics of colonization of BALB/c nose, trachea, and lungs by B. bronchiseptica and B. pertussis. Groups of four female 4-week-old BALB/c mice were inoculated with 5 × 105 CFU of either B. bronchiseptica (RB50, open circles) or B. pertussis (Tohama I, open squares) delivered in a 50-μl volume of PBS into the nares. Datum points are the means ± the standard error. ∗, P ≤ 0.001.
Lung pathology and polymorphonuclear leukocyte (PMN) recruitment in response to B. bronchiseptica and B. pertussis.
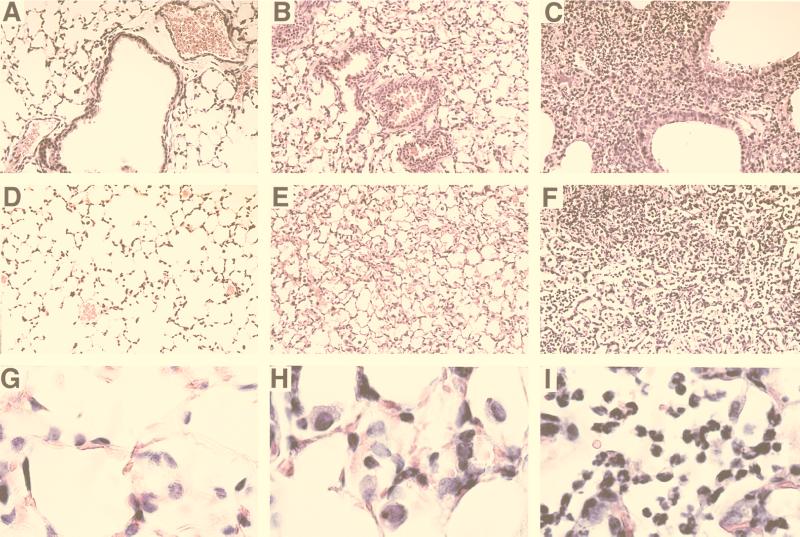
To compare the inflammatory responses to B. pertussis or B. bronchiseptica infection, BALB/c mice were sacrificed 3 days postinoculation for bronchoalveolar lavage (BAL) or histological examination of trachea and lungs. Compared to control mice inoculated with PBS (Fig. 2A, D, and G), the lungs of mice inoculated with B. pertussis contained little perivascular and peribronchiolar inflammation (Fig. 2B) but did contain a modest hypercellularity of alveoli consisting of mostly macrophages (Fig. 2E and H). Cells recovered in BAL fluids from B. pertussis-infected lungs on day 3 postinoculation consisted mostly of macrophages (67%) with fewer PMNs (32%) (data not shown). B. bronchiseptica-infected lungs, in marked contrast, showed extensive perivascular and peribronchiolar inflammation (Fig. 2C), with large numbers of infiltrating cells, primarily PMNs, located throughout the lungs, and some areas were consolidated and necrotic (Fig. 2F and I). Greater numbers of cells were recovered in BAL fluids from B. bronchiseptica-infected lungs and consisted predominantly of PMNs (74%) with a smaller proportion of macrophages (25%). These results demonstrate dramatically different innate immune responses to B. bronchiseptica and B. pertussis infections.
FIG. 2.
Comparison of mouse lung inflammation on day 3 postinoculation. Groups of five 4-week-old female BALB/c mice were inoculated with a 50-μl volume of PBS (A, D, and G) or PBS containing 5 × 105 CFU of either B. pertussis (B, E, and H) or B. bronchiseptica (C, F, and I) delivered to the nares. Animals were sacrificed on day 3 postinoculation, and histological sections of lung tissues were prepared as described in Materials and Methods. Magnification, ×200 (A to F) or ×1,000 (G to I).
Mouse antibody response to B. bronchiseptica and B. pertussis.
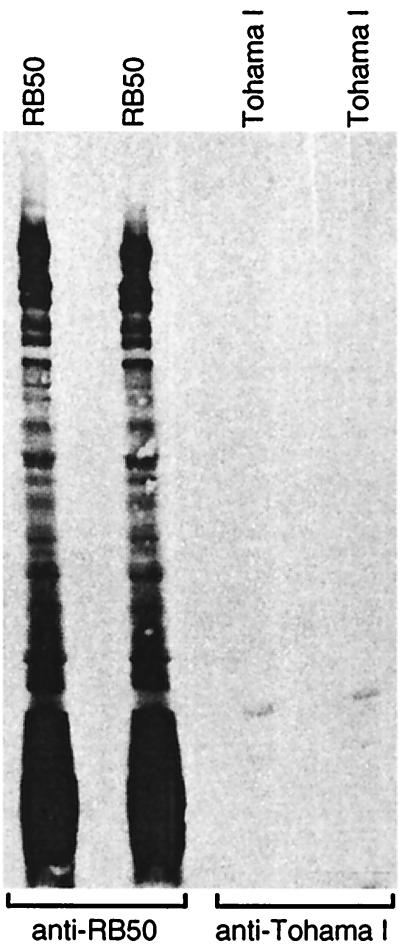
To compare the antibody response to B. bronchiseptica and B. pertussis infection, serum was taken from mice 28 days postinoculation. High titers of anti-Bordetella antibodies were detected by ELISA in sera from B. bronchiseptica-infected mice (data not shown). Western blot analysis showed that antibodies recognized many different B. bronchiseptica polypeptides (Fig. 3). In contrast, anti-Bordetella antibodies were not detected by ELISA or Western blot analysis of sera from mice infected with B. pertussis (Fig. 3 and data not shown). These and other results (1, 5) demonstrate that a strong antibody response was mounted against B. bronchiseptica. We found no evidence of a serum antibody response against B. pertussis after the infection of BALB/c mice.
FIG. 3.
Infected BALB/c mice mount an antibody response to B. bronchiseptica but not to B. pertussis. Total bacterial cell proteins of the indicated strain were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes. Two membranes each were probed with sera from mice infected for 28 days with B. bronchiseptica (anti-RB50) or B. pertussis (anti-Tohama I).
Virulence of B. bronchiseptica and B. pertussis in B-cell- and T-cell-deficient mice.
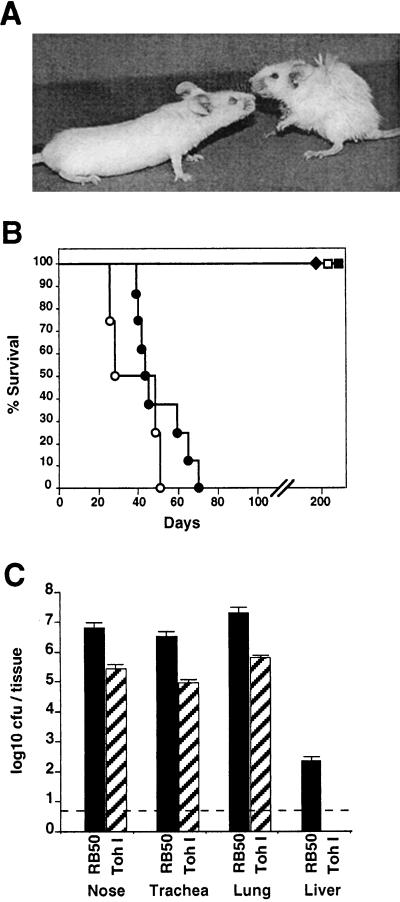
The innate and adaptive immune responses to B. bronchiseptica and B. pertussis appear to differ in BALB/c mice. To examine the role of innate immunity more closely, we used SCID-beige mice (BALB/c genetic background) which are deficient in B and T cells, as well as natural killer cell activities (9, 23). A low-dose–low-volume inoculum of 500 CFU in 5 μl was delivered to the external nares of these mice, and their survival was monitored over time. Both groups of mice remained healthy for 30 days. As we have previously shown (12), after 30 days animals infected with B. bronchiseptica began to display signs of illness, such as piloerection, weight loss, hunched stature, labored breathing, listlessness (Fig. 4A), and eventually, loss of responsiveness followed by death. All of the animals died between days 40 and 70 postinoculation (Fig. 4B). In striking contrast, B. pertussis-inoculated SCID-beige mice showed no signs of illness for more than 200 days, although B. pertussis was recovered from the respiratory tracts of these animals throughout the time course of the experiment (data not shown). We repeated the experiment with the same inoculation regimen as described for BALB/c mice above (5 × 105 CFU in 50 μl of PBS). Although this regimen deposits approximately 105 CFU directly in the lungs at day 0, B. pertussis still did not cause a lethal infection (Fig. 4B, open symbols). The time to death was only marginally accelerated by this high-dose inoculation of B. bronchiseptica, indicating that disease progression is not limited by slow growth in the nose and the rate of spread down the trachea to the lungs.
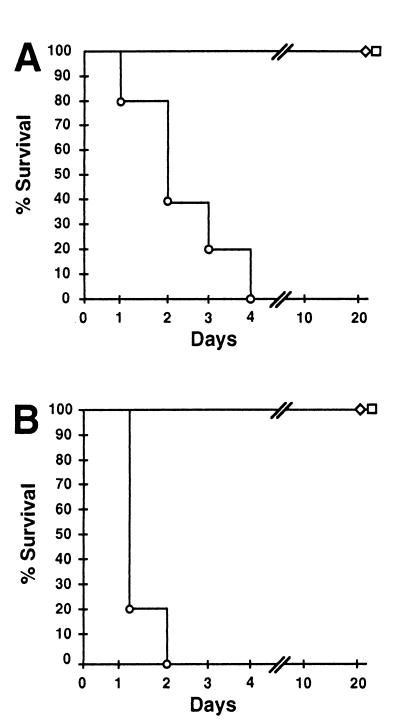
FIG. 4.
Comparison of B. bronchiseptica and B. pertussis infection of SCID-beige mice. (A) Representative mice were photographed on day 40 after intranasal inoculation with 50 μl of PBS containing 5 × 105 CFU of B. bronchiseptica (right) or B. pertussis (left). (B) Survival of SCID-beige mice inoculated with either B. bronchiseptica (circles) or B. pertussis (squares) is presented as a function of time. Bacteria were delivered intranasally at either a low-dose–low-volume of 500 CFU in a 5-μl PBS droplet (solid symbols) or a high-dose–high-volume of 5 × 105 CFU in 50 μl of PBS (open symbols). (C) Colonization of various tissues by B. bronchiseptica or B. pertussis. Groups of four 4-week-old female SCID-beige mice were inoculated with 5 × 105 CFU in 50 μl of PBS of either B. bronchiseptica (solid bars) or B. pertussis (hatched bars) and sacrificed at 45 days postinoculation, and the colonization levels in the nasal cavity, trachea, lungs, and liver were determined. The mean log10 CFU per organ or tissue is shown. The dashed line represents the lower limit of detection.
Separate groups of SCID-beige mice inoculated with either B. bronchiseptica or B. pertussis (5 × 105 CFU in 50 μl of PBS) were sacrificed at day 45 postinoculation to determine the numbers and distribution of bacteria in these animals. B. bronchiseptica was recovered at more than 10-fold higher numbers than B. pertussis throughout the respiratory tract (Fig. 4C). B. bronchiseptica was also consistently recovered from various other organs, including the liver, spleen, kidney, and heart, and from blood. Each of 10 moribund animals euthanized between days 40 and 70 were systemically infected in all organs examined, indicating that systemic infection consistently accompanies the final stages of B. bronchiseptica infection. B. pertussis, however, was limited to the respiratory tract and was recovered in lower numbers on days 45 and 200 postinoculation (Fig. 4C and data not shown). B. bronchiseptica also killed SCID mice (both BALB/c and C3H background) and RAG-1−/− mice (C57BL/6 background), but not mice carrying the beige mutation alone (C57BL/6 background), indicating that T cells and/or B cells are required to limit B. bronchiseptica infection. B. pertussis, however, was controlled by immune mechanisms active in all of these T-cell- and B-cell-deficient mice, suggesting it may be less efficient in modulating innate immune mechanisms retained in these immunodeficient animals.
Virulence of B. bronchiseptica and B. pertussis in neutropenic mice.
In the lungs of BALB/c mice, proliferation of B. pertussis was about 100-fold higher than that of B. bronchiseptica by day 3 but B. bronchiseptica induced dramatically more neutrophil infiltrate. To investigate the importance of neutrophils in controlling Bordetella infections, we inoculated neutropenic mice intranasally with either B. bronchiseptica or B. pertussis at the same dose as was used for BALB/c mice above (5 × 105 CFU in 50 μl of PBS). G-CSF−/− mice (B6,129 genetic background) lack both chromosomal copies of the G-CSF gene and have >90% reduction in neutrophil numbers and a severe defect in neutrophil chemotaxis (15). B. bronchiseptica rapidly killed these mice between days 1 and 4 postinoculation, indicating that neutrophils are a critically important component of the primary immune response to B. bronchiseptica infection (Fig. 5A). B. bronchiseptica killed G-CSF−/− mice even at doses as low as 5,000 CFU (data not shown). B. pertussis, in contrast, did not kill neutropenic mice, suggesting recruitment of these inflammatory cells is not required to control B. pertussis infection in mice.
FIG. 5.
Comparison of B. bronchiseptica and B. pertussis infection of neutropenic mice. Groups of five G-CSF−/− mice (A) or BALB/c mice (B) rendered neutropenic by cyclophosphamide treatment were inoculated with 5 × 105 CFU of either B. bronchiseptica (circles), B. pertussis (squares), or Bvg−-phase locked B. bronchiseptica (RB54, diamonds) delivered in a 50-μl volume. The percentages of animals surviving over time are indicated.
Since G-CSF−/− mice may be deficient in additional immune functions, we also used mice rendered neutropenic by intraperitoneal injection of cyclophosphamide. These mice were confirmed to be depleted of >90% of peripheral blood neutrophils by microscopic examination of blood smears. Mice were inoculated intranasally with either B. bronchiseptica or B. pertussis (5 × 105 CFU in 50 μl). Consistent with the results for G-CSF−/− mice, B. bronchiseptica rapidly killed these mice but B. pertussis did not (Fig. 5B).
Serum resistance of B. bronchiseptica and B. pertussis.
Unlike B. pertussis, B. bronchiseptica has been isolated from the blood of human patients (usually aged or immunocompromised) and SCID-beige mice, suggesting it can survive the antimicrobial agents present in blood and lymph fluids (11, 12). We therefore compared the susceptibility of B. pertussis and B. bronchiseptica to factors in serum. Naive serum obtained from Bordetella-free rabbits contained no detectable antibodies recognizing Bordetella antigens as determined by Western immunoblot (data not shown). We compared Bvg+- and Bvg−-phase derivatives of B. bronchiseptica RB50 (RB53 and RB54, respectively) and B. pertussis Tohama I (370 and 369, respectively) for their ability to survive in serum. A total of 1,000 bacteria taken from mid-log-growth-phase liquid cultures was incubated in 100 μl of 90% serum to ensure that serum components were not limiting. Both Bvg+- and Bvg−-phase B. bronchiseptica organisms were resistant to naive serum, while Bvg+- and Bvg−-phase B. pertussis organisms were highly sensitive (Fig. 6). Inhibition of complement with EDTA prevented the serum killing of B. pertussis. B. bronchiseptica, but not B. pertussis, is therefore resistant to the innate antimicrobial activities of serum, including the alternative pathway of complement.
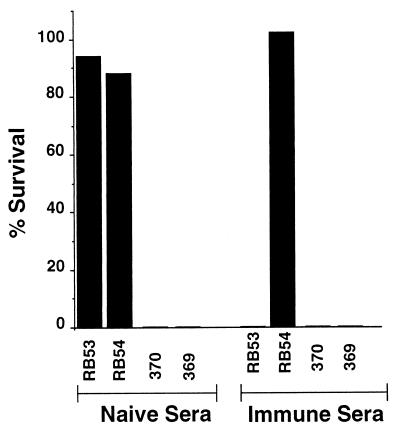
FIG. 6.
Serum resistance of B. bronchiseptica and B. pertussis. Bvg+ and Bvg− derivatives of RB50 (RB53 and RB54, respectively) and Tohama I (370 and 369, respectively) were grown to mid-log phase in Stainer-Scholte broth and diluted in PBS. A total of 1,000 bacteria was incubated at 37°C for 1 h in 100 μl of 90% serum. Serum resistance is presented as the percent survival. Naive and immune serum are described in Materials and Methods.
Immune sera were obtained from a rabbit that had been infected with B. bronchiseptica for 6 months. Only RB54, the Bvg−-phase B. bronchiseptica strain, was resistant to killing by this postinfection serum (Fig. 6). Similar results were obtained with all four strains with immune serum from infected rabbits, rats, and mice or from vaccinated rabbits and humans. In all cases B. pertussis was killed by naive serum but B. bronchiseptica was only killed by immune serum.
Immune serum, which killed only Bvg+-phase B. bronchiseptica, recognized predominantly Bvg+-phase-specific polypeptides, as detected by immunoblot (Fig. 7). Minimal reactivity was detected to antigens common to the Bvg+ and Bvg− phases. The low-molecular-weight smear observed in both Bvg+ and Bvg− phases was not present in a strain containing a deletion of the wlb locus, suggesting these antigens consist of lipopolysaccharide (LPS) (11a). Consistent with previous observations, antibodies to Bvg−-phase-specific antigens were not detected in immune serum by ELISA (11a), and this serum did not kill Bvg−-phase B. bronchiseptica, suggesting that antigens common to the Bvg+ and Bvg− phases are not surface exposed. Immunoelectron microscopy studies showed that immune serum recognized antigens exposed on the surface of Bvg+-phase, but not Bvg−-phase, B. bronchiseptica (Fig. 7), a result consistent with other data indicating that the Bvg− phase is not expressed in vivo (1, 16). Together, these data indicate that B. bronchiseptica, but not B. pertussis, is able to resist killing by the alternative pathway of complement. However, antibodies specific for B. bronchiseptica surface antigens mediate killing via the classical pathway of complement activation.
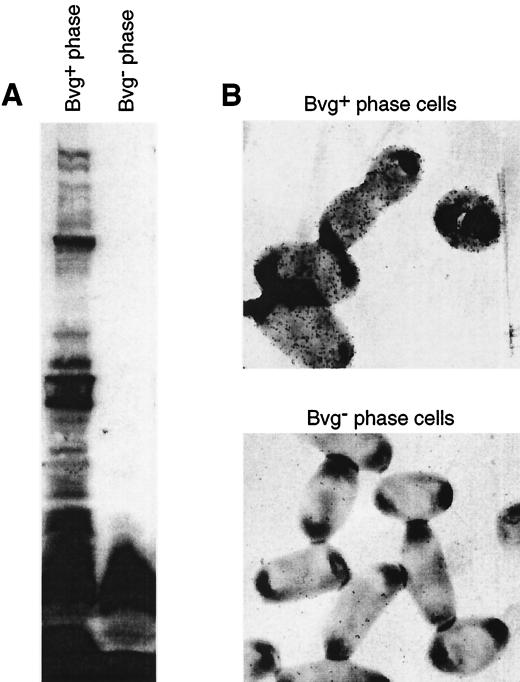
FIG. 7.
Convalescent serum recognizes surface antigens specific to the Bvg+ phase. (A) Whole-cell extracts of Bvg+- or Bvg−-phase bacteria were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and probed with immune serum from a rabbit infected for 6 months with B. bronchiseptica (RB50). (B) Immunoelectron micrograph showing that convalescent serum recognized the surface of Bvg+- but not Bvg−-phase B. bronchiseptica.
The ability of B. bronchiseptica to resist serum complement may relate to the ability of this organism to survive in blood and cause bacteremia in immunocompromised mice and humans. Since B. bronchiseptica is sensitive to antibody-mediated complement activation, we hypothesized that the addition of anti-B. bronchiseptica antibodies to SCID-beige mice might protect these mice from bacteremia. We intranasally inoculated groups of mice with 5 × 105 CFU of B. bronchiseptica in 50 μl of PBS and adoptively transferred naive or immune serum by intraperitoneal injection on postinoculation days 1, 45, 90, and 135. The animals given immune serum were healthy for more than 200 days, whereas the group given naive serum died between days 20 and 32 postinoculation (Fig. 8). Adoptive transfer of serum antibodies is therefore sufficient to limit B. bronchiseptica infection in SCID-beige mice.
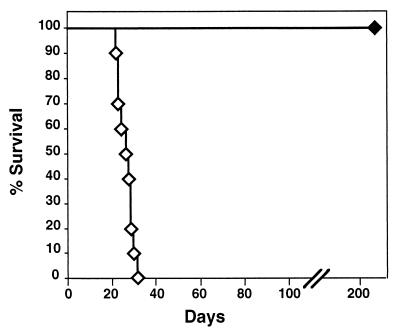
FIG. 8.
Adoptive transfer of antibody protects SCID-beige mice from B. bronchiseptica lethal infection. Survival of SCID-beige mice intranasally inoculated with B. bronchiseptica, 5 × 105 CFU in 50 μl of PBS. On postinoculation days 1, 45, 90, and 135, mice were given 0.2-ml intraperitoneal injections of either naive serum (open symbols) or immune serum (solid symbols). Sera are described in Materials and Methods. Survival is presented as the percentage of animals alive at the given day postinoculation.
Macrophage cytotoxicity of B. bronchiseptica and B. pertussis.
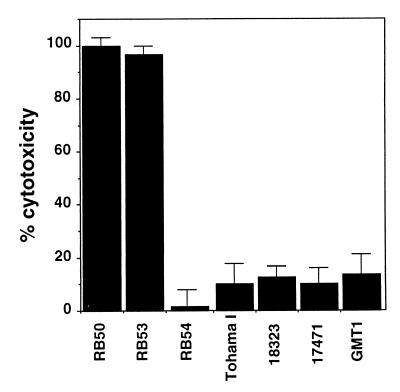
Neutropenic mice died rapidly after B. bronchiseptica inoculation, whereas SCID-beige mice died over an extended time period, suggesting neutrophils are critical to the early response to B. bronchiseptica infection. In contrast, B. pertussis did not kill neutropenic mice, indicating that other aspects of innate immunity were sufficient to contain this infection. To investigate the possibility that the different virulence characteristics of these two subspecies in neutropenic mice were due to differing interactions with resident macrophages in the lungs, we compared their macrophage cytotoxicity in vitro. J774 cell cultures were inoculated with either organism by adding bacteria at an MOI of 10 and centrifuging the mixture at 500 × g for 5 min to synchronize bacterial contact with cultured cells. At this time similar numbers of each strain of bacteria were in contact with macrophages. The plate was then incubated at 37°C for 4 h, after which we assessed total cellular lysis by measuring lactate dehydrogenase release. In this assay a high proportion (95% ± 2%) of J774 cells was consistently killed by B. bronchiseptica, but B. pertussis was minimally cytotoxic (12% ± 5%) (P < 0.001) (Fig. 9). B. bronchiseptica macrophage cytotoxicity is a Bvg+-phase-specific characteristic, since RB53 killed these macrophages but RB54 did not. A filamentous hemagglutinin-reversed strain (FHAr), expressing FHA in the Bvg− phase (6), was not cytotoxic, indicating that FHA-mediated adherence was not sufficient and that other B. bronchiseptica Bvg+-phase factors are required (11a). In addition to Tohama I, we tested other B. pertussis strains, including 18-323 and two recent clinical isolates, GMT1 and 17471, and found all three to have minimal cytotoxic activity in this assay. B. pertussis strains were cytotoxic only after a longer incubation with macrophages at a higher MOI (results not shown). These results indicate B. bronchiseptica and B. pertussis differ dramatically in their cytotoxicity for J774 cells in vitro.
FIG. 9.
Cytotoxicity of Bordetella species for the mouse macrophage-like cell line J774. Bacteria were added at an MOI of 10 to J774 cells in culture medium in a 96-well plate. The plate was spun at 500 × g for 10 min and then incubated at 37°C for 4 h. Cytotoxicity was assessed by using the Cytotox96 Kit according to manufacturer's instructions. The average and standard error of three samples are presented as a percentage of total lysis with detergent. Bacterial strains include RB50 and its Bvg+ and Bvg− derivatives (RB53 and RB54, respectively) and a Bvg− strain ectopically expressing FHA (FHAr). Tohama I and three other B. pertussis strains (18-323, 17471, and GMT1) are compared.
Induction of apoptosis in mouse lungs by B. bronchiseptica and B. pertussis.
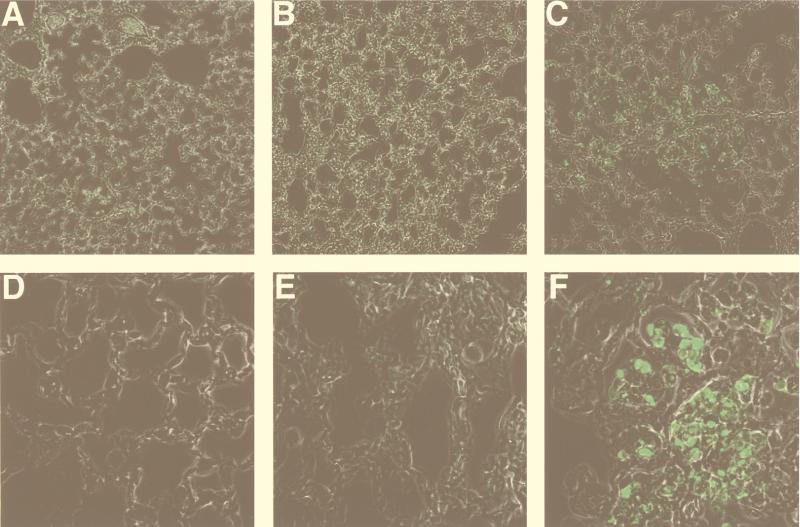
In a concurrent report we show that the B. bronchiseptica type III secretion system mediates cytotoxicity for macrophages by inducing apoptosis in vitro and in vivo (29a). We have previously shown that Tohama I does not express the type III secretion system under laboratory growth conditions (30), although B. pertussis has been shown to induce apoptosis under conditions that involve longer incubation at a higher MOI (10, 14). To compare B. bronchiseptica and B. pertussis for their ability to induce apoptosis in vivo, lung sections of mice infected with RB50 or Tohama I were assayed for the presence of apoptotic nuclei by using the TUNEL reaction. Fluorescent (apoptotic) nuclei were not detected in lungs from mice inoculated with PBS (Fig. 10A and D) and only rarely in lung sections from mice infected with B. pertussis (5 × 105 in 50 μl of PBS) (Fig. 10B and E). Lung sections from mice inoculated with B. bronchiseptica (5 × 105 in 50 μl of PBS), however, showed fluorescent nuclei throughout the lungs with dense areas containing many apoptotic nuclei (Fig. 10C and F). Although on day 3 B. pertussis was present in higher numbers in infected mouse lungs (Fig. 1), B. bronchiseptica induced a dramatic increase in the number of apoptotic bodies.
FIG. 10.
B. bronchiseptica, but not B. pertussis, induces apoptosis in mouse lungs. Mice were inoculated with PBS (A and D), B. pertussis (B and E), or B. bronchiseptica (C and F), and 3 days later lungs were excised, fixed, paraffin embedded, and sectioned as in Fig. 2. Sections were stained for apoptosis as described in Materials and Methods. Magnification, ×200 (A to C) or ×1,000 (D to F).
DISCUSSION
B. pertussis infection of humans is characteristically an upper respiratory infection involving the nasopharynx and trachea. Although the infection appears to be limited to the epithelial surface, systemic effects, such as leukocytosis, are common. A marked antibody response is induced that typically persists for life and is thought to be protective. Vaccination also induces antibodies that correlate with protection from disease but do not necessarily protect against subclinical infection (8). In addition to the antibody response, T-cell responses have been described (7, 24). The relative roles of humoral and cell-mediated immunity in the control of pertussis, however, are not well understood. Experimental models for pertussis have focused primarily on mouse lung colonization after high-dose–high-volume inoculation. Correlating observations made with B. pertussis in mouse lungs with natural infection of human trachea compound problems of host specificity with those of tissue tropism. The immune response of mice to high-dose B. pertussis inoculation may also differ from that of infected humans. Dissecting the immune response by adoptive transfer of its components from immune to naive BALB/c mice suggested a dominant role for T cells in immunity (19). However, only a small fraction of the level of protection observed in convalescent mice could be transferred by this approach, indicating that the most effective immune functions are not yet understood. In stark contrast to the human response, mice produce little or no antibody response to B. pertussis infection (21, 22). Murine colonization by B. pertussis, and the resulting immune response, bear little resemblance to B. pertussis infection of humans.
We have previously described models of Bordetella respiratory tract infection by using B. bronchiseptica and several of its natural hosts: rabbits, rats, and mice (1, 5, 12). Like B. pertussis infection of humans, B. bronchiseptica efficiently establishes respiratory tract infections in these hosts. In mice, for example, ID50 values are as low as 10 organisms in a 5-μl droplet delivered to the external nares (12). The bacteria grow to high numbers in the nasal cavity and trachea and induce a strong serum antibody response that correlates with protection against superinfection (unpublished results). Therefore, by these measures of efficiency of bacterial infection and the immune response, B. bronchiseptica infection of rodents appears to be similar to B. pertussis infection of humans. Unlike B. pertussis infection of humans, B. bronchiseptica typically persists in the upper respiratory tracts of its animal hosts for life. Furthermore, B. bronchiseptica infections are often asymptomatic, whereas B. pertussis causes acute disease.
Similarities and differences between closely related Bordetella subspecies provide fertile grounds for comparative studies of pathogenesis. We therefore initiated a side-by-side comparison of B. bronchiseptica RB50 and B. pertussis Tohama I in murine models of respiratory tract infection. Tohama I was initially isolated from a child with whooping cough, and it has been used in numerous studies since that time (27). RB50 is a recent clinical isolate obtained from the nasal cavity of a New Zealand White rabbit (5), and it has also been subjected to considerable experimental analysis (1, 2, 12). The complete genomic sequence of Tohama I has been determined, and the sequence of RB50 is well underway (20a).
Both Tohama I and RB50 are capable of multiplying in the respiratory tracts of BALB/c mice. The two strains differ, however, in that RB50 grows to higher numbers in the nasal cavity and persists there indefinitely. Tohama I, in contrast, grows to higher numbers in the lungs but is cleared from all sites by day 50. Histological examination of respiratory tract tissue shows that RB50 induces considerably greater lung pathology than Tohama I, mostly due to infiltration by large numbers of neutrophils. Significantly, an antibody response to Tohama I infection was not observed, whereas a vigorous response to RB50 was noted.
We also compared the role of adaptive immunity in modulating the course of infection by RB50 and Tohama I. In SCID-beige, SCID, and RAG-1−/− mice the differences between B. bronchiseptica and B. pertussis were striking. B. pertussis caused a persistent infection, confirming earlier reports that B and T cells are required for the clearance of high doses of B. pertussis delivered to the lungs (19). Interestingly, infection was asymptomatic. B. bronchiseptica, however, grew progressively within the respiratory tract and reached 10-fold-higher numbers, causing bacteremia and death of these animals. We have previously shown that the virulence of B. bronchiseptica strain RB50 in SCID mice is dependent on the expression of the adenylate cyclase toxin (12). Since B. pertussis strains also express this toxin, there is apparently some other factor, unique to B. bronchiseptica, that is responsible for virulence in these immunodeficient mice. In all of the animals examined, lethal infection by B. bronchiseptica correlated with bacteremia, which requires resistance to the antimicrobial activities present in serum. In vitro, B. bronchiseptica was highly resistant to antimicrobial components in naive serum, whereas B. pertussis was killed. Immune serum, however, killed Bvg+-phase B. bronchiseptica in vitro and protected SCID-beige mice from B. bronchiseptica infection. LPS structures are known to affect serum resistance in other organisms, and a recent report correlates the LPS structure of these two subspecies with serum resistance phenotypes (28). Furthermore, we have shown that deletion of genes involved in LPS assembly affects serum resistance as well as virulence in SCID-beige mice (unpublished results). Together, these data suggest that one or more structural features of B. bronchiseptica LPS may promote serum resistance, resulting in a highly virulent phenotype in B-cell- and T-cell-deficient mice. The relationship, if any, between serum resistance and colonization of the respiratory epithelium is currently unclear.
Similar to results obtained with B-cell- and T-cell-deficient mice, neutropenic mice were also found to be highly susceptible to lethal infection by B. bronchiseptica at doses as low as 5,000 CFU. Although B. pertussis grew more rapidly in the lungs of BALB/c mice, neutropenic mice were not killed by doses of B. pertussis as high as 5 × 105 CFU. Neutrophils are therefore critical components of resistance to lethal infection by B. bronchiseptica but not by B. pertussis. It is possible that neutrophils may be more important in B. bronchiseptica infection because this subspecies may be able to kill resident and recruited phagocytes in the lungs. This suggestion is supported by the cytotoxicity of B. bronchiseptica for J774 cells in vitro and by the dramatically higher number of apoptotic nuclei in the lungs of B. bronchiseptica-inoculated mice. It is also possible that resident alveolar macrophages, which can be immunomodulatory, are sufficient to control B. pertussis infection with little additional inflammation or immune response.
Although some of the differences observed may reflect the fact that mice are natural hosts for B. bronchiseptica but not for B. pertussis, others may reflect inherent differences in the pathogenic strategies of these subspecies. Discriminating between these and other possibilities will improve both our understanding of the mechanistic basis for host specificity and our ability to apply the power of the B. bronchiseptica natural host model to the analysis of infection by a human-adapted pathogen. The availability of the genomic sequence of the strains used here will also allow the phenotypic differences we have described to be correlated with genotypic differences identified in silico and gene expression differences identified by using genome-based assays such as microarrays. Traditional methods have already revealed differences between B. bronchiseptica and B. pertussis in their expression of pertussis toxin, LPS, and the type III secretion system (2, 3, 30). We have begun to determine which of these virulence factors may be involved in species-specific characteristics by testing genetically deleted strains in the assays we have described here. In this way we have shown that the type III secretion system is required for persistence in the rat and mouse trachea (reference 29 and unpublished results) and that wlb genes involved in LPS synthesis are required for serum resistance of B. bronchiseptica (unpublished results). By swapping genes between B. bronchiseptica and B. pertussis we are currently determining whether these genes are sufficient to confer the virulence characteristics of one organism on the other. Genome-based approaches will reveal other genes differentially expressed by these two organisms that may likewise be tested for their roles in species-specific characteristics. The close phylogenetic relationship between Bordetella subspecies and the growing availability of phenotypic and genotypic methods for their analysis make these organisms excellent models for understanding comparative pathogenesis and the evolution of bacterium-host interactions.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant AI38417 (to J.F.M.) and U.S. Department of Agriculture grant 960-1856 (to E.T.H.).
REFERENCES
- 1.Akerley B J, Cotter P A, Miller J F. Ectopic expression of the flagellar regulon alters development of the Bordetella-host interaction. Cell. 1995;80:611–620. doi: 10.1016/0092-8674(95)90515-4. [DOI] [PubMed] [Google Scholar]
- 2.Allen A G, Thomas R M, Cadisch J T, Maskell D J. Molecular and functional analysis of the lipopolysaccharide biosynthesis locus wlb from Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Mol Microbiol. 1998;29:27–38. doi: 10.1046/j.1365-2958.1998.00878.x. [DOI] [PubMed] [Google Scholar]
- 3.Arico B, Rappuoli R. Bordetella parapertussis and Bordetella bronchiseptica contain transcriptionally silent pertussis toxin genes. J Bacteriol. 1987;169:2847–2853. doi: 10.1128/jb.169.6.2847-2853.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arico B, Scarlato V, Monack D M, Falkow S, Rappuoli R. Structural and genetic analysis of the bvg locus in Bordetella species. Mol Microbiol. 1991;5:2481–2491. doi: 10.1111/j.1365-2958.1991.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 5.Cotter P A, Miller J F. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect Immun. 1994;62:3381–3390. doi: 10.1128/iai.62.8.3381-3390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotter P A, Yuk M H, Mattoo S, Akerley B J, Boschwitz J, Relman D A, Miller J F. Filamentous hemagglutinin of Bordetella bronchiseptica is required for efficient establishment of tracheal colonization. Infect Immun. 1998;66:5921–5929. doi: 10.1128/iai.66.12.5921-5929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Magistris M T, Romano M, Nuti S, Rappuoli R, Tagliabue A. Dissecting human T cell responses against Bordetella species. J Exp Med. 1988;168:1351–1362. doi: 10.1084/jem.168.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deville J G, Cherry J D, Christenson P D, Pineda E, Leach C T, Kuhls T L, Viker S. Frequency of unrecognized Bordetella pertussis infections in adults. Clin Infect Dis. 1995;21:639–642. doi: 10.1093/clinids/21.3.639. [DOI] [PubMed] [Google Scholar]
- 9.Dorshkind K, Keller G M, Phillips R A, Miller R G, Bosma G C, O'Toole M, Bosma M J. Functional status of cells from lymphoid and myeloid tissues in mice with severe combined immunodeficiency disease. J Immunol. 1984;132:1804–1808. [PubMed] [Google Scholar]
- 10.Gueirard P, Druilhe A, Pretolani M, Guiso N. Role of adenylate cyclase-hemolysin in alveolar macrophage apoptosis during Bordetella pertussis infection in vivo. Infect Immun. 1998;66:1718–1725. doi: 10.1128/iai.66.4.1718-1725.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gueirard P, Weber C, Le Coustumier A, Guiso N. Human Bordetella bronchiseptica infection related to contact with infected animals: persistence of bacteria in host. J Clin Microbiol. 1995;33:2002–2006. doi: 10.1128/jcm.33.8.2002-2006.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11a.Harvill, E. T. Unpublished results.
- 12.Harvill E T, Cotter P A, Yuk M H, Miller J F. Probing the function of Bordetella bronchiseptica adenylate cyclase toxin by manipulating host immunity. Infect Immun. 1999;67:1493–1500. doi: 10.1128/iai.67.3.1493-1500.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasuga T, Nakase Y, Ukishima K, Takatsu K. Studies on Haemophilus pertussis. V. Relation between the phase of bacilli and the progress of the whooping-cough. Kitasato Arch Exp Med. 1954;27:57–62. [PubMed] [Google Scholar]
- 14.Khelef N, Zychlinsky A, Guiso N. Bordetella pertussis induces apoptosis in macrophages: role of adenylate cyclase-hemolysin. Infect Immun. 1993;61:4064–4071. doi: 10.1128/iai.61.10.4064-4071.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lieschke G J, Grail D, Hodgson G, Metcalf D, Stanley E, Cheers C, Fowler K J, Basu S, Zhan Y F, Dunn A R. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84:1737–1746. [PubMed] [Google Scholar]
- 16.Martinez de Tejada G, Cotter P A, Heininger U, Camilli A, Akerley B J, Mekalanos J J, Miller J F. Neither the Bvg-phase nor the vrg6 locus of Bordetella pertussis is required for respiratory infection in mice. Infect Immun. 1998;66:2762–2768. doi: 10.1128/iai.66.6.2762-2768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez de Tejada G, Miller J F, Cotter P A. Comparative analysis of the virulence control systems of Bordetella pertussis and Bordetella bronchiseptica. Mol Microbiol. 1996;22:895–908. doi: 10.1046/j.1365-2958.1996.01538.x. [DOI] [PubMed] [Google Scholar]
- 18.Miller J F, Johnson S A, Black W J, Beattie D T, Mekalanos J J, Falkow S. Constitutive sensory transduction mutations in the Bordetella pertussis bvgS gene. J Bacteriol. 1992;174:970–979. doi: 10.1128/jb.174.3.970-979.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mills K H, Barnard A, Watkins J, Redhead K. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect Immun. 1993;61:399–410. doi: 10.1128/iai.61.2.399-410.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musser J M, Hewlett E L, Peppler M S, Selander R K. Genetic diversity and relationships in populations of Bordetella spp. J Bacteriol. 1986;166:230–237. doi: 10.1128/jb.166.1.230-237.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.Parkhill, J. 6 September 1999, revision date. Sequences. [Online.] http://www.sanger.ac.uk/Projects/B_pertussis/. [17 September 1999, last date accessed.]
- 21.Petersen J W, Ibsen P H, Haslov K, Heron I. Proliferative responses and gamma interferon and tumor necrosis factor production by lymphocytes isolated from tracheobroncheal lymph nodes and spleen of mice aerosol infected with Bordetella pertussis. Infect Immun. 1992;60:4563–4570. doi: 10.1128/iai.60.11.4563-4570.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redhead K, Watkins J, Barnard A, Mills K H. Effective immunization against Bordetella pertussis respiratory infection in mice is dependent on induction of cell-mediated immunity. Infect Immun. 1993;61:3190–3198. doi: 10.1128/iai.61.8.3190-3198.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roder J, Duwe A. The beige mutation in the mouse selectively impairs natural killer cell function. Nature. 1979;278:451–453. doi: 10.1038/278451a0. [DOI] [PubMed] [Google Scholar]
- 24.Ryan M, Murphy G, Gothefors L, Nilsson L, Storsaeter J, Mills K H. Bordetella pertussis respiratory infection in children is associated with preferential activation of type 1 T helper cells. J Infect Dis. 1997;175:1246–1250. doi: 10.1086/593682. [DOI] [PubMed] [Google Scholar]
- 25.Stibitz S, Aaronson W, Monack D, Falkow S. Phase variation in Bordetella pertussis by frameshift mutation in a gene for a novel two-component system. Nature. 1989;338:266–269. doi: 10.1038/338266a0. [DOI] [PubMed] [Google Scholar]
- 26.Stibitz S, Black W, Falkow S. The construction of a cloning vector designed for gene replacement in Bordetella pertussis. Gene. 1986;50:133–140. doi: 10.1016/0378-1119(86)90318-5. [DOI] [PubMed] [Google Scholar]
- 27.Stibitz S, Yang M S. Genomic fluidity of Bordetella pertussis assessed by a new method for chromosomal mapping. J Bacteriol. 1997;179:5820–5826. doi: 10.1128/jb.179.18.5820-5826.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Akker W M. Lipopolysaccharide expression within the genus Bordetella: influence of temperature and phase variation. Microbiology. 1998;144:1527–1535. doi: 10.1099/00221287-144-6-1527. [DOI] [PubMed] [Google Scholar]
- 29.van der Zee A, Mooi F, Van Embden J, Musser J. Molecular evolution and host adaptation of Bordetella spp.: phylogenetic analysis using multilocus enzyme electrophoresis and typing with three insertion sequences. J Bacteriol. 1997;179:6609–6617. doi: 10.1128/jb.179.21.6609-6617.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29a.Yuk, M. H., E. T. Harville, P. A. Cotter, and J. F. Miller. Submitted for publication.
- 30.Yuk M H, Harvill E T, Miller J F. The BvgAS virulence control system regulates type III secretion in Bordetella bronchiseptica. Mol Microbiol. 1998;28:945–959. doi: 10.1046/j.1365-2958.1998.00850.x. [DOI] [PubMed] [Google Scholar]