Abstract
Background
A novel hematologic parameter, systemic coagulation-inflammation (SCI) index reflecting inflammation and coagulation pathways could be easily obtained from clinically routine laboratory findings. We hypothesize that the SCI index has prognostic implication in predicting operative mortality for patients with acute type A aortic dissection (ATAAD).
Objectives
This study aims to investigate the prognostic value of the SCI index and to establish an SCI-adding nomogram for mortality prediction in ATAAD patients.
Methods
A total of 1,967 ATAAD patients surgically repaired were collected from 12 Chinese cardiovascular centers by the 5A (Additive Anti-inflammatory Action for Aortopathy & Arteriopathy [Multicenter Retrospective Study]) study III (2016-2020). SCI index was calculated as platelet count × fibrinogen/white blood cell count on admission. By adding SCI index, a nomogram was developed and evaluated for 90-day mortality prediction with conventional predictors via the Cox model with 10-fold cross-validation.
Results
Patients were stratified with low SCI (<40), middle SCI (40-100), or high SCI (>100). The 90-day survival rates increased with SCI index (low 86.9%; [95% CI: 84.9%-89.0%], middle 92.7% [95% CI: 90.9%-94.9%], and high 96.4% [95% CI: 94.2%-98.6%]; log-rank P < 0.001). SCI index is independently associated with 90-day mortality (adjusted hazard ratio: 0.549; 95% CI: 0.424-0.710; P < 0.001). The addition of SCI index provided significantly incremental prognostic value to base model including age, serum creatinine, DeBakey class, and location of intimal entry (area under the curve: 0.677; 95% CI: 0.641-0.716 vs 0.724; 95% CI: 0.685-0.760; P = 0.002), which was confirmed by net reclassification improvement index (0.158; 95% CI: 0.065-0.235; P < 0.001) and integrated discrimination improvement index (0.070; 95% CI: 0.007-0.036; P < 0.001).
Conclusions
SCI index is easily obtainable, performs moderately well as a predictor of short-term mortality in ATAAD patients, and may be useful for risk stratification in emergency settings. (Additive Anti-inflammatory Action for Aortopathy & Arteriopathy [Multicenter Retrospective Study] III NCT04918108)
Key Words: coagulation, inflammation, mortality, type A aortic dissection
Abbreviations and Acronyms: ATAAD, acute type A aortic dissection; AUC, the area under the curve; DHCA, deep hypothermic circulatory arrest; SCI, systemic coagulation-inflammation index; SII, systemic immune-inflammation index
Central Illustration
Acute type A aortic dissection (ATAAD) is a life-threatening emergency which comprises a subset of patients characterized by major anatomopathological changes, systemic inflammatory response, and other devastating comorbid conditions that potentially can contribute to significant morbidity and mortality.1,2 It is well recognized that the initiation and progression of aortic dissection is closely associated with systemic inflammatory and coagulopathic disorders.3,4 Risk stratification for short-term mortality is recommended to drive clinical management in the acute phase of this disease; however, it does not account for the inflammatory predisposition.5
There has been a recent emphasis on the systemic immune-inflammation (SII) index that shows favorable predictive value of clinical outcome in a noncardiac setting and in some cardiac settings such as coronary heart diseases and valvular diseases.6,7 However, different from these aforementioned settings, the SII index will lose its capacity for predicting prognosis of aortic dissection because the alteration of the SII index might be offset against lower platelet and higher neutrophile counts in this exclusive disease.8
Moreover, white blood cells, platelets, and fibrinogen as well as systemic inflammatory and coagulopathic biomarkers, which are simple and rapidly available assessments, have been shown to correlate with adverse outcome in patients with ATAAD.9, 10, 11 For better prediction of short-term mortality, we have proposed a novel hematologic parameter called the systemic coagulation-inflammation (SCI) index; calculated as platelet count × fibrinogen level/white blood cell count) that organically reflects coagulopathic and inflammatory pathways. Therefore, the aim of this study was to investigate the characteristic and prognostic value of the SCI index in ATAAD patients.
Methods
Patients
From an investigator-initiated 5A (Additive Anti-inflammatory Action for Aortopathy & Arteriopathy) III project, we retrospectively identified patients with ATAAD from 12 Chinese cardiovascular centers between January 2016 and December 2020. Patients aged 18 years or older were included in this study provided that counts for white blood cells, platelets, and fibrinogen were available on hospital admission, they presented symptom duration within 72 hours, and they received open surgical repair for the dissected aorta. In particular, we also included DeBakey type III patients (retrograde ATAAD) in our study who failed in medical management along with important cardiovascular risk factors including total aortic diameter >50 mm, hematoma thickness >11 millimeters, pericardial effusion, or aortic regurgitation, and whose anatomy was not favorable for thoracic endovascular aortic repair without an adequate proximal landing zone for the stent graft. Patients were excluded if they had received anticoagulants or antiplatelet therapy in the most recent 3 months or if they had hematological or immune system diseases (Figure 1). The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board of Aortic Collaborative Institutions involved (2021-SR-381). This study was registered with ClinicalTrials.gov number NCT04918108. Informed consent was waived for this observational study.
Figure 1.
Patient Selection Flow Chart
Patients were from 12 Chinese university cardiovascular centers: the First Affiliated Hospital of Nanjing Medical University, Nanjing; the First Affiliated Hospital of Guangzhou Medical University, Guangzhou; the First Affiliated Hospital of Shantou University Medical College, Shantou; Nanjing First Hospital, Nanjing Medical University, Nanjing; the Affiliated Hospital of Qingdao University, Qingdao; Xiamen Cardiovascular Hospital, Xiamen University, Xiamen; Teda International Cardiovascular Hospital, Chinese Academy of Medical Sciences, Tianjin; Shanghai East Hospital, Tongji University, Shanghai; Xiangya Hospital, Central South University, Changsha; Beijing Anzhen Hospital, Capital Medical University, Beijing; the First Affiliated Hospital of Bengbu Medical College, Bengbu; the First Affiliated Hospital of Guilin Medical College, Guilin. ICD 10 = International Classification of Diseases, 10th revision.
Data collection
The laboratory signatures were evaluated using the first venous blood samples obtained during admission to the emergency department. All laboratories involved among 12 Chinese cardiovascular centers were certificated via the China National Accreditation Service for Conformity Assessment, which ensures consistency and homogeneity of laboratory results.
We collected baseline data, including sex, age, and previous medical histories (hypertension, diabetes mellitus, hyperlipidemia, chronic lung diseases, stroke), and smoking and drinking status from structured electronic medical records. Other recorded characteristics included the dissected features and procedural characteristics. The rationale and strategy of surgical techniques were determined by surgeons in the department of cardiovascular surgery at each hospital. The main surgical techniques used in this study have been described previously.12
For each patient the following, the parameters were calculated: SCI index (platelet count × fibrinogen level/white blood cell count), SII index (platelet count × neutrophil count/lymphocyte count), platelet lymphocyte ratio (PLR) (platelet count/lymphocyte count) and neutrophil to lymphocyte ratio (NLR) (neutrophil count/lymphocyte count). All patients were followed up from the date of index admission until at least 3 months after surgery. Outcome data were obtained by reviewing medical records for all deaths recorded following discharge.
Outcome
The primary outcome was 90-day mortality, defined as any death, regardless of cause, occurring within 90 days after surgery in or out of the hospital, according to the Society of Thoracic Surgeons criteria.13 Secondary outcomes included 30-day, hospital, and intensive care unit (ICU) mortality as well as mechanical ventilation duration, ICU length of stay, bleeding, and stroke. All outcomes were adjudicated independently by an event collaborative team.
Statistical analysis
The continuous data were presented as the mean ± SD or median (IQR) and compared using a Student t-test (parametric) for normal distribution or the Kruskal–Wallis test (nonparametric) for skewed distribution, and the categorical data were reported as percentages and compared using chi square or Fisher exact testing. The correlation was evaluated using the Pearson coefficient test. In this study, C-reaction protein (CRP) levels on admission were incomplete (398 of 1,967 patients without CRP on admission). Missing CRP levels were assumed to be missing at random and were imputed using multiple imputation of chained equations.14 Based on the proportion of incomplete cases, we determined that 5 imputed datasets and 10 iterations were needed to minimize the simulation error (Monte Carlo).
We fitted the functional relationship between SCI index and 90-day mortality using restricted cubic spline curves in the multiple Cox regression model.15,16 The thresholds for SCI index that were output from the predictive model that was used to classify patients into different clusters were defined as SCI index that gave the largest log-likelihood value in the piecewise regression model.17 Accordingly, patients were stratified by the threshold (inflection point) of SCI index.
Survival curves were assessed using the Kaplan-Meier method with log-rank test (time-to-death).
We used unadjusted and multivariable-adjusted Cox proportional hazards regression models to estimate HRs and 95% CIs of 90-day mortality associated with each category of SCI index vs the lowest category as the reference group. We performed tests for linear trend by entering the median value of each category of SCI index as a continuous variable in the model.18 In addition to the crude model (unadjusted for any covariates), the adjusted model was constructed including relevant variables as confounders which appeared in any of the existing clinical scores for mortality prediction (ie, the additive and logistic EuroSCORE [European system for cardiac operative risk evaluation], the Parsonnet score, the Cleveland score, OPR[Ontario Province Risk] score, SinoSCORE [Sino System For Coronary Operative Risk Evaluation], and the GERAADA [German Registry of Acute Aortic Dissection Type A] score) such as demographics, baseline, clinical and procedural characteristics.19, 20, 21, 22, 23, 24, 25
The prognostic value of the individual and combined laboratory signatures for predicting primary outcome was analyzed by calculating the area under the curve (AUC) from time-dependent receiver operating characteristics.26 Moreover, the Cox hazards model was used to identify the potential risk factors correlated with 90-day mortality in addition to clinical importance criteria for variable selection. Variables with a P < 0.10 in the univariable analysis were then entered into a multivariable Cox regression, in which covariates showing significant associations (P < 0.05) with were modeled together to develop a nomogram model for predicting 90-day mortality using multivariate Cox regression.
Subsequently, we compared the prognostic value of SCI adding the prediction model with this base risk prediction model in predicting 90-day mortality. The performance of the predictive models was evaluated with a 10-fold cross-validation scheme, including discrimination (time-dependent AUC, net reclassification index, and integrated discrimination index) and calibration (calibration plots).27,28 Meanwhile, we generated the functional curves to assess the association of AUC with the number of days after surgery based on the method proposed by Chambless and Diao.29 We also plotted decision curves to assess the net benefit of inflammation-based decisions.30
We calculated 95% CIs of the AUC and then compared the model predictive accuracies using the 10 AUCs obtained from the 10 cross-validation folds following the method of DeLong et al.31 Overall performance was reported as the mean AUC and SD over 10 cross-validation folds.
Additional analysis was performed to investigate the effect modification of deep hypothermic circulatory arrest (DHCA) on the association between SCI index as a continuous variable and 90-day mortality using interaction terms in a multivariable mode, in which heterogeneity was evaluated using the likelihood ratio test.
We did analyses using Stata version 14 (Stata Corp) and R software (version 3.2.0). A P values <0.05 was considered statistically significant.
Results
Patients characteristics
A total of 1,967 patients with ATAAD were included in the final analysis, with median age of 54 (IQR: 45-62) years, 1,399 male (71.1%), and median body mass index of 25.2 (22.7-27.7) kg/m2. There were no significant differences regarding baseline demographics and medical history between each group. A flow chart describing participants is presented in Figure 1. Baseline demographic and clinical characteristics are summarized in Table 1. Preoperative malperfusion was significantly negatively correlated with SCI index (correlation coefficient: -0.107; 95% CI: -0.152 to 0.063; P < 0.001).
Table 1.
Patients Characteristics and Outcomes by Stratification of SCI Index
| High SCI (N1 = 275) | Middle SCI (N2 = 658) | Low SCI (N3 = 1,034) | P Valuea | |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 55 (44-63) | 53 (43-62) | 54 (46-62) | 0.162 |
| Male | 189 (68.7) | 479 (72.8) | 731 (70.7) | 0.415 |
| Body mass index, kg/m2 | 24.9 (22.5-27.0) | 25.2 (22.4-27.9) | 25.2 (22.7-27.7) | 0.151 |
| Body surface area, m2 | 1.89 (1.76-2.01) | 1.92 (1.78-2.07) | 1.92 (1.79-2.05) | 0.096 |
| Comorbidities | ||||
| Smoking | 116 (44.8) | 267 (43.3) | 398 (44.4) | 0.879 |
| Alcohol drinking | 72 (27.8) | 191 (30.9) | 263 (29.3) | 0.628 |
| Hypertension | 225 (81.8) | 509 (77.4) | 822 (79.6) | 0.281 |
| Diabetes mellitus | 17 (6.2) | 38 (5.8) | 61 (5.9) | 0.971 |
| Hyperlipidemia | 38 (13.8) | 65 (9.9) | 105 (10.2) | 0.168 |
| Cerebrovascular accident | 23 (8.4) | 31 (4.7) | 57 (5.5) | 0.085 |
| Chronic pulmonary disease | 12 (4.4) | 14 (2.1) | 35 (3.4) | 0.148 |
| Arrhythmia | 31 (11.3) | 50 (7.6) | 75 (7.3) | 0.084 |
| Creatinine, μmoI/L | 77 (60-98) | 84 (66-110) | 94 (72-136) | <0.001 |
| Hematologic signatures | ||||
| White blood cell, ×109/L | 7.87 (5.99-10.24) | 10.16 (7.79-12.70) | 13.52 (10.91-16.35) | <0.001 |
| Neutrophils, ×109/L | 5.84(3.82-7.96) | 8.20 (5.78-10.60) | 11.52 (9.28-14.38) | <0.001 |
| Lymphocytes, ×109/L | 1.28(0.95-1.78) | 1.12 (0.73-1.60) | 0.88 (0.58-1.23) | <0.001 |
| Monocytes, ×109/L | 0.60 (0.41-0.82) | 0.69 (0.47-0.97) | 0.75 (0.51-1.04) | <0.001 |
| Platelets, ×109/L | 247 (205-294) | 177 (143-215) | 134 (100-165) | <0.001 |
| Fibrinogen, g/L | 4.36 (3.52-5.96) | 3.47 (2.80-4.35) | 2.10 (1.63-2.74) | <0.001 |
| C-reaction protein, mg/L | 26.4 (12.5-42.7) | 38.1 (17.4-51.3) | 48.8 (22.5-66.2) | <0.001 |
| D-dimer, μg/mL | 5.1 (1.2-15.9) | 8.3 (1.9-18.6) | 13.6 (2.5-22.8) | <0.001 |
| Platelet lymphocyte ratio | 192 (142-290) | 161 (110-247) | 148 (98-218) | <0.001 |
| Neutrophils lymphocyte ratio | 4.3 (2.6-7.7) | 7.8 (4.7-12.6) | 13.6 (9.2-19.9) | <0.001 |
| SII index | 826 (402-2,065) | 1,201 (523-3,145) | 2,033 (956-4,052) | <0.001 |
| SCI index | 140 (116-176) | 60 (50-76) | 21 (14-30) | <0.001 |
| Disease-specific conditions | ||||
| DeBakey class | 0.015 | |||
| Type I | 206 (74.9) | 511 (77.7) | 849 (82.1) | |
| Type II | 65 (23.6) | 128 (19.5) | 163 (15.8) | |
| Type IIIb | 4 (1.5) | 19 (2.9) | 22 (2.1) | |
| Extension of the dissection | ||||
| Arch | 217 (78.9) | 544 (82.7) | 917 (88.7) | <0.001 |
| Descending | 185 (67.3) | 487 (74.0) | 846 (81.8) | <0.001 |
| Location of primary entry tear | <0.001 | |||
| Ascending | 188 (68.4) | 455 (69.1) | 709 (68.6) | |
| Arch | 25 (9.1) | 50 (7.6) | 152 (14.7) | |
| Descending | 11 (4.0) | 15 (2.3) | 32 (3.1) | |
| Invisible | 51 (18.5) | 138 (21.0) | 141 (13.6) | |
| Cardiac tamponade | 24 (8.6) | 59 (9.0) | 98 (9.5) | 0.899 |
| Malperfusionc | 85 (30.9) | 235 (35.7) | 444 (42.9) | <0.001 |
| Any pulse deficit | 41 (15.0) | 125 (19.0) | 241 (23.3) | |
| Acute renal failure | 24 (8.9) | 68 (10.3) | 155 (15.0) | |
| MI at presentation | 9 (3.2) | 24 (3.6) | 45 (4.4) | |
| TIA in the last 24 hours | 11 (3.9) | 33 (5.0) | 64 (6.2) | |
| Cardiogenic shock | 28 (10.2) | 90 (13.9) | 188 (18.2) |
Values are median (IQR) or n (%).
MI = myocardial infarction; SCI = systemic coagulation-inflammation; SII = systemic immune-inflammation index; TIA = transient ischemic attack.
P value represents chi square analysis for categorical variables and Kruskal-Wallis rank sum for continuous variables.
Indicated those retrograde Stanford type A acute aortic dissection.
Defined as one of the following conditions: coronary malperfusion, renal malperfusion, cardiogenic shock (cardiac tamponade, low cardiac output), cerebral perfusion (cerebrovascular accident in the previous 24 hours), and any pulse deficit/limb ischemia.
Patients were divided into 3 groups based on the threshold of SCI index of the cubic spline curve of the HR for 90-day mortality: lower SCI (<40, n = 1,034), middle SCI (40-100, n = 658), or high SCI (>100, n = 275) (Figure 2A, Supplemental Table 1). Patients with low SCI were more likely to have more extensive dissected involvement compared to those with middle and high SCI. Significant differences were found in individual laboratory signature as well as combined laboratory variables across groups (all P < 0.05) (Table 1, Supplemental Figure 1).
Figure 2.
Functional Relationship Between SCI Index and the Risk of 90-Day Mortality
(A) Crude model for SCI grouping. (B) Adjusted for demographics. (C) Adjusted for demographics and comorbidities. (D) Adjusted for demographics, comorbidities, and disease-specific conditions. (E) Adjusted for demographics, comorbidities, disease-specific conditions, and procedures. SCI = systemic coagulation-inflammation.
Procedural characteristics of the 3 groups are summarized in Table 2. With respect to procedure, patients with low SCI were more likely to have higher rates of arch replacement, frozen elephant trunk implantation, and DHCA than those with middle and high SCI (Table 1).
Table 2.
Procedural Characteristics and Outcomes by Stratification of SCI Index
| High SCI (N1 = 275) | Middle SCI (N2 = 658) | Low SCI (N3 = 1,034) | P Valuea | |
|---|---|---|---|---|
| Procedural characteristics | ||||
| Root procedure | 0.201 | |||
| Root repair only | 45 (16.4) | 143 (21.7) | 250 (24.2) | |
| Aortic valve replacement only | 15 (5.4) | 45 (6.8) | 50 (4.8) | |
| Bentall | 57 (20.4) | 123 (18.5) | 214 (20.5) | |
| David | 4 (1.5) | 5 (0.8) | 8 (0.8) | |
| Arch procedure | <0.001 | |||
| Hemiarch replacement | 25 (9.1) | 53 (8.1) | 66 (6.4) | |
| Total arch replacement | 192 (69.8) | 491 (74.6) | 851 (82.3) | |
| ET implantation | 186 (67.6) | 490 (74.5) | 848 (82.0) | <0.001 |
| Hypothermic circulatory arrest | 213 (77.5) | 537 (81.6) | 910 (88.0) | <0.001 |
| Total arch replacement plus FET implantation | 185 (67.3) | 481 (73.1) | 842 (81.4) | <0.001 |
| Concomitant operation | ||||
| CABG | 10 (3.6) | 41 (6.2) | 55 (5.3) | 0.275 |
| Valve surgeryb | 8 (2.9) | 12 (1.8) | 26 (2.5) | 0.523 |
| Primary outcome | ||||
| 90-day mortality | 10 (3.6) | 47 (7.1) | 135 (13.1) | <0.001 |
| Secondary outcomes | ||||
| 30-day mortality | 10 (3.6) | 40 (6.1) | 118 (11.4) | <0.001 |
| Hospital mortality | 10 (3.6) | 47 (7.1) | 136 (13.2) | <0.001 |
| Cardiac death | 5 | 19 | 44 | |
| Neurologic death | 2 | 13 | 36 | |
| Respiratory death | 0 | 3 | 7 | |
| ICU stay, days | 4 (2-7) | 4 (2-7) | 5 (3-11) | <0.001 |
| Ventilation support, h | 26 (17-78) | 33 (17-81) | 43 (19-115) | <0.001 |
| Hospital stay, days | 20 (14-28) | 18 (13-25) | 18 (12-27) | 0.367 |
| Re-exploration for bleeding | 13 (4.8) | 35 (5.3) | 65 (6.3) | 0.520 |
| Stroke | 16 (5.8) | 41 (6.2) | 71 (6.9) | 0.772 |
Values are n (%) or median (IQR).
AVSRR = aortic valve-sparing root replacement; CABG = coronary artery bypass grafting; FET = frozen elephant trunk; ICU = intensive care unit; SCI = systemic coagulation-inflammation; SII = systemic immune-inflammation index.
P value represents chi square analysis for categorical variables and Kruskal-Wallis rank sum for continuous variables.
Indicated the surgery including mitral, tricuspid, pulmonary valve surgery, but not aortic valve surgery.
Primary and secondary outcome
Overall, 9.8% (n = 196 of 1,967) patients died within 90 days of surgery. In Kaplan-Meier analysis, overall survival was 90.2% (95% CI: 88.9%-91.6%) at 90 days (Figure 3A). SCI index subgroups showed a significant trend for 90-day mortality: low 86.9% (95% CI: 84.9%-89.0%), middle 92.7% (95% CI: 90.9%-94.9%); and high 96.4% (95% CI: 94.2%-98.6%; log-rank P < 0.001) (Figure 3B). After additional adjustment for demographics, comorbidities, disease-specific conditions, and procedural confounders, the risk of 90-day mortality remained significant higher in the low and middle SCI group than in the high SCI group, respectively (Table 3). Similarly, there were significant differences regarding 30-day mortality, hospital mortality, ICU stay, ventilation support, and re-exploration for bleeding among 3 groups (all P < 0.05). Secondary outcomes are summarized in Table 2.
Figure 3.
Kaplan-Meier Curves Overall and by Stratification of SCI Index
(A) Kaplan-Meier survival plot of overall patients. (B) Kaplan-Meier survival plot by stratification of SCI index. Abbreviation as in Figure 2.
Table 3.
Multivariable Cox Models of 90-day Mortality Associated With SCI Index Among Groups
| High SCI Group | Middle SCI Group | Low SCI Groupa | Per-SD Increasea | |
|---|---|---|---|---|
| Crude | 1.000 | 1.984 (1.003-3.927) | 3.773 (1.985-7.172) | 0.514 (0.407-0.649) |
| Adjusted for demographicsb | 1.000 | 2.312 (1.1293-4.732) | 4.058 (2.060-7.987) | 0.527 (0.416-0.667) |
| Adjusted for demographics and comorbiditiesc,d | 1.000 | 2.874 (1.284-6.434) | 4.868 (2.256-10.505) | 0.519 (0.403-0.668) |
| Adjusted for demographics, comorbidities, and dissection | 1.000 | 2.492 (1.10-5.595) | 3.976 (1.833-8.623) | 0.557 (0.431-0.721) |
| Adjusted for demographics, comorbidities, dissection, and procedurese | 1.000 | 2.426 (1.078-5.458) | 4.048 (1.862-8.799) | 0.549 (0.424, 0.710) |
Values are HR (95% CI).
P for trend <0.001.
Demographics including age, sex, and body mass index.
Comorbidities including smoking, drinking, diabetes mellitus, hypertension, hyperlipidemia, chronic lung disease, arrhythmia, and stroke.
Disease-specific conditions including DeBakey classification, the extent of the dissected involvement, and location of entry.
Procedures including root procedure, arch procedure, frozen elephant trunk implantation, hypothermic circulatory arrest, and concomitant coronary artery bypass graft as well as concomitant valve surgery.
Association of SCI index with outcome
Restricted cubic spline model showed a significant inverse linear trend between 90-day mortality risk and SCI index on a continuous scale among all patients in a crude multivariate Cox model (HR: 0.514; 95% CI: 0.407-0.649 per-SD increase; P < 0.0001), which is consistent with the main analysis (Figure 2A). With adjustments of confounders, the inverse linear trend remained significant between 90-day mortality and SCI index (all P for trend <0.01) (Table 3, Figures 2B to 2E). At subgroup-specific analysis, the risk reduction of 90-day mortality is greatest in those with low SCI index (HR: 0.085; 95% CI: 0.037-0.198 per-SD increase; P < 0.0001) but was modest in those patients with middle and high SCI index (HR: 0.741; 95% CI: 0.537-1.022 per-SD increase; P = 0.068), with significant threshold effect in piecewise regression model (P < 0.001) (Figure 2A).
Prognostic value of SCI index alone
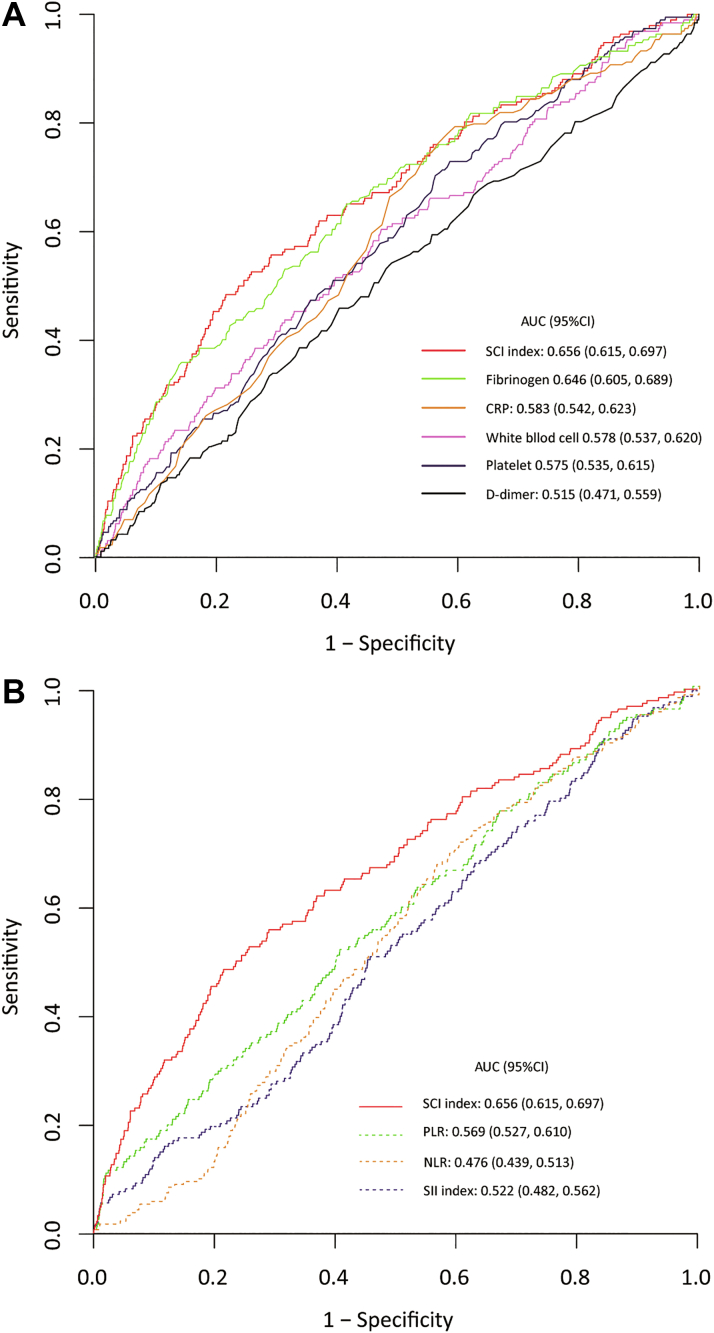
For the prediction of 90-day mortality, the AUC of SCI index (AUC: 0.662; 95% CI: 0.626-0.703) was significantly greater than that of individual hematologic signature (CRP: 0.583, 95% CI: 0.542-0.623; D-dimer: 0.515, 95% CI: 0.471-0.559; white blood cell count: 0.581, 95% CI: 0.538-0.621; platelet count: 0.611, 95% CI: 0.568-0.651; and fibrinogen level: 0.640, 95% CI: 0.598-0.680; SCI index vs each variable, P < 0.05, respectively) and collaborative hematologic signatures (SII: 0.531, 95% CI: 0.488-0.572; PLR: 0.579, 95% CI: 0.535-0.623; and NLR: 0.513, 95% CI: 0.473-0.551; SCI index vs each variable, P < 0.05, respectively) (Figure 4). For predicting secondary outcomes, the AUCs of SCI index were 0.661 (95% CI: 0.616-0.707) for 30-day mortality and 0.662 (95% CI: 0.620-0.705) for hospital mortality, respectively (Supplemental Figure 2).
Figure 4.
Comparative Performance of Hematologic Signatures
(A) Receiver operating characteristic (ROC) curves of individual hematologic signatures. (B) ROC curves of collaborative hematologic signatures. AUC = area under the curve; CRP = C-reactive protein; NLR = neutrophil to lymphocyte ratio; PLR = platelet to lymphocyte ratio; SII = systemic immune-inflammation (index); other abbreviation as in Figure 2.
Incremental prognostic value of adding SCI index
Based on the conventional independent risk factors (age, creatinine, DeBakey class, and location of primary entry tear) (Supplemental Table 2), we developed a nomogram (base model) using multivariable Cox regression to predict the probability of 90-day mortality (Supplemental Figure 3). By addition of SCI index to this base model, we further developed an inflammation-adding nomogram for risk prediction (Figure 5A). When we compared the base model and the inflammation-adding model (base model plus SCI index), a significant improvement in 90-day mortality prediction was noted regarding mean AUC (base model: 0.677, 95% CI: 0.641-0.716; SD: 0.018 vs inflammation-adding model: 0.724, 95% CI: 0.685-0.760; SD: 0.012; P = 0.002) (Figure 5B), which was also confirmed by net reclassification improvement index (0.158; 95% CI: 0.065-0.235; P < 0.001) and integrated discrimination improvement index (0.070; 95% CI: 0.007-0.036; P < 0.0001). The performances of the model including accuracy, and false-positives and false-negatives were also shown (Supplemental Table 3). A calibration plot was generated for evaluation of calibration regarding both models (Figure 5C). The time-dependent AUC of the inflammation-adding model maintained better performances ranging from 0.68 to 0.85 since the number of days after surgery, which indicates that our distributed Cox model works better for prediction relative to base model ranging from 0.62 to 0.75 (both AUC >0.50) (Figure 5D). Furthermore, the decision curves showed that the inflammation-adding model had better performance in clinical application (Figure 5E).
Figure 5.
Establishment and Evaluation of Inflammation-Based Nomogram for 90-Day Mortality Prediction
(A) Inflammation-based nomogram for 90-day mortality prediction. (B) ROC curves of base model vs inflammation-based nomogram. (C) Calibration plot of base model vs inflammation-based nomogram. (D) The time-dependent AUC of base model vs inflammation-based nomogram. (E) The decision curves of base model vs inflammation-based nomogram. Abbreviations as in Figures 2, 3, and 4.
Additional analysis
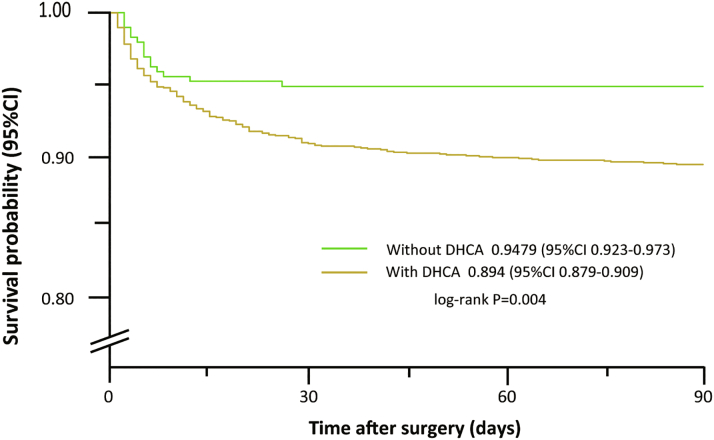
Overall, the cumulative incidence of 90-day mortality was higher for those with DHCA relative to those without DHCA (n = 16 of 307 vs n = 176 of 1,660; HR: 2.076; 95% CI: 1.245-3.464; P = 0.005) (Figure 6). At subgroup-specific analysis, the risk of 90-day mortality was significantly associated with SCI index in both those without DHCA (HR: 0.428; 95% CI: 0.195-0.940 per-SD increase; P = 0.035) and those with (HR: 0.541; 95% CI: 0.424-0.689 per-SD increase; P < 0.0001), with no evidence of interaction between DHCA and SCI index (P for interaction = 0.537).
Figure 6.
Kaplan-Meier Survival Plot by DHCA Use
Differences was observed in 90-day survival after surgery between patients with and without deep hypothermic circulatory arrest (DHCA).
Discussion
The main findings of the study are: 1) SCI index is independently associated with 90-day mortality in ATAAD patients; 2) SCI index provided incremental prognostic value to base risk prediction model that included age, serum creatinine, DeBakey classification, and location of primary entry tear (Central Illustration); and 3) a significant inverse trend existed between SCI index and 90-day mortality, in which significant threshold effect is observed at the 40 of SCI index. These findings highlight that the hematologic parameter SCI index provides additional prognostic value for ATAAD patients.
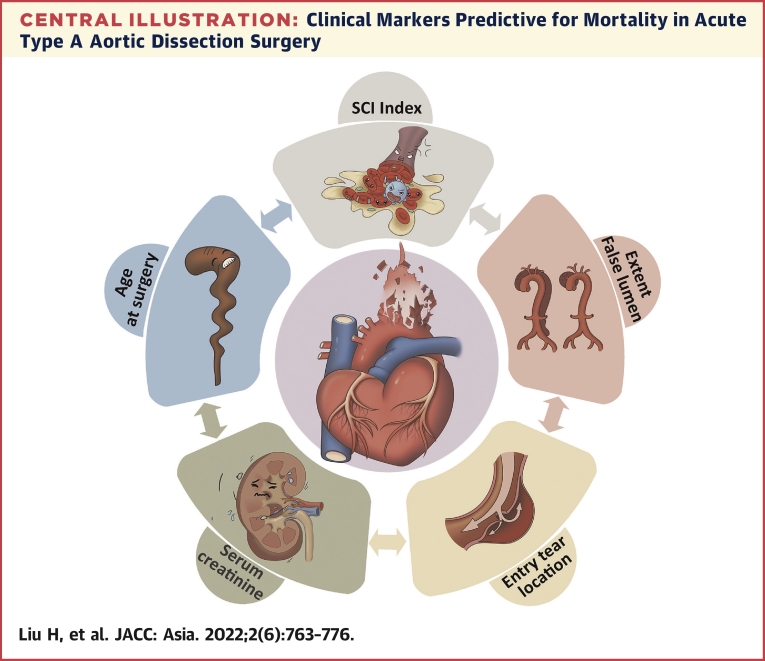
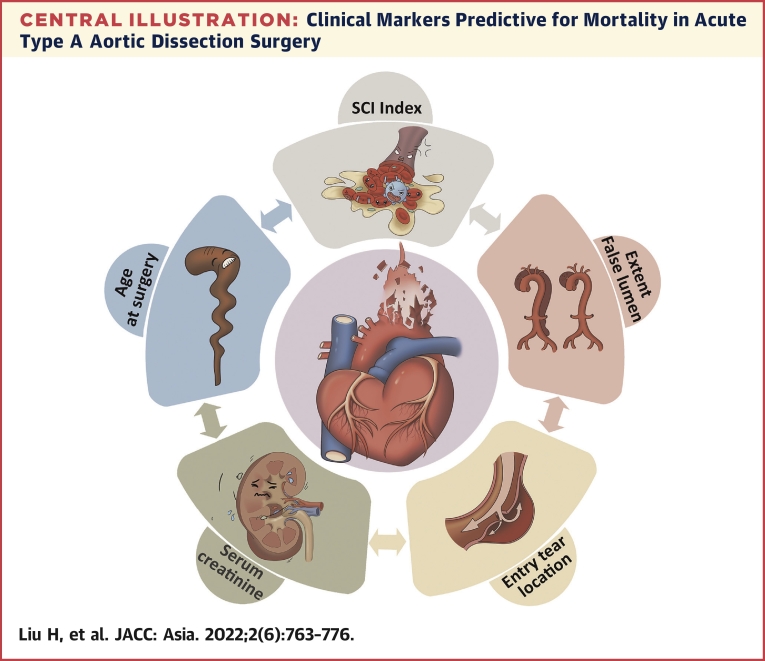
Central Illustration.
Clinical Markers Predictive for Mortality in Acute Type A Aortic Dissection Surgery
We focused on routine preoperative baseline and clinical features to develop a risk score model for predicting 90-day mortality in Chinese patients with acute type A aortic dissection who underwent surgical repair, composed of 1 novel derived hematologic marker (systemic coagulation-inflammation [SCI] index) and conventional model 4 conventional factors (age at surgery, serum creatinine, DeBakey class, and location of intimal entry tear). Our results suggest that this inflammation-based model is useful for early prediction of operative mortality risk in that patient population. SCI index was calculated as peripheral platelet count × fibrinogen/white blood cell count on admission.
Previous studies had found that platelet and fibrinogen were primary determinants of thrombosis and coagulopathy and plays an important role in coagulopathic function.10 Dey et al6 found that SII index could predict major adverse cardiac and cerebrovascular events after elective off-pump coronary artery bypass grafting. Wang et al32 established the risk prediction model including age, D-dimer, red cell distribution width, and platelet distribution width to predict in-hospital death in ATAAD patients. However, the hematologic parameter that can well reflect inflammation and coagulation pathway is still limited in predicting outcome for ATAAD patients. The coexistence of increased inflammation and decreased coagulation function in ATAAD patients has been well known.5,10,11,33 Therefore, we proposed a novel hematologic parameter SCI index that could proportionately integrate inflammation and coagulation and can easily be derived and obtained from clinically routine laboratory findings. To the best of our knowledge, this is the first study to investigate the clinical value of the novel hematologic parameter SCI index in ATAAD patients.
Our findings have shown that this novel hematologic parameter SCI index is superior to both individual and collaborative hematologic parameters in predicting short-term mortality in ATAAD patients. The remarkable advantage of this index is the proposition that the addition of fibrinogen to platelet and leucocyte to compute SCI index bestows a simultaneous evaluation of the fibrinolysis pathway besides the aggregation and inflammation pathway.34 The results suggest that a comprehensive assessment of both inflammatory and coagulopathic characteristic changes is necessary to risk-stratify ATAAD patients. The underlying mechanisms of the relationship between inflammation, coagulation, fibrinolysis, and prognosis require further research.
At present, some selected retrograde ATAAD patients could have a better prognosis after being treated with medical management or endovascular coverage of the primary entry tear.35,36 However, these retrograde patients included in our study were those who failed in medical management along with important cardiovascular risk factors including total aortic diameter >50 mm, hematoma thickness >11 mm, pericardial effusion, or aortic regurgitation, and those whose anatomy was not favorable for thoracic endovascular aortic repair on cross-sectional imaging without an adequate proximal landing zone for the stent graft.37 Therefore, our results must be interpreted in the context of dissection characteristics of patients included.
The composite contribution of the corpuscular lines in the occurrence, development, and progression process of aortic dissection highlights a collaborative hematologic biomarker SCI index. Despite the confirmatory relationship between leukocytes and the underlying inflammatory responses, a better understanding of dissection-mediated thrombocytopenia and fibrinogenopenia was advanced and emphasized as a result of systemic fibrinolytic hyperactivity, regional thrombosis, and peripheral thrombocyte redistribution.3,10,11,38 Furthermore, platelets and fibrinogens are intriguing regarding the diversity of pathophysiological roles beyond hemostasis-thrombosis-fibrinolysis to inflammation mediators, compelling the multiple interactions between inflammation, immune, and coagulation pathways.39 Finally, we did not find a significant effect of DHCA on outcomes, although it is plausible that both hypothermia and circulatory arrest may still play important roles in determining patient outcomes.
In addition to 90-day mortality, the SCI index also showed adequate discrimination with respect to 30-day mortality, hospital mortality, as well as ICU stay, ventilation support time, re-exploration for bleeding, and stroke. Thus, our model has the potential to evaluate the risk of perioperative mortality and morbidity more objectively than conventional models, even in emergency clinical settings, due to the clinical accessibility and availability of the hematological parameters used for derivation of SCI index.
Study limitations
This study is subject to the limitations of its retrospective, observational design, in which treatment was left to the discretion of the attending physicians and this could have somehow influenced the patients’ outcome. Because the present study was observational in nature, unknown confounders may have affected the results, even after adjustments were made. And because data were not available for the identification of cardiovascular-specific mortality, future studies with more specific survival analysis such as heart failure death and sudden cardiac death are needed. Although blood samples were collected upon admission, the interval between symptom onset and the first blood examination could not be accurately evaluated. Important operative details that might influence patient outcome (such as cannulation sites, cross clamp vs open distal anastomosis or core temperature) were not recorded. Finally, the inflammation-based prediction model will need external validation in case series from other countries before it can be adopted outside China.
Conclusions
SCI index is a novel hematologic marker available from routine laboratory examinations and is associated with systemic inflammation and coagulation disorders reflected by leukocytosis, thrombocytopenia, and fibrinogenopenia in ATAAD patients. There was a significantly negative dose-response relationship between the SCI index and 90-day mortality in ATAAD patients, highlighting that anti-inflammatory therapy should be individualized to patients according SCI index. Furthermore, the addition of SCI index will significantly improve mortality prediction into the conventional risk mode composed of age, serum creatinine, DeBakey class, and location of primary entry tear, indicating the clinical application of SCI measurement in risk assessment of short-term mortality in ATAAD patients.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: We propose a novel hematologic parameter SCIindex (calculated as platelet count × fibrinogen level/white blood cell count). This SCI adding model might be helpful for early prediction of the risk of 90-mortality in patients with acute type A aortic dissection patients who undergo surgical repair. Furthermore, its overall accuracy is superior to that of the conventional predictors-based risk score model.
TRANSLATIONAL OUTLOOK: Prospective clinical trials are needed to validate the predictive accuracy of our inflammatory model.
Funding Support and Author Disclosures
This work was supported by the National Natural Science Foundation of China (82000305, 82070483), Scientific Research Common Program of Beijing Municipal Commission of Education (KM202110025014), and Beijing Municipal Science and Technology Commission (Z211100002921010). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgement
The authors thank Dr Bu-qing Ni for constructive comments and suggestions in manuscript revision. The data that support the findings of this study are available on request to the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and figures as well as a listing of the 5A investigators, please see the online version of this paper.
Contributor Information
Yong-feng Shao, Email: yfshaojph@sina.com.
Hai-yang Li, Email: ocean0203@163.com.
Appendix
References
- 1.Erbel R., Aboyans V., Boileau C., et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the European Society of Cardiology (ESC) Eur Heart J. 2014;35(41):2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 2.Bossone E., Eagle K.A. Epidemiology and management of aortic disease: aortic aneurysms and acute aortic syndromes. Nat Rev Cardiol. 2021;18(5):331–348. doi: 10.1038/s41569-020-00472-6. [DOI] [PubMed] [Google Scholar]
- 3.Morello F., Cavalot G., Giachino F., et al. White blood cell and platelet count as adjuncts to standard clinical evaluation for risk assessment in patients at low probability of acute aortic syndrome. Eur Heart J Acute Cardiovasc Care. 2017;6(5):389–395. doi: 10.1177/2048872615600097. [DOI] [PubMed] [Google Scholar]
- 4.Zindovic I., Sjögren J., Bjursten H., et al. The coagulopathy of acute type A aortic dissection: a prospective, observational study. J Cardiothorac Vasc Anesth. 2019;33(10):2746–2754. doi: 10.1053/j.jvca.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Benedetto U., Dimagli A., Kaura A., et al. Determinants of outcomes following surgery for type A acute aortic dissection: the UK National Adult Cardiac Surgical Audit. Eur Heart J. 2021 doi: 10.1093/eurheartj/ehab586. ehab586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dey S., Kashav R., Kohli J.K., et al. Systemic immune-inflammation index predicts poor outcome after elective off-pump CABG: a retrospective, single-center study. J Cardiothorac Vasc Anesth. 2021;35(8):2397–2404. doi: 10.1053/j.jvca.2020.09.092. [DOI] [PubMed] [Google Scholar]
- 7.Agus H.Z., Kahraman S., Arslan C., et al. Systemic immune-inflammation index predicts mortality in infective endocarditis. J Saudi Heart Assoc. 2020;32(1):57–64. doi: 10.37616/2212-5043.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allaire E., Schneider F., Saucy F., et al. New insight in aetiopathogenesis of aortic diseases. Eur J Vasc Endovasc Surg. 2009;37(5):531–537. doi: 10.1016/j.ejvs.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Sbarouni E., Georgiadou P., Kosmas E., et al. Platelet to lymphocyte ratio in acute aortic dissection. J Clin Lab Anal. 2018;32(7) doi: 10.1002/jcla.22447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan X.L., Wang X.L., Liu Y.Y., et al. Changes in the hemostatic system of patients with acute aortic dissection undergoing aortic arch surgery. Ann Thorac Surg. 2016;101(3):945–951. doi: 10.1016/j.athoracsur.2015.08.047. [DOI] [PubMed] [Google Scholar]
- 11.Ma M., Shi J., Feng X., et al. The elevated admission white blood cell count relates to adverse surgical outcome of acute Stanford type A aortic dissection. J Cardiothorac Surg. 2020;15(1):48. doi: 10.1186/s13019-020-1078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun L., Qi R., Zhu J., et al. Total arch replacement combined with stented elephant trunk implantation: a new "standard" therapy for type a dissection involving repair of the aortic arch? Circulation. 2011;123(9):971–978. doi: 10.1161/CIRCULATIONAHA.110.015081. [DOI] [PubMed] [Google Scholar]
- 13.Overman D.M., Jacobs J.P., Prager R.L., et al. Report from the Society of Thoracic Surgeons National Database Workforce: clarifying the definition of operative mortality. World J Pediatr Congenit Heart Surg. 2013;4(1):10–12. doi: 10.1177/2150135112461924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White I.R., Royston P., Wood A.M. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 15.Liu H., Zheng S.Q., Li X.Y., et al. Derivation and validation of a nomogram to predict in-hospital complications in children with tetralogy of Fallot repaired at an older age. J Am Heart Assoc. 2019;8(21) doi: 10.1161/JAHA.119.013388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herndon J.E., 2nd, Harrell F.E., Jr. The restricted cubic spline as baseline hazard in the proportional hazards model with step function time-dependent covariables. Stat Med. 1995;14(19):2119–2129. doi: 10.1002/sim.4780141906. [DOI] [PubMed] [Google Scholar]
- 17.Piepho H.P., Ogutu J.O. Inference for the break point in segmented regression with application to longitudinal data. Biometrical J. 2003;45:591–601. [Google Scholar]
- 18.Park S.Y., Freedman N.D., Haiman C.A., et al. Association of coffee consumption with total and cause-specific mortality among nonwhite populations. Ann Intern Med. 2017;167(4):228–235. doi: 10.7326/M16-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nashef S.A., Roques F., Michel P., Gauducheau E., Lemeshow S., Salamon R. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999;16(1):9–13. doi: 10.1016/s1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 20.Roques F., Michel P., Goldstone A.R., Nashef S.A. The logistic EuroSCORE. Eur Heart J. 2003;24(9):881–882. doi: 10.1016/s0195-668x(02)00799-6. [DOI] [PubMed] [Google Scholar]
- 21.Parsonnet V., Dean D., Bernstein A.D. (1989) A method of uniform stratification of risk for evaluating the results of surgery in acquired adult heart disease. Circulation. 1989;79:3–12. [PubMed] [Google Scholar]
- 22.Higgins T.L., Estafanous F.G., Loop F.D., Beck G.J., Blum J.M., Paranandi L. Stratification of morbidity and mortality outcome by preoperative risk factors in coronary artery bypass patients. A clinical severity score. JAMA. 1992;267(17):2344–2348. [PubMed] [Google Scholar]
- 23.Tu J.V., Jaglal S.B., Naylor C.D. Multicenter validation of a risk index for mortality, intensive care unit stay, and overall hospital length of stay after cardiac surgery. Steering Committee of the Provincial Adult Cardiac Care Network of Ontario. Circulation. 1995;91(3):677–684. doi: 10.1161/01.cir.91.3.677. [DOI] [PubMed] [Google Scholar]
- 24.Zheng Z., Zhang L., Li X., Hu S., et al. SinoSCORE: a logistically derived additive prediction model for post-coronary artery bypass grafting in-hospital mortality in a Chinese population. Front Med. 2013;7(4):477–485. doi: 10.1007/s11684-013-0284-0. [DOI] [PubMed] [Google Scholar]
- 25.Czerny M., Siepe M., Beyersdorf F., et al. Prediction of mortality rate in acute type A dissection: the German Registry for acute type A aortic dissection score. Eur J Cardiothorac Surg. 2020;58(4):700–706. doi: 10.1093/ejcts/ezaa156. [DOI] [PubMed] [Google Scholar]
- 26.Beyene K.M., El Ghouch A. Smoothed time-dependent receiver operating characteristic curve for right censored survival data. Stat Med. 2020;39(24):3373–3396. doi: 10.1002/sim.8671. [DOI] [PubMed] [Google Scholar]
- 27.Samad M.D., Ulloa A., Wehner G.J., et al. Predicting survival from large echocardiography and electronic health record datasets: optimization with machine learning. J Am Coll Cardiol Img. 2019;12(4):681–689. doi: 10.1016/j.jcmg.2018.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alba A.C., Agoritsas T., Walsh M., et al. Discrimination and calibration of clinical prediction models: users' guides to the medical literature. JAMA. 2017;318(14):1377–1384. doi: 10.1001/jama.2017.12126. [DOI] [PubMed] [Google Scholar]
- 29.Chambless L.E., Diao G. Estimation of time-dependent area under the ROC curve for long-term risk prediction. Stat Med. 2006;25(20):3474–3486. doi: 10.1002/sim.2299. [DOI] [PubMed] [Google Scholar]
- 30.Vickers A.J., Elkin E.B. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26(6):565–574. doi: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 32.Wang M., Luo L., Xia X., et al. A simple model predicting in-hospital death in patients with type A acute aortic dissection. Perfusion. Published online July 5, 2021 doi: 10.1177/02676591211029762. [DOI] [PubMed] [Google Scholar]
- 33.Cifani N., Proietta M., Tritapepe L., et al. Stanford-A acute aortic dissection, inflammation, and metalloproteinases: a review. Ann Med. 2015;47(6):441–446. doi: 10.3109/07853890.2015.1073346. [DOI] [PubMed] [Google Scholar]
- 34.Zhou C., Li Y., Yan Y., et al. Changes in coagulation and fibrinolysis systems during the perioperative period of acute type A aortic dissection. Heart Surg Forum. 2021;24(2):E223–E230. doi: 10.1532/hsf.3503. [DOI] [PubMed] [Google Scholar]
- 35.Omura A., Matsuda H., Matsuo J., et al. Thoracic endovascular repair for retrograde acute type A aortic dissection as an alternative choice. Gen Thorac Cardiovasc Surg. 2020;68(12):1397–1404. doi: 10.1007/s11748-020-01397-0. [DOI] [PubMed] [Google Scholar]
- 36.Shu C., Wang T., Li Q.M., et al. Thoracic endovascular aortic repair for retrograde type A aortic dissection with an entry tear in the descendingaorta. J Vasc Interv Radiol. 2012;23(4):453–460. doi: 10.1016/j.jvir.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 37.Malaisrie S.C., Szeto W.Y., Halas M., et al. 2021 The American Association for Thoracic Surgery expert consensus document: surgical treatment of acute type A aortic dissection. J Thorac Cardiovasc Surg. 2021;162(3):735–758. doi: 10.1016/j.jtcvs.2021.04.053. [DOI] [PubMed] [Google Scholar]
- 38.Sayed A., Munir M., Bahbah E.I. Aortic dissection: a review of the pathophysiology, management and prospective advances. Curr Cardiol Rev. 2021;17(4) doi: 10.2174/1573403X16666201014142930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wortmann M., Peters A.S., Erhart P., et al. Inflammasomes in the pathophysiology of aortic disease. Cells. 2021;10(9):2433. doi: 10.3390/cells10092433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.