Abstract
Elevated concentration of lipoprotein(a) [Lp(a)] is an independent risk factor for atherosclerotic cardiovascular disease, including coronary artery disease, stroke, peripheral artery disease, and so on. Emerging data suggest that Lp(a) contributes to the increased risk for cardiovascular events even in the setting of effective reduction of plasma low-density lipoprotein cholesterol. Nevertheless, puzzling issues exist covering potential genetic factors, Lp(a) assay, possible individuals for analysis, a cutoff point of increased risk, and clinical interventions. In the Chinese population, Lp(a) exhibited a distinctive prevalence and regulated various cardiovascular diseases in specific ways. Hence, it is valuable to clarify the role of Lp(a) in cardiovascular diseases and explore prevention and control measures for the increase in Lp(a) prevalence in the Chinese population. This Beijing Heart Society experts' scientific statement will present the detailed knowledge concerning Lp(a)-related studies combined with Chinese population observations to provide the key points of reference.
Key Words: atherosclerotic cardiovascular disease, calcific aortic value stenosis, lipoprotein(a), scientific statement
Abbreviations and Acronyms: AMI, acute myocardial infarction; Apo, apolipoprotein; ASCVD, atherosclerotic cardiovascular disease; CAD, coronary artery disease; CAVS, calcific aortic valve stenosis; CVD, cardiovascular disease; CVE, cardiovascular event; FH, familial hypercholesterolemia; GWAS, genome-wide association analysis; KIV, Kringle IV; LA, lipoprotein apheresis; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein(a); MACE, major adverse cardiovascular events; OxPL, oxidized phospholipids; PCSK9, proprotein convertase subtilisin/kexin type 9; SNP, single nucleotide polymorphism; T2DM, type 2 diabetes mellitus
Central Illustration
Atherosclerotic cardiovascular disease (ASCVD) is the most common cardiovascular disease (CVD) worldwide and has become a leading cause of death.1 Dyslipidemia is considered the core pathogenesis of ASCVD and deemed as a reversible risk factor. It has been generally recognized that low-density lipoprotein cholesterol (LDL-C) is a critical blood lipid indicator and the primary target for blood lipid intervention in ASCVD. However, randomized controlled and real-world studies have shown that a residual risk of cardiovascular events (CVEs) still exists even when LDL-C levels are controlled within an optimal range specified by current guidelines.2 It has been found that some novel blood lipid indicators are still associated with residual risk. Much evidence has indicated that lipoprotein(a) [Lp(a)] is a potential target for blood lipids intervention, which has attracted eyes.
In fact, Lp(a) was first discovered and named about 60 years ago.3 Later, clinical observational studies with a small sample size reported that a 3.5-fold higher Lp(a) level might increase the risk of coronary artery disease (CAD) and general CVEs, particularly in those with LDL-C ≥130 mg/dL.4 It was not until the Mendelian Randomization Study in 2009 that Lp(a) was associated with an increased risk of myocardial infarction (MI).5 Since then, the significance of Lp(a) has evoked growing attention. Recently, various studies have been conducted on Lp(a) from different perspectives or using different approaches, including pathophysiology,6 epidemiology,7 Mendelian randomization,5 whole-genome analysis,8 post hoc analysis of randomized controlled clinical trials,9 in specific populations such as those with familial hypercholesterolemia (FH),10 or through meta-analyses.11 The findings consistently indicate that Lp(a) is probably a pathological risk factor of ASCVD, independent of LDL-C, attracting scholars' eyes to Lp(a).
Current evidence indicates that higher Lp(a) levels predict a higher risk of CVE. This prediction holds true in either the primary or secondary prevention populations already taking statins or proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors.11 However, we are still uncertain or even confused about many aspects of Lp(a). Several recent guidelines published in various countries and regions have proposed recommendations concerning Lp(a) cutoff, target populations for Lp(a) testing, and intervention strategies, although they are different.
Compared with other populations, the epidemiology of Lp(a) in the Chinese population shows a unique differentiation. Dong et al12 found a genetic variation in Lp(a) at single nucleotide polymorphisms (SNPs) rs6415084, rs3798221, and rs7770628, which may cause the differentiation of high Lp(a) plasma levels among the Han Chinese population. A cross-sectional study of 3,462 case and 6,125 control subjects also suggested that the Lp(a) distribution in the Chinese Han population differs from that in Caucasian populations, and high Lp(a) levels can be modified by some risk factors in the Chinese Han population.13 Therefore, elevated Lp(a) levels may be affected by many distinct factors, and the epidemiology of Lp(a) is different in the Chinese population. However, current guidelines on the prevention and treatment of CVD in China are deficient in systematic recommendations and guidance based on the properties and significance of Lp(a). Moreover, the underlying mechanisms of elevated Lp(a) in the Chinese population are still uncertain.
Given the facts in the previous text, we aimed to provide a scientific statement on the relationship between Lp(a) and CVD risk and management in this research. A modified Delphi method was applied to develop scientific statement. A Central Illustration of Lp(a) in Chinese population was designed. A comprehensive literature review was performed regarding Lp(a) in Chinese population using PubMed databases. We screened as many people as possible from a Chinese population for a comprehensive and overall understanding. We used the search terms: “cardiovascular diseases,” “ischemic stroke,” “calcific aortic valve diseases,” “lipid metabolism,” “lipoprotein(a),” or “Lp(a).” The statements, along with literature, were then presented to a panel of 24 cardiovascular specialists. They are key experts in the field of Lp(a) and CVD with years of clinical experience and deep understanding of the mechanisms and treatment who are from different provinces in China. The expert panel anonymously rated the statements on a 1 to 10 scale (1 = strongly disagree and 10 = strongly agree). An expert scientific statement development meeting was held virtually to review, discuss, refine, and reformulate statements that did not meet the criteria for agreement or were ambiguous. During the meeting, additional statements were proposed. Panelists then confidentially revoted, and statements rated ≥6 by 80% or more of the participants were accepted. Our expert panel summarized the current knowledge about Lp(a) and reviewed the research data on the Chinese population. Lp(a) was systematically reviewed, and we have presented some key points (Table 1) concerning the clinical management of Lp(a) in the Chinese population to guide clinical practice.
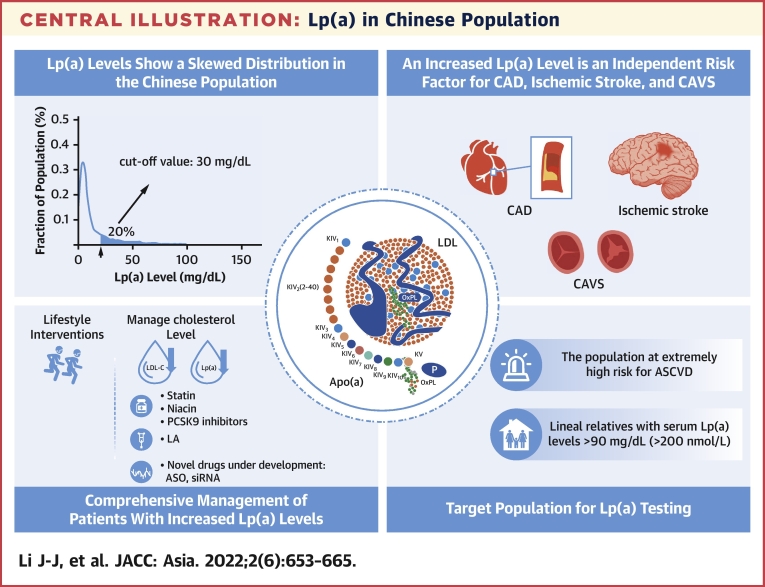
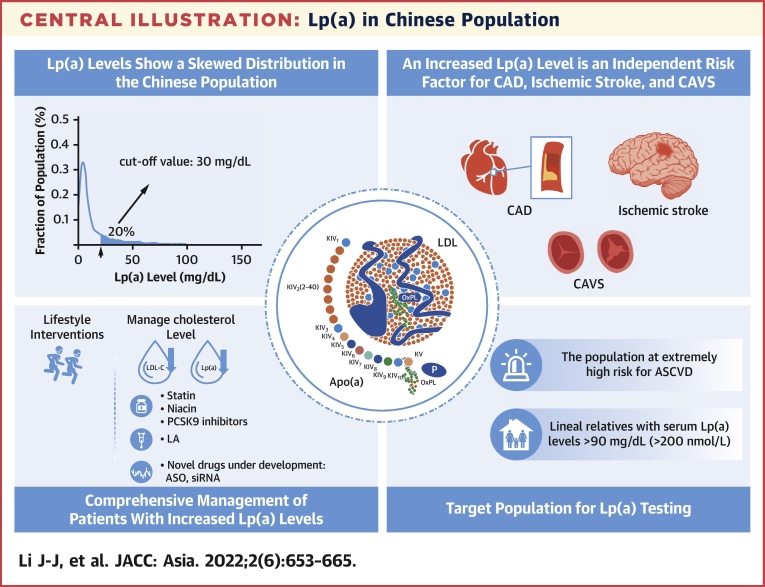
Central Illustration.
Lp(a) in Chinese Population
A summary of lipoprotein(a) [Lp(a)] in Chinese population and the key recommendations of the statements of this review, including Lp(a) level distribution, independent risk factors, comprehensive management, and Lp(a) testing. ASCVD = atherosclerotic cardiovascular disease; ASO = Antisense Oligomer; CAD = coronary artery disease; CAVS = calcific aortic valve stenosis; LA = lipoprotein apheresis; LDL-C = low-density lipoprotein cholesterol; PCSK9 = proprotein convertase subtilisin/kexin type 9; siRNA = small interfering RNA.
Table 1.
A Summary of 7 Expert Opinions
| Expert Opinion No. | Recommendation |
|---|---|
| 1 | Lp(a) levels show a skewed distribution, and geographic and racial variations have been observed. Generally speaking, Lp(a) levels are lower in the Chinese population than in populations in other countries and regions. |
| 2 | Although serum Lp(a) levels are primarily determined by genetic factors, they may also be related to some nongenetic factors. |
| 3 | Increased Lp(a) levels are an independent risk factor for CAD, ischemic stroke, and CAVS. |
| 4 | Lp(a) testing is recommended for the following populations: 1) populations at extremely high risk for ASCVD; 2) individuals with a family history of early-onset ASCVD (men <55 y of age, women <65 y of age); 3) individuals whose lineal relatives have Lp(a) levels ≥90 mg/dL (200 nmol/L); 4) FH or other types of hereditary dyslipidemia; and 5) CAVS patients. |
| 5 | Guidelines and consensuses in different countries recommend different Lp(a) cutoff values for the increased cardiovascular risk. But, a cutoff value of 50 mg/dL is most common. Based on the existing data from the Chinese population, we also favor the Lp(a) cutoff value of 30 mg/dL for an increased cardiovascular risk. |
| 6 | Lp(a) laboratory test: 1) a monoclonal antibody insensitive to apo(a) isomerism and not cross-reacting with plasminogen should be used; 2) a calibrator material traceable to WHO/IFCC SRM-2B should be used; 3) the Lp(a) can be reported in values of either mass concentration or molar concentration (molar concentration is preferred). However, no fixed value is used as a conversion factor between the mass concentration and the molar concentration. |
| 7 | There are 2 major management principles for increased Lp(a) levels: the first is to lower the overall ASCVD risk, and the second is to control all associated types of dyslipidemia. |
Apo = apolipoprotein; ASCVD = atherosclerotic cardiovascular disease; CAD = coronary artery disease; CAVS = calcific aortic valve stenosis; FH = familial hypercholesterolemia; Lp(a) = lipoprotein(a); WHO = World Health Organization.
Structural and Epidemiological Features of Lp(a)
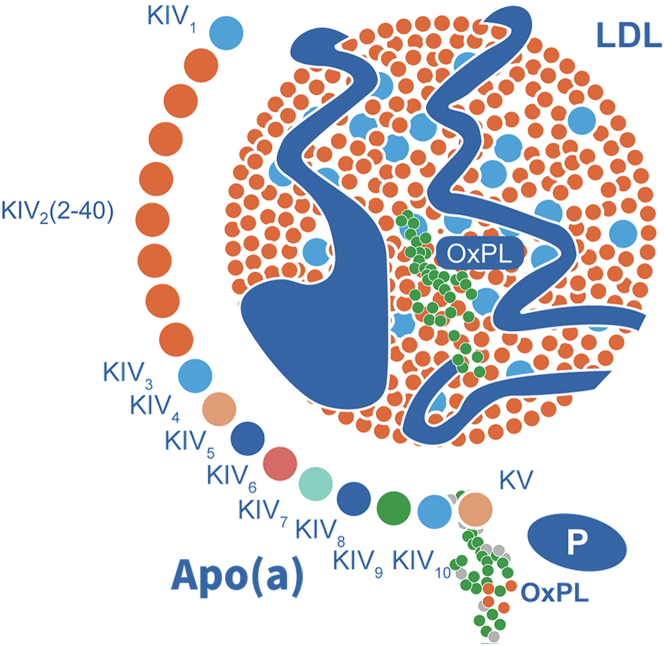
Lp(a) is an LDL-like particle with a single apolipoprotein (apo) B100, which is covalently linked by a disulfide bond to a single apo(a).14 Under an electron microscope, Lp(a) is a spherical particle with a diameter of about 21 nm and a density of about 1.05 to 1.10 g/mL. The structural diagram of Lp(a) is shown in Figure 115: the LDL-like particle contains roughly 30% to 46% cholesterol, apoB100, and oxidized phospholipids (OxPL). Apo(a) is a highly glycated hydrophilic protein, accounting for approximately 25% to 40% of Lp(a). Lp(a) is known for its noticeable polymorphism, which is derived from varying lengths of apo(a) peptide. Apo(a) contains 10 homologous plasminogen Kringle IV (KIV) domains. The similar Kringle in the second domain of KIV has 2 to 40 copies, whereas there is only 1 copy for other domains, leading to large individual variations in the molecular weight of Lp(a) and plasma Lp(a) levels. In addition, the polymorphism of apo(a) also depends on its degree of glycation.
Figure 1.
Schematic Diagram of the Lp(a) Composition
Lp(a) comprises apolipoprotein (apo) (a) covalently attached to the apoB-100 component of a low-density lipoprotein (LDL)–like moiety by a single disulfide bond. The organization of the 10 types of Kringle IV (KIV) in apo(a) are shown, with KIV 2 being present in different numbers of copies in different apo(a) isoforms. Also depicted is the presence of Kringle V (KV) and the protease-like domain (P) in apo(a). The LDL-like moiety comprises a single molecule of apoB-100, an outer amphipathic shell of phospholipids and free cholesterol, and a neutral lipid core of cholesteryl esters, and triglycerides. OxPL = oxidized phospholipids.
Unlike LDL, Lp(a) cannot be converted from very-low-density lipoprotein. Neither can it be turned into other lipoproteins. In a word, Lp(a) is exclusively synthesized in the liver. However, the site for Lp(a) assembly is not fully identified. The assembly may be in the liver cells, space of Disse, or blood circulation. The steps of assembly include apo(a) docking to LDL and then the formation of a covalent disulfide bond between KIV-9 of apo(a) and apoB of LDL. However, it is still unclear whether this process is reversible. Moreover, little is known about the clearance pathway and mechanism of Lp(a). So far, it is believed that Lp(a) is cleared primarily by LDL receptors in the liver and partially by kidneys and other pathways.14 Studies have shown that scavenger receptor B type 1, plasminogen receptor, low-density lipoprotein receptor-related protein 1, and other receptors are also involved in the Lp(a) clearance in the liver.
Unlike LDL-C levels obeying a normal distribution in the population, Lp(a) levels are usually featured by a skewed distribution. In most individuals (about 70%), Lp(a) levels are <30 mg/dL. One large-scale epidemiological survey in the United States recruited 531,144 outpatients. The Lp(a) data were all collected from the CVD risk screening by general practitioners among the general outpatients. The results showed a skewed distribution of Lp(a) levels, with an average of 34.0 ± 40.0 mg/dL and a median of 17 mg/dL (quartile: 7-47 mg/dL). Lp(a) levels ≥30 and ≥50 mg/dL were found in 35% and 24% of outpatients, respectively.16 Similarly, Lp(a) levels also show a skewed distribution in the Chinese population, with a relatively lower 80th percentile. Among 9,238 healthy people receiving routine physical check-ups in Jiangsu Province, the median Lp(a) levels were 5.6 mg/dL. The 80th percentile was 20.7 mg/dL among women and 14.5 mg/dL among men.17
Apo(a) polymorphism largely explains racial and geographic differences in plasma Lp(a) levels. One study compared Lp(a) levels among Chinese, Caucasians, and African Americans, and their results showed that median Lp(a) levels were 8.0, 9.0, and 33 mg/dL, respectively. It was also found that plasma Lp(a) levels of the Chinese and Caucasians were slightly different (P = 0.06). However, the difference was much more noticeable when compared with African Americans (P < 0.001).18 As for the geographical variation of populations with increased Lp(a) levels throughout the world, a working group under the National Heart, Lung, and Blood Institute pointed out in 201819 that over 1.4 billion people had Lp(a) levels above 50 mg/dL. About 30% of such people live in Africa; about 20% in Europe, North America, and Oceania; and about 15% in South America. Except for South Asia, where Lp(a) levels above 50 mg/dL were found in about 25% of the population, Lp(a) levels above 50 mg/dL were found in about 10% of populations in other parts of Asia. These results indicated racial variation of Lp(a) levels.
Expert opinion 1
Lp(a) levels show a skewed distribution, and geographic and racial variations have been observed. Generally speaking, Lp(a) levels are lower in the Chinese population than in populations in other countries and regions.
Genomics Research and Factors Related to Increased Lp(a) Levels
The genomics research has verified the correlation between Lp(a) and CAD and ischemic stroke. A Mendelian randomization study suggests that Lp(a) is related to CAD.5 Data from the PROCARDIS (Precocious Coronary Artery Disease) cohort and the genome-wide association analysis (GWAS) showed that 2 LPA SNPs (SNP rs10455872 and rs3798220) were associated with an increased risk of Lp(a) elevation and CAD.8 One GWAS recruited 1,403 CAD patients receiving percutaneous coronary intervention. The results showed that LPA polymorphisms rs7770628, rs73596816, and rs6926458 and SLC22A2 polymorphism rs144217738 were independently correlated with Lp(a) levels. Among them, LPA polymorphisms rs7770628 and rs73596816 were significantly correlated with the severity of CAD.20 Another GWAS recruited 3 cohorts of European Caucasians, including 6,942 patients with aortic valve calcification and 3,795 patients with mitral annular calcification. Genetic testing showed that LPA SNP (rs10455872) was associated with aortic valve calcification (for each allele, OR: 2.05; P = 9 × 10−10), which was also verified in other cohorts.21
Although plasma Lp(a) levels mainly depend on LPA polymorphisms (about 70%-90%), it is already recognized that some nongenetic factors may also be related to Lp(a) levels. In those with chronic renal diseases, serum Lp(a) levels increase as the glomerular filtration rate decreases, which is presumed to be related to reduced catabolism of large isomers. Besides, serum Lp(a) levels decrease in most patients with liver diseases but increase in those with overt hypothyroidism. Although serum Lp(a) levels are unrelated to age or sex, hormone replacement therapy in postmenopausal women significantly reduces serum Lp(a) levels.14 Therefore, identifying secondary factors that possibly increase Lp(a) levels may also be clinically significant.
Expert opinion 2
Although serum Lp(a) levels are primarily determined by genetic factors, they may also be related to some nongenetic factors.
Possible Pathogenic Mechanisms of Lp(a)
The pathophysiological function of Lp(a) is not fully understood yet. Considering its biological structure, Lp(a) may induce ASCVD more easily than LDL-C. First, Lp(a) can easily penetrate the arterial lining and bind to the extracellular matrix components, thereby promoting macrophage infiltration and smooth muscle cell proliferation.22 Lp(a) is enriched in plaques throughout the progression of ASCVD. Second, Lp(a) contains a unique protein, apo(a), which is critical for the atherogenic effect.23 Because Apo(a) has a similar structure as plasminogen, Lp(a) is speculated to be involved in the early repair of wounds. The extracellular matrix is exposed to the blood upon tissue damage. In that case, Lp(a) may be recruited to the wounds to promote the inflow of proinflammatory cells (eg, monocytes) beneath the vascular endothelium, where it exerts a prothrombotic effect by inhibiting fibrinolysis.15 Finally, among all lipoproteins, Lp(a) is the major carrier of OxPL. OxPL shows important proinflammatory and atherogenic properties, inducing the inflammatory response of the arterial wall by activating the proinflammatory signaling in endothelial cells, smooth muscle cells, and macrophages.24 Therefore, compared with LDL-C, Lp(a) can also facilitate the occurrence and development of ASCVD caused by its prothrombotic and proinflammatory properties. Recent studies have indicated that Lp(a) is involved in the occurrence and development of valvular and vascular calcification. However, the working mechanism has not been elucidated yet.
Correlation Between Lp(a) Levels and CVD
Genomics, epidemiological, and Mendelian randomization studies have shown that increased Lp(a) levels are an independent risk factor for various CVD, including CAD, ischemic stroke, and calcific aortic valve stenosis (CAVS). Increased Lp(a) levels are also a risk factor predicting CVD in patients with FH and type 2 diabetes mellitus (T2DM).
Coronary artery disease
Several observational studies and genomics studies have demonstrated that increased Lp(a) levels are an independent risk factor for CAD. The AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes) trial indicated that the risk of major adverse cardiovascular events (MACE) increased by 90% in patients with LDL-C levels reaching 65.2 mg/dL (1.62 mmol/L) and Lp(a) ≥50 mg/dL compared with those with similar LDL-C levels but lower Lp(a) levels15 One meta-analysis included 7 randomized controlled trials with 29,069 patients who received statin therapy, and their results showed that increased Lp(a) levels still increased the risk of CVD even when the LDL-C levels were reduced after the statin therapy.11 The Copenhagen City Heart Study analyzed the data from 7,524 subjects who were followed up for 16 years and reported that increased Lp(a) levels were significantly positively correlated with a higher risk of CVD.11 A large-scale observational study in a Chinese population showed that Lp(a) was an independent risk factor for CAD. A retrospective analysis based on the case collection and scientific research system for clinical cardiology database included 1,522 patients with the first episode of acute myocardial infarction (AMI) and 1,691 control subjects without CAD, and their findings revealed that compared with the control group (LDL-C <2.6 mmol/L, the first quintile in term of Lp(a) levels), the OR values for the first episode of AMI in those with Lp(a) levels in the second, third, fourth, and fifth quintiles were 1.51, 1.84, 1.86, and 2.66, respectively.25
Ischemic stroke
Whether in observational studies or genetic analyses, Lp(a) is found to be related to a higher risk of ischemic stroke. The Copenhagen General Population Study and the Copenhagen City Heart Study included more than 60,000 patients with ischemic stroke, and the results showed that higher Lp(a) levels were related to a higher risk of ischemic stroke. Compared with individuals with Lp(a) levels <10 mg/dL, the risk ratio of ischemic stroke corrected by multiple variables was 1.60 in those with Lp(a) levels ≥93 mg/dL. In 1 observational study, the risk ratio of ischemic stroke after correction for age and sex was 1.20 in those with Lp(a) levels ≥50 mg/dL.26 In another study, a cohort included 10,375 randomly selected citizens aged above 40 years from Jiading District, Shanghai. Among them, 8,500 subjects were included in the final analysis, and it was found that serum Lp(a) levels were correlated with the risk of ischemic stroke (P < 0.05). A multivariate correction was performed, and it was revealed that compared with the low-Lp(a) group, the HR of ischemic stroke was 1.34 in the high-Lp(a) group.27 In a Mendelian randomization study, the data from over 400,000 people indicated that Lp(a) levels were positively correlated with ischemic stroke induced by aortic occlusion and negatively correlated with ischemic stroke induced by small vessel occlusion.28
Calcific aortic valve stenosis
Lp(a) is also a risk factor for CAVS.29 The ASTRONOMER (Aortic Stenosis Progression Observation: Measuring Effects of Rosuvastatin) study measured Lp(a) levels in 220 patients with mild to moderate aortic stenosis who were followed up for 3.5 years on average. The results showed that increased Lp(a) levels were related to the progression of CAVS.30 Another study selected Lp(a) 58.5 mg/dL as the cutoff value, and it was reported that in patients with increased Lp(a) levels, aortic stenosis progressed faster, suggesting that Lp(a) might predict the progression from mild to moderate CAVS.29 Liu et al31 detected serum Lp(a) levels in 652 Chinese CAVS patients who were followed up for an average of 3.16 ± 2.74 years. The clinical endpoint was defined as the endpoint of aortic valve replacement and cardiac death. The results showed that compared with patients in the first and second quartiles in terms of Lp(a) levels, the proportion of patients with severe atherosclerosis was higher in those with the third quartile (46.2% vs 33.9%; P = 0.005). In addition, the highest quartile in terms of Lp(a) levels was an independent predictor of severe atherosclerosis (OR: 1.78; 95% CI: 1.18-2.66; P = 0.006). However, the third quartile in terms of Lp(a) levels was not significantly correlated with clinical events.31
It is noteworthy that elevated Lp(a) levels are also a risk factor for CVD in patients with FH and T2DM. The SAFEHEART (Spanish Familial Hypercholesterolemia Cohort Study) Registry recruited 2,404 FH patients without prior episodes of ASCVD, and the median follow-up period was 5.5 years. It was revealed that Lp(a) levels were an independent predictor of ASCVD in FH patients.10 Another Chinese study recruited a consecutive series of 393 HeFH patients who were divided into 3 groups based on baseline Lp(a) levels (<19.7, 19.8-51.9, and ≥52.0 mg/dL). The average follow-up period was 36.5 months. It was found that higher Lp(a) levels were associated with a significant reduction in the event-free survival rate (P = 0.004). For each unit increase in Lp(a) levels after the logarithmic transformation, the risk of CVEs doubled.32 Furthermore, increased Lp(a) levels were significantly correlated with an increase in the ASCVD events in patients with prediabetes (pre-DM) and DM.33,34 A research team at Fuwai Hospital recruited a consecutive series of 2,284 T2DM patients with prior CVE from April 2011 to March 2017, who were followed up for an average of 3 years. The results showed that when CVD was combined with T2DM, the cardiovascular risks associated with Lp(a) might further increase.35 There are also occasional reports indicating that increased Lp(a) levels are associated with arterial calcification and venous thrombotic disease, but more evidence is needed to confirm this finding.
Expert opinion 3
Increased Lp(a) levels are an independent risk factor for CAD, ischemic stroke, and CAVS.
Target Population for Lp(a) Testing
Plasma Lp(a) levels in individuals are primarily determined by genetic factors and, therefore, remain relatively stable throughout life. 1) The scientific statement presented herein recommends Lp(a) testing at least once for the general population, which is consistent with the 2018 European Guidelines for the Management of Dyslipidemia36: Measurement of Lp(a) should be considered at least once in each person's lifetime, if available, to identify people who have inherited extremely increased levels of Lp(a) ≥180 mg/dL (≥430 nmol/L) and, therefore, have a very high lifetime risk of ASCVD that is approximately equivalent to the risk associated with HeFH. 2) Based on evidence from the previously mentioned guidelines, “the relationship between Lp(a) levels and CVD,” the scientific statement presented herein recommends Lp(a) testing in the following target populations:
-
1.
The population at extremely high risk for ASCVD (2019 European Society of Cardiology/European Atherosclerosis Society guidelines for the definition). The PRIME (Prospective Epidemiological Study of Myocardial Infarction) study showed that Lp(a) was a predictor of coronary heart disease.37 Moreover, Lp(a) levels are independently correlated with the risk of recurrent CVE; Lp(a) measurement is helpful for timely intervention so as to reduce the recurrence of CVEs.38
-
2.
Family history of early-onset ASCVD (men <55 years of age, women <65 years of age). The risk of CVD is increased for individuals with a family history of early-onset ASCVD.39 These individuals are also associated with a higher atherosclerosis burden.40 Increased Lp(a) levels can further aggravate such risk, and Lp(a) testing is thus necessary for those with a family history of early-onset ASCVD.
-
3.
Lineal relatives with serum Lp(a) levels ≥90 mg/dL (≥200 nmol/L). Inheritance of the Lp(a) molecule is dominant. Lp(a) testing may be useful for lineal relatives of patients with a severely increased Lp(a) level (≥90 mg/dL or ≥200 nmol/L).
-
4.
FH and other types of hereditary dyslipidemia. Lp(a) levels are one of the independent predictors of ASCVD in FH patients,10 which is an important precipitating factor for ASCVD and also an indicator during the cascade screening for FH. Previous studies have shown that Lp(a) measurement is beneficial for FH patients.
-
5.
CAVS patients. Increased Lp(a) levels are a potential risk factor for CAVS,29 and earlier interventions may be required for those with increased Lp(a) levels. Lp(a) testing may guide treatment and prolong follow-up intervals.
Expert opinion 4
Lp(a) testing is recommended for the following populations: 1) populations at extremely high risk for ASCVD; 2) individuals with a family history of early-onset ASCVD (men <55 years of age, women <65 years of age); 3) individuals whose lineal relatives have Lp(a) levels ≥90 mg/dL (200 nmol/L); 4) FH or other types of hereditary dyslipidemia; and 5) CAVS patients.
Lp(a) Cutoff Values
A large number of studies have pointed out the correlation between increased Lp(a) levels and CAD, ischemic stroke, and CAVS, which is associated with an increased MACE risk. Unfortunately, there has been no consensus on the Lp(a) cutoff values for higher risk of CVD. In fact, data from studies with a large sample size are required to determine Lp(a) cutoff values. As mentioned in the previous text, there are racial differences in Lp(a) levels. Therefore, the Lp(a) cutoff values vary from one race to another. Based on the existing studies and meta-analyses, the expert panel suggests that the Lp(a) cutoff value for the risk of atherosclerosis is about 30 to 50 mg/dL (or 75-125 nmol/L). However, recommendations from guidelines and consensus in different countries vary in the Lp(a) cutoff for the risk of CVD. According to the 2016 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in Adults,41 Lp(a) ≥30 mg/dL is considered a risk factor for CVD. The guidelines recommend Lp(a) measurement for treatment decisions, especially among young moderate-risk populations who have a family history of early-onset CAD but do not yet meet the high-risk criteria. The 2016 Chinese Guidelines for the Prevention and Treatment of Dyslipidemia in Adults42 recommend that after excluding other potential factors induced by irritability, increased Lp(a) levels are considered an independent risk factor for ASCVD. The Lp(a) cutoff value is set to 30 mg/dL, above which the CAD risk increases significantly. The European Society of Cardiology guidelines for the management of dyslipidemia and the National Lipid Association (NLA) recommendations have both proposed that in clinical practice,36,43 Lp(a) levels ≥50 mg/dL (about 100-125 nmol/L) are associated with a significantly higher risk for ASCVD. After treatment and management of LDL-C, Lp(a) testing is recommended for those with a moderate to high risk for CVD to achieve the target of LDL <50 mg/dL (125 nmol/L). Several studies on Lp(a) cutoff values in Chinese populations are summarized in Table 2.17,27,38,44,45 Most seem to suggest that Lp(a) ≥30 mg/dL is an independent predictor of CAD and ischemic stroke. Based on the existing data on the Chinese population, we also favor the Lp(a) cutoff value of 30 mg/dL.
Table 2.
A Summary of Several Studies on Lp(a) Cutoff Values in Chinese Populations
| Population | Sample Size | Primary Endpoint | High Lp(a) vs Low Lp(a) | Main Conclusions | Lp(a) Cutoff |
|---|---|---|---|---|---|
| Healthy population receiving routine physical checkup16 | 9,238 | Myocardial infarction | Lp(a) <16.7 mg/dL vs Lp(a) ≥16.7 mg/dL | High Lp(a) is significantly correlated with myocardial infarction | 17 mg/dL |
| Patients with a history of CAD37 | 7,562 | Recurrent CVE | The first tertile of Lp(a) levels (<8.88 mg/dL) vs the third tertile (≥26.45 mg/dL) | High Lp(a) is independently correlated with the risk of recurrent CVE | 26.45 mg/dL |
| Stable CAD patients with a history of PCI43 | 4,078 | CVE | Lp(a) <15 mg/dL vs Lp(a) ≥30 mg/dL | High Lp(a) is correlated with CVE | 30 mg/dL |
| Patients with a history of MI45 | 3,864 | CVE | The first quartile of Lp(a) levels (<8.19 mg/dL) vs the third quartile (18.84-41.43 mg/dL) | The cumulative rates of CVEs and cardiac mortality were significantly higher in patients with high Lp(a) levels | 18.84 mg/dL |
| Population aged >40 y and receiving routine physical check-up26 | 8,500 | Stroke | Lp(a) <26 mg/dL vs Lp(a) ≥26 mg/dL | High Lp(a) is significantly correlated with stroke | 26 mg/dL |
| Patients with ischemic stroke (ischemic and hemorrhagic)38 | 2,149 | Stroke | The first quartile of Lp(a) levels (<4.6 mg/dL) vs the fourth quartile (≥23.2 mg/dL) | High Lp(a) is positively correlated with ischemic and hemorrhagic ischemic stroke | 23.2 mg/L |
CVE = cardiovascular event; MI = myocardial infarction; PCI = percutaneous coronary intervention; other abbreviations as in Table 1.
Expert opinion 5
Guidelines and consensuses in different countries recommend different Lp(a) cutoff values for the increased cardiovascular risk. But a cutoff value of 50 mg/dL is most common. Based on the existing data on the Chinese population, we also favor the Lp(a) cutoff value of 30 mg/dL for an increased cardiovascular risk.
Lp(a) Lab Test
Many studies have shown that Lp(a) testing facilitates the diagnosis and classification of patients with a risk of atherosclerosis and thrombosis caused by increased Lp(a) levels. The cardiovascular risk conferred by Lp(a) may be graded depending on the lipoprotein(a) particle concentration. HEART UK (Hyperlipidemia Education and Atherosclerosis Research Trust UK) has employed data from the ongoing Copenhagen General Population Study5 to grade this risk based on percentile distributions as follows: 32 to 90 nmol/L, minor; 90 to 200 nmol/L, moderate; 200 to 400 nmol/L, high; and ≥400 nmol/L, very high. For the Chinese population, further studies are required to derive ethnicity-specific ranges appropriate to the Chinese population because Lp(a) concentration varies by race/ethnicity.
As plasma concentrations of Lp(a) are predominantly genetically determined, they are relatively stable over a lifetime. Therefore, Lp(a) may only need to be measured once unless a secondary cause is suspected or specific treatment is instituted to lower its plasma concentration. Increased Lp(a) levels are an independent risk factor for ASCVD. However, there is still a lack of standardized Lp(a) laboratory test procedures. Therefore, the correlation between Lp(a) and CVD can hardly be evaluated accurately. Standardization of Lp(a) laboratory tests has become a crucial topic in clinical practice. At present, the Northwest Lipid Research Laboratories at the University of Washington has used the monoclonal antibodies against apo(a) KIV-9 epitope a-40 and KIV-8 epitope a1-1 for the Sandwich enzyme-linked immunosorbent assay procedure. This procedure is accepted as the gold standard for detection and is independent of the heterogeneity of apo(a). In 2003, IFCC SRM 2B was accepted by the World Health Organization (WHO) as the secondary standard material for Lp(a) for immunoassay (WHO/IFCC SRM-2B). This standard material, traceable to the primary standard material, has an Lp(a) content of 107.1 ± 8.6 nmol/L. At present, several guidelines proposed by different countries have recommended that the detection methods used in clinical laboratories should be traceable to WHO/IFCC SRM-2B.14,43 It is noteworthy that no fixed value is used as a conversion factor between the mass concentration method (mg/dL) and the molar concentration method (nmol/L). So far, the Lp(a) level is still reported in the unit of mg/dL in clinical practice and studies. Before Lp(a) can be uniformly reported in mmol/L, both 2 units are acceptable (the molecular concentration method traceable to SRM-2B is more favored).
Expert opinion 6
Lp(a) laboratory test: 1) A monoclonal antibody insensitive to apo(a) isomerism and not cross-reacting with plasminogen should be used; 2) a calibrator material traceable to WHO/IFCC SRM-2B should be used; and 3) the Lp(a) can be reported in values of either mass concentration or molar concentration (molar concentration is preferred). However, no fixed value is used as a conversion factor between the mass concentration and the molar concentration.
Comprehensive Management of Patients With Increased Lp(a) Levels
Lp(a)-lowering therapies should be more broadly applied in clinical care settings in patients with or without ASCVD in the future.46 Effective methods to lower Lp(a) levels are still deficient, and neither are there any specific drugs approved for this purpose. For those with increased Lp(a) levels, there are 2 major management principles: the first is to lower the overall ASCVD risk, and the second is to control all associated types of dyslipidemia.
-
1.
Lifestyle interventions: Lp(a) levels cannot be directly lowered by dietary and sports interventions. However, taking active measures to control other reversible cardiovascular risk factors is critical for lowering the overall cardiovascular risk for patients with increased Lp(a) levels. In the EPIC-Norfolk Study, compared with the participants with the lowest cardiovascular health scores, those with increased Lp(a) levels (≥50 mg/dL) and the highest cardiovascular health scores (including body mass index, healthy diet, physical activities, smoking status, blood pressure, DM, and cholesterol levels) had a dramatically reduced CVD risk (corrected HR: 0.33; 95% CI: 0.17-0.63; P = 0.001). These results indicated that a healthy lifestyle is critical for those with increased Lp(a) levels.47 For those with increased Lp(a) levels but at low risk for ASCVD, lifestyle intervention should be encouraged. For those with a moderate or severe risk for ASCVD, LDL-C–lowering therapy is recommended to be prescribed in addition to lifestyle intervention.
-
2.
Statin therapy: Lowering LDL-C levels can also help reduce the CVD risk induced by increased Lp(a) levels.48,49 Recommendations from the 2018 ACC/AHA cholesterol guideline50: For the primary prevention, populations at moderate risk for ASCVD (10-year ASCVD risk 7.5%-19.9%), Lp(a) ≥50 mg/dL or ≥100 nmol/L is a reasonable risk factor, and moderate- or high-intensity statin therapy may be considered. It is noteworthy that even with LDL-C kept at a low level, the MACE risk in patients with increased Lp(a) levels is still significantly higher compared with those with low Lp(a) levels,11,51 indicating that the risk associated with high Lp(a) levels cannot be completely eliminated by lipid-lowering drugs (eg, statins) or keeping normal LDL-C levels. Therefore, some patients with increased Lp(a) levels may benefit from more active LDL-C-lowering therapy (eg, statins). Some recent studies have shown that statin therapy can mildly increase Lp(a) levels. However, whether such an increase will further increase the CVE risk remains unclear. Given the facts in the previous text, our expert panel recommends statin therapy for ASCVD patients with Lp(a) ≥30 mg/dL until LDL-C levels are below 55 mg/dL.
-
3.
Niacin: Niacin can reduce Lp(a) levels by about 23%.52 However, the AIM-HIGH and HPS2-THRIVE (Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events) studies indicate that the use of niacin based on statin therapy did not reduce the CVE risk in patients at high risk for ASCVD, including those with increased Lp(a) levels. Instead, the risk of severe adverse events was increased.53,54 It remains unknown whether it is attributed to an insignificant increase of Lp(a) levels in the participants in the AIM-HIGH and HPS2-THRIVE studies. Therefore, the expert panel suggests further investigation on the clinical value of niacin in reducing Lp(a) levels. Therefore, the expert panel suggests that the clinical significance of niacin reducing LP (a) needs to be further discussed.
-
4.
PCSK9 inhibitors: Existing data have indicated that the monoclonal antibodies against PCSK9 and the small-interference RNA targeting the PCSK9 messenger RNA can reduce Lp(a) levels by about 20% to 30%.55, 56, 57 The FOURIER (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk) and ODYSSEY (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) studies indicated that subjects with increased Lp(a) levels seemed to have a greater reduction in the CVE risk.58, 59, 60 However, since these patients usually have a higher absolute risk, whether the benefits are entirely attributed to lower Lp(a) levels and the degree needs further investigation. It should be pointed out that according to the Mendelian randomization study, a large absolute reduction in Lp(a) levels (65.7-100 mg/dL) was required for a clinically significant decrease in cardiovascular risk.61,62 Therefore, the expert panel recommends that for ASCVD patients with Lp(a) ≥30 mg/dL, the combination of statins and ezetimibe at the moderate intensity and maximal doses is considered for keeping normal LDL-C levels, including additional use of PCSK9 inhibitors. But, based on pharmacoeconomics and existing evidence, we do not recommend using PCSK9 inhibitors to lower Lp(a) as the primary goal in any population.
-
5.
Lipoprotein apheresis (LA): Lp(a) levels can be reduced by 50% to 70% immediately after LA, and the average reduction within 1 week after LA is about 30% to 35%.63 The HEART UK guidelines recommend LA for those with progressive CAD deterioration after the maximum-intensity lipid-lowering therapy, Lp(a) ≥60 mg/dL, and LDL-C still above 125 mg/dL (3.3 mmol/L).64 Since 2008, about 1,500 patients with Lp(a) levels above 60 mg/dL have received LA in Germany.65 Two previous studies have indicated that regular LA can dramatically lower the CVD risk. Compared with before LA, the CVE risk was reduced by about 80% on average after LA.66,67 A recent randomized controlled study showed that for refractory angina pectoris patients with Lp(a) ≥60 mg/dL, LA could dramatically improve myocardial perfusion, reduce atherosclerosis burden and lower the incidence of angina pectoris.68 Considering the limitations of LA (complex procedures, potential complications, low cost-effectiveness ratio, and very small number of domestic centers qualified for LA), the expert panel does not recommend LA as a routine treatment for increased Lp(a) levels. According to foreign experience and recommendations, LA may be considered if Lp(a) ≥60 mg/dL with progressive aggravation of atherosclerosis despite sufficient LDL-C–lowering therapy and controlling for other risk factors.
-
6.
Novel drugs under development: RNA-targeting therapy displays high selectivity and affinity to target mRNAs, which has been successfully applied to many diseases.69 RNA-targeting therapy is also the intervention most likely to be approved for lowering Lp(a). Antisense oligonucleotides targeting the liver LPA RNA can silence the apo(a) gene expression, thereby blocking the apo(a) synthesis and thus reducing Lp(a) levels in the circulation. In phase I and II clinical trials that have already been completed, antisense oligonucleotides lowered Lp(a) levels by over 80% and displayed favorable safety and tolerance profiles.70,71 At present, a global phase III clinical trial evaluating the effects of antisense oligonucleotides on cardiovascular outcomes of patients with increased Lp(a) levels is currently underway (HORIZON [Assessing the Impact of Lipoprotein (a) Lowering With Pelacarsen (TQJ230) on Major Cardiovascular Events in Patients With CVD], NCT04023552). This study is the first global study focusing on the cardiovascular endpoints in patients with increased Lp(a) levels. Moreover, the small-interference RNA therapy targeting LPA RNA is still in the early development stage (NCT03626662). We are eager to know whether the RNA-targeting therapy for Lp(a) reduction can actually reduce the CVE risk.
-
7.
Other drugs: According to the reports, cholesteryl ester transfer protein inhibitor Mipomersen can reduce plasma Lp(a) levels by about 20% to 30%.72,73 However, there is a shortage of evidence supporting its cardiovascular benefits.
Expert opinion 7
There are 2 major management principles for increased Lp(a) levels: the first is to lower the overall ASCVD risk, and the second is to control all associated types of dyslipidemia.
Future Directions of Research on Lp(a) in Chinese Populations
Although some knowledge has been gained about Lp(a) in recent years, more remains to be explored in the future, such as the synthetic and metabolic pathways and the pathophysiological features of Lp(a). These aspects of Lp(a) will be the topics of interest in the future. Given the racial variation of Lp(a) levels, epidemiological studies with higher levels, larger sample sizes, and better representative populations are required to identify the Lp(a) level distribution in the Chinese population and Lp(a) cutoff values. How to improve the accuracy and promote the standardization of Lp(a) testing in the Chinese population will be prioritized by researchers in the future. It is urgent to detect the effect of interventions that can specifically lower Lp(a) levels through randomized controlled trials, such as antisense oligonucleotides, on the prognosis of CVD patients with increased Lp(a) levels. Clinical intervention trials may be considered for primary and secondary prevention populations and those with specific diseases, such as FH and CAVS.
Finally, the authors of the scientific statement herein declare no conflicting interests concerning this paper. The authors share one common goal, which is to educate Chinese health care workers and the general public about the current knowledge of Lp(a) and to promote Chinese studies in this field.
Conclusions
Previous studies have reported that an elevated Lp(a) concentration is an independent risk factor for CVD. This review summarizes recent research and focuses on novel evolutionary insights of Lp(a)-related studies in the Chinese population. Compared with other populations, Lp(a) showed specific characteristics in the Chinese population. These distinct risk factors contribute to disparities in the genetic landscape, molecular subtypes, and clinical phenotypes of high Lp(a) cases between China and Western countries. Many studies have shown that the Lp(a) cutoff value of the Asian population is lower than that of Caucasian and Black populations. This scientific recommendation cites large Chinese population studies to further confirm lower Lp(a) cutoff values and recommends an Lp(a) cutoff value of 30 mg/dL instead of 50 mg/dL. Repeated Lp(a) testing is not recommended, although it is recommended to test at least once in a lifetime. The 5 groups recommended by this committee are also based on the data of the Chinese population. Although presently there is no global standardization of Lp(a) measurement, the preferred measurement unit is nmol/L; however, mg/dL is dominantly used in the clinical setting in China, so it is recommended that both units can be used. The principle of management of Lp(a) elevation is a comprehensive treatment to reduce overall ASCVD risk. Although lifestyle interventions and statins do not reduce Lp(a) levels, they are beneficial in lowering ASCVD risk. In patients with elevated Lp(a) and insufficient LDL-C lowering, it is reasonable to add ezetimibe and, in selected cases, PCSK9 inhibitors, whereas niacin and LA therapy should be avoided. What is more, we also provided the future direction of Lp(a) research in the Chinese population. This scientific statement would be helpful in understanding the role and function of Lp(a) in CVD in Chinese population and improve health and outcomes of elevated Lp(a) patients.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Michael Shapiro, MD, served as Guest Associate Editor for this paper. Nathan Wong, PhD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a list of the members of the Expert Committee (sorted by contribution), please see the online version of this paper.
Contributor Information
Jian-Jun Li, Email: lijianjun938@126.com.
Chang-Sheng Ma, Email: chshma@vip.sina.com.
Beijing Heart Society and Expert Committee:
Jianjun Li, Changsheng Ma, Dong Zhao, Xiaowei Yan, Ping Ye, Hong Chen, Yong Li, Zuyi Yuan, Ruiyan Zhang, Shuiping Zhao, Guoping Lu, Chun Liang, Yugang Dong, Zhenyue Chen, Daoquan Peng, Yida Tang, Fang Wang, Zhou Zhou, Yihong Sun, Jing Liu, Yuanlin Guo, Hui Yuan, Naqiong Wu, and Ye Zhu
Supplemental Appendix
References
- 1.Libby P., Buring J.E., Badimon L., et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56. doi: 10.1038/s41572-019-0106-z. [DOI] [PubMed] [Google Scholar]
- 2.Sabatine M.S., Giugliano R.P., Keech A.C., et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 3.Berg K. A new serum type system in man—the Lp system. Acta Pathol Microbiol Scand. 1963;59:369–382. doi: 10.1111/j.1699-0463.1963.tb01808.x. [DOI] [PubMed] [Google Scholar]
- 4.Bucci M., Tana C., Giamberardino M.A., Cipollone F. Lp(a) and cardiovascular risk: Investigating the hidden side of the moon. Nutr Metab Cardiovasc Dis. 2016;26:980–986. doi: 10.1016/j.numecd.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Kamstrup P.R., Tybjaerg-Hansen A., Steffensen R., Nordestgaard B.G. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–2339. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 6.Anuurad E., Enkhmaa B., Berglund L. Enigmatic role of lipoprotein(a) in cardiovascular disease. Clin Transl Sci. 2010;3:327–332. doi: 10.1111/j.1752-8062.2010.00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kral B.G., Kalyani R.R., Yanek L.R., et al. Relation of plasma lipoprotein(a) to subclinical coronary plaque volumes, three-vessel and left main coronary disease, and severe coronary stenoses in apparently healthy African-Americans with a family history of early-onset coronary artery disease. Am J Cardiol. 2016;118:656–661. doi: 10.1016/j.amjcard.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke R., Peden J.F., Hopewell J.C., et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 9.Nordestgaard B.G., Chapman M.J., Ray K., et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–2853. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pérez de Isla L., Alonso R., Mata N., et al. Predicting cardiovascular events in familial hypercholesterolemia: the SAFEHEART registry (Spanish familial hypercholesterolemia cohort study) Circulation. 2017;135:2133–2144. doi: 10.1161/CIRCULATIONAHA.116.024541. [DOI] [PubMed] [Google Scholar]
- 11.Willeit P., Ridker P.M., Nestel P.J., et al. Baseline and on-statin treatment lipoprotein(a) levels for prediction of cardiovascular events: individual patient-data meta-analysis of statin outcome trials. Lancet. 2018;392:1311–1320. doi: 10.1016/S0140-6736(18)31652-0. [DOI] [PubMed] [Google Scholar]
- 12.Dong H., Cong H., Wang J., et al. Correlations between lipoprotein(a) gene polymorphisms and calcific aortic valve disease and coronary heart disease in Han Chinese. J Int Med Res. 2020;48 doi: 10.1177/0300060520965353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao A.J., Shao R., Wang Y. Progress in search for traditional Chinese medicine treating prostate cancer via androgen receptor signaling regulation. Chin J Clin Pharmacol. 2017;33:1263–1266. [Google Scholar]
- 14.Cegla J., Neely R.D.G., France M., et al. HEART UK consensus statement on Lipoprotein(a): A call to action. Atherosclerosis. 2019;291:62–70. [Google Scholar]
- 15.Tsimikas S. A test in context: Lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69:692–711. doi: 10.1016/j.jacc.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 16.Varvel S., McConnell J.P., Tsimikas S. Prevalence of elevated Lp(a) mass levels and patient thresholds in 532,359 patients in the United States. Arterioscler Thromb Vasc Biol. 2016;36:2239–2245. doi: 10.1161/ATVBAHA.116.308011. [DOI] [PubMed] [Google Scholar]
- 17.Cui F.M., Fang F., He Y.M., Cai D.P., He J., Yang X.J. Establishing age and sex dependent upper reference limits for the plasma lipoprotein (a) in a Chinese health check-up population and according to its relative risk of primary myocardial infarction. Clinica Chimica Acta. 2018;484:232–236. doi: 10.1016/j.cca.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Gaw A., Boerwinkle E., Cohen J.C., Hobbs H.H. Comparative analysis of the apo(a) gene, apo(a) glycoprotein, and plasma concentrations of Lp(a) in 3 ethnic groups. Evidence for no common “null” allele at the apo(a) locus. J Clin Invest. 1994;93:2526–2534. doi: 10.1172/JCI117263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsimikas S., Fazio S., Ferdinand K.C., et al. NHLBI working group recommendations to reduce Lipoprotein(a)-mediated risk of cardiovascular disease and aortic stenosis. J Am Coll Cardiol. 2018;71:177–192. doi: 10.1016/j.jacc.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y.B., Ma H.K., Zhu Q., et al. A genome-wide association study on lipoprotein (a) levels and coronary artery disease severity in a Chinese population. J Lipid Res. 2019;60:1440–1448. doi: 10.1194/jlr.P091009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thanassoulis G., Campbell C.Y., Owens D.S., et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–512. doi: 10.1056/NEJMoa1109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruscica M., Sirtori C.R., Corsini A., Watts G.F., Sahebkar A. Lipoprotein(a): knowns, unknowns and uncertainties. Pharmacol Res. 2021;173 doi: 10.1016/j.phrs.2021.105812. [DOI] [PubMed] [Google Scholar]
- 23.Gencer B., Kronenberg F., Stroes E.S., Mach F. Lipoprotein(a): the revenant. Eur Heart J. 2017;38:1553–1560. doi: 10.1093/eurheartj/ehx033. [DOI] [PubMed] [Google Scholar]
- 24.Boffa M.B., Koschinsky M.L. Oxidized phospholipids as a unifying theory for lipoprotein(a) and cardiovascular disease. Nat Rev Cardiol. 2019;16:305–318. doi: 10.1038/s41569-018-0153-2. [DOI] [PubMed] [Google Scholar]
- 25.Hu Y., Tao J.Y., Cai D.P., He Y.M. Interaction of lipoprotein(a) with low-density lipoprotein cholesterol on first incident acute myocardial infarction. Clin Chim Acta. 2020;501:1–5. doi: 10.1016/j.cca.2019.10.044. [DOI] [PubMed] [Google Scholar]
- 26.Langsted A., Nordestgaard B.G., Kamstrup P.R. Elevated lipoprotein(a) and risk of ischemic stroke. J Am Coll Cardiol. 2019;74:54–66. doi: 10.1016/j.jacc.2019.03.524. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J., Du R., Peng K., et al. Serum lipoprotein (a) is associated with increased risk of stroke in Chinese adults: a prospective study. Atherosclerosis. 2019;289:8–13. doi: 10.1016/j.atherosclerosis.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 28.Pan Y.S., Li H., Wang Y.L., Meng X., Wang Y.J. Causal effect of Lp(a) [Lipoprotein(a)] level on ischemic stroke and alzheimer disease: A mendelian randomization study. Stroke. 2019;50:3532–3539. doi: 10.1161/STROKEAHA.119.026872. [DOI] [PubMed] [Google Scholar]
- 29.Capoulade R., Chan K.L., Yeang C., et al. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J Am Coll Cardiol. 2015;66:1236–1246. doi: 10.1016/j.jacc.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 30.Capoulade R., Yeang C., Chan K.L., Pibarot P., Tsimikas S. Association of mild to moderate aortic valve stenosis progression with higher lipoprotein(a) and oxidized phospholipid levels: Secondary analysis of a randomized clinical trial. JAMA Cardiol. 2018;3:1212–1217. doi: 10.1001/jamacardio.2018.3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu S.L., Rynat R., Shi H.W., et al. Association of serum lipoprotein(a) level with the severity and prognosis of calcific aortic valve stenosis: a Chinese cohort study. J Geriatr Cardiol. 2020;17:133–140. doi: 10.11909/j.issn.1671-5411.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao Y.X., Jin J.L., Guo Y.L., et al. Baseline and on-statin treatment lipoprotein(a) levels for predicting cardiovascular events in patients with familial hypercholesterolemia. Atherosclerosis. 2019;291:27–33. doi: 10.1016/j.atherosclerosis.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Saeed A., Sun W., Agarwala A., et al. Lipoprotein(a) levels and risk of cardiovascular disease events in individuals with diabetes mellitus or prediabetes: The Atherosclerosis Risk In Communities study. Atherosclerosis. 2019;282:52–56. doi: 10.1016/j.atherosclerosis.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waldeyer C., Makarova N., Zeller T., et al. Lipoprotein(a) and the risk of cardiovascular disease in the European population: results from the BiomarCaRE consortium. Eur Heart J. 2017;38:2490–2498. doi: 10.1093/eurheartj/ehx166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y., Jin J.L., Cao Y.X., et al. Lipoprotein (a) predicts recurrent worse outcomes in type 2 diabetes mellitus patients with prior cardiovascular events: a prospective, observational cohort study. Cardiovasc Diabetol. 2020;19:111. doi: 10.1186/s12933-020-01083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mach F., Baigent C., Catapano A.L., et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 37.Luc G., Bard J.M., Arveiler D., et al. Lipoprotein (a) as a predictor of coronary heart disease: the PRIME Study. Atherosclerosis. 2002;163:377–384. doi: 10.1016/s0021-9150(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 38.Liu H.H., Cao Y.X., Jin J.L., et al. Association of lipoprotein(a) levels with recurrent events in patients with coronary artery disease. Heart. 2020;106:1228–1235. doi: 10.1136/heartjnl-2020-316586. [DOI] [PubMed] [Google Scholar]
- 39.Lloyd-Jones D.M., Nam B.H., D'Agostino R.B., Sr., et al. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: a prospective study of parents and offspring. JAMA. 2004;29:2204–2221. doi: 10.1001/jama.291.18.2204. [DOI] [PubMed] [Google Scholar]
- 40.Parikh N.I., Hwang S.J., Larson M.G., et al. Parental occurrence of premature cardiovascular disease predicts increased coronary artery and abdominal aortic calcification in the Framingham Offspring and Third Generation cohorts. Circulation. 2007;116:1473–1481. doi: 10.1161/CIRCULATIONAHA.107.705202. [DOI] [PubMed] [Google Scholar]
- 41.Anderson T.J., Grégoire J., Pearson G.J., et al. 2016 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in the Adult. Can J Cardiol. 2016;32:1263–1282. doi: 10.1016/j.cjca.2016.07.510. [DOI] [PubMed] [Google Scholar]
- 42.Joint Committee for Guideline Revision 2016 Chinese guideline for the management of dyslipidemia in adults. J Geriatr Cardiol. 2018;15:1–29. doi: 10.11909/j.issn.1671-5411.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson D.P., Jacobson T.A., Jones P.H., et al. Use of lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol. 2019;13:374–392. doi: 10.1016/j.jacl.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 44.Liu H.H., Cao Y.X., Jin J.L., et al. Predicting cardiovascular outcomes by baseline lipoprotein(a) concentrations: a large cohort and long-term follow-up study on real-world patients receiving percutaneous coronary intervention. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.014581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu H.H., Zhang D.D., Zhu R., et al. Association between lipoprotein(a) concentration and the risk of stroke in the Chinese Han population: a retrospective case-control study. Ann Transl Med. 2020;8:212. doi: 10.21037/atm.2020.01.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsimikas S. Lipoprotein(a) and coronary calcium: Clinical management and potential design of primary prevention trials. J Am Coll Cardiol. 2022;79:769–771. doi: 10.1016/j.jacc.2021.12.018. [DOI] [PubMed] [Google Scholar]
- 47.Perrot N., Verbeek R., Sandhu M., et al. Ideal cardiovascular health influences cardiovascular disease risk associated with high lipoprotein(a) levels and genotype: the EPIC-Norfolk prospective population study. Atherosclerosis. 2017;256:47–52. doi: 10.1016/j.atherosclerosis.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicholls S.J., Tang W.H., Scoffone H., et al. Lipoprotein(a) levels and long-term cardiovascular risk in the contemporary era of statin therapy. J Lipid Res. 2010;51:3055–3061. doi: 10.1194/jlr.M008961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khera A.V., Everett B.M., Caulfield M.P., et al. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) Circulation. 2014;129:635–642. doi: 10.1161/CIRCULATIONAHA.113.004406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grundy S.M., Stone N.J., Bailey A.L., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/ NLA/PCNA guideline on the management of blood cholesterol: a report of the American college of Cardiology/ American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285–e350. doi: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 51.Albers J.J., Slee A., O'Brien K.D., et al. Relationship of apolipoproteins A-1 and B, and lipoprotein(a) to cardiovascular outcomes: the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride and Impact on Global Health Outcomes) J Am Coll Cardiol. 2013;62:1575–1579. doi: 10.1016/j.jacc.2013.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sahebkar A., Reiner Ž., Simental-Mendía L.E., Ferretti G., Cicero A.F. Effect of extended-release niacin on plasma lipoprotein(a) levels: a systematic review and meta-analysis of randomized placebo-controlled trials. Metabolism. 2016;65:1664–1678. doi: 10.1016/j.metabol.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Boden W.E., Probstfield J.L., Anderson T., et al. for the AIM-HIGH Investigators Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 54.Landray M.J., Haynes R., Hopewell J.C., et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 55.Raal F.J., Giugliano R.P., Sabatine M.S., et al. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J Am Coll Cardiol. 2014;63:1278–1288. doi: 10.1016/j.jacc.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 56.Gaudet D., Watts G.F., Robinson J.G., et al. Effect of alirocumab on lipoprotein(a) over ≥1.5 years (from the phase 3 ODYSSEY program) Am J Cardiol. 2017;119:40–46. doi: 10.1016/j.amjcard.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 57.Ray K.K., Wright R.S., Kallend D., et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382:1507–1519. doi: 10.1056/NEJMoa1912387. [DOI] [PubMed] [Google Scholar]
- 58.O'Donoghue M.L., Fazio S., Giugliano R.P., et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation. 2019;139:1483–1492. doi: 10.1161/CIRCULATIONAHA.118.037184. [DOI] [PubMed] [Google Scholar]
- 59.Ray K.K., Vallejo-Vaz A.J., Ginsberg H.N., et al. Lipoprotein(a) reductions from PCSK9 inhibition and major adverse cardiovascular events: pooled analysis of alirocumab phase 3 trials. Atherosclerosis. 2019;288:194–202. doi: 10.1016/j.atherosclerosis.2019.06.896. [DOI] [PubMed] [Google Scholar]
- 60.Szarek M., Bittner V.A., Aylward P., et al. Lipoprotein(a) lowering by alirocumab reduces the total burden of cardiovascular events independent of low-density lipoprotein cholesterol lowering: ODYSSEY OUTCOMES trial. Eur Heart J. 2020;41:4245–4255. doi: 10.1093/eurheartj/ehaa649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burgess S., Ference B.A., Staley J.R., et al. Association of LP(A) variants with risk of coronary disease and the implications for lipoprotein(a)-lowering therapies: A mendelian randomization analysis. JAMA Cardiol. 2018;3:619–627. doi: 10.1001/jamacardio.2018.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lamina C., Kronenberg F. Estimation of the required lipoprotein(a)-lowering therapeutic effect size for reduction in coronary heart disease outcomes. JAMA Cardiol. 2019;4:575–579. doi: 10.1001/jamacardio.2019.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moriarty P.M., Hemphill L. Lipoprotein apheresis. Endocrinol Metab Clin North Am. 2016;45:39–54. doi: 10.1016/j.ecl.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 64.Thompson G.R. Recommendations for the use of LDL apheresis. Atherosclerosis. 2008;198:247–255. doi: 10.1016/j.atherosclerosis.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 65.Schettler V.J.J., Neumann C.L., Peter C., et al. The German lipoprotein apheresis registry (GLAR) - almost 5 years on. Clin Res Cardiol Suppl. 2017;12:44–49. doi: 10.1007/s11789-017-0089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stefanutti C., Vivenzio A., Di Giacomo S., Mazzarella B., Ferraro P.M., Abbolito S. Treatment of symptomatic HyperLp(a)lipoproteinemia with LDL-apheresis: a multicentre study. Atheroscler Suppl. 2009;10:89–94. doi: 10.1016/S1567-5688(09)71819-7. [DOI] [PubMed] [Google Scholar]
- 67.Jaeger B.R., Richter Y., Nagel D., et al. Longitudinal cohort study on the effectiveness of lipid apheresis treatment to reduce high lipoprotein(a) levels and prevent major adverse coronary events. Nat Clin Pract Cardiovasc Med. 2009;6:229–239. doi: 10.1038/ncpcardio1456. [DOI] [PubMed] [Google Scholar]
- 68.Khan T.Z., Hsu L.Y., Arai A.E., et al. Apheresis as novel treatment for refractory angina with raised lipoprotein(a): a randomized controlled cross-over trial. Eur Heart J. 2017;38:1561–1569. doi: 10.1093/eurheartj/ehx178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu A.M., Jian C., Yu A.H., Tu M.J. RNA therapy: Are we using the right molecules? Pharmacol Ther. 2019;196:91–104. doi: 10.1016/j.pharmthera.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Viney N.J., van Capelleveen J.C., Geary R.S., et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): 2 randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388:2239–2253. doi: 10.1016/S0140-6736(16)31009-1. [DOI] [PubMed] [Google Scholar]
- 71.Tsimikas S., Karwatowska-Prokopczuk E., Gouni-Berthold I., et al. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med. 2020;382:244–255. doi: 10.1056/NEJMoa1905239. [DOI] [PubMed] [Google Scholar]
- 72.Bowman L., Hopewell J.C., Chen F., et al. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377:1217–1227. doi: 10.1056/NEJMoa1706444. [DOI] [PubMed] [Google Scholar]
- 73.Bell D.A., Hooper A.J., Watts G.F., Burnett J.R. Mipomersen and other therapies for the treatment of severe familial hypercholesterolemia. Vasc Health Risk Manag. 2012;8:651–659. doi: 10.2147/VHRM.S28581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.