Abstract
Avian metapneumovirus (aMPV) is an important causative agent that causes acute respiratory disease and egg-dropping in chickens and turkeys. Here, we characterized an aMPV subgroup C (aMPV/C) from 320-day-old broiler breeder chickens with severe respiratory diseases in Beijing, China, as evidenced by RT-PCR typing and confirmation of the nucleoprotein (N) gene sequence. The N gene sequence of the aMPV/C strain (designated BJ17) exhibited no deletions or insertions and possessed 94.6% to 99.6% identity to those of published aMPV/C isolates. The phylogenetic tree of the nucleotide sequences constructed using the neighbor-joining clustering method showed that the BJ17 strain formed one cluster with other aMPV/C viruses and formed one subcluster with published Chinese aMPV/C isolates regardless of Muscovy duck or chicken origins. Comparative analysis of the N proteins showed that a unique amino acid residue D at position 110 might be associated with regional distribution due to its occurrence in all the Chinese aMPV/C isolates only. Strain BJ17 was successfully isolated by cultured Vero cell passage and further inoculated in 3-wk-old specific-pathogen-free chickens for the examination of pathogenicity. Animal experimental results showed that BJ17-inoculated chickens had severe respiratory diseases and inflammatory lesions, as demonstrated by pathological changes and aMPV antigen in the nasal turbinate, tracheae, and lung tissues. These results enrich the available information regarding the epidemiology and pathogenicity of aMPV/C in chickens, which may facilitate the development of effective measures against aMPV/C infection in China.
Key words: avian metapneumovirus subgroup C (aMPV/C), isolation and characterization, respiratory disease, nucleoprotein gene sequencing, pathogenicity
INTRODUCTION
Avian metapneumovirus (aMPV) is an important and widely distributed pathogen in turkeys, chickens, and other avian species such as wild birds (Jones, 1996; Cook, 2000; Cecchinato et al., 2012). Infection with aMPV may cause severe respiratory diseases as well as impaired egg production due to infections by secondary pathogens, such as Escherichia coli (Droual and Woolcock, 1994; Giovanardi et al., 2014), thereby leading to severe economic losses to both turkey and chicken farming in many regions of the world (Mase et al., 2003; Banet-Noach et al., 2005; Roussan et al., 2008; Catelli et al., 2010). aMPV, a member of the genus Metapneumovirus, belongs to the subfamily Pneumovirinae within the family Paramyxoviridae. Its genome is a non-segmented, single-stranded, negative-sense RNA of approximately 13 kb in length, and is ordered as 3-leader-N-P-M-F-M2-SH-G-L-trailer-5′. Four subgroups, namely A, B, C, and D, have been recognized to be dependent on genetic and antigenic properties (Gough and Jones, 2008). Subgroup A (aMPV/A) was first described in turkey flocks in South Africa in 1978 (Buys and Du Preez, 1980), and since then, aMPV/A and subgroup B (aMPV/B) have been reported in Europe (Jones et al., 1991; Hafez et al., 2000; D'Arce et al., 2005; Banet-Noach et al., 2009; Cecchinato et al., 2013a, Cecchinato et al., 2013b; Listorti et al., 2014), Asia (Mase et al., 2003; Banet-Noach et al., 2005; Kwon et al., 2010; Yu et al., 2019), and other parts of the world (Dani et al., 1999; Mahmoud et al., 2008; Chacon et al., 2011; Abdel-Azeem et al., 2014; Rivera-Benitez et al., 2014). Subgroup C aMPV (aMPV/C) was first found in turkeys in the USA (Seal, 1998; Cook et al., 1999) and subsequently isolated from Mallard ducks, Muscovy ducks, pheasants, guinea fowl, ostriches, geese, and some wild birds in other parts of the world (Toquin et al., 1999; Shin et al., 2002; Toquin et al., 2006; Lee et al., 2007; Turpin et al., 2008). Subgroup D (aMPV/D) has been detected in turkeys in France (Bäyon-Auboyer et al., 2000).
In China, since aMPV was first found in 1999 (Shen et al., 1999), a series of serological surveys have shown that aMPV infection has become prevalent in chicken flocks (Guo and Qu, 2009; Zhang et al., 2017a,b). Infection of broiler or breeder chickens with aMPV/A or aMPV/B infection has been detected or isolated in China (Owoade et al., 2008; Yu et al., 2019). In a previous report, we first isolated an aMPV/C (strain JC) from Chinese local 60-day-old meat-type chickens with acute respiratory disease in South East China in 2013 (Wei et al., 2013).
In this study, we identified an aMPV/C strain using a typing RT-PCR method to examine nasal turbinate samples taken from 320-day-old broiler breeder chickens with severe respiratory clinical signs in Beijing, China, in 2017. This aMPV/C strain has been successfully adapted to Vero cell culture. To understand the genetic and pathogenic characteristics of aMPV/C isolated in the present study, we further analyzed the phylogenetic relationships based on nucleoprotein (N) gene sequences and evaluated the pathogenicity of the isolate in 3-wk-old specific-pathogen-free chickens.
MATERIALS AND METHODS
Ethics Statement
The chicken experiment was conducted according to the animal welfare guidelines of the Institutional Animal Care and Use Committee (IACUC) of the Institute of Animal Husbandry and Veterinary Medicine, Beijing Academy of Agriculture and Forestry Sciences.
Case History and Sample Collection
In August 2017, ten nasal turbinate samples were collected from 320-day-old broiler breeder chickens with severe respiratory diseases at a breeder farm in Beijing, China. The morbidity rates were 10% to 30% in different chicken flocks, but no mortality was observed. Collected samples were homogenized in PBS, centrifuged at 3,000 × g for 15 min, and the supernatant was further passed through a 0.45 μM Millipore membrane for extraction of RNA and isolation of virus.
Sequencing of the Nucleoprotein Gene
Total RNA was extracted from the treated samples using the RNeasy Mini Kit (Qiagen, Germantown, MD,) according to the manufacturer's instructions and subjected to typing reverse transcription-polymerase chain reaction (RT-PCR) with aMPV subgroup-specific primers as described (Gharaibeh and Algharaibeh, 2007). The positive samples were further exploited to amplify the whole nucleoprotein (N) gene sequences using the sense primer (5’-CGCATATAAGACAACTTCCAAACA-3’) and antisense primer (5’-CTTTCCCCTCAGGAAAGGAC-3’). The RT-PCR protocol followed the instructions of the TaKaRa PrimeScript one-step RT-PCR Kit Ver.2 (TaKaRa, Dalian, China). After sequencing, the nucleotide (nt) and amino acid (aa) sequences were predicted using DNAStar (Madison, WI). Multiple-sequence alignments were generated using ClustalX 2.0, and nucleotide sequence identities were further obtained using the ClustalW method of DNAStar. All sequence data were assembled using the DNAStar software. Phylogenetic trees were constructed by the neighbor-joining tree method using MEGA 5.1 software, and bootstrap values were calculated on 1,000 replicates of the alignment (Tamura et al., 2011).
Cell Culture and Virus Isolation
Vero cells were cultured in Dulbecco's modified Eagle's medium (Gibco, 11995) supplemented with 100 units/mL penicillin, 100 mg/mL streptomycin, and 5% heat-inactivated fetal bovine serum (FBS; Gibco, 10099-141) at 37°C in an incubator with 5% CO2. Vero cell monolayers in a 25 × 25 cm2 flask were inoculated with 1 mL each supernatant of nasal turbinate tissue homogenates and incubated with a maintenance medium containing 2% FBS at 37°C. The Vero cells were checked for cytopathic effects (CPE) each day.
TCID50 Assay
The amount of aMPV/C produced was assayed using Vero cell monolayers. Briefly, 96-well plates were seeded with Vero cells and infected with 100 μL of serial 10-fold dilutions of the aMPV/C strain BJ17. After 5 days of incubation, plates were fixed with 4% paraformaldehyde in PBS and an indirect fluorescence assay (IFA) was conducted using aMPV/C hyperimmune chicken serum. Titers were assayed by the Reed and Muench method and expressed as 50% tissue culture infective dose per milliliter (TCID50/mL). Virus growth was determined in Vero cell cultures at 0.1 multiplicity of infection (MOI). At the indicated times following BJ17 infection, the culture supernatants were harvested for virus titration as described above.
Indirect Immunofluorescence
Monolayer Vero cells were inoculated with the aMPV/C strain BJ17 for 72 h, and the expression of N protein was detected by IFA under a microscope. In brief, the aMPV/C-infected cells were fixed with 4% paraformaldehyde in PBS followed by incubation with rabbit polyclonal antibody against an aMPV N protein polypeptide (Alvarez et al., 2004) and goat anti-rabbit fluorescein isothiocyanate (FITC)-conjugated antibody (DAKO). The cells were washed with PBS and examined by fluorescence microscopy.
Western Blotting
Western blotting was used to analyze the expression levels of aMPV nucleoprotein from the whole cell lysates. The protein concentrations were assayed. Samples were loaded with 1 × sample loading buffer, boiled for 10 min at 100°C, separated by 12% SDS-PAGE, and then transferred to a PE membrane. The membrane was then washed and blocked for 2 h in TBST blocking buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.1% Tween 20) with 5% nonfat dried milk, reacted with rabbit antibody against an aMPV N protein polypeptide (Alvarez et al., 2004) overnight at 4°C, and diluted in TBST. The membrane was then washed 3 times in TBST and incubated with horseradish peroxidase (HRP)-conjugated secondary antibody for 45 min at room temperature. The membrane was washed and the N protein expression was detected using a SuperSignal West Femto Substrate Trial Kit (Thermo Scientific, Waltham, MA; 34096) and then exposed to a chemiluminescence apparatus (Proteinsample, Santa Clara, CA).
Chicken Experiment
Thirty 3-wk-old White Leghorn chickens, purchased from Beijing Boehringer Ingelheim Vital Biotechnology Co., Ltd, Beijing, China, were assigned randomly to 2 groups. Chickens from group 1 received 200 μL of Vero cell supernatant as a sham-infected group (n = 15). Birds in group 2 (n = 15) were intranasally infected with the aMPV/C strain BJ17 at a dose of 104.5 TCID50 inocula. Oropharyngeal swabs were harvested at 3, 5, and 7 d postinoculation (dpi), and viral replication was determined using quantitative RT-PCR. All nasal turbinate, tracheae, and lung tissue samples were collected from all birds 7 d after inoculation for histopathological observation and immunohistochemical staining.
Histopathology and Immunohistochemical (IHC) Staining
Turbinate, tracheae, and lung tissue samples of the BJ17-inoculated chickens were harvested for histopathological examination and detection of aMPV viral antigens by IHC staining as described previously (Wei et al., 2013).
Quantitative RT-PCR (qRT-PCR)
The viral RNA levels in the nasal turbinate, tracheae, and lung tissues were assessed by qRT-PCR at the indicated times following aMPV/C inoculation. Total RNA was extracted from the nasal turbinate, tracheae, and lung tissues of the inoculated- or sham-inoculated chickens using the RNeasy Mini Kit (Qiagen) for qRT-PCR. The sense primer (5’-AGATGTAGGGACAACCACTGCT-3’) and antisense primer (5’-GTCAATACTGCCTGCACTTCAC-3’) designed were used to amplify a 204-bp nucleotide region of the N gene. The qRT-PCR protocol was performed following the instructions of the iTaq Universal SYBR Green One-Step Kit (Bio-Rad, Hercules, CA). The qRT-PCR parameters comprised cDNA synthesis at 50°C for 10 min and inactivation of reverse transcriptase at 95°C for 5 min, followed by 40 cycles of denaturation at 95°C for 10 s and annealing at 55°C for 30 s. Serial dilutions of a plasmid pCMV-N (N gene cloned into pCMV-HA) were utilized to determine the aMPV genomic copy number.
Statistics
Statistical analyses were performed using Student's t-test, and the results are shown as means ± standard deviations or errors of the means as indicated.
RESULTS
Isolation and Characterization of aMPV/C Strain BJ17
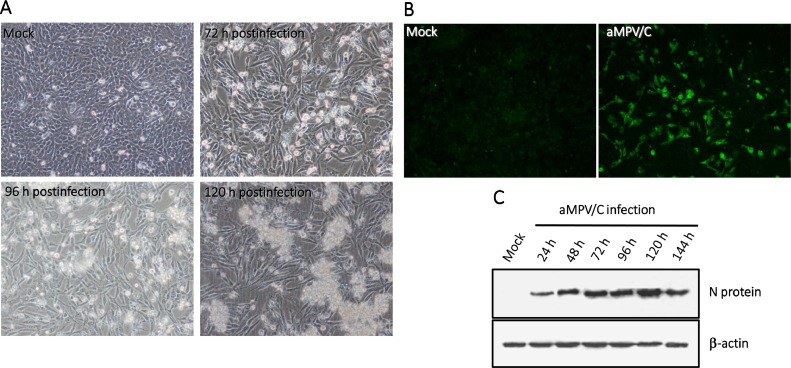
Total RNA in the turbinate tissues of chickens with clinical respiratory disease was extracted and subjected to typing RT-PCR with aMPV subgroup-specific primers as previously described (Gharaibeh and Algharaibeh, 2007). The typing RT-PCR results indicated that all 10 turbinate samples corresponded to aMPV/C but not aMPV/A, aMPV/B, or aMPV/D. Additionally, no infections of other related viruses (influenza virus H5N1, H7N9, and H9N2, Newcastle disease virus, infectious bronchitis virus, and infectious laryngotracheitis virus) were confirmed by PCR or RT-PCR. The turbinate homogenate supernatants were inoculated into cultured Vero cells for 10 blind passages. As shown in Figure 1A, the turbinate homogenate-inoculated Vero cells showed severe cytopathic effects (CPEs) at 120 h postinoculation from three passages onward, as compared to that in the noninfected cells. The isolate was designated as strain BJ17. We examined aMPV/C replication in Vero cells using IFA under a microscope. N protein expression (green) was predominantly distributed in the cytoplasm of aMPV/C-infected cells at 72 h after infection (Figure 1B), but not in the sham-infected cells (Figure 1B). We also determined aMPV/C replication in Vero cells by detecting the level of N protein expression by Western blotting after infection, with the expression peak of the N protein being at 96 h after infection (Figure 1C). Taken together, these data confirmed that aMPV/C might be a major etiological agent in these chickens with severe respiratory signs.
Figure 1.
Infection of aMPV/C strain BJ17 in cultured Vero cells. (A) Cytopathic effects were observed in the Vero cells after strain BJ17 infection. (B) Vero cells at 72 h following aMPV/C infection were incubated with a rabbit antibody raised against aMPV N protein polypeptide followed by incubation with a FITC-conjugated anti-rabbit IgG antibody. Vero cells expressing N protein stained in green. (C) aMPV/C-infected cell lysates at various time points after infection were collected, electrophoresed by SDS-PAGE, and immunoblotted for analysis of the N protein expression. β-actin was acted as the loading control.
N Gene Sequencing and Phylogenetic Tree Analysis
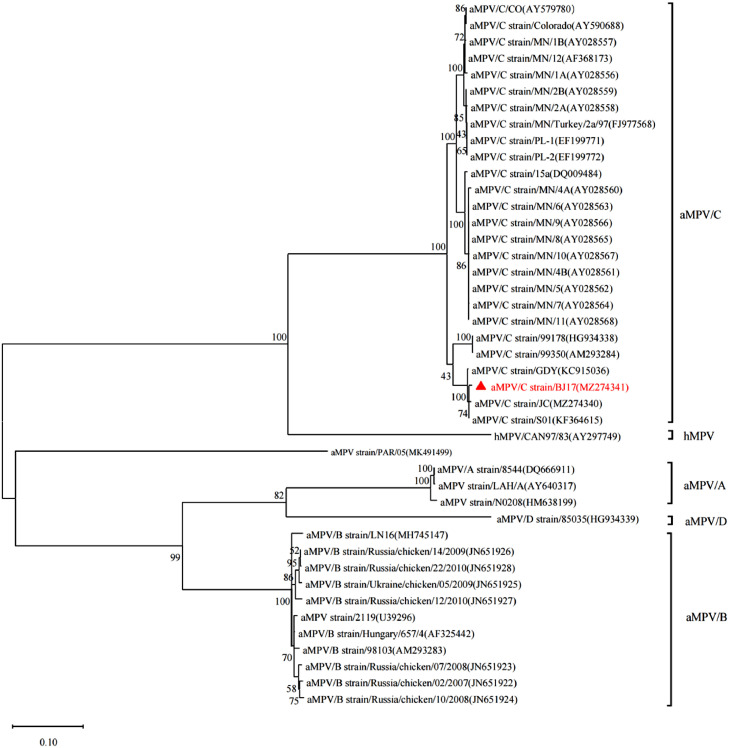
The full-length sequences of the N gene from these nasal turbinate tissue samples were determined and no difference of N gene sequences among these samples were found. The N gene sequence from an aMPV/C isolate (designated as strain BJ17) was submitted to GenBank under the accession number MZ274341. Analyses on phylogenetic and molecular evolution were performed using MEGA 5.10 (www.megasoftware.net). This N gene has a length of 1,185 nucleotides encoding 395 amino acids, which shared 94.6% to 99.6% nt identity with other aMPV/C isolates from ducks, turkeys, pheasants, and wild birds, but 65.8% to 69.3% nt identity with the aMPV subgroups A, B, and D isolates. The isolate BJ17 was more closely linked to human metapneumovirus (hMPV) isolate CAN97.83 (75.9%) than other aMPV/A, aMPV/B, and aMPV/D isolates. Notably, the BJ17 isolate showed higher identities (99.2%–99.6%) to Chinese aMPV/C isolates from native meat-type chickens and white Muscovy ducks (JC, S01, and GDY) than other aMPV/C isolates (94.9%–95.4%) outside China. The phylogenetic tree of the nucleotide sequences of the N gene from all aMPV subgroups as well as one hMPV strain was generated using the neighbor-joining clustering method (Figure 2). Strain BJ17 formed one cluster with other aMPV/C isolates. Within the cluster, all Chinese aMPV/C isolates and Franch Muscovy duck aMPV/C isolates 99178 and 99350 (Brown et al., 2014) formed a separate subcluster as compared to other subclusters, including other aMPV/C isolates, suggesting that all the Chinese aMPV/C isolates, regardless of Muscovy duck or chicken origins, might be derived from French Muscovy ducks.
Figure 2.
Phylogenetic analysis of aMPV viruses based upon the nucleotide sequences of the nucleoprotein gene by MAGE7. Isolate BJ17 is highlighted by a marked symbol. The GenBank accession number of each aMPV sequence is listed as the accession number in parenthesis.
No amino acid deletions or insertions were found in the N protein of strain BJ17 compared with that of other aMPV/C isolates. The N protein of isolate BJ17 shared 98.0% to 99.2% aa identity with the other aMPV/C isolates, but 70.7% to 73.2% aa identity with the aMPV/A, aMPV/B, and aMPV/D isolates. The nucleoprotein of aMPV/C strain BJ17 showed 98.2% to 99.2% amino acid homology to that of other Chinese and French aMPV/C isolates. The BJ17 isolate showed 88.6% aa identity of N protein to that of the hMPV strain CAN97.83. Three amino acid mutations were found at positions 11 (L–S), 46 (L–H), and 77 (E–G) in the nucleoprotein of the BJ17 strain compared to those of other aMPV/C. In addition, a conservative position 110D of the N protein of the BJ17 strain was observed only for the aMPV/C strains JC (Wei et al., 2013), S01 (Sun et al., 2014), and GDY (KC915036) isolated from Chinese duck and chicken farms. In contrast, the protein 110E amino acid residue of the other aMPV/C isolates outside China, including the French Muscovy duck isolates 99178 and 99350 or turkey or wild bird isolates. Whether this amino acid mutation at position 110 is associated with the distribution of aMPV/C isolates requires further investigation.
Pathogenicity of aMPV/C Strain BJ17 in 2-Wk-Old SPF Chickens
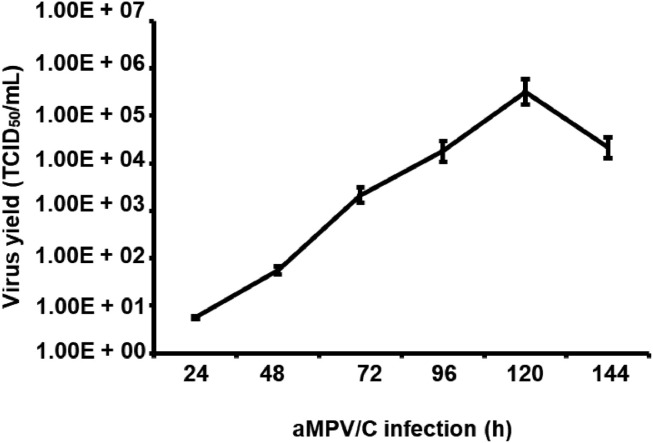
The aMPV/C strain BJ17 was continuously passaged 10 times in Vero cells to increase viral titers. 3, 5, and 10 passages of the BJ17 strain were collected for sequencing aMPV N gene and no nucleotide mutations were found in the N gene (data not shown), suggesting the genetic stability of strain BJ17 during continued passage in Vero cells. The titer of isolate BJ171 after 10 passages was 105.5 TCID50/mL at 120 h post infection. To assess the growth kinetics of aMPV/C in cultured cells, we infected Vero cells with strain BJ17 (MOI = 0.1 TCID50) and collected the culture supernatants from these cells at the indicated times to measure progeny virus production. Figure 3 shows the growth of aMPV/C (expressed as TCID50/mL) in Vero cells at different time points postinfection. Vero cells displayed increased titers at 24 h postinfection, peaked (105–106) at 120 h postinfection thereafter decreased. These results showed that aMPV/C replication in cultured Vero cells peaked at 120 h postinfection.
Figure 3.
Growth kinetics of the aMPV/C strain BJ17 in cultured cells. Vero cells were infected with BJ17 at an MOI of 0.1 and collected at the indicated time points for the viral titration. Virus titers were shown as TCID50/mL, and values are expressed as the mean ± SD of the results of triple independent experiments.
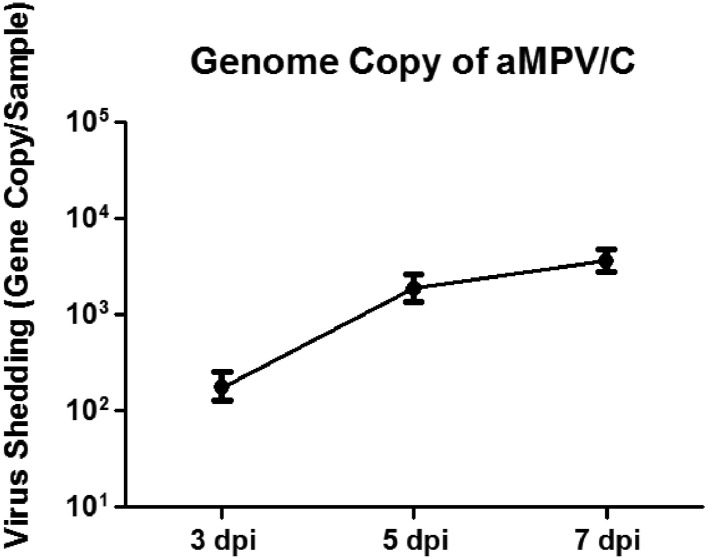
We further determined the pathogenicity of strain BJ17 in 3-wk-od SPF chickens. qRT-PCR was used to determine viral shedding from oropharyngeal swabs of chickens inoculated with strain BJ17 at 3 to 7 dpi. Sham-inoculated chickens were negative for aMPV/C-shedding. The aMPV/C genomic copy numbers in oropharyngeal swabs ranged from 1.76 × 102 to 3.65 × 103 copies/sample for the BJ17-inoculated chickens (Figure 4).
Figure 4.
aMPV shedding in oropharyngeal swabs of chickens inoculated with strain BJ17 was quantitatively detected by real-time RT-PCR. The values are expressed as the means of the results for all 15 BJ17-inoculated chickens at 3, 5, and 7 dpi; error bars represent the standard deviations.
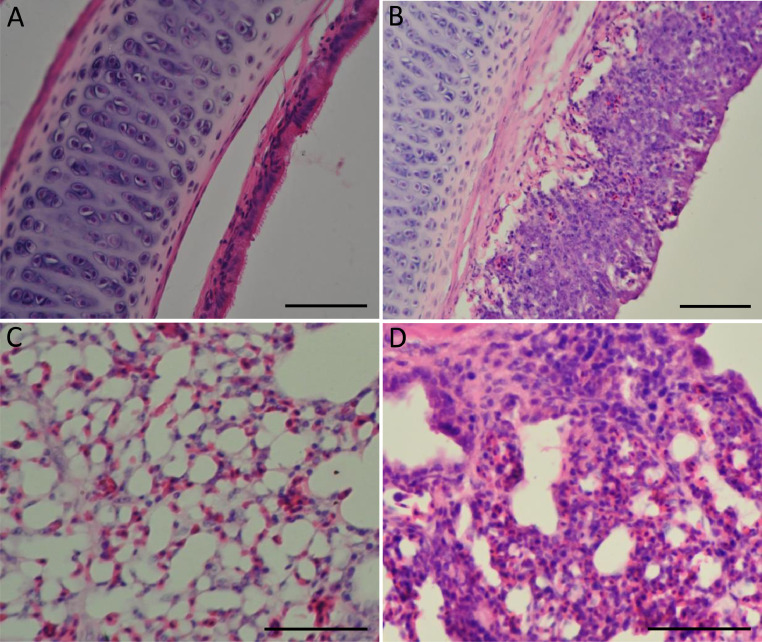
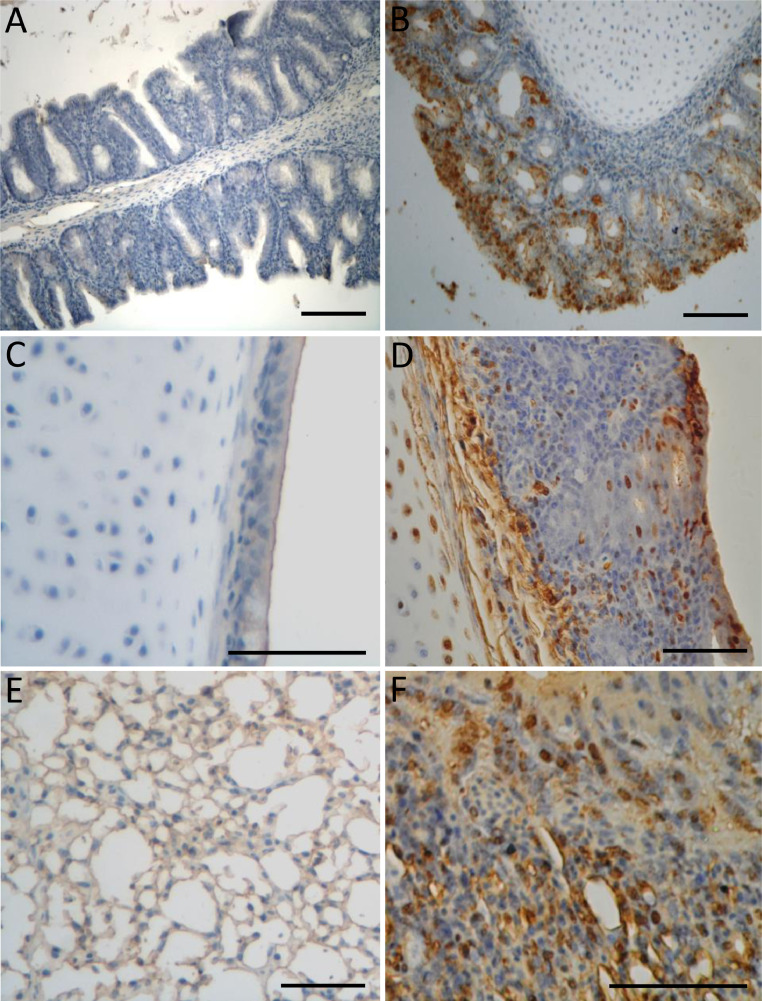
No clinical signs were seen in the sham-inoculated chickens during the animal experiment. At 3, 5, and 7 dpi, 6 of 15, 13 of 15, and 14 of 15 chickens, respectively, showed clinical signs such as depression, nasal discharge, sinus swelling, watery or frothy eyes, or gasping and deeply seated gurgling (data not shown). None of the sham-inoculated chickens showed any histopathological changes. The presence of clinical disease in the BJ17-inoculated chickens was related to histopathological alterations characterized by moderate to severe inflammatory lesions in the turbinate, tracheae, and lungs, including loss of ciliation, rupture of epithelial architecture, shedding of epithelial cells, and inflammatory cell infiltration (Figure 5 and data not shown) after 7 dpi. For the nasal turbinate, diffuse and severe infiltration of inflammatory cells, including macrophages, lymphocytes, and heterophils, was observed in the nasal epithelium, mucosal lamina propria, and submucosa (data not shown). Hemorrhage, lymphocyte infiltration, and hyperplasia were observed in the tracheal epithelium and lamina propria (Figure 5B). For the lungs, infiltration of macrophages, lymphocytes, and heterophils, as well as sloughed epithelial cells were observed in the bronchial lumens, and edema of the bronchial submucosa was present in the lungs (Figure 5D). IHC staining using rabbit polyclonal antibody raised against an aMPV nucleoprotein polypeptide (Alvarez et al., 2004) showed that viral antigen was present in morphologically normal or disrupted respiratory epithelial cells (Figure 6). Moreover, basal cells, mucus cells, as well as luminal cellular debris that contained sloughed epithelial cells and macrophages stained positive for the aMPV antigen.
Figure 5.
Histological lesions of respiratory tract samples from the BJ17-inoculated SPF chickens. (A) Trachea section from a non-inoculated chicken shows intact ciliated epithelium. (B) Trachea section of a BJ17-inoculated chicken displaying loss of cilia, rupture of architenture, and infiltrated inflammatory cells in the epithelium and submucosa. (C) Lung section from a non-inoculated chicken shows no overt inflammation. (D) Lung section of a BJ17-inoculated chicken displaying infiltration of inflammatory cells, such as lymphocytes, disffused heterophils and macrophages in the lung. Bars, 20 μm.
Figure 6.
Immunohistochemical staining of respiratory tract samples from the aMPV/C strain BJ17-inoculated SPF chickens. Nasal turbinate (A), tracheae (C), and lung tissue (E) from a non-inoculated chicken displays no positive cells for aMPV/C antigen. Nasal turbinate (B), tracheae (D), and lung tissue (F) of a BJ17-inoculated chicken displays large amount of positive cells for aMPV/C antigen. Bars, 20 μm.
DISCUSSION
aMPV-mediated turkey rhinotracheitis was first described in South Africa in 1978. Since then, rhinotracheitis in turkeys as well as swollen head syndrome in chickens mediated by aMPV infection have been reported in many poultry rearing countries around the world (Tanaka et al., 1995; Jones, 1996; Chockalingam et al., 2010; Kwon et al., 2010; Abdel-Azeem et al., 2014), causing severe economic losses. In China, a series of serological and molecular epidemiological surveys have shown that aMPV has widely infected chickens (Owoade et al., 2008; Zhu et al., 2016; Zhang et al., 2017a,b). However, only 2 reports on aMPV/C isolation and characterization have been reported for local meat-type commercial chickens in southeastern China in 2012 (Wei et al., 2013) and Muscovy ducks in South China in 2012 (Sun et al., 2014), and one report on aMPV/B isolation and pathogenicity in chickens in northern China in 2018 (Yu et al., 2019). In this study, we successfully characterized an aMPV subgroup C strain BJ17 isolated from an outbreak of broiler breeder chickens with severe respiratory disease in 2017 in northern China and found that the strain BJ17 is severely pathogenic to 3-wk-old SPF chickens. Together with the data on previous characterization of aMPV/C in native meat-type chickens in eastern China (Wei et al., 2013), the present study suggests that aMPV/C infection might be widespread in Chinese chicken flocks.
In the present study, the BJ17 isolate from breeder chickens was classified as aMPV subgroup C based upon phylogenetic analysis of the N gene (Figure 2). The phylogenetic tree of all aMPV subgroup isolates indicated that all the aMPV/C isolates from ducks, turkeys, chickens, pheasants, and wild birds formed an independent cluster (Figure 2). Previous studies in the USA have revealed the possible involvement of wild birds in aMPV/C virus transmissions (Shin et al., 2000; Bennett et al., 2004,2005; Velayudhan et al., 2005; Turpin et al., 2008), suggesting that wild birds can act as a reservoir of subgroup C aMPV and may possess a potential mechanism for spreading aMPVs to poultry. The results in the present study clearly show the possibility of aMPV/C transmission from wild birds to chickens.
The N gene sequence of strain BJ17 exhibited no deletions or insertions, as observed for the aMPV/C isolates deposited in GenBank under accession number MZ274341. The N protein of strain BJ17 showed high identity to those of the Chinese aMPV/C strains GDY, S01 (Sun et al., 2014), and JC (Wei et al., 2013), regardless of chicken or duck origins. Moreover, the N protein of these aMPV/C isolates from China showed a conserved amino acid residue D at position 110 compared to that of the other aMPV/C isolates outside China, including French Muscovy duck isolates 99178 and 99350, as well as turkey, pheasant, or wild bird isolates (Shin et al., 2000; Bennett et al., 2002; Bennett et al., 2004; Velayudhan et al., 2005; Toquin et al., 2006; Lee et al., 2007). These results indicate that the nucleoproteins of aMPV/C are highly conserved across isolates from different periods, different countries, and different host species (turkeys, ducks, chickens, and wild birds). Further analysis of N protein amino acid sequences showed that conserved amino acid residue at position E110 occurred in the aMPV/A, aMPV/B, non-Chinese aMPV/C, and aMPV/D viruses (data not shown). However, the contribution of a unique putative genetic marker to the distribution or other characteristics of the Chinese aMPV/C isolates will need further investigation using a reverse genetic system for identifying the critical sites required for the specific characteristics of the Chinese aMPV/C isolates.
In the present study, we used aMPV/C strain BJ17 to inoculate 3-wk-old SPF chickens and found that strain BJ17 caused clinicopathological alterations as evidenced by nasal discharge and symptoms of depression, sinus swelling, watery or frothy eyes, gasping and deeply seated gurgling, and inflammation in the turbinate, trachea, and lungs, as observed for aMPV/C strain JC in chickens (Wei et al., 2013). Severe histological lesions, which were characterized by rupture of the epithelial architecture, loss of cilia, hemorrhage, edema of the bronchial submucosa, or infiltration of inflammatory cells including macrophages, lymphocytes, and heterophils, were observed in various organs or tissues, such as the nasal turbinate tissue, trachea tissue, and lungs of chickens after aMPV/C strain BJ17 infection (Figure 5). IHC staining showed that aMPV antigen was detected in various cells, such as epithelial cells, mucus cells, basal cells, and macrophages, after aMPV/C BJ17 infection (Figure 6). In contrast, infection with aMPV/A or aMPV/B in chickens was considered to induce no apparent or mild clinical signs, virus shedding, and histological lesions (Gough et al., 1988; Kwon et al., 2010), which might be due to only transient replication of aMPV in upper respiratory tissues of chickens or a low tropism of aMPV in chickens (Cook et al., 1993; Jones, 1996). However, the exact mechanism by which chicken aMPV/C isolate possesses more severe pathogenic than aMPV/A or aMPV/B isolates for chickens requires further study.
In summary, we successfully characterized an aMPV/C strain isolated from chickens in Beijing, China, and found high conservation among subgroup C aMPV isolates regardless of turkey, duck, chicken, or wild bird origins. This chicken isolate is highly pathogenic and able to initiate aMPV/C clinical disease outbreaks in susceptible broiler breeder chickens, which might be due to direct transmission of the virus between ducks and chickens. Our results facilitate better understanding the molecular characteristics of aMPV/C thereby helping lay the groundwork for developing effective measures to prevent aMPV/C infection in China.
ACKNOWLEDGMENTS
This work was granted by the National Science Foundation of China (31830095), the National Key Research and Development Program of China (2016YFD0500806), China Agriculture Research System (CARS-41), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- Abdel-Azeem A-A.S., Franzo G., Zotte A.D., Drigo M., Catelli E., Lupini C., Martini M., Cecchinato M. First evidence of avian metapneumovirus subtype A infection in turkeys in Egypt. Trop. Anim. Health Prod. 2014;46:1093–1097. doi: 10.1007/s11250-014-0591-8. [DOI] [PubMed] [Google Scholar]
- Alvarez R., Njenga M.K., Scott M., Seal B.S. Development of a nucleoprotein-based enzyme-linked immunosorbent assay using a synthetic peptide antigen for detection of avian metapneumovirus antibodies in turkey sera. Clin. Diagn. Lab. Immunol. 2004;11:245–249. doi: 10.1128/CDLI.11.2.245-249.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banet-Noach C., Simanov L., Laham-Karam N., Perk S., Bacharach E. Longitudinal survey of avian metapneumoviruses in poultry in Israel: infiltration of field strains into vaccinated flocks. Avian Dis. 2009;53:184–189. doi: 10.1637/8466-090408-Reg.1. [DOI] [PubMed] [Google Scholar]
- Banet-Noach C., Simanov L., Perk S. Characterization of Israeli avian metapneumovirus strains in turkeys and chickens. Avian Pathol. 2005;34:220–226. doi: 10.1080/03079450500112625. [DOI] [PubMed] [Google Scholar]
- Bäyon-Auboyer M.H., Arnauld C., Toquin D., Eterradossi N. Nucleotide sequences of the F, L and G protein genes of two non-A/non-B avian pneumoviruses (APV) reveal a novel APV subgroup. J. Gen. Virol. 2000;81:2723–2733. doi: 10.1099/0022-1317-81-11-2723. [DOI] [PubMed] [Google Scholar]
- Bennett R.S., LaRue R., Shaw D., Yu Q., Nagaraja K.V., Halvorson D.A., Njenga M.K. A wild goose metapneumovirus containing a large attachment glycoprotein is avirulent but immunoprotective in domestic turkeys. J. Virol. 2005;79:14834–14842. doi: 10.1128/JVI.79.23.14834-14842.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R.S., McComb B., Shin H.J., Njenga M.K., Nagaraja K.V., Halvorson D.A. Detection of avina pneumovirus in wild Canada (Branta canadensis) and blue-winged teal (Anas discors) geese. Avian Dis. 2002;46:1025–1029. doi: 10.1637/0005-2086(2002)046[1025:DOAPIW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bennett R.S., Nezworski J., Velayudhan B.T., Nagaraja K.V., Zeman D.H., Dyer N., Graham T., Lauer D.C., Njenga M.K., Halvorson D.A. Evidence of avian pneumovirus spread beyond Minnesota among wild and domestic birds in central North America. Avian Dis. 2004;48:902–908. doi: 10.1637/7208-051804R. [DOI] [PubMed] [Google Scholar]
- Brown P.A., Lemaitre E., Briand F.X., Courtillon C., Guionie O., Allee C., Toquin D., Bayon-Auboyer M.H., Jestin V., Eterradossi N. Molecular comparisons of full length metapneumovirus (MPV) genomes, including newly determined French AMPV-C and -D isolates, further supports possible subclassification within the MPV genus. PLoS One. 2014;9 doi: 10.1371/journal.pone.0102740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buys S., Du Preez J. A preliminary report on the isolation of a virus causing sinusitis in turkeys in South Africa and attempts to attenuate the virus. Turkeys. 1980;28:36. [Google Scholar]
- Catelli E., Lupini C., Cecchinato M., Ricchizzi E., Brown P., Naylor C.J. Field avian metapneumovirus evolution avoiding vaccine induced immunity. Vaccine. 2010;28:916–921. doi: 10.1016/j.vaccine.2009.10.149. [DOI] [PubMed] [Google Scholar]
- Cecchinato M., Drigo M., Lupini C., Martini M., Listorti V., Franzo G., Bonci M., Laconi A., Morandini E., Catelli E. Field survey of avian metapneumovirus in northern Italy. Large Anim. Rev. 2013;19:267–270. [Google Scholar]
- Cecchinato M., Lupini C., Munoz Pogoreltseva O.S., Listorti V., Mondin A., Drigo M., Catelli E. Development of a real-time RT-PCR assay for the simultaneous identification, quantitation and differentiation of avian metapneumovirus subtypes A and B. Avian Pathol. 2013;42:283–289. doi: 10.1080/03079457.2013.788130. [DOI] [PubMed] [Google Scholar]
- Cecchinato M., Lupini C., Ricchizzi E., Falchieri M., Meini A., Jones R.C., Catelli E. Italian field survey reveals a high diffusion of avian metapneumovirus subtype B in layers and weaknesses in the vaccination strategy applied. Avian Dis. 2012;56:720–724. doi: 10.1637/10202-041312-Reg.1. [DOI] [PubMed] [Google Scholar]
- Chacon J.L., Mizuma M., Vejarano M.P., Toquin D., Eterradossi N., Patnayak D.P., Goyal S.M., Ferreira A.J. Avian metapneumovirus subtypes circulating in Brazilian vaccinated and nonvaccinated chicken and turkey farms. Avian Dis. 2011;55:82–89. doi: 10.1637/9501-081310-Reg.1. [DOI] [PubMed] [Google Scholar]
- Chockalingam A.K., Chander Y., Halvorson D.A., Goyal S.M. Stability of the glycoprotein gene of avian metapneumovirus (Canada goose isolate 15a/01) after serial passages in cell cultures. Avian Dis. 2010;54:915–918. doi: 10.1637/9016-081909-RESNOTE.1. [DOI] [PubMed] [Google Scholar]
- Cook J.K.A. Avian pneumovirus infections of turkeys and chickens. Vet. J. 2000;160:118–125. doi: 10.1053/tvjl.2000.0486. [DOI] [PubMed] [Google Scholar]
- Cook J.K.A., Huggins M.B., Orbell S.J., Senne D.A. Preliminary antigenic characterization of an avian Pneumovirus isolated from a commercial turkeys in Colorado, USA. Avian Pathol. 1999;28:607–617. doi: 10.1080/03079459994407. [DOI] [PubMed] [Google Scholar]
- Cook J.K.A., Kinloch S., Ellis M.M. In vitro and in vivo studies in chickens and turkeys on strains of turkey rhinotracheitis virus isolated from the two species. Avian Pathol. 1993;22:157–170. doi: 10.1080/03079459308418907. [DOI] [PubMed] [Google Scholar]
- Dani M.A., Arns C.W., Durigon E.L. Molecular characterization of Brazilian avian pneumovirus isolates using reverse transcription-polymerase chain reaction, restriction endonuclease analysis and sequencing of a G gene fragment. Avian Pathol. 1999;28:473–476. doi: 10.1080/03079459994498. [DOI] [PubMed] [Google Scholar]
- D'Arce R.C.F., Coswig L.T., Almeida R.S., Trevisol I.M., Monteiro M.C.B., Rossini L.I., Fabio J.D., Hafez H.M., Arns C.W. Subtyping of new Brazilian avian metapneumovirus isolates from chickens and turkeys by reverse transcriptase-nested-polymerase chain reaction. Avian Pathol. 2005;34:133–136. doi: 10.1080/03079450500059180. [DOI] [PubMed] [Google Scholar]
- Droual R., Woolcock P.R. Swollen head syndrome associated with E. coli and infectious bronchitis virus in the Central Valley of California. Avian Pathol. 1994;23:733–742. doi: 10.1080/03079459408419042. [DOI] [PubMed] [Google Scholar]
- Gharaibeh S.M., Algharaibeh G.R. Serological and molecular detection of avian pneumovirus in chickens with respiratory disease in Jordan. Poult. Sci. 2007;86:1677–1681. doi: 10.1093/ps/86.8.1677. [DOI] [PubMed] [Google Scholar]
- Giovanardi D., Lupini C., Pesente P., Rossi G., Ortali G., Catelli E. Longitudinal field studies of avian metapneumovirus and turkey hemorrhagic enteritis virus in turkeys suffering from colibacillosis associated mortality. Vet. Res. Commun. 2014;38:129–137. doi: 10.1007/s11259-014-9596-z. [DOI] [PubMed] [Google Scholar]
- Gough R.E., Collins M.S., Cox W.J., Chettle N.J. Experimental infection of turkeys, chickens, ducks, geese, guinea fowl, pheasants and pigeons with turkey rhinotracheitis virus. Vet. Rec. 1988;123:58–59. doi: 10.1136/vr.123.2.58. [DOI] [PubMed] [Google Scholar]
- Gough R.E., Jones R.C. In: Diseases of Poultry. Saif Y.M., Fadly A.M., Glisson J.R., McDougald L.R., Nolan L.K., Swayne D.E., editors. Blackwell; Ames: 2008. Avian metapneumoviruses; pp. 100–110. [Google Scholar]
- Guo L., Qu L. Serologic investigation of pneumovirus infection in breeding birds. China Anim. Husb. Vet. Med. 2009;36:149–150. (In Chinese) [Google Scholar]
- Hafez H.M., Hess M., Prusas C., Naylor C.J., Cavanagh D. Presence of avian pneumovirus type A in continental Europe during the 1980s. J. Vet. Med. B Infect. Dis. Vet. Public Health. 2000;47:629–633. doi: 10.1046/j.1439-0450.2000.00398.x. [DOI] [PubMed] [Google Scholar]
- Jones R.C. Avian pneumovirus infection: questions still unanswered. Avian Pathol. 1996;25:639–648. doi: 10.1080/03079459608419171. [DOI] [PubMed] [Google Scholar]
- Jones R.C., Naylor C.J., Bradbury J.M., Savage C.E., Worthington K., Williams R.A. Isolation of a turkey rhinotracheitis-like virus from broiler breeder chickens in England. Vet. Rec. 1991;129:509–510. [PubMed] [Google Scholar]
- Kwon J-S., Lee H-J., Jeong S-H., Park J-Y., Hong Y-H., Lee Y-J., Youn H-S., Lee D-W., Do S-H., Park S-Y., Choi I-S., Lee J-B., Song C-S. Isolation and characterization of avian metapneumovirus from chickens in Korea. J. Vet. Sci. 2010;11:59–66. doi: 10.4142/jvs.2010.11.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E., Song M.S., Shin J.Y., Lee Y.M., Kim C.J., Lee Y.S., Kim H., Choi Y.K. Genetic characterization of avian metapneumovirus subtype C isolated from pheasants in a live bird market. Virus Res. 2007;128:18–25. doi: 10.1016/j.virusres.2007.03.029. [DOI] [PubMed] [Google Scholar]
- Listorti V., Lupini C., Cecchinato M., Pesente P., Rossi G., Giovanardi D., Naylor C.J., Catelli E. Rapid detection of subtype B avian metapneumoviruses using RT-PCR restriction endonuclease digestion indicates field circulation of vaccine-derived viruses in older turkeys. Avian Pathol. 2014;43:51–56. doi: 10.1080/03079457.2013.866212. [DOI] [PubMed] [Google Scholar]
- Mahmoud A.H., Fahmy H.A., Gafer J.A.M., Arafa A. In: 7th International Symposium on Turkey Diseases, Berlin, 19th-21th May 2008. Hafez H.M., editor. Verlag der DVG Service GmbH; Giessen, Germany: 2008. Investigation on Turkey Rhinotracheitis in commercial Turkeys in Egypt; pp. 186–196. [Google Scholar]
- Mase M., Yamaguchi S., Tsukamoto K., Imada T., Imai K., Nakamura K. Presence of avian pneumovirus subtypes A and B in Japan. Avian Dis. 2003;47:481–484. doi: 10.1637/0005-2086(2003)047[0481:POAPSA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Owoade A.A., Ducatez M.F., Hubschen J.M., Sausy A., Chen H., Guan Y., Muller C.P. Avian metapneumovirus subtype A in China and subtypes A and B in Nigeria. Avian Dis. 2008;52:502–506. doi: 10.1637/8266-021208-Reg.1. [DOI] [PubMed] [Google Scholar]
- Rivera-Benitez J.F., Martinez-Bautista R., Rios-Cambre F., Ramirez-Mendoza H. Molecular detection and isolation of avian metapneumovirus in Mexico. Avian Pathol. 2014;43:217–223. doi: 10.1080/03079457.2014.903557. [DOI] [PubMed] [Google Scholar]
- Roussan D.A., Haddad R., Khawaldeh G. Molecular survey of avian respiratory pathogens in commercial broiler chicken flocks with respiratory diseases in Jordan. Poult. Sci. 2008;87:444–448. doi: 10.3382/ps.2007-00415. [DOI] [PubMed] [Google Scholar]
- Seal B.S. Matrix protein gene nucleotide and predicted amino acid sequence demonstrate that the first US avian pneumovirus isolate is distinct from European strains. Virus Res. 1998;58:45–52. doi: 10.1016/s0168-1702(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Shen R.S., Qu L., Yu K., Li J., Zhang J., Gu S., Xu Y., Tang G. Isolation and characterization of an avian pneumovirus from chickens in China. Chin. J. Prev. Vet. Med. 1999;21:76–77. (In Chinese) [Google Scholar]
- Shin H.J., Nagaraja K.V., McComb B., Halvorson D.A., Jirjis F.F., Shaw D.P., Seal B.S., Njenga M.K. Isolation of avian pneumovirus from mallard ducks that is genetically similar to viruses isolated from neighboring commercial turkeys. Virus Res. 2002;83:207–212. doi: 10.1016/s0168-1702(01)00402-6. [DOI] [PubMed] [Google Scholar]
- Shin H.J., Njenga M.K., McComb B., Halvorson D.A., Nagaraja K.V. Avian pneumovirus (APV) RNA from wild and sentinel birds in the United States has genetic homology with RNA from APV isolates from domestic turkeys. J. Clin. Microbiol. 2000;38:4282–4284. doi: 10.1128/jcm.38.11.4282-4284.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S., Chen F., Cao S., Liu J., Lei W., Li G., Song Y., Lu J., Liu C., Qin J., Li H. Isolation and characterization of a subtype C avian metapneumovirus circulating in Muscovy ducks in China. Vet. Res. 2014;45:74. doi: 10.1186/s13567-014-0074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Takuma H., Kokumai N., Oishi E., Obi T., Hiramatsu K., Shimizu Y. Turkey rhinotracheitis virus isolated from broiler chicken with swollen head syndrome in Japan. J. Vet. Med. Sci. 1995;57:939–941. doi: 10.1292/jvms.57.939. [DOI] [PubMed] [Google Scholar]
- Toquin D., Bayon-Auboyer M.H., Eterradossi N., Jestin V. Isolation of pneumovirus from a Muscovy duck. Vet. Rec. 1999;145:680. [PubMed] [Google Scholar]
- Toquin D., Guionie Q., Jestin V., Zwingelstein F., Allee C., Eterradossi N. European and American subgroup C isolates of avian metapneumovirus belong to different genetic lineages. Virus Genes. 2006;32:97–103. doi: 10.1007/s11262-005-5850-3. [DOI] [PubMed] [Google Scholar]
- Turpin E.A., Stallknecht D.E., Slemons R.D., Zsak L., Swayne D.E. Evidence of avian metapneumovirus subtype C infection of wild birds in Georgia, South Carolina, Arkansas and Ohio USA. Avian Pathol. 2008;37:343–351. doi: 10.1080/03079450802068566. [DOI] [PubMed] [Google Scholar]
- Velayudhan B.T., McComb B., Bennett R.S., Lopes V.C., Shaw D.P., Halvorson D.A., Nagaraja K.V. Emergence of a virulent type C avian metapneumovirus in turkeys in Minnesota. Avian Dis. 2005;49:520–526. doi: 10.1637/7388-052805R.1. [DOI] [PubMed] [Google Scholar]
- Wei L., Zhu S., Yan X., Wang J., Zhang C., Liu S., She R., Hu F., Quan R., Liu J. Avian metapneumovirus subgroup C infection in chickens. China. Emerg. Infect. Dis. 2013;19:1092–1094. doi: 10.3201/eid1907.121126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., Xing L., Chang F., Bao Y., Wang S., He X., Wang J., Wang S., Liu Y., Farooque M., Pan Q., Wang Y., Gao L., Qi X., Hussain A., Li K., Liu C., Zhang Y., Cui H., Wang X., Gao Y. Genomic sequence and pathogenicity of the first avian metapneumovirus subtype B isolated from chicken in China. Vet. Microbiol. 2019;228:32–38. doi: 10.1016/j.vetmic.2018.11.009. [DOI] [PubMed] [Google Scholar]
- Zhang D., Dai Y., Zhao R., Hu X., Shen X., Hou H., Pan X., Zhou X., Zhu C. Serologic investigation of pneumoviral infection in chickens in parts of anhui province. Adv. Small Anim. Med. Surg. 2017;38:126–129. [Google Scholar]
- Zhang L., Li H., Yuan Y., Wang D., Tang N., Wei P., Wei T., Huang T., Tang M. Survey of avian metapneumovirus in chickens of Guangxi region. Chin. J. Prev. Vet. Med. 2017;39:439–442. [Google Scholar]
- Zhu Y., Gong X., Guo W., Xu B., Li L., Lang F., Liu H., Liu D., Fang G. Molecular epidemiological analysis of avian partial lung virus in some areas of China from 2012 to 2015. Prog. Vet. Med. 2016;37:30–34. [Google Scholar]