Abstract
Left atrial appendage (LAA) closure (LAAC) device implantation may be challenging in cases with difficult LAA anatomy. A deflectable sheath to approach multiple LAA positions may be helpful. We used a deflectable sheath to implant LAAC devices in 20 patients and included 60 cases using the standard sheaths for comparison. The procedures were successful in all patients without peri-procedural complications. After a median follow-up of 1.52 (IQR: 0.76-2.33) years, none of the patients in the deflectable sheath group had peri-device leak ≥3 mm and experienced thromboembolic stroke. In the standard sheath group, after follow-up of 2.03 (IQR: 0.87-3.19) years, 2 had peri-device leak ≥3 mm and 1 experienced thromboembolic stroke. We first proved the idea of using a universal steerable sheath for LAAC device implantation in difficult LAA anatomy, which also allows rapid switching of different LAAC devices.
Key Words: deflectable sheath, left atrial appendage closure device, steerable sheath
Abbreviations and Acronyms: AF, atrial fibrillation; LAA, left atrial appendage; LAAC, left atrial appendage closure
Central Illustration
The PROTECT-AF study showed that percutaneous closure of the left atrial appendage (LAA) by an LAA closure (LAAC) device was noninferior to use of warfarin for the prevention of thromboembolic event in patients with atrial fibrillation (AF).1 Nevertheless, because of the high variability and complexity of LAA morphology, a deflectable sheath may facilitate LAAC device implantation in difficult cases. However, the sheaths of the most commonly used LAAC devices are not deflectable and steerable. This study is the first to investigate the feasibility and safety of using a deflectable and steerable sheath for implanting LAAC devices.
Methods
Study population
We selected patients from our National Taiwan University Atrial Fibrillation Registry.2 and finally 20 consecutive patients agreed to participate in this study; 10 of them underwent Watchman (the first LAAC device) and the other 10 Amulet (the second LAAC device) implantation. The study was approved by the ethics committee and institutional review board on human research of the Medical Research Department of National Taiwan University Hospital, Taipei, Taiwan, and all patients provided written informed consent.
Use of a universal steerable sheath in laac device implantation
The FlexCath Steerable Sheath is a unidirectional, deflectable sheath with 135° of deflection initially designed to guide a cryoablation catheter and it facilitates better catheter positioning. It comes with an inner diameter of 12 French and an outer diameter of 15 French, and is 65 cm long. After successful trans-septal puncture, the sheath was advanced into the LAA with a pigtail catheter positioned in front of it.
For optimal device positioning, the sheath was deflected to a coaxial alignment between sheath and LAA. Then, the device was loaded into and advanced through the sheath distally. The device was positioned into the landing zone and deployed. After the procedure, all patients received either single antiplatelet plus oral anticoagulant or dual antiplatelet therapy with aspirin and clopidogrel for 1-3 months, followed by single antiplatelet alone.
Outcome definitions and follow-up
Implantation success was defined as correct placement of the LAAC device in the LAA with the axis of the device aligned with the LAA axis and without significant peri-device leak (≥3 mm) as in our previous report. The safety and efficacy during the procedure and follow-up were defined as in our previous report. Clinical follow-up was arranged at 1 week, 1 month, and then every 3 months after device implantation to assess the clinical outcomes, whereas a follow-up transesophageal echocardiography was scheduled at 2-4 months and 12 months after the procedure.
Statistical analysis
The data were reported as median with IQR (quartile 1 and quartile 3). Nonparametric Mann-Whitney test was used to compare continuous variables between groups and Fisher exact test was used for comparison of categorical variables between groups. The log-rank test was used to compare outcome differences between groups. All statistical analyses were performed using SPSS 25 (IBM Corp.).
Results
A total of 20 patients underwent implantation of LAAC devices using the steerable sheaths. There was no significant difference in the baseline characteristics between the 2 groups with 2 difference LAAC devices.
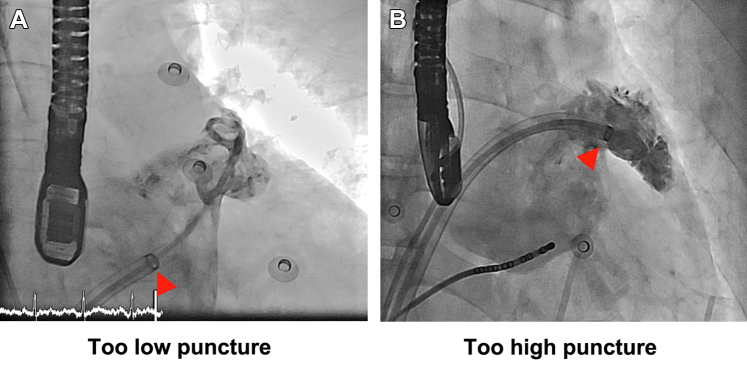
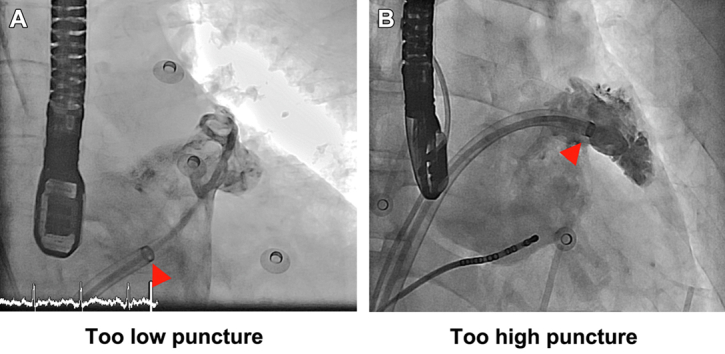
In the beginning of the procedure, the initial sheath location relative to the level of the LAA ostium could be too low (too low septal puncture or superior LAA location) (Figure 1A) or too high (high septal puncture or low LAA) (Figure 1B). With the advantage of a steerable sheath, we could adjust the deflection and curvature of the sheath, advance the sheath into LAA, and achieve a good coaxiality between the sheath and the LAA. In most of the cases, the puncture position was relatively high and it might be difficult to advance the sheath into the LAA directly. We then bent or deflected the sheath downward and advanced the sheath into the LAA.
Figure 1.
Angiography
Angiography showing different trans-septal puncture positions in performing LAAC device implantation. (A) The sheath location is too low relative to the LAA ostium (or too high LAA). (B) The sheath location is too high relative to the LAA ostium (or too low LAA). In most of the scenarios, the trans-septal puncture is too high or the LAA is low. Then the sheath could be bent downward to achieve a good coaxiality between the sheath and the LAA. Arrowheads indicate the distal marker of the steerable sheath. LAA = left atrial appendage; LAAC = left atrial appendage closure.
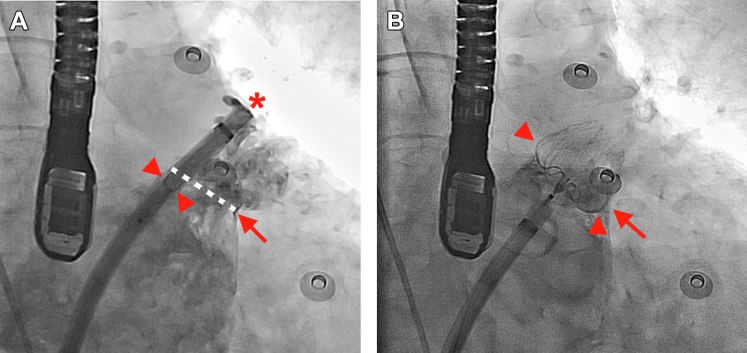
The implantation procedure of the first LAAC device using a steerable sheath was different from that using standard access and delivery sheathes of the first LAAC device. The steerable sheath should first be advanced into the LAA as deep as possible. Then the delivery sheath has to be cut short, loaded into the steerable sheath together with the LAAC device, and then the LAAC device was advanced to the distal end of the steerable sheath. Under fluoroscopic guidance, the distal tip of the loaded LAAC device in the sheath should be aligned with the distal tip of the sheath (asterisk in Figure 2A).
Figure 2.
Implantation
Implantation of the first LAAC using the steerable sheath. (A) The loaded LAAC device is advanced distally and the distal tip of the device should be aligned with the distal tip of the steerable sheath (asterisk). Then dye injection shows that the proximal border of the loaded device (arrowheads) is close to the LAA ostial plane (arrow and white dashed line). (B) After deployment, the proximal waist of the device (arrowheads) remains in the same position and is close to the LAA ostial plane (arrow). Abbreviations as in Figure 1.
There are no 3 marker bands in the distal part of the sheath, but we could see the proximal border of the loaded LAAC device within the sheath under fluoroscope (arrowheads in Figure 2A). Then we can inject contrast to show the whole LAA and ensure whether the most proximal border of the device (arrowheads in Figure 2A with the loaded LAAC device) is close to the LAA ostial plane (arrow and white dashed line in Figure 2A). After deployment, the proximal border/waist of the device (arrowhead in Figure 2B with the deployed LAAC device) generally remains in the same position and, therefore, is close to the LAA ostial plane (arrow in Figure 2B).
The implantation of the second LAAC device is more straightforward. We first cut the loader of the device to a short length. Then the loader together with the LAAC device was pushed into the sheath and the LAAC device was advanced to the distal part of the sheath. The implantation procedure was then very similar to that using the standard sheath of the second LAAC device.
Comparison with patients receiving laac device using the conventional sheaths
For comparison, we included 30 consecutive cases using the standard sheath of the first LAAC device and 30 cases using the standard sheath of the second LAAC device. There was no significant difference in the baseline characteristics between the steerable sheath group and the standard sheath group. The procedure success rate was similar between the 2 groups (100% in the steerable sheath group and 98% [59 of 60] in the standard sheath group; P = 0.750). The failure case in the standard sheath group was a patient in whom the implantation of the first LAAC device failed due to a very low and anterior LAA and the access sheath location was always too high even when we did a very low trans-septal puncture.
In the steerable sheath group, after a median follow-up of 1.52 (IQR: 0.76-2.33) years, none of the patients had peri-device lead ≥3 mm in transesophageal echocardiography follow-up. None of them experienced a thromboembolic event during follow-up. In the standard sheath group, after a median follow-up of 2.03 (IQR: 0.87-3.19) years, 2 had peri-device leak ≥3 mm and 1 experienced thromboembolic stroke. There was no statistical difference in terms of these outcomes (P = 0.934 for peri-device leak and .763 for thromboembolic event).
Discussion
In selected cases like the infero-posteriorly directed LAA, small-angle or reverse chicken wing, complex lobe structure, or in those with a huge left atrium in which the location and morphology of LAA may be distorted, implantation of a LAAC device using the conventional sheath can be a challenging procedure. A deflectable sheath could adjust the orientation of sheath tip to better align the axis of the LAA. The most common scenario is for inadvertently high septal puncture, in which case a deflectable sheath could be bent downward to improve the coaxiality between the LAA and the sheath. In reverse chicken wing, the deflectable sheath is advanced into the LAA neck and first rotated upward and posteriorly with decreased curvature in the small space of the LAA neck. Then the sheath is deflected and thus could be pushed into the distal posterosuperior lobe. In complex lobe structure, the sheath direction is steerable and could be advanced to any target lobe.
Kleinecke et al3 reported the first-in-human experience of implanting Lambre LAAC device using the steerable FuStar delivery sheath in 20 patients with nonvalvular AF and contraindications to oral anticoagulation. Successful device implantation was achieved in all patients (100%). No periprocedural complications were observed. They showed the feasibility and safety of the steerable sheath for implanting the LAAC device. In the present study, we reported the first-in-human experience of implanting both commonly used LAAC devices using the steerable sheath, which had never been reported before our study.
There are other advantages of using a steerable or deflectable sheath for LAAC device implantation. The initial design of the steerable sheath was for the catheter ablation of AF and, using a single steerable sheath, catheter ablation or cryoablation of pulmonary veins and implantation of LAAC device could be combined without the need to change the sheath during the procedure. For pulmonary vein isolation, the trans-septal puncture site should be middle or anterior, which is not suitable for LAAC device implantation. If the sheath is steerable, this anterior puncture is not a problem for LAAC device implantation. Finally, we first demonstrated a universal steerable sheath could be used for both commonly used LAAC devices, which allows rapid switching to the other LAAC device if one device is found inappropriate in the first attempt.
There are limitations in the present study. Sample size was small and the retrospective and observational nature of the study design may introduce selection bias. The cohort was also too small to detect even large differences between the groups in the risk of outcomes (eg, thromboembolic events). This is a single-center experience and the results cannot be applied to other scenarios or catheterization laboratories with operators still in the learning curve. The procedure was modified to some extent and in vitro testing or practice was recommended before doing this procedure in real patients.
Funding Support and Author Disclosures
The work was supported by grants from the Ministry of Science and Technology in Taiwan (MOST 109-2314-B-002-244-MY3 and MOST 110-2314-B-002-198 -MY3). The Ministry of Science and Technology had no role in the conduct of this study. All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Holmes D.R., Reddy V.Y., Turi Z.G., et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534–542. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 2.Chang S.N., Lai L.P., Chiang F.T., Lin J.L., Hwang J.J., Tsai C.T. C-reactive protein gene polymorphism predicts the risk of thromboembolic stroke in patients with atrial fibrillation: a more than 10-year prospective follow-up study. J Thromb Haemost. 2017;15:1541–1546. doi: 10.1111/jth.13735. [DOI] [PubMed] [Google Scholar]
- 3.Kleinecke C., Gomez Monterrosas O., Scalone G., et al. First-in-human experience of left atrial appendage occlusion with the steerable FuStar sheath. J Interv Cardiol. 2018;31:532–537. doi: 10.1111/joic.12509. [DOI] [PubMed] [Google Scholar]