Abstract
We present the case of a 42-year-old male patient with ST-segment elevation myocardial infarction and pericardial effusion due to rupture of the left anterior descending artery most likely secondary to polyarteritis nodosa. Successful surgery was performed under cardiopulmonary bypass using antegrade and retrograde cardioplegia combined. (Level of Difficulty: Intermediate.)
Key Words: coronary artery bypass, myocardial infarction, myocardial ischemia, rheumatic heart disease
Abbreviations and Acronyms: CPB, cardiopulmonary bypass; CT, computed tomography; LAD, left anterior descending
Central Illustration
History of presentation
A 42-year-old Mauritanian male patient had admitted himself with chest and neck pain and fever for 3 days. There had been no history of trauma or relevant infection in the past weeks. Analgesic medication with ibuprofen had been inefficient.
Learning Objectives
-
•
To understand the connection of polyarteritis nodosa or other rheumatic vessel diseases with possible coronary artery complications in patients with unclear symptoms of chest pain and rheumatic or infectious medical history.
-
•
To gain knowledge of the clinical presentation of coronary artery aneurysm rupture and therefore to develop an effective diagnostic pattern with special emphasis on computed tomography and echocardiography first before other diagnostic steps.
-
•
To develop strategies in fast and effective myocardial protection during surgical treatment of ruptured coronary arteries considering combined forms of cardioplegia
The patient presented pleuritic chest pain and dyspnea, pulse was elevated at 110 to 120 beats/min, and body temperature was 38 °C. He presented no signs of gastrointestinal or urinary tract infection.
Past medical history
The patient had a long medical history with intermittent fever and exhaustion, recurrent thrombophlebitis, recurrent deep vein thrombosis, and sinus vein thrombosis in 2015. Because of intolerance to apixaban, anticoagulation therapy was switched to phenprocoumon in 2020. He also had a history of hepatitis B. In August 2021, polyarteritis nodosa was diagnosed by histological examination of a biopsy specimen (including arterial tissue) from a left lower extremity lesion. Since then he was treated with mycophenolate mofetil 500 mg twice daily.
Differential diagnosis
As for the fever and respiratory chest pain, pneumonia or atypical infection of the bronchial system was suspected.
Investigations
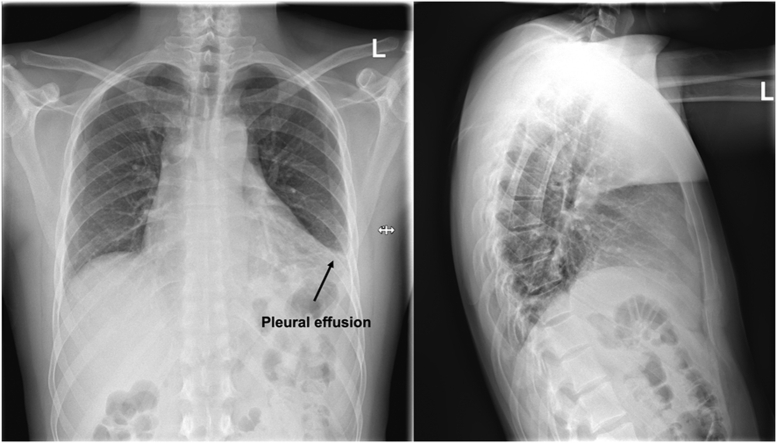
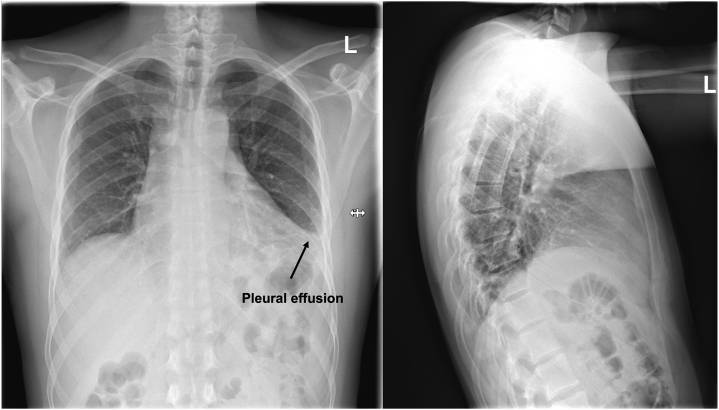
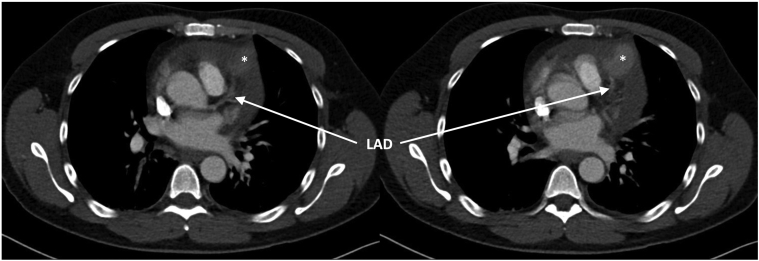
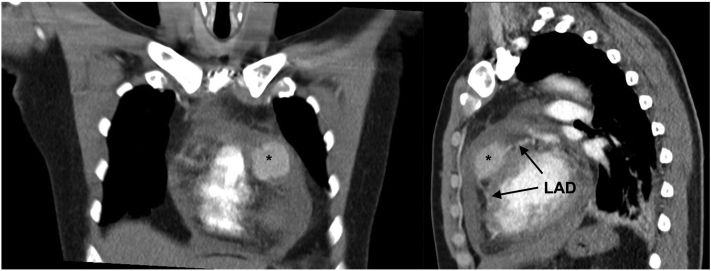
Chest x-ray showed left pleural marginal effusion (Figure 1). Chest computed tomography (CT) indicated for detection of a potential pneumonia showed circular pericardial effusion (maximum width of 2 cm) and leakage of contrast agent in the left anterior descending artery (LAD) territory (maximum width of 35.4 mm). Differential diagnosis was either covered myocardial rupture or rupture of an LAD aneurysm, the latter being more likely because the course of LAD towards and away from the suspected aneurysm was evident (Figures 2 and 3).
Figure 1.
Chest X-Ray
Left pleural effusion.
Figure 2.
Computed Tomography Scans, Axial Plane
Course of left anterior descending artery (LAD) (→) to aneurysm/LAD rupture (∗).
Figure 3.
Computed Tomography Scans, Coronal and Sagittal Plane
Course of LAD (→) to aneurysm/LAD rupture (∗). Abbreviation as in Figure 2.
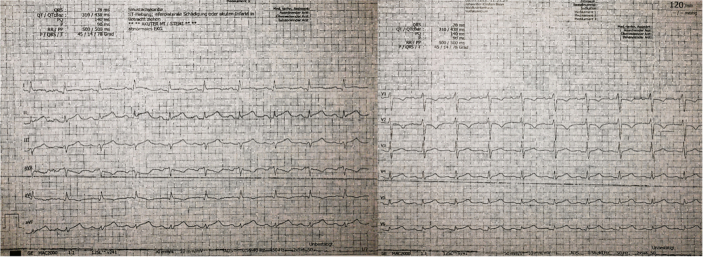
Electrocardiogram revealed ST-segment elevation in I, II, III, aVF, and V4 to V6 (Figure 4). Transthoracic echocardiography detected the pericardial effusion and a hyperechoic structure in LAD territory (Figure 5). Leukocytes and C-reactive protein were elevated (12.7 g/L, 77 mg/L) and hemoglobin was normal (13.2 g/dL).
Figure 4.
Electrocardiogram
ST-segment elevation in I, II, III, aVF, and V4 to V6.
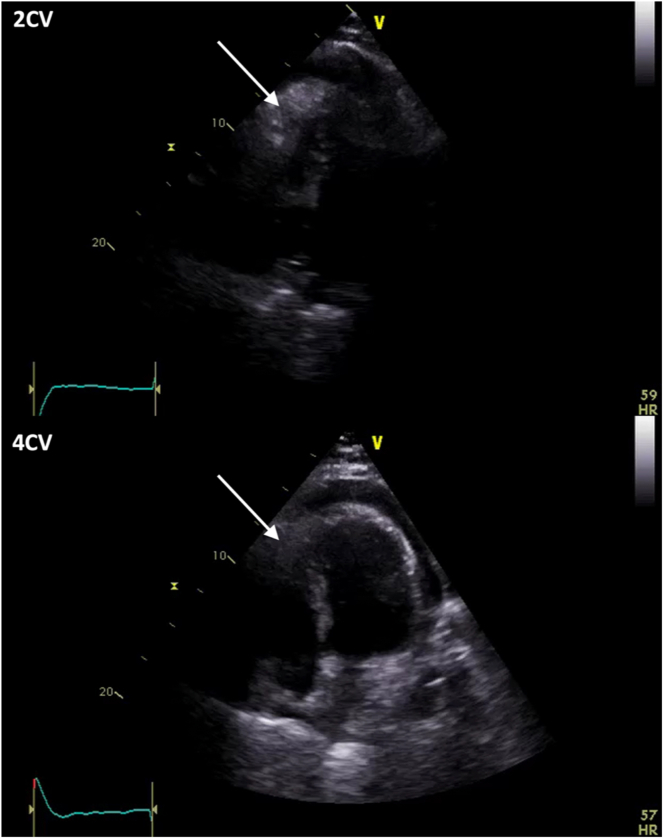
Figure 5.
Transthoracic Echocardiography
2-chamber view (2CV) and 4-chamber view (4CV) with pericardial effusion and hyperechoic structure (→).
Management
We diagnosed an LAD rupture with consecutive posterior myocardial infarction due to left-dominant coronary circulation, resulting in cardiac tamponade. The following therapeutic options were discussed: Pericardial tab via drainage to relieve the tamponade was ruled out as we expected catastrophic pericardial bleeding. Intracoronary covered stent placement was ruled out because of the potential infectious context and because stenting was not expected to be sufficient in covering the LAD rupture.
Thus, the patient was emergently transferred to our cardiovascular surgery department for surgical therapy.
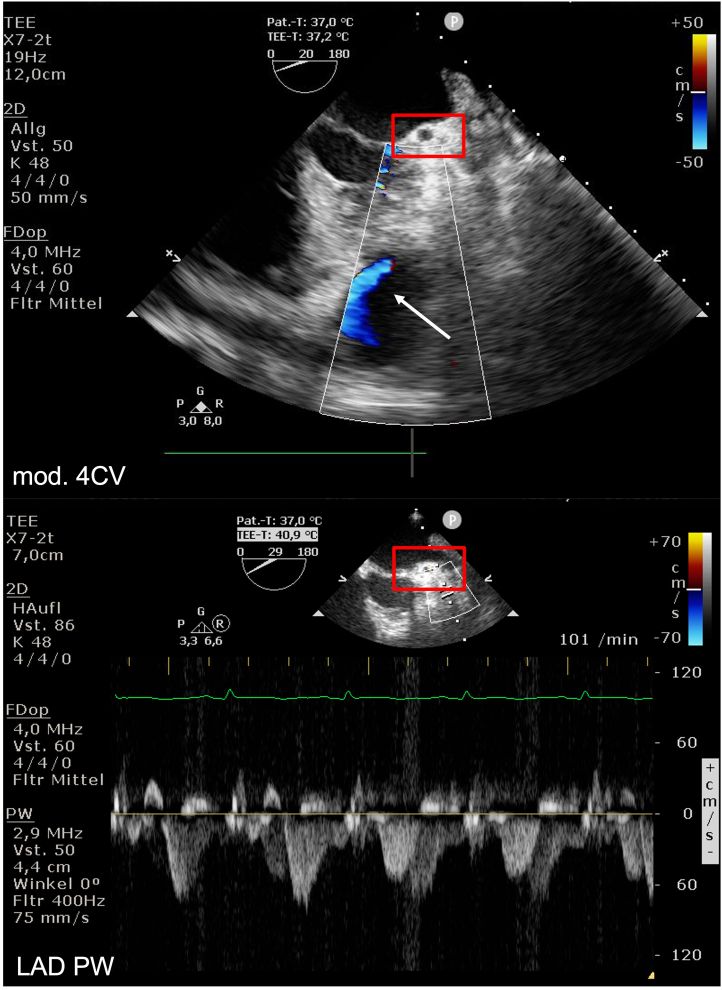
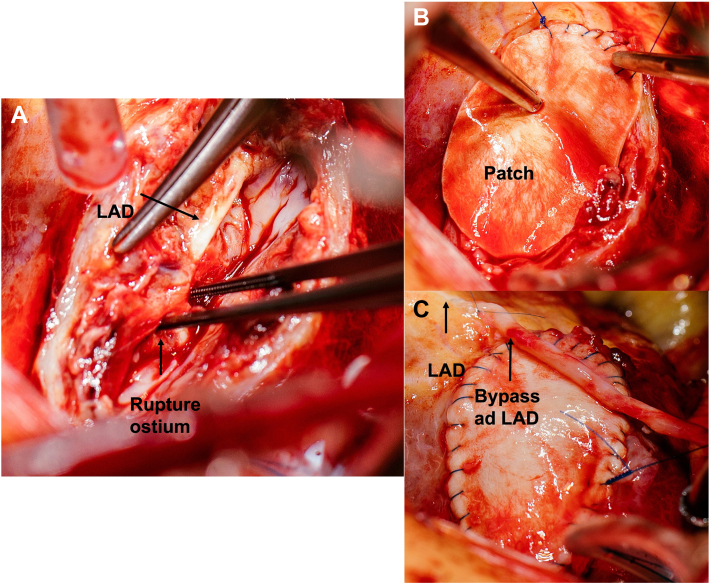
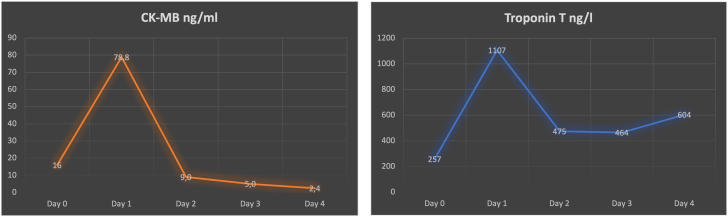
On admission, the patient presented signs of now manifest cardiogenic shock with blood pressure 75/50 mm Hg and pulse 130 beats/min and was directly transferred to the operating room. Using transesophageal echocardiography we observed a coronary cavity with blood flow coming from the LAD representing a possible LAD aneurysm, pulsed-wave Doppler showed normal flow over the left coronary artery ostium (Figure 6). After median sternotomy, we found massive hemorrhagic pericardial effusion; the anterior myocardium was ruptured but covered with pericardium. The pericardium was removed under mechanical decompression of the heart via cardiopulmonary bypass (CPB). We used Calafiore (warm blood) cardioplegia retrograde (through coronary sinus) and antegrade (through aortic root) combined, which resulted in fast cardiac arrest. The anticipated diagnosis of myocardial rupture secondary to rupture of LAD due to LAD aneurysm was confirmed (Figure 7A). The tissue was massively destroyed, which made it impossible to identify more anatomical anomalies. Torn off septal branches were sutured. The myocardial cavity around the rupture itself was filled with tissue glue, then reconstructed using a bovine pericardial patch (Figure 7B). A single venous aortocoronary bypass to distal LAD was performed (Figure 7C). Procedural time was 120 minutes, CPB time was 60 minutes, and cross clamp time was 42 minutes. After extubation, the patient was transferred to the intensive care unit without vasopressor therapy. On the first postoperative day, he was transferred to intermediate care and on the second postoperative day to the normal ward. The cardiac enzymes (troponin and CK-MB) peaked on the first postoperative day and then decreased adequately (Figure 8). Postoperative echocardiography showed only a mildly impaired left ventricular function despite the massive myocardial infarction. After consultation with our rheumatology department, the preoperative medication with mycophenolate mofetil was not sufficient and was therefore increased to 1,000 mg 3 times daily. The patient was discharged at day 8 without any adverse events.
Figure 6.
Transesophageal Echocardiography
Modified 4CV with perfusion of coronary cavity. Pulsed-wave (PW) Doppler with normal flow over left coronary artery ostium (red rectangle). TEE = transesophageal echocardiography; other abbreviations as in Figures 1 and 5.
Figure 7.
Surgical Repair
(A) LAD with rupture ostium. (B) Patch reconstruction with bovine pericardial patch. (C) Completed repair with patch and bypass ad LAD. Abbreviation as in Figure 2.
Figure 8.
Postoperative Cardiac Enzyme Course
Day 0 is day of surgery. Troponin T and CK-MB levels were increasing at postoperative day 1.
Discussion
Rupture of a coronary artery spontaneous or due to previous coronary artery aneurysm is very rare. In most cases it presents as acute coronary syndrome with pericardial tamponade and requires emergency surgery.1,2
The development of coronary artery aneurysms has a wide pathogenesis including different rheumatic and vascular diseases, such as, in very rare cases, polyarteritis nodosa.3, 4, 5
The rupture of coronary artery aneurysms secondary to polyarteritis nodosa is seldom described in children but not described for adults until now.6,7 Furthermore, until now there have been no reports of patients with ruptured coronary artery due to polyarteritis nodosa with successful surgical treatment. Because we could not harvest tissue from the destroyed coronary artery, we can only speculate whether it was definitively polyarteritis nodosa which led to the rupture. However, considering all of the clinical findings (especially the previous tissue sample with arterial wall which confirmed the diagnosis of polyarteritis nodosa in August 2021) it is most likely.
In this case, the crucial factor leading to sufficient therapy was preoperative imaging because of the rheumatic history of the patient. The normal treatment in acute myocardial infarction and intrapericardial rupture with pericardial tamponade would have been taping the pericardial effusion and treatment with intracoronary stent placing, which likely would have resulted in the patient’s death. Using CT we were able to diagnose LAD aneurysm rupture with resulting myocardial rupture. Because of this CT scan, the pathway of clinical decision making was steered towards surgical treatment.
With establishment of CPB resulting in total mechanical decompression, a cautious approach to then uncover the pericardium from the ruptured cavity and the destroyed tissue was secured. A few clinical trials classify the simultaneous application of retrograde and antegrade cardioplegia as successful in bypass surgery.8,9 We decided to use both antegrade and retrograde cardioplegia combined to achieve fast cardiac arrest in a patient with interrupted LAD perfusion because antegrade cardioplegia alone would not have achieved myocardial protection of the distal LAD territory. Therefore, further ischemia was prevented after the massive myocardial infarction the patient had suffered.
Regarding the use of saphenous vein graft rather than left internal mammary artery (LIMA), 2 objectives led to the decision. 1) Because the patient had presented his untypical symptoms for several days, we were not sure about the outcome of LAD territory because we could not estimate the exact time span of myocardial ischemia. Therefore, internal mammary artery use could have been without advantages and would have had higher risk of wound complications. 2) The operative situs was very fragile. The ruptured cavity was covered by pericardial tissue which at that point prevented free rupture. Therefore, we decided against preparation of LIMA because the manipulation during LIMA harvesting could have uncovered the cavity leading to massive bleeding.
Follow-up
After medical rehabilitation, the patient was able to return to normal home and work environment without physical handicap.
Conclusions
Rupture of coronary artery aneurysms results in pericardial tamponade and myocardial infarction and requires emergency surgery. In patients with suspected or possible coronary anomalies due to rheumatic or infectious history, imaging with CT and transthoracic as well as transesophageal echocardiography is decisive to optimal decision making. Otherwise, the treatment of only the symptoms of myocardial infarction can be catastrophic. Simultaneous application of antegrade and retrograde cardioplegia proofed effective to achieve sufficient myocardial protection in our case of extensive anterior myocardial rupture with no subsequent impairment of left ventricular function. Until this report, no cases of adults with ruptured coronary arteries, polyarteritis nodosa, and successful operative treatment have been described in the literature.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Longobardi A., Iesu S., Baldi C., et al. Spontaneous coronary artery rupture presenting as an acute coronary syndrome evolved in pseudoaneurysm and cardiac tamponade: case report and literature review. Eur Heart J Acute Cardiovasc Care. 2017;6(7):666–669. doi: 10.1177/2048872615617043. [DOI] [PubMed] [Google Scholar]
- 2.Kondo T., Takahashi M., Nakagawa K., et al. Rupture of massive coronary artery aneurysm resulting in cardiac tamponade. Leg Med (Tokyo) 2015;17(5):388–390. doi: 10.1016/j.legalmed.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Gori T. Coronary vasculitis. Biomedicines. 2021;9(6):622. doi: 10.3390/biomedicines9060622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeudy J., White C.S., Kligerman S.J., et al. Spectrum of coronary artery aneurysms: from the Radiologic Pathology Archives. Radiographics. 2018;38(1):11–36. doi: 10.1148/rg.2018170175. [DOI] [PubMed] [Google Scholar]
- 5.Nichols L., Lagana S., Parwani A. Coronary artery aneurysm: a review and hypothesis regarding etiology. Arch Pathol Lab Med. 2008;132(5):823–828. doi: 10.5858/2008-132-823-CAAARA. [DOI] [PubMed] [Google Scholar]
- 6.Sinclair W., Jr., Nitsch E. Polyarteritis nodosa of the coronary arteries; report of a case in an infant with rupture of an aneurysm and intrapericardial hemorrhage. Am Heart J. 1949;38(6):898–904. doi: 10.1016/0002-8703(49)90890-x. [DOI] [PubMed] [Google Scholar]
- 7.Holt S., Jackson P. Ruptured coronary aneurysm and valvulitis in an infant with polyarteritis nodosa. J Pathol. 1975;117(2):83–87. doi: 10.1002/path.1711170204. [DOI] [PubMed] [Google Scholar]
- 8.Radmehr H., Soleimani A., Tatari H., Salehi M. Does combined antegrade-retrograde cardioplegia have any superiority over antegrade cardioplegia? Heart Lung Circ. 2008;17(6):475–477. doi: 10.1016/j.hlc.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Bhayana J.N., Kalmbach T., Booth F.V., et al. Combined antegrade/retrograde cardioplegia for myocardial protection: a clinical trial. J Thorac Cardiovasc Surg. 1989;98(5 pt 2):956–960. [PubMed] [Google Scholar]