Abstract
Gaucher type 3C disease with porcelain aorta can cause severe hemodynamic impairment. We report the first case, to our knowledge, of a 13-year-old Mexican girl with a GBA1 homozygous c.1342G>C [p.Asp448His] (commonly known as p.D409H) pathogenic variant who underwent extensive aortic replacement. She has been on enzyme replacement therapy and is alive 5 years after surgery. (Level of Difficulty: Intermediate.)
Key Words: acute heart failure, aortic valve, Gaucher disease, pediatric surgery, porcelain aorta
Abbreviations and Acronyms: EF, ejection fraction; ERT, enzyme replacement therapy; PTFE, polytetrafluoroethylene
Central Illustration
History of Presentation
A 13-year-old Mexican girl was referred to our hospital with a 6-month history of marked dyspnea on exertion and orthopnea.
Learning Objectives
-
•
To recognize the severe cardiovascular involvement in Gaucher disease 3C in children.
-
•
To be able to develop a differential diagnosis for porcelain aorta in children and acknowledge the benefit of prompt surgical referral.
On examination, she was tachycardic and tachypneic. She had a suprasternal thrill; a right upper sternal, midsystolic grade V/VI murmur; and an apical, pansystolic grade III/VI murmur. She had significant hepatomegaly and splenomegaly.
Past Medical History
The patient was the first pregnancy of young, healthy parents. Hepatosplenomegaly and slightly impaired eye movements were detected at age 2 years, but a comprehensive workup had not been carried out. Historical laboratory records showed anemia and thrombocytopenia.
Investigations
Initial electrocardiogram showed sinus rhythm with left ventricular hypertrophy. A posteroanterior chest x-ray film showed cardiomegaly, and a transthoracic echocardiogram revealed severe mitral and tricuspid regurgitation, aortic valve Doppler mean gradient of 98 mm Hg, limited visualization of the aortic arch, and left ventricular ejection fraction (EF) of 35% (Figure 1, Videos 1 and 2). Cardiac computed tomography demonstrated calcification of the aortic and mitral valves and concentric calcification of the aortic root, with ostial calcification of the coronary arteries and the brachiocephalic, left carotid, and left subclavian arteries (Figure 2). Cardiac magnetic resonance confirmed biventricular systolic dysfunction and aortic stenosis (Videos 3, 4, and 5).
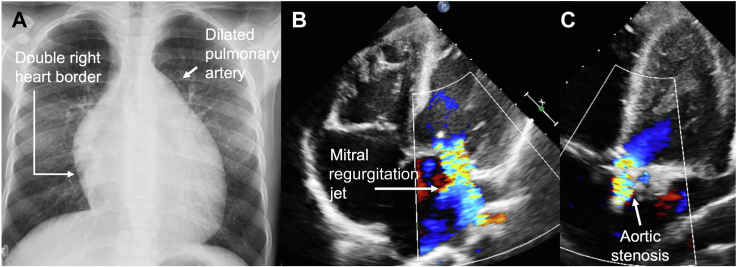
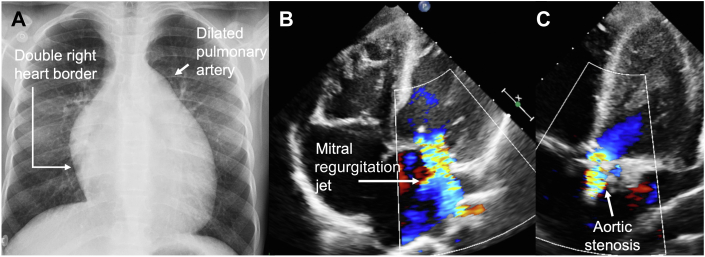
Figure 1.
Chest Radiography and Transthoracic Echocardiogram
(A) Global cardiomegaly and dilated pulmonary trunk. (B) Apical 4-chamber view demonstrates global dilation of cavities and mitral regurgitation. (C) Apical 5-chamber view shows aortic valve calcification and stenosis.
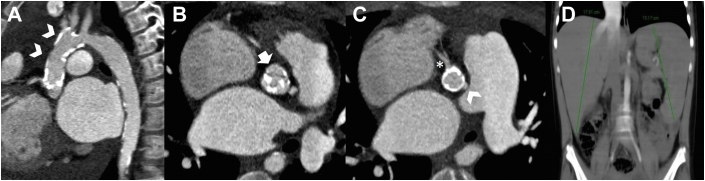
Figure 2.
Cardiac Computed Tomography Before Aortic Replacement Surgery With Prospective Electrocardiogram Triggering
(A) Double oblique sagittal view of the aortic root, ascending aorta, and origin of supra-aortic vessels (arrow heads) with calcification. (B) Double oblique transverse views showing aortic valve calcification (arrow) and (C) concentric calcification involving coronary ostia of the right (asterisk) and left coronary artery (arrowhead). (D) Sagittal view showing liver length of 19 cm and spleen length of 15 cm (>97th percentile for age).
Test results for erythrocyte sedimentation rate, serum C-reactive protein, and autoantibodies came back normal. Ophthalmologic assessment showed oculomotor apraxia.
Differential Diagnosis
Pediatric causes of aortic calcification were sought, and our first suspicion was Takayasu arteritis; however, the atypical course compelled us to rule out other disorders.
We deemed infectious aortitis very unlikely because inflammatory signs, risk exposures, and immunodeficiency were lacking, and no pathogens were retrieved from biopsy samples. Systemic lupus erythematosus and rheumatoid arthritis were also ruled out.
Finally, we conducted a literature search and found 2 monogenic childhood-onset disorders that can feature porcelain aorta: Singleton-Merten syndrome and Gaucher type 3C. The first one is a rare interferonopathy caused by mutations in genes IFIH1 and DDX58.1 Autosomal recessive Gaucher type 3C matches the multisystem involvement seen in our case report: longstanding hepatosplenomegaly, bicytopenia, oculomotor apraxia, and porcelain aorta.2
Management
Thoracic aorta was replaced using a 16-mm polytetrafluoroethylene (PTFE) graft with reimplantation of the 3 supra-aortic trunks. Using the Konno procedure, the aortic annulus was enlarged and sutured to a valved PTFE conduit (21-mm St. Jude Medical mechanical valve). Coronary arteries were reimplanted through the Cabrol technique with a 4-mm PTFE graft; severe coronary ostial calcification precluded a button Bentall procedure. The mitral valve was replaced with a 25-mm St. Jude Medical mechanical valve (Figure 3).
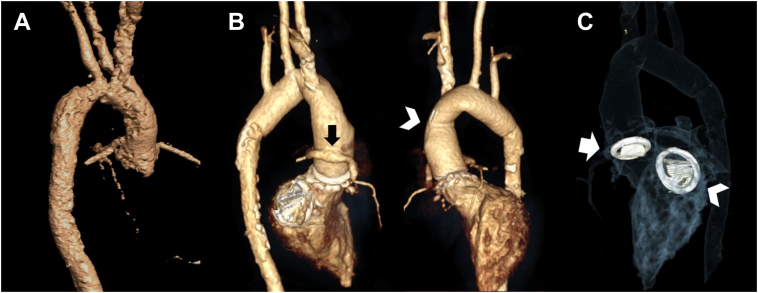
Figure 3.
Cardiac Computed Tomography After Aortic Replacement Surgery With Ultrafast, High-Pitch Spiral Acquisition
3-dimensional volume-rendering reconstructions of the aorta (A) before and (B) after aortic replacement, showing coronary reimplantation to the valved conduit using the Cabrol technique (arrow) and anastomosis (arrowhead) between the 21-mm valved conduit and 16-mm graft. (C) Aortic (arrow) and mitral (arrowhead) mechanical valves in adequate positions.
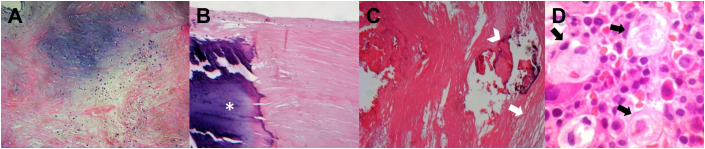
Pathology report indicated calcification and fibrosclerotic degeneration of the aortic wall (Figure 4).
Figure 4.
Histopathologic Images of the Aortic Wall, Mitral Valve, and Bone Marrow
(A) Hematoxylin and eosin stain shows the mitral valve with mixoid degeneration and sclerosis. (B) Dystrophic calcification in the mitral valve (asterisk). (C) Aortic wall with sclerosis (arrow) and calcification (arrowhead). (D) Bone marrow biopsy sample showing classical “wrinkled tissue paper” Gaucher cells (arrows).
Pharmacologic therapy included digoxin, captopril, furosemide, spironolactone, acetylsalicylic acid, and acenocoumarin. After surgery, hepatosplenomegaly and bicytopenia were still present, so bone marrow examination and next-generation sequencing were requested. We found a c.1342G>C [p.Asp448His] (commonly known as p.D409H) homozygous pathogenic variant and several Gaucher cells in the bone marrow. Low glucocerebrosidase activity of 0.41 nmol/mL/h (normal: 1.69-22.6) in peripheral leucocytes confirmed the diagnosis. Both parents were confirmed to be carriers. Imiglucerase enzyme replacement therapy (ERT) therapy was started at 60 U/kg once every 2 weeks.
Discussion
Porcelain aorta is defined as diffuse concentric calcification of the thoracic aorta. There are several causes of pediatric porcelain aorta to bear in mind (Table 1).1,3
Table 1.
Differential Diagnoses in Childhood Porcelain Aorta
| Diagnosis | Features |
|---|---|
| Takayasu arteritis | Chronic granulomatous large vessel panarteritis |
| Systemic lupus erythematous and rheumatoid arthritis | Autoimmune diseases, atherosclerotic plaque calcification |
| Infectious aortitis | Diverse pathogens, aneurysms mostly described |
| Gaucher disease 3C | Autosomal recessive lysosomal storage disease |
| Singleton Merten syndrome | Autosomal dominant interferonopathy |
Gaucher disease is an autosomal recessive lysosomal storage disorder caused by a deficiency of the enzyme glucocerebrosidase, caused by GBA1 gene pathogenic homozygous or compound heterozygous variants. Most cases share 2 clinical features: hepatosplenomegaly and cytopenias. Gaucher type 3C (Online Mendelian Inheritance in Man number 231005) features mitral and aortic calcification, oculomotor apraxia, and hepatosplenomegaly. It is caused by the homozygous mutation c.1342G>C [p.Asp448His], also known as p.D409H. Even though it is the most frequent lysosomal storage disease, subtype 3C is extremely rare.2
Few other cases of Gaucher 3C with porcelain aorta have been reported, with a median age at diagnosis of 14 years and a 1.5:1 female-to-male ratio (Table 2).4, 5, 6, 7, 8, 9 All cases report cardiovascular symptoms at diagnosis. Premature cardiovascular death occurred in patients who did not undergo surgical correction.8,9
Table 2.
Pediatric Reports of Gaucher Disease 3C With Porcelain Aorta
| First Author, Year | Ethnicity/Sex | Age at Diagnosis, y | Clinical Overview | Cardiovascular Calcification | Diagnostic Method | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| George et al, 20014 | Palestinian Male |
17 | Dyspnea, chest pain Splenomegaly Corneal deposits |
Aorta (ascending, transverse, isthmus) Aortic/mitral valves Coronaries |
Homozygous D409H Low enzyme activity Gaucher cells in mitral valve |
Aortic/mitral valve and ascending aorta replacement Enzyme therapy |
Stable 20 months after surgery |
| Shah et al, 20085 | Indian Female |
12 | Dyspnea on exertion Epistaxis Splenomegaly Oculomotor apraxia Corneal deposits |
Aorta (ascending, transverse) Aortic/mitral valves |
Homozygous D409H Gaucher cells in bone marrow |
— | — |
| Talluto and Silverman, 20106 | Mexican American Female |
13 | Acute heart failure | Aorta (ascending, transverse) Aortic/mitral valves Coronaries |
Gaucher cells in cardiac valve | Mitral/aortic valve and aortic replacement Patch augmentation |
Stable after surgery |
| Mireles et al, 20107 | Hispanic Female |
13 | Intermittent chest pain | Aorta (ascending, transverse, isthmus) Aortic/mitral valves Coronaries |
Homozygous D409H | Replacement: mitral/aortic valves, ascending aorta Enzyme therapy |
Died 14 months after surgery |
| Altunbas et al, 20158 | Not reported | 17 | Palpitations Syncope |
Thoracic aorta Aortic/mitral valves Brachiocephalic trunk |
— | Inotropic therapy | Died 8 hours after symptom onset |
| Kör et al, 20179 | Male | 15 | Syncope Splenomegaly Oculomotor apraxia Mild osteoporosis |
Aorta (ascending, transverse) Aortic/mitral valves Left subclavian, left carotid Left coronary |
Homozygous D409H Gaucher cells in bone marrow Low enzyme activity |
Enzyme therapy Beta blocker Angiotensin-converting enzyme inhibitor |
Died of myocardial infarction 6 months after enzyme therapy |
There is a clear genotype-phenotype correlation, and rare interferonopathies were also suspected; thus, next-generation sequencing proved useful to confirm the diagnosis.
Genetic counseling is mandatory to inform about the risk of developing the disease. As for our report, both parents are unaffected carriers and normal enzyme activity was reported in both dizygotic twin siblings.
ERT improves visceral manifestations; however, there are no clear data regarding cardiovascular sequels, which account for high mortality rates. The International Gaucher Registry described outcomes among Gaucher disease type 3 patients after ERT initiation, including 11 children with homozygous p.D409H mutation. Six patients died of cardiac disease, 4 of whom were p.D409H homozygous, reaching a cardiac mortality of 36% in type 3C.10
Follow-Up
After surgery, the patient’s functional class improved dramatically, and she continues ERT. Serial echocardiograms have reported recovered left ventricular systolic function and normal functioning of the mechanical valves. A control computed tomography angiography after surgery did not show signs of new vascular damage, and her last cardiac magnetic resonance 5 years after surgery demonstrated left ventricular EF of 61% and right ventricular EF of 42%, with a slight increase in T1 values at the basal septum of 1,109 ms and 28% extracellular volume (Figure 3, Videos 4, 5, and 6).
Conclusions
Porcelain aorta in Gaucher type 3C carries high mortality, and aortic replacement is a lifesaving approach. Raising awareness of the early manifestations of Gaucher type 3C is crucial to improve diagnostic yield whenever newborn screening and molecular techniques are not available. Lifelong cardiovascular follow-up is mandatory to monitor enzyme therapy response, anticoagulation therapy, and prosthetic valve functioning. Longitudinal studies need to clarify the role of enzyme therapy in detaining or even preventing cardiovascular compromise in these patients.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Transthoracic Echocardiogram 4-chamber and 2-chamber Views. Mitral valve with thickened leaflets and restricted motion causing severe mitral regurgitation.
Transthoracic Echocardiogram 5-Chamber and Parasternal Short-Axis Views. Aortic valve calcification with severe aortic stenosis.
Cardiac Magnetic Resonance Assessment of the Aortic Valve. Phase-contrast velocity mapping shows severe stenosis of the aortic valve.
Cardiac Magnetic Resonance Short-Axis Cine Images. Improved left ventricular wall motility on follow-up cardiac magnetic resonance.
Cardiac Magnetic Resonance 4-Chamber Cine Images. Improved left ventricular wall motility on follow-up cardiac magnetic resonance. The mechanical valve can be appreciated.
Follow-up Cardiac Magnetic Resonance. The aortic root with mechanical valve can be appreciated in a cine sequence. Three-dimensional volume-rendered image allows visualization of the replaced thoracic aorta without stenosis.
References
- 1.Nitschke Y., Rutsch F. Inherited arterial calcification syndromes: etiologies and treatment concepts. Curr Osteoporos Rep. 2017;15:255–270. doi: 10.1007/s11914-017-0370-3. [DOI] [PubMed] [Google Scholar]
- 2.Stirnemann J., Belmatoug N., Camou F., et al. A review of Gaucher disease pathophysiology, clinical presentation and treatments. Int J Mol Sci. 2017;18:1–30. doi: 10.3390/ijms18020441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abramowitz Y., Jilaihawi H., Chakravarty T., Mack M.J., Makkar R.R. Porcelain aorta: a comprehensive review. Circulation. 2015;131:827–836. doi: 10.1161/CIRCULATIONAHA.114.011867. [DOI] [PubMed] [Google Scholar]
- 4.George R., McMahon J., Lytle B., Clark B., Lichtin A. Severe valvular and aortic arch calcification in a patient with Gaucher’s disease homozygous for the D409H mutation. Clin Genet. 2001;59:360–363. doi: 10.1034/j.1399-0004.2001.590511.x. [DOI] [PubMed] [Google Scholar]
- 5.Shah S., Misri A., Bhat M., Maheshwari S. Gaucher’s disease type III C: unusual cause of intracardiac calcification. Ann Pediatr Cardiol. 2008;1:144–146. doi: 10.4103/0974-2069.43883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talluto C.J., Silverman N.H. Aortic and mitral valve stenosis with regurgitation: not due to rheumatic heart disease. Echocardiography. 2011;28:E24–E27. doi: 10.1111/j.1540-8175.2010.01253.x. [DOI] [PubMed] [Google Scholar]
- 7.Mireles S.A., Seybold J., Williams G. Undiagnosed type IIIc Gaucher disease in a child with aortic and mitral valve calcification: perioperative complications after cardiac surgery. J Cardiothorac Vasc Anesth. 2010;24 doi: 10.1053/j.jvca.2009.05.006. 471-447. [DOI] [PubMed] [Google Scholar]
- 8.Altunbas G., Ercan S., Inanç I.H., Ozer O., Kervancıoglu S., Davutoglu V. Extensive vascular and valvular involvement in Gaucher disease. Asian Cardiovasc Thorac Ann. 2015;23:446–448. doi: 10.1177/0218492313513598. [DOI] [PubMed] [Google Scholar]
- 9.Kör Y., Keskin M., Başpınar O. Severe cardiac involvement in Gaucher type IIIC: a case report and review of the literature. Cardiol Young. 2017;27:1426–1429. doi: 10.1017/S1047951117000579. [DOI] [PubMed] [Google Scholar]
- 10.El-Beshlawy A., Tylki-Szymanska A., Vellodi A., et al. Long-term hematological, visceral, and growth outcomes in children with Gaucher disease type 3 treated with imiglucerase in the International Collaborative Gaucher Group Gaucher Registry. Mol Genet Metab. 2017;120(1-2):47–56. doi: 10.1016/j.ymgme.2016.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transthoracic Echocardiogram 4-chamber and 2-chamber Views. Mitral valve with thickened leaflets and restricted motion causing severe mitral regurgitation.
Transthoracic Echocardiogram 5-Chamber and Parasternal Short-Axis Views. Aortic valve calcification with severe aortic stenosis.
Cardiac Magnetic Resonance Assessment of the Aortic Valve. Phase-contrast velocity mapping shows severe stenosis of the aortic valve.
Cardiac Magnetic Resonance Short-Axis Cine Images. Improved left ventricular wall motility on follow-up cardiac magnetic resonance.
Cardiac Magnetic Resonance 4-Chamber Cine Images. Improved left ventricular wall motility on follow-up cardiac magnetic resonance. The mechanical valve can be appreciated.
Follow-up Cardiac Magnetic Resonance. The aortic root with mechanical valve can be appreciated in a cine sequence. Three-dimensional volume-rendered image allows visualization of the replaced thoracic aorta without stenosis.