Abstract
Mesenchymal stem cells are multipotent stem cells that reside in many human tissues and organs. Mesenchymal stem cells are widely used in experimental and clinical regenerative medicine due to their capability to transdifferentiate into various lineages. However, when transplanted, they lose part of their multipotency and immunomodulatory properties, and most of them die after injection into the damaged tissue. In this review, we discuss the potential utility of melatonin in preserving mesenchymal stem cells’ survival and function after transplantation. Melatonin is a pleiotropic molecule regulating critical cell functions including apoptosis, endoplasmic reticulum stress, and autophagy. Melatonin is also synthesized in the mitochondria where it reduces oxidative stress, the opening of the mitochondrial permeability transition pore and the downstream caspase activation, activates uncoupling proteins, and curtails the proinflammatory response. In addition, recent findings showed that melatonin also promotes the formation of tunneling nanotubes and the transfer of mitochondria between cells through the connecting tubules. As mitochondrial dysfunction is a primary cause of mesenchymal stem cells death and senescence and a critical issue for survival after transplantation, we propose that melatonin by favoring mitochondria functionality and their transfer through tunneling nanotubes from healthy to suffering cells could improve mesenchymal stem cell-based therapy in a large number of diseases for which basic and clinical trials are underway.
Keywords: brain ischemia, cell transplantation, melatonin, mesenchymal stem cell, mitochondria, mitochondrial transplantation, regenerative therapy, senescence, tunneling nanotubes
Introduction
Mesenchymal stem cells (MSCs) are non-hematopoietic stem cells originating from the mesoderm. MSCs may be readily isolated from several different adult tissues, such as liver, muscle, bone marrow, adipose tissue, and umbilical cord, and are being incorporated as an alternative cell source in regenerative therapy. Since they originate from adult tissues, their utilization bypasses the ethical, political, and fundamental moral issues raised by pluripotent embryonic stem cells isolated from the inner mass of the blastocyst (Frankel, 2000; Zuk et al., 2001; Zheng, 2016). Currently, many clinical trials are evaluating MSCs therapy in a variety of diseases, including osteoarthritis, wound healing, degenerative diseases, and autoimmune disorders (US National Institutes of Health; ClinicalTrials.gov). Once transplanted into the recipient tissue, the effects of MSCs are influenced by three main factors, i.e. (i) the expansion and differentiation into tissues of mesodermal origin; (ii) the close interactions with neighboring cells through cell-to-cell contact, and the release of paracrine factors and extracellular vesicles, which regulate the microenvironmental changes affecting immune effector cells, vessels, and other cell types; and (iii) the apoptotic phenomena involving both MSCs, immune, and tissue cells, eventually leading to efferocytosis of cellular debris, microenvironmental reorganization, and functional polarisation of phagocytic cells. While the first factor is far from being elucidated, the last two factors seem to mediate the long-lasting therapeutic effects that MSCs have been showing in different animal models (Liu et al., 2019).
A growing body of evidence has shown that the biological activity of MSC is mediated by the paracrine release of bioactive factors. When MSCs are transplanted, they find a hostile environment and may lose part of their multipotency and immunomodulatory properties. Most of them, in addition, die after injection into the damaged tissue. Thus, it is important to develop effective strategies for improving the efficacy of MSCs-based therapies. In this perspective, we briefly review recent evidence showing that melatonin possesses properties that could have the potential to improve MSCs-based cell therapy.
Search Strategy and Eligibility Criteria
This review was constructed using the information gathered, assembled, and selected from publications found using PubMed and Google Scholar. Searches were performed for studies published up to 2022. The search strategy utilized the MeSH terms melatonin, tunneling nanotubes, mitochondria, sirtuins, mitochondrial transplantation, mesenchymal stem cell, cell transplantation, regenerative therapy, brain ischemia, senescence. In some cases, bibliographic entries cited in the most relevant studies of this narrative were used. No limit was placed on the year of publication or authorship.
Mitochondria Transfer through Tunneling Nanotubes May Represent a Key Process for Survival in Seriously-Damaged Cells
Intercellular connections through tunneling nanotubes (TNTs) represent a novel direct way of communication between distant cells. TNTs are thin-extended membrane protrusions connecting cells over long distances allowing them to share components of their cytoplasm such as small molecules (e.g., calcium ions), macromolecules (e.g., nucleic acids and proteins), and organelles (e.g., mitochondria, vesicles, lysosomes, and autophagosomes). TNTs physical connections occur under both physiological and pathological conditions, leading to changes in cell energy metabolism and functions among multiple involved cells. Through TNT, cells can form dynamic transient networks allowing the exchange of information which ultimately allows these cells to respond more efficiently to cellular challenges, including tissue repair following injuries (Wang and Gerdes, 2015; Nasoni et al., 2021).
By regulating energy production, intermediary metabolism, calcium signaling, and apoptosis, mitochondria are essential organelles for maintaining cellular homeostasis; dysfunction of these organelles is responsible for numerous acute and chronic neurodegenerative disorders including ischemia/reperfusion injury. In cells, mitochondria establish a dynamic network that changes continuously in size, shape, and location; these changes are mainly controlled through biogenesis, fission, and fusion. Mitochondrial fission is characterized by the division of a mitochondrion into two daughter organelles, whereas mitochondrial fusion is the union of two mitochondria resulting in the formation of a large new mitochondrion. The balance between fission and fusion is crucial to ensure a steady energy supply and cellular homeostasis. Whereas mitochondrial fission causes mitochondrial fragmentation and is generally associated with metabolic dysfunctions, fusion permits the exchange of contents between two mitochondria, allowing them to maintain optimal biochemical and genetic uniformity via elimination of damaging reactive oxygen species (ROS) and mutated DNA, and reducing the polarization of membranes (Youle and van der Bliek, 2012).
It has been shown that healthy mitochondria can be harvested from healthy cells and transplanted into injured tissue (McCully et al., 2009). They can also be transferred between healthy and injured cells through TNTs (Nasoni et al., 2021), thereby improving recipient cell survival. Mitochondrial trafficking via TNTs seems to be an essential mechanism used by MSCs to promote tissue regeneration and improve organ functions. Through TNT formation, for example, MSCs repaired postischemic endothelial cells by transferring functional mitochondria and protecting endothelial cells from apoptosis (Liu et al., 2018). MSCs from TNTs between different target cells, including renal tubular, endothelial, pulmonary alveolar epithelial, neuronal, and cancer cells. In addition to TNTs, MSCs also form gap junctions, release exosomes, and transfer other constituents that affect target cells; these processes are important signaling processes for stem cell-based regenerative therapy.
The removal of MSCs from their original environment, unfortunately, causes them to quickly lose their vitality and become senescent, reducing their stemness and hindering the differentiation and their repair efficiency. Two types of cellular senescence have been described in mammalian cells (Sikora et al., 2011), i.e., the replicative senescence that occurs with the arrest of cellular proliferation after a number of divisions due to the telomerase attrition, and the stress-induced premature senescence that occurs when the cells stop proliferating under conditions such as oxidative stress, DNA damage or oncogene activation (Kuilman et al., 2010). The ex vivo expansion of MSCs requires massive numbers of these cells because replicative senescence causes decreased therapeutic efficacy (Lee et al., 2020).
Senescent MSCs no longer undergo cell division and their multilineage differentiation potential is markedly impaired thus rendering them less useful for many situations (Zhou et al., 2015). During senescence, cell size and mitochondrial mass significantly increase but the mitochondria often exhibit a reduced membrane potential and at the same time produce increased quantities of destructive ROS. Senescent MSCs, in addition, remain metabolically active but develop a senescence-associated secretory phenotype (SASP) characterized by augmented oxidative damage and the secretion of proinflammatory cytokines, which promotes chronic inflammation and deleterious changes in the tissue microenvironment (Watanabe et al., 2017). Because of the harsh microenvironment and the slowly-developing blood supply that cannot provide sufficient nutrition or growth factors, after their in vivo injection most implanted cells become senescent and undergo apoptosis or necrosis.
Melatonin May Represent an Effective Adjuvant Treatment for Mesenchymal Stem Cells-Based Therapy
Melatonin is a pleiotropic molecule with diverse and potent antioxidant actions and it regulates a number of critical cellular functions including apoptosis and autophagy, and also curtails the amount of endoplasmic reticulum stress and the proinflammatory response (Tan et al., 2016; Reiter et al., 2022). Melatonin is also synthesized in the mitochondria. It has been recently reported that these organelles can release melatonin, which can bind to high-affinity MT1 receptors located in the outer mitochondrial membrane (“automitocrine” stimulation). By activating the mitochondrial MT1/G protein signal system, melatonin inhibits the release of cytochrome c and apoptosis (Suofu et al., 2017). Thus, mitochondrial melatonin functions in the maintenance of optimal membrane potential controlling their reductive state by neutralizing ROS inhibiting the mitochondrial permeability transition pore, and activating uncoupling proteins (Tan et al., 2016; Suofu et al., 2017; Reiter et al., 2022). When ex vivo MSCs are treated with melatonin, senescence and oxidative stress are reduced due to the attenuation of p-p38, inhibition of p16, and augmentation of SIRT1 (Zhou et al., 2015); it also prevents aberrant differentiation and an iron imbalance which normally would contribute to the generation of the highly destructive hydroxyl radicals and membrane potential depolarization (Yang et al., 2017). Moreover, incubating MSCs with melatonin attenuates their long-term expansion-mediated cellular senescence, likely modulating the autophagic process (Tan et al., 2021).
Melatonin enhances the therapeutic potential of MSCs in myocardial infarction, chronic kidney disease, hindlimb ischemia, and focal cerebral ischemia (Tang et al., 2014). In ischemic and chronic kidney disease, melatonin increases the regenerative potential of MSCs through upregulation of the cellular prion protein, which is involved in self-renewal, differentiation, and angiogenesis in stem and/or progenitor cells (Doeppner et al., 2015). Melatonin promotes the survival of MSCs in vitro and prevents them from undergoing apoptosis after transplantation into the ischemic brain resulting in increased angiogenesis, neurogenesis, and expression of vascular endothelial growth factor. Luzindole, a melatonin receptor antagonist, and the ERK phosphorylation inhibitor U0126 completely reversed the protective effects of melatonin documenting the involvement of membrane melatonin receptors (Tang et al., 2014). In cultured placenta-derived MSCs, melatonin increased cell proliferation and survival rate and enhanced the degree of neuronal differentiation by acting on the MT1 receptor (Hu and Li, 2019).
We recently reported that in cultured hippocampal HT22 neuronal cells subjected to oxygen/glucose deprivation, the addition of melatonin during the reperfusion phase improved cell survival and prevented mitochondrial dysfunction by reducing ROS formation and restoring the mitochondrial fusion/fission dynamics affected by oxygen/glucose deprivation. In this model, melatonin favored mitochondrial fusion and, in addition, mitochondria conserved their shapes and parallel cristae comparable to the control condition. Melatonin also preserved the membrane translocases TOM20 and TIM23 and the matrix protein Hsp60, proteins involved in mitochondrial biogenesis (Nasoni et al., 2021). However, the most novel and important finding that emerged from this study was the observation that, besides preserving mitochondrial function, melatonin also promoted the formation of TNTs and the transfer of mitochondria between cells through the connecting tubules (Nasoni et al., 2021). Yip et al. (2021) reported a similar effect in a rat model of brain ischemia. These authors showed that purified healthy mitochondria pre-treated with melatonin and injected after reperfusion into the infarcted site enhanced the number of intact and functional mitochondria in the damaged neurons reduced mitochondrial DNA damage, and lowered oxidative stress, apoptosis, and the infarct volume. Using mitochondrial trackers, the authors showed that the injected healthy mitochondria were transferred from healthy to apoptotic cells through TNTs.
Other evidence linking melatonin to mitochondrial function preservation and cell viability involves sirtuins. Sirtuins, a NAD-dependent class III histone deacetylase family, play pivotal roles in preventing cellular senescence and age-associated diseases (O’Callaghan and Vassilopoulos, 2017). For example, SIRT1 overexpression delays human bone marrow-derived MSCs and adipose tissue-derived MSCs senescence (Yuan et al., 2012). Similarly, depletion of Sirtuin 3, the major mitochondrial deacetylase involved in reducing oxidative stress, accelerates aging and inhibits MSCs differentiation into osteoblasts and adipocytes. Overexpression of Sirtuin 3 can restore their differentiation capacity, reduce oxidative stress and defer senescence (Denu, 2017). Melatonin has marked effects on sirtuins. We previously reported a marked and rapid increase in SIRT1 expression after neonatal hypoxia/ischemia (Carloni et al., 2017); this effect also has been observed in different systems (Song et al., 2020). Likewise, melatonin is a potent stimulator of mitochondrial Sirtuin 3, which influences numerous beneficial downstream targets (Reiter et al., 2022).
Concluding Remarks and Perspective
Because of their self-renewal and multilineage differentiation capabilities, MSCs hold enormous potential for the treatment of many pathological conditions including degenerative diseases; as a result, they represent an expanding frontier of regenerative medicine. MSCs form TNTs and gap junctions between cells, and transfer mitochondria and other constituents to target cells. In addition, MSCs secrete soluble factors including hormones and proteins, and release exosomes or micro-vesicles that contain immunoregulatory agents and other molecules.
Melatonin exerts important effects on mitochondria, and mitochondrial dysfunction is a primary cause of cell death and senescence of MSCs (Wang et al., 2020) and it is a critical issue related to their survival after transplantation. We propose that melatonin, for the reasons described here and in our recent publication (Luchetti et al., 2022), may represent a potentially effective pharmacological agent to improve both MSCs-based and mitochondrial transplantation-based therapy. The latter is a therapeutic approach already tested in pediatric patients with myocardial ischemia, although its efficacy needs further documentation (Bertero et al., 2018). Several pieces of evidence support our hypothesis, the first of which relates to preserving mitochondria survival and reducing inflammation. Indeed, maintaining oxidative/reductive homeostasis represents a well-known strategy to extend the survival and reduce the senescence of MSCs. In addition, melatonin also stimulates TNTs formation, and mitochondrial trafficking via TNTs seems to be a key mechanism by which MSCs promote tissue regeneration and improve organ functions. If the reported findings are further confirmed, melatonin could prove to have great therapeutic potential in MSCs-based therapy as it applies to a large number of diseases, including neurological and immune disorders, cerebral and cardiac ischemia, diabetes, and bone and cartilage diseases (Figure 1). The effective dose, as well as the possible limitation of the melatonin-based pharmacological approach, need to be addressed and, indeed, one purpose of this narrative is to stimulate further research in this critically important field.
Figure 1.
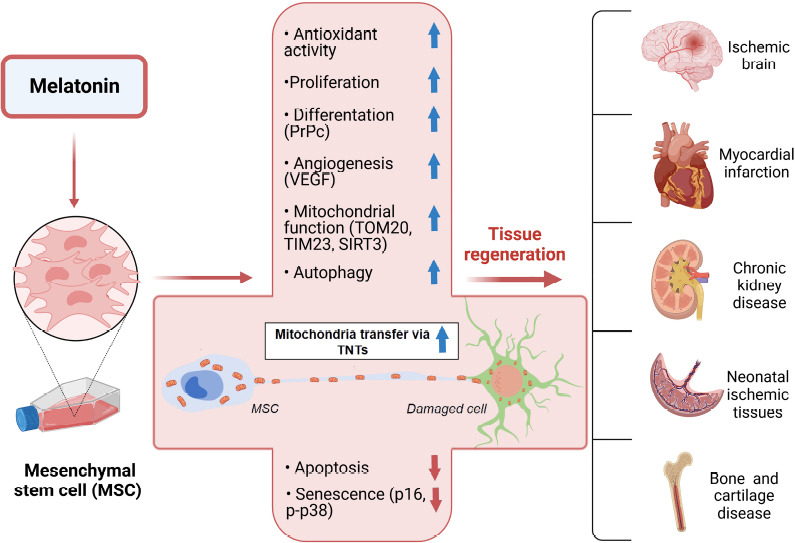
The different mechanisms by which melatonin may improve MSCs-based cell transplantation.
The upper part of the figure shows the main effects stimulated by melatonin and responsible for the reduction of apoptosis and senescence, as shown in the lower part of the figure. The improved mitochondrial functions and trafficking via TNTs may represent the key mechanisms by which melatonin may improve MSCs cell transplantation and organ functions. MSCs: Mesenchymal stem cells; PrPc: cellular prion protein; SIRT3: Sirtuin 3; TIM23: translocase of inner membrane 23; TNTs: tunneling nanotubes; TOM20: translocase of outer membrane 20; VEGF: vascular endothelial growth factor. Created with BioRender.com.
Additional file: Open peer review reports 1 (88.6KB, pdf) and 2 (86.1KB, pdf) .
Footnotes
Funding: This work was supported by the University of Urbino Carlo Bo (No. DR-473_2018) to WB.
Conflicts of interest: The authors declare no conflicts of interest.
Availability of data and materials: All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open peer reviewers: Pavel Montes de Oca Balderas, Instituto nacional de Neurologia y Neurocirugia, Mexico; Chengbin Xue, Affiliated Hospital of Nantong University, China.
P-Reviewers: Montes de Oca Balderas P, Xue C; C-Editors: Zhao M, Liu WJ, Wang Lu; T-Editor: Jia Y
References
- 1.Bertero E, Maack C, O'Rourke B. Mitochondrial transplantation in humans:“magical”cure or cause for concern? J Clin Invest. 2018;128:5191–5194. doi: 10.1172/JCI124944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carloni S, Riparini G, Buonocore G, Balduini W. Rapid modulation of the silent information regulator 1 by melatonin after hypoxia-ischemia in the neonatal rat brain. J Pineal Res. 2017 doi: 10.1111/jpi.12434. doi:10.1111/jpi.12434. [DOI] [PubMed] [Google Scholar]
- 3.Denu RA. SIRT3 enhances mesenchymal stem cell longevity and differentiation. Oxid Med Cell Longev. 2017;2017:5841716. doi: 10.1155/2017/5841716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doeppner TR, Kaltwasser B, Schlechter J, Jaschke J, Kilic E, Bähr M, Hermann DM, Weise J. Cellular prion protein promotes post-ischemic neuronal survival angioneurogenesis and enhances neural progenitor cell homing via proteasome inhibition. Cell Death Dis. 2015;6:e2024. doi: 10.1038/cddis.2015.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frankel MS. In search of stem cell policy. Science. 2000;287:1397. doi: 10.1126/science.287.5457.1397. [DOI] [PubMed] [Google Scholar]
- 6.Hu C, Li L. Melatonin plays critical role in mesenchymal stem cell-based regenerative medicine in vitro and in vivo. Stem Cell Res Ther. 10. 2019:13. doi: 10.1186/s13287-018-1114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JH, Yoon YM, Song KH, Noh H, Lee SH. Melatonin suppresses senescence-derived mitochondrial dysfunction in mesenchymal stem cells via the HSPA1L-mitophagy pathway. Aging Cell. 2020;19:e13111. doi: 10.1111/acel.13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu F, Lu J, Manaenko A, Tang J, Hu Q. Mitochondria in ischemic stroke:new insight and implications. Aging Dis. 2018;9:924–937. doi: 10.14336/AD.2017.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu L, Wong CW, Han M, Farhoodi HP, Liu G, Liu Y, Liao W, Zhao W. Meta-analysis of preclinical studies of mesenchymal stromal cells to treat rheumatoid arthritis. EBioMedicine. 2019;47:563–577. doi: 10.1016/j.ebiom.2019.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luchetti F, Carloni S, Nasoni MG, Reiter RJ, Balduini W. Tunneling nanotubes and mesenchymal stem cells:New insights into the role of melatonin in neuronal recovery. J Pineal Res. 2022;13:e12800. doi: 10.1111/jpi.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCully JD, Cowan DB, Pacak CA, Toumpoulis IK, Dayalan H, Levitsky S. Injection of isolated mitochondria during early reperfusion for cardioprotection. Am J Physiol Heart Circ Physiol. 2009;296:H94–105. doi: 10.1152/ajpheart.00567.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nasoni MG, Carloni S, Canonico B, Burattini S, Cesarini E, Papa S, Pagliarini M, Ambrogini P, Balduini W, Luchetti F. Melatonin reshapes the mitochondrial network and promotes intercellular mitochondrial transfer via tunneling nanotubes after ischemic-like injury in hippocampal HT22 cells. J Pineal Res. 2021;71:e12747. doi: 10.1111/jpi.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Callaghan C, Vassilopoulos A. Sirtuins at the crossroads of stemness aging and cancer. Aging Cell. 2017;16:1208–1218. doi: 10.1111/acel.12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reiter RJ, Sharma R, Rosales-Corral S, de Campos Zuccari DAP, de Almeida Chuffa LG. Melatonin:A mitochondrial resident with a diverse skill set. Life Sci. 2022;301:120612. doi: 10.1016/j.lfs.2022.120612. [DOI] [PubMed] [Google Scholar]
- 16.Sikora E, Arendt T, Bennett M, Narita M. Impact of cellular senescence signature on ageing research. Ageing Res Rev. 2011;10:146–152. doi: 10.1016/j.arr.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Song YJ, Zhong CB, Wu W. Cardioprotective effects of melatonin:Focusing on its roles against diabetic cardiomyopathy. Biomed Pharmacother. 2020;128:110260. doi: 10.1016/j.biopha.2020.110260. [DOI] [PubMed] [Google Scholar]
- 18.Suofu Y, Li W, Jean-Alphonse FG, Jia J, Khattar NK, Li J, Baranov SV, Leronni D, Mihalik AC, He Y, Cecon E, Wehbi VL, Kim J, Heath BE, Baranova OV, Wang X, Gable MJ, Kretz ES, Di Benedetto G, Lezon TR, et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc Natl Acad Sci U S A. 2017;114:E7997–8006. doi: 10.1073/pnas.1705768114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan DX, Manchester LC, Qin L, Reiter RJ. Melatonin:a mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int J Mol Sci. 2016;17:2124. doi: 10.3390/ijms17122124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan YZ, Xu XY, Dai JM, Yin Y, He XT, Zhang YL, Zhu TX, An Y, Tian BM, Chen FM. Melatonin induces the rejuvenation of long-term ex vivo expanded periodontal ligament stem cells by modulating the autophagic process. Stem Cell Res Ther. 2021;12:254. doi: 10.1186/s13287-021-02322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Y, Cai B, Yuan F, He X, Lin X, Wang J, Wang Y, Yang GY. Melatonin pretreatment improves the survival and function of transplanted mesenchymal stem cells after focal cerebral ischemia. Cell Transplant. 2014;23:1279–1291. doi: 10.3727/096368913x667510. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Gerdes HH. Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ. 2015;22:1181–1191. doi: 10.1038/cdd.2014.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Liu Y, Chen E, Pan Z. The role of mitochondrial dysfunction in mesenchymal stem cell senescence. Cell Tissue Res. 2020;382:457–462. doi: 10.1007/s00441-020-03272-z. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe S, Kawamoto S, Ohtani N, Hara E. Impact of senescence-associated secretory phenotype and its potential as a therapeutic target for senescence-associated diseases. Cancer Sci. 2017;108:563–569. doi: 10.1111/cas.13184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang F, Yang L, Li Y, Yan G, Feng C, Liu T, Gong R, Yuan Y, Wang N, Idiiatullina E, Bikkuzin T, Pavlov V, Li Y, Dong C, Wang D, Cao Y, Han Z, Zhang L, Huang Q, Ding F, et al. Melatonin protects bone marrow mesenchymal stem cells against iron overload-induced aberrant differentiation and senescence. J Pineal Res. 2017;63:e12422. doi: 10.1111/jpi.12422. [DOI] [PubMed] [Google Scholar]
- 26.Yip HK, Dubey NK, Lin KC, Sung PH, Chiang JY, Chu YC, Huang CR, Chen YL, Deng YH, Cheng HC, Deng WP. Melatonin rescues cerebral ischemic events through upregulated tunneling nanotube-mediated mitochondrial transfer and downregulated mitochondrial oxidative stress in rat brain. Biomed Pharmacother. 2021;139:111593. doi: 10.1016/j.biopha.2021.111593. [DOI] [PubMed] [Google Scholar]
- 27.Youle RJ, van der Bliek AM. Mitochondrial fission fusion and stress. Science. 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan HF, Zhai C, Yan XL, Zhao DD, Wang JX, Zeng Q, Chen L, Nan X, He LJ, Li ST, Yue W, Pei XT. SIRT1 is required for long-term growth of human mesenchymal stem cells. J Mol Med (Berl) 2012;90:389–400. doi: 10.1007/s00109-011-0825-4. [DOI] [PubMed] [Google Scholar]
- 29.Zheng YL. Some ethical concerns about human induced pluripotent stem cells. Sci Eng Ethics. 2016;22:1277–1284. doi: 10.1007/s11948-015-9693-6. [DOI] [PubMed] [Google Scholar]
- 30.Zhou L, Chen X, Liu T, Gong Y, Chen S, Pan G, Cui W, Luo ZP, Pei M, Yang H, He F. Melatonin reverses H2O2 -induced premature senescence in mesenchymal stem cells via the SIRT1-dependent pathway. J Pineal Res. 2015;59:190–205. doi: 10.1111/jpi.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue:implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


