The brain has a high metabolic rate and consumes approximately 20% of the total energy in the body at any given time. Although it requires a large amount of energy to function, the brain cannot store significant amounts of energy making it exquisitely dependent on constant nutrient supply via blood flow. When blood flow to the brain is impaired, such as during stroke, there are rapid and severe consequences for the neurons that depend on this constant energy supply.
Stroke is an acute cerebrovascular disorder and a leading cause of death and long-term disability. Although stroke can be ischemic or hemorrhagic, the majority of strokes are caused by ischemic events interrupting blood flow to a region of the brain. Acute ischemic stroke therapy aims to remove the occluding factor (usually a blood clot) through either pharmacological (tissue plasminogen activator) or physical means (endovascular thrombectomy) (Campbell et al., 2019). The overall goal of both approaches is to re-open the occluded vessels to enable blood flow to return to the ischemic region – this is termed recanalization. Importantly, even after recanalization, brain damage continues to spread resulting in the expansion of the ischemic core into the surrounding brain regions known as the penumbra – this ultimately increases brain damage and resulting disability (Campbell et al., 2019). While clot removal is an effective strategy that restores large artery flow, the extent to which blood flow returns to the microvasculature, termed reperfusion, in the ischemic regions and how reperfusion changes over time remains to be fully determined. Although the mechanisms that contribute to the expansion of brain damage after successful recanalization are not clear, the role of dysregulated microvascular responses and blood flow may contribute to this.
Decades of research have focused on finding new strategies to reduce neural damage after stroke. However, despite the incredibly huge investment, almost all strategies targeting neuroprotection have failed to demonstrate clinical efficacy. Today, treatment for stroke consists of dealing with the cause, attempting to remove the occluding blood clot, and recanalize the vessel. However, clinical evidence suggests that the beneficial effect of post-stroke recanalization may be hampered by abnormal microvascular reperfusion which influences long-term recovery (Campbell et al., 2019). In this perspective, we provide a rationale for therapeutic targeting of the microvasculature in both the acute and long-term timeframes after ischemic stroke.
Acute vascular and blood flow responses after ischemic stroke: An adequate restoration of brain perfusion during the first hours after a stroke is the main strategy employed to reduce brain injury. This approach is especially effective when salvageable brain tissue is present and prior to the formation of the ischemic core. Indeed, data from humans and experimental rodents indicate that rapid recanalization following stroke favors improved acute and long-term vascular reperfusion and remodeling, contributing to functional recovery (Campbell et al., 2019). However, rapid recanalization in the clinical setting is not common and usually occurs after many hours of ischemia. In this setting, recanalization does not always improve recovery and multiple mechanisms that acutely impact blood flow restoration are likely to contribute (Figure 1).
Figure 1.
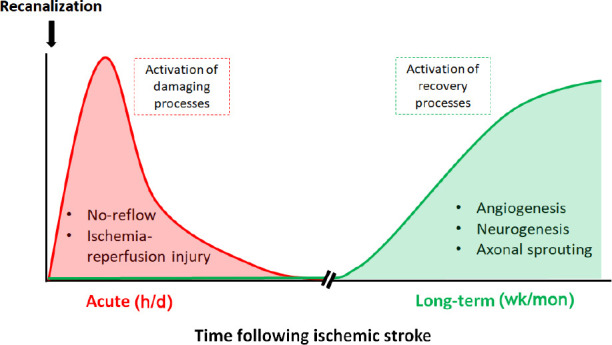
Time-course of acute or recovery processes activated after a stroke.
In the acute phase (hours/days) after recanalization, abnormal vascular processes are activated that impair or exceed normal perfusion of the damaged brain tissue contributing to further damage and the spread of damage beyond the core to the penumbral regions. In the long-term phase (weeks/months), repair mechanisms are activated to help drive vascular and neural recovery in the damaged brain areas.
In the acute phase after recanalization, studies have shown that reperfusion is not always complete at the capillary level contributing to the no-reflow phenomenon (Hall et al., 2014; Hill et al., 2015; Ter Schiphorst et al., 2021). Although human studies regarding microvascular no-reflow are now emerging (Ter Schiphorst et al., 2021), most of the evidence regarding this phenomenon comes from work in experimental rodents where invasive techniques are required for direct microvascular imaging. In this setting, studies show that as many as 30–40% of capillaries in the ischemic region remain closed after recanalization due to sustained constriction of contractile vascular cells (Hall et al., 2014; Hill et al., 2015) or obstruction of capillaries by neutrophils (El Amki et al., 2020). Whether vascular occlusion occurs at the level of the arteriole or the capillary, or both remains a point of contention in the literature (Hall et al., 2014; Hill et al., 2015), but the overall effect is similar – loss of capillary perfusion and therefore continued tissue ischemia and damage. With time, the net result of no-reflow is capillary degradation, permanent loss of these vessels, and a reduction in the energy supplied to the brain. Whether the no-reflow phenomenon spreads to adjacent capillaries and contributes to increasing brain damage in the penumbra over time is not known. It is tantalizing to suggest that re-opening these closed capillaries could rescue and reverse the brain damage after an ischemic stroke. However, whether this is a feasible therapeutic option remains to be determined.
Beyond the no-reflow phenomenon, another challenge within the acute setting after stroke is ischemia/reperfusion injury. While rapid recanalization is needed for tissue survival after ischemia, perhaps surprisingly, restoring blood flow and reoxygenation of the tissue can lead to exacerbation of tissue injury and a profound inflammatory response (Eltzschig and Eckle, 2011). Although this reperfusion injury has been well characterized in the heart, over the past two decades the significance of this mechanism in the context of ischemic stroke has also become clearer. Ischemia-reperfusion has been shown to exacerbate endogenous damaging mechanisms that can affect the survival of brain vessels such as oxidative stress, activation of coagulation and the complement system, neuroinflammation, and increased blood-brain barrier leakage and vasogenic edema (Eltzschig and Eckle, 2011). We have also shown that the acute cerebral blood flow response post-stroke is markedly increased above baseline levels (Premilovac et al., 2020), adding support to the notion of ischemia-reperfusion driving further injury potentially through excessive activation of dilatory mechanisms. Preclinical studies aiming to reduce the impact of these mechanisms through ischemic conditioning, antioxidant therapy, or inhibition of nucleotide signaling pathways have shown therapeutic potential in reducing brain damage. Unfortunately, clinical studies have failed to provide evidence for a protective effect of specific therapeutic approaches targeting ischemia-reperfusion injury.
Although recanalization is the mainstay of acute ischemic stroke treatment, two major vascular mechanisms impact the recovery process in the acute setting. No-reflow phenomenon leads to continued hypoperfusion in some regions of the already damaged brain while other regions where microvascular blood flow is restored are exposed to ischemia-reperfusion damage. The challenge of combatting these competing mechanisms after ischemic stroke has proven a difficult prospect. What is needed is a better understanding of the timeline of these deleterious events and how they evolve in adjacent brain regions so that therapeutic interventions can be more tailored and specific. Although there is much to be done, emerging technologies that enable targeted delivery of drugs to specific regions of the brain provide a unique opportunity to target the microvasculature to restore normal blood flow and reducing brain damage in the acute phase after stroke.
Long-term vascular and blood flow responses after ischemic stroke: The acute changes that occur following ischemic stroke are substantial, but subsequent to this the brain undergoes a long-term phase of recovery. Ischemic damage can trigger several repair processes that begin approximately seven days following stroke, including angiogenesis, neurogenesis, cell migration, and axonal sprouting (Figure 1). These processes can last months. However, it remains unknown how vascular function contributes to the long-term recovery of the brain following ischemic injury.
Human studies have shown that in some patients there are long-term blood flow changes post stroke, particularly in the infarcted region. Harston et al. (2017) performed longitudinal imaging in stroke patients over one month and found a gradient of decreasing perfusion from outside to inside the ischemic core, which remained persistent with time. Interestingly, in patients who spontaneously recanalized, perfusion deficits remained within the infarct area (Harston et al., 2017). Despite this, not all patients have post-stroke perfusion abnormalities, with Beaulieu et al. (1999) identifying that 69% of patients no longer had perfusion abnormalities 1-month post stroke. Although it is not clear whether hypoperfusion drives further tissue injury, emerging work indicates that hypoperfusion is associated with impaired motor recovery following stroke, suggesting that recovery of the vasculature is critical for post-stroke functional recovery. However, more work needs to be conducted to better understand the long-term consequences of hypoperfusion on post-stroke brain regeneration in humans and whether this response can be targeted for therapeutic gain.
Given that functional perfusion is extremely important for the recovery of ischemic tissue, animal studies can provide valuable pathophysiological information as well as novel therapeutic assessment to determine the value of vascular recovery post stroke. It has been demonstrated that ischemia can lead to blood vessel loss and reduced vascular density in the brain acutely (Brown et al., 2007). However, the blood vessels that remain are larger in diameter most likely due to a vasodilatory response to recruiting more blood flow to this region, as well as being leakier, indicative of blood-brain barrier impairment. Following this, chronic remodeling processes lead to an increase in microvessel number that is associated with neuroplasticity (Brown et al., 2007). Furthermore, this vascular remodeling can be aided through the use of a customized angiogenic biomaterial, which appears to improve functional recovery (Nih et al., 2018), providing a novel therapeutic avenue for the long-term treatment of stroke. However, in and around the lesion area, communication between neurons and the vasculature remains dissociated, suggesting these vessels may lack appropriate neurovascular regulation. Therefore, the mere presence of blood vessels in ischemic lesions does not mean that this tissue will become functional. Lesioned tissue is devoid of neurons due to the initial widespread cell death, and so having blood vessels to provide blood flow to a region that has no neuronal function may mean that the vasculature has more importance in supporting the recovery of the surrounding tissue and ensuring those regions remain functional.
Long-term changes in vascular function may be dependent on the mural cells that encircle the brain’s blood vessels. Pericytes are contractile cells that regulate cerebral blood flow by modulating capillary diameter and have been implicated in the no-reflow phenomenon acutely post stroke (Hall et al., 2014). However, it has been shown that pericytes can also have multiple roles that may aid in the long-term recovery of the brain after stroke including angiogenesis, phagocytosis, and stem-cell-like properties that enable repopulation of glia and neurons in the damaged area and coverage of newly formed vessels to ensure blood-brain barrier integrity (reviewed in Courtney and Sutherland, 2020). Therefore, mural cells such as pericytes have a large repertoire of functions that could be harnessed to aid in the long-term repair processes of the brain following a stroke. This is an exciting area of regeneration research, and we need to better understand the biology of pericytes to effectively take advantage of their therapeutic potential to aid in neural repair following a stroke.
Summary and future directions: Stroke is a major cause of death and long-term disability and there is a paucity of therapeutic options available to reduce brain damage. Here, we review and highlight the potential of targeting the microvasculature in the acute and longer-term phases after stroke. In the acute setting, the challenge is dealing with competing pathophysiological mechanisms in adjacent regions of the brain where there is too little or potentially too much blood flow. In the long-term phase, revascularization and functional recovery of the damaged brain tissue is key, but importance must be placed on ensuring blood vessels are functional (i.e., vessels can respond to signals/mediators to regulate blood flow). While much remains unknown about the role of the brain’s vasculature in the development of and recovery from ischemic injury, enhanced technology and advanced targeting strategies may lead to the vasculature, and possibly the surrounding mural cells, as a critical therapeutic target for improving functional brain recovery following stroke.
This work was supported by the National Health and Medical Research Council (NHMRC; application id: 2003351) of Australia.
Footnotes
Open peer reviewer: Xiang Mao, The First Affiliated Hospital of Anhui Medical University, China.
P-Reviewer: Mao X; C-Editors: Zhao M, Zhao LJ, Wang Lu; T-Editor: Jia Y
References
- 1.Beaulieu C, De Crespigny A, Tong DC, Moseley ME, Albers GW, Marks MP. Longitudinal magnetic resonance imaging study of perfusion and diffusion in stroke:evolution of lesion volume and correlation with clinical outcome. Ann Neurol. 1999;46:568–578. doi: 10.1002/1531-8249(199910)46:4<568::aid-ana4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 2.Brown CE, Li P, Boyd JD, Delaney KR, Murphy TH. Extensive turnover of dendritic spines and vascular remodeling in cortical tissues recovering from stroke. J Neurosci. 2007;27:4101–4109. doi: 10.1523/JNEUROSCI.4295-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell BC, De Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM, Donnan GA. Ischaemic stroke. Nat Rev Dis Primers. 2019;5:70. doi: 10.1038/s41572-019-0118-8. [DOI] [PubMed] [Google Scholar]
- 4.Courtney JM, Sutherland BA. Harnessing the stem cell properties of pericytes to repair the brain. Neural Regen Res. 2020;15:1021–1022. doi: 10.4103/1673-5374.270301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Amki M, Glück C, Binder N, Middleham W, Wyss MT, Weiss T, Meister H, Luft A, Weller M, Weber B. Neutrophils obstructing brain capillaries are a major cause of no-reflow in ischemic stroke. Cell Rep. 2020;33:108260. doi: 10.1016/j.celrep.2020.108260. [DOI] [PubMed] [Google Scholar]
- 6.Eltzschig HK, Eckle T. Ischemia and reperfusion—from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O'Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harston GW, Okell TW, Sheerin F, Schulz U, Mathieson P, Reckless I, Shah K, Ford GA, Chappell MA, Jezzard P, Kennedy J. Quantification of serial cerebral blood flow in acute stroke using arterial spin labeling. Stroke. 2017;48:123–130. doi: 10.1161/STROKEAHA.116.014707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill RA, Tong L, Yuan P, Murikinati S, Gupta S, Grutzendler J. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron. 2015;87:95–110. doi: 10.1016/j.neuron.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nih LR, Gojgini S, Carmichael ST, Segura T. Dual-function injectable angiogenic biomaterial for the repair of brain tissue following stroke. Nat Mater. 2018;17:642–651. doi: 10.1038/s41563-018-0083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Premilovac D, Blackwood SJ, Ramsay CJ, Keske MA, Howells DW, Sutherland BA. Transcranial contrast-enhanced ultrasound in the rat brain reveals substantial hyperperfusion acutely post-stroke. J Cereb Blood Flow Metab. 2020;40:939–953. doi: 10.1177/0271678X20905493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ter Schiphorst A, Charron S, Hassen WB, Provost C, Naggara O, Benzakoun J, Seners P, Turc G, Baron JC, Oppenheim C. Tissue no-reflow despite full recanalization following thrombectomy for anterior circulation stroke with proximal occlusion:A clinical study. J Cereb Blood Flow Metab. 2021;41:253–266. doi: 10.1177/0271678X20954929. [DOI] [PMC free article] [PubMed] [Google Scholar]


