Keywords: alpha2-adrenoceptors, ambulatory behavior, clonidine, cortical injury, efaroxan, functional recovery, immunohistochemistry, motor deficit, norepinephrine, sensorimotor score
Abstract
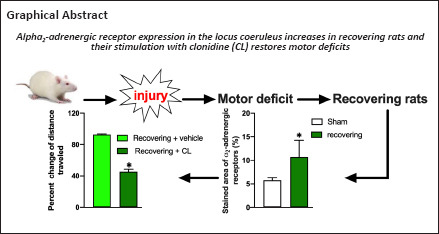
Norepinephrine plays an important role in motor functional recovery after a brain injury caused by ferrous chloride. Inhibition of norepinephrine release by clonidine is correlated with motor deficits after motor cortex injury. The aim of this study was to analyze the role of α2-adrenergic receptors in the restoration of motor deficits in recovering rats after brain damage. The rats were randomly assigned to the sham and injury groups and then treated with the following pharmacological agents at 3 hours before and 8 hours, 3 days, and 20 days after ferrous chloride-induced cortical injury: saline, clonidine, efaroxan (a selective antagonist of α2-adrenergic receptors) and clonidine + efaroxan. The sensorimotor score, the immunohistochemical staining for α2A-adrenergic receptors, and norepinephrine levels were evaluated. Eight hours post-injury, the sensorimotor score and norepinephrine levels in the locus coeruleus of the injured rats decreased, and these effects were maintained 3 days post-injury. However, 20 days later, clonidine administration diminished norepinephrine levels in the pons compared with the sham group. This effect was accompanied by sensorimotor deficits. These effects were blocked by efaroxan. In conclusion, an increase in α2-adrenergic receptor levels was observed after injury. Clonidine restores motor deficits in rats recovering from cortical injury, an effect that was prevented by efaroxan. The underlying mechanisms involve the stimulation of hypersensitive α2-adrenergic receptors and inhibition of norepinephrine activity in the locus coeruleus. The results of this study suggest that α2 receptor agonists might restore deficits or impede rehabilitation in patients with brain injury, and therefore pharmacological therapies need to be prescribed cautiously to these patients.
Introduction
Evidence has suggested that motor disturbances might result from iron accumulation in specific brain areas (Kastman et al., 2012; Apostolakis and Kypraiou, 2017; Devos et al., 2020). At the cerebral level, iron-induced injury is related to oxidative stress, which is increased through the Fenton reaction and leads to neurodegeneration and neuronal death (Zhao, 2019). Iron accumulation potentiates the formation of free radicals that produce lipoperoxidation (Willmore and Rubin, 1984; Bueno-Nava et al., 2010; Carocci et al., 2018). Clinically, iron accumulation is related to neurodegenerative disorders such as neuropsychiatric abnormalities (dementia), neuroferritinopathy, Huntington’s, Alzheimer’s and Parkinson’s diseases (Apostolakis and Kypraiou, 2017; Carocci et al., 2018; Galaris et al., 2019; Zhao, 2019). On the other hand, studies have shown that oxidative damage at the synaptic level affects neurotransmitter transport, release, and turnover (Rafalowska et al., 1989). Additionally, iron injection into the motor cortex (MC) causes motor deficit through a remote inhibitory mechanism (Bueno-Nava et al., 2008) that is apparently produced by a restriction of certain distant intact brain regions anatomically related to the region of the injury (Carrera and Tononi, 2014). This remote inhibition is known as diaschisis, and the damage within a focal area of the brain is presumed to affect distant brain regions. Brain injury caused by an intracerebral infusion of ferrous chloride (FeCl2) decreases norepinephrine (NE) levels in the pons to an approximately functional pontine depletion (Bueno-Nava et al., 2008). Additionally, the damaged central nervous system has the potential for functional recovery that varies according to the site, size, and time after the initial brain injury (Higo, 2014; Kolb and Gibb, 2015; Hofer and Schwab, 2019). Likewise, recovery might result from the restoration of the basal conditions that were present prior to the injury (Ramos-Languren et al., 2016b). Researchers have proposed that catecholamines, such as NE, play an important role in the recovery after brain injury (Osier and Dixon, 2016; Ahmed-Farid et al., 2019; Zahrai et al., 2020). Following this logical sequence, the locus coeruleus (LC) is the main noradrenergic reservoir (Benarroch, 2018) and a structure anatomically connected through adrenergic neurons to the MC that is associated with motor function within the cerebellum (Steindler, 1981; Benarroch, 2018). In the LC, sympathetic neuron functions are mediated by seven transmembrane domain G protein-coupled adrenergic receptors (Berridge and Waterhouse, 2003; Alcántara-Hernández and Hernández-Méndez, 2018). Currently, these three types of adrenergic receptors are designated α1, α2, and β. Each type consists of three subtypes: α1A, α1B, α1D, α2A, α2B, α2C, β1, β2, and β3 (Berridge and Waterhouse, 2003; Ramos and Arnsten, 2007). α1 and β receptors are mainly distributed in postsynaptic sites, whereas α2 receptors exist both at pre- and postsynaptic sites (Langer and Angel, 1991; Steinberg, 2018; Perez, 2020). Studies have shown that presynaptic α2A-adrenergic autoreceptors play an important role in the modulation of NE released in the rat LC (Starke, 2001; Aston-Jones and Cohen, 2005; Abe et al., 2020).
In this context, an increase in NE levels promotes functional recovery after cortical injury (Stibick and Feeney, 2001; Ramos-Languren et al., 2016a). Other than D-amphetamine (indirectly acting as a sympathomimetic drug), treatment protects against cortex injury-induced effects (Kikuchi et al., 2000; Hylin et al., 2017), and α2-adrenergic antagonists exert a similar effect (Mair et al., 2005; Hylin et al., 2017), while α2-adrenergic agonists exert the opposite effect (Rizk et al., 2006; Calderón et al., 2018). Thus, the presence of NE is key to maintaining recovery after brain injury in rats. Based on studies of adrenergic antagonist-induced reinstatement of motor deficits in recovered rats, researchers proposed that noradrenergic system integrity is necessary to maintain functional recovery (Stibick and Feeney, 2001; Feeney et al., 2004; Ramos-Languren et al., 2016a). Clonidine (CL) is an antihypertensive drug with a central mechanism of action (Yasaei and Saadabadi, 2021) that is associated with a decrease in the cytosolic Ca2+ concentration and inhibition of NE release through the activation of presynaptic α2-adrenergic receptors that subsequently inhibit adenyl cyclase activity and reduce cAMP levels. In contrast, efaroxan (EF) is a selective antagonist of α2-adrenergic receptors, and their activation enhances noradrenergic transmission (Rascol et al., 1998; Philipp et al., 2002). The administration of EF in combination with ephedrine increases motor and antioxidant activity (Rusu-Zota et al., 2021). Other effects of EF are associated with short-term memory and reference memory (Rusu-Zota et al., 2019). Therefore, the aim of the present study was to analyze the role of α2-adrenergic receptors in the restoration of motor deficits in recovering rats by assessing the pontine norepinephrinergic response after activation or blockade of α2-adrenergic receptors with CL and EF.
Methods
Animals
Seventy-four male adult Wistar rats weighing 280–320 g (3 months old) were born in the vivarium of the Instituto Nacional de Neurología y Neurocirugía Manuel Velasco Suárez. The animals were housed in acrylic cages (5 rats per cage) and maintained on a 12-hour light/dark cycle, provided ad libitum access to food (LabDiet® rodent laboratory chow; Richmond, IN, USA) and water, and acclimated to the laboratory conditions for at least 1 week prior to surgical procedures. During this period, the rats were handled daily to habituate them to the experimental manipulations. The rodents were treated according to the Guide for the Care and Use of Experimental Animals (National Research Council, 2011) and the Mexican Official Standard for the Production, Care and Use of Laboratory Animals (NOM-062-ZOO-1999, 2001). The protocol was approved by the Instituto Nacional de Neurología y Nerocirugía Manuel Velasco Suárez Research Committee (approval number 08/17) on September 9, 2017.
Groups
The animals (n = 74) were randomly allocated into one of two groups: the FeCl2-induced cortical injury (n = 37) and the sham group (n = 37). Both groups were then subdivided to immunohistochemically detect the α2A-adrenergic receptors (n = 5) and to determine pontine NE levels after intraperitoneal (i.p.) administration of the following pharmacological agents: saline (n = 8; 1 mL/kg), CL hydrochloride ((2-2.6-dichloroanilino)-2-imidazoline hydrochloride, Merck, San Francisco, CA, USA; CAS number: 4205-91-8; n = 8; 6 μg/kg), EF ((2-ethyl-2-imidazolin-2-yl)-2.3-dihydrobenzofuran hydrochloride, Merck, San Francisco, CA, USA; CAS number: 89197-00-2; n = 8; 0.63 mg/kg), and CL + EF (n = 8). Doses of both CL (6 μg/kg) and EF (0.63 mg/kg) were based on previous studies (Martel et al., 1998; Mair et al., 2005; Calderón et al., 2018). EF and CL were chosen since they have been shown to be effective at blocking or stimulating the α2-adrenoreceptor, respectively (Mair et al., 2005; Calderón et al., 2018; Rusu-Zota et al., 2019, 2021). Both drugs (CL and EF) were freshly prepared in 0.9% NaCl before use and administered i.p. in a volume of 10 mL/kg. The study design is shown in Figure 1.
Figure 1.

Experimental timeline.
CSF: Cerebrospinal fluid; HPLC: high-performance liquid chromatography; NE: norepinephrine.
Surgery and injury
After the first session of motor testing, in both sham (infusion of artificial cerebrospinal fluid [CSF]) and injured (artificial cerebrospinal fluid containing FeCl2) groups, animals were anesthetized with a mixture of ketamine (100 mg/kg; i.p.; Pisa, Atitalaquia, Hgo., México) and xylazine (5 mg/kg; i.p.; Pisa), and then mounted on a stereotaxic frame (Stoelting Corp., Wood Dale, IL, USA) and administered an intracortical infusion of an artificial cerebrospinal fluid composed of 125 mM NaCl (Sigma-Aldrich, St. Louis, MO, USA), 3 mM KCl (Sigma-Aldrich), 1.3 mM CaCl2 (Sigma-Aldrich), 1 mM MgCl2 (Sigma-Aldrich), 2.3 mM NaHCO3 (Sigma-Aldrich), pH 7.2 or artificial cerebrospinal fluid containing FeCl2 (10 μL, 50 mM, Sigma-Aldrich), respectively. The infusions were injected unilaterally into the motor cortical area representing the hind limb (posterior: +2 mm, and lateral –2 mm with respect to the bregma (Paxinos and Watson, 2013)) according to Bueno-Nava et al. (2008). The unilateral FeCl2 infusion is directly related to the amount of extravasated blood that may occur in patients with cerebral vascular disease (Triggs and Willmore, 1984; Bueno-Nava et al., 2010). Meloxicam (2 mg/kg) was administered subcutaneously 30 minutes before and every 12 hours for 24 hours after surgery. Topical lidocaine was applied to the incision site. The FeCl2 infusion into the MC was performed to mimic cortical injury reminiscent of subarachnoid hemorrhage. Twenty days after the injection, the animals were decapitated to quantify NE levels and the localization of α2-adrenergic receptors at the pontine level.
Spontaneous motor activity
Spontaneous motor activity was recorded according to the study of Ramos-Languren et al. (2016a) using an AutoTrack Opto-Varimex activity monitoring system (Columbus Instruments, Columbus, OH, USA) placed inside an anechoic chamber. The system was comprised of one chamber (42.2 × 42.5 × 20.5 cm3). The activity was monitored in four sessions of 5 minutes each at –3 hours, 8 hours, 3 days, and 20 days after FeCl2-induced cortical injury. Additionally, another session was conducted 30 minutes after the administration of CL, EF, or CL + EF 20 days after FeCl2-induced cortical injury. Spontaneous motor activity was evaluated by determining the following parameters: distance traveled (cm), resting time (RT, seconds), stereotypic time (ST, seconds), ambulatory time (AT, seconds), and total time in the chamber (TTC, seconds). The resting time was calculated according to the equation RT = TTC– (AT + ST), where TTC = 300 seconds. The distance traveled was converted into percentages (100% represents the average of the first session, the distance traveled in the following sessions is divided by the distance covered in the first session and multiplied by 100).
Sensorimotor deficits
Similar to applied treatments to assess spontaneous motor activity, the sensorimotor deficit was measured using a previously described neurological evaluation (Bueno-Nava et al., 2010). Briefly, symmetry in the movement of the four limbs, climbing, body proprioception, and response to vibrissae touch were recorded. A score was assigned for each evaluation ranging from three to zero points, which indicate the least and greatest deficits, respectively. The score obtained for each subject was calculated as the sum of the four individual evaluations. In effect, the minimum and maximum scores obtained were three and twelve points, respectively, and a higher score indicates lower deficits. The animals were evaluated over five sessions after spontaneous motor activity was determined.
Chromatographic analysis of norepinephrine
At 20 days after FeCl2-induced injury, eight animals from each group were sacrificed by decapitation, and the pons was removed from both hemispheres. The weight was measured and the tissue was stored at –80°C. On the day of analysis, the samples were thawed and homogenized in 0.4 N perchloric acid containing 0.1% sodium metabisulfite as previously reported (Bueno-Nava et al., 2010). Homogenates were centrifuged (20,000 × g, 20 minutes, 4°C) and supernatants were incubated on ice during the analysis. Samples were analyzed using a high-performance liquid chromatography (HPLC) system coupled to an electrochemical detector as previously described (Montes et al., 2001). The samples were injected into an isocratic pump (LC250, PerkinElmer, Waltham, MA, USA) using a Rheodyne valve with a 20 µL loop. The mobile phase consisted of potassium phosphate buffer (0.1 mM, pH 3.2) containing 0.2 mM sodium octyl sulfate and 0.1 mM ethylenediaminetetraacetic acid with 15.5% HPLC-grade absolute methanol pumped at a 1.2 mL/min flow rate. The signals were recorded with an electrochemical detector (Mod. 656 Metrohm) and integrated using Turbochrome software v.4.10 (PerkinElmer).
Immunohistochemical detection of α2A-adrenergic receptors using immunofluorescence staining
At 20 days post-surgery, the injured and sham animals were euthanized with pentobarbital sodium (vial with 6.3 g/100 mL, Pisa; using a dose of 43 mg/kg, i.p.) and sequentially intracardially perfused with 0.1 M phosphate-buffered saline (PBS, pH 7.4) and 4% paraformaldehyde. Brains were removed, fixed with a 4% paraformaldehyde solution at 4°C overnight, dehydrated, and embedded in paraffin using standard histological techniques. A microtome (Leica Biosystems, RM 2125, Deer Park, IL, USA) was used to cut 7 μm thick histological sections. Nissl staining was performed to locate the study area (Parra-Cid et al., 2020), and sections were incubated with 8% bovine serum albumin and 0.1% Tween X-100 in 0.1 M PBS at room temperature for 30 minutes for immunohistochemical staining. Next, the sections were incubated overnight at 4°C in the presence of the primary antibody rabbit anti-α2A-adrenergic receptor (a rabbit anti-α2A-adrenergic receptor primary antibody; 1:200 dilution; Abcam, Cambridge, MA, USA, RRID: AB_85570). Twenty-four hours later, the samples were washed three times with 0.1 M PBS and incubated for 2 hours at 37°C with the corresponding goat anti-rabbit Alexa Fluor 488 (Alexa Fluor 488-conjugated goat anti-rabbit secondary antibody; 1:100 dilution; Abcam, RRID: AB_150077,) secondary antibody. The nuclei were stained with Hoechst dye (H3570, 1:1000 dilution; Thermo Fisher Scientific, Waltham, MA, USA) and covered with an aqueous mounting medium (FluoroCare Mountant, Biocare, Pacheco, CA. USA). Finally, sections were analyzed using an Imager Axiostar A1 epifluorescence microscope (Carl Zeiss, Oberkochen, Germany). Images were captured with an AxioCamHRc camera (Carl Zeiss) and processed with REL.4.8 Axio vision software (Carl Zeiss). The images were converted to grayscale by adjusting the image using the threshold to select the area stained with the α2A antibody, and the area with positive α2A staining in the locus coeruleus was analyzed by performing a particle analysis using ImageJ software (v. 1.48; Schneider et al., 2012), which is an open-access program developed by the National Institutes of Health of the USA. Estimates were obtained from three sections per animal.
Sample size
The sample size was calculated using the method described by Festing (2018). Assuming that the standard deviation for pontine NE contents would be similar to those reported by Avila-Luna et al. (2016), a power analysis showed that a sample size of eight rats per group has 90% power to detect a predicted effect size of 11%, assuming a 5% significance level and one-sided test. For immunohistochemical localization of α2A-adrenergic receptors, the sample size was established in five rats per condition, because most researchers conducted immunohistochemical staining for adrenergic receptors in the brain of three to five rats per group and achieved successful results.
Statistical analysis
All data were compiled as the means ± SEM of eight, six, or five rats from the corresponding experimental group. The results for sensorimotor deficits were analyzed with the nonparametric Kruskal-Wallis test followed by the Mann-Whitney U test to compare the mean rank of the treatment groups. Distance traveled, the resting time, stereotypic time, ambulatory time, and NE levels were analyzed with a one-way analysis of variance followed by Tukey’s post hoc test. Immunohistochemical detection was analyzed with Student’s t-test. Statistical significance was set to P < 0.05. The statistical analysis was performed with IBM SPSS version 23.0.0 (IBM Corp., Armonk, NY, USA).
Results
Sensorimotor evaluation after brain damage
The evolution of changes in sensorimotor function before and after FeCl2-induced MC injury is shown in Figure 2. The administration of FeCl2 in the MC induced a significant decrease in the sensorimotor score 8 hours after surgery (P < 0.001) compared with that of the sham group, an effect that was maintained for up to 3 days post-lesioning (P = 0.0154). No significant differences were observed between the sham and injured groups at 20 days after FeCl2 administration (P > 0.05). Importantly, no significant differences were observed in the sensorimotor scores of the sham group at different time points (P > 0.05).
Figure 2.

Effect of FeCl2-induced MC injury on the temporal evolution of rat sensorimotor activity at 3 hours pre-injury and 8 hours, 3 days, and 20 days post-injury.
Values are presented as means ± SEM (n = 8 per group). Statistical analyses were performed with the nonparametric Kruskal-Wallis test followed by the Mann-Whitney U test to compare the mean rank of the drug treatment groups. *P < 0.05, vs. sham group.
Spontaneous motor activity
The effect of FeCl2-induced MC injury on the distance traveled is shown in Figure 3A and B. A decrease was noted in the mean percentage of motor spontaneous activity 8 hours after injury compared with the sham group (P = 0.0002), and this effect was maintained over the following 3 days (P = 0.0002). At 20 days post-injury, functional recovery was observed (P > 0.05). Figure 3B shows that the injured rats were able to walk, despite having displayed diminished spontaneous motor activity.
Figure 3.

Percent change with respect to basal values obtained for the distance traveled by the sham and injured rats.
(A) Effect of FeCl2-induced MC injury on the distance traveled by rats in each group at 3 hours pre-injury and 8 hours, 3 and 20 days post-injury. (B) Representative maps of the distance traveled under the abovementioned conditions are shown. Values are presented as means ± SEM (n = 8 per group). Statistical analyses were performed with the nonparametric Kruskal-Wallis test followed by the Mann-Whitney U test to compare the mean rank of the drug treatment groups. ***P < 0.001, vs. sham group.
Additionally, resting time, stereotypic time, and ambulatory time are shown in Table 1. The resting time at 8 hours post-injury was significantly increased compared with the sham group (F(1, 63) = 4.01, P = 0.003). However, the stereotypic time and ambulatory time at 8 hours post-injury were both reduced in the injured group compared with the sham group (F(1, 63) = 4.01, P = 0.0008 and F(1, 63) = 4.01, P = 0.001, respectively). Similar results were obtained for the resting time, stereotypic time and ambulatory time at 3 days post-injury (F(1, 63) = 4.01, P = 0.0008, P = 0.004, and P = 0.008 compared with the sham group, respectively). Only an increase in ambulatory time was observed at 20 days post-injury compared with the sham group (F(1, 63) = 4.01, P = 0.009), suggesting a partial functional recovery in this period.
Table 1.
Spontaneous motor activity was assessed by determining the resting time (second), stereotypic time (second), and ambulatory time (second) at 3 hours preinjury and at 8 hours, 3 and 20 days post-injury
| Parameter | Group | Pre-injury | Post-injury | ||
|---|---|---|---|---|---|
|
|
|
||||
| 3 h | 8 h | 3 d | 20 d | ||
| Resting time | Sham | 71±3 | 102±3 | 121±6 | 72±7 |
| Injured | 75±2 | 223±4** | 226±4** | 79±2 | |
| Stereotypic time | Sham | 156±6 | 126±5 | 104±7 | 159±12 |
| Injured | 148±10 | 38±2** | 38±4** | 134±4 | |
| Ambulatory time | Sham | 73±5 | 71±4 | 74±9 | 68±8 |
| Injured | 76±10 | 38±3** | 35±2 | 86±4** | |
Values are presented as means ± SEM (n = 8 per group). Statistical analyses were performed using a one-way analysis of variance followed by Tukey’s post hoc test. **P < 0.01, vs. sham group.
Effects of the administration of CL, EF, or EF + CL on the sensorimotor scores
As shown in Figure 4, the sham and injured animals that received the vehicle solution showed no significant changes in the sensorimotor scores at 20 days after FeCl2 administration. The systemic administration of CL restored FeCl2-induced sequelae and diminished the sensorimotor score compared with the sham group (P = 0.0002), and this effect was blocked by the coadministration of the antagonist EF. The administration of EF alone did not exert a significant effect on sensorimotor scores at 20 days after FeCl2-induced injury.
Figure 4.
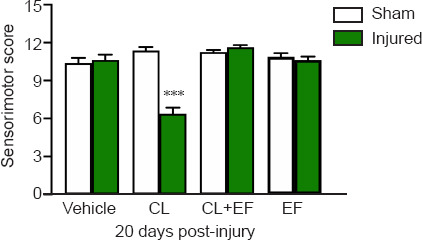
Effects obtained 30 minutes after the systemic administration of clonidine (CL), efaroxan (EF), and the coadministration of CL + EF on the functional recovery of sensorimotor activity at 20 days after FeCl2-induced cortical injury.
Values are presented as means ± SEM (n = 8 per group). Statistical analyses were performed with the nonparametric Kruskal-Wallis test followed by the Mann-Whitney U test to compare the mean rank of the drug treatment groups. ***P < 0.001, vs. sham group.
The effect of CL, EF, or EF + CL treatment on spontaneous motor activity
Figure 5A shows the effect of the CL, EF, and EF + CL treatments on spontaneous motor activity evaluated as distance traveled at 20 days post-injury. Systemic administration of the agonist CL significantly decreased the distance traveled by the injured group (P = 0.0003) compared with the sham group, and this effect was blocked by the coadministration of the antagonist EF. In the sham group, the administration of CL significantly decreased the distance traveled compared with the sham group that received the vehicle solution (P = 0.0002), and this effect was blocked by the coadministration of the antagonist EF. In both the sham and injured groups, the administration of EF alone did not exert a significant effect on the distance traveled at 20 days after FeCl2-induced injury (Figure 5A). Additionally, the effects of all treatments (vehicle, CL, EF, and CL + EF) mentioned above are shown in Figure 5B, with representative maps of the distance traveled at 20 days post-injury.
Figure 5.

Percent change with respect to basal values obtained for the distance traveled by sham and injured rats.
(A) Effects of the administration of clonidine (CL), efaroxan (EF), and CL + EF on the distance traveled at 20 days post-injury. (B) Representative maps of the distance traveled under the abovementioned conditions are shown. Values are presented as means ± SEM (n = 8 per group). Statistical analyses were performed with the nonparametric Kruskal-Wallis test followed by the Mann-Whitney U test to compare the mean rank of the drug treatment groups. ***P < 0.001, vs. sham group; ###P < 0.001, vs. sham (vehicle) group.
Moreover, resting, stereotypic and ambulatory times were also analyzed after the administration of CL, EF, and CL + EF at 20 days post-injury, and the results are summarized in Table 2. The administration of CL produced a significant increase in resting time (F(1, 79) = 7.42, P = 0.008) and a significant decrease in stereotypic (F(1, 79) = 7.42, P = 0.016 compared with the sham group) and ambulatory (F(1, 79) = 7.42, P = 0.029) times compared with the sham group. The effects of CL on the ambulatory time were blocked by the coadministration of the antagonist EF compared with the sham group (F(1, 79) = 7.42, P = 0.001). Importantly, CL treatment increased the resting time and reduced the stereotypic and ambulatory times. The administration of EF alone did not exert a significant effect on the stereotypic and ambulatory times at 20 days after FeCl2-induced injury (F(1, 79) = 7.42, P = 0.0001, and P = 0.032, respectively).
Table 2.
Effects of the administration of clonidine (CL), efaroxan (EF), and CL+EF on spontaneous motor activity, as assessed by measuring the resting time (second), stereotypic time (second), and ambulatory time at 20 days post-injury
| Test | Group | Vehicle (saline) | CL (6 µg/kg) | CL + EF | EF (0.63 mg/kg) |
|---|---|---|---|---|---|
| Resting time | Sham | 77±6 | 118±3 | 71±5 | 71±6 |
| Injured | 85±4 | 194±7* | 66±6 | 96±8 | |
| Stereotypic time | Sham | 76±8 | 111±3 | 91±9 | 125±6 |
| Injured | 80±4 | 64±3* | 77±3 | 78±3* | |
| Ambulatory time | Sham | 147±2 | 71±2 | 139±2 | 104±4 |
| Injured | 135±2* | 42±1* | 157±3* | 126±8* |
Values are presented as means ± SEM (n = 8 per group). Statistical analyses were performed using a one-way analysis of variance followed by Tukey’s post hoc test. *P < 0.05, vs. sham group.
Effect of the administration of CL, EF, or CL + EF on the pontine NE content after FeCl2-induced cortical lesions
As shown in Figure 6, the systemic administration of CL reduced the pontine NE levels at 20 days after cortical injury compared with the sham group (F(7, 56) = 4.05, P = 0.0012), and this effect was blocked by EF. However, the pontine NE levels detected in the presence of CL/EF, EF, or saline were not significantly different from those in the corresponding sham groups.
Figure 6.

Effects of the administration of clonidine (CL), efaroxan (EF), and CL + EF on pontine NE levels at 20 days after cortical injury (chromatographic analysis).
Values are presented as means ± SEM (n = 8 per group). Statistical analyses were performed using a one-way analysis of variance followed by Tukey’s post hoc test. **P < 0.01, vs. sham group. NE: Norepinephrine.
Immunofluorescence staining for the α2A-adrenergic receptor
Within the sham animals, α2A-positive NE cells were observed in the LC (Figure 7). In the injured rats, the immune signals for α2A-positive NE cells seemed more evident in the peripheral layers adjacent to the LC (Figure 7B and C). An increased staining area of α2A-positive NE cells was observed in the LC of injured rats (t = 2.3, P = 0.039; Figure 7D).
Figure 7.

Histological analysis of the locus coeruleus (LC).
(A) Nissl staining was performed to show the site of the LC (indicated with a white arrow) that was considered in the analysis. Scale bar: 200 μm. (B, C) Tissues were immunostained with an α2A antibody (green), and Hoechst was used for core staining (blue). α2A-adrenergic receptors are indicated by white arrows in the LC. Scale bars: 50 μm. (D) Immunohistochemical analysis of α2A-adrenergic receptors indicated increased levels in the injured group compared to the sham group (P < 0.05). Values are presented as means ± SEM (n = 5 per group). *P = 0.039, vs. sham group
Discussion
Brain damage represents the cause of the dysfunction of several brain processes, which affects the patient both physically and emotionally. Primarily, these injuries cause significant brain tissue damage that results in neuronal death in a majority of instances, as well as changes in afferent and efferent connections that communicate with different brain areas (Heredia et al., 2019). In this sense, speech, learning, and motor function are some of the main affected skills. Functional recovery both in humans and animal models may occur after brain damage (Gonzalez-Pina et al., 2006; Ramos-Languren et al., 2016b; Viale et al., 2018).
Several studies have documented that recovery after cerebral damage is potentially mediated by changes in central nervous system structures that were not affected by the injury. Several studies have also reported compensatory effects due to changes in brain anatomical characteristics (Ramos-Languren et al., 2016b; Guggisberg et al., 2019).
Additionally, several studies using other paradigms have been conducted, for example, balance beam, in which survivors of hemiparetic stroke were studied, footprint analysis, and studies where a significant decrease in motor capacity has been reported (Bates et al., 2013; Mace et al., 2017). In animal models, FeCl2-induced cortical injury leads to effects in areas remote from the lesion site, for example, in the cerebellum (Bueno-Nava et al., 2008).
Sensorimotor and spontaneous activities after FeCl2-induced injury
The results obtained in the present study have shown that the administration of FeCl2 in the MC induced a decrease in the sensorimotor score 8 hours after surgery compared with that of the sham group, an effect that was maintained for up to 3 days post-lesioning. These data suggest that the behavioral sequelae of FeCl2-induced neuronal injury occur almost immediately. Similar results were obtained in another previous study, which showed that a lesion in the MC induced biochemical changes, such as an increase in lipid peroxidation levels and a decrease in pontine NE total content at 3 days post-injury and subsequent restoration at 20 days post-injury (Bueno-Nava et al., 2008). The results obtained in the present study and the above-mentioned study are consistent with other findings (Ramos-Languren et al., 2016a), in which motor deficits were observed at 3 hours after FeCl2-induced MC injury, the NE content was reduced in the pons and cerebellum, and functional recovery also occurred (Gonzalez-Pina et al., 2006; Ramos-Languren et al., 2016a). In clinical studies, hemiparesis is the result of damage to the efferent pyramidal fibers in the internal capsule in patients with stroke, and edema and diaschisis may contribute to damage (Ramachandran, 2005; Carrasco-Moro et al., 2019).
Thus, functional recovery occurs 20 days after FeCl2-induced MC injury. In fact, the sensorimotor score and motor activity were not different compared to the corresponding sham group. In this regard, evidence from several studies has shown that nerve connections are modified as a result of brain activity. This finding is now fundamental for understanding functional recovery (Bueno-Nava et al., 2010; Kolb and Gibb, 2015; Hylin et al., 2017; Crozier et al., 2018; Viale et al., 2018; Crofts et al., 2020). For example, sensory and motor stimuli elicit biochemical and structural modifications in the hippocampus that potentially reorganize the system and lead to the recovery process by modulating structural and functional plasticity.
Effect of the administration of CL and EF on the norepinephrine content after FeCl2-induced injury
The NE contents and the granule cell number in the dentate gyrus are reduced in rats with MC ablation, and sensory and motor stimulation provoked a reversion of these reductions (Ramos-Languren et al., 2016b). Thus, researchers proposed that NE contents play an important role in brain recovery. On the one hand, MC lesions in rats elicit an important motor deficit, and reduced NE contents in the pons were observed. The content of pontine NE and motor deficits in recovering animals are similar to those observed in the control rats (Ramos-Languren et al., 2016b).
Based on our results, the administration of CL restored FeCl2-induced sequelae, diminished the sensorimotor score compared with the sham group, and decreased the pontine NE levels in recovering rats. These effects were blocked by the coadministration of EF, an α2-adrenergic receptor antagonist. Notably, presynaptic α2-adrenergic receptor activation by CL reduces the release of NE (Goldstein and Davis, 1990; Mair et al., 2005). Although three main types of noradrenergic receptors are expressed in the brain (α1, α2, and β), α2 receptors have the highest affinity for NE. Presynaptic α2 receptors function as autoreceptors, and their activation increases potassium conductance and inhibits calcium channels. Thus, we expected that NE levels would decrease after CL stimulation. In contrast, α1 and β receptors have a lower affinity for NE, and their activation decreases potassium conductance (Ramos and Arnsten, 2007). We found that the NE total contents in the pons decreased after CL administration. According to our results, we postulate that the sensorimotor and spontaneous ambulatory deficits reinstated by CL are mediated by α2A receptors. In this context, we observed a remarkable increase in the area of α2A receptor immunofluorescent staining using an anti-α2 receptor antibody within the microscopic field of the LC, the main noradrenergic reservoir in the central nervous system.
The possible mechanism involved in the restoration of the behavioral deficits
Our data allow us to explain a possible mechanism that is involved in the restoration of behavioral deficits after the administration of CL in recovering subjects. Diaschisis is a theory based on remote functional inhibition in sites located remotely from but anatomically related to an injured site (Lee et al., 2020; Puderbaugh and Emmady, 2021). Our results have revealed that the process after brain injury is reversible, leading to functional recovery, which we observed at 20 days after lesioning. Additionally, NE concentrations decreased to undetectable levels in both sections of the pons after FeCl2-induced cortical injury (Bueno-Nava et al., 2008), and we observed changes in α2A receptors in the LC when injured rats were recovering. The LC sends projections to the sensorimotor cortex (Lindvall and Björklund, 1974). The organization of ascending catecholamine neurons in the rat brain was revealed using the glyoxylic acid fluorescence method (Benarroch, 2018). Thus, our experimental model has shown that diaschisis occurs after MC injury because of the presence of focalized cerebral injury, a neuronal basis for functional depression, the participation of a remote structure related to the lesion site (the LC) and the reversibility of the process [see also Gonzalez-Pina et al. (2006), Bueno-Nava et al. (2008)]. An intracortical infusion of FeCl2 is expected to lead to a local increase in oxidative stress, producing neuronal death and deafferentation. In response, the neurons in the LC might shift NE synthesis to protein production to restore deafferented fibers, which may explain the important decrease in the NE content observed in the pons after brain injury. Although this hypothesis has not yet been clarified, the plastic mechanism of restoration of neuronal pathways is known as sprouting and occurs after a stroke in large pathways (Chen et al., 2002; Zai et al., 2009). Moreover, another response induced by deafferentation might be an increase in levels of NE receptors, such as the increase we observed in the α2A receptor, to increase the sensitivity to diminished levels of NE. We propose that behavioral effects are observed during the process of pathway restoration and receptor synthesis in the LC. In this case, the LC is in the OFF state. When pathway restoration is complete, functional recovery begins to occur, and the LC is in the ON state. However, an increase in α2 receptor levels remains that might also constitute a switch between the ON and OFF states of the LC because of stimulation with CL, an α2 receptor agonist, and restoration of the behavioral deficits in recovering rats (LC OFF) after cortical injury. This effect is prevented by efaroxan, a selective α2 receptor antagonist (LC ON).
A potential limitation was in the pontine NE analysis, which was determined in a mixture of both pontine sides. This does not allow comparison between injured and intact sides in the pons. Another limitation was the difficulty to analyze NE released, our results of NE were obtained in both extracellular NE and compartmentalized NE present in both cytosolic and vesicular pools in the noradrenergic axons.
In conclusion, FeCl2-induced MC injury causes a motor deficit after a few hours, and 20 days later, recovery is observed. Motor deficits are restored in recovering rats by CL administration through a mechanism that is apparently mediated by a high density of α2A receptors in the LC, where stimulation decreases the total NE contents in the pons. This change is prevented by EF administration.
Our findings have important clinical implications because pharmacological therapies must be prescribed cautiously to brain-injured patients: α2 receptor agonists might restore deficits or complicate rehabilitation.
At a theoretical level, this experimental model may have the potential for studies of the plastic mechanisms involved in diaschisis. More research is needed because we did not clearly determine whether the post-injury increase in α2 receptor levels was temporary or permanent. The roles of other neurotransmitters involved in recovery also must be clarified. This knowledge will allow us to develop stronger pharmacological strategies to facilitate recovery after brain injury.
Acknowledgments:
We would like to thank Dr. Guillermo Aquino-Miranda (McGobern Medical School, University of Texas Health Science Center at Houston, USA) and Dr. Paul Carrillo-Mora (National Institute of Rehabilitation LGII Mexico) for their collaboration and support.
Footnotes
Funding: This study was supported by Consejo Nacional de Ciencia y Tecnología (CONACyT) project CB 2016-287614 (to RGP and ABN) and by Scholarship Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica de la Universidad Nacional Autónoma de México (PAPIIT-UNAM) IA203319 and PAPIIT-UNAM IN216221 to (LERL).
Conflicts of interest: The authors have no relevant financial or nonfinancial interests to disclose.
C-Editor: Zhao M; S-Editor: Li CH; L-Editors: Li CH, Song LP; T-Editor: Jia Y
References
- 1.Abe N, Toyama H, Ejima Y, Saito K, Tamada T, Yamauchi M, Kazama I. α1-Adrenergic receptor blockade by prazosin synergistically stabilizes rat peritoneal mast cells. Biomed Res Int. 2020;2020:3214186. doi: 10.1155/2020/3214186. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Ahmed-Farid OA, Haredy SA, Niazy RM, Linhardt RJ, Warda M. Dose-dependent neuroprotective effect of oriental phyto-derived glycyrrhizin on experimental neuroterminal norepinephrine depletion in a rat brain model. Chem Biol Interact. 2019;308:279–287. doi: 10.1016/j.cbi.2019.05.045. [DOI] [PubMed] [Google Scholar]
- 3.Alcántara-Hernández R, Hernández-Méndez A. Adrenergic signaling molecular complexes. Gac Med Mex. 2018;154:223–235. doi: 10.24875/GMM.18002390. [DOI] [PubMed] [Google Scholar]
- 4.Apostolakis S, Kypraiou AM. Iron in neurodegenerative disorders:being in the wrong place at the wrong time? Rev Neurosci. 2017;28:893–911. doi: 10.1515/revneuro-2017-0020. [DOI] [PubMed] [Google Scholar]
- 5.Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus–norepinephrine system in optimal performance. J Comp Neurol. 2005;493:99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- 6.Avila-Luna A, Verduzco-Mendoza A, Bueno-Nava A. Effects of dopamine D-1 receptor activation and blockade on dopamine and noradrenaline levels in the rat brain. Neurosci Lett. 2016;612:116–120. doi: 10.1016/j.neulet.2015.11.040. [DOI] [PubMed] [Google Scholar]
- 7.Bates KT, Savage R, Pataky TC, Morse SA, Webster E, Falkingham PL, Ren L, Qian Z, Collins D, Bennett MR, McClymont J, Crompton RH. Does footprint depth correlate with foot motion and pressure? J R Soc Interface. 2013;10:20130009. doi: 10.1098/rsif.2013.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benarroch EE. Locus coeruleus. Cell Tissue Res. 2018;373:221–232. doi: 10.1007/s00441-017-2649-1. [DOI] [PubMed] [Google Scholar]
- 9.Berridge CW, Waterhouse BD. The locus coeruleus–noradrenergic system:modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 10.Bueno-Nava A, Montes S, DelaGarza-Montano P, Alfaro-Rodriguez A, Ortiz A, Gonzalez-Pina R. Reversal of noradrenergic depletion and lipid peroxidation in the pons after brain injury correlates with motor function recovery in rats. Neurosci Lett. 2008;443:32–36. doi: 10.1016/j.neulet.2008.07.046. [DOI] [PubMed] [Google Scholar]
- 11.Bueno-Nava A, Gonzalez-Pina R, Alfaro-Rodriguez A, Nekrassov-Protasova V, Durand-Rivera A, Montes S, Ayala-Guerrero F. Recovery of motor deficit cerebellar serotonin and lipid peroxidation levels in the cortex of injured rats. Neurochem Res. 2010;35:1538–1545. doi: 10.1007/s11064-010-0213-4. [DOI] [PubMed] [Google Scholar]
- 12.Calderón P, Deltenre P, Stany I, Kaleeta Maalu J-P, Stevens M, Lamoureux J, Bellemans M, Dujardin S, Van der Linden P, Dachy B. Clonidine administration during intraoperative monitoring for pediatric scoliosis surgery:Effects on central and peripheral motor responses. Neurophysiol Clin. 2018;48:93–102. doi: 10.1016/j.neucli.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Carocci A, Catalano A, Sinicropi MS, Genchi G. Oxidative stress and neurodegeneration:the involvement of iron. BioMetals. 2018;31:715–735. doi: 10.1007/s10534-018-0126-2. [DOI] [PubMed] [Google Scholar]
- 14.Carrasco-Moro R, Castro-Dufourny I, Martínez-San Millán JS, Cabañes-Martínez L, Pascual JM. Ipsilateral hemiparesis:the forgotten history of this paradoxical neurological sign. Neurosurg Focus. 2019;47:E7. doi: 10.3171/2019.6.FOCUS19337. [DOI] [PubMed] [Google Scholar]
- 15.Carrera E, Tononi G. Diaschisis:past present future. Brain. 2014;137:2408–2422. doi: 10.1093/brain/awu101. [DOI] [PubMed] [Google Scholar]
- 16.Chen P, Goldberg DE, Kolb B, Lanser M, Benowitz LI. Inosine induces axonal rewiring and improves behavioral outcome after stroke. Proc Natl Acad Sci U S A. 2002;99:9031–9036. doi: 10.1073/pnas.132076299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crofts A, Kelly ME, Gibson CL. Imaging functional recovery following ischemic stroke:clinical and preclinical fMRI studies. J Neuroimaging. 2020;30:5–14. doi: 10.1111/jon.12668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crozier J, Roig M, Eng JJ, MacKay-Lyons M, Fung J, Ploughman M, Bailey DM, Sweet SN, Giacomantonio N, Thiel A, Trivino M, Tang A. High-intensity interval training after stroke:an opportunity to promote functional recovery cardiovascular health and neuroplasticity. Neurorehabil Neural Repair. 2018;32:543–556. doi: 10.1177/1545968318766663. [DOI] [PubMed] [Google Scholar]
- 19.Devos D, Cabantchik ZI, Moreau C, Danel V, Mahoney-Sanchez L, Bouchaoui H, Gouel F, Rolland AS, Duce JA, Devedjian JC FAIRPARK-II and FAIRALS-II studygroups. Conservative iron chelation for neurodegenerative diseases such as Parkinson's disease and amyotrophic lateral sclerosis. J Neural Transm (Vienna) 2020;127:189–203. doi: 10.1007/s00702-019-02138-1. [DOI] [PubMed] [Google Scholar]
- 20.Feeney DM, De Smet AM, Rai S. Noradrenergic modulation of hemiplegia:Facilitation and maintenance of recovery. Restor Neurol Neurosci. 2004;22:175–190. [PubMed] [Google Scholar]
- 21.Festing MF. On determining sample size in experiments involving laboratory animals. Lab Anim. 2018;52:341–350. doi: 10.1177/0023677217738268. [DOI] [PubMed] [Google Scholar]
- 22.Galaris D, Barbouti A, Pantopoulos K. Iron homeostasis and oxidative stress:An intimate relationship. Biochim Biophys Acta Mol Cell Res. 2019;1866:118535. doi: 10.1016/j.bbamcr.2019.118535. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein LB, Davis JN. Clonidine impairs recovery of beam-walking after a sensorimotor cortex lesion in the rat. Brain Res. 1990;508:305–309. doi: 10.1016/0006-8993(90)90413-6. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Pina R, Bueno-Nava A, Montes S, Alfaro-Rodriguez A, Gonzalez-Maciel A, Reynoso-Robles R, Ayala-Guerrero F. Pontine and cerebellar norepinephrine content in adult rats recovering from focal cortical injury. Neurochem Res. 2006;31:1443–1449. doi: 10.1007/s11064-006-9196-6. [DOI] [PubMed] [Google Scholar]
- 25.Guggisberg AG, Koch PJ, Hummel FC, Buetefisch CM. Brain networks and their relevance for stroke rehabilitation. Clin Neurophysiol. 2019;130:1098–1124. doi: 10.1016/j.clinph.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heredia M, Rodríguez N, Sánchez Robledo V, Criado JM, de la Fuente A, Devesa J, Devesa P, Sánchez Riolobos A. Factors involved in the functional motor recovery of rats with cortical ablation after GH and rehabilitation treatment:cortical cell proliferation and nestin and actin expression in the striatum and thalamus. Int J Mol Sci. 2019;20:5770. doi: 10.3390/ijms20225770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higo N. Effects of rehabilitative training on recovery of hand motor function:A review of animal studies. Neurosci Res. 2014;78:9–15. doi: 10.1016/j.neures.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Hofer AS, Schwab ME. Enhancing rehabilitation and functional recovery after brain and spinal cord trauma with electrical neuromodulation. Curr Opin Neurol. 2019;32:828–835. doi: 10.1097/WCO.0000000000000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hylin MJ, Brenneman MM, Corwin JV. Noradrenergic antagonists mitigate amphetamine-induced recovery. Behav Brain Res. 2017;334:61–71. doi: 10.1016/j.bbr.2017.07.035. [DOI] [PubMed] [Google Scholar]
- 30.Kastman EK, Willette AA, Coe CL, Bendlin BB, Kosmatka KJ, McLaren DG, Xu G, Canu E, Field AS, Alexander AL, Voytko ML, Beasley TM, Colman RJ, Weindruch RH, Johnson SC. A calorie-restricted diet decreases brain iron accumulation and preserves motor performance in old rhesus monkeys. J Neurosci. 2012;32:11897–11904. doi: 10.1523/JNEUROSCI.2553-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kikuchi K, Nishino K, Ohyu H. Increasing CNS norepinephrine levels by the precursor l-DOPS facilitate beam-walking recovery after sensorimotor cortex ablation in rats. Brain Res. 2000;860:130–135. doi: 10.1016/s0006-8993(00)02034-5. [DOI] [PubMed] [Google Scholar]
- 32.Kolb B, Gibb R. Plasticity in the prefrontal cortex of adult rats. Front Cell Neurosci. 2015;9:15. doi: 10.3389/fncel.2015.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langer SZ, Angel I. Pre- and postsynaptic alpha-2 adrenoceptors as target for drug discovery. J Neural Transm Suppl. 1991;34:171–177. doi: 10.1007/978-3-7091-9175-0_22. [DOI] [PubMed] [Google Scholar]
- 34.Lee MC, Kim RG, Lee T, Kim JH, Lee KH, Choi YD, Kim HS, Cho J, Park JY, Kim HI. Ultrastructural dendritic changes underlying diaschisis after capsular infarct. J Neuropathol Exp Neurol. 2020;79:508–517. doi: 10.1093/jnen/nlaa001. [DOI] [PubMed] [Google Scholar]
- 35.Lindvall O, Björklund A. The organization of the ascending catecholamine neuron systems in the rat brain as revealed by the glyoxylic acid fluorescence method. Acta Physiol Scand Suppl. 1974;412:1–48. [PubMed] [Google Scholar]
- 36.Mace M, Kinany N, Rinne P, Rayner A, Bentley P, Burdet E. Balancing the playing field:collaborative gaming for physical training. J Neuroeng Rehabil. 2017;14:116. doi: 10.1186/s12984-017-0319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mair RD, Zhang Y, Bailey KR, Toupin MM, Mair RG. Effects of clonidine in the locus coeruleus on prefrontal- and hippocampal-dependent measures of attention and memory in the rat. Psychopharmacology (Berl) 2005;181:280–288. doi: 10.1007/s00213-005-2263-x. [DOI] [PubMed] [Google Scholar]
- 38.Martel JC, Chopin P, Colpaert F, Marien M. Neuroprotective effects of the α2-adrenoceptor antagonists (+)-efaroxan and (±)-idazoxan against quinolinic acid-induced lesions of the rat striatum. Exp Neurol. 1998;154:595–601. doi: 10.1006/exnr.1998.6942. [DOI] [PubMed] [Google Scholar]
- 39.Montes S, Alcaraz-Zubeldia M, Muriel P, Ríos C. Striatal manganese accumulation induces changes in dopamine metabolism in the cirrhotic rat. Brain Res. 2001;891:123–129. doi: 10.1016/s0006-8993(00)03208-x. [DOI] [PubMed] [Google Scholar]
- 40.National Research Council. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, DC: The National Academies Press; 2011. [Google Scholar]
- 41.NOM-062-ZOO-1999 (|y2001) Especificaciones técnicas para la producción cuidado y uso de los animales de laboratorio In:Diario Oficial de la Federación [Google Scholar]
- 42.Osier ND, Dixon CE. Catecholaminergic based therapies for functional recovery after TBI. Brain Res. 2016;1640:15–35. doi: 10.1016/j.brainres.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parra-Cid C, Orozco-Castillo E, García-López J, Contreras-Figueroa E, Ramos-Languren EL, Ibarra C, Carreón-Rodríguez A, Aschner M, Königsberg M, Santamaría A. Early expression of neuronal dopaminergic markers in a Parkinson's disease model in rats implanted with enteric stem cells (ENSCs) CNS Neurol Disord Drug Targets. 2020;19:148–162. doi: 10.2174/1871527319666200417123948. [DOI] [PubMed] [Google Scholar]
- 44.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 7th ed. London, UK: Academic Press; 2013. [Google Scholar]
- 45.Perez DM. α1-Adrenergic receptors in neurotransmission synaptic plasticity and cognition. Front Pharmacol. 2020;11:581098. doi: 10.3389/fphar.2020.581098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Philipp M, Brede M, Hein L. Physiological significance of alpha(2)-adrenergic receptor subtype diversity:one receptor is not enough. Am J Physiol Regul Integr Comp Physiol. 2002;283:R287–295. doi: 10.1152/ajpregu.00123.2002. [DOI] [PubMed] [Google Scholar]
- 47.Puderbaugh M, Emmady PD. In:StatPearls [Internet]:StatPearls Publishing; 2021. Neuroplasticity. [PubMed] [Google Scholar]
- 48.Rafalowska U, Liu GJ, Floyd RA. Peroxidation induced changes in synaptosomal transport of dopamine and γ-aminobutyric acid. Free Radic Biol Med. 1989;6:485–492. doi: 10.1016/0891-5849(89)90041-5. [DOI] [PubMed] [Google Scholar]
- 49.Ramachandran VS. Plasticity and functional recovery in neurology. Clin Med (Lond) 2005;5:368–373. doi: 10.7861/clinmedicine.5-4-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition:focus on the prefrontal cortex. Pharmacol Ther. 2007;113:523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramos-Languren LE, González-Piña R, Montes S, Chávez-García N, Ávila-Luna A, Barón-Flores V, Ríos C. Sensorimotor recovery from cortical injury is accompanied by changes on norepinephrine and serotonin levels in the dentate gyrus and pons. Behav Brain Res. 2016a;297:297–306. doi: 10.1016/j.bbr.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Ramos-Languren LE, Garcia-Diaz G, Gonzalez-Maciel A, Rosas-Lopez LE, Bueno-Nava A, Avila-Luna A, Ramirez-Anguiano H, Gonzalez-Pina R. Sensorimotor intervention recovers noradrenaline content in the dentate gyrus of cortical injured rats. Neurochem Res. 2016b;41:3261–3271. doi: 10.1007/s11064-016-2054-2. [DOI] [PubMed] [Google Scholar]
- 53.Rascol O, Sieradzan K, Peyro-Saint-Paul H, Thalamas C, Brefel-Courbon C, Senard JM, Ladure P, Montastruc JL, Lees A. Efaroxan an alpha-2 antagonist in the treatment of progressive supranuclear palsy. Mov Disord. 1998;13:673–676. doi: 10.1002/mds.870130411. [DOI] [PubMed] [Google Scholar]
- 54.Rizk P, Salazar J, Raisman-Vozari R, Marien M, Ruberg M, Colpaert F, Debeir T. The alpha2-adrenoceptor antagonist dexefaroxan enhances hippocampal neurogenesis by increasing the survival and differentiation of new granule cells. Neuropsychopharmacology. 2006;31:1146–1157. doi: 10.1038/sj.npp.1300954. [DOI] [PubMed] [Google Scholar]
- 55.Rusu-Zota G, Vasile Timofte D, Albu E, Nechita P, Sorodoc V. The effects of idazoxan and efaroxan improves memory and cognitive functions in rats experimental research. Rev Chim. 2019;70:1411–1415. [Google Scholar]
- 56.Rusu-Zota G, Burlui A, Rezus E, Paduraru L, Sorodoc V. Idazoxan and efaroxan potentiate the endurance performances and the antioxidant activity of ephedrine in rats. Medicina (Kaunas) 2021;57:194. doi: 10.3390/medicina57030194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ:25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Starke K. Presynaptic autoreceptors in the third decade:focus on alpha2-adrenoceptors. J Neurochem. 2001;78:685–693. doi: 10.1046/j.1471-4159.2001.00484.x. [DOI] [PubMed] [Google Scholar]
- 59.Steinberg SF. Beta1-adrenergic receptor regulation revisited. Circ Res. 2018;123:1199–1201. doi: 10.1161/CIRCRESAHA.118.313884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steindler DA. Locus coerules neurons have axons that branch to the forebrain and cerebellum. Brain Res. 1981;223:367–373. doi: 10.1016/0006-8993(81)91149-5. [DOI] [PubMed] [Google Scholar]
- 61.Stibick DL, Feeney DM. Enduring vulnerability to transient reinstatement of hemiplegia by prazosin after traumatic brain injury. J Neurotrauma. 2001;18:303–312. doi: 10.1089/08977150151070955. [DOI] [PubMed] [Google Scholar]
- 62.Triggs WJ, Willmore LJ. In vivo lipid peroxidation in rat brain following intracortical Fe2+ injection. J Neurochem. 1984;42:976–980. doi: 10.1111/j.1471-4159.1984.tb12699.x. [DOI] [PubMed] [Google Scholar]
- 63.Viale L, Catoira NP, Di Girolamo G, González CD. Pharmacotherapy and motor recovery after stroke. Expert Rev Neurother. 2018;18:65–82. doi: 10.1080/14737175.2018.1400910. [DOI] [PubMed] [Google Scholar]
- 64.Willmore LJ, Rubin JJ. Effects of antiperoxidants on FeCl2-induced lipid peroxidation and focal edema in rat brain. Exp Neurol. 1984;83:62–70. doi: 10.1016/0014-4886(84)90046-3. [DOI] [PubMed] [Google Scholar]
- 65.Yasaei R, Saadabadi A. Clonidine. In:StatPearls [Internet]:StatPearls Publishing; 2021. [Google Scholar]
- 66.Zahrai A, Vahid-Ansari F, Daigle M, Albert PR. Fluoxetine-induced recovery of serotonin and norepinephrine projections in a mouse model of post-stroke depression. Transl Psychiatry. 2020;10:334. doi: 10.1038/s41398-020-01008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zai L, Ferrari C, Subbaiah S, Havton LA, Coppola G, Strittmatter S, Irwin N, Geschwind D, Benowitz LI. Inosine alters gene expression and axonal projections in neurons contralateral to a cortical infarct and improves skilled use of the impaired limb. J Neurosci. 2009;29:8187–8197. doi: 10.1523/JNEUROSCI.0414-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao Z. Iron and oxidizing species in oxidative stress and Alzheimer's disease. Aging Med (Milton) 2019;2:82–87. doi: 10.1002/agm2.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]


