Keywords: cerebral ischemia, microglia, neuroprotection, nuclear factor kappa-B, pro-inflammatory phenotype, regulatory phenotype, reperfusion, Toll-like receptor 4, vagus nerve stimulation, α7 nicotinic acetylcholine receptor
Abstract
Microglia are the brain’s primary innate immune cells, and they are activated and affect pro-inflammatory phenotype or regulatory phenotype after ischemic stroke. Vagus nerve stimulation was shown to activate microglial phenotypic changes and exhibit neuroprotective effects in ischemia/reperfusion injury. In this study, we established rat models of ischemic stroke by occlusion of the middle cerebral artery and performed vagus nerve stimulation 30 minutes after modeling. We found that vagus nerve stimulation caused a shift from a pro-inflammatory phenotype to a regulatory phenotype in microglia in the ischemic penumbra. Vagus nerve stimulation decreased the levels of pro-inflammatory phenotype markers inducible nitric oxide synthase and tumor necrosis factor α and increased the expression of regulatory phenotype markers arginase 1 and transforming growth factor β through activating α7 nicotinic acetylcholine receptor expression. Additionally, α7 nicotinic acetylcholine receptor blockade reduced the inhibition of Toll-like receptor 4/nuclear factor kappa-B pathway-associated proteins, including Toll-like receptor 4, myeloid differentiation factor 88, I kappa B alpha, and phosphorylated-I kappa B alpha, and also weakened the neuroprotective effects of vagus nerve stimulation in ischemic stroke. Vagus nerve stimulation inhibited Toll-like receptor 4/nuclear factor kappa-B expression through activating α7 nicotinic acetylcholine receptor and regulated microglial polarization after ischemic stroke, thereby playing a role in the treatment of ischemic stroke. Findings from this study confirm the mechanism underlying vagus nerve stimulation against ischemic stroke.
Introduction
Reperfusion therapy is the best treatment for patients after an acute ischemic stroke (Jahan et al., 2019). Over 90% of ischemic stroke patients cannot receive reperfusion therapy for various reasons (Ma et al., 2021). Additionally, over 40% of individuals who receive reperfusion therapy experience serious sequela and long-term disability (Yaghi et al., 2017). Clinically, rehabilitation is an effective method to improve motor function after ischemic stroke (Richards and Cramer, 2021), but current rehabilitation strategies need to be further validated and optimized (Stinear et al., 2020). Therefore, development of new rehabilitation therapies is critically important for individuals with stroke caused by an ischemic condition.
Vagus nerve stimulation (VNS), which is approved by the United States Food and Drug Administration, is used as a substitute treatment for intractable epilepsy, but its therapeutic efficacy for other neurological diseases is being investigated (Ryvlin et al., 2021; Wang et al., 2021). Animal studies have shown that VNS is a potential treatment for cerebral ischemia (Zhang et al., 2017a; Meyers et al., 2018). VNS effectively improved motor recovery in patients with stroke (Dawson et al., 2021; Li et al., 2022). However, although VNS significantly reduces the severity of stroke-induced damage to brain parenchyma, the underlying mechanism of this beneficial effect remains largely unknown.
Neuroinflammation, which generally refers to inflammatory responses inside the central nervous system, has been demonstrated to play a key part in the pathogenesis of stroke (Jayaraj et al., 2019). Microglia are among the most essential cells in neuroinflammation after ischemic stroke (Borst et al., 2021). Currently, these immune cells are considered to have dual effects on neurological recovery because they can switch towards the deleterious pro-inflammatory phenotype or the neuroprotective regulatory phenotype. The change in the microglial phenotype towards a classical pro-inflammatory phenotype is induced by toxins, lipopolysaccharide, and/or interferon-gamma, and microglia with a pro-inflammatory phenotype release harmful inflammatory mediators. Conversely, microglia with a regulatory phenotype, which secrete anti-inflammatory cytokines such as transforming growth factor beta1 (TGF-β1) and interleukin (IL)-10, clear cell debris and rectify deleterious neuroinflammation (Hu et al., 2015; Xue et al., 2021). Our previous research (Zhang et al., 2021) showed that VNS can affect the microglial phenotypic change, thereby promoting an improvement in motor function in rats after stroke, but the regulatory mechanism remains unclear. The α7 nicotinic acetylcholine receptor (α7nAChR) is a specific acetylcholine receptor that represents an essential function in the central nervous system and in the cholinergic inflammatory pathway (Noviello et al., 2021). VNS increases α7nAChR activity and promotes recovery following ischemic injury (Li et al., 2020a), and the activated α7nAChR induces pro-inflammatory phenotype microglial transformation to the microglial regulatory phenotype. Conversely, both α7nAChR knockout and blockade with specific antagonists abolish the receptor’s ability to induce microglial conversion, which suggests that α7nAChR may activate microglial phenotype transformation (Hoover, 2017). Therefore, the goal of this research was to investigate whether VNS is a new potential therapeutic option for ischemic stroke and whether α7nAChR is associated with the VNS-mediated shift in the microglial phenotype after ischemic brain injury.
Methods
Animals
All animal experiments were approved by Chongqing Medical University’s Experimental Ethics Committee (approval No. 2021(103) on February 21, 2021) and performed in compliance with the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines. Because estrogen is an independent factor affecting stroke outcomes and inflammatory signaling pathways (Koellhoffer and McCullough, 2013), 120 specific-pathogen-free adult male Sprague-Dawley rats (7–8 weeks old, 250–280 g) were acquired from Chongqing Medical University’s Experimental Animal Center (Chongqing, China; license No. 0011233). All rats were housed in a controlled environment with a 12-hour light/dark cycle, a temperature of 22–23°C, a relative humidity of 55% to 60%, and free access to food and drink. Additionally, the heart rate, blood gas levels, and tail blood vessel pressure were calculated. Every effort was made to minimize their pain and suffering. During the experiment, the rats were randomized into the following five groups: sham (n = 24), ischemia/reperfusion (I/R) (n = 24), I/R + VNS (n = 24), I/R + VNS + targeting adeno-associated virus (AAV)-short hairpin RNA (shRNA)-α7nAchR (I/R + VNS + AAV-shRNA-α7nAchR) (n = 24), and I/R + VNS + AAV-enhanced green fluorescent protein (eGFP) (n = 24) groups. The study protocol is presented in Figure 1A. In the experiments, anesthesia included an intraperitoneal injection of 1% pentobarbital sodium (45 mg/kg, intraperitoneally, Tocris, Bristol, UK).
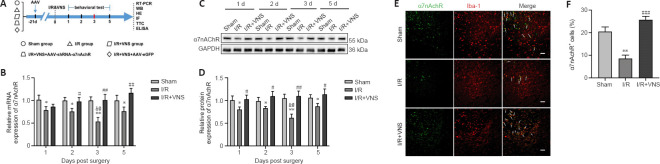
Figure 1.
VNS upregulates α7nAchR expression in the ischemic penumbra after cerebral I/R injury.
(A) Schematic illustration of the experimental design. (B) α7nAChR mRNA levels were measured by RT-PCR (normalized by sham group) at 1, 2, 3, and 5 days after I/R. (C, D) (C) α7nAChR protein levels at 1, 2, 3, and 5 days after I/R were measured using western blot (C) and quantified by optical density (normalized by the sham group) (D). (E) Double immunofluorescence staining of α7nAchR (Alexa Fluor 488 labeled green) and Iba-1 (Alexa Fluor cy3 labeling red) and areas of overlap appear yellow in the merged image (white arrow) at 3 days. α7nAchR colocalized with Iba-1 in the peri-infarct region. The α7nAchR(+) and Iba-1(+) microglia were reduced in the I/R group (normalized by the sham group), but following VNS, the decrease in the number of these cells was reversed. Scale bars: 50 μm. (F) The percentage of α7nAChR-positive cells. Results are expressed as the mean ± SD (n = 6). *P < 0.05, **P < 0.01, vs. sham group; #P < 0.05, ##P < 0.01, ###P < 0.001, vs. I/R group; &P < 0.05, vs. I/R group at day 2; @P < 0.05, vs. I/R group on day 5 (one-way analysis of variance followed by Tukey’s post hoc test). α7nAchR: α7 nicotinic acetylcholine receptor; AAV: targeting adeno-associated virus; ELISA: enzyme-linked immunosorbent assay; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; HE: hematoxylin-eosin staining; IF: immunofluorescence staining; I/R: ischemia/reperfusion; RT-PCR: real-time polymerase chain reaction; shRNA: short hairpin RNA; TTC: 2,3,5-triphenyltetrazolium chloride; VNS: vagus nerve stimulation; WB: western blot.
Stereotaxic injection of adeno-associated virus
AAV-shRNA-α7nAchR was purchased from Bio Technology Co., Ltd. (Shanghai, China). AAV encoding GFP served as a negative control (AAV-eGFP). Twenty-one days before cerebral I/R, 5 µL of AAV-shRNA-α7nAchR or AAV-eGFP (approximately 1 × 1013 infection units/mL) was stereotaxically administered into the left lateral ventricle at a pace of 2 µL/min (–0.8 mm anteroposterior and –1.5 mm mediolateral from the bregma, at a depth of 3.5 mm from the surface) (Seyer et al., 2016). After injection, the syringe was left in place for at least 5 minutes before removal to prevent reflux and ensure that the virus was completely dispersed.
Cerebral I/R model establishment and VNS treatment
The procedure for middle cerebral artery occlusion (MCAO) was performed as described previously (Sun et al., 2018). The rats were sedated and their body temperature was maintained at 37°C using a thermostatic pad (Globalebio, Beijing, China). The left common carotid, internal carotid, and external carotid arteries were all exposed through a midline skin incision. To block the origin of the middle cerebral artery, a silicone-coated nylon monofilament (diameter 0.32 ± 0.02 mm, Cinontech, Beijing, China) was gently introduced through the midline skin incision into the internal carotid artery lumen. Reperfusion was initiated 90 minutes after MCAO by removing the suture. After 30 minutes of ischemia, left cervical VNS was applied using a Grass model S48 stimulator (Grass Technologies, Warwick, RI, USA), which administered a 20-Hz, 0.5-ms (0.5 mA) square pulse train lasting 30 seconds, every 4 minutes for 1 hour, in accordance with the method used in a previous study (Li et al., 2020b). The control rats’ cervical skin was opened and wrapped with moist gauze, but these rats were not subjected to MCAO or stimulation.
Neurobehavioral assessment
Neurological function was assessed before and 1, 2, 3, and 5 days after MCAO. Those who performed the neurobehavioral assessment were blinded to the treatment groups.
Modified neurologic severity score
The modified neurologic severity score is a composite of motor and sensory system function as well as balance and reflexes (Chen et al., 2001). The modified neurologic severity score ranges from 0 to 18, with 0 representing normal function and higher scores indicating increasingly more severe damage.
Rotarod test
The rotarod test was used to assess alterations in motor coordination and balance (Wang et al., 2014). Each rat underwent the rotarod test at least three times daily at intervals of 20 minutes. The speed of the rotating rod was slowly increased from 20 to 40 revolutions/minute over 5 minutes. The duration that the rats stayed on the accelerated rotating rod was recorded.
Real-time polymerase chain reaction
To detect changes in the α7nAchR mRNA expression during the acute phase, TRIzol (Invitrogen, Carlsbad, CA, USA) was used to extract total RNA from ischemic penumbral brain tissue at 1, 2, 3, and 5 days after I/R, which was then reverse-transcribed into complementary DNA using HiScript II Q RT SuperMix for use in real-time polymerase chain reaction (Vazyme, Nanjing, China). The AceQ® qPCRSYBR® GreenMaster Mix kit was used for real-time polymerase chain reaction (Vazyme). The comparative Ct (ΔΔCt) approach (Zhao et al., 2019a) was used to standardize relative gene expression levels to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression level, which was used as an endogenous control. The primer sequences were as follows: α7nAchR, 5′-ACC TCG TGT GAT CCA AAG CC-3′ (forward primer) and 5′-GGT TTC CTC TTG CTC AGG GT-3′ (reverse primer); and GAPDH, 5′-ACA GCA ACA GGG TGG TGG AC-3′ (forward primer) and 5′-TTT GAG GGT GCA GCG AAC TT-3′ (reverse primer).
Western blot analysis
To detect changes in α7nAchR protein expression, inflammatory factors, and the TLR4/NF-κB pathway during the acute phase, protein was isolated from ischemic penumbral brain tissue using a whole-protein extraction kit (Beyotime, Shanghai, China) at 1, 2, 3, and 5 days after I/R. Subsequently, 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis was used to separate 30 μg of protein from each sample, which was then transferred onto PVDF membranes (Bio-Rad, Hercules, CA, USA). The membranes were blocked with Tris-buffered saline with Tween-20 (TBST) containing 5% nonfat milk powder for 1 hour before being incubated with the following the primary antibodies overnight at 4°C: α7nAchR (rat, 1:200, Santa Cruz Biotechnology, Dallas, TX, USA, Cat# sc-58607, RRID: AB_784835); microglia regulatory phenotype markers arginase (Arg)-1 (mouse, 1:100, Santa Cruz Biotechnology, Cat# sc-271430, RRID: AB_10648473), and TGF-β1 (rabbit, 1:1000, Proteintech, Wuhan, China, Cat# 21898-1-AP, RRID: AB_2811115), microglia pro-inflammatory phenotype markers inducible nitric oxide synthase (iNOS) (rabbit, 1:1000, Proteintech, Cat# 18985-1-AP, RRID: AB_2782960), and tumor necrosis factor alpha (TNF-α) (rabbit, 1:500, Proteintech, Cat# 17590-1-AP, RRID: AB_2271853). Toll-like receptor (TLR)4/nuclear factor kappa-B (NF-κB) pathway-associated proteins, including TLR4 (mouse, 1:200, Santa Cruz Biotechnology, Cat# sc-293072, RRID: AB_10611320), myeloid differentiation factor 88 (MyD88) (rabbit, 1:1000, Abcam, Cambridge, UK, Cat# ab133739, RRID: AB_2637005), I kappa B alpha (IκBα) (mouse, 1:1000, Cell Signaling Technology, Danvers, MA, USA, Cat# 4814, RRID: AB_390781), phosphorylated (p)-IκBα (rabbit, 1:1000, Cell Signaling Technology, Cat# 2859, RRID: AB_561111), NF-κB (rabbit, 1:1000, Cell Signaling Technology, Cat# 8242, RRID: AB_10859369), p-NF-κB (rabbit, 1:1000, Cell Signaling Technology, Cat# 3033, RRID: AB_331284) were also used. To ensure the correctness of experimental data, the internal controls β-tubulin (rabbit, 1:1000, Proteintech, Cat# 10094-1-AP, RRID: AB_2210695) and GAPDH (mouse, 1:5000, Proteintech, Cat# 60004-1-Ig, RRID: AB_2107436) were used. The membranes were treated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:5000, Proteintech, Cat# SA00001-2, RRID: AB_2722564), goat anti-mouse secondary antibody (1:5000, Proteintech, Cat# SA00001-1, RRID: AB_2722565), or goat anti-rat secondary antibody (1:1000, Proteintech, Cat# SA00001-15, RRID:AB_2864369) for 1 hour at 25°C after washing three times in TBST. A Fusion FX5 analysis device (BP 31, Vilber, Marne-la-Vallée, France) was used to capture the images, which were then quantified using ImageJ 1.8.0_282 (National Institutes of Health, Bethesda, MD, USA) (Schneider et al., 2012).
Immunofluorescence staining
Immunofluorescence staining was used to detect target protein expression in microglia. The ischemic penumbral brain tissue was fixed with 4% paraformaldehyde on day 3 after I/R. The brains were sliced into 12-μm-thick pieces after fixation and dehydration in graded ethanol solutions. The brain slices were washed in PBS, blocked with 5% bovine serum albumin blocking buffer at 25°C for 1 hour, and then incubated overnight at 4°C with the primary antibodies as follows: microglia marker Iba-1 (mouse, 1:100, Fujifilm Wako Shibayagi, Shibukawa, Japan, Cat# 016-26721, RRID: AB_2811160), α7nAchR (rabbit, 1:200, Bioss, Beijing, China, Cat# bs-1049R, RRID: AB_10855397), microglia regulatory phenotype marker Arg-1 (rabbit, 1:100, Proteintech, Cat# 16001-1-AP, RRID: AB_2289842), and microglia pro-inflammatory phenotype marker iNOS (rabbit, 1:100, Proteintech, Cat# 18985-1-AP, RRID: AB_2782960). Then, the samples were incubated with a mixture of goat anti-rabbit secondary antibody with Alexa Fluor 488 labeling (1:500, Abcam, Cat# ab150077, RRID: AB_2630356), and goat anti-mouse secondary antibody with Alexa Fluor cy3 labeling (1:500, Abcam, Cat# ab97035, RRID: AB_10680176) for 1 hour in the dark at 25°C. A fluorescence microscope (Nikon, Tokyo, Japan) was used to image three locations of the ischemic penumbra in the cortex.
2,3,5-Triphenyltetrazolium chloride and hematoxylin-eosin staining
For infarct volume measurement, rats were sacrificed by decapitation on day 3 after I/R, and their brains were rapidly removed and stored at –20°C for 20 minutes. The tissues were cut into 2-mm-thick coronal slices and then stained with 2% 2,3,5-triphenyltetrazolium chloride (Sigma, St. Louis, MO, USA) for 20 minutes at 37°C, followed by 24 hours of fixation in 4% paraformaldehyde at 4°C. ImageJ software was used to examine the infarct volume. The infarct volume percentage was estimated as follows: [total infarct volume – (left hemisphere volume – right hemisphere volume)]/right hemisphere volume × 100.
Hematoxylin-eosin staining was used to assess pathological alterations in the ischemic penumbra. Briefly, whole brains were fixed on day 3 after I/R in 4% paraformaldehyde overnight. The brain tissues were equilibrated in 30% sucrose-phosphate buffer, embedded in paraffin, and cut into coronal slices. All slices were then dewaxed in xylene, dehydrated in gradient alcohol, and rinsed in distilled water. The slices were then stained with hematoxylin for 5 minutes at 25°C before being rinsed in tap water. After that, the portions were soaked in ethanol hydrochloride for 30 seconds followed by washing in tap water. The slides were dehydrated in alcohol using a concentration gradient and then stained with alcohol eosin for 3 minutes. The portions were dehydrated again before treatment with xylene to make them transparent. Finally, neutral resin droplets were used to seal the slices. Under a light microscope (Nikon, Tokyo, Japan), morphological alterations in the slices were detected.
Enzyme-linked immunosorbent assay
On day 3 after I/R injury, the levels of pro- and anti-inflammatory cytokines (IL-1β and IL-10) and microglial phenotype markers (CD86 and CD206) were determined in the peri-infarction cortex using enzyme-linked immunosorbent assay (ELISA; R&D, Emeryville, CA, USA). The study procedures were performed in accordance with the manufacturer’s instructions.
Statistical analysis
The evaluators were blinded to the group assignments. The loss rate was approximately 16% owing to MCAO model failure, animal injury, and other factors in the pilot experiment. The effect size and standard deviation of each group of animals were obtained in the pilot experiment and the required sample number was then estimated to obtain sufficient efficacy for statistical analysis and to obtain the most reliable conclusions. During the modeling process, 18 rats died. After reperfusion, three rats died on the first day, one died on the second day, and two died on the third day, and thus 96 rats were included in the final analysis. All data are shown as the mean ± standard deviation (SD). Graphing was performed using GraphPad Prism 9.0.1 (GraphPad, San Diego, CA, USA, www.GraphPad.com), and statistical analysis was performed using SPSS version 26.0.0.0 (IBM Corp., Armonk, NY, USA). Differences between groups were compared using Mann-Whitney U test and one-way analysis of variance followed by Tukey’s post hoc test. Statistical significance was indicated by a value of P < 0.05.
Results
VNS has no significant effect on the physiological parameters after cerebral I/R injury
The physiological parameters, including the mean values for blood pressure, heart rate, and blood gas were all within normal limits in all groups (Table 1). These findings revealed that VNS treatment had no significant effect on physiological parameters during the experiment, which is consistent with earlier research (Li et al., 2020b).
Table 1.
Effect of VNS on physiological parameters during the experiment
| Group | Mean blood pressure (mmHg) | Heat rate (beats/min) | pH in blood | PCO2 in blood (mmHg) | PO2 in blood (mmHg) |
|---|---|---|---|---|---|
| Sham | 86±4.6 | 365±9 | 7.37±0.03 | 46.8±1.0 | 115±11.7 |
| I/R | 84±5.8 | 360±11 | 7.38±0.01 | 45.9±0.9 | 113±12.2 |
| I/R+VNS | 85±7.2 | 367±12 | 7.41±0.02 | 47.2±1.2 | 109±9.8 |
| I/R+VNS+AAV-shRNA-α7nAChR | 86±6.4 | 362±8 | 7.39±0.03 | 45.6±1.3 | 110±10.3 |
| I/R+VNS+AAV-eGFP | 87±5.8 | 366±10 | 7.40±0.01 | 46.4±0.9 | 112±11.4 |
All date are shown as the mean ± SD (n = 24). α7nAChR: α7 nicotinic acetylcholine receptor; AAV: targeting adeno-associated virus; I/R: ischemia/reperfusion; PCO2: partial pressure of carbon dioxide; pH: potential of hydrogen; PO2: partial pressure of oxygen; shRNA: short hairpin RNA; VNS: vagus nerve stimulation.
VNS upregulates α7nAchR expression in the ischemic penumbra after cerebral I/R injury
α7nAchR expression was evaluated 1, 2, 3, and 5 days after MCAO. Our results showed that α7nAchR mRNA and protein expression levels in the ischemic penumbra were lower than those in the sham group, especially on day 3 (both P < 0.01, respectively; I/R group vs. sham group; Figure 1B–D). However, VNS reversed the reduction in α7nAchR expression caused by I/R injury, and α7nAchR expression gradually reached normal levels after VNS (both P < 0.01; I/R + VNS group vs. I/R group; Figure 1B–D). On day 3 after cerebral I/R was the key time point for microglial transformation from the pro-inflammatory phenotype to the regulatory phenotype (Ma et al., 2019). Thus, we chose day 3 after reperfusion as the time point of interest for subsequent experiments. To further explore the cellular localization of α7nAchR in the central nervous system, we performed double immunofluorescence staining, and the results suggested that α7nAchR was colocalized with Iba-1, a marker of microglia (Figure 1E). The immunofluorescence results suggested that the percentage of α7nAchR+ cells was decreased after I/R compared with that in the sham group (P < 0.01; I/R group vs. sham group), but this decline in the cell number in the I/R group was attenuated following VNS (P < 0.001; I/R + VNS group vs. I/R group; Figure 1F).
VNS attenuates brain injury and improves neurological outcomes after cerebral I/R injury via an α7nAChR-dependent pathway
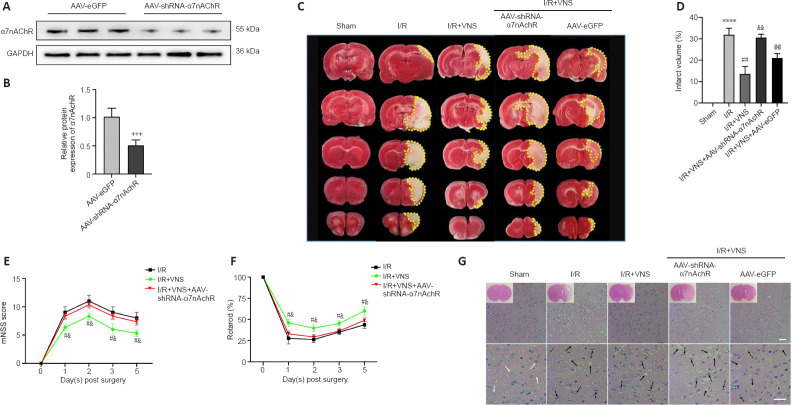
To verify the role of α7nAchR in VNS-induced neuroprotective effects, an α7nAchR-targeting AAV-shRNA was directly injected into the lateral ventricles 3 weeks before MCAO. α7nAchR knockdown efficiency was confirmed by western blot assay (P < 0.001; AAV-shRNA α7nAchR group vs. AAV-eGFP group; Figure 2A and B). The 2,3,5-triphenyltetrazolium chloride staining results indicated that VNS significantly reduced the infarct volume on day 3 after MCAO compared with that in the I/R group (P < 0.01; I/R + VNS group vs. I/R group; Figure 2C and D). However, the effects of VNS were inhibited by knockdown of α7nAchR compared with that in the I/R + VNS group (P < 0.01; I/R + VNS + AAV-shRNA-α7nAchR group vs. I/R + VNS group; Figure 2C and D). The modified neurologic severity score assessment and the rotarod test were conducted before MCAO and again 1, 2, 3, and 5 days after MCAO to determine neurological function recovery. Our results showed that the I/R + VNS group outperformed the model group in terms of neurological scores at all time points after perfusion compared with that in the I/R group (all P < 0.05, I/R + VNS group vs. I/R group; Figure 2E and F). However, injection of AAV-shRNA-α7nAchR drastically attenuated the improvement of VNS on neurological function at the same time point compared with that in the I/R + VNS group (all P < 0.05, I/R + VNS + AAV-shRNA-α7nAchR group vs. I/R + VNS group; Figure 2E and F).
Figure 2.
VNS attenuates brain injury and improves neurological outcomes after cerebral I/R injury via an α7nAChR-dependent pathway.
(A, B) The AAV-shRNA-α7nAchR transfection efficacy was confirmed by western blot assay (n = 5). α7nAchR was normalized by the AAV-eGFP group. (C) 2,3,5-Triphenyltetrazolium chloride staining and infarct volume in the brain (n = 6 per group). An obvious infarct was present in the I/R group, and VNS dramatically decreased the infarct volume in the I/R + VNS group compared with that in the I/R group. However, the effect of VNS was reduced when α7nAchR was suppressed in the I/R + VNS + AAV-shRNA-α7nAchR group. Infarct volume is indicated by the yellow dotted boxes. (D) Brain infarct volume is shown as a percentage of the ischemia volumes (one-way analysis of variance followed by Tukey’s post hoc test). (E, F) The mNSS scores (E) and rotarod test (F) were performed to examine neurobehavioral outcomes of I/R, I/R + VNS and I/R + VNS + AAV-shRNA-α7nAchR-treated rats on day 3 after MCAO (Mann-Whitney U test, n = 6 per group). (G) Effects of α7nAChR on the morphological changes of ischemic penumbra by hematoxylin-eosin staining. The number of cells with nuclear contractions and vacuolation decreased in the VNS group compared with that in the I/R group. Additionally, AAV-shRNA-α7nAchR negated the favorable effects of VNS, as shown by an increase in the number of compacted nuclei, strong staining, and shrinking in the I/R + VNS + AAV-shRNA-α7nAchR group compared with that in the I/R + VNS group. The blue arrow refers to the normal neurons, the dotted arrow refers to the post-ischemic nuclear pyknosis deep-stained neurons, and the black arrow refers to vacuolation (100× [upper] and 400× [lower] magnification, scale bars: 50 μm, n = 4 per group). Data are expressed as the mean ± SD. +++P < 0.001, vs. AAV-eGFP group; ****P < 0.0001, vs. sham group; #P < 0.05, ##P < 0.01, vs. I/R group; &P < 0.05, &&P < 0.01, vs. I/R + VNS group; @P < 0.05, @@P < 0.01, vs. I/R + VNS + AAV-shRNA-α7nAchR group. α7nAchR: α7 nicotinic acetylcholine receptor; AAV: targeting adeno-associated virus; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; I/R: ischemia/reperfusion; MCAO: middle cerebral artery occlusion; mNSS: modified neurologic severity score; shRNA: short hairpin RNA; VNS: vagus nerve stimulation.
For hematoxylin-eosin staining, brain cells were swollen and nuclei were condensed and vacuolized in the I/R group compared with that in the sham group. Following VNS, inflammatory cell infiltration and neuronal necrosis were dramatically alleviated. AAV-shRNA-α7nAchR was also found to counteract the beneficial effects of VNS, as shown by an increasing number of condensed nuclei, intense staining, and shrinkage (Figure 2G). On the basis of these results, we hypothesized that VNS exerts neuroprotection against cerebral I/R injury through α7nAchR.
VNS promotes the regulatory phenotype of microglia through α7nAchR after cerebral I/R injury
Microglia are transformed towards the classic pro-inflammatory phenotype or the regulatory phenotype under optimal conditions (Wang et al., 2018). To explore whether VNS affects the change in the microglial phenotype via α7nAchR, western blot assay, immunofluorescence, and ELISA were used to evaluate specific microglial marker expression.
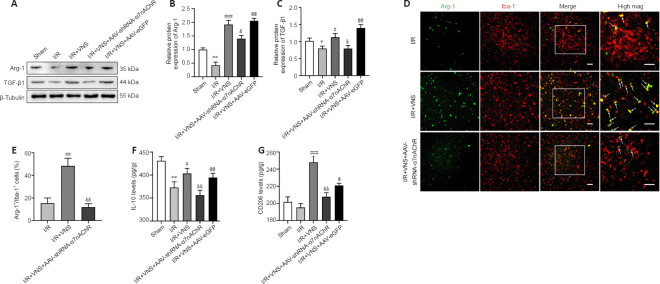
Western blot results showed that the levels of regulatory phenotype markers (Arg-1 and TGF-β1) were notably increased after VNS treatment compared with that in the I/R group (Arg-1, P < 0.001; TGF-β1, P < 0.05; I/R + VNS group vs. I/R group; Figure 3A–C). However, the aforementioned effects of VNS on the change in the microglial phenotype were attenuated after α7nAchR knockdown compared with that in the I/R + VNS group (Arg-1, P < 0.05; TGF-β1, P < 0.05; I/R + VNS + AAV-shRNA-α7nAchR group vs. I/R + VNS group; Figure 3A–C). Immunofluorescence staining of microglia in the cerebral cortex further indicated that VNS exerted a protective effect. Immunofluorescence analysis of the ischemic penumbral brain tissue indicated that VNS increased the Arg-1+/Iba-1+ microglial density compared with that in the I/R group (P < 0.01; I/R + VNS group vs. I/R group; Figure 3D and E). The effect of VNS on the Arg-1+/Iba-1+ cell density in the ischemic penumbra was abolished after inhibition of α7nAchR compared with that in the I/R + VNS group (P < 0.01; I/R + VNS + AAV-shRNA-α7nAchR group vs. I/R + VNS group; Figure 3D and E).
Figure 3.
VNS promotes the microglial regulatory phenotype through α7nAchR after cerebral I/R injury.
(A–C) Protein level of the microglial regulatory phenotype markers Arg-1 and TGF-β1 at day 3 after MCAO. (D) Immunofluorescence images showed Arg-1 expression in the peri-infarction cortex. Arg-1 (Alexa Fluor 488 labeled green) localized to the microglia marker Iba-1 (Alexa Fluor cy3 labeling red) with areas of overlap appearing yellow in the merged image (white arrow). Increased Arg-1(+) and Iba-1(+) microglia were noted in the I/R + VNS group compared with that in the I/R group, and the increase in the number of these cells in the I/R + VNS group was reversed in the I/R + VNS + AAV-shRNA-α7nAchR group. Scale bars: 50 μm. (E) The percentage of Arg-1+/Iba-1+ cells. (F, G) Effect of I/R, VNS + I/R, and I/R + VNS + AAV-shRNA-α7nAchR treatments on IL-10 (F) and CD206 (G) levels in the peri-infarction cortex at 3 days after MCAO. Data are expressed as the mean ± SD (n = 6). *P < 0.05, **P < 0.01, vs. sham group; #P < 0.05, ##P < 0.01, ###P < 0.001, vs. I/R group; &P < 0.05, &&P < 0.01, vs. I/R + VNS group; @P < 0.05, @@P < 0.01, vs. I/R + VNS + AAV-shRNA-α7nAchR group (one-way analysis of variance followed by Tukey’s post hoc test). α7nAchR: α7 nicotinic acetylcholine receptor; AAV: targeting adeno-associated virus; Arg-1: Arginase-1; IL-10: interleukin-10; I/R: ischemia/reperfusion; mag.: image; MCAO: middle cerebral artery occlusion; shRNA: short hairpin RNA; TGF-β1: transforming growth factor beta1; VNS: vagus nerve stimulation.
ELISA was used to assess changes in inflammatory cytokines and specific microglial markers. VNS increased IL-10 and CD206 expression levels compared with those in the I/R group (IL-10, P < 0.05; CD206, P < 0.001; I/R + VNS group vs. I/R group; Figure 3F and G). Compared with that in VNS, α7nAchR inhibition attenuated the VNS-mediated regulatory microglial phenotype after MCAO (IL-10, P < 0.01; CD206, P < 0.01; I/R + VNS + AAV-shRNA-α7nAchR group vs. I/R + VNS group; Figure 3F and G).
VNS inhibits the microglial pro-inflammatory phenotype through α7nAchR after cerebral I/R injury
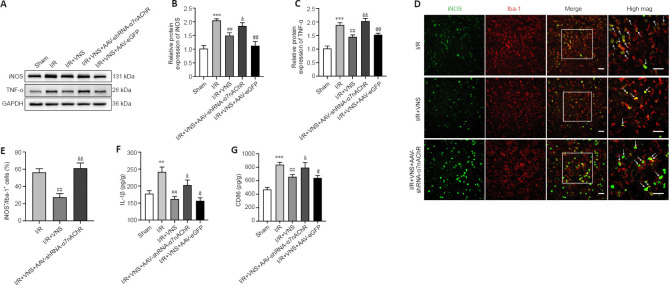
The pro-inflammatory microglial phenotype was also assessed on day 3 after MCAO. Western blot results revealed that VNS reduced the expression of pro-inflammatory phenotype markers iNOS and TNF-α (iNOS, P < 0.01; TNF-α, P < 0.01; I/R + VNS group vs. I/R group; Figure 4A–C). However, lateral ventricle injection of AAV-shRNA-α7nAchR considerably upregulated iNOS and TNF-α expression (iNOS, P < 0.05; TNF-α, P < 0.01; I/R + VNS + AAV-shRNA-α7nAchR group vs. I/R + VNS group; Figure 4A–C). Similarly, immunofluorescence staining suggested that knockdown of α7nAchR abolished the beneficial effect of VNS on the density of iNOS+/Iba-1+ (P < 0.01; I/R + VNS + AAV-shRNA-α7nAchR group vs. I/R + VNS group; Figure 4D and E). Additionally, the IL-1β and CD86 levels were significantly decreased after VNS compared with that in the I/R group (IL-1β, P < 0.01; CD86, P < 0.01; I/R + VNS group vs. I/R group). However, this trend was reversed considerably through AAV-shRNA-α7nAchR injection (IL-1β, P < 0.05; CD86, P < 0.05; I/R + VNS + AAV-shRNA-α7nAchR group vs. I/R + VNS group; Figure 4F and G).
Figure 4.
VNS inhibits the pro-inflammatory phenotype of microglia through α7nAchR after cerebral I/R injury.
(A–C) Protein level of the microglia pro-inflammatory phenotype markers iNOS and TNF-α on day 3 after stroke. (D) Immunofluorescence images show iNOS expression in the peri-infarction cortex. iNOS (Alexa Fluor 488 labeled green) is localized to the microglia marker Iba-1 (Alexa Fluor cy3 labeling red) with areas of overlap appearing yellow in the merged image (white arrow). The I/R + VNS group had less iNOS+ expression that colocalized with Iba-1+ microglia than that in the I/R group. In the I/R + VNS + AAV-shRNA-α7nAchR group, the decrease in the number of these cells was reversed compared with that in the I/R + VNS group. Scale bars: 50 μm. (E) The percentage of iNOS+/Iba-1+ cells. (F, G) Effect of I/R, I/R + VNS, and I/R + VNS + AAV-shRNA-α7nAchR treatments on IL-1β (F) and CD86 (G) levels in the peri-infarction cortex on day 3 after MCAO. Data are expressed as the mean ± SD (n = 6 per group). **P < 0.01, ***P < 0.001, vs. sham group; ##P < 0.01, vs. I/R group; &P < 0.05, &&P < 0.01, vs. I/R + VNS group; @P < 0.05, @@P < 0.01, vs. I/R + VNS + AAV-shRNA-α7nAchR group (one-way analysis of variance followed by Tukey’s post hoc test). α7nAchR: α7 nicotinic acetylcholine receptor; AAV: targeting adeno-associated virus; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; iNOS: inducible nitric oxide synthase; IL-1β: interleukin-1beta; I/R: ischemia/reperfusion; mag: image; MCAO: middle cerebral artery occlusion; shRNA: short hairpin RNA; TNF-α: tumor necrosis factor alpha; VNS: vagus nerve stimulation.
Taken together, these data indicated that VNS may affect the microglia change from the pro-inflammatory phenotype to the regulatory phenotype via activating α7nAchR, a protein that may be involved in preventing brain injury after a stroke.
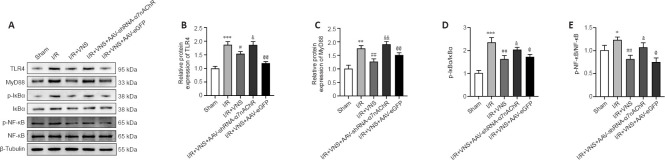
VNS regulates the TLR4/NF-κB pathway through α7nAchR after cerebral I/R injury
Our previous study found that VNS mediates the change in the microglial phenotype by blockade of the TLR4/NF-κB pathway after stroke (Zhang et al., 2021). To explore whether α7nAChR is involved in mediating this signaling pathway, TLR4/NF-κB pathway-associated protein expression levels in the penumbra area were measured in all groups. VNS treatment significantly reduced TLR4, MyD88, p-IκBα/IκBα, and p-NF-κB/NF-κB protein expression after stroke compared with that in the I/R group (TLR4, P < 0.05; MyD88, P < 0.01; p-IκBα/IκBα, P < 0.01; p-NF-κB/NF-κB, P < 0.01; I/R + VNS group vs. I/R group; Figure 5A–E). However, inhibiting α7nAchR prevented VNS’s effects on the TLR4/NF-κB pathway compared with that in the I/R + VNS group (TLR4, P < 0.05; MyD88, P < 0.01; p-IκBα/IκBα, P < 0.05; p-NF-κB/NF-κB, P < 0.05; I/R + VNS + AAV-shRNA-α7nAchR group vs. I/R + VNS group; Figure 5A–E). Collectively, these data suggested that α7nAchR may contribute to the neuroprotective effect of VNS by activating the TLR4/NF-κB pathway.
Figure 5.
VNS regulates the TLR4/NF-κB pathway through α7nAchR after cerebral I/R injury.
(A–E) Protein level for the TLR4/NF-κB pathway after knockdown of α7nAchR. Data are expressed as the mean ± SD (n = 6 per group). *P < 0.05, **P < 0.01, ***P < 0.001, vs. sham group; #P < 0.05, ##P < 0.01, vs. I/R group; &P < 0.05, &&P < 0.01, vs. I/R + VNS group; @P < 0.05, @@P < 0.01, vs. I/R + VNS + AAV-shRNA-α7nAchR group (one-way analysis of variance followed by Tukey’s post hoc test). α7nAchR: α7 nicotinic acetylcholine receptor; AAV: targeting adeno-associated virus; IκBα: IkappaBalpha; I/R: ischemia/reperfusion; mag: image; mag: image; MCAO: middle cerebral artery occlusion; MyD88: myeloiddifferentiationfactor88; NF-κB: nuclear factor kappa-B; p-IκBα: phosphorylated-IkappaBalpha; p-NF-κB: phosphorylated-nuclear factor kappa-B; shRNA: short hairpin RNA; TLR4: Toll-like receptor 4; VNS: vagus nerve stimulation.
Discussion
In this study, we found that α7nAChR is essential for the effect of VNS on the change in microglial phenotype after reperfusion injury following stroke. This was supported by the observations that VNS inhibited the change in the pro-inflammatory microglial phenotype and promoted the change in the anti-inflammatory microglial phenotype. Nevertheless, these effects were abrogated by inhibiting α7nAChR. We verified that the TLR4/NF-κB pathway is downstream of α7nAChR and that it is negatively regulated by α7nAChR. Thus, these results provide evidence that VNS exerts a neuroprotective effect through modulating the changes within the microglial phenotype after cerebral I/R damage, which may mediate the TLR4/NF-κB pathway via α7nAChR.
Recently, a clinical trial indicated that the application of VNS results in clinically meaningful alleviation of functional impairment during the long-term phase of stroke (Dawson et al., 2021). Our pilot study revealed that VNS is also beneficial for improving motor function in those who have a subacute ischemic stroke (Wu et al., 2020). These clinical studies suggest that VNS might be a novel strategy for achieving improvement in motor function after stroke. However, its therapeutic mechanism remains unclear. VNS is effective during the early and late ischemic stages (Hiraki et al., 2012; Sun et al., 2012). Animal studies have revealed that VNS confers strong protection against the neuroinflammatory response in the ischemic brain and neurological deficits (Lu et al., 2017; Li et al., 2020b). In accordance with previously published research, our results revealed that VNS inhibited microglia-mediated neuroinflammation and reduced the level of pro-inflammatory phenotype microglial markers (iNOS, TNF-α, IL-1β, and CD86), while increasing the level of regulatory phenotype microglial markers (Arg-1, TGF-β1, IL-10, and CD206). Furthermore, the beneficial effects of VNS described above were reversed by α7nAChR inhibition. Transcutaneous vagal stimulation (ta-VNS) is an altered VNS procedure that involves non-invasive stimulation of the vagus nerve’s auricular branch. Previous research has suggested that ta-VNS and VNS may share the same pathway (Kraus et al., 2007; Capone et al., 2015). In accordance with previous research findings, we recently published a meta-analysis on the efficacy of combined VNS on stroke recovery, and our subgroup analyses between VNS and ta-VNS revealed that both VNS and ta-VNS combined with rehabilitation have a good effect in those with motor impairment following stroke (Liu et al., 2022). Although both VNS and ta-VNS enhance the prognosis for patients with motor dysfunction, ta-VNS has become more widely used because of its safety and non-invasive nature.
Phenotypic changes in microglia are critical in stroke (Lu et al., 2021). A previous study showed that the microglial regulatory phenotype reduces neurological injury by regulating Janus kinase 1/signal transducer and activator of transcription 6 signaling (He et al., 2020). Studies have also found that transformation of the microglia phenotype by activation of α7nAChR markedly rescues lipopolysaccharide-inhibited activation of Janus kinase 2/signal transducer and activator of transcription 3 and phosphoinositide 3-kinases/protein kinase B (Zhang et al., 2017b). Furthermore, increasing numbers of studies have reported that VNS can modulate the phenotypic transformation of microglia to promote post-stroke recovery, but the mechanism requires further clarification (Zhao et al., 2019b).
α7nAChR is a ligand-gated ion channel with many protein subunits, and it is mainly expressed in the central and peripheral nervous systems (Piovesana et al., 2021). Convincing evidence indicates that α7nAChR activation cross-talks with the pro-inflammatory microglial phenotype (Carandina et al., 2021; Zhao et al., 2022). α7nAchR agonists and VNS protect against cerebral I/R, but α7nAChR antagonists can decrease the effects of VNS (Hoover, 2017). However, the involvement of α7nAChR in regulating the phenotypic change in microglia in the brain has not been elucidated. In this study, we showed that VNS induced microglia switching from the pro-inflammatory phenotype to the regulatory phenotype via α7nAChR after cerebral I/R injury. We speculate that VNS-induced alleviation of cerebral stroke was largely dependent on α7nAchR because VNS prevented the downregulation of α7nAchR expression and subsequently suppressed the inflammatory response.
TLRs are activated by pathogen-associated molecular patterns and damage-associated molecular patterns, and their signaling serves as a central target in the brain during ischemic injury. Our recent study found that suppression of the TLR4/NF-κB pathway alleviates brain injury and decreases the inflammatory response after ischemic stroke (Zhang et al., 2021). Few previous studies have assessed the relationship between α7nAChR and TLR4. Researchers have found that activation of α7nAChR by a partial selective receptor (GTS-21) inhibits the TLR4/NF-κB signaling during diabetic cardiomyopathy (Youssef et al., 2021). They also found that α7nAChR inhibits the TLR4/MyD88/NF-κB signaling pathway to protect against lipopolysaccharide-induced liver injury. Moreover, the hepatoprotective effect of α7nAChR was abolished by α-BGT, a specific α7nAChR antagonist (Hoover, 2017). However, it is unclear whether VNS has a neuroprotective effect against ischemic stroke via α7nAChR in association with TLR4 suppression. The obtained data showed that α7nAChR dramatically reduces TLR4/NF-κB expression in rats with cerebral I/R. In this study, AAV-shRNA-α7nAchR reversed the suppression of TLR4/NF-κB signaling and the reparative effect of VNS, thus amplifying the inflammatory cascade.
Our research has some limitations. First, the effect of α7nAChR on microglial phenotype alterations can be better verified by a rat model with conditional knockout of microglia α7nAChR. Second, the effect of α7nAchR agonist on microglial phenotypes was not examined. Moreover, both α7nAChR and TLR4 are transmembrane receptors, and α7nAChR may promote TLR4 degradation by activating adenylyl cyclase-6, which inhibits inflammation in macrophages (Zhu et al., 2021). Further investigation is required to determine how α7nAChR regulates TLR4 activation. Nevertheless, our findings revealed that VNS contributes to the neuroinflammatory response during brain diseases through the TLR4/NF-κB pathway by activating α7nAChR. Activation of α7nAChR induces microglial transformation from the pro-inflammatory phenotype to the regulatory phenotype. These data show that VNS could be a promising treatment for ischemic stroke recovery.
Acknowledgments:
We express special gratitude to The Second Affiliated Hospital of Chongqing Medical University for providing technical support.
Footnotes
Funding: This work was supported by the Natural Science Foundation of Chongqing, No. cstc2019jcyj-msxmX0026; the Medical Scientific Research Projects Foundation of Chongqing, No. 2021ZY023818, and the Natural Science Foundation of Chongqing, No. cstc2018jcyjAX0180 (all to GWJ).
Conflicts of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Yu J, Song LP; T-Editor: Jia Y
References
- 1.Borst K, Dumas AA, Prinz M. Microglia:immune and non-immune functions. Immunity. 2021;54:2194–2208. doi: 10.1016/j.immuni.2021.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Capone F, Assenza G, Di Pino G, Musumeci G, Ranieri F, Florio L, Barbato C, Di Lazzaro V. The effect of transcutaneous vagus nerve stimulation on cortical excitability. J Neural Transm (Vienna) 2015;122:679–685. doi: 10.1007/s00702-014-1299-7. [DOI] [PubMed] [Google Scholar]
- 3.Carandina A, Lazzeri G, Villa D, Di Fonzo A, Bonato S, Montano N, Tobaldini E. Targeting the autonomic nervous system for risk stratification outcome prediction and neuromodulation in ischemic stroke. Int J Mol Sci. 2021;22:2357. doi: 10.3390/ijms22052357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 5.Dawson J, Liu CY, Francisco GE, Cramer SC, Wolf SL, Dixit A, Alexander J, Ali R, Brown BL, Feng W, DeMark L, Hochberg LR, Kautz SA, Majid A, O'Dell MW, Pierce D, Prudente CN, Redgrave J, Turner DL, Engineer ND, et al. Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB):a randomised, blinded, pivotal, device trial. Lancet. 2021;397:1545–1553. doi: 10.1016/S0140-6736(21)00475-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He Y, Gao Y, Zhang Q, Zhou G, Cao F, Yao S. IL-4 switches microglia/macrophage M1/M2 polarization and alleviates neurological damage by modulating the JAK1/STAT6 pathway following ICH. Neuroscience. 2020;437:161–171. doi: 10.1016/j.neuroscience.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Hiraki T, Baker W, Greenberg JH. Effect of vagus nerve stimulation during transient focal cerebral ischemia on chronic outcome in rats. J Neurosci Res. 2012;90:887–894. doi: 10.1002/jnr.22812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoover DB. Cholinergic modulation of the immune system presents new approaches for treating inflammation. Pharmacol Ther. 2017;179:1–16. doi: 10.1016/j.pharmthera.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, Chen J. Microglial and macrophage polarization—new prospects for brain repair. Nat Rev Neurol. 2015;11:56–64. doi: 10.1038/nrneurol.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jahan R, Saver JL, Schwamm LH, Fonarow GC, Liang L, Matsouaka RA, Xian Y, Holmes DN, Peterson ED, Yavagal D, Smith EE. Association between time to treatment with endovascular reperfusion therapy and outcomes in patients with acute ischemic stroke treated in clinical practice. JAMA. 2019;322:252–263. doi: 10.1001/jama.2019.8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA. Neuroinflammation:friend and foe for ischemic stroke. J Neuroinflammation. 2019;16:142. doi: 10.1186/s12974-019-1516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koellhoffer EC, McCullough LD. The effects of estrogen in ischemic stroke. Transl Stroke Res. 2013;4:390–401. doi: 10.1007/s12975-012-0230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kraus T, Hösl K, Kiess O, Schanze A, Kornhuber J, Forster C. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J Neural Transm (Vienna) 2007;114:1485–1493. doi: 10.1007/s00702-007-0755-z. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Zhang Q, Li S, Niu L, Ma J, Wen L, Zhang L, Li C. α7nAchR mediates transcutaneous auricular vagus nerve stimulation-induced neuroprotection in a rat model of ischemic stroke by enhancing axonal plasticity. Neurosci Lett. 2020a;730:135031. doi: 10.1016/j.neulet.2020.135031. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Zhang K, Zhang Q, Zhou X, Wen L, Ma J, Niu L, Li C. PPAR-γmediates Ta-VNS-induced angiogenesis and subsequent functional recovery after experimental stroke in rats. Biomed Res Int. 2020b;2020:8163789. doi: 10.1155/2020/8163789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li JN, Xie CC, Li CQ, Zhang GF, Tang H, Jin CN, Ma JX, Wen L, Zhang KM, Niu LC. Efficacy and safety of transcutaneous auricular vagus nerve stimulation combined with conventional rehabilitation training in acute stroke patients:a randomized controlled trial conducted for 1 year involving 60 patients. Neural Regen Res. 2022;17:1809–1813. doi: 10.4103/1673-5374.332155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Zhang L, Zhang X, Ma J, Jia G. Effect of combined vagus nerve stimulation on recovery of upper extremity function in patients with stroke:a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2022;31:106390. doi: 10.1016/j.jstrokecerebrovasdis.2022.106390. [DOI] [PubMed] [Google Scholar]
- 18.Lu XX, Hong ZQ, Tan Z, Sui MH, Zhuang ZQ, Liu HH, Zheng XY, Yan TB, Geng DF, Jin DM. Nicotinic acetylcholine receptor alpha7 subunit mediates vagus nerve stimulation-induced neuroprotection in acute permanent cerebral ischemia by α7nAchR/JAK2 pathway. Med Sci Monit. 2017;23:6072–6081. doi: 10.12659/MSM.907628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu Y, Zhou M, Li Y, Li Y, Hua Y, Fan Y. Minocycline promotes functional recovery in ischemic stroke by modulating microglia polarization through STAT1/STAT6 pathways. Biochem Pharmacol. 2021;186:114464. doi: 10.1016/j.bcp.2021.114464. [DOI] [PubMed] [Google Scholar]
- 20.Ma Y, Yang S, He Q, Zhang D, Chang J. The role of immune cells in post-stroke angiogenesis and neuronal remodeling:the known and the unknown. Front Immunol. 2021;12:784098. doi: 10.3389/fimmu.2021.784098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma Z, Zhang Z, Bai F, Jiang T, Yan C, Wang Q. Electroacupuncture pretreatment alleviates cerebral ischemic injury through α7 nicotinic acetylcholine receptor-mediated phenotypic conversion of microglia. Front Cell Neurosci. 2019;13:537. doi: 10.3389/fncel.2019.00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyers EC, Solorzano BR, James J, Ganzer PD, Lai ES, Rennaker RL, 2nd , Kilgard MP, Hays SA. Vagus nerve stimulation enhances stable plasticity and generalization of stroke recovery. Stroke. 2018;49:710–717. doi: 10.1161/STROKEAHA.117.019202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noviello CM, Gharpure A, Mukhtasimova N, Cabuco R, Baxter L, Borek D, Sine SM, Hibbs RE. Structure and gating mechanism of the α7 nicotinic acetylcholine receptor. Cell. 2021;184:2121–2134. doi: 10.1016/j.cell.2021.02.049. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piovesana R, Salazar Intriago MS, Dini L, Tata AM. Cholinergic modulation of neuroinflammation:focus on α7 nicotinic receptor. Int J Mol Sci. 2021;22:4912. doi: 10.3390/ijms22094912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richards LG, Cramer SC. Advances in stroke:therapies targeting stroke recovery. Stroke. 2021;52:348–350. doi: 10.1161/STROKEAHA.120.033231. [DOI] [PubMed] [Google Scholar]
- 26.Ryvlin P, Rheims S, Hirsch LJ, Sokolov A, Jehi L. Neuromodulation in epilepsy:state-of-the-art approved therapies. Lancet Neurol. 2021;20:1038–1047. doi: 10.1016/S1474-4422(21)00300-8. [DOI] [PubMed] [Google Scholar]
- 27.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ:25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seyer B, Pham V, Albiston AL, Chai SY. Cannula implantation into the lateral ventricle does not adversely affect recognition or spatial working memory. Neurosci Lett. 2016;628:171–178. doi: 10.1016/j.neulet.2016.06.034. [DOI] [PubMed] [Google Scholar]
- 29.Stinear CM, Lang CE, Zeiler S, Byblow WD. Advances and challenges in stroke rehabilitation. Lancet Neurol. 2020;19:348–360. doi: 10.1016/S1474-4422(19)30415-6. [DOI] [PubMed] [Google Scholar]
- 30.Sun R, Song Y, Li S, Ma Z, Deng X, Fu Q, Qu R, Ma S. Levo-tetrahydropalmatine attenuates neuron apoptosis induced by cerebral ischemia-reperfusion injury:involvement of c-Abl activation. J Mol Neurosci. 2018;65:391–399. doi: 10.1007/s12031-018-1063-9. [DOI] [PubMed] [Google Scholar]
- 31.Sun Z, Baker W, Hiraki T, Greenberg JH. The effect of right vagus nerve stimulation on focal cerebral ischemia:an experimental study in the rat. Brain Stimul. 2012;5:1–10. doi: 10.1016/j.brs.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Xing H, Wan L, Jiang X, Wang C, Wu Y. Treatment targets for M2 microglia polarization in ischemic stroke. Biomed Pharmacother. 2018;105:518–525. doi: 10.1016/j.biopha.2018.05.143. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Shi Y, Zhang L, Zhang F, Hu X, Zhang W, Leak RK, Gao Y, Chen L, Chen J. Omega-3 polyunsaturated fatty acids enhance cerebral angiogenesis and provide long-term protection after stroke. Neurobiol Dis. 2014;68:91–103. doi: 10.1016/j.nbd.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Zhan G, Cai Z, Jiao B, Zhao Y, Li S, Luo A. Vagus nerve stimulation in brain diseases:therapeutic applications and biological mechanisms. Neurosci Biobehav Rev. 2021;127:37–53. doi: 10.1016/j.neubiorev.2021.04.018. [DOI] [PubMed] [Google Scholar]
- 35.Wu D, Ma J, Zhang L, Wang S, Tan B, Jia G. Effect and safety of transcutaneous auricular vagus nerve stimulation on recovery of upper limb motor function in subacute ischemic stroke patients:a randomized pilot study. Neural Plast. 2020;2020:8841752. doi: 10.1155/2020/8841752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xue Y, Nie D, Wang LJ, Qiu HC, Ma L, Dong MX, Tu WJ, Zhao J. Microglial polarization:novel therapeutic strategy against ischemic stroke. Aging Dis. 2021;12:466–479. doi: 10.14336/AD.2020.0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yaghi S, Willey JZ, Cucchiara B, Goldstein JN, Gonzales NR, Khatri P, Kim LJ, Mayer SA, Sheth KN, Schwamm LH American Heart Association Stroke Council Council on Cardiovascular and Stroke Nursing Council on Clinical Cardiology and Council on Quality of Care and Outcomes Research. Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke:a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48:e343–361. doi: 10.1161/STR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 38.Youssef ME, Abdelrazek HM, Moustafa YM. Cardioprotective role of GTS-21 by attenuating the TLR4/NF-κB pathway in streptozotocin-induced diabetic cardiomyopathy in rats. Naunyn Schmiedebergs Arch Pharmacol. 2021;394:11–31. doi: 10.1007/s00210-020-01957-4. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L, Ma J, Jin X, Jia G, Jiang Y, Li C. L-PGDS mediates vagus nerve stimulation-induced neuroprotection in a rat model of ischemic stroke by suppressing the apoptotic response. Neurochem Res. 2017a;42:644–655. doi: 10.1007/s11064-016-2121-8. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Liu Y, Wang S, Long L, Zang Q, Ma J, Yu L, Jia G. Vagus nerve stimulation mediates microglia M1/2 polarization via inhibition of TLR4 pathway after ischemic stroke. Biochem Biophys Res Commun. 2021;577:71–79. doi: 10.1016/j.bbrc.2021.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Q, Lu Y, Bian H, Guo L, Zhu H. Activation of the α7 nicotinic receptor promotes lipopolysaccharide-induced conversion of M1 microglia to M2. Am J Transl Res. 2017b;9:971–985. [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao JJ, Wang ZH, Zhang YJ, Wang WJ, Cheng AF, Rong PJ, Shan CL. The mechanisms through which auricular vagus nerve stimulation protects against cerebral ischemia/reperfusion injury. Neural Regen Res. 2022;17:594–600. doi: 10.4103/1673-5374.320992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao R, Ying M, Gu S, Yin W, Li Y, Yuan H, Fang S, Li M. Cysteinyl leukotriene receptor 2 is involved in inflammation and neuronal damage by mediating microglia M1/M2 polarization through NF-κB pathway. Neuroscience. 2019a;422:99–118. doi: 10.1016/j.neuroscience.2019.10.048. [DOI] [PubMed] [Google Scholar]
- 44.Zhao XP, Zhao Y, Qin XY, Wan LY, Fan XX. Non-invasive vagus nerve stimulation protects against cerebral ischemia/reperfusion injury and promotes microglial M2 polarization Via interleukin-17A inhibition. J Mol Neurosci. 2019b;67:217–226. doi: 10.1007/s12031-018-1227-7. [DOI] [PubMed] [Google Scholar]
- 45.Zhu S, Huang S, Xia G, Wu J, Shen Y, Wang Y, Ostrom RS, Du A, Shen C, Xu C. Anti-inflammatory effects of α7-nicotinic ACh receptors are exerted through interactions with adenylyl cyclase-6. Br J Pharmacol. 2021;178:2324–2338. doi: 10.1111/bph.15412. [DOI] [PubMed] [Google Scholar]