Keywords: miR-124, neural stem cell, neurogenesis, oscillating field stimulation, recovery, spinal cord injury, Tal1, tissue repair, transplantation
Abstract
Spinal cord injury often leads to severe motor and sensory deficits, and prognosis using the currently available therapies remains poor. Therefore, we aimed to explore a novel therapeutic approach for improving the prognosis of spinal cord injury. In this study, we implanted oscillating field stimulation devices and transplanted neural stem cells into the thoracic region (T9–T10) of rats with a spinal cord contusion. Basso-Beattie-Bresnahan scoring revealed that oscillating field stimulation combined with neural stem cells transplantation promoted motor function recovery following spinal cord injury. In addition, we investigated the regulation of oscillating field stimulation on the miR-124/Tal1 axis in neural stem cells. Transfection of lentivirus was performed to investigate the role of Tal1 in neurogenesis of neural stem cells induced by oscillating field stimulation. Quantitative reverse transcription-polymerase chain reaction, immunofluorescence and western blotting showed that oscillating field stimulation promoted neurogenesis of neural stem cells in vitro and in vivo. Hematoxylin and eosin staining showed that oscillating field stimulation combined with neural stem cells transplantation alleviated cavities formation after spinal cord injury. Taking the results together, we concluded that oscillating field stimulation decreased miR-124 expression and increased Tal1 content, thereby promoting the neurogenesis of neural stem cells. The combination of oscillating field stimulation and neural stem cells transplantation improved neurogenesis, and thereby promoted structural and functional recovery after spinal cord injury.
Introduction
Effective treatment of spinal cord injury (SCI) is one of the foremost clinical challenges because SCI often leads to permanent deficits in sensory and motor function (Samira and Marios, 2021). The main obstacles to SCI repair include neuroinflammation activation, glial scar and cavity formation, neuronal damage and death, and axonal demyelination (Ilyas et al., 2021; Steven et al., 2021). Although numerous clinical strategies to treat SCI exist, their effectiveness remains far from ideal (Chio et al., 2021). Thus, there is thus an urgent need for more effective therapies to improve SCI outcomes.
In view of the strong proliferation and pluripotency of neural stem cells (NSCs), NSCs transplantation has been considered promising in SCI research (Houman et al., 2021; De Gioia et al., 2020). NSCs are able to differentiate into three forms of neural cells: neurons, astrocytes and oligodendrocytes. The processes of forming the different types are known as neurogenesis and gliogenesis, respectively, which are regulated by multiple intrinsic and external signal factors (Farokhi et al., 2021). Because of the complex local microenvironment after SCI, the fate of transplanted NSCs is unpredictable (Taeko and Ryoichiro, 2021). To be useful in therapy, directed differentiation of NSCs is crucial (Zakrzewski et al., 2019).
Oscillating field stimulation (OFS) is a physical therapy for SCI, which can automatically switch the polarity of an electric field every 15 minutes to promote the bidirectional growth of nerve fibers. OFS has been shown to effectively promote nerve repair and regeneration after SCI (Li, 2019). Our previous study demonstrated that OFS promotes the neurogenesis of endogenous NSCs in spinal cord, thereby accelerating neural functional recovery following SCI (Chao et al., 2021). However, the limited reserve of NSCs in the adult central nervous system (CNS) is not enough to achieve the ideal therapeutic effect. Whether OFS has a similar effect on transplanted NSCs to play a therapeutic role in SCI has not been reported.
MiR-124 is abundant in the CNS and neuronal cells, and plays an important role in repairing damage after SCI (Yao et al., 2019). An increasing number of studies have shown that miR-124 is involved in regulating autophagy, and reducing apoptosis and inflammation in neurodegenerative diseases (Liu et al., 2019). Tal1 is a TCF3-related transcription factor that is expressed in the developing CNS (Livia et al., 2018). Recent studies have shown that Tal1 is a target of miR-124 and is necessary for the neurogenesis of NSCs or other neuronal precursors in the CNS (Wang et al., 2017; Andrzejczuk et al., 2018; Morello et al., 2020). Briefly, the expression of Tal1 was inhibited by miR-124, and the reduction of Tal1 was considered to maintain pluripotency of neuronal precursors in development (Wang et al., 2020). Interestingly, initial experiments by our research group found that OFS downregulates miR-124 expression in NSCs in vitro. However, it is unclear whether OFS regulates the neurogenesis of NSCs to promote neuronal regeneration after SCI though the miR-124/Tal1 axis.
Recently, a multi-therapeutic approach in SCI has attracted growing attention. In this study, OFS combined with NSCs transplantation was applied in a rat model of SCI. The present study aimed to explore whether OFS is able to regulate the miR-124/Tal1 axis and induce the neuronal differentiation of transplanted NSCs, so as to promote SCI repair.
Methods
NSCs culture and transfection
NSCs were derived from the forebrain of a 12.5-embryonic day-old C57BL/6 mouse carrying a green fluorescent protein (GFP) reporter gene (Stock No. MUBNF-01102, Cyagen Biosciences Inc., Guangzhou, Guangdong, China). Cells were cultured in a T25 flask containing 5 mL of growth medium for strain C57BL/6 mouse NSCs (96% NSC basal medium containing 2% B27, 1% glutamine, 0.2 μL/mL bFGF, 0.1 μL/mL EGF and 1 μL/mL heparin sodium; MUBNF-90011, Cyagen Biosciences Inc.), and incubated in a humidified incubator (Thermo Fisher Scientific, Waltham, MA, USA) with 5% CO2 at 37°C. To knockdown Tal1 expression in NSCs, a TAL1 shRNA-ZsGreen lentivirus (LV-Tal1) was synthesized by Hanbio (LV61110952, Shanghai, China) to silence Tal1 genes. ZsGreen-NC lentivirus (LV-NC) was synthesized as a negative control vector. These lentiviruses were transfected into NSCs according to the manufacturer’s instructions.
NSCs differentiation
Approximately 1 × 105 NSCs per well (Control group) were cultured with differentiation medium (DMEM/F12 medium [PM150312, Procell, Wuhan, Hubei, China] supplemented with N2 supplement [17502048, Gibco, New York, NY, USA] and 10% fetal bovine serum [164210, Procell]) for 7 days. NSCs in the OFS group, LV-Tal1 group and LV-NC group were transferred into stainless steel cell culture dishes with differentiation medium and were continuously stimulated by OFS for 7 days (Figure 1). The differentiation medium was changed every 2 days.
Figure 1.
Diagram of experiments in vitro and in vivo.
NSCs: Neural stem cells; OFS: oscillating field stimulation; SCI: spinal cord injury.
Animals
All surgical interventions and animal care were performed in accordance with the Animal Ethics Committee of Anhui Medical University (approval No. LLSC20190736, approval date April 16, 2019). This study was reported in accordance with the Animal Research: Reporting of In Vivo Experiments 2.0 guidelines (Percie du Sert et al., 2020). Seventy-eight adult female Sprague-Dawley rats, approximately 8 weeks of age, weighing 220–240 g, were purchased from Laboratory Animal Center of Anhui Medical University (license No. SCXK (Wan) 2017-001). Male rats were kept separate to avoid fighting, which increases the difficulty of the experiment. Female rats are more docile (Taylor et al., 1987). Rats were raised (four per cage) in a specific pathogen-free environment, maintained at a temperature of 23°C under a 12:12-hour dark and light cycle with adequate food and water supplies. All rats used in this study were naive and did not undergo drug tests. All experimental design was aimed to minimize the suffering and number of animals used. Animals were randomly divided into three groups by the random number table method: SCI group (culture medium transplantation after experimental SCI), NSC group (NSCs transplantation after experimental SCI) and NSC + OFS group (OFS combined with NSCs transplantation after experimental SCI). Each group consisted of 26 rats, and the rats that died postoperatively were replaced. Four rats in each group were executed at 3 and 7 days post-operation, and 13 rats in each group were executed at 14 days post-operation. In addition, five rats in each group were used for behavioral analysis (Figure 2).
Figure 2.
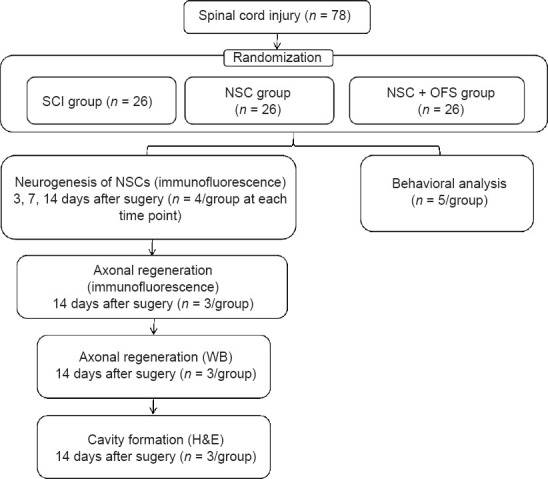
Flowchart of the animal experiment.
H&E: Hematoxylin and eosin; NSCs: neural stem cells; OFS: oscillating field stimulation; SCI: spinal cord injury; WB: western blotting.
SCI induction and OFS treatment
Rats were anesthetized via intraperitoneal injection of 1% pentobarbital sodium (40 mg/kg; P3761, Sigma, St. Louis, MO, USA). Throughout the surgery, the body temperature of animals was kept at 37°C. After a longitudinal incision of the median dorsal skin was made, spinous processes and laminae were removed using a spinal rongeur to expose the spinal cord tissue at the level of the T9–T10 vertebral body. Additional damage was avoided during the exposure procedure. Afterwards, SCI was induced using a NYU impactor (New York University, New York, NY, USA) with a weight of 10 g, hitting the central part of the dura mater from a height of 3 cm (Basso et al., 1996). Then, culture medium (MUBNF-90011, Cyagen Biosciences Inc.) or NSCs were immediately transplanted into the injured spinal cord by micro syringe (Hamilton, Bonadutz, Switzerland). Passages 3–5 NSCs were used for transplantation. After induction of SCI, the SCI group received 3 μL culture medium, whereas the NSC and NSC + OFS groups received 2 × 105 NSCs in 3 μL of culture medium, injected into the center of the lesions. After injection, the needle of the micro syringe was kept in the spinal cord for 5 minutes. All operations were performed under sterile conditions.
For animals in the OFS condition, immediately following SCI the OFS device electrodes were sutured using 4-0 silk in close proximity to the paravertebral muscles of the upper and lower segments of the lesion site (Figure 1). The OFS device (developed by the Beijing Key Laboratory of Bioelectromagnetism, Institute of Electrical Engineering, Chinese Academy of Sciences) was then stitched to the back epidermis to secure it in place. The design of the OFS device and the details of its application were described in a previous study by our group (Jing et al., 2015). Briefly, the OFS device produces electrical field stimulation between positive and negative electrodes, with an intensity of 400 µV/mm and an output current of 40 µA. The device was powered by a 3.0 V battery (CR1220), and the power supply was controlled by an induction system (74HC4060, Texas Instruments, Dallas, TX, USA). During the stimulation process, the battery power was checked twice per day.
To prevent infection and reduce postoperative mortality, animals were intraperitoneally injected with 105 units/kg penicillin G once per day until the third day after surgery. Manual bladder squeezing was performed every 8 hours following SCI, until rats regained autonomic micturition.
Behavioral analysis
Basso-Beattie-Bresnahan (BBB) scoring is widely used to evaluate functional motor recovery in rats following SCI (Guo et al., 2019). Scoring ranges from 0 to 21, with 0 indicating complete paralysis, and each additional point representing a gradual recovery of motor function. In the present study, each rat was scored at 3, 7, 14, 21 and 28 days after surgery by two examiners to reduce subjective errors. The mean scores of each group were calculated and recorded.
Hematoxylin and eosin staining
A 1-cm section of spinal cord tissue centered on the lesion site was collected at 14 days after SCI, fixed in 4% paraformaldehyde for 24 hours, and then embedded in paraffin. Tissue of the lesion segment was cut into 10-µm-thick transverse sections with a microtome (RM2255, Leica, Heidelberg, Germany) for hematoxylin and eosin (H&E) staining. All staining followed standard procedures. Images were acquired on a microscope (Eclipse E100, Nikon, Tokyo, Japan). The relative area of cavities was measured by using Image-Pro Plus 6.0 (Media Cybernetics, Rockville, MD, USA).
Quantitative reverse transcription-polymerase chain reaction
The NSCs differentiated for 7 days in vitro were collected and total RNA was extracted using an RNAprep Pure Micro Kit (DP420, TianGen, Beijing, China) following the manufacturer’s instructions. After cDNA was synthesized by RT Plus Kit (AG0304, SparkJade, Jinan, Shandong, China), quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed with qPCR Mix (AH0104, SparkJade) on ABI QuantStudio 6 Flex (Thermo Fisher Scientific) according to the manufacturer’s instructions. The qRT-PCR conditions were as follows: 95°C for 3 minutes denaturation, followed by 40 cycles of 95°C for 15 seconds, 60°C for 15 seconds, and 72°C for 25 seconds. The relative expression levels were normalized to Gapdh gene expression using the 2–∆∆Ct method (Livak and Schmittgen, 2001). Related specific primers were as shown in Table 1.
Table 1.
The primers used in quantitative reverse transcription-polymerase chain reaction
| Target | Primer sequence (5’–3’) |
|---|---|
| miR-124 | F: GCG AGG ATC TGT GAA TGC CAA A |
| R: AGA TGG TGA TGG GCT TCC C | |
| Tal1 | F: GAG CGC TGC TCT ATA GCC T |
| R: ACC CGG TTG TTG TTG GTG AA | |
| β-Tubulin III | F: CCA GAT CTT TCG GCC AGA CAA |
| R: CAG GAC GGC ATC CAC TAA CT | |
| Gapdh | F: GGG TGG AGC CAA ACG GGT C |
| R: GGA GTT GCT GTT GAA GTC GCA |
F: Forward; Gapdh: glyceraldehyde-3-phosphate dehydrogenase; R: reverse.
Immunofluorescence staining
After 3, 7, and 14 days of OFS stimulation in vivo, spinal cord tissue centered on the lesion site was collected and fixed in 4% paraformaldehyde for 24 hours. The tissue was embedded in paraffin and then cut into 10-µm-thick transverse and longitudinal sections with a microtome (RM2255, Leica). These sections were fixed in 4% paraformaldehyde for 15 minutes, and permeabilized with 1% Triton X-100 (T8200, Solarbio, Beijing, China), followed by washing in phosphate buffered saline (PBS) three times for 5 minutes each. For immunofluorescence (IF) staining, the transverse sections were incubated with primary antibodies against neuronal marker β-tubulin III (GTX130245, polyclonal antibody, rabbit anti-rat, 1:500, GeneTex, RRID: AB_2886220, Alton Pkwy Irvine, CA, USA) at 4°C overnight. Longitudinal sections were incubated with primary antibody against neurofilament marker NF-H (ab8135, polyclonal antibody, rabbit anti-rat, 1:500, Abcam, RRID: AB_306298, Cambridge, MA, USA) at 4°C overnight. Each section was washed with PBS three times and incubated with secondary antibody Cyanine 3 (Cy3)-conjugated goat anti-rabbit IgG (GB21303, 1:500, Servicebio, RRID: AB_2861435, Wuhan, Hubei, China) for 1 hour at room temperature. Then, transverse sections were incubated with FITC-conjugated donkey anti-goat IgG (GB22404, 1:200, Servicebio, RRID: AB_2818950) at room temperature for 10 minutes. EDTA was used to remove excess antibodies. After that, the sections were incubated with primary antibody against GFP (ab290, polyclonal antibody, rabbit anti-rat, 1:500, Abcam, RRID: AB_303395) at 4°C overnight. After washing in PBS, sections were incubated with Alexa Fluor 488-conjugated goat anti-rabbit IgG (AS053, 1:200, ABclonal, RRID: AB_2768320, Wuhan, Hubei, China) at room temperature for 1 hour. Finally, nuclei were stained using 4′,6-diamidino-2-phenylindole (DAPI; G1012, Servicebio).
For NSCs differentiated for 7 days in vitro, the cells were seeded on a poly-D-lysine-coated 24-well plate with cell-climbing slices overnight, and fixed in 4% paraformaldehyde for 20 minutes. After the cells were permeabilized with 0.3% Triton X-100 for 20 minutes and blocked with 5% goat serum for 30 minutes, the cells were incubated with primary antibodies against β-tubulin III (GTX130245, GeneTex) at 4°C overnight. Then, Alexa Fluor 488-conjugated goat anti-rabbit IgG (AS053, ABclonal), followed by DAPI (G1012, Servicebio) were each added for 1 hour at room temperature. Images were acquired on a fluorescence microscope (Eclipse C1; Nikon) and quantitatively analyzed using Image-Pro Plus 6.0 (Media Cybernetics). Three sites in the lesion center were randomly selected from each sample and the number of IF-positive cells was counted.
Western blotting
NSCs differentiated for 7 days in vitro were lysed with RIPA lysis buffer (R0010, Solarbio) to obtain total protein. At 14 days post-SCI surgery, the spinal cord lesion site was dissected out and protein was extracted for WB. After quantification of protein content in each group by bicinchoninic acid assay (Liu et al., 2021), equal concentrations of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (WIX, Beijing, China), followed by transfer to a polyvinylidene fluoride membrane (IPVH00010, Merck Millipore, Boston, MA, USA). Then, 5% nonfat milk powder was used to block the membranes for 1 hour. Afterwards, membranes were incubated with primary antibodies against NF-H (ab8135, polyclonal antibody, rabbit anti-rat, 1:10,000, Abcam, RRID: AB_306298), GFP (ab290, polyclonal antibody, rabbit anti-rat, 1:1000, Abcam, RRID: AB_303395) or β-tubulin III (GTX130245, polyclonal antibody, rabbit anti-rat, 1:2000, GeneTex, RRID: AB_2886220) at 4°C overnight. After washing in PBS, membranes were incubated with horseradish peroxidase-labeled goat anti-rabbit IgG antibody (E-AB-1003, 1:2000, Elabscience, Wuhan, Hubei, China) at room temperature for 1 hour. Finally, a chemiluminescence (ECL) kit was used to reveal protein bands, which were quantitatively analyzed using ImageJ 1.8.0 (National Institutes of Health, Bethesda, MD, USA) (Schneider et al., 2012). Relative protein expression was normalized to GAPDH (A19056, rabbit anti-rat, 1:20,000, ABclonal, RRID: AB_2862549) or β-actin (AC026, rabbit anti-rat, 1:50,000, ABclonal, RRID: AB_2768234).
Statistical analysis
No statistical methods were used to predetermine sample sizes; however, our sample sizes were similar to those reported in a previous publication (Khan et al., 2021). Behavioral and histological analyses were conducted by experimenters blind to treatment status, whereas in vivo microscopy and biochemical analysis were not performed blind to the conditions of the experiments. A one-way analysis of variance with repeated measures, followed by Tukey’s post hoc test, was used to assess statistical significance between the groups. SPSS 19.0 (IBM, Armonk, NY, USA) was used to perform statistical analysis. A P value < 0.05 was considered statistically significant. All data are presented as the mean ± standard error of the mean.
Results
OFS combined with NSCs transplantation therapy enables remarkable functional recovery in SCI rats
BBB scores were measured to explore the effect of combination therapy on functional motor recovery post-SCI. In all groups, paralyzed rats moved in a manner fully dependent on their forelimbs, and suffered from incontinence early in the post-SCI stage. In the NSC and NSC + OFS groups, BBB scores gradually increased over time. Compared with the NSC group, the NSC + OFS group showed significantly higher BBB scores at 21 and 28 days post-SCI (P < 0.05; Figure 3).
Figure 3.
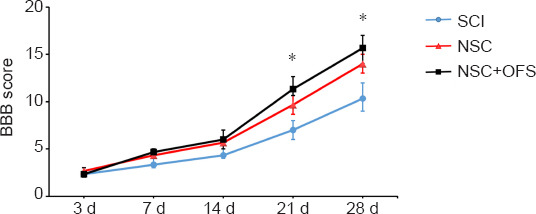
Recovery of motor function assessed by Basso-Beattie-Bresnahan (BBB) score after spinal cord injury (SCI).
The BBB score was assessed at 3, 7, 14, 21, and 28 days after surgery. The experiments were repeated three times. *P < 0.05 NSC + OFS group vs. NSC group (one-way analysis of variance with repeated measures followed by Tukey’s post hoc test). All data are presented as the mean ± standard error of the mean (n = 5/group). NSC: Neural stem cell; OFS: oscillating field stimulation.
OFS combined with NSCs transplantation therapy alleviates cavities formation after SCI
To assess the integrity of spinal cord tissue, H&E staining was performed at 14 days after surgery. Cavities were formed in the epicenter following SCI. The area of cavities formed in NSC + OFS group was significantly smaller than that of the SCI and NSC groups (Figure 4).
Figure 4.
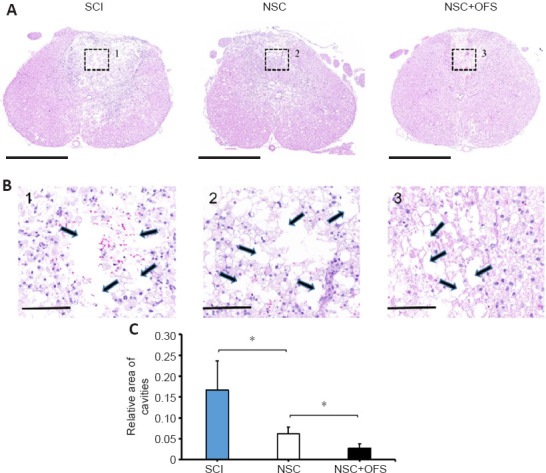
OFS combined with NSCs transplantation alleviates cavities formation after SCI.
(A) Hematoxylin and eosin staining was used for histological analysis at 14 days after SCI. Scale bars: 1000 µm. (B) Enlarged images from boxes 1–3 in A. Cavities were labeled by arrows. Scale bars: 100 µm. (C) Quantitative analysis demonstrating that the area of cavities formed in the NSC + OFS group was significantly smaller than that of the NSC group. The experiments were repeated three times. *P < 0.05 (one-way analysis of variance with repeated measures followed by Tukey’s post hoc test). All data are presented as the mean ± standard error of the mean (n = 3/group). NSCs: Neural stem cells; OFS: oscillating field stimulation; SCI: spinal cord injury.
OFS promotes the neurogenesis of NSCs in vitro
Following the stimulation of differentiation medium for 7 days, all cells were collected. β-Tubulin III IF staining showed that neurons in the OFS group exhibited a more complex, elongated and branched morphology than those in the Control group (Figure 5). WB showed a significant increase of β-tubulin III protein levels in the OFS group compared with those in the Control group (P < 0.05; Figure 6A and B). Additionally, qRT-PCR analysis showed a significant increase of β-tubulin III gene expression in the OFS group compared with that in the Control group (P < 0.05; Figure 6C).
Figure 5.
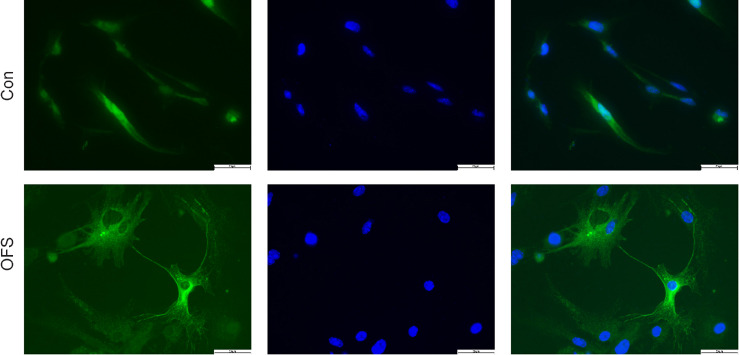
OFS promotes the neurogenesis of neural stem cells in vitro.
Neurons differentiated from neural stem cells for 7 days were identified by β-tubulin III immunostaining (green). Nuclei were identified by 4′,6-diamidino-2-phenylindole (blue). Scale bars: 50 µm. OFS: Oscillating field stimulation.
Figure 6.
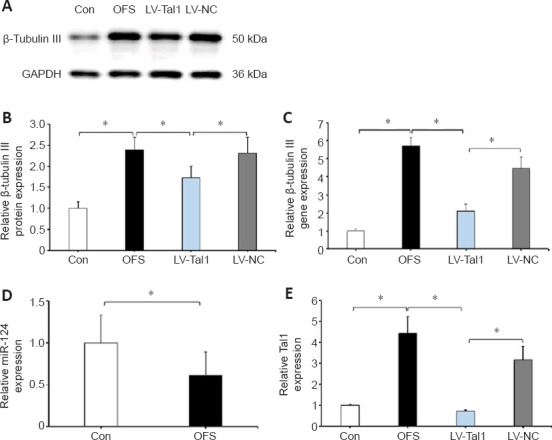
OFS promotes the neurogenesis of NSCs though the miR-124/Tal1 axis.
Neurons differentiated from NSCs for 7 days were identified by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and western blotting. (A) WB was used to assess β-tubulin III protein levels. (B) Quantitative analysis showed a significant decrease of β-tubulin III expression levels in the LV-Tal1 group compared with those in the OFS group. (C) Tal1-shRNA lentivirus transfection decreased the expression of neuronal marker β-tubulin III, as detected by qRT-PCR. (D) OFS decreased the expression of miR-124 in NSCs, as detected by qRT-PCR. (E) The expression of Tal1 was detected by qRT-PCR. NSCs were transfected with Tal1-shRNA or NC lentivirus. The experiments were repeated three times. *P < 0.05 (one-way analysis of variance with repeated measures followed by Tukey’s post hoc test). All data are presented as the mean ± standard error of the mean (n = 4/group). Gapdh: Glyceraldehyde-3-phosphate dehydrogenase; LV-NC: ZsGreen-NC lentivirus; LV-Tal1: TAL1 shRNA-ZsGreen lentivirus; NSCs: Neural stem cells; OFS: oscillating field stimulation; WB: western blotting.
Knockdown of Tal1 attenuates neurogenesis of NSCs induced by OFS
As previously mentioned (Wang et al., 2017; Andrzejczuk et al., 2018; Morello et al., 2020), Tal1 is a target gene of miR-124, and the miR-124/Tal1 axis is necessary for neurogenesis in the CNS. The expression of miR-124 in NSCs was decreased following OFS stimulation compared with that in the Control group (P < 0.05; Figure 6D), whereas, the expression of Tal1 was increased significantly (P < 0.05; Figure 6E). To further verify whether Tal1 overexpression participated in NSCs neurogenesis, NSCs were transfected with Tal1-shRNA lentivirus. WB and qRT-PCR analysis showed a significant decrease of β-tubulin III level in the LV-Tal1 group compared with that in the OFS and LV-NC groups (P < 0.05; Figure 6A and C). These results indicated that OFS enhanced the neurogenesis of NSCs though the miR-124/Tal1 axis.
OFS promotes the neurogenesis of NSCs in vivo
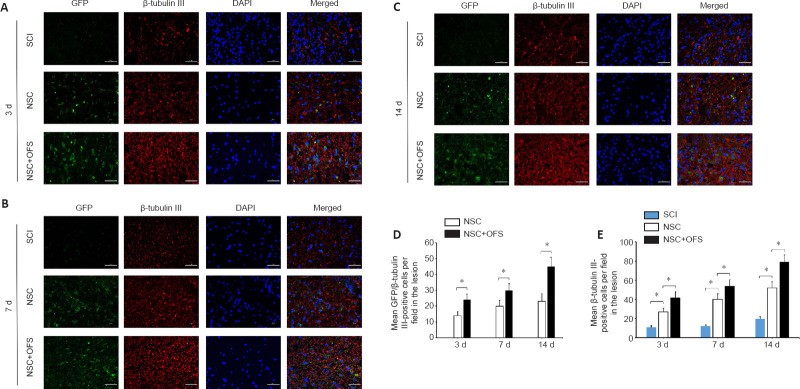
To assess whether OFS regulates the neurogenesis of transplanted NSCs in vivo, IF double staining of GFP and neuron marker β-tubulin III was performed at 3, 7 and 14 days after surgery. The number of GFP- and β-tubulin III-positive cells in the lesion area of the NSC and NSC + OFS groups increased over time, and there were higher numbers of NSC-derived neurons in the NSC + OFS group than in the NSC group at each time point after surgery (P < 0.05; Figure 7). Furthermore, WB results showed that there were higher GFP protein levels in the NSC + OFS group compared with those in the NSC group (P < 0.05; Figure 8A and B), which suggested that OFS treatment is beneficial to the vitality of transplanted NSCs.
Figure 7.
OFS promotes the neurogenesis of transplanted NSCs after spinal cord injury in vivo.
(A–C) Neurons differentiated from transplanted NSCs were identified by β-tubulin III (red) and green fluorescent protein (GFP; green) immunostaining. Nuclei of all cells were identified by 4′,6-diamidino-2-phenylindole (DAPI; blue). Scale bars: 50 µm. (D) Quantitative analysis indicated a greater number of β-tubulin III/GFP-positive cells in the NSC + OFS group compared with those in the NSC group. (E) Quantitative analysis showed that there were more β-tubulin III-positive cells in the NSC + OFS group than in the other groups. The experiments were repeated three times. *P < 0.05 (one-way analysis of variance with repeated measures followed by Tukey’s post hoc test). All data are presented as the mean ± standard error of the mean (n = 4/group). NSCs: Neural stem cells; OFS: oscillating field stimulation; SCI: spinal cord injury.
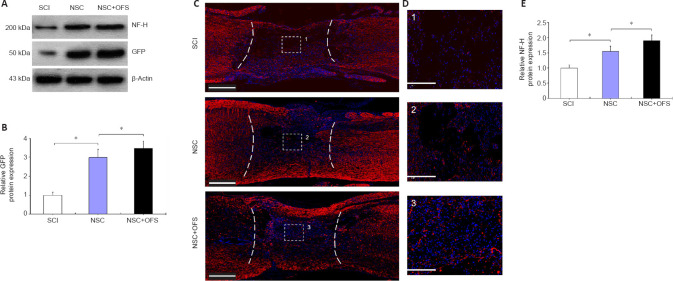
Figure 8.
OFS combined with NSCs transplantation promotes axonal regeneration after SCI.
Axons in the lesion site were identified by immunostaining at 14 days after SCI. (A) Western blotting was used to assess NF-H and green fluorescent protein (GFP) protein levels. (B) Quantitative analysis indicated a higher level of GFP protein in the NSC + OFS group than in the NSC group. (C) Nerve fibers were identified by neurofilament H (NF-H) immunostaining (red). Nuclei of all cells were identified by 4′,6-diamidino-2-phenylindole (DAPI; blue). Scale bars: 500 µm. (D) 1–3 enlargements of the images within the corresponding boxes. Scale bars: 100 µm. (E) Quantitative analysis indicated a higher level of NF-H protein in the NSC + OFS group than in the NSC group. The experiments were repeated three times. *P < 0.05 (one-way analysis of variance with repeated measures followed by Tukey’s post hoc test). All data are presented as the mean ± standard error of the mean (n = 3/group). NSCs: Neural stem cells; OFS: oscillating field stimulation; SCI: spinal cord injury.
OFS combined with NSCs transplantation therapy promotes axonal regeneration in the lesion site after SCI
To assess the regeneration of axons, IF staining of neurofilament marker NF-H was performed at 14 days post-surgery. In the SCI and NSC groups, there were few NF-H-positive axonal fibers in the lesion site, and these axonal fibers did not grow into the cavities formed after SCI (Figure 8C and D). However, NF-H-positive axonal fibers in the NSC + OFS group were widely distributed in the visual field and penetrated into the lesion (Figure 8C and D). In addition, WB showed that the expression of NF-H protein level was significantly higher in the NSC + OFS group compared with that in the SCI and NSC groups (P < 0.05; Figure 8A and E).
Discussion
Structural discontinuity in the injury site after SCI results in an interruption of nerve impulse conduction, which leads to a loss of various bodily functions (Angeli et al., 2018). Current therapeutic options for SCI are mainly symptomatic treatments, and reconstructing the interrupted neural structure or restoring neural function remains a challenge (Vismara et al., 2017). In general, the targets of SCI therapy involve the regulation of neuronal viability and survival, promotion of neuronal regeneration, anatomical and functional recovery of axons, and suppression of inflammatory responses (Bramlett and Dietrich, 2015; David, 2017). Our data indicated that the OFS treatment combined with NSCs transplantation effectively improved neurogenesis after SCI in rats.
Recently, NSCs transplantation has been considered to have broad prospects in the treatment of neurodegenerative diseases (De et al., 2020). It is becoming clear that Tal1 is necessary for neurogenesis from NSCs or other neuronal precursors in the CNS (Achim et al., 2013). In addition, Tal1 is targeted and reverse regulated by miR-124 in humans and rats (Wang et al., 2017, 2020). In this study, we found that OFS downregulated miR-124 of NSCs in vitro. We speculated that OFS regulates the neurogenesis of NSCs through the miR-124/Tal1 axis. The RT-qPCR analysis revealed a significant increase of β-tubulin III and Tal1 levels following OFS, whereas the LV-Tal1 group showed the opposite trend. These results demonstrated that OFS enhanced the neurogenesis of NSCs in vitro through the miR-124/Tal1 axis. In summary, a reduced level of miR-124 following OFS caused a remarkable increase in the expression of Tal1 and a higher level of NSCs neurogenesis compared with those levels in the Control group. In contrast, Tal1 knockdown by lentivirus attenuated neurogenesis of NSCs in vitro. Furthermore, in vitro IF staining showed that OFS promoted the growth of NSC-differentiated neurons.
Although numerous studies have shown the therapeutic potential of NSCs, cell transplantation for SCI still suffers from many obstacles in the damaged microenvironment (Taeko and Ryoichiro, 2021). The current research is committed to further improving the repair capacity of NSCs though various means (Jiao et al., 2017). Interestingly, OFS shows its therapeutic potential in synergy with other treatment means (Maria et al., 2019). In this study, we also conducted in vivo experiments to verify the synergistic effect of OFS and NSCs transplantation. The results of H&E staining showed that the NSC + OFS group had better structural integrity in the lesion site. In vivo IF staining showed that OFS enhanced neurogenesis of transplanted NSCs after SCI, which is consistent with our in vitro study. Moreover, WB results suggested that more NSCs survived in the lesion site following OFS after SCI. The relevant mechanism of this effect remains to be explored.
The most intuitive manifestation of SCI repair is the recovery of motor function. We used BBB scoring to assess the short-term improvement of motor function after surgery, and found a greater improvement in hind limb locomotor recovery in the NSC + OFS group than in the other groups. Reconstructing the discontinuous regions of the injured spinal cord is a challenge and a focus of many studies. Axonal regeneration is an important factor in reconstructing the structure and function of discontinuous regions (Byron and Cahyono, 2015). In the present study, IF staining showed that more axonal fibers extended to the lesion site in the NSC + OFS group than in the other groups. The role of these axonal fibers in motor function recovery needs to be further explored. These findings suggested that OFS combined with NSCs transplantation is a safe and effective treatment strategy for SCI.
This study has some limitations. First, we only explored the effect of OFS on the neurogenesis of NSCs, whereas gliogenesis also plays an important role in the pathological process of SCI. Additionally, the repair of SCI is a complex process, which requires more in-depth study of the relevant treatment mechanisms.
In conclusion, this study suggested that OFS enhances the neurogenesis of NSCs through the miR-124/Tal1 axis. Our data suggest that the combination of OFS and NSCs transplantation enhanced neurogenesis, and promoted structural integrity and motor function recovery after SCI.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, Nos. 81471273 (to JQ), and 81472088 (to CLS); the Natural Science Research Projects in Colleges and Universities of Anhui Province, No. KJ2020ZD23 (to JQ); the Natural Science Foundation of Anhui Province, No. 2208085MH210 (to JQ).
Conflicts of interest: All authors declare that there is no conflict of interest.
C-Editor: Zhao M; S-Editor: Li CH; L-Editors: MeCollum L, Li CH, Song LP; T-Editor: Jia Y
References
- 1.Achim K, Peltopuro P, Lahti L, Tsai HH, Zachariah A, Astrand M, Salminen M, Rowitch D, Partanen J. The role of Tal2 and Tal1 in the differentiation of midbrain GABAergic neuron precursors. Biol Open. 2013;2:990–997. doi: 10.1242/bio.20135041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrzejczuk L, Banerjee S, England SJ, Voufo C, Kamara K, Lewis KE. Tal1 Gata2a and Gata3 Have distinct functions in the development of V2b and cerebrospinal fluid-contacting KA spinal neurons. Front Neurosci. 2018;12:170. doi: 10.3389/fnins.2018.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angeli CA, Boakye M, Morton RA, Vogt J, Benton K, Chen Y, Ferreira CK, Harkema SJ. Recovery of Over-ground walking after chronic motor complete spinal cord injury. N Engl J Med. 2018;379:1244–1250. doi: 10.1056/NEJMoa1803588. [DOI] [PubMed] [Google Scholar]
- 4.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 5.Bramlett HM, Dietrich WD. Long-term consequences of traumatic brain injury:current status of potential mechanisms of injury and neurological outcomes. J Neurotrauma. 2015;32:1834–1848. doi: 10.1089/neu.2014.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byron AK, Cahyono K. The neuropathological foundations for the restorative neurology of spinal cord injury. Clin Neurol Neurosurg. 2015;129(Suppl 1):S1–7. doi: 10.1016/j.clineuro.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Chao F, Jian S, Laifu W, Fei G, Jun Q. Oscillating field stimulation promotes recovery from spinal cord injury in rats by regulating the differentiation of endogenous neural stem cells. Exp Ther Med. 2021;22:979. doi: 10.3892/etm.2021.10411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chio JCT, Xu KJ, Popovich P, David S, Fehlings MG. Neuroimmunological therapies for treating spinal cord injury:Evidence and future perspectives. Exp Neurol. 2021;341:113704. doi: 10.1016/j.expneurol.2021.113704. [DOI] [PubMed] [Google Scholar]
- 9.David H. Spinal-cord injury:spurring regrowth. Nature. 2017;552:S49. doi: 10.1038/d41586-017-07550-9. [DOI] [PubMed] [Google Scholar]
- 10.De Gioia R, Biella F, Citterio G, Rizzo F, Abati E, Nizzardo M, Bresolin N, Comi GP, Corti S. Neural stem cell transplantation for neurodegenerative diseases. Int J Mol Sci. 2020;21:3103. doi: 10.3390/ijms21093103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farokhi M, Mottaghitalab F, Saeb MR, Shojaei S, Zarrin NK, Thomas S, Ramakrishna S. Conductive biomaterials as substrates for neural stem cells differentiation towards neuronal lineage cells. Macromol Biosci. 2021;21:e2000123. doi: 10.1002/mabi.202000123. [DOI] [PubMed] [Google Scholar]
- 12.Guo S, Perets N, Betzer O, Ben-Shaul S, Sheinin A, Michaelevski I, Popovtzer R, Offen D, Levenberg S. Intranasal delivery of mesenchymal stem cell derived exosomes loaded with phosphatase and tensin homolog siRNA repairs complete spinal cord injury. ACS Nano. 2019;13:10015–10028. doi: 10.1021/acsnano.9b01892. [DOI] [PubMed] [Google Scholar]
- 13.Houman K, Bahman R, Hamid M, Mohammad RS, Hajar J, Fatemeh R. The role of Nrf2 in neural stem/progenitors cells:From maintaining stemness and self-renewal to promoting differentiation capability and facilitating therapeutic application in neurodegenerative disease. Ageing Res Rev. 2021;65:101211. doi: 10.1016/j.arr.2020.101211. [DOI] [PubMed] [Google Scholar]
- 14.Ilyas E, David PL, Zoher G. Acute traumatic spinal cord injury. Neurol Clin. 2021;39:471–488. doi: 10.1016/j.ncl.2021.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Jiao S, Liu Y, Yao Y, Teng J. miR-124 promotes proliferation and differentiation of neuronal stem cells through inactivating Notch pathway. Cell Biosci. 2017;7:68. doi: 10.1186/s13578-017-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jing JH, Qian J, Zhu N, Chou WB, Huang XJ. Improved differentiation of oligodendrocyte precursor cells and neurological function after spinal cord injury in rats by oscillating field stimulation. Neuroscience. 2015;303:346–351. doi: 10.1016/j.neuroscience.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Khan NZ, Cao T, He J, Ritzel RM, Li Y, Henry RJ, Colson C, Stoica BA, Faden AI, Wu J. Spinal cord injury alters microRNA and CD81+exosome levels in plasma extracellular nanoparticles with neuroinflammatory potential. Brain Behav Immun. 2021;92:165–183. doi: 10.1016/j.bbi.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J. Weak direct current (DC) electric fields as a therapy for spinal cord injuries:review and advancement of the oscillating field stimulator (OFS) Neurosurg Rev. 2019;42:825–834. doi: 10.1007/s10143-018-01068-y. [DOI] [PubMed] [Google Scholar]
- 19.Liu D, Wang B, Qiu M, Huang Y. MiR-19b-3p accelerates bone loss after spinal cord injury by suppressing osteogenesis via regulating PTEN/Akt/mTOR signalling. J Cell Mol Med. 2021;25:990–1000. doi: 10.1111/jcmm.16159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Feng Z, Du L, Huang Y, Ge J, Deng Y, Mei Z. The potential role of microRNA-124 in cerebral ischemia injury. Int J Mol Sci. 2019;21:120. doi: 10.3390/ijms21010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Livia AA, Santanu B, Samantha JE, Christiane V, Kadiah K, Katharine EL. Tal1 Gata2a and Gata3 have distinct functions in the development of V2b and cerebrospinal fluid-contacting KA spinal neurons. Front Neurosci. 2018;12:170. doi: 10.3389/fnins.2018.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maria B, Katarina B, Jana F, Nadezda L, Jan G. Epidural oscillating field stimulation as an effective therapeutic approach in combination therapy for spinal cord injury. J Neurosci Methods. 2019;311:102–110. doi: 10.1016/j.jneumeth.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 24.Morello F, Borshagovski D, Survila M, Tikker L, Sadik-Ogli S, Kirjavainen A, Estartús N, Knaapi L, Lahti L, Törönen P, Mazutis L, Delogu A, Salminen M, Achim K, Partanen J. Molecular fingerprint and developmental regulation of the tegmental GABAergic and Glutamatergic neurons derived from the anterior hindbrain. Cell Rep. 2020;33:108268. doi: 10.1016/j.celrep.2020.108268. [DOI] [PubMed] [Google Scholar]
- 25.Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, et al. The ARRIVE guidelines 2.0:Updated guidelines for reporting animal research. PLoS Biol. 2020;18:e3000410. doi: 10.1371/journal.pbio.3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samira S, Marios CP. Acute severe traumatic spinal cord injury:monitoring from the injury site and expansion duraplasty. Neurosurg Clin N Am. 2021;32:365–376. doi: 10.1016/j.nec.2021.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ:25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steven K, Brittany S, Fatma E, James G. Characterizing natural recovery after traumatic spinal cord injury. J Neurotrauma. 2021;38:1267–1284. doi: 10.1089/neu.2020.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taeko K, Ryoichiro K. Lysosomes and signaling pathways for maintenance of quiescence in adult neural stem cells. FEBS J. 2021;288:3082–3093. doi: 10.1111/febs.15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor GT, Weiss J, Rupich R. Male rat behavior endocrinology and reproductive physiology in a mixed-sex socially stressful colony. Physiol Behav. 1987;39:429–433. doi: 10.1016/0031-9384(87)90368-4. [DOI] [PubMed] [Google Scholar]
- 31.Vismara I, Papa S, Rossi F, Forloni G, Veglianese P. Current options for cell therapy in spinal cord injury. Trends Mol Med. 2017;23:831–849. doi: 10.1016/j.molmed.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Wang F, Song W, Zhao H, Ma Y, Li Y, Zhai D, Pi J, Si Y, Xu J, Dong L, Su R, Zhang M, Zhu Y, Ren X, Miao F, Liu W, Li F, Zhang J, He A, Shan G, et al. The RNA-binding protein QKI5 regulates primary miR-124-1 processing via a distal RNA motif during erythropoiesis. Cell Res. 2017;27:416–439. doi: 10.1038/cr.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Li H, Chen L, Dong J, Yang J, Gong Z, Wang B, Zhao X. mRNA profiling for miR-124-mediated repair in spinal cord injury. Neuroscience. 2020;438:158–168. doi: 10.1016/j.neuroscience.2020.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Yao L, Zhu Z, Wu J, Zhang Y, Zhang H, Sun X, Qian C, Wang B, Xie L, Zhang S, Lu G. MicroRNA-124 regulates the expression of p62/p38 and promotes autophagy in the inflammatory pathogenesis of Parkinson's disease. FASEB J. 2019;33:8648–8665. doi: 10.1096/fj.201900363R. [DOI] [PubMed] [Google Scholar]
- 35.Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells:past present and future. Stem Cell Res Ther. 2019;10:68. doi: 10.1186/s13287-019-1165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]