Abstract
A cardinal process in bacterial endocarditis (BE) is the activation of the clotting system and the formation of a fibrin clot on the inner surface of the heart, the so-called endocardial vegetation. The processes that lead to the activation of the clotting system on endothelial surfaces upon exposure to bacteria are largely unknown. In the present study, we investigated in an in vitro model whether infection of human endothelial cells (EC) with bacteria that are relevant to BE, such as Staphylococcus aureus, Streptococcus sanguis, and Staphylococcus epidermidis, leads to induction of tissue factor (TF)-dependent procoagulant activity (TFA) and whether this process is influenced by host factors, such as interleukin-1 (IL-1), that are produced in response to the bacteremia in vivo. The results show that S. aureus binds to and is internalized by EC, resulting in expression of TF mRNA and TF surface protein as well as generation of TFA within 4 to 8 h after infection. No TFA was found when EC were exposed to UV-irradiated S. aureus or bacterial cell wall fragments. S. sanguis and S. epidermidis, although also binding to EC, did not induce endothelial TFA. This indicates a species and strain dependency. EC also expressed TFA after exposure to IL-1. The enhanced TFA of EC after exposure to S. aureus was not prevented by IL-1 receptor antagonist, arguing against an auto- or paracrine contribution of endogenous IL-1. When IL-1 was applied together with bacteria, this had a synergistic effect on the induction of EC TFA. This was found in particular with S. aureus but also, although to a lesser degree, with S. sanguis and S. epidermidis. This influence of IL-1 on the species- and strain-dependent induction of EC TFA suggests that bacterial factors as well as host factors orchestrate the induction of coagulation in an early stage in the pathogenesis of endovascular disease, such as BE.
In the pathogenesis of endovascular infections, such as bacterial endocarditis (BE), pathogenic bacteria attach to and colonize vascular endothelium. Depending on the infecting microorganism, such contact will either lead to direct endothelial cell (EC) damage, followed by exposure of the thrombogenic subendothelial matrix, or induce EC activation, causing a local inflammatory reaction. In BE, the inflammatory reaction is localized on the heart valves and the mural endocardium. Staphylococcus aureus is one of the major causative pathogens of the disease (16, 51). The incidence of S. aureus endocarditis now accounts for 25 to 35% of cases (32). It often causes an acute and massive valvular destruction that affects patients with previously intact, undamaged heart valves (16, 32). By contrast, viridans group streptococci, e.g., Streptococcus sanguis, cause a prolonged subacute clinical course in patients with already damaged heart valves (44). With this microorganism, the endocardial lesion is characterized by the so-called vegetations, consisting of a fibrin-platelet matrix, in which bacterial colonies are embedded. Finally, Staphylococcus epidermidis often is isolated in prosthetic valve endocarditis (12, 36). Bacteria frequently causing BE, such as S. aureus, S. sanguis, and S. epidermidis, were found in vitro to adhere more avidly to cultured ECs (4, 39, 41, 52) and aortic valves (28) than bacteria uncommon to the disease. Also, significantly smaller inocula of these bacteria are needed to induce endocarditis in vivo (25). Specific human endothelial and bacterial surface molecules as well as plasma proteins have been identified as being involved in the interaction between the bacteria and ECs (13, 48, 49). However, less well understood are the events subsequent to adherence as well as the bacterial or host cell factors that directly contribute to the initiation of the disease. So far, in vitro studies have shown that S. aureus, after its adherence to human ECs, is phagocytosed by these cells (7, 33, 39). In vivo, this may lead to a persistent or recurrent infection (42). Subsequent studies, including ours, revealed that various S. aureus strains induce EC activation that resulted in production of the chemokines interleukin-8 (IL-8) and monocyte chemotactic protein-1, surface expression of intercellular adhesion molecule-1 (ICAM-1; CD54) and vascular cell adhesion molecule-1 (VCAM-1; CD106), and enhanced monocyte adhesion (7, 46, 50, 54). In addition, S. aureus has been shown to enhance endothelial secretion of the proinflammatory cytokines IL-1 and IL-6 (53, 54) and to elicit procoagulant activity in these cells (21). These studies provide convincing arguments for an active role of S. aureus-infected ECs in thrombosis associated with inflammation and, with regard to BE, may explain why S. aureus already at a low inoculum causes infection of previously intact heart valves.
For the formation of the endocardial vegetations, the clotting system has to be activated. This occurs along the extrinsic pathway, indicating the involvement of tissue factor (TF) (11, 19). As a result of this activation, fibrin is formed, which both serves as an adhesion surface for blood-borne bacteria and covers adhered microorganisms, making them inaccessible to host defense factors. TF is a 45-kD transmembrane cell surface protein. Its expression can be induced on cells situated within the vasculature in contact with blood, such as monocytes and ECs, under various pathological conditions (40, 47) and also in vitro upon activation by a variety of stimuli, such as proinflammatory cytokines, bacterial lipopolysaccharides, or bacteria (1, 9, 15, 22, 40, 45). It is an obligate cofactor for coagulation factor VII/VIIa (FVIIa). The formed TF-FVIIa complex proteolytically activates coagulation factor X (FX), resulting in formation of FXa, which in turn triggers downstream coagulation pathways ultimately leading to the generation of thrombin and fibrin (11, 19, 35).
Numerous investigations on the pathogenesis of BE focused on TF-dependent procoagulant activity (TFA) of vegetations that were already formed after mechanical damage to the endocardium. We have shown that in these vegetations TF, which is needed to activate the clotting cascade, is generated by monocytes, settling from the circulation on the (infected) vegetational surface (2, 3). However, the events that start the formation of a vegetation on an intact endothelial surface when exposed to pathogenic bacteria are still largely unknown. Most likely, this process is influenced by certain characteristics of the infecting microorganism, such as its type, virulence, and ability to interact with vascular endothelium. We hypothesized that host factors, such as cytokines that are produced in response to the bacteremia, could be involved, rendering ECs more prone to infection and its consequences.
Therefore, the present in vitro study was undertaken to compare the abilities of bacteria relevant to BE to induce expression of TF in human ECs. Furthermore, the modulating effect of IL-1, as a representative cytokine produced at sites of bacterial infection and as a potent stimulator of EC function, on bacteria-induced endothelial TFA was investigated. This might provide a better insight into the crucial events during the initial stage of BE and, in particular, into the bacterial and host factors that orchestrate the induction of coagulation.
MATERIALS AND METHODS
Reagents.
Fetal calf serum, culture medium M199, and RPMI 1640 were purchased from GIBCO Laboratories (Grand Island, N.Y.). Penicillin was obtained from Brocades Pharma BV (Leiderdorp, The Netherlands), streptomycin was obtained from Gist-brocades NV (Delft, The Netherlands), amphothericin B was obtained from Squibb BV (Rijswijk, The Netherlands), and l-glutamine was obtained from Flow Laboratories (Irvine, United Kingdom). EC growth factor was prepared from calf hypothalamus as described previously (5). Lysostaphin and agarose were obtained from Sigma Chemical Co. (St. Louis, Mo.), gelatin and trypsin were obtained from Difco Laboratories (Detroit, Mich.), and EDTA was obtained from Boehringer (Mannheim, Germany). Human serum was collected from healthy donors and inactivated at 56°C for 30 min (heat-inactivated human serum [HuSi]).
FVII was prepared from human plasma as previously described (1); and the chromogenic substrate PefachromeFXa were purchased from Kordia (Leiden, The Netherlands). Acetic acid, CaCl2, chloroform, isopropanol, and Tris base were obtained from Merck (Darmstadt, Germany). Rusel Viper Venom was obtained from Chromogenix (Mölndal, Sweden). GIBCO supplied the M-MLV reverse transcriptase (RT) enzyme, the buffer for the cDNA reaction, dithiothreitol (DTT), oligo deoxyribosylthymine, deoxynucleoside triphosphates (dNTPs), and a 100-bp DNA leader. Rnasin was purchased from Promega (Madison, Wis.), and Rnazol was purchased from Campro Scientific (Veenendaal, The Netherlands). For PCR, AmpliTaq from Perkin-Elmer Cetus (Nieuwerkerk a/d IJssel, The Netherlands) was used with the supplied buffer.
MAbs and cytokines.
The monoclonal antibody (MAb) TFg-10H10 (immunoglobulin G1) against human TF (CD142) (38) was obtained from OMNILabo International BV (Breda, The Netherlands). Human recombinant IL-1 (further referred to as IL-1) was obtained from P. Lomedico (Hoffmann-La Roche, Nutley, N.J.). Human recombinant IL-1 receptor antagonist (rIL-1ra) was obtained from ITK Diagnostics BV (Uithoorn, The Netherlands).
Cells.
ECs were isolated from human umbilical veins as described previously (8). The cells were cultured in M199 culture medium supplemented with 100 U of penicillin G per ml, 0.1 mg of streptomycin per ml, 100 U of amphotericin B per ml, 0.1 mg of EC growth factor per ml, 5 U of heparine per ml, 1 mM l-glutamine, and 10% HuSi in a 5% CO2 incubator at 37°C. The cells were grown to confluence in plastic tissue culture dishes (Falcon; Becton Dickinson, Lincoln Park, N.J.) coated with 0.75% gelatin in pyrogen-free water. Primary cultures of ECs were harvested with 0.05% (wt/vol) trypsin and 0.01% (wt/vol) EDTA and subsequently subcultured in culture medium. In most experiments, confluent monolayers of secondary cultured ECs, i.e., cultured after one passage, were used. In some experiments, secondary cultures of ECs were grown to confluence on 0.75% gelatin-coated glass coverslips (5) in 24-well tissue culture plates (Costar, Cambridge, Mass.).
Bacteria.
The bacteria used in this study were S. aureus 42D, S. epidermidis ATCC 149900, and S. sanguis NCTC 7864. Bacterial suspensions were stored at −70°C until thawed for use. S. aureus and S. epidermidis were routinely grown overnight in brain heart infusion broth, and S. sanguis was grown in Todd-Hewitt broth at 37°C. The bacteria were harvested by centrifugation, resuspended in M199 plus 0.1% (wt/vol) gelatin and 10% (vol/vol) fresh human serum, and incubated for 30 min under rotation (4 rpm) for opsonization. The bacteria were then diluted in M199 plus 10% HuSi at the desired concentration prior to use in the infection assay. In some experiments, bacteria were killed by exposure to UV light for 60 min before opsonization. The number of bacteria used in the infection assay as well as the number of viable bacteria after UV irradiation was measured by colony counts after plating serial dilutions on blood agar plates and overnight incubation at 37°C.
Infection of ECs with bacteria.
Confluent monolayers of about 2 × 105 ECs were washed once with culture medium without antibiotics. Then various inocula of opsonized bacteria in M199 with 10% HuSi were added, and plates were incubated for different periods of time at 37°C in a 5% CO2 incubator, as previously described (7). After this, the monolayers were washed with warm phosphate-buffered saline (PBS). In some experiments, this was followed by an incubation with 10 U of lysostaphin per ml for 5 min at room temperature, which lysed all extracellular but not the intracellular bacteria. For the determination of the percentage of infected ECs, monolayers grown on gelatin-coated glass coverslips were used. After the infection period, these coverslips were washed with warm PBS. Then the ECs were fixed in methanol for 15 min and stained with Giemsa stain. The percentage of infected ECs, i.e., cells with at least one cell-associated bacterium, was determined under a light microscope.
Flow cytometric analysis of endothelial TF antigen expression.
For fluorescence-activated cell sorter (FACS) analyses, ECs were collected and prepared as previously described (7). Briefly, ECs were grown to confluence in a 0.75% gelatin-coated tissue culture flask and washed with culture medium without antibiotics. These cultures were then incubated with approximately 5 × 108 CFU of opsonized bacteria, i.e., about 200 bacteria per EC, in 10 ml of M199 with 10% HuSi or culture medium alone for 24 h at 37°C in a 5% CO2 incubator. After a wash in PBS, the cells were harvested after mild trypsinization and collected and washed in cold (4°C) PBS with 1% heat-inactivated fetal calf serum (wash buffer). Subsequently, these cells were incubated for 30 min at 4°C in PBS supplemented with 1% goat serum and 1% HuSi. All further incubations were done on ice. The cells were washed twice in wash buffer and incubated with 1 μg of MAb TF10H10 per ml for 30 min. After two washes, the cells were incubated for 30 min with phycoerythrin-conjugated goat anti-mouse immunoglobulin (Southern Biotechnology Associates Inc., Birmingham, Ala.) according to the suppliers manual, washed once, and then analyzed by flow cytometry with a FACScan (Becton Dickinson). In each sample, 10,000 cells were analyzed. ECs treated with conjugated Ab alone served as a control to set background fluorescence.
TF assay.
Confluent secondary EC cultures on gelatin-coated coverslips were incubated with opsonized bacteria or IL-1 in M199 with 10% HuSi, as described above, for different periods of time. Then the coverslips were transferred to a 24-well plate. TFA was measured as described by Bancsi et al. (1). Briefly, the coverslip was washed once with warm PBS and incubated with 125 μl of buffer containing 0.125 pmol of purified FVII and 0.125 nmol of CaCl2 for 20 min at 37°C under rotation at 200 rpm to allow formation of a TF-FVII-Ca complex. Next, 20 μl of 10 U of FX per ml was added. After 5 min at 37°C, 100 μl of the mixture was removed and added to 100 μl of buffer containing EDTA, to stop FXa formation. Then the sample mixture was warmed to 37°C. Subsequently, 25 μl of 1 mg of PefachromeFXa, a chromogenic substrate for FXa, was added. After 20 min at 37°C, the conversion of the substrate was stopped by the addition of 200 μl of 50% (vol/vol) acetic acid. The optical density at 405 nm was measured and converted into FXa concentrations. For this calculation, a calibration curve was used from purified FX that was fully activated with Rusel Viper Venom. Data are expressed as mU of FXa per well containing approximately 2 × 105 ECs.
RNA isolation and RT-PCR.
RNA was isolated with RNazol according to the provider's manual from noninfected ECs or from ECs infected for different periods of time with an optimal concentration of S. aureus, S. epidermidis, or S. sanguis or from ECs stimulated with IL-1. From this RNA, cDNA was made. Therefore, 1 μl of 5 pmol of oligo deoxyribosylthymine was incubated with approximately 1 μg of total RNA for 10 min at 70°C. Subsequently, a mixture of 0.5 μl of RT, 4 μl of 5× cDNA reaction buffer, 2 μl of 0.1 M dithiothreitol, 2 μl of dNTPs, 0.25 μl of RNasin, and 1.25 μl of water was added, followed by incubation for 1 h at 37°C. The cDNA-containing solution was stored at −20°C until further use. For PCR, the following primers, obtained from GIBCO BRL (Breda, The Netherlands), were used: human TF, 5′-ATGGAGACCCCTGCCTGG-3′ (sense) and 5′-CCAGCAGAACCGGTGCTC-3′ (antisense), and β2-microglobulin, 5′-CCAGCAGAGAATGGAAAGTC-3′ (sense) and 5′-GATGCTGCTTACATGTCTCG-3′ (antisense). One microliter of the cDNA-containing solution was mixed with 5 μl of supplied buffer, 2 μl of dNTPs, 1 μl of 10 pmol of sense primer, 1 μl of 10 pmol of antisense primer, and 0.16 μl of AmpliTaq, to a total volume of 50 μl with aquadest. To avoid evaporation, mixtures were covered with paraffin. After 30 cycles, a 5-μl sample was put on a 2% agarose gel with ethidium bromide. After electrophoresis, the PCR product was measured with UV light (EagleEye II; Stratagene, Westburg BV, Leusden, The Netherlands).
Statistical analysis.
Differences between the results of the various experiments were evaluated by means of the Wilcoxon signed-rank test.
RESULTS
Infection of ECs after bacterial challenge.
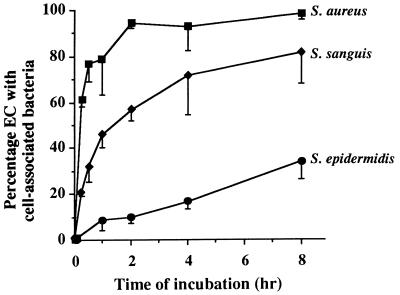
To determine whether different species of bacteria that represent the main causative bacteria in bacterial endocarditis differ in their ability to attach to ECs, monolayers were exposed to opsonized S. aureus, S. sanguis, or S. epidermidis for different periods of time. The bacteria to EC ratio was approximately 200:1. The percentage of ECs with cell-associated bacteria, i.e., bacteria bound to the EC surface or localized intracellularly, increased in a time-dependent fashion (Fig. 1). This was already shown for S. aureus in a previous study (7). For both S. aureus and S. sanguis, the binding to ECs occurred within 15 min after the addition of the bacteria. For S. aureus, a plateau level of approximately 95% of infection was reached in 2 h. With S. sanguis, about 80% of the ECs were infected after 8 h (Fig. 1). A longer exposure of ECs did not further increase the number of infected cells; 24 h after the addition of bacteria, the percentages of ECs infected with S. aureus or S. sanguis were 99.9% ± 0.24% (n = 3) and 83.2% ± 4.4% (n = 3), respectively. For S. epidermidis, a consistently lower infection (7% at 1 h) of the ECs was found. This gradually increased to 54.2% ± 12.9% (n = 3) after 24 h.
FIG. 1.
Time course of bacterial numbers associated with ECs for different species or strains of bacteria. Monolayers of ECs (∼2 × 105 cells/well) were incubated at 37°C with 5 × 106 S. aureus, S. sanguis, or S. epidermidis strains for the indicated periods of time. After washing, the percentage of ECs with cell-associated bacteria, i.e., membrane-bound as well as internalized bacteria, was determined. Data are expressed as the means ± standard deviations of three to four experiments with ECs from different donors.
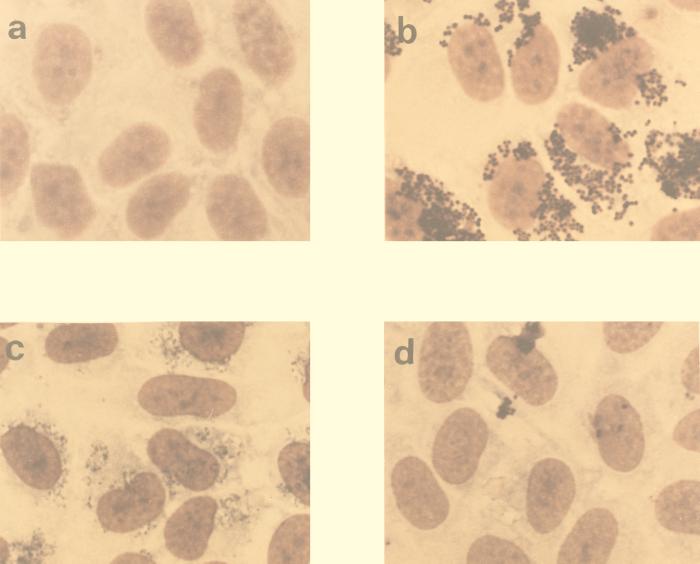
By microscopy, EC-bound bacteria were found to be located randomly over the EC monolayer (Fig. 2). This was observed with all three bacteria. Exposure of ECs to bacteria, in particular S. aureus, for periods longer than 24 h resulted in cell detachment and loss of EC monolayer integrity.
FIG. 2.
Light microscopic evaluation of bacterial infection of ECs. Monolayers of ECs (a) were exposed for 8 h to similar numbers of S. aureus (b), S. sanguis (c), or S. epidermidis (d) organisms at 37°C. Photographs show the degree of infection after staining with Giemsa stain. Magnification, ×80.
For all three bacteria, outgrowth in M199 containing 10% HuSi, i.e., the medium that was used in the infection assay, was negligible during 24 h (data not shown). Thus, the time-dependent increase in EC-associated bacteria cannot be explained by extracellular proliferation during the infection assay.
Species- and strain-dependent induction of TFA of bacteria-infected ECs.
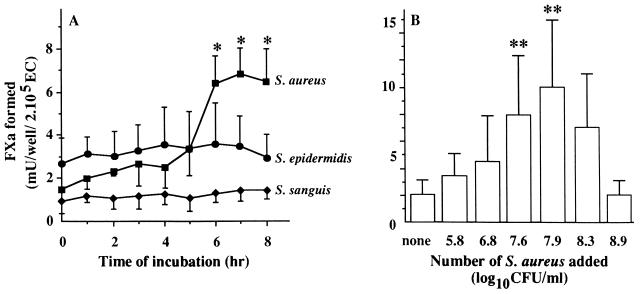
Noninfected, unstimulated monolayers of ECs expressed little TF-dependent procoagulant activity. This confirmed earlier findings by Maynard et al. (37). Values for TFA ranged from 0.7 to 4.1 mU per well of about 2 × 105 cells, representing variation between EC donors. Exposure of these ECs to S. aureus at a ratio of about 250 bacteria per single EC resulted in an increase of the low basal TFA. Time course experiments (Fig. 3A) showed this increase to be significant (P < 0.001; n = 5) from 6 h onward, with the level gradually rising to 13.23 ± 4.94 mU of FXa/well at 24 h of bacterial exposure. The increase of endothelial TFA was dependent on the number of added S. aureus (Fig. 3B). Incubation of ECs for 7 h with at least 107 S. aureus organisms per well (n = 4), i.e., more than 50 bacteria per single EC, resulted in an increase of TFA. A maximum of about 10 mU of FXa/well was achieved by incubation with 8 × 107 bacteria (n = 4). Incubation with more than 2 × 108 S. aureus organisms, i.e., 1,000 bacteria per EC, resulted in a decline of the TFA, most probably due to EC damage as apparent from disruption of the monolayer integrity. S. aureus in suspension did not by itself express TFA (data not shown). Experiments with S. sanguis or S. epidermidis showed that neither of these microorganisms induced TFA at any bacterial concentration (data not shown) with any incubation period (Fig. 3A).
FIG. 3.
(A) Course of TFA in bacteria-infected ECs. Monolayers of 2 × 105 ECs were incubated at 37°C with 3 × 107 to 5 × 107 S. aureus, S. sanguis, or S. epidermidis organisms. At the indicated time points, the monolayers were washed and assessed for TFA by measuring FVIIa-dependent FX activation as described in Materials and Methods. ∗, P < 0.001 versus 0 h. (B) Dose dependency of the S. aureus-induced endothelial TFA. Monolayers of ∼2 × 105 ECs were incubated for 7 h at 37°C with medium alone (none) or 1 ml of the indicated S. aureus numbers. After washing, TFA was assessed as described above. ∗∗, P < 0.05 versus none. Values represent the means ± standard deviations of five (A) or four (B) experiments with ECs from different donors.
Influence of extracellular bacteria, bacterial products, or bacterial viability on endothelial TFA.
In an earlier study, it was demonstrated that cultured ECs internalize S. aureus within 60 min after bacterial exposure (7). This resulted in the induction of a variety of proinflammatory properties, such as expression of adhesion molecules and leukocyte binding. This could be detected after an additional 23 h of culture and could not be abrogated by removal of the extracellular bacteria after 60 min. However, when in the present study the extracellular S. aureus strains were removed after 60 min, either by washing or by treatment with lysostaphin, the increase in EC TFA after 23 h of culture did not occur in the absence of extracellular bacteria (Table 1). Thus, either the bacteria, when present only intracellularly, are unable to induce EC TFA or their presence is insufficient in this respect.
TABLE 1.
Effect of S. aureus on TFA of ECsa
| Time of culture (h) | TFA (mU of FXa/2 × 105 ECs) for:
|
||
|---|---|---|---|
| S. aureusb | Cell-associated S. aureusc | Intracellular S. aureusd | |
| 0 (no bacteria) | 1.52 ± 0.65e | 0.70 ± 0.43 | 0.79 ± 0.33 |
| 5 | 3.33 ± 1.59 | 0.70 ± 0.37 | 0.88 ± 0.41 |
| 6 | 6.39 ± 1.32 | 0.92 ± 0.78 | 0.92 ± 0.46 |
| 7 | 6.83 ± 1.17 | 0.70 ± 0.57 | 0.96 ± 0.49 |
| 8 | 6.48 ± 1.75 | 0.92 ± 0.79 | 0.96 ± 0.41 |
Monolayers of 2 × 105 ECs were incubated with 4 × 107 S. aureus strains for the indicated periods of time. All values represent the means ± standard deviations of at least three individual experiments with ECs from different donors.
Values of continuous incubation with S. aureus (Fig. 3A).
ECs were incubated with 4 × 107 S. aureus organisms for 1 h, washed with warm PBS to remove non-cell-associated bacteria, and then cultured for the indicated additional periods of time.
ECs were incubated with 4 × 107 S. aureus organisms for 1 h and then washed, treated with lysostaphin to lyse extracellular but not intracellular bacteria, washed, and cultured for the indicated additional periods of time.
The value of P was <0.001 for S. aureus at 0 h versus S. aureus at 6, 7, and 8 h.
As for live S. aureus, it was also found in a previous study that UV-killed S. aureus could adhere to and be internalized by ECs as well as induce proinflammatory properties in these cells after 23 h of culture (7). By contrast, these killed bacteria did not induce EC TFA, as shown by incubation with 5 × 107 UV-irradiated S. aureus (Table 2). Neither was TFA induced by supernatants of overnight cultures of S. aureus containing cell wall fragments and soluble bacteria-derived factors (Table 2).
TABLE 2.
Influence of bacterial viability, bacterial fragments, and bacterium-derived factors on TFA of ECsa
| Time of incubation (h) | TFA (mU of FXa/2 × 105 ECs) for:
|
|||
|---|---|---|---|---|
| Medium | Live S. aureus | UV-killed S. aureus | Supernatant from S. aureus culture | |
| 7 | NDb | 4.95 ± 3.27 | 2.47 ± 1.69 | 2.58 ± 2.69 |
| 24 | 0.73 ± 0.79c | 8.68 ± 4.09 | 1.78 ± 2.65 | 0.76 ± 0.79 |
Monolayer of 2 × 105 ECs were incubated with M199 and 10% HuSi (medium), 5 × 107 live or UV-killed S. aureus organisms, or supernatants from overnight S. aureus cultures that were passed through a 0.45-μm-pore-size filter. After 7 or 24 h of incubation, ECs were washed and endothelial TFA was measured. Values represent means of three to five individual experiments with ECs from different donors.
ND, not determined, but values are expected to be similar to those at 24 h.
The value of P was <0.05 for medium at 24 h versus live S. aureus at 7 and 24 h.
Together, these findings indicate that the presence and uptake of substantial numbers of live S. aureus organisms intracellularly as well as extracellularly are needed to induce endothelial TFA.
Influence of exogenous or endogenous IL-1 on TFA by bacteria-infected ECs.
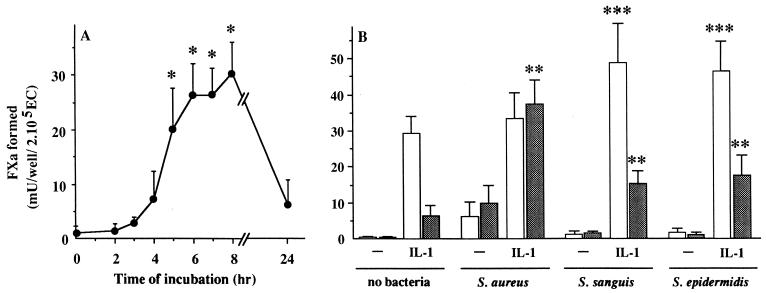
Stimulation of ECs by IL-1 can lead to expression of functional TF antigen (9, 10). This observation was confirmed in this study (Fig. 4A). In addition, we found that the time course and level of TFA during exposure of ECs to IL-1 were different from those observed during infection with S. aureus (Fig. 3A). With IL-1, TFA was already detectable after 5 h (20.26 ± 7.29 mU of FXa/well; P = 0.013; n = 4), had reached the highest level (30.5 ± 5.6 mU of FXa/well) after 8 h, and had returned to a near basal level (6.9 ± 3.0 mU of FXa/well; n = 4) after 24 h of stimulation (Fig. 4A), the time point at which TFA was maximal with S. aureus.
FIG. 4.
Effect of IL-1 on EC TFA during exposure to different species of bacteria. (A) Time course of TFA in 2 × 105 ECs after incubation with 5 ng of IL-1 per ml. ∗, P < 0.015 versus 0 h (i.e., nonstimulated ECs). (B) TFA of 2 × 105 ECs incubated with 3 × 107 to 5 × 107 of the indicated bacteria species in the absence or presence of 5 ng of IL-1 per ml. TFA was determined at 7 h (white bars) or 24 h (gray bars) as described in Material and Methods. ∗∗, P < 0.01 versus IL-1 alone at 24 h; ∗∗∗, P < 0.05 versus IL-1 alone at 7 h. Values represent the means ± standard deviations of four (A) or five (B) experiments with ECs from different donors.
It could be that the enhanced TFA of S. aureus-infected ECs was due to stimulation by IL-1, produced by the infected ECs. To test this possibility, the infection assay was performed in the presence of rIL-1ra. This did not prevent the increase of S. aureus-induced EC TFA, whereas TFA induced by IL-1 was effectively abolished (Table 3). Neither did RT-PCR give evidence for induction of IL-1 mRNA in ECs infected with S. aureus. The time points tested were 4, 7, and 20 h after infection. As a control, ECs were stimulated with IL-1, which resulted in IL-1 mRNA at 20 h (data not shown), demonstrating the validity of the selected PCR primers. Thus, although IL-1 could induce TFA in EC, the above findings strongly argue against an auto- or paracrine stimulation by IL-1 in the induction of TF in ECs by staphylococcal infection. RT-PCR also did not reveal induction of mRNA for tumor necrosis factor alpha (TNF-α) in ECs after stimulation with bacteria (data not shown).
TABLE 3.
Effect of endogenous IL-1 on EC TFAa
| Stimulus | rIL-1ra | TFA (mU of FXa/2 × 105 ECs) at the following timeb
|
|
|---|---|---|---|
| 7 h | 24 h | ||
| None | − | 0.74 ± 0.63 | 0.74 ± 0.63 |
| + | 4.28 ± 3.91 | 1.82 ± 1.94 | |
| S. aureus | − | 6.45 ± 0.33 | 14.82 ± 5.38 |
| + | 8.11 ± 2.55 | 14.03 ± 5.01 | |
| IL-1 | − | 56.06 ± 8.00 | 12.69 ± 5.84 |
| + | 17.74 ± 10.28c | 8.37 ± 5.69 | |
Treatment of ECs: 2 × 105 ECs were incubated at 37°C with M199 plus 10% HuSi (none) or 5 × 107 S. aureus organisms in the absence (−) or presence (+) of 30 ng of rIL-1ra per ml for 7 or 24 h. Control monolayers were stimulated with 5 ng of IL-1 per ml. Then ECs were washed, and TFA was determined as described in Material and Methods.
Values represent means ± standard deviations of at least four experiments with ECs from different donors.
The value of P was 0.03 for the TFA with IL-1 as the stimulus in the presence of rIL-1ra at 7 h versus that in the absence of rIL-1ra at 7 h.
Next, we tested whether exogenous IL-1 could influence the induction of TFA in ECs during simultaneous exposure to bacteria. Incubation of ECs with S. aureus together with IL-1 was found to have a synergistic effect on TFA induction (Fig. 4B). TFA reached a high level (33.51 ± 7.13 mU of FXa/well) after 7 h, as it did with IL-1 alone, but remained at this level (37.39 ± 6.71 mU of FXa/well; n = 5) up till 24 h. Exposure of ECs to IL-1 together with S. sanguis or S. epidermidis, i.e., the bacteria that by themselves did not induce TFA (Fig. 3A), resulted in TFA that up till 24 h remained significantly higher (P < 0.04; n = 5) than the TFA induced after stimulation with IL-1 alone, although at this time point it was significantly lower than the TFA induced with the combination of IL-1 and S. aureus (Fig. 4B).
TF mRNA and TF surface antigen expression.
To assess whether the increase in TF functional activity after bacterial and/or cytokine stimulation was accompanied by an increase in mRNA and/or surface protein expression, bacteria-infected ECs were prepared for RT-PCR and FACS analysis. In noninfected control ECs, the PCR product for TF mRNA was undetectable over a period of at least 20 h (Fig. 5). Also, flow cytometry revealed no TF antigen on the EC membranes (data not shown).
FIG. 5.
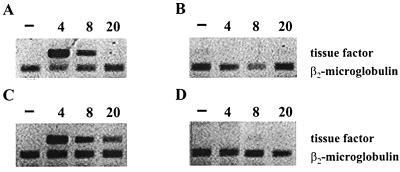
Analysis of TF mRNA in ECs. Monolayers of cultured ECs were incubated with 5 ng of IL-1 per ml (A) or with S. sanguis (B), S. aureus (C), or S. epidermidis (D) at a bacteria to cell ratio of ∼250:1. At the indicated time points, EC cultures were washed, lysed, and analyzed for the presence of TF mRNA by RT-PCR. β2-Microglobulin mRNA was used as a control.
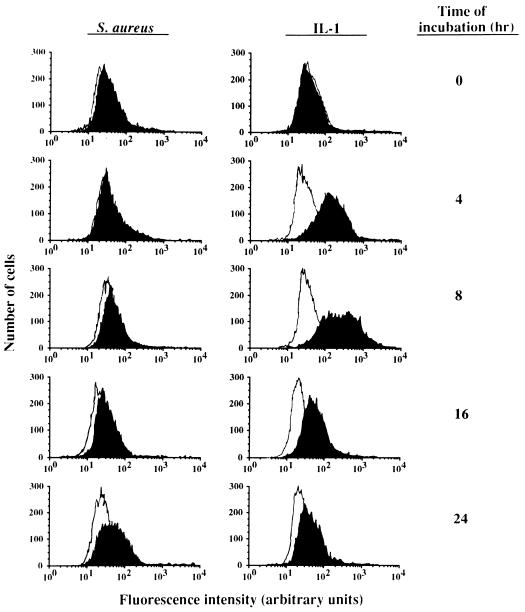
Stimulation with IL-1 as well as infection with S. aureus resulted in a time-dependent TF mRNA (Fig. 5) and TF antigen expression (Fig. 6). With IL-1 stimulation, TF mRNA could already be detected at 4 and 8 h but no longer at 20 h, while the expression of TF antigen on the cell surface followed a similar course. With S. aureus stimulation, TF mRNA could be detected at 4 h but persisted at least up to 20 h after infection of the ECs. TF antigen could be detected at 8 h and persisted at least up till 24 h, being the latest time point assayed. In S. sanguis- or S. epidermidis-infected ECs, neither TF mRNA (Fig. 5) nor TF surface antigen (data not shown) was found over an infection period of at least 20 h.
FIG. 6.
Flow cytometric analysis of TF antigen expression on the surface of cultured ECs. A monolayer of 8 × 105 ECs was incubated for the indicated periods of time at 37°C with 108 S. aureus organisms or 5 ng of IL-1 (closed graphs) per ml. Then the cell cultures were washed and prepared for analysis of TF antigen by FACS. Background fluorescence was established by incubation of ECs with the phycoerythrin-conjugated MAb alone (open graphs).
DISCUSSION
The present study shows that in addition to the induction of a variety of proinflammatory properties (7, 46, 53, 54) cultured human ECs upon exposure to S. aureus respond by the induction of TF mRNA, followed by surface expression of TF antigen as well as TFA. By contrast, neither S. epidermidis nor S. sanguis, bacteria with a low propensity to cause infection on naive endocardium, had such an effect in these cells. IL-1, a cytokine produced by the host in response to bacteremia, could induce TFA in ECs by itself and, in a synergistic manner, enhance endothelial TFA in S. aureus, as well as in S. epidermidis- or S. sanguis-infected cells. The influence of IL-1 on the species- and strain-dependent induction of EC TFA is in accord with our hypothesis that both bacterial and host factors orchestrate the induction of coagulation in an early stage in the pathogenesis of intravascular infections, such as BE.
The ability of invasive microorganisms to infect and colonize vascular endothelium has been studied in relation to many types of infectious diseases. Pathogens like Neisseria meningitidis (30), Streptococcus pneumoniae (27), Rickettsia rickettsii (14), and Chlamydia pneumoniae (26) have been reported to directly induce TFA in cultured human ECs upon infection. Drake and Pang (21) found that S. aureus also induces TFA in cultures of human valvular ECs. As an extension to this, the present study shows not only that monolayers of human venous ECs express TFA upon exposure to S. aureus but also that this was accompanied by an induction of TF mRNA. This began somewhat earlier and was accompanied by an increase of surface expression of TF molecules. Thus, TFA of S. aureus-infected ECs is controlled at the level of TF gene transcription and a de novo synthesis of TF. Our data, however, do not exclude the possibility that some TFA results from TF de-encryption, a process causing an increase in TFA due to changes in the quaternary structure of the TF molecule or alterations in the endothelial outer cell surface membrane (29, 43). For induction of endothelial TFA, at least a ratio of approximately 30 bacteria per cell was needed, while maximal TFA was attained after incubation at a ratio of 200 to 400 bacteria per EC, resulting in an infection of 95 to 100% of the cells. It is not unlikely that this degree of infection can be achieved locally during S. aureus bacteremia, in particular when the well-described propensity of these organisms to colonize ECs is also taken into account (4, 39, 41; this study).
ECs are known to synthesize and secrete cytokines, including IL-1, upon bacterial infection (53). Thus, it could be that such endogenous IL-1 could activate ECs in an auto- or paracrine manner. However, a contribution of endogenous IL-1 with respect to TFA by S. aureus-infected ECs could be excluded, since it was not affected by rIL-1ra. Also, by RT-PCR no IL-1 mRNA could be detected in S. aureus-infected ECs up to at least 20 h after infection (data not shown). Neither did S. aureus-infected ECs express TNF-α mRNA, arguing against a possible contribution of endogenous TNF-α in the induction of TFA of infected ECs.
From our data, we conclude that the increase in endothelial TFA is dependent on specific properties of the bacteria that infect the ECs. While S. aureus induces endothelial TFA, neither S. sanguis nor S. epidermidis under similar experimental conditions does so. Also, incubation of ECs with soluble S. aureus-derived factors or cell wall fragments does not influence TFA. The inability of S. epidermidis to induce TFA in human umbilical vein ECs is in line with the findings of Drake and Pang with human valvular ECs (22). That S. sanguis and S. epidermidis do not induce TFA in ECs is not explained by their inability to interact with ECs, because we found that these bacteria do associate with the EC surface membrane, although the magnitude of their adherence to ECs is somewhat lower than that of S. aureus. Moreover, in a separate study, with the same experimental procedure, we found that ECs upon contact with S. epidermidis or S. sanguis become activated and express elevated numbers of ICAM-1 and VCAM-1 molecules on their surface, resulting in an enhanced monocyte adhesion (unpublished data). For these bacteria, this indicates that the process of bacterial adhesion by itself is insufficient to induce TFA in ECs and furthermore suggests that this induction is regulated in bacteria-infected ECs by processes that differ from those regulating endothelial proinflammatory activity, such as cytokine production and leukocyte recruitment. Also, the lack of TFA in ECs after incubation with UV-killed, and therefore inactive, S. aureus, is in agreement with this. These killed bacteria are still able to effectively induce endothelial proinflammatory activity (7, 46). Although the experiments give no evidence about the nature of the relevant bacterial structures, they do indicate that endothelial TFA is induced only in the presence of cell-associated live S. aureus, suggesting an active participation of these microorganisms. With regard to the pathogenesis of BE, this propensity of S. aureus to interact with ECs and consequently induce TFA in these cells might explain why S. aureus already at a low inoculum can cause an infection on previously intact heart valves.
A further important finding was that the generation of EC TFA by bacteria is enhanced by IL-1. This inflammatory cytokine is produced during bacteremia and is a potent activator of EC function (6, 20, 34). It is conceivable that the interaction between bacteria and ECs is facilitated by the presence of this host-derived cytokine. The results of this study indeed demonstrate that exposure of ECs to a combination of IL-1 and bacteria leads to a marked induction of TFA, which was more than the sum of TFA induced by either stimulus alone. This synergism was most clear from the experiments with S. aureus but, surprisingly, was also observed with S. sanguis and S. epidermidis, two bacterial species that by themselves did not induce endothelial TFA. Apparently, the inflammatory stimulus changes the response of ECs to bacterial infection. We surmise that, in vivo, in the situation that the vascular endothelium is still undamaged, cytokines precondition ECs in a way that promotes the induction of endothelial TFA by bacteria. This finding may be important for the induction of coagulation in BE and other intravascular infections that leads to fibrin formation and, consequently, the colonization of (valvular) endothelium. This conclusion is supported by results from a study in rabbits demonstrating that induction of BE could be promoted by intravenous injection of this cytokine prior to S. sanguis inoculation (18). The mechanism underlying this preconditioning effect of IL-1 is currently under investigation.
The data presented in this study were obtained in vitro by using ECs from human umbilical veins. Although conditions in vivo for endocardial ECs may be somewhat different, we presume that these data are relevant for the pathogenesis of BE. Therefore, in summary, we show that binding to and/or internalization of bacteria by ECs have an effect on endothelial TFA that depends on the type of infecting microorganism but also on the inflammatory status of the ECs. With regard to the pathogenesis of BE, this has prompted us to consider the following hypothesis for the events that can start coagulation and fibrin formation on intact endothelial surfaces. Bacteria with a high affinity for ECs, such as S. aureus, induce EC TFA as well as monocyte adhesion by surface expression of ICAM-1 and VCAM-1. By contrast, S. sanguis or S. epidermidis induce EC activation in a manner that results only in monocyte adhesion but not in generation of TFA. We assume that (surface) proteins of S. aureus responsible for mediating their binding to ECs and their induction of endothelial TFA may be different or lacking in S. sanguis or S. epidermidis. The binding of monocytes to bacteria-infected ECs through the engagement of their β1- and β2-integrin receptors, being the natural ligands for endothelial VCAM-1 and ICAM-1, initiate signal transduction leading to generation and expression of TF (17, 24) as well as the release of TNF-α (17, 23) or IL-1 (31, 55). These cytokines and possibly other so far unknown humoral host factors may precondition ECs in a way that renders them more susceptible to the consequences of bacterial interaction. This hypothesis is currently under investigation. These studies that focus on the contribution of monocytes and specific species- or strain-dependent bacterial factors as well as host cell factors to the initiation of endothelial procoagulant activity may open new ways to a more specific and targeted approach in the treatment and prevention of BE.
ACKNOWLEDGMENTS
Part of this work was supported by grant 3.2.13 from the Institute of Radiopathology and Radiation Protection, J. A. Cohen Institute, Leiden, The Netherlands.
We gratefully acknowledge the coworkers of the Department of Gynecology at the Leiden University Medical Center, Leiden, The Netherlands, for providing human umbilical cords and J. S. van de Gevel for assistance in the preparation of EC cultures.
REFERENCES
- 1.Bancsi M J L M F, Thompson J, Bertina R M. Stimulation of monocyte tissue factor expression in an in vitro model of bacterial endocarditis. Infect Immun. 1994;62:5669–5672. doi: 10.1128/iai.62.12.5669-5672.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bancsi M J L M F, Veltrop M H A M, Bertina R M, Thompson J. Influence of monocytes and antibiotic treatment on tissue factor activity of endocardial vegetations in rabbits infected with Streptococcus sanguis. Infect Immun. 1996;64:448–451. doi: 10.1128/iai.64.2.448-451.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bancsi M J L M F, Veltrop M H A M, Bertina R M, Thompson J. Role of monocytes and bacteria in Staphylococcus epidermidis endocarditis. Infect Immun. 1998;66:448–450. doi: 10.1128/iai.66.2.448-450.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker R C, DiBello P M, Lucas F V. Bacterial tissue tropism: an in vitro model for infective endocarditis. Cardiovasc Res. 1987;21:813–820. doi: 10.1093/cvr/21.11.813. [DOI] [PubMed] [Google Scholar]
- 5.Beekhuizen H, Corsel-van Tilburg A J, van Furth R. Characterization of monocyte adherence to human macrovascular and microvascular endothelial cells. J Immunol. 1990;145:510–518. [PubMed] [Google Scholar]
- 6.Beekhuizen H, van de Gevel J S. Endothelial cell adhesion molecules in inflammation and postischemic reperfusion injury. Transplant Proc. 1998;30:4251–4256. doi: 10.1016/s0041-1345(98)01405-5. [DOI] [PubMed] [Google Scholar]
- 7.Beekhuizen H, van de Gevel J S, Olsson B, van Benten I J, van Furth R. Infection of human vascular endothelial cells with Staphylococcus aureus induces hyperadhesiveness for human monocytes and granulocytes. J Immunol. 1997;158:774–782. [PubMed] [Google Scholar]
- 8.Beekhuizen H, van Furth R. Growth characteristics of cultured human macrovascular venous and arterial and microvascular endothelial cells. J Vasc Res. 1994;31:230–239. doi: 10.1159/000159048. [DOI] [PubMed] [Google Scholar]
- 9.Bevilacqua M P, Pober J S, Majeau G R, Fiers W, Cotran R S, Gimbrone M A. Recombinant tumor necrosis factor induces procoagulant activity in cultured vascular endothelium: characterization and comparison with the actions of interleukin-1. Proc Natl Acad Sci USA. 1986;83:4533–4537. doi: 10.1073/pnas.83.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bevilacqua M P, Pober J S, Majeau G R, Cotran R S, Gimbrone M A. Interleukin 1 (IL-1) induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J Exp Med. 1984;160:618–623. doi: 10.1084/jem.160.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camerer E, Kolsto A-B, Prydz H. Cell biology of tissue factor, the principal initiator of blood coagulation. Thromb Res. 1996;81:1–41. doi: 10.1016/0049-3848(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 12.Chastre J, Trouillet J L. Early infective endocarditis on prosthetic valves. Eur Heart J. 1995;16:32–38. doi: 10.1093/eurheartj/16.suppl_b.32. [DOI] [PubMed] [Google Scholar]
- 13.Cheung A L, Krishnan M, Jaffe E A, Fischetti V A. Fibrinogen acts as a bridging molecule in the adherence of Staphylococcus aureus to cultured human endothelial cells. J Clin Investig. 1991;87:2236–2245. doi: 10.1172/JCI115259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Courtney M-A, Haidaris P J, Marder V J, Sporn L A. Tissue factor mRNA expression in endothelium of an intact umbilical vein. Blood. 1996;87:174–179. [PubMed] [Google Scholar]
- 15.Crossman D C, Carr D P, Tuddenham E G D, Pearson J D, McVey J M. The regulation of tissue factor mRNA in human endothelial cells in response to endotoxin or phorbol ester. J Biol Chem. 1990;265:9782–9787. [PubMed] [Google Scholar]
- 16.Cunha B A, Gill V, Lazar J M. Acute infective endocarditis. Diagnostic and therapeutic approach. Infect Dis Clin N Am. 1996;10:811–834. doi: 10.1016/s0891-5520(05)70328-7. [DOI] [PubMed] [Google Scholar]
- 17.Dackiw A P B, Nathens A B, Marshall J C, Rotstein O D. Integrin engagement induces monocyte procoagulant activity and tumor necrosis factor production via induction of tyrosine phosphorylation. J Surg Res. 1996;64:210–215. doi: 10.1006/jsre.1996.0330. [DOI] [PubMed] [Google Scholar]
- 18.Dankert J, van de Werff J, Joldersma W. Abstracts of the 31st Interscience Conference on Antimicrobial Agents and Chemotherapy 1991. Washington, D.C: American Society for Microbiology; 1991. Interleukin-1 (IL-1) enhances the onset of experimental bacterial endocarditis, abstr. 535; p. 18. [Google Scholar]
- 19.Davie E W, Fujikawa K, Kisiel W. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry. 1991;30:10363–10370. doi: 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- 20.Dinarello C A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991;77:1627–1652. [PubMed] [Google Scholar]
- 21.Drake T A, Pang M. Staphylococcus aureus induces tissue factor expression in human cardiac valve endothelial cells. J Infect Dis. 1988;157:749–756. doi: 10.1093/infdis/157.4.749. [DOI] [PubMed] [Google Scholar]
- 22.Drake T A, Pang M. Effects of interleukin-1, lipopolysaccharide, and streptococci on procoagulant activity of cultured human cardiac valve endothelial cells and stromal cells. Infect Immun. 1989;57:507–512. doi: 10.1128/iai.57.2.507-512.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan S-T, Edgington T S. Integrin regulation of leukocyte inflammatory functions. CD11b/CD18 enhancement of the tumor necrosis factor-α responses of monocytes. J Immunol. 1993;150:2972–2980. [PubMed] [Google Scholar]
- 24.Fan S-T, Mackman N, Cui M-Z, Edgington T S. Integrin regulation of an inflammatory effector gene. Direct induction of tissue factor promoter by engagement of β1 or α4 integrin chains. J Immunol. 1995;154:3266–3274. [PubMed] [Google Scholar]
- 25.Freedman L R, Valone J. Experimental infective endocarditis. Prog Cardiovasc Dis. 1979;22:169–180. doi: 10.1016/0033-0620(79)90021-5. [DOI] [PubMed] [Google Scholar]
- 26.Fryert R H, Schwobe E P, Woods M L, Rodgers G M. Chlamydia species infect human vascular endothelial cells and induce procoagulant activity. J Investig Med. 1997;45:168–174. [PubMed] [Google Scholar]
- 27.Geelen S, Bhattacharyya C, Tuomanen E. Induction of procoagulant activity on human endothelial cells by Streptococcus pneumoniae. Infect Immun. 1992;60:4179–4183. doi: 10.1128/iai.60.10.4179-4183.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gould K, Ramirez-Ronda C H, Holmes R K, Sanford J P. Adherence of bacteria to heart valves in vitro. J Clin Investig. 1975;56:1364–1370. doi: 10.1172/JCI108216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greeno E W, Bach R R, Moldow C F. Apoptosis is associated with increased cell surface tissue factor procoagulant activity. Lab Investig. 1996;75:281–289. [PubMed] [Google Scholar]
- 30.Heyderman R S, Klein N J, Daramola O A, Hammerschmidt S, Frosch M, Robertson B D, Levin M, Ison C A. Induction of human endothelial tissue factor expression by Neisseria meningitidis: the influence of bacterial killing and adherence to the endothelium. Microb Pathog. 1997;22:265–274. doi: 10.1006/mpat.1996.0112. [DOI] [PubMed] [Google Scholar]
- 31.Lin T H, Rosales C, Mondal K, Bolen J B, Haskill S, Juliano R L. Integrin-mediated tyrosine phosphorylation and cytokine message induction in monocytic cells: a possible role for the syk tyrosine kinase. J Biol Chem. 1995;270:16189–16197. doi: 10.1074/jbc.270.27.16189. [DOI] [PubMed] [Google Scholar]
- 32.Lowy F D. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 33.Lowy F D, Fant J, Higgins L L, Ogawa S K, Hatcher V B. Staphylococcus aureus-human endothelial cell interactions. J Ultrastruct Mol Struct Res. 1988;98:137–146. doi: 10.1016/s0889-1605(88)80906-6. [DOI] [PubMed] [Google Scholar]
- 34.Mantovani A, Garlanda C, Introna M, Vecchi A. Regulation of endothelial cell function by pro- and anti-inflammatory cytokines. Transplant Proc. 1998;30:4239–4243. doi: 10.1016/s0041-1345(98)01402-x. [DOI] [PubMed] [Google Scholar]
- 35.Martin D M A, Boys C W G, Ruf W. Tissue factor: molecular recognition and cofactor function. FASEB J. 1995;9:852–859. doi: 10.1096/fasebj.9.10.7615155. [DOI] [PubMed] [Google Scholar]
- 36.Masur H, Johnson W D. Prosthetic valve endocarditis. J Thorac Cardiovasc Surg. 1980;80:31–37. [PubMed] [Google Scholar]
- 37.Maynard J R, Dreyer B E, Stemerman M B, Pitlick F A. Tissue factor coagulant activity of cultured endothelial and smooth muscle cells and fibroblasts. Blood. 1977;50:387–396. [PubMed] [Google Scholar]
- 38.Morrissey J H, Fair D S, Edgington T S. Monoclonal antibody analysis of purified and cell-associated tissue factor. Thromb Res. 1988;52:247–261. doi: 10.1016/0049-3848(88)90084-9. [DOI] [PubMed] [Google Scholar]
- 39.Ogawa S K, Yurberg E R, Hatcher V B, Levitt M A, Lowy F D. Bacterial adherence to human endothelial cells in vitro. Infect Immun. 1985;50:218–224. doi: 10.1128/iai.50.1.218-224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osterud B. Tissue factor expression by monocytes: regulation and pathophysiological roles. Blood Coagul Fibrinolysis. 1998;9:S9–S14. [PubMed] [Google Scholar]
- 41.Peterson L R, Sinha A A, Gruninger R P. Selective bacterial adherence to cardiac endothelial cells in tissue culture. Cardiovasc Res. 1981;15:404–410. doi: 10.1093/cvr/15.7.404. [DOI] [PubMed] [Google Scholar]
- 42.Proctor R A, van Langevelde P, Kristjansson M, Maslow J N, Arbeit R D. Persistent and relapsing infections associated with small colony variants of Staphylococcus aureus. Clin Infect Dis. 1995;20:95–102. doi: 10.1093/clinids/20.1.95. [DOI] [PubMed] [Google Scholar]
- 43.Rao L V M, Pendurthi U R. Tissue factor on cells. Blood Coagul Fibrinolysis. 1998;9:S27–S35. [PubMed] [Google Scholar]
- 44.Roberts R B. Streptococcal endocarditis. In: Kaye D, editor. Infective endocarditis. New York, N.Y: Raven Press; 1995. pp. 191–208. [Google Scholar]
- 45.Schwager I, Jungi T W. Effect of recombinant cytokines on the induction of macrophage procoagulant activity. Blood. 1994;83:152–160. [PubMed] [Google Scholar]
- 46.Tekstra, J., H. Beekhuizen, J. S. van de Gevel, I. J. van Benten, C. W. Tuk, and R. H. J. Beelen. Infection of human endothelial cells with Staphylococcus aureus induce the production of monocyte chemotactic protein-1 and monocyte chemotaxis. Clin. Exp. Immunol., in press. [DOI] [PMC free article] [PubMed]
- 47.Thiruvikraman S V, Guha A, Roboz J, Taubman M B, Nemerson Y, Fallon J T. In situ localization of tissue factor in human atherosclerotic plaques by binding of digoxigenin-labeled factors VIIa and X. Lab Investig. 1996;75:451–461. [PubMed] [Google Scholar]
- 48.Tompkins D C, Blackwell L J, Hatcher V B, Elliott D A, O'Hagan-Sotsky C, Lowy F D. Staphylococcus aureus proteins that bind to human endothelial cells. Infect Immun. 1992;60:965–969. doi: 10.1128/iai.60.3.965-969.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tompkins D C, Hatcher V B, Patel D, Orr G A, Higgins L L, Lowy F D. A human endothelial cell membrane protein that binds Staphylococcus aureus in vitro. J Clin Investig. 1990;85:1248–1254. doi: 10.1172/JCI114560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van de Gevel J S, Veltrop M H A M, Thompson J, Tekstra J, Beekhuizen H. Vascular endothelium displays pro-inflammatory-like characteristics upon binding and ingestion of bacteria. Immunol Lett. 1997;56:436. . (Abstract.) [Google Scholar]
- 51.Van der Meer J T M, Thompson J, Michel M F, Valkenburg H A. Epidemiology of bacterial endocarditis in the Netherlands. Part I. Patient characteristics. Arch Intern Med. 1992;152:1863–1868. doi: 10.1001/archinte.152.9.1863. [DOI] [PubMed] [Google Scholar]
- 52.Vercellotti G M, Lussenhop D, Peterson P K, Furcht L T, McCarthy J B, Jacob H S, Moldow C F. Bacterial adherence to fibronectin and endothelial cells: a possible mechanism for bacterial tissue tropism. J Lab Clin Med. 1984;103:34–43. [PubMed] [Google Scholar]
- 53.Yao L, Bengualid V, Lowy F D, Gibbons J J, Hatcher V B, Berman J W. Internalization of Staphylococcus aureus by endothelial cells induces cytokine gene expression. Infect Immun. 1995;63:1835–1839. doi: 10.1128/iai.63.5.1835-1839.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yao L, Lowy F D, Berman J W. Interleukin-8 gene expression in Staphylococcus aureus-infected endothelial cells. Infect Immun. 1996;64:3407–3409. doi: 10.1128/iai.64.8.3407-3409.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yurochko A D, Liu D Y, Eierman D, Haskill S. Integrins as a primary signal transduction molecule regulating monocyte immediate-early gene induction. Proc Natl Acad Sci USA. 1992;89:9034–9038. doi: 10.1073/pnas.89.19.9034. [DOI] [PMC free article] [PubMed] [Google Scholar]