Abstract
Background
Renal cell carcinoma (RCC) can be complicated by a venous tumor thrombus (TT), of which the optimal management is unknown.
Objectives
This study sought to assess the prevalence of TT in RCC, its current management, and its association with venous thromboembolism (VTE), arterial thromboembolism (ATE), major bleeding (MB), and mortality.
Methods
Patients diagnosed with RCC between 2010 and 2019 in our hospital were included and followed from RCC diagnosis until 2 years after, or until an outcome of interest (VTE, ATE, and MB) or death occurred, depending on the analysis. Cumulative incidences were estimated with death as a competing risk. Cause-specific hazard models were used to identify predictors and the prognostic impact.
Results
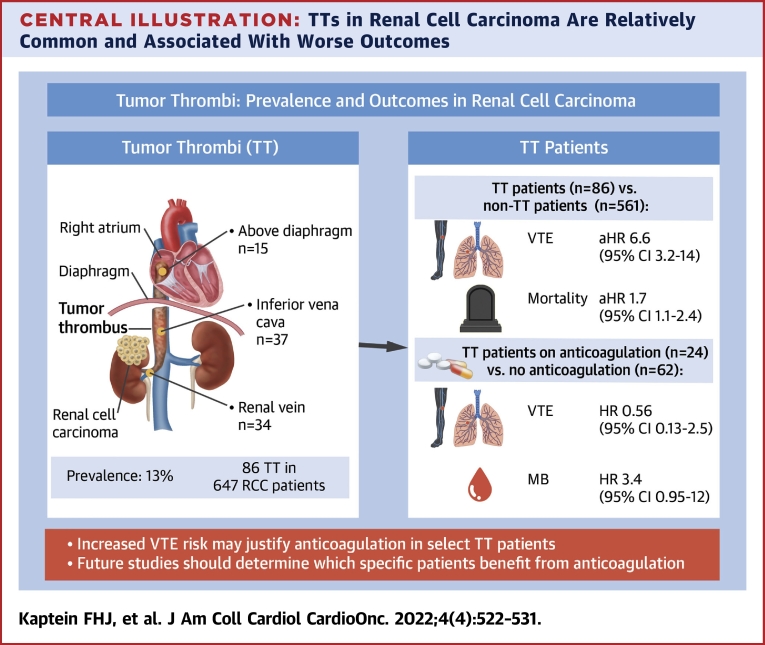
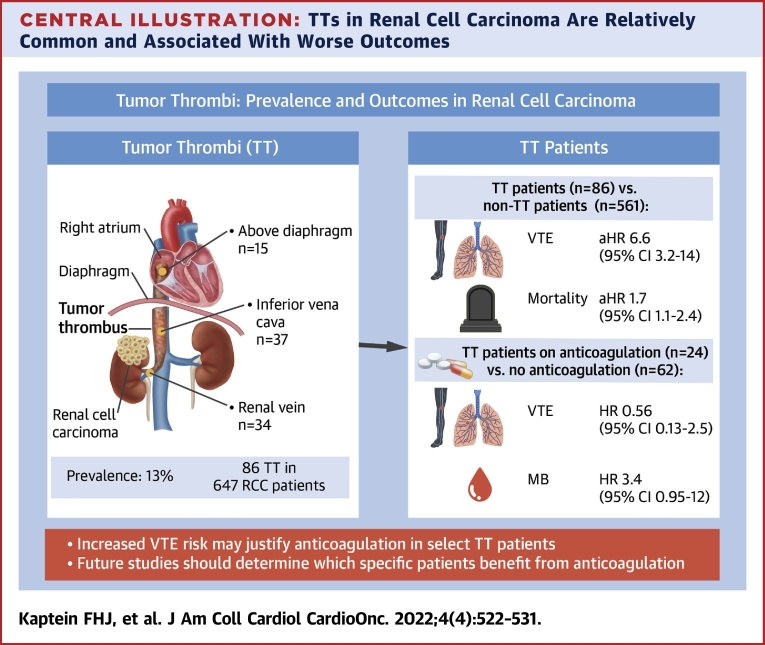
Of the 647 patients, 86 had a TT (prevalence 13.3%) at RCC diagnosis, of which 34 were limited to the renal vein, 37 were limited to the inferior vena cava below the diaphragm, and 15 extended above the diaphragm; 20 patients started therapeutic anticoagulation and 45 underwent thrombectomy with/without anticoagulation. During follow-up (median 24.0 [IQR: 7.0-24.0] months), 17 TT patients developed a VTE, 0 developed an ATE, and 11 developed MB. TT patients were more often diagnosed with VTE (adjusted HR: 6.61; 95% CI: 3.18-13.73) than non-TT patients, with increasing VTE risks in more proximal TT levels. TT patients receiving anticoagulation still developed VTE (HR: 0.56; 95% CI: 0.13-2.48), at the cost of more MB events (HR: 3.44; 95% CI: 0.95-12.42) compared with those without anticoagulation.
Conclusions
Patients with RCC-associated TT were at high risk of developing VTE. Future studies should establish which of these patients benefit from anticoagulation therapy.
Key Words: anticoagulants, hemorrhage, inferior vena cava, renal cell carcinoma, prognosis, venous thrombosis
Abbreviations and Acronyms: aHR, adjusted HR; ATE, arterial thromboembolism; CT, computed tomography; DVT, deep vein thrombosis; IVC, inferior vena cava; LMWH, low-molecular-weight heparin; MB, major bleeding; PE, pulmonary embolism; RCC, renal cell carcinoma; TT, tumor thrombus; VKA, vitamin K antagonist; VTE, venous thromboembolism
Central Illustration
Intravascular tumor extension, also known as tumor thrombus (TT), can occur in different tumor types, but in adult patients it is most common in renal cell carcinoma (RCC). RCC represents around 3% of all cancers, with an age-standardized incidence of 10 per 100,000 persons in Western countries.1,2 The reported prevalence of venous TT in RCC is 10% to 18% invading the renal vein, 4% to 23% involving the inferior vena cava (IVC), and 1% extending into the right atrium.3, 4, 5 Studies on the prognostic value of the presence and extension of a TT in RCC patients show conflicting results, but as TTs are associated with larger tumors, higher grading, and staging and more often distant metastases,5 this biologically more aggressive tumor behavior is likely to contribute to prognosis.6
In the absence of distant metastases, the indicated treatment for a TT is surgical resection to obtain local control.6 The use of anticoagulation remains debated mostly because a TT behaves differently from a bland thrombus, as it contains organized tumor cells rather than only a fibrin clot.7 Large vena cava TTs may rarely embolize to the pulmonary arteries (1.5%-3.4% of the cases),8 in particular intraoperatively, with high mortality rates.9,10 These pulmonary emboli may be thromboembolic (in case of concurrent bland thrombus, which are less stable and embolize more easily), actual pulmonary tumor emboli, or a combination of both.7,8,11 Although several radiographic characteristics have been identified to discriminate tumor embolism from thromboembolism, this distinction remains challenging in practice, and a biopsy would be required for definitive diagnosis, albeit rarely performed.11, 12, 13 Because high-level evidence is unavailable, anticoagulation is scarcely addressed in current international RCC guidelines, if at all.6,14,15
In the absence of randomized controlled trials comparing the safety and efficacy of anticoagulation vs placebo in patients with RCC associated TT, the best available evidence guiding treatment decisions are accurate incidence estimates of the TT associated thrombotic and bleeding complications, and the impact of anticoagulation on this incidence. Current literature on TT associated thrombosis mainly focuses on surgical methods and the perioperative period, and the few studies that do include long-term outcomes do not assess anticoagulation or bleeding complications.16,17 Therefore, the aim of our study was to establish the prevalence and incidence of TT in RCC patients and the proportion and type of TT that were treated with anticoagulation therapy. Furthermore, we sought to determine the association of TT with venous and arterial thrombosis, major bleeding (MB), and overall survival.
Methods
Study design, patients, and data collection
We performed a single-center retrospective cohort study of consecutive adult patients diagnosed with RCC between January 2010 and December 2019 at the Leiden University Medical Center (Leiden, the Netherlands). Patients were included when they had a histologically proven RCC, or when patients with metastatic disease were treated according to RCC protocols regardless of histological confirmation.
Standard of care for localized RCC involved (partial) nephrectomy. Thermal ablation was indicated in small tumors in which partial nephrectomy was technically impossible or patient performance status was insufficient for invasive surgery. No adjuvant therapy in locoregional disease was given, but follow-up imaging was performed every 6 months for the first 3 years (thereafter depending on tumor and patient characteristics). In metastatic disease, targeted therapy was initiated as indicated by tumor type and prognostic risk group (International Metastatic RCC Database Consortium criteria).18 Nephrectomy with metastatic disease was considered when feasible for cytoreduction and/or symptom reduction. Of note, TTs (regardless of their extension) are not considered to be metastases.
Thrombectomy was the standard of care when a TT was present, unless unfeasible due to technical or patient-related factors. Whether or not anticoagulation therapy was started was left to the discretion of the treating physician; this was not dictated by the local hospital protocol.
Patients were followed between 2 weeks before RCC diagnosis until 2 years after, until their last follow-up visit before March 2021, or until a thrombotic or bleeding complication or death occurred (depending on the analysis), whichever came first. Patients were considered lost to follow-up if their treatment in the Leiden University Medical Center was stopped before the end of the observation period.
Data collection were performed by reviewing the patient charts for baseline characteristics (demographics, Karnofsky performance score, tumor characteristics, and treatment details) and outcomes of interest, using a standardized electronic case report form. The study was approved by the local Institutional Review Board (the Medical Research Ethics Committee Leiden-The Hague-Delft), and the need for informed consent was waived. However, patients who were alive and still under the care of a Leiden University Medical Center physician during data collection were informed about the study and were offered the possibility to refuse the use of their medical data for study purposes.
Outcomes
The main study outcomes were TT at time of RCC diagnosis or during follow-up, venous thromboembolism (VTE), arterial thromboembolism (ATE), and MB. The endpoints were adjudicated by 2 independent experts (M.C.B. and F.A.K).
TTs were defined as: 1) histopathological proof of tumor cells in surgical specimen removed from the venous vasculature; or 2) extension of the tumor into the ipsilateral renal vein (and into the IVC and further) on computed tomography (CT), with the arguments in favor of a tumor instead of bland thrombus being enhancement of the thrombus after contrast administration, expansion of the vein, and increase of the thrombus under anticoagulation. TT extension was classified in 3 groups, conforming to the T stage of the TNM classification of RCC,19 that is: 1) limited to the renal vein; 2) extension into the IVC below the diaphragm; and 3) with supradiaphragmatic localization or invasion of the wall of the vena cava. The presence of TT was assessed by expert radiologists (M.C.B. and E.L.v.P.v.M.), who had no knowledge of the clinical course and the occurrence of the main study outcomes during follow-up.
VTE consisted of symptomatic or incidental pulmonary embolism (PE), deep vein thrombosis (DVT) of the upper or lower extremities, cerebral sinus vein thrombosis, or splanchnic vein thrombosis, confirmed by CT, magnetic resonance, or ultrasound imaging.20, 21, 22 ATE included ischemic strokes, myocardial infarction, and peripheral arterial embolism. Ischemic strokes were confirmed by CT or magnetic resonance imaging of the brain. Myocardial infarction was confirmed by corresponding electrocardiogram, echocardiogram, cardiac enzymes, and preferably coronary angiography. Peripheral arterial embolism was diagnosed with (CT) angiography or Doppler ultrasound of the extremities. The International Society on Thrombosis and Haemostasis definition of major bleeding was used, defining MB as: 1) fatal bleeding; 2) symptomatic bleeding in a critical area or organ; or 3) bleeding causing a fall in hemoglobin level of ≥1.24 mmol/L, or leading to a transfusion of ≥2 units of blood.23
Statistical analysis
Data are presented as mean ± SD or median (IQR) for continuous variables and counts with percentages for categorical variables. The Karnofsky score was divided into good (≥80%) vs moderate to poor (<80%) performance status. The tumor grade was separated as high (Fuhrman grade 3-4) vs low (Fuhrman grade 1-2). The index date was 2 weeks before the date of histopathological diagnosis of RCC (or in absence of histological confirmation the date of radiological evidence of metastatic disease).
Cumulative incidence was estimated using the cumulative incidence competing risks method,24 to adjust for competing risk of death, and were presented with a 95% CI. Cumulative incidence was calculated both for the total cohort and in TT patients separately. Incidence rates were calculated as number of events divided by the total person-time of observation, and are presented with 95% CIs. Outcome predictors were determined with univariable binary logistic (presented as OR with 95% CI) or cause-specific hazard models (presented as HR with 95% CI), depending on whether the time-to-event factor was relevant. To assess the prognostic impact of the outcomes, cause-specific hazard models with time-dependent covariates (ie, TT, VTE, ATE, and MB in the respective analyses) were performed,25 with adjustment for age, sex, performance score, tumor grade, and distant metastases (presented as adjusted HRs [aHRs] with 95% CI).25,26 For the interpretation of the prognostic impact of specific outcomes on VTE or MB, we considered cause-specific death models (with VTE respectively MB as censoring event) as well, to assess death as a competing risk.25,26 For univariable analyses, only complete cases were included. For the multivariable analyses, to assess the prognostic impact of the outcomes, missing values were included as a separate category per variable, to allow all cases to be analyzed. Statistical significance was determined when the CI did not include 1.
Since in PEs no differentiation between thromboembolism and tumor embolism was possible with the imaging performed for regular clinical care,27 we performed a sensitivity analysis with only non-PE VTE events.
Statistical analyses were carried out in SPSS Statistics version 25.0 (IBM) and RStudio version 1.3.1056 (RStudio).
Results
Patients
A total of 647 patients with RCC were included. Their mean age was 64.3 ± 11.1 years, and 436 (67.4%) patients were men (Table 1). The majority had a clear cell RCC (n = 457 [70.6%]). At diagnosis, 475 (73.4%) patients had locoregional disease, of which the majority underwent nephrectomy (n = 364 [76.6%]) or thermal ablation (n = 94 [19.8%]). Distant metastases were present at diagnosis in 172 (26.6%) patients, of which 156 (90.7%) received any type of palliative treatment, predominantly systemic therapy (n = 117 [75.0%]). At the end of the observation period, 378 (58.4%) patients were still alive, 132 (20.4%) had died, and 137 (21.2%) were lost to follow-up. The majority of those latter patients (77.4%) were referred back to the referring hospital. Another 3.6% were referred to a different expertise center for second/third opinion, and 8.0% were transferred to a palliative care unit. The median follow-up time was 24.0 (IQR: 7.0-24.0) months.
Table 1.
Baseline and Follow-Up Characteristics (N = 647)
| Age at diagnosis, y | 64.3 ± 11.1 |
| Male | 436 (67.4) |
| Anticoagulation use at diagnosis | 65 (10.0) |
| Histopathological diagnosis | |
| PA not performed | 20 (3.1) |
| Clear cell | 457 (70.6) |
| Mixed histology | 29 (4.5) |
| Non–clear cell | 114 (17.6) |
| PA performed, but results unknown | 27 (4.2) |
| Tumor grade | |
| Fuhrman grade 1 | 84 (13.0) |
| Fuhrman grade 2 | 254 (39.3) |
| Fuhrman grade 3 | 95 (14.7) |
| Fuhrman grade 4 | 37 (5.7) |
| Unknown | 177 (27.4) |
| Stage at diagnosis | |
| Local disease | 457 (70.6) |
| Regional lymph node metastases | 18 (2.8) |
| Distant metastases | 172 (26.6) |
| Performance status (Karnofsky score) | |
| <80% | 96 (14.8) |
| ≥80% | 451 (69.7) |
| unknown | 100 (15.4) |
| Anticancer treatment started at diagnosis | |
| (Partial) nephrectomy | 395 (61.1) |
| Radiofrequency ablation | 98 (15.1) |
| Systemic therapy | 118 (18.2) |
| No treatment | 29 (4.5) |
| Total follow-up, mo | 24.0 (7.0-24.0) |
| Status at the end of follow-up | |
| Alive | 378 (58.4) |
| Died | 132 (20.4) |
| Lost to follow-up | 137 (21.2) |
Values are mean ± SD, n (%), or median (IQR).
PA = pathological analysis.
Tumor thrombi
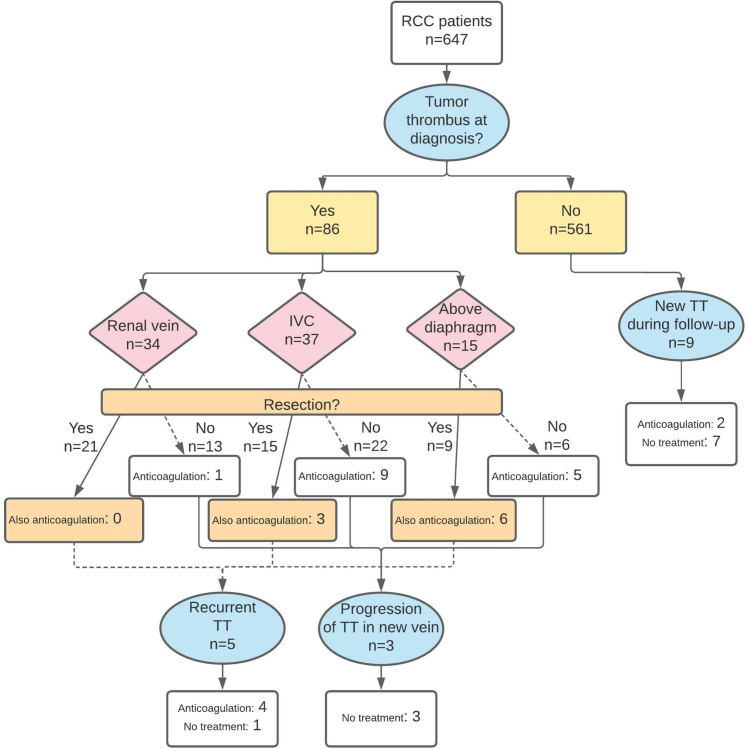
At RCC diagnosis, a TT was present in 86 patients (prevalence: 13.3%; 95% CI: 10.9%-16.1%), of which 37 were located in the IVC and 15 extended above the diaphragm, corresponding to an overall prevalence of 5.7% (95% CI: 4.0%-7.9%) and 2.3% (95% CI: 1.3%-3.8%), respectively (Figure 1). In 36 patients, only thrombectomy was performed; 9 patients received both thrombectomy and anticoagulation; and 15 patients only received anticoagulation. Six of these patients were already on chronic anticoagulation for a different indication, and continued this treatment (5 used vitamin K antagonist [VKA] and 1 low-molecular-weight heparin [LMWH]). Of the patients that initiated anticoagulation for the TT, 16 of 18 received LMWH and the others received VKA. Twenty-six patients did not receive any specific TT treatment. If no thrombectomy was performed and/or if the TT level was more proximal, anticoagulation was numerically more often prescribed (Figure 1).
Figure 1.
TT Diagnosis and Treatment
Flow chart with the number of renal cell carcinoma (RCC) patients diagnosed with a tumor thrombus (TT), and the treatment of the TT according to their extension (resection and/or long-term anticoagulation, or none). IVC = inferior vena cava.
During the observation period, 9 patients developed a new TT (incidence rate 0.98 per 100 person-years; 95% CI: 0.51-1.89 per 100 person-years), and 5 patients had a recurrent TT after resection at initial TT diagnosis. Furthermore, in 3 other patients the TT extended to a higher TT classification (none had received anticoagulation). TTs at RCC diagnosis were associated with a high tumor grade (OR: 5.54; 95% CI: 3.14-9.76), a low Karnofsky performance status (OR: 2.45; 95% CI: 1.43-4.22), and distant metastases at RCC diagnosis (OR: 3.01; 95% CI: 1.92-4.72) (Table 2).
Table 2.
Variables Associated With TT at Baseline and Adverse Outcomes (Univariable Analysis)
| Na | TT OR (95% CI) |
VTE HR (95% CI) |
MB HR (95% CI) |
Mortality HR (95% CI) | |
|---|---|---|---|---|---|
| Age, y | 647 | 1.02 (0.99-1.04) | 0.99 (0.96-1.02) | 1.02 (0.99-1.05) | 1.01 (1.00-1.03) |
| Sex (male vs female) | 647 | 1.26 (0.78-2.03) | 1.45 (0.68-3.08) | 1.48 (0.75-2.92) | 1.14 (0.78-1.65) |
| Histological type (clear cell vs nonclear cell) | 620 | 1.58 (0.90-2.77) | 1.11 (0.50-2.48) | 1.76 (0.78-3.96) | 0.68 (0.47-0.99) |
| Fuhrman grade (3-4 vs 1-2) | 469 | 5.54 (3.14-9.76) | 1.48 (0.66-3.36) | 1.63 (0.79-3.36) | 2.92 (1.80-4.75) |
| Karnofsky score (<80% vs ≥80%) | 547 | 2.45 (1.43-4.22) | 2.76 (1.34-5.69) | 3.01 (1.53-5.92) | 5.47 (3.73-8.04) |
| Distant metastases (yes vs no) | 647 | 3.01 (1.92-4.72) | 2.56 (1.32-4.96) | 2.12 (1.16-3.86) | 5.82 (4.10-8.27) |
Number of patients with known value for corresponding variable.
Venous thromboembolism
Thirty-six patients in the total cohort were diagnosed with VTE during the observation period: 27 with acute PE, 6 with DVT of the lower extremity, and 3 with splanchnic thrombosis. Nearly half of the VTE events (14 PEs and 3 DVTs) were in TT patients, corresponding to a proportion of 19.8% (n = 17 of 86). Three of the 36 patients (8.3%) developed recurrent VTE (all non-TT patients), of which 2 stopped anticoagulation for the initial VTE due to high bleeding risk.
Two VTEs were fatal: both were PEs that occurred during thrombectomy (1 of a TT in the right atrium and 1 of an IVC TT below the diaphragm). Three VTEs occurred during therapeutic anticoagulation (1 using LMWH, 1 using VKA, and 1 using a direct oral anticoagulant), of which 2 patients received anticoagulation because of a TT. The 2-year cumulative incidence of VTE in the total cohort was 5.8% (95% CI: 4.1%-7.8%) (Table 3), whereas this was 22.4% (95% CI: 13.3%-32.9%) in the TT cohort.
Table 3.
Adverse Outcomes in the Total Cohort and in TT Patients
| Cumulative Incidence at 2 Years |
|||
|---|---|---|---|
| VTEa | Major Bleedinga | Mortality | |
| Overall population, % | 5.8 (4.1-7.9) | 7.7 (5.7-10.0) | 24.6 (21.0-28.4) |
| No TT, %b | 3.4 (2.1-5.3) | 6.6 (4.6-8.9) | 20.9 (17.3-24.7) |
| TT, %b | 22.4 (13.3-32.9) | 15.5 (8.1-25.2) | 51.3 (38.0-63.0) |
| HR (yes vs no)b | 7.16 (3.71-13.82) | 2.46 (1.25-4.87) | 2.78 (1.88-4.13) |
| No anticoagulation, % | 23.9 (13.7-35.7) | 11.9 (5.1-21.9) | 45.7 (31.5-58.8) |
| Anticoagulation, %c | 17.6 (1.5-48.5) | 32.5 (8.2-60.3) | 77.5 (30.2-94.7) |
| HR (yes vs no) | 0.56 (0.13-2.48) | 3.44 (0.95-12.42) | 2.13 (0.98-4.62) |
| No thrombectomy, % | 17.3 (6.6-32.1) | 18.6 (6.9-34.6) | 63.4 (41.3-79.1) |
| Thrombectomy, % | 26.6 (13.4-41.8) | 12.9 (4.6-25.7) | 42.0 (25.3-57.9) |
| HR (yes vs no) | 1.09 (0.41-2.88) | 0.53 (0.16-1.77) | 1.49 (0.25-0.98) |
| TT in renal vein, %d | 7.4 (1.2-21.7) | 12.8 (3.8-27.4) | 51.3 (30.5-68.7) |
| TT in IVC (below diaphragm), %d | 22.0 (9.4-38.1) | 23.2 (9.8-39.8) | 53.9 (33.1-70.8) |
| HR (IVC vs renal vein) | 2.27 (0.59-8.78) | 1.60 (0.47-5.46) | 1.93 (0.44-1.92) |
| TT above diaphragm, %d | 55.3 (8.8-86.6) | N/A | 37.5 (8.9-67.2) |
| HR (above diaphragm vs IVC) | 4.07 (1.40-11.89) | N/A | 0.90 (0.30-2.71) |
| HR (above diaphragm vs renal vein) | 9.24 (2.33-36.58) | N/A | 0.83 (0.27-2.53) |
Values are % (95% CI) or HR (95% CI).
MB = major bleeding; N/A = not available; TT = tumor thrombus; VTE = venous thromboembolism.
Cumulative incidences adjusted for competing risk of death.
Only TTs at RCC diagnosis included in this analysis.
TT patients who received therapeutic anticoagulation at TT diagnosis for any indication (eg, TT, VTE, atrial fibrillation).
Upper level of the tumor thrombus.
Distant metastasis and a low Karnofsky score (<80%) at RCC diagnosis were associated with incident VTE (Table 2). A TT at baseline or a new TT during follow-up was associated with more VTE events (adjusted HR: 6.61; 95% CI: 3.18-13.73) (Table 4, Figure 2, Supplemental Table 1). In the sensitivity analysis with only non-PE VTE events, the association with TT was not statistically significant (aHR: 1.51; 95% CI: 0.32-7.22). Furthermore, patients with TTs extending above the diaphragm were more likely to develop VTE than those with more limited TTs. Thrombectomy did not lead to a lower VTE incidence, whereas TT patients treated with anticoagulation tended to have lower VTE risk than those without (Table 3). Notably, TT patients using anticoagulation still frequently developed a VTE (2-year cumulative incidence: 17.6%; 95% CI: 1.5%-48.5%). It was not possible to assess whether treatment outcomes differed between the TT level, as the number of events was too low.
Table 4.
Prognostic Impact Of Presence Of Tumor Thrombi as Well as Thrombotic and Bleeding Events
| VTE HR (95% CI) |
MB HR (95% CI) |
Mortality HR (95% CI) |
||||
|---|---|---|---|---|---|---|
| Unadjusteda | Adjusteda | Unadjusteda | Adjusteda | Unadjusteda | Adjusteda | |
| TT (yes vs no)b | 7.77 (4.03-14.95) | 6.61 (3.18-13.73) | 2.38 (1.21-4.70) | 1.69 (0.82-3.50) | 3.18 (2.18-4.64) | 1.65 (1.12-2.45) |
| ATE (yes vs no)b | N/Ac | N/Ac | 4.59 (0.63-33.58) | 3.49 (0.46-26.57) | 3.49 (1.11-11.00) | 6.29 (1.90-20.84) |
| VTE (yes vs no)b | N/A | N/A | 6.08 (2.56-14.45) | 4.67 (1.91-11.41) | 4.61 (2.77-7.67) | 2.87 (1.70-4.84) |
| MB (yes vs no)b | 1.93 (0.46-8.07) | 1.64 (0.39-6.94) | N/A | N/A | 2.49 (1.38-4.52) | 1.96 (1.08-3.57) |
ATE = arterial thromboembolism; other abbreviations as in Table 3.
Unadjusted HR derived from univariable Cox regression analysis. Adjusted HR derived from multivariable Cox regression, with adjustment for age, sex, Fuhrman grade, Karnofsky score and distant metastases at diagnosis.
Time-dependent variable.
Insufficient ATE events to perform statistical analysis.
Figure 2.
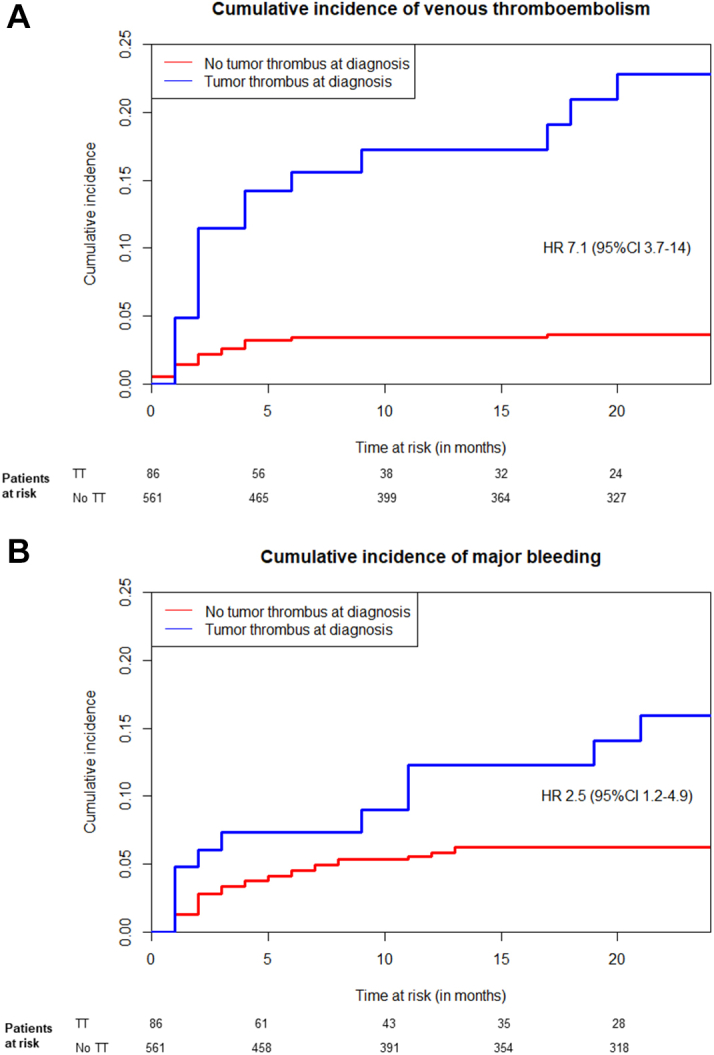
Cumulative Incidence of Venous Thromboembolism and Major Bleeding
Cumulative incidence graphs, accounting for the competing risk of death, of (A) venous thromboembolism and (B) major bleeding among patients with a tumor thrombus (TT) at renal cell carcinoma diagnosis (blue line) vs those without (red line). No adjustments for baseline characteristics or TT treatment were included in this graph, resulting in unadjusted HRs.
Major bleeding
A total of 45 patients developed MB during the observation period. The main bleeding location was (peri)renal, occurring in 22 patients (18 of 22 were postoperative bleedings), intracerebral (n = 8), and gastrointestinal bleeding (n = 5). Seven MBs were fatal, predominantly intracerebral (n = 4; 1 of these patients had cerebral metastases), of which 2 were receiving therapeutic anticoagulation. A total of 13 (28.9%) MBs were associated with anticoagulation therapy. In patients with a TT at baseline, 11 MBs occurred, of which 6 were associated with anticoagulation (2 for a TT, 2 for a concurrent TT and VTE, and 2 for atrial fibrillation). The adjusted 2-year cumulative incidence of MB in the total cohort was 7.7% (95% CI: 5.7%-10.0%) (Table 3), and 15.5% (95% CI: 8.1%-25.2%) in the TT cohort (Figure 2). TT patients that were treated with anticoagulation had a 32.5% 2-year cumulative incidence (95% CI: 8.2%-60.3%) of MB, vs 11.9% (95% CI: 5.1%-21.9%) in those without anticoagulation. Distant metastases and an impaired performance score at baseline were predictors of MB during follow-up (Table 2), whereas nephrectomy at RCC diagnosis was not (HR: 0.89; 95% CI: 0.44-1.40). A TT at diagnosis or during follow-up was not associated with MB (aHR: 1.69; 95% CI: 0.82-3.50) (Table 4, Supplemental Table 1), either. Incident VTE during follow-up, on the other hand, was strongly associated with risk of MB (aHR: 4.67; 95% CI: 1.91-11.41) (Table 4, Supplemental Table 1).
Arterial thromboembolism
In the total cohort, 11 patients developed an ATE during the observation period, all of which were ischemic strokes. Two ATEs were fatal. No patient with a TT developed an ATE. The 2-year adjusted cumulative incidence in the total cohort was 2.1% (95% CI: 1.2%-3.6%).
Mortality
Of the 132 patients that died during the observation period, the median time to death was 7.0 (IQR: 4.2-14.3) months. In the total cohort, predictors for mortality were non–clear cell RCC (HR: 0.68; 95% CI: 0.47-0.99), distant metastases at diagnosis (HR: 5.82; 95% CI: 4.10-8.27), a higher Fuhrman grade (HR: 2.92; 95% CI: 1.80-4.75), and a lower Karnofsky score (HR: 5.47; 95% CI: 3.73-8.04) (Table 2). TT (aHR: 1.65; 95% CI: 1.12-2.45), VTE (aHR: 2.87; 95% CI: 1.70-4.84), MB (aHR: 1.96; 95% CI: 1.08-3.57), and ATE (aHR: 6.29; 95% CI: 1.90-20.84) during the observation period were all independently associated with higher mortality as well (Table 4).
Discussion
We found a considerable 13.3% prevalence of TT in RCC patients (Central Illustration). In our cohort, only half of the TTs were resected, and anticoagulation was initiated in one-quarter. TTs were associated with a more than 6-fold higher incidence of VTE but not of ATE. A TT is thought to disrupt vascular integrity and disturb venous blood flow leading to a local procoagulant state, explaining this excess VTE risk.7 TT patients in our study treated with anticoagulation still frequently developed VTE, even though anticoagulant therapy prevented half of the events. It has to be noted that the group of TT patients either treated or not treated with anticoagulation differed per TT level and that the sample size was too small to accurately evaluate the impact of anticoagulation on thrombotic and bleeding events per TT level. Nevertheless, our findings suggest that TT patients have a hypercoagulable state in general, which could partly be related to the association of TT with more advanced cancer (ie, higher RCC grade and tumor stage).5
Central Illustration.
TTs in Renal Cell Carcinoma Are Relatively Common and Associated With Worse Outcomes
A venous tumor thrombus (TT) is relatively common in patients with a renal cell carcinoma (RCC), and is associated with poorer outcomes. Currently it is unknown which patient subgroups may benefit from anticoagulation therapy. aHR = adjusted HR; MB = major bleeding; VTE = venous thromboembolism.
Of note, since TT was mostly associated with PE and less with other VTE presentations, some of these VTE events may have been tumor embolism, rather than bland thromboembolism. Previous studies have suggested that the risk of tumor embolization in TT patients is limited (overall incidence 1.5%) but increases when the TT is located in the IVC or beyond, and is highest intraoperatively (up to 4%, with a mortality rate of 75%).7,8,10 This is in line with our findings, although none of the PEs were pathologically examined. Various studies on the best surgical technique for thrombectomy are available, primarily to accomplish complete resection (as incomplete resection results in a lower survival rate).28, 29, 30 The common perception is that anticoagulation does not mitigate the tumor embolization risk, yet TTs are regularly accompanied by less stable, bland thrombi (20%-40% of cases), in which situation anticoagulation can protect against regular thromboembolism.7,31,32 Hence, although antitumor therapy and surgery are the keystone of TT management, considering anticoagulation is reasonable to prevent further progression/embolization of the bland thrombus component.
We did not observe a strong correlation between TT and major bleeding but found a high incidence of bleeding in patients receiving anticoagulant treatment for TT. Of note, in the total cohort, more than 70% of all major bleeding complications were not associated with anticoagulant treatment, suggesting that the background bleeding incidence in patients with RCC is high, which should be taken into account when considering anticoagulant therapy. Although the literature on the bleeding risk associated with TT in RCC in general is scarce, it has been previously suggested that more proximal TTs are associated with a higher perioperative bleeding risk,33 which increases when a concomitant bland thrombus is present.31 We also observed a tendency toward more major bleeding in TT patients treated with anticoagulation compared with those without, emphasizing the difficult balance between the benefits and harms of anticoagulation in these patients.
Based on current guidelines, primary thromboprophylaxis might be considered in patients with cancer and a high risk of thrombosis. To identify such ambulant cancer patients, prediction scores have been developed, of which the Khorana score, designed for patients with solid tumors starting chemotherapy,34 is the most widely studied.35, 36, 37 Notably, as the standard systemic therapy for RCC includes targeted therapy or immunotherapy instead of chemotherapy, it is unknown whether the Khorana score is applicable in this setting. A cohort study in patients with metastatic RCC receiving immunotherapy showed that the Khorana score did not accurately predict thromboembolic events.38 Meta-analyses have shown that a Khorana score threshold of ≥2 points equals an 8.9% 6-month cumulative VTE incidence, which may be an argument to consider pharmacological thromboprophylaxis according to recent guideline updates.39 The observed VTE incidence in TT patients in our cohort (6-month cumulative incidence of 13.8%) and the observation that the cumulative incidence of VTE was numerically higher than of major bleeding in our TT patients, opposed to the reverse in non-TT patients (22.4% vs 15.5% and 3.4% vs 6.6%, respectively), underline the indication of anticoagulation at (at least) a prophylactic dose.
No definite answers regarding the optimal use of anticoagulation in TT patients can be provided based on our study results, and the optimal management of TT should be decided on a case-to-case basis depending on the patient’s individual thrombosis and bleeding assessment. It seems reasonable to guide anticoagulation therapy by TT level, given their associated VTE risks. Renal vein thrombi were hardly associated with incident VTE and may be left untreated, whereas extension into the vena cava and beyond requires at least the consideration of anticoagulation. We cannot comment on the preferred type of anticoagulation, as in our cohort predominantly LMWH was used, nor can we comment on the optimal dose. In an isolated TT (ie, without bland thrombus), prophylactic anticoagulation might be sufficient in preventing VTE events. Furthermore, the use of low-dose apixaban or rivaroxaban,40,41 or alternatively factor XI inhibitors (as they primarily inhibit contact pathway activation),42,43 might be promising options for VTE prevention in patients with TT. This should be the focus of future studies.
Study strengths
To our knowledge, this is the first cohort study focusing on the use of anticoagulation in adult RCC patients with a TT. We used a standardized and predefined radiographic definition of a TT in order to differentiate from a bland thrombus, which can be easily applied in daily practice.
Study limitations
There were a small number of cases per TT treatment and/or thrombus level, leading to wide CIs and resulting in the inability to analyze the treatment modalities per TT level. The study was performed in a university medical center, which primarily provided general care for RCC but also served as a regional referral center for certain treatment modalities (thermal ablation, laparoscopic partial nephrectomy, and thoracic surgery), which could have led to selection bias. Furthermore, a substantial proportion of the cohort was lost to follow-up (21.2%).
Conclusions
TT are common in RCC and are associated with poorer outcomes. Although we found no definitive evidence that anticoagulation therapy improves outcomes in TT patients, their increased VTE risk might justify its use in selected patients, for example based on TT level, associating bland thrombus or concomitant VTE risk factors. Future prospective studies are needed to identify patient subgroups that may benefit from anticoagulation therapy, including the appropriate drug class and dose.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: A venous TT is relatively common in patients with a RCC, and is associated with poorer outcomes. Clinicians should be particularly aware of the increased VTE risk, which might justify anticoagulation therapy in selected patients.
TRANSLATIONAL OUTLOOK: Currently, it is unknown which patient subgroups may benefit from anticoagulation therapy, which should be the focus of future prospective studies. Furthermore, the appropriate drug class and dose of anticoagulation need to be evaluated.
Funding Support and Author Disclosures
Dr Klok has received research grants from Bayer, Merck Sharpe & Dohme, the Netherlands Organisation for Health Research and Development, Actelion, the Dutch Heart Foundation, and the Dutch Thrombosis Association, all outside the submitted work. Dr Huisman has received unrestricted grant support from The Netherlands Organisation for Health Research and Development (ZonMW); and received unrestricted grant support and fees for presentations from Boehringer Ingelheim, Pfizer-BMS, Bayer Health Care, Aspen, and Daiichi Sankyo, all outside the submitted work. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental table, please see the online version of this paper.
Appendix
References
- 1.Chow W.-H., Devesa S.S., Warren J.L., Fraumeni J., Joseph F. Rising incidence of renal cell cancer in the United States. JAMA. 1999;281(17):1628–1631. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 2.Padala S.A., Barsouk A., Thandra K.C., et al. Epidemiology of renal cell carcinoma. World J Oncol. 2020;11(3):79–87. doi: 10.14740/wjon1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabbani F., Hakimian P., Reuter V.E., Simmons R., Russo P. Renal vein or inferior vena caval extension in patients with renal cortical tumors: impact of tumor histology. J Urol. 2004;171(3):1057–1061. doi: 10.1097/01.ju.0000112885.66352.e2. [DOI] [PubMed] [Google Scholar]
- 4.Ficarra V., Righetti R., D’Amico A., et al. Renal vein and vena cava involvement does not affect prognosis in patients with renal cell carcinoma. Oncology. 2001;61(1):10–15. doi: 10.1159/000055346. [DOI] [PubMed] [Google Scholar]
- 5.Zisman A., Wieder J.A., Pantuck A.J., et al. Renal cell carcinoma with tumor thrombus extension: biology, role of nephrectomy and response to immunotherapy. J Urol. 2003;169(3):909–916. doi: 10.1097/01.ju.0000045706.35470.1e. [DOI] [PubMed] [Google Scholar]
- 6.Ljungberg B., Albiges L., Bedke J., et al. EAU Guidelines on Renal Cell Carcinoma. https://uroweb.org/guidelines/renal-cell-carcinoma
- 7.Serena G., Gonzalez J., Gaynor J.J., Salerno T., Verzaro R., Ciancio G. Pulmonary tumor embolization as early manifestation in patients with renal cell carcinoma and tumor thrombus: perioperative management and outcomes. J Card Surg. 2019;34(10):1018–1023. doi: 10.1111/jocs.14182. [DOI] [PubMed] [Google Scholar]
- 8.Shuch B., Larochelle J.C., Onyia T., et al. Intraoperative thrombus embolization during nephrectomy and tumor thrombectomy: critical analysis of the University of California-Los Angeles Experience. J Urol. 2009;181(2):492–499. doi: 10.1016/j.juro.2008.10.036. [DOI] [PubMed] [Google Scholar]
- 9.Woodruff D.Y., Van Veldhuizen P., Muehlebach G., Johnson P., Williamson T., Holzbeierlein J.M. The perioperative management of an inferior vena caval tumor thrombus in patients with renal cell carcinoma. Urol Oncol. 2013;31(5):517–521. doi: 10.1016/j.urolonc.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Yokom D.W., Ihaddadene R., Moretto P., et al. Increased risk of preoperative venous thromboembolism in patients with renal cell carcinoma and tumor thrombus. J Thromb Haemost. 2014;12(2):169–171. doi: 10.1111/jth.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.González J., Andrés G., Martínez-Salamanca J.I., Ciancio G. Improving surgical outcomes in renal cell carcinoma involving the inferior vena cava. Expert Rev Anticancer Ther. 2013;13(12):1373–1387. doi: 10.1586/14737140.2013.858603. [DOI] [PubMed] [Google Scholar]
- 12.Bassiri A.G., Haghighi B., Doyle R.L., Berry G.J., Rizk N.W. Pulmonary tumor embolism. Am J Respir Crit Care Med. 1997;155(6):2089–2095. doi: 10.1164/ajrccm.155.6.9196119. [DOI] [PubMed] [Google Scholar]
- 13.Goldhaber S.Z., Dricker E., Buring J.E., et al. Clinical suspicion of autopsy-proven thrombotic and tumor pulmonary embolism in cancer patients. Am Heart J. 1987;114(6):1432–1435. doi: 10.1016/0002-8703(87)90548-5. [DOI] [PubMed] [Google Scholar]
- 14.Campbell S., Uzzo R.G., Allaf M.E., et al. Renal mass and localized renal cancer: AUA guideline. J Urol. 2017;198(3):520–529. doi: 10.1016/j.juro.2017.04.100. [DOI] [PubMed] [Google Scholar]
- 15.Landelijke Werkgroep Urologische Tumoren . Integraal Kankercentrum Nederland; 2010. Landelijke richtlijn Niercelcarcinoom versie 2.0. [Google Scholar]
- 16.Park H., Jeong C.W., Yuk H., et al. Influence of tumor thrombus on occurrence of distant venous thromboembolism and survival in patients with renal cell carcinoma after surgery. Clin Appl Thromb Hemost. 2019;25 doi: 10.1177/1076029618823288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ihaddadene R., Yokom D.W., Le Gal G., et al. The risk of venous thromboembolism in renal cell carcinoma patients with residual tumor thrombus. J Thromb Haemost. 2014;12(6):855–859. doi: 10.1111/jth.12580. [DOI] [PubMed] [Google Scholar]
- 18.Heng D.Y.C., Xie W., Regan M.M., et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 2013;14(2):141–148. doi: 10.1016/S1470-2045(12)70559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunnick N.R. Renal cell carcinoma: staging and surveillance. Abdom Radiol. 2016;41(6):1079–1085. doi: 10.1007/s00261-016-0692-0. [DOI] [PubMed] [Google Scholar]
- 20.Huisman M.V., Klok F.A. Diagnostic management of acute deep vein thrombosis and pulmonary embolism. J Thromb Haemost. 2013;11(3):412–422. doi: 10.1111/jth.12124. [DOI] [PubMed] [Google Scholar]
- 21.Huisman M.V., Klok F.A. Diagnostic management of clinically suspected acute pulmonary embolism. J Thromb Haemost. 2009;7(suppl 1):312–317. doi: 10.1111/j.1538-7836.2009.03386.x. [DOI] [PubMed] [Google Scholar]
- 22.Dronkers C.E., Klok F.A., Huisman M.V. Current and future perspectives in imaging of venous thromboembolism. J Thromb Haemost. 2016;14(9):1696–1710. doi: 10.1111/jth.13403. [DOI] [PubMed] [Google Scholar]
- 23.Kaatz S., Ahmad D., Spyropoulos A.C., Schulman S. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119–2126. doi: 10.1111/jth.13140. [DOI] [PubMed] [Google Scholar]
- 24.Verduijn M., Grootendorst D.C., Dekker F.W., Jager K.J., le Cessie S. The analysis of competing events like cause-specific mortality—beware of the Kaplan-Meier method. Nephrol Dial Transplant. 2010;26(1):56–61. doi: 10.1093/ndt/gfq661. [DOI] [PubMed] [Google Scholar]
- 25.Austin P.C., Putter H., Lee D.S., Steyerberg E.W. Estimation of the absolute risk of cardiovascular disease and other events: issues with the use of multiple Fine-Gray subdistribution hazard models. Circ Cardiovasc Qual Outcomes. 2022;15(2) doi: 10.1161/CIRCOUTCOMES.121.008368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szychowski J.M., Roth D.L., Clay O.J., Mittelman M.S. Patient death as a censoring event or competing risk event in models of nursing home placement. Stat Med. 2010;29(3):371–381. doi: 10.1002/sim.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Unal E., Balci S., Atceken Z., Akpinar E., Ariyurek O.M. Nonthrombotic pulmonary artery embolism: imaging findings and review of the literature. AJR Am J Roentgenol. 2016;208(3):505–516. doi: 10.2214/AJR.16.17326. [DOI] [PubMed] [Google Scholar]
- 28.Haddad A.Q., Wood C.G., Abel E.J., et al. Oncologic outcomes following surgical resection of renal cell carcinoma with inferior vena caval thrombus extending above the hepatic veins: a contemporary multicenter cohort. J Urol. 2014;192(4):1050–1056. doi: 10.1016/j.juro.2014.03.111. [DOI] [PubMed] [Google Scholar]
- 29.Noguchi K., Hori D., Nomura Y., Tanaka H. Renal cell carcinoma with tumor-thrombus extension into the right ventricle. Ann Vasc Dis. 2012;5(3):376–380. doi: 10.3400/avd.cr.11.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirkali Z., Van Poppel H. A critical analysis of surgery for kidney cancer with vena cava invasion. Eur Urol. 2007;52(3):658–662. doi: 10.1016/j.eururo.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Liu Z., Zhang L., Hong P., et al. The influence of venous tumor thrombus combined with bland thrombus on the surgical treatment and prognosis of renal cell carcinoma patients. Cancer Med. 2020;9(16):5860–5868. doi: 10.1002/cam4.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hutchinson R., Rew C., Chen G., et al. The adverse survival implications of bland thrombus in renal cell carcinoma with venous tumor thrombus. Urology. 2018;115:119–124. doi: 10.1016/j.urology.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 33.Suggs W.D., Smith R.B., Dodson T.F., Salam A.A., Graham S.D. Renal cell carcinoma with inferior vena caval involvement. J Vasc Surg. 1991;14(3):413–418. doi: 10.1067/mva.1991.29912. [DOI] [PubMed] [Google Scholar]
- 34.Khorana A.A., Kuderer N.M., Culakova E., Lyman G.H., Francis C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902–4907. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Key N.S., Khorana A.A., Kuderer N.M., et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2020;38(5):496–520. doi: 10.1200/JCO.19.01461. [DOI] [PubMed] [Google Scholar]
- 36.Wang T.-F., Zwicker J.I., Ay C., et al. The use of direct oral anticoagulants for primary thromboprophylaxis in ambulatory cancer patients: Guidance from the SSC of the ISTH. J Thromb Haemost. 2019;17(10):1772–1778. doi: 10.1111/jth.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farge D., Frere C., Connors J.M., et al. 2019 International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20(10):e566–e581. doi: 10.1016/S1470-2045(19)30336-5. [DOI] [PubMed] [Google Scholar]
- 38.Sheng I.Y., Gupta S., Reddy C.A., et al. Thromboembolism in patients with metastatic renal cell carcinoma treated with immunotherapy. Target Oncol. 2021;16(6):813–821. doi: 10.1007/s11523-021-00852-z. [DOI] [PubMed] [Google Scholar]
- 39.Mulder F.I., Candeloro M., Kamphuisen P.W., et al. The Khorana score for prediction of venous thromboembolism in cancer patients: a systematic review and meta-analysis. Haematologica. 2019;104(6):1277–1287. doi: 10.3324/haematol.2018.209114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Horani R.A. Factor XI(a) inhibitors for thrombosis: an updated patent review (2016-present) Expert Opin Ther Pat. 2020;30(1):39–55. doi: 10.1080/13543776.2020.1705783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen A.T., Hunt B.J. Is there a role for low-dose DOACs as prophylaxis? Hematology Am Soc Hematol Educ Program. 2019;2019(1):187–193. doi: 10.1182/hematology.2019000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gailani D., Bane C.E., Gruber A. Factor XI and contact activation as targets for antithrombotic therapy. J Thromb Haemost. 2015;13(8):1383–1395. doi: 10.1111/jth.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verhamme P., Yi B.A., Segers A., et al. Abelacimab for prevention of venous thromboembolism. N Engl J Med. 2021;385(7):609–617. doi: 10.1056/NEJMoa2105872. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.