Abstract
Background
Trametinib is a MEK1 (mitogen-activated extracellular signal-related kinase kinase 1) inhibitor used in the treatment of BRAF (rapid accelerated fibrosarcoma B-type)–mutated metastatic melanoma. Roughly 11% of patients develop cardiomyopathy following long-term trametinib exposure. Although described clinically, the molecular landscape of trametinib cardiotoxicity has not been characterized.
Objectives
The aim of this study was to test the hypothesis that trametinib promotes widespread transcriptomic and cellular changes consistent with oxidative stress and impairs cardiac function.
Methods
Mice were treated with trametinib (1 mg/kg/d). Echocardiography was performed pre- and post-treatment. Gross, histopathologic, and biochemical assessments were performed to probe for molecular and cellular changes. Human cardiac organoids were used as an in vitro measurement of cardiotoxicity and recovery.
Results
Long-term administration of trametinib was associated with significant reductions in survival and left ventricular ejection fraction. Histologic analyses of the heart revealed myocardial vacuolization and calcification in 28% of animals. Bulk RNA sequencing identified 435 differentially expressed genes and 116 differential signaling pathways following trametinib treatment. Upstream gene analysis predicted interleukin-6 as a regulator of 17 relevant differentially expressed genes, suggestive of PI3K/AKT and JAK/STAT activation, which was subsequently validated. Trametinib hearts displayed elevated markers of oxidative stress, myofibrillar degeneration, an 11-fold down-regulation of the apelin receptor, and connexin-43 mislocalization. To confirm the direct cardiotoxic effects of trametinib, human cardiac organoids were treated for 6 days, followed by a 6-day media-only recovery. Trametinib-treated organoids exhibited reductions in diameter and contractility, followed by partial recovery with removal of treatment.
Conclusions
These data describe pathologic changes observed in trametinib cardiotoxicity, supporting the exploration of drug holidays and alternative pharmacologic strategies for disease prevention.
Key Words: cardiomyopathy, cardiotoxicity, MEK1, trametinib
Abbreviations and Acronyms: APJ, apelin receptor; BRAF, rapid accelerated fibrosarcoma B-type; CX43, connexin-43; ECM, extracellular matrix; ERK1/2, extracellular signal-regulated protein kinase 1/2; hCO, human cardiac organoid; hERG, human ether-ago-go; IL, interleukin; MEK1, mitogen-activated extracellular signal-related kinase kinase 1
Central Ilustration
Cutaneous malignant melanoma is a form of skin cancer that develops from pigment-producing melanocytes. In the United States, melanoma is the fifth most common cancer, with nearly 200,000 adults diagnosed annually.1,2 The prognosis for patients with metastatic stage IV melanoma is poor, with a 5-year survival rate of roughly 30%.3 Approximately 60% of melanomas harbor activating mutations in BRAF (rapid accelerated fibrosarcoma B-type), a serine/threonine protein kinase, leading to constitutive activation of the mitogen activation protein kinase pathway.4 Roughly 90% of BRAF mutations involve the substitution of glutamic acid for valine at amino acid 600. As such, the BRAF inhibitors dabrafenib, vemurafenib, and encorafenib are routinely used to treat patients with unresectable stage III or stage IV BRAF-mutated metastatic melanoma.5 The use of vemurafenib as monotherapy in metastatic melanoma was associated with a relative reduction of 63% in the risk for death and of 74% in the risk for either death or disease progression compared to dacarbazine.6 Although promising, BRAF inhibition quickly leads to compensatory activation of MEK1 (mitogen-activated extracellular signal-related kinase kinase 1), promoting drug resistance.5 As such, the MEK1 inhibitors trametinib, cobimetinib, and binimetinib have been approved for concomitant use in patients with advanced disease.
Concurrent BRAF/MEK1 inhibition has led to dramatic improvements in response rate, progression-free survival, and overall survival in comparison with historical treatments.7 Clinical data suggest the use of MEK1 inhibitors may be limited by cardiovascular toxicities, such as systemic hypertension, cardiomyopathy, venous thromboembolism, atrial fibrillation, and QT interval prolongation.5,8 Of note, roughly 11% of patients treated with trametinib develop cardiomyopathy, defined as a reduction in ejection fraction >10% as determined by echocardiography.9 Evidence suggests that MEK1/ERK1/2 (extracellular signal-regulated protein kinase 1/2) signaling is critical for cardiomyocyte homeostasis and cardiac stress responses; however, the cellular and molecular changes that occur during MEK1 inhibitor–induced cardiotoxicity have not yet been characterized.8,9 Understanding the mechanisms of MEK1 inhibitor cardiotoxicity may prove beneficial in implementing early screening tools and/or adjuvant therapies for prevention.5 In this study, we used in vitro and in vivo tools to determine the histopathologic changes associated with trametinib cardiotoxicity, as well as the underlying mechanisms involved.
Methods
Details on animal methods, as well as histology, cell culture, analytical techniques, and statistics are available in the Supplemental Appendix. All experiments on mice were approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina. A total of 40 mice (1-5 per cage) were kept in a 12-hour light/dark cycle with food and water ad libitum. Control mice received an open-source diet (D11112201) produced by Research Diets. Starting at 2 months of age, experimental mice received the same base diet supplemented with trametinib (10 mg/kg of food). On average, 20-g animals consumed 3 g of food per day, amounting to 1 mg/kg/d of trametinib. No differences in food consumption were noted between groups. All data are presented as mean ± SD. Statistical analysis was performed using Prism 9 (GraphPad Prism Software). For normally distributed data, unpaired data were compared using Student’s t-test or 2-way analysis of variance (ANOVA) with the Bonferroni post hoc test for multiple pairwise comparisons. For nonparametric data, the Mann-Whitney U test was used. Survival was compared using the log-rank test. Values are P < 0.05 were considered to indicate statistical significance.
Results
Health evaluation of mice
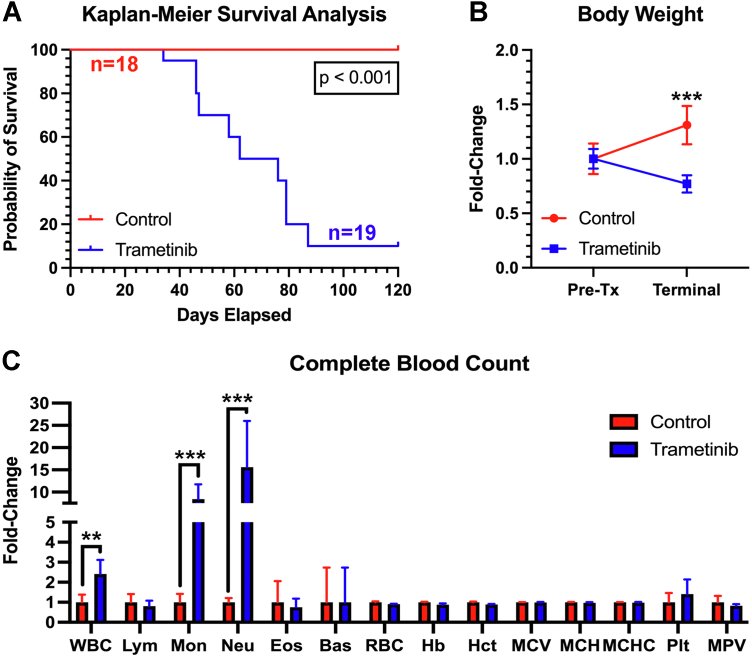
Body weights and detailed clinical observations were made thrice weekly. Endpoint criteria included noticeable signs of toxicity or 20% body weight reduction. The survival rate of trametinib-treated mice was markedly lower compared with vehicle (Figure 1A). Upon euthanasia, 88% of mice (16 of 18) exhibited reductions in body weight, consistent with clinical findings (Figure 1B).10 Terminal complete blood counts were assessed for signs of immunotoxicity. Trametinib-treated animals exhibited leukocytosis, with marked neutrophilia and monocytosis, with no detectable changes in lymphocyte count (Figure 1C). These results are consistent with clinical reports of patients chronically administered trametinib.11,12
Figure 1.
Trametinib Treatment Promotes Changes in Survival and Routine Health Measurements
(A) Comparison of survival curves demonstrated a statistically significant reduction in survival (69.4 days) in trametinib-treated animals (n = 15-20/group). (B) Trametinib-treated animals experienced a significant weight reduction following long-term treatment. Data were analyzed using a 2-way analysis of variance with a Bonferroni test (n = 10/group). (C) Complete blood counts were procured and normalized to vehicle control (n = 5/group). Data were analyzed using unpaired Student’s t-tests. Data are represented as mean fold change. Bas = basophils; Eos = eosinophils; Hb = hemoglobin; Hct = hematocrit; Lym = lymphocytes; MCH = mean corpuscular hemoglobin; MCHC = mean corpuscular hemoglobin concentration; MCV = mean corpuscular volume; Mon = monocytes; MPV = mean platelet volume; Neu = neutrophils; Plt = platelets; RBC = red blood cells; Tx = treatment; WBC = white blood cells.
Echocardiography assessment of left ventricular function
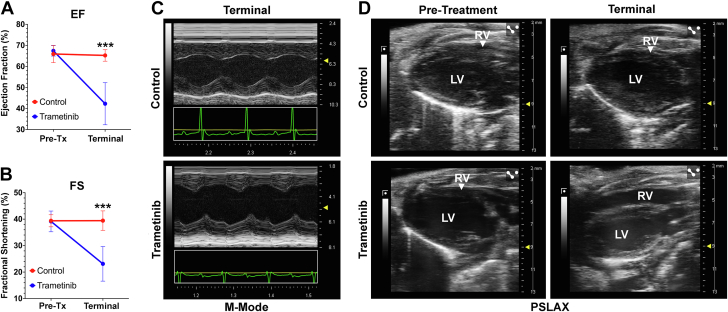
A comprehensive assessment of the left ventricle by echocardiography was made prior to treatment at 2 months of age and upon terminal health assessment. Trametinib-treated mice exhibited significant reductions in ejection fraction and fractional shortening (Figures 2A and 2B). Of the mice treated with trametinib, 57% (4 of 7) exhibited wall motion abnormalities of the left ventricle (Figure 2C). Additionally, 28% (2 of 7) exhibited right ventricular dilation on terminal assessment (Figure 2D).
Figure 2.
Echocardiographic Assessment of Left Ventricular Function
Left ventricular function was assessed on echocardiography at 2 months (pretreatment [Tx]) and upon terminal health assessment (n = 7/group). Comparison of ejection fraction (EF) (A) and fractional shortening (FS) (B). (C) Representative M-mode images demonstrating wall motion abnormalities exclusively seen in trametinib-treated animals on terminal assessment. (D) Representative images demonstrating normal parasternal long-axis images on pretreatment assessment, with right ventricular enlargement on terminal assessment in a fraction of trametinib-treated animals. Data were analyzed using 2-way analysis of variance with a Bonferroni test. LV = left ventricle; RV = right ventricle.
Histologic analysis
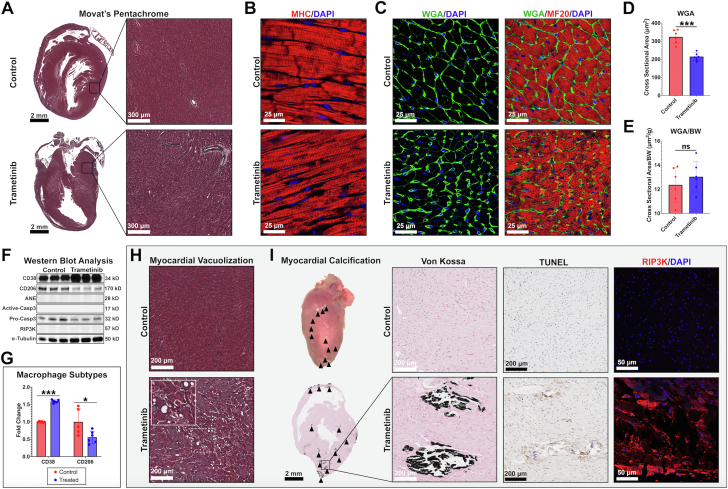
Necroscopic and histologic analyses were performed on control and trametinib-treated animals. Grossly, hearts from trametinib-treated animals appeared normal except for white lesions observed in 23% of samples (3 of 13). No significant histopathologic changes were observed in most hearts (Figure 3A). Grossly, no changes in collagen content were observed between groups. Immunohistochemical analysis examining cardiac myosin heavy chain confirmed preserved sarcomere structure (Figure 3B), with evidence of myofibrillar breakdown (Supplemental Figure 1). As other cancer therapy cardiomyopathies (eg, doxorubicin) can be associated with cardiac atrophy,13 we stained hearts with wheat germ agglutinin, a lectin that binds to glycoproteins of the cell membrane, allowing quantification of cardiomyocyte cross-sectional area (Figure 3C). Trametinib mice exhibited an overall reduction in cardiomyocyte cross-sectional area (Figure 3D). The reduction in cardiomyocyte size was proportional to the observed body weight reduction (Figure 3E). These data are consistent with doxorubicin-induced cardiotoxicity, in which cardiomyocytes size decreases occur concomitantly with weight loss. Next, we probed whole-heart tissue lysate for markers of cell death and inflammation using western blot analysis (Figure 3F). No changes in active caspase-3, a marker of apoptosis, or RIP3K, a marker of necrosis, were observed in a majority of hearts. Significant changes were observed in CD38, an M1 proinflammatory macrophage marker, and CD206, an M2 reparative macrophage marker, in the absence of changes in neutrophil markers (Figures 3F and 3G).14 Specifically, CD38 was elevated and CD206 was reduced, suggestive of M1 macrophage polarization.
Figure 3.
Histopathologic Analysis of Trametinib-Treated Hearts
(A) Movat’s pentachrome staining of trametinib-treated hearts showed no differences between groups. (B) Cardiac myosin heavy chain (MHC) staining confirmed normal sarcomeric architecture between groups. (C) Wheat germ agglutinin (WGA) and myosin heavy chain (MF20) staining of cardiac tissue demonstrated (D) a reduction in cardiomyocyte cross-sectional area in the trametinib group prior to normalization (E), with no differences observed when normalized to body weight (BW). (F) Western blot analysis examining markers for immune cells and cell death. No changes in markers of cell death or antineutrophil elastase (ANE) were observed. (G) CD38 was elevated, whereas CD206 was reduced. Data were compared using unpaired Student’s t-tests. (H) Myocardial vacuolization and (I) calcification were seen in a small subset of trametinib samples. Von Kossa staining confirmed calcification. Calcific regions stained positive for RIP3K in the absence of TUNEL positivity. N = 5-6/group.
In a subset of hearts (23% [3 of 13]), we observed myocardial vacuolization (Figure 3H), a reversible morphologic manifestation of cardiotoxicity.15 Additionally, 23% of hearts (3 of 13) demonstrated intramyocardial calcification (Figure 3I). These lesions exhibited an increase in RIP3K staining in the absence of TUNEL positivity, suggestive of a necrotic process. In examining tissues from all other major organ systems, no noticeable gross or histologic changes were appreciated (Supplemental Figure 2). These data, in the presence of heart failure, suggest cardiomyopathy may be responsible for the observed symptoms of terminal illness, such as poor body score, lethargy, and hunched posture.
Transcriptomic analysis of trametinib-induced cardiotoxicity
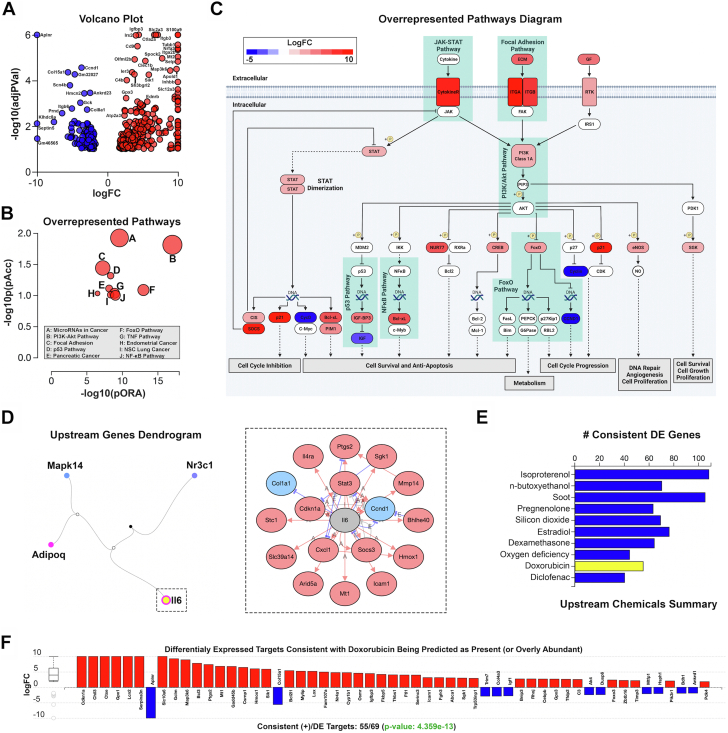
To further examine the cellular and molecular changes that occur in the setting of trametinib-induced cardiotoxicity, bulk RNA sequencing was performed on murine cardiac tissue. In this experiment, 435 differentially expressed genes were identified among a total of 57,719 genes in Advaita Knowledge Base (Figure 4A). One hundred sixteen were pathways found to be significantly affected. Of note, PI3K/AKT and JAK/STAT signaling were among the top over-represented pathways discovered (Figures 4B and 4C). Downstream genes of these pathways are associated with the regulation of cell survival, cell cycle progression, DNA repair, cell growth, and metabolism (Figure 4C). Following Bonferroni correction, 4 upstream regulators predicted as activated were discovered (Figure 4D). Of the 20 genes known to be regulated by interleukin (IL)-6, 17 genes were differentially expressed in our dataset, consistent with IL-6 activation of the PI3K/AKT and JAK/STAT pathways (P = 6.70 × 10−4). Following Bonferroni correction, several changes in biological function (n = 297), molecular function (n = 19), and cellular components (n = 29) were observed (Supplemental Figures 3A to 3C). An upstream chemicals analysis was performed to identify chemicals with related transcriptomic changes (Figure 4E). Of note, doxorubicin was identified as an upstream chemical, with 80% of changes in downstream differentially expressed genes consistent with findings from our dataset (P = 4.36 × 10−13) (Figure 4F). These data suggest a strong relationship between doxorubicin- and trametinib-induced cardiotoxicity.
Figure 4.
Transcriptomic Analysis of Hearts Following Long-Term Trametinib Exposure
(A) Volcano plot: 435 significantly differentially expressed (DE) genes represented by expression change (x-axis) and the significance of the change (y-axis). Up-regulated genes are shown in red and down-regulated genes in blue. (B) Top 10 pathways plotted by over-representation on the x-axis (pORA) and total pathway accumulation on the y-axis (pAcc). Pathways are represented by a single dot, with dot size corresponding to the size of the pathway it represents. (C) Diagram depicting major over-represented pathways overlayed with the computed perturbation of each gene. (D) Top upstream regulators predicted as activated. Of note, interleukin-6 was predicted to be present. (E) Of note, doxorubicin was identified as a related upstream chemical, with 80% of changes in downstream DE genes being consistent with findings from our dataset (P = 4.356 × 10−13). N = 3/group. FC = fold change; NSC = non-small-cell.
Elevated IL-6 activates pathways associated with mitochondrial biogenesis, mitophagy, and oxidative stress
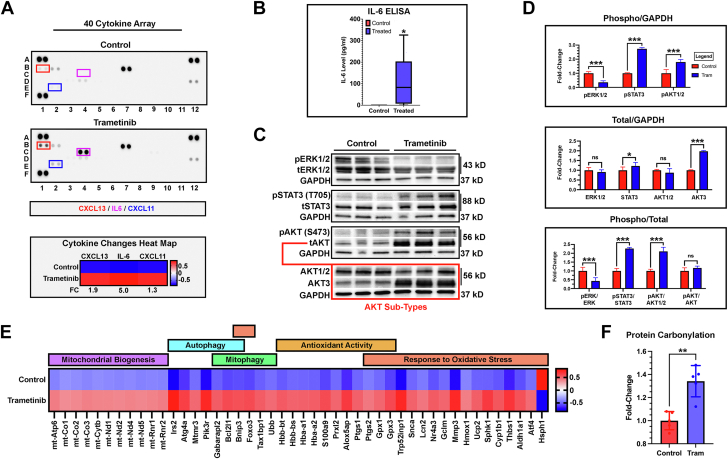
Differentially expressed genes from our RNA sequencing experiment suggest proinflammatory cytokine activation of PI3K/AKT and JAK/STAT signaling pathways. To validate this prediction, we performed a 40-cytokine array on plasma procured from control and trametinib-treated animals. Results confirmed elevated IL-6, as well increases in CXCL11 and CXCL13 (Figure 5A). An enzyme-linked immunosorbent assay further confirmed statistically elevated plasma IL-6 levels (110.6 ± 127.4 pg/mL) (Figure 5B). There was considerable variability in plasma IL-6 levels, ranging from 0.0 to 326 pg/mL. IL-6 transcripts were not statistically increased in our dataset, suggesting extracardiac production of the cytokine. Next, we probed cardiac tissue lysate for the presence of activated ERK1/2, STAT3, and AKT (Figure 5C). Trametinib significantly reduced cardiac ERK1/2 activation, as indicated by a reduction in ERK1/2 phosphorylation (Figure 5D). Additionally, trametinib-treated animals exhibited increased activation of STAT3 and AKT, as previously predicted. Interestingly, long-term trametinib exposure led to significant elevations in total STAT3 and AKT3 protein levels.
Figure 5.
Elevated IL-6 Activates Pathways Associated With Oxidative Stress
(A) Forty-cytokine panel demonstrating elevations in interleukin (IL)-6, CXCL11, and CXCL13 (n = 2/group). (B) IL-6 enzyme-linked immunosorbent assay confirming statistically elevated plasma levels. Data were compared using a Mann-Whitney U test (n = 6/group). (C) Cardiac tissue western blots examining phosphorylated ERK1/2 (pERK1/2), total ERK1/2 (tERK1/2), phosphorylated STAT3 (pSTAT3), total STAT3 (tSTAT3), phosphorylated AKT (pAKT), total AKT (tAKT), AKT3, and GAPDH. (D) pERK1/2 was reduced in trametinib-treated animals. pSTAT3 and pAKT1/2 were elevated. Total protein changes were detected in STAT3 and AKT3. No changes in tERK1/2 and AKT1/2 protein were observed. Significant differences were observed in pERK1/2:tERK1/2, pSTAT3:tSTAT3, and pAKT:tAKT ratios. No difference was observed in pAKT:tAKT (n = 6/group). (E) Changes in mitochondrial genes were detected. Data are represented as z-scores in a heat map (n = 3/group). (F) Oxyblot analysis demonstrating increased protein carbonylation, a marker for oxidative stress, in trametinib heart lysate (n = 6/group).
Given the observed statistically significant elevations in AKT3 total protein and IL-6, we probed our Advaita dataset for mitochondrial Gene Ontology annotations. AKT3 is a regulator of mitochondrial biogenesis, whereas IL-6 activates autophagy and mitophagy.16,17 Significant elevations in genes associated with mitochondrial biogenesis (11 genes), autophagy (7 genes), and mitophagy (6 genes) were observed (Figure 5E). In addition to PI3K/AKT activation, previous studies have shown that mitogen activation protein kinase inhibition promotes disturbances in mitochondrial bioenergetics, leading to increased oxidative phosphorylation, mitochondrial respiration, and oxidative stress.18, 19, 20 We observed significant changes in the expression of antioxidant genes (11 genes) and genes associated with response to oxidative stress (19 genes). Next, we examined cardiac tissue lysate for the presence of protein carbonylation, a well-established marker of oxidative stress. Trametinib tissue lysate demonstrated elevated levels of protein carbonylation, suggestive of pathologic oxidative tissue modification (Figure 5F).
Transcriptomic changes associated with disturbances in cardiac function
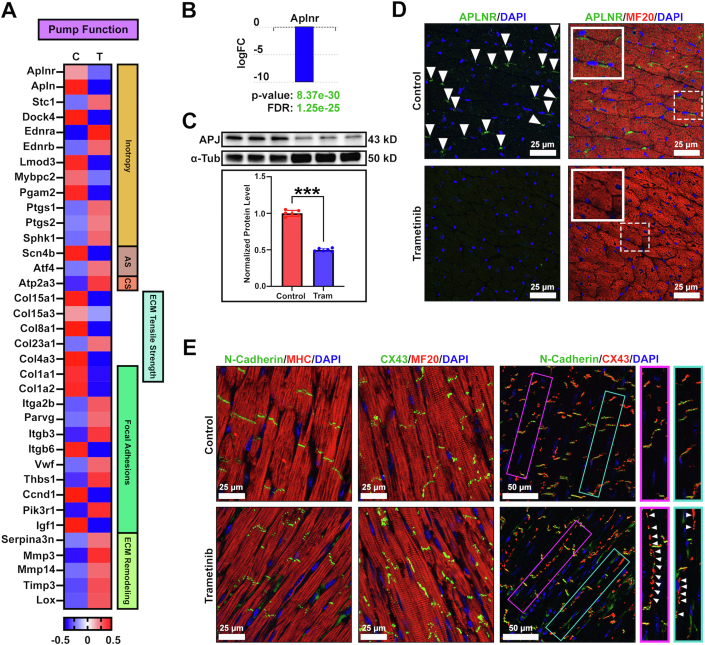
Transcriptomic changes associated with cardiac contraction and function were assessed. Twelve differentially expressed genes were identified that directly affect cardiac inotropy, as well as 2 genes associated with adrenergic signaling and 1 gene associated with calcium signaling (Figure 6A). Several transcriptional changes in extracellular matrix (ECM) genes were observed. Of note, changes in tensile strength (6 genes), focal adhesions (11 genes), and ECM remodeling (5 genes) were discovered. Interestingly, transcription of fibrillar collagen genes were reduced, a finding that is atypical for drug-induced cardiomyopathy, in which increased collagen production and interstitial fibrosis are commonly observed. These changes, taken together, may explain the reduction in systolic function observed with long-term trametinib exposure.
Figure 6.
Molecular and Cellular Changes Affecting Cardiac Function
(A) Transcriptomic changes relating to cardiac function (n = 3/group). Changes in genes related to inotropic action (n = 12), adrenergic signaling (AS) (n = 2) calcium signaling (n = 1), extracellular matrix (ECM) tensile strength (n = 6), focal adhesions (n = 11), and ECM remodeling (n = 5) were discovered. Data are represented as z-scores in a heat map. (B) The apelin receptor (Aplnr/APJ) was our top hit from RNA sequencing. A log fold change of −11.31 in APJ expression was observed in trametinib-treated hearts. (C) Western blot analysis showing a 2-fold reduction in APJ protein levels (n = 6/group). Data were compared using an unpaired Student’s t-test. (D) Immunohistochemical (IHC) analysis demonstrating a loss of APJ at the surface of cardiomyocytes (n = 4/group). (E) IHC stains examining N-cadherin and connexin-43 (CX43). N-cadherins remain present at the intercalated discs between cardiomyocytes. CX43 mislocalizes to the lateral surface of cardiomyocytes (n = 5/group). α-Tub = α-tubulin; FC = fold change; FDR = false discovery rate; MF20 = myosin heavy chain; MHC = myosin heavy chain.
Next, we followed up on our top hit from RNA sequencing: the apelin receptor (APJ) (P = 8.37 × 10−30). The APJ is a G protein–coupled receptor expressed within the myocardium and vascular endothelium, known for regulating cardiac myocyte contraction, cardioprotection, and vascular homeostasis.13,21 Down-regulation of the apelin/apelin receptor system has been extensively characterized in doxorubicin-induced cardiotoxicity.13,20,21 Furthermore, APJ knockout mice demonstrate accelerated myocardial damage, protein carbonylation, and mitochondrial dysfunction with doxorubicin administration.20 Consistent with doxorubicin-induced cardiomyopathy, a log fold change of −11.31 in APJ expression was observed in trametinib-treated hearts (Figure 6B). Additionally, a 2-fold reduction in APJ protein levels was observed (Figure 6C), with a loss of receptor presence on the surface of cardiomyocytes (Figure 6D). Next, we examined the major cardiac gap junction proteins N-cadherin and connexin-43 (CX43) to probe for deficits in cell-to-cell communication. Trametinib-treated hearts demonstrated normal N-cadherin staining within the intercellular space of the myocardial cells (Figure 6E). In addition, hearts from trametinib-treated animals exhibited extensive CX43 mislocalization to the lateral surface of cardiomyocytes. A reduction in N-cadherin/CX43 colocalization was appreciated, potentially explaining the wall motion abnormalities present on functional assessment.
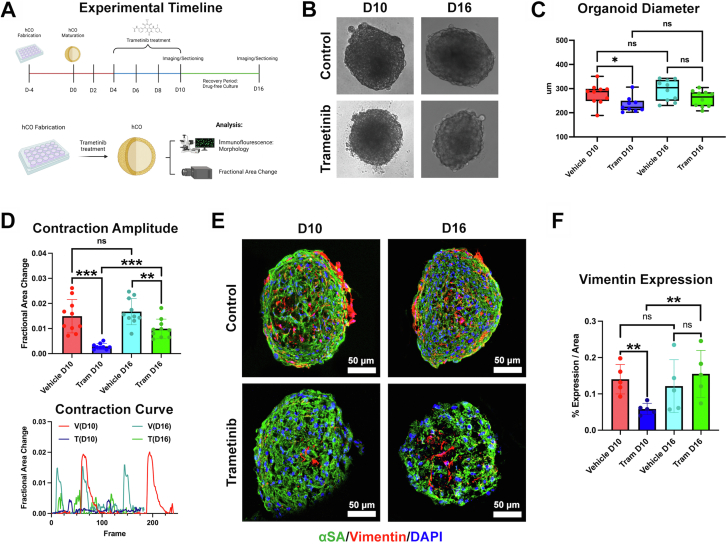
Trametinib alters human cardiac organoid morphology and reduces contraction amplitude
Human cardiac organoids (hCOs) have been used for modeling common cardiac pathologies and drug-induced toxicities.22,23 To assess the direct cardiotoxic potential of trametinib, hCOs were treated with trametinib for 6 days (Figure 7A). A dose of 40 nM was selected as the treatment dose to mirror plasma levels found in patients actively undergoing trametinib treatment.24,25 After 6 days of treatment, organoid diameter was significantly reduced compared with vehicle controls (Figures 7B and 7C). To investigate if trametinib altered hCO function, we examined fractional area change (contraction amplitude) after trametinib treatment. Trametinib significantly reduced fractional area change in the hCOs (Figure 7D). Given the gross reduction in diameter and function, we questioned whether hCO morphology was altered. hCOs treated with 40 nM trametinib had significantly reduced vimentin expression per area compared with vehicle controls, indicating a reduction in fibroblast content (Figures 7E and 7F).
Figure 7.
Trametinib-Treated Human Cardiac Organoids
Trametinib promotes partially reversible changes in human cardiac organoid (hCO) morphology and reduces contraction amplitude and fibroblast content. (A) Timeline summarizing hCO fabrication (day 4 [D4] to D0), maturation (D0-D4), drug treatment (D4-D10), and hCO recovery (D10-D16). (B) Representative images of hCO at D10 and D16. (C) Trametinib promotes a reduction in hCO diameter at D10, with partial size recovery observed at D16. (D) Trametinib treatment reduces contraction amplitude at D10, with partial recovery by D16. (E) Trametinib-treated hCO demonstrated a reduction in fibroblast content, with recovery of fibroblast content by D16. All statistical analysis was performed using unpaired Student’s t-tests. N = 10/group. A was made using BioRender.
Trametinib-induced cardiotoxicity is partially reversible in hCOs
Given the cardiotoxicity profile of trametinib in patients, there is a strong interest in understanding if the toxicity is reversible. To this end, hCOs were treated with trametinib for 6 days and then allowed to recover in drug-free media for 6 days. Upon 6 days of recovery, hCO diameter was still reduced compared with vehicle controls (P = 0.060) (Figures 7B and 7C). Although fractional area change was still significantly reduced compared with vehicle controls on day 16, the initial functional effects of trametinib treatment on hCOs had significantly improved compared with fractional area change after 6 days of trametinib treatment (Figure 7D). To determine if hCO morphology changed upon recovery, we examined the α-sarcomeric actinin and vimentin content on day 16. Vimentin expression per area was no longer significant compared with vehicle controls, indicating a rebound in the fibroblast content of the hCOs (Figures 7E and 7F).
Discussion
More than 1 million Americans are currently living with melanoma.2 For patients with BRAF-mutated metastatic melanoma, MEK1 inhibitors have dramatically improved patient survival and outcomes. Despite impressive efficacy, the long-term use of MEK1 inhibitors has been limited by cardiovascular toxicity. The exact mechanisms of trametinib-induced cardiotoxicity remain elusive, presenting challenges in predicting or preventing adverse events in patients.
In this study, several cellular and molecular changes that occur in the setting of trametinib-induced cardiotoxicity were unveiled (Central Illustration). Findings from this study suggest that trametinib promotes immunotoxicity, leading to expansion of the proinflammatory CD38 monocyte population. These cells are likely in part responsible for the observed elevations in IL-6, a potent activator of the PI3K/AKT and JAK/STAT signaling pathways. Studies have characterized IL-6 as a pleiotropic cytokine, providing a cardioprotective benefit in the short term while contributing to pathologic remodeling in the long-term.26 Activation of PI3K/AKT and JAK/STAT signaling promotes hypertrophy, cell survival, and DNA damage repair. Our data in hCOs suggest that trametinib treatment promotes cardiomyocyte atrophy, a finding that is consistent with our in vivo data. Additionally, a reduction in hCO fibroblast content was observed following long-term trametinib exposure. IL-6, as well as other circulating factors, may prevent fibroblast cell death in vivo, explaining the absence of these observations in our animal model (Supplemental Figure S4). Clinical trials are ongoing examining the efficacy of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in the treatment of trametinib-induced pyrexia.27 Results from the trial may help further characterize IL-6 as either a cardioprotective or pathologic effector in the setting of trametinib-induced cardiotoxicity. Patients with trametinib-induced pyrexia had statistically elevated IL-6 levels.25 Retrospective studies examining the incidence of cardiomyopathy in the presence of pyrexia may also help illustrate the role of IL-6 in trametinib-induced heart failure. In the event IL-6 is a driver of trametinib-induced cardiac remodeling and oxidative stress, tocilizumab may emerge as an effective strategy in preventing heart failure and drug resistance.
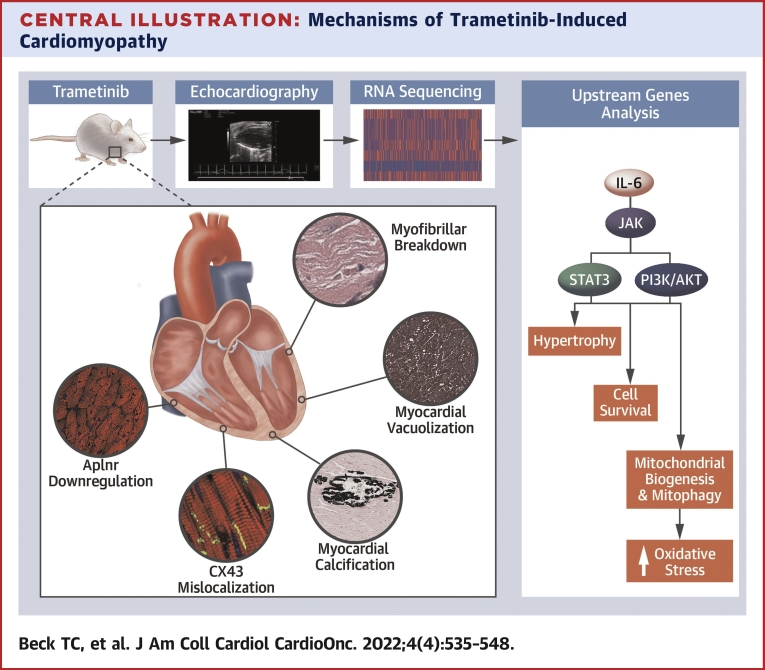
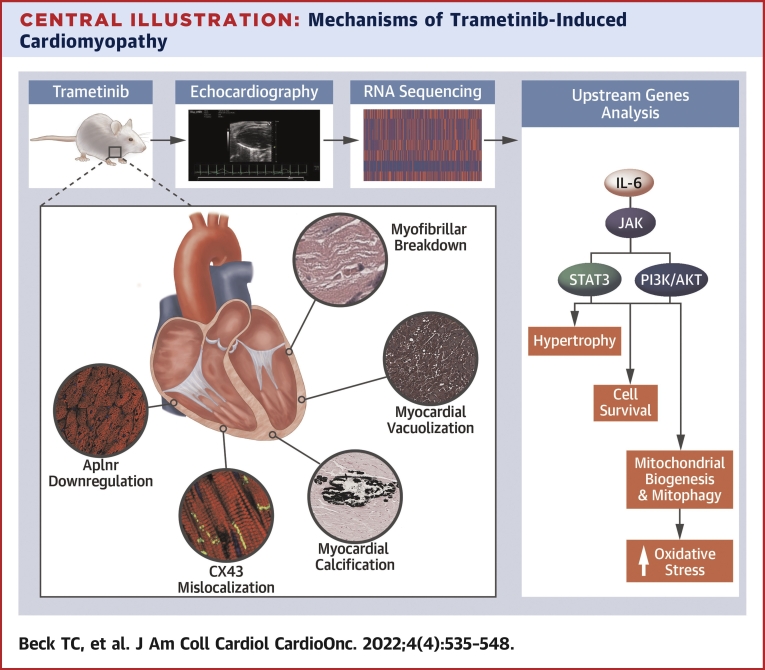
Central Illustration.
Mechanisms of Trametinib-Induced Cardiomyopathy
Trametinib-treated animals experienced a decline in cardiac function, as determined by echocardiography. RNA sequencing was performed to help further characterize the cellular and molecular changes associated with trametinib cardiotoxicity. Transcriptomic and iPathway analysis identified interleukin-6 as an activator of PI3K/AKT and JAK/STAT signaling pathways, with downstream changes in genes associated with hypertrophy, cell survival, mitochondrial biogenesis, mitophagy, and oxidative stress. Key histologic changes included a loss of cardiomyocyte Aplnr expression, connexin-43 (CX43) mislocalization, myofibrillar breakdown, and, in 23% of cases, myocardial calcification and vacuolization.
In addition to immunotoxicity, trametinib-treated animals demonstrated transcriptomic and protein-level changes consistent with increased mitochondrial turnover and oxidative phosphorylation, as well as reduced cardiac function. Transcriptional changes in genes associated with inotropy, adrenergic signaling, calcium signaling, ECM tensile strength, focal adhesions, and ECM remodeling were observed. Taken together, these data represent complex changes in signaling, ECM environment, and overall cardiac structure, explaining the decline in cardiac function following long-term MEK1 inhibition. Of note, CX43 mislocalization was observed in the hearts of trametinib-treated animals. CX43 is the primary gap junction protein in the myocardium and exhibits increased mislocalization to the lateral surface of cardiomyocytes in the setting of various cardiac pathologies.28, 29, 30 Specifically, CX43 lateralization is associated with atrial fibrillation, conduction abnormalities, and reduced ejection fraction.30,31 These data may explain the high incidence of arrythmias observed in trametinib-treated patients. In addition to CX43 mislocalization, trametinib and cobimetinib inhibit KCNH2 (Kv11.1) or the human ether-ago-go (hERG) channel.32,33 hERG conducts potassium out of the cardiac cell during repolarization. Inhibition of hERG can cause QT interval prolongation and is associated with an increased risk for arrhythmias.8 Thus, a need exists for novel MEK1 inhibitors devoid of hERG interactions.
Following withdrawal of trametinib, hCO function and morphology began to trend toward baseline. These data suggest trametinib-induced cardiotoxicity may be reversible, consistent with clinical reports suggesting most patients achieve recovery in left ventricular function.34 Studies examining the use of drug holidays (brief treatment breaks) and the incidence of adverse events, as well as the impact on treatment efficacy, are warranted. Drug holidays may help abrogate cardiac remodeling and drug resistance if implemented properly. In addition to assessing the use of tocilizumab and drug holidays, antioxidant drugs, such as dexrazoxane, may warrant clinical investigation to assess for efficacy in the prevention of trametinib-induced cardiotoxicity.
Last, 10% to 15% of trametinib-treated patients develop hypertension.35 Perturbance of 2 key pathways that signal through MEK/ERK has been hypothesized to play a role trametinib-induced hypertension: vascular endothelial growth factor/nitric oxide and renin-angiotensin-aldosterone. Moreover, it has been suggested that nitric oxide production may be impaired by blocking MEK1. Our transcriptomic data suggest, by activation of AKT, that nitric oxide production may be compensated for via downstream up-regulation of endothelial nitric oxide synthase (Figure 4C, Supplemental Figures 5A and 5B). Future studies examining nitric oxide levels may yield beneficial data. IL-6, in the setting of systemic inflammation, has been shown to regulate angiotensin II–mediated hypertension.36 Interestingly, transcription of angiotensin-converting enzyme 2, a known regulator of blood pressure homeostasis by inhibiting the renin-angiotensin-aldosterone system, was statistically elevated (log fold change = 5.95) (Supplemental Figure 5C). In the context of statistically elevated plasma IL-6, future studies should examine angiotensin-II levels in patients following long-term trametinib exposure.
Study limitations
Trametinib-treated animals were not tumor bearing. Cancers, such as melanoma, promote changes in immunologic function and thus may reduce or amplify the toxicologic effects observed. Additionally, post-treatment echocardiography was performed on the onset of toxicities or at 120 days of treatment, whichever came first. For animals that underwent echocardiography before 120 days of treatment, an age-matched wild-type control was used in comparison. Serial echocardiographic and transcriptomic analyses were not performed. These data may have provided insight on exactly when left ventricular function was in a state of decline to inform future preclinical studies of rescue or dose adjustment.
One limitation of the organoid model is that on the basis of the present model, not every aspect of trametinib-induced heart failure can be mimicked with the organoids. Furthermore, the organoids do not contain inflammatory cell and use immature human induced pluripotent stem cell cardiomyocytes. The goal of using hCOs was to assess the direct cardiotoxic potential of trametinib in the absence of systemic exposure. Our data suggest that hCOs are an exciting new tool that allows researchers to study the cardiotoxic potential of drug candidates on human-derived cell populations. Another limitation was the use of trametinib-incorporated food. Drug-incorporated food was used to eliminate the need for daily injections or oral gavage of drug, minimizing stress on the animals. The use of trametinib-incorporated diet may have increased the risk for toxicities; however, the animals demonstrated histopathologic, molecular, and cellular changes consistent with previously reported data. The impact of trametinib on mitochondrial function and overall cardiac collagen content was not examined in vivo. Future studies should examine these changes using advanced techniques such as tissue electron microscopy. The absence of data on blood pressure is a limitation. The development of hypertension in the animals could have induced M2-M1 polarization and changes in ECM remodeling transcriptomics. Additionally, 14 different complete blood count tests were compared. Subsequent statistical analyses, such as a Benjamini-Hochberg to adjust for multiple comparisons, could have been used to correct for multiple comparisons. Future studies should evaluate the intrinsic molecular factors driving trametinib-induced cardiomyocyte atrophy. Last, in vivo reversibility studies would have helped confirm the reversibility of the observed functional deficits. Unfortunately, we did not have Institutional Animal Care and Use Committee approval to perform reversibility studies in vivo, as a reduction in ejection fraction and onset of toxicologic findings were endpoints of the study.
Conclusions
These data define a unique pathology: drug-induced heart failure in the absence of interstitial fibrosis. Pedersen et al37 argued against the clinical significance of ejection fraction reduction in patients with melanoma treated with BRAF/MEK inhibitors; however, recent data suggest the incidence of trametinib-induced cardiomyopathy is underestimated and patients may benefit adjuvant therapy and/or improved screening tools for prevention.5 Data from this study motivate studies investigating our hypothesis that early intervention may prevent irreversible damage. Moreover, this may be coupled with systematic cardiac assessment to better determine cardiac risk at baseline and longitudinally.
Funding Support and Author Disclosures
Dr Norris was supported by grants from the National Institutes of Health (NIH) (R01-HL131546, R01-HL149696, R01-HL122906, R01-HL162913, and P30-1034444), the American Heart Association (19TPA34850095, 20SRG35540029, and 19TPA34900016), and the South Carolina Translational Research Institute (TD1612). Dr Mei was supported by grants from the NIH (R01HL133308), the National Science Foundation (EPS- 0903795), and the U.S. Department of Veterans Affairs Merit Review (I01 BX002327). Dr Beck was supported by training grants from the NIH (F31-HL158243 and T32-HL007260). Mr Arhontoulis and Mr Morningstar were supported by a training grant from the NIH (T32-HL007260). Dr Gensemer was supported by grants from the NIH (T32GM132055 and T32HL007260). The work at Medical University of South Carolina was performed in a facility constructed with support from NIH grant C06 RR018823 from the Extramural Research Facilities Program of the National Center for Research Resources. Additional funding support was provided by the Translational Sciences Lab in part by pilot research funding (Hollings Cancer Center’s Cancer Center Support Grant P30 CA138313 at the Medical University of South Carolina). The views expressed in this paper are those of the authors and do not necessarily represent the view of the National Heart, Lung, and Blood Institute, the NIH, or the American Heart Association. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Trametinib produces a cardiomyopathy characterized by elevated IL-6, increased oxidative stress, decreased cardiac contractility, loss of cell-to-cell communication, and myofibrillar breakdown. Our data support that these changes may be reversible over time.
TRANSLATIONAL OUTLOOK: Elevations in IL-6 and oxidative stress, as well as transcriptomic changes associated with cardiac function, were observed. Future studies should examine the effects of tocilizumab on cardiotoxicity, drug resistance, and anticancer efficacy. Drug holidays may be used to prevent pathologic remodeling, as well as drug resistance. Last, the use of antioxidant drugs, including agents such as dexrazoxane, could be studied in the prevention of trametinib-induced cardiotoxicity.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental methods, figures, and references, please see the online version of this paper.
Appendix
References
- 1.American Society of Clinical Oncology Melanoma: statistics. Published 2022. https://www.cancer.net/cancer-types/melanoma/statistics
- 2.Skin cancer. Published 2022. Accessed April 1, 2022. https://seer.cancer.gov/statfacts/html/melan.html
- 3.SEER∗Explorer: An Interactive Website for SEER Cancer Statistics. Surveillance Research Program, National Cancer Institute. Survival rates for melanoma skin cancer. Accessed April 1, 2022. https://seer.cancer.gov/explorer
- 4.Ascierto P.A., Kirkwood J.M., Grob J.J., et al. The role of BRAF V600 mutation in melanoma. J Transl Med. 2012;10:85. doi: 10.1186/1479-5876-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glen C., Tan Y.Y., Waterston A., et al. Mechanistic and clinical overview cardiovascular toxicity of BRAF and MEK inhibitors. J Am Coll Cardiol CardioOnc. 2022;4(1):1–18. doi: 10.1016/j.jaccao.2022.01.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman P.B., Hauschild A., Robert C., et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broman K.K., Dossett L.A., Sun J., Eroglu Z., Zager J.S. Update on BRAF and MEK inhibition for treatment of melanoma in metastatic, unresectable, and adjuvant settings. Expert Opin Drug Saf. 2019;18(5):381–392. doi: 10.1080/14740338.2019.1607289. [DOI] [PubMed] [Google Scholar]
- 8.Banks M., Crowell K., Proctor A., Jensen B.C. Cardiovascular effects of the MEK inhibitor, trametinib: a case report, literature review, and consideration of mechanism. Cardiovasc Toxicol. 2017;17(4):487–493. doi: 10.1007/s12012-017-9425-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert C.J., Longenecker J.Z., Accornero F. ERK1/2: an integrator of signals that alters cardiac homeostasis and growth. Biology (Basel) 2021;10(4) doi: 10.3390/biology10040346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sengul Samanci N., Celik E., Bagcilar O., et al. Loss of skeletal muscle area and fat-free mass during dabrafenib/trametinib and vemurafenib/cobimetinib treatments in patients with BRAF-mutant metastatic malignant melanoma. Melanoma Res. 2020;30(5):477–483. doi: 10.1097/CMR.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 11.Maeda T., Yoshino K., Yamashita C., et al. Dynamics of neutrophil and C-reactive protein reflect the clinical course of pyrexia during combination therapy with dabrafenib and trametinib. J Dermatol. 2019;46(8):716–719. doi: 10.1111/1346-8138.14949. [DOI] [PubMed] [Google Scholar]
- 12.Dummer R., Biette K., Gusenleitner D., et al. Effect of first-line spartalizumab + dabrafenib + trametinib on immunosuppressive features detected in peripheral blood and clinical outcome in patients (pts) with advanced BRAF V600–mutant melanoma. J Clin Oncol. 2020;38(15) [Google Scholar]
- 13.Hamada J., Baasanjav A., Ono N., et al. Possible involvement of downregulation of the apelin-APJ system in doxorubicin-induced cardiotoxicity. Am J Physiol Heart Circ Physiol. 2015;308(8):H931–H941. doi: 10.1152/ajpheart.00703.2013. [DOI] [PubMed] [Google Scholar]
- 14.Jablonski K.A., Amici S.A., Webb L.M., et al. Novel markers to delineate murine M1 and M2 macrophages. PLoS ONE. 2015;10(12) doi: 10.1371/journal.pone.0145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.U.S. Department of Health and Human Services, National Toxicology Program Heart, myocardium—vacuolation, cytoplasmic. Published 2017. https://ntp.niehs.nih.gov/nnl/cardiovascular/heart/myvacuol/index.htm
- 16.Corum D.G., Tsichlis P.N., Muise-Helmericks R.C. AKT3 controls mitochondrial biogenesis and autophagy via regulation of the major nuclear export protein CRM-1. FASEB J. 2014;28(1):395–407. doi: 10.1096/fj.13-235382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu F., Song D., Yan Y., et al. IL-6 regulates autophagy and chemotherapy resistance by promoting BECN1 phosphorylation. Nat Commun. 2021;12(1):3651. doi: 10.1038/s41467-021-23923-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McQuade J.L., Vashisht Gopal Y. Counteracting oxidative phosphorylation-mediated resistance of melanomas to MAPK pathway inhibition. Mol Cell Oncol. 2015;2(3) doi: 10.4161/23723556.2014.991610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vashisht Gopal Y.N., Gammon S., Prasad R., et al. A novel mitochondrial inhibitor blocks MAPK pathway and overcomes MAPK inhibitor resistance in melanoma. Clin Cancer Res. 2019;25(21):6429–6442. doi: 10.1158/1078-0432.CCR-19-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trotta A.P., Gelles J.D., Serasinghe M.N., Loi P., Arbiser J.L., Chipuk J.E. Disruption of mitochondrial electron transport chain function potentiates the pro-apoptotic effects of MAPK inhibition. J Biol Chem. 2017;292(28):11727–11739. doi: 10.1074/jbc.M117.786442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saleme B., Das S.K., Zhang Y., et al. p53-mediated repression of the PGC1A (PPARG coactivator 1alpha) and APLNR (apelin receptor) signaling pathways limits fatty acid oxidation energetics: implications for cardio-oncology. J Am Heart Assoc. 2020;9(15) doi: 10.1161/JAHA.120.017247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richards D.J., Coyle R.C., Tan Y., et al. Inspiration from heart development: biomimetic development of functional human cardiac organoids. Biomaterials. 2017;142:112–123. doi: 10.1016/j.biomaterials.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards D.J., Li Y., Kerr C.M., et al. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat Biomed Eng. 2020;4(4):446–462. doi: 10.1038/s41551-020-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Infante J.R., Fecher L.A., Falchook G.S., et al. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13(8):773–781. doi: 10.1016/S1470-2045(12)70270-X. [DOI] [PubMed] [Google Scholar]
- 25.Kim H.Y., Duong J.K., Gonzalez M., et al. Pharmacokinetic and cytokine profiles of melanoma patients with dabrafenib and trametinib-induced pyrexia. Cancer Chemother Pharmacol. 2019;83(4):693–704. doi: 10.1007/s00280-019-03780-y. [DOI] [PubMed] [Google Scholar]
- 26.Fontes J.A., Rose N.R., Cihakova D. The varying faces of IL-6: from cardiac protection to cardiac failure. Cytokine. 2015;74(1):62–68. doi: 10.1016/j.cyto.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pyrexia management using an IL-6 antibody in BRAF+ melanoma patients treated with dabrafenib/ trametinib ± immunotherapy (Nov IIT- Pyrex) https://clinicaltrials.gov/ct2/show/NCT04652258
- 28.Hesketh G.G., Shah M.H., Halperin V.L., et al. Ultrastructure and regulation of lateralized connexin43 in the failing heart. Circ Res. 2010;106(6):1153–1163. doi: 10.1161/CIRCRESAHA.108.182147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colussi C., Rosati J., Straino S., et al. Nepsilon-lysine acetylation determines dissociation from GAP junctions and lateralization of connexin 43 in normal and dystrophic heart. Proc Natl Acad Sci U S A. 2011;108(7):2795–2800. doi: 10.1073/pnas.1013124108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jennings M.M., Donahue J.K. Connexin remodeling contributes to atrial fibrillation. J Atr Fibrillation. 2013;6(2):839. doi: 10.4022/jafib.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucero C.M., Andrade D.C., Toledo C., et al. Cardiac remodeling and arrhythmogenesis are ameliorated by administration of Cx43 mimetic peptide Gap27 in heart failure rats. Sci Rep. 2020;10(1):6878. doi: 10.1038/s41598-020-63336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leighton J.K. U.S. Food and Drug Administration; Maryland: 2015. Cotellic (cobimetinib): pharmacology/toxicology NDA review and evaluation. Silver Spring. [Google Scholar]
- 33.Leighton J.K. U.S. Food and Drug Administration; Maryland: 2013. Mekinist (trametinib): pharmacology/toxicology NDA review and evaluation. Silver Spring. [Google Scholar]
- 34.Thakur A., Witteles R.M. Cancer therapy-induced left ventricular dysfunction: interventions and prognosis. J Card Fail. 2014;20(3):155–158. doi: 10.1016/j.cardfail.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 35.Mincu R.I., Mahabadi A.A., Michel L., et al. Cardiovascular adverse events associated with BRAF and MEK inhibitors: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(8) doi: 10.1001/jamanetworkopen.2019.8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chamarthi B., Williams G.H., Ricchiuti V., et al. Inflammation and hypertension: the interplay of interleukin-6, dietary sodium, and the renin-angiotensin system in humans. Am J Hypertens. 2011;24(10):1143–1148. doi: 10.1038/ajh.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pedersen S., Larsen K.O., Christensen A.H., Svane I.M., Zerahn B., Ellebaek E. Cardiotoxicity in metastatic melanoma patients treated with BRAF and MEK inhibitors in a real-world setting. Acta Oncol. 2022;61(1):45–51. doi: 10.1080/0284186X.2021.1992010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.