Abstract
Background
Performance status (PS) is a reliable prognostic tool for overall survival in patients with cancer-associated pulmonary embolism (PE). However, its association with venous thromboembolism (VTE) recurrence and bleeding remains unclear.
Objectives
The aim of this study was to investigate whether PS at the time of PE diagnosis and its course during follow-up are linked to VTE-related outcomes.
Methods
In this post hoc analysis of the Hokusai-VTE Cancer study, multivariable survival analysis was used to examine the association of PS with anticoagulation discontinuation and the composite primary outcome of VTE recurrence or major bleeding in patients with cancer-associated PE. PS was assessed using the Eastern Cooperative Oncology Group (ECOG) scale at baseline and at predefined study follow-up visits.
Results
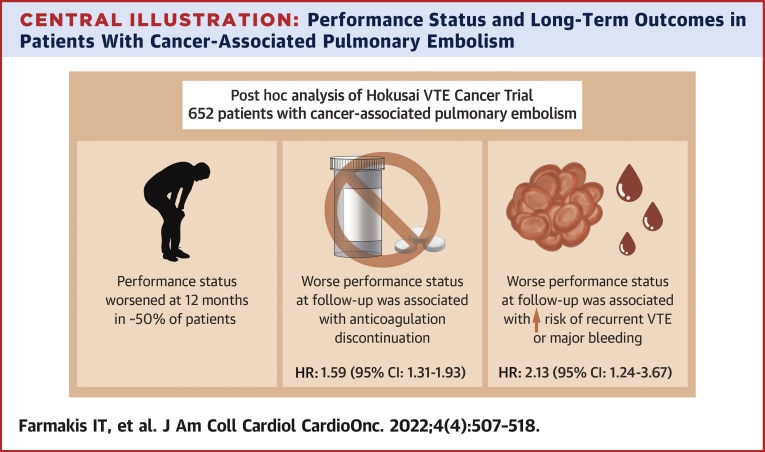
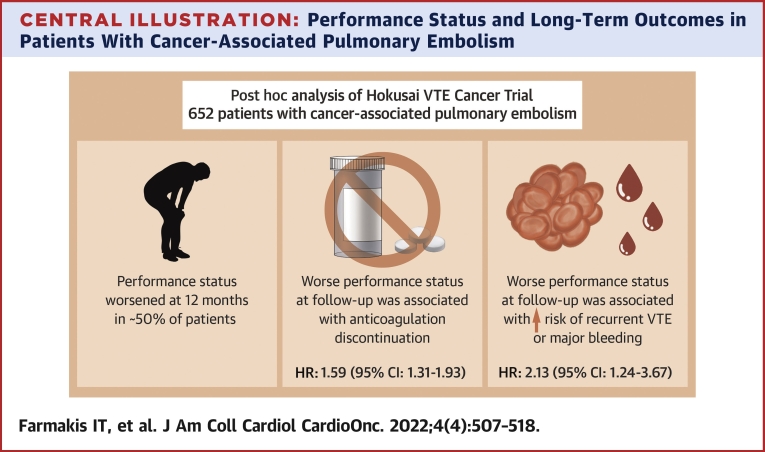
Overall, 652 patients with cancer-associated PE were included. During 12-month follow-up, PS worsened in 317 of 642 patients (49.4%) with complete ECOG data at the end of follow-up. Those with worse ECOG values over follow-up were more likely to discontinue anticoagulation for any reason apart from death (adjusted HR: 1.59; 95% CI: 1.31-1.93). The composite primary outcome occurred in 57 of 500 patients with baseline ECOG status 0 or 1 and in 32 of 152 patients with ECOG status 2 (cumulative incidence at 12 months 10.7% [95% CI: 8.2%-13.9%] vs 14.4% [95% CI: 9.7%-21.3%]). Worse ECOG values during follow-up were associated with greater risk for the composite outcome (adjusted HR: 2.13; 95% CI: 1.24-3.67).
Conclusions
ECOG PS is a valuable indicator for predicting VTE-related outcomes and may inform decision making regarding anticoagulation during follow-up in patients with cancer-associated PE.
Key Words: anticoagulation, Eastern Cooperative Oncology Group, ECOG, edoxaban, performance status, cancer, pulmonary embolism
Abbreviations and Acronyms: DVT, deep vein thrombosis; ECOG, Eastern Cooperative Oncology Group; PE, pulmonary embolism; VTE, venous thromboembolism
Central Illustration
Pulmonary embolism (PE) is a frequent complication in patients with cancer and a major cause of death, second only to the tumor itself.1 However, its severity spectrum varies largely, both in the acute phase of PE and in the long term.2 In the case of cancer-associated PE, prediction of PE-related outcomes may be a particularly difficult task, as the risk for competing mortality directly related to cancer is often a substantial confounder. Traditional stratification scores for short-term prognosis in PE, including the (simplified) Pulmonary Embolism Severity Index or the Geneva prognostic score, take into account the history of cancer itself but fail to account for cancer-specific disease characteristics.3,4 Other clinical classifiers specifically devised for patients with cancer, such as the Pulmonary Embolism Mortality and Computerized Registry of Patients With Venous Thromboembolism tools, may not identify low-risk groups better than simple dichotomous decision rules.5, 6, 7 Moreover, the original and modified Ottawa risk scores have moderate sensitivity and specificity for the identification of recurrent venous thromboembolism (VTE) in patients with cancer-associated VTE during the first 6 months of anticoagulation.8
Performance status in patients with cancer can be assessed by validated scales such as the Eastern Cooperative Oncology Group (ECOG) scale in a reproducible way to define prognosis and guide treatment.9 Cohort studies have shown an increased risk for incident VTE in patients with cancer and a poor performance status.10,11 Baseline performance status is also independently associated with death in patients with both symptomatic and incidental cancer-associated PE.4,12,13 However, previous studies have assessed only short-term outcomes; were not powered to detect differences in long-term disease-specific outcomes such as discontinuation of anticoagulation, VTE recurrence, and major bleeding; or focused only on specific subgroups of patients, such as ambulatory patients with cancer with incidental PE. In addition, little is known regarding the temporal pattern of changes in the performance status over time and the potential prognostic value of these changes in patients with cancer-associated PE with regard to outcomes of interest both to treating oncologists and to cardiologists or pulmonologists following patients after PE.
The aim of this study was to examine the course of patients’ performance status after a diagnosis of cancer-associated PE and to investigate whether performance status assessed at baseline as well as over follow-up is associated with outcomes related to VTE in patients with cancer-associated PE from a large randomized clinical trial.14
Methods
This was a post hoc analysis of the Hokusai-VTE Cancer phase III study.14,15 Briefly, Hokusai-VTE Cancer was a multicenter, prospective, randomized, open-label, blinded endpoint trial that compared edoxaban (60 mg once daily or 30 mg once daily) with dalteparin (200 IU/kg once daily for 30 days and 150 IU/kg thereafter) in patients with cancer with acute VTE (ie, presenting with acute PE or deep vein thrombosis [DVT]). The treatment duration was at least 6 months and up to 12 months, at the treating physician’s discretion. Patients were followed for 12 months or until the global end of the study. Predefined follow-up visits were performed at 1, 3, 6, 9, and 12 months after enrollment. All participants provided written informed consent prior to randomization. The ethics committees of the participating centers approved the study.
Patient population
This analysis included adult patients presenting with symptomatic or incidental PE of a segmental or a more proximal pulmonary artery confirmed by diagnostic imaging (as defined in the Hokusai-VTE Cancer protocol) and associated with cancer. Incidental PE was defined as PE detected by imaging tests performed for reasons other than the clinical suspicion of PE. At diagnosis, cancer (excluding basal-cell or squamous-cell skin cancer) had to be active (diagnosed or on therapy within 6 months prior to randomization; recurrent, regionally advanced or metastatic; hematologic malignancy with no complete remission), or diagnosed and objectively confirmed within the previous 2 years. Patients had to be intended for long-term therapy (at least 6 months) with low–molecular weight heparin. The exclusion criteria are described in the Supplemental Appendix. Patients with missing baseline ECOG values were excluded from the present analysis.
Definitions and outcomes
The aim of the present analysis was to investigate the impact of performance status, both at diagnosis and at the follow-up visits, on long-term outcomes related to VTE and its management, namely: 1) the discontinuation of anticoagulation therapy during follow-up; and 2) the composite outcome of VTE recurrence or major bleeding (as well as its components) at 12 months. VTE recurrence was defined as DVT or PE recurrence. Performance status was assessed using the ECOG scale, a validated measure commonly used in the clinical assessment of patients with cancer.9 The ECOG scale ranges from 0, indicating ability to carry out activities without any restriction, to 5, indicating death (Table 1). Hokusai-VTE Cancer included patients with ECOG status 0 to 2 at the time of PE diagnosis, and ECOG status was reevaluated at each prespecified follow-up visit (1, 3, 6, 9, and 12 months). Other clinical outcomes included VTE recurrence (recurrence of PE or DVT recurrence), bleeding events (major bleeding and clinically relevant nonmajor bleeding), all-cause mortality, hospitalization for VTE, and bleeding events, and event-free survival.
Table 1.
ECOG Scale of Performance Status
| ECOG Scale Value | Definition |
|---|---|
| 0 | Fully active, able to carry on all predisease performance without restriction |
| 1 | Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature (eg, light housework, office work) |
| 2 | Ambulatory and capable of all self-care but unable to carry out any work activities; up and about more than 50% of waking hours |
| 3 | Capable of only limited self-care; confined to bed or chair more than 50% of waking hours |
| 4 | Completely disabled; cannot carry on any self-care; totally confined to bed or chair |
| 5 | Death |
ECOG = Eastern Cooperative Oncology Group.
A detailed description of the definitions of clinical events and the eligibility criteria for low-dose edoxaban are provided in the Supplemental Appendix.
Statistical analysis
Baseline characteristics of patients were summarized by ECOG status (0 or 1 vs 2) using proportions for categorical variables and medians and IQRs for continuous variables. We performed 3 main types of analysis.
First, we described the course of ECOG status during the study period (baseline visit, 6-month follow-up, 12-month follow-up, or latest available) by constructing an alluvial plot. We dealt with missing values as follows: if there was at least 1 recorded visit after the visit with a missing value, the patient was not considered lost to follow-up, and the missing ECOG value was filled by imputing the value from an available previous follow-up, assuming a steady performance status; if there was no recorded next visit, the missing value was regarded as a true missing value. An alluvial plot with complete cases was constructed as a sensitivity analysis.
Second, a Cox proportional hazards model was used to estimate cause-specific HRs with 95% CIs for the association of ECOG status both at baseline and at each follow-up visit with the discontinuation of anticoagulation treatment at any time during follow-up, for any reason other than death. ECOG performance status at each follow-up visit was an integer scale ranging from 0 to 5 and was treated as a time-dependent covariate in the Cox model, with time intervals based on the recorded study day of each patient’s study visit. The model was adjusted for the following variables: 1) ECOG status at baseline (time-independent covariate); 2) age; 3) risk factors for bleeding at baseline; 4) bleeding events at follow-up (time-dependent covariate); 5) creatinine clearance <50 mL/min, hemoglobin <8 g/dL, or platelet count <50,000/mL at any time during follow-up; and 6) study intervention (edoxaban vs dalteparin), with an interaction term between ECOG status (time-dependent variable) and assigned study treatment. In a secondary model, a Fine-Gray multivariable model was constructed to account for the competing risk for death, while adjusting for the same confounders.
Third, we plotted survival curves with: 1) cumulative incidence function for the primary composite outcome of the Hokusai-VTE Cancer study, namely, VTE recurrence or major bleeding; and 2) Kaplan-Meier estimates for all-cause death, by ECOG baseline status (0 or 1 vs 2). A Cox proportional hazards model was used to associate the composite outcome as well as its components with the time-dependent ECOG variable, as described earlier. Additional variables included for adjustment in this model were ECOG status at baseline (time-independent covariate), age, VTE history, no prior surgery for cancer treatment, regionally advanced disease or metastatic disease, discontinuation of anticoagulation not because of death during follow-up (time-dependent covariate), incidental or symptomatic PE, and study intervention. Interaction terms between ECOG status (time-dependent variable) and assigned study treatment as well as the type of the index PE event (incidental or symptomatic) were introduced into the model. The first term was introduced because this was a post hoc analysis of a randomized study and the second in light of previous research in the field indicating an important role for performance status, especially in incidental PE.13 In a secondary model, a Fine-Gray multivariable model was constructed to account for the competing risk for death not due to VTE recurrence, while adjusting for the same confounders. We graphically compared log(−log) curves and used a goodness-of-fit test based on scaled Schoenfeld residuals to test the proportional hazards assumption in all models. A 2-sided P value <0.05 was considered to indicate statistical significance. All statistical analyses were performed by I.T.F. using R version 4.1.1 (R Project for Statistical Computing) in a remote computer environment provided by the Vivli data platform and were independent from the trial sponsor.
Data sharing statement
Access to the trial datasets was provided by Daiichi Sankyo through the data sharing platform Vivli.org.
Results
Among the entire Hokusai-VTE Cancer population, which included patients with either PE or DVT, the present analysis was focused on 652 patients who presented with acute PE (with or without DVT), thus excluding patients with DVT alone. The patient selection flowchart is depicted in Supplemental Figure 1. The baseline characteristics of the included patient population are shown in Table 2 and those of excluded patients with missing baseline ECOG values (n = 7) in Supplemental Table 1. Overall, 319 of the patients (48.9%) analyzed in the present study presented with unsuspected PE, and 100 (23%) had concomitant diagnoses of DVT. Compared with patients with baseline ECOG status 0 or 1 (n = 500), those with baseline ECOG status 2 (n = 152) more often had metastatic disease, a higher prevalence of individual bleeding risk factors, and a higher likelihood of presenting with symptomatic PE and receiving reduced-dose edoxaban according to the dose reduction criteria recommended by the trial protocol. Bleeding risk factors and types of baseline cancer diagnosis can be seen in Supplemental Table 2.
Table 2.
Baseline Characteristics of the Included Population
| Overall (N = 652) | ECOG Status 0 or 1 (n = 500) | ECOG Status 2 (n = 152) | P Value | |
|---|---|---|---|---|
| Age ≥65 years | 311 (47.7) | 234 (46.8) | 77 (50.7) | 0.40 |
| Female | 327 (50.2) | 247 (49.4) | 80 (52.6) | 0.48 |
| Randomized to edoxaban | 328 (50.3) | 247 (49.4) | 81 (53.3) | 0.40 |
| Received low-dose edoxabana | 88 (26.8) | 58 (23.5) | 30 (37.0) | 0.017 |
| Concomitant presence of DVT | 0.016 | |||
| Symptomatic DVT | 87 (13.3) | 60 (12.0) | 27 (17.8) | |
| Unsuspected DVT | 63 (9.7) | 42 (8.4) | 21 (13.8) | |
| Index PE event | 0.013 | |||
| Symptomatic PE | 333 (51.1) | 242 (48.4) | 91 (59.9) | |
| Unsuspected PEb | 319 (48.9) | 258 (51.6) | 61 (40.1) | |
| Type of primary cancer | 0.50 | |||
| Hematological malignancy | 54 (8.3) | 44 (8.8) | 10 (6.6) | |
| Solid tumor | 595 (91.3) | 454 (90.8) | 141 (92.8) | |
| Both | 3 (0.5) | 2 (0.4) | 1 (0.7) | |
| Active cancer | 625 (95.9) | 477 (95.4) | 148 (97.4) | 0.29 |
| Metastatic disease | 354 (54.3) | 261 (52.2) | 93 (61.2) | 0.052 |
| Recurrent cancer | 153 (23.5) | 119 (23.8) | 34 (22.4) | 0.71 |
| Surgery for cancer (curative or palliative) | 258 (39.6) | 198 (39.6) | 60 (39.5) | >0.99 |
| Cancer treatment within the previous 4 wk | 488 (74.8) | 376 (75.2) | 112 (73.7) | 0.71 |
| Cardiovascular disease | 265 (40.6) | 205 (41.0) | 60 (39.5) | 0.74 |
| Pulmonary disease (excluding PE) | 107 (16.4) | 74 (14.8) | 33 (21.7) | 0.044 |
| Diabetes mellitus | 97 (14.9) | 67 (13.4) | 30 (19.7) | 0.055 |
| Previous VTE | 64 (9.8) | 54 (10.8) | 10 (6.6) | 0.13 |
| Hemoglobin <8 g/dL | 12 (1.9) | 7 (1.4) | 5 (3.3) | 0.16 |
| Platelet count <50,000 per μL | 2 (0.3) | 0 (0.0) | 2 (1.3) | 0.054 |
| Creatinine clearance <50 mL/min | 41 (6.4) | 28 (5.7) | 13 (8.7) | 0.17 |
| Risk factors for bleedingc | 0.048 | |||
| 0 | 203 (31.1) | 166 (33.2) | 37 (24.3) | |
| 1 | 305 (46.8) | 227 (45.4) | 78 (51.3) | |
| 2 | 135 (20.7) | 98 (19.6) | 37 (24.3) | |
| 3 | 9 (1.4) | 9 (1.8) | 0 (0.0) |
Values are n (%).
DVT = deep vein thrombosis; ECOG = Eastern Cooperative Oncology Group; PE = pulmonary embolism; VTE = venous thromboembolism.
Low-dose edoxaban was administered at a dose of 30 mg once daily (instead of 60 mg once daily) in patients with creatinine clearance of 30 to 50 mL/min or body weight ≤ 60 kg and in those receiving concomitant treatment with potent P-glycoprotein inhibitors.
Unsuspected PE was defined as PE detected by means of imaging tests performed for reasons other than clinical suspicion of PE.
These include surgery within 2 weeks before randomization, the use of antiplatelet agents, a primary or metastatic brain tumor at randomization, regionally advanced or metastatic cancer, gastrointestinal or urothelial cancer that was present at randomization or had been diagnosed within 6 months before randomization, and treatment with bevacizumab within the 6-week period before randomization.
Changes of ECOG performance status during the trial period
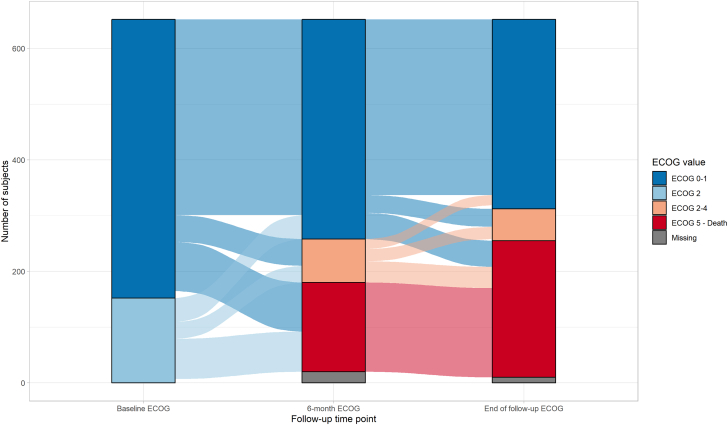
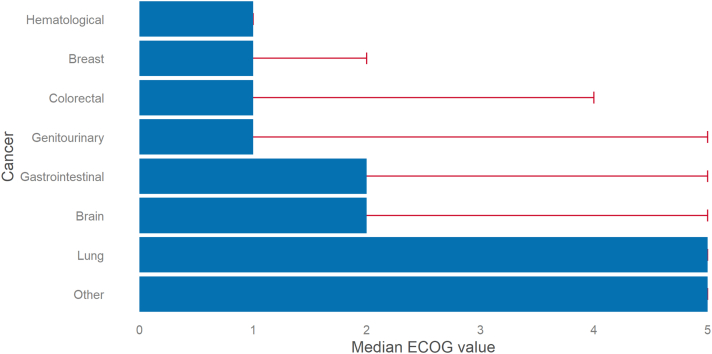
Of 642 patients with complete data at the end of follow-up (10 of 652 had missing follow-up ECOG values), 340 (53%) were classified as ECOG status 0 or 1, 42 (6.5%) as ECOG status 2, 10 (1.6%) as ECOG status 3, 5 (0.8%) as ECOG status 4, and 245 (38.2%) as ECOG status 5. ECOG performance status at the end of follow-up was worse than baseline in 317 (49.4%), steady in 200 (31.2%), and better in 125 (19.5%) patients. The frequency distribution across the ECOG performance status categories through baseline, 6 months, and 12 months or the end of follow-up can be seen in Figure 1 and for the subgroups with edoxaban and dalteparin separately in Supplemental Figures 2 and 3, respectively. ECOG status values were missing in 60 of 652 patients (9.2%) at 1 or more follow-up visits. A complete case sensitivity analysis can be seen in Supplemental Figure 4. Among the different cancer types, brain, lung, and gastrointestinal cancer had the highest (4, 3.3, and 2.6, respectively) and breast and hematological cancer the lowest (1.6 and 1.1, respectively) mean values of ECOG performance status at the end of follow-up (Figure 2). In the stratification by sex, men showed worse ECOG status for gastrointestinal carcinomas and women for genitourinary carcinomas (Supplemental Figure 5).
Figure 1.
Alluvial Plot of ECOG Performance Status Course During the Trial
The plot depicts the distribution of patients across 3 categories of Eastern Cooperative Oncology Group (ECOG) performance status (0 or 1, 2-4, and 5) captured at 3 distinct time points and the flow from baseline to month 6 and from month 6 to month 12 or end of follow-up.
Figure 2.
Bar Plot of ECOG Performance Status for Different Cancer Subtypes
For each subtype of cancer (represented by a single bar), the Eastern Cooperative Oncology Group (ECOG) scale ranges from 0 to 5 (death). Each bar indicates the median of ECOG scale values for the specific cancer subtype at the end of follow-up (12 months or global end of treatment), and the error bar extends to the quartile 3 value.
Discontinuation of anticoagulant therapy during the trial period
Patients with ECOG status 2 at baseline received anticoagulation for a median of 116 days (IQR: 40-269 days) and those with baseline ECOG status 0 or 1 for a median of 248 days (IQR: 121-358 days) (P < 0.001). Overall, 425 patients (65.2%) discontinued anticoagulation during follow-up, 207 of 328 patients (63.1%) in the edoxaban arm and 218 of 324 patients (67.3%) in the dalteparin arm. Reasons for discontinuation included adverse events or clinical outcomes in 23.3%, cancer progression in 8%, cured cancer in 4.2%, concomitant use of a drug not allowed by the study protocol in 0.2%, and other reasons (eg, investigator decision, patient decision, withdrawal of consent) in 33.6%. Death was the reason for anticoagulation discontinuation in 124 of 425 (29.2%). The reasons for discontinuation in each treatment arm, stratified by baseline ECOG status, are displayed in Table 3. Both high baseline ECOG values and higher ECOG values during follow-up were associated with a discontinuation of anticoagulation therapy for any reason other than death (adjusted cause-specific HRs: 1.46 [95% CI: 1.06-1.99] and 1.59 [95% CI: 1.31-1.93], respectively) (Table 4). These associations were significant in the model with death as a competing risk, along with the presence of risk factors for bleeding at baseline and the bleeding events at follow-up (Table 4). A significant interaction for higher chance of discontinuation was found for higher ECOG performance status through follow-up and randomization to edoxaban (as opposed to dalteparin; P for interaction <0.001).
Table 3.
Reasons for Discontinuation of Anticoagulation in the 2 Treatment Arms Stratified by Baseline ECOG Status
| ECOG Status 0 or 1 | ECOG Status 2 | |
|---|---|---|
| Dalteparin arm | (n = 253) | (n = 71) |
| Overall | 160 (63) | 58 (82) |
| Death | 36 (22.5) | 28 (48.3) |
| Adverse event or clinical outcome | 27 (16.9) | 9 (15.5) |
| Cancer cured | 9 (5.6) | 3 (5.2) |
| Cancer progression | 11 (6.9) | 5 (8.6) |
| Subject withdrew consent | 3 (1.9) | 1 (1.7) |
| Prohibited concomitant medication use | 1 (0.6) | 0 (0.0) |
| Othera | 73 (45.6) | 12 (20.7) |
| Edoxaban arm | (n = 247) | (n = 81) |
| Overall | 139 (56) | 68 (84) |
| Death | 39 (28.1) | 21 (30.9) |
| Adverse event or clinical outcome | 39 (28.1) | 24 (35.3) |
| Cancer cured | 4 (2.9) | 2 (2.9) |
| Cancer progression | 10 (7.2) | 8 (11.8) |
| Subject withdrew consent | 1 (0.7) | 1 (1.5) |
| Prohibited concomitant medication use | 0 (0.0) | 0 (0.0) |
| Othera | 46 (33.1) | 12 (17.6) |
Values are n (%).
ECOG = Eastern Cooperative Oncology Group.
Other reasons included, among others, the investigator’s decision (benefit/risk judgement, palliative treatment only, patient noncompliance), the patient’s decision (inconvenience of dosing), the start of new chemotherapy, and platelet count < 50,000/μL.
Table 4.
Multivariable Regression Models for Anticoagulation Discontinuation (for Reason Other Than Death) During the Trial
| Cause-Specific HR (95% CI) With Censoring at Death | Subdistribution HR (95% CI) Using Fine-Gray Method | |
|---|---|---|
| Baseline covariates | ||
| ECOG status at baseline (2 vs 0 or 1) | 1.46 (1.06-1.99) | 1.49 (1.11-2.01) |
| Age ≥65 y (vs <65 y) | 0.92 (0.72-1.18) | 0.93 (0.73-1.17) |
| Bleeding risk factor (≥1) at baseline | 1.24 (0.96-1.61) | 1.30 (1.01-1.67) |
| Allocation to dalteparin (vs edoxaban) | 1.92 (1.33-2.75) | 1.77 (1.25-2.52) |
| Anemia at follow-up | 1.17 (0.75-1.81) | 1.05 (0.68-1.61) |
| Thrombocytopenia at follow-up | 2.04 (0.94-4.45) | 1.87 (0.91-3.87) |
| Creatinine clearance < 60 mL/min at follow-up | 0.80 (0.56-1.16) | 0.78 (0.55-1.12) |
| Time-dependent covariates | ||
| ECOG status at follow-up (0-5) | 1.59 (1.31-1.93) | 1.54 (1.28-1.86) |
| Bleeding event at follow-up | 1.39 (0.84-2.29) | 1.51 (1.13-2.03) |
| Interaction terms | ||
| ECOG status at follow-up (0-5) × randomization arm (dalteparin vs edoxaban) | 0.57 (0.43-0.75) | 0.58 (0.44-0.76) |
ECOG = Eastern Cooperative Oncology Group.
Clinical outcomes in relation to ECOG status
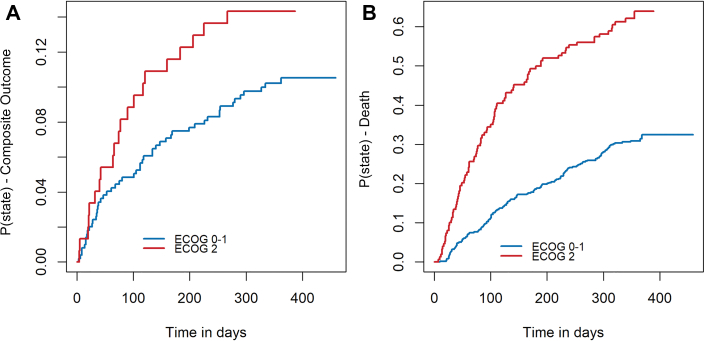
In the population analyzed in the present study, the cumulative incidence rate of the composite outcome of VTE recurrence or major bleeding at 12 months was 11.4% (95% CI: 8.9%-13.8%). Randomization to edoxaban was associated with a reduced risk for VTE recurrence (adjusted HR: 0.28; 95% CI: 0.11-0.69) and a tendency toward increased risk for major bleeding (adjusted HR: 2.04; 95% CI: 0.99-4.19) compared with dalteparin. The cumulative incidence curves for the composite outcome and Kaplan-Meier estimates for all-cause death can be seen in Figure 3. Patients with baseline ECOG values of 2 had increased cumulative rates of the composite outcome at 12 months compared with patients with baseline values of 0 or 1 (14.4% [95% CI: 9.7%-21.3%] vs 10.7% [95% CI: 8.2%-13.9%]). However, only time-dependent deterioration of performance status (increase in ECOG value) was independently associated with a higher risk for the primary composite outcome (adjusted cause-specific HR: 2.13; 95% CI: 1.24-3.67), whereas baseline ECOG status was not (adjusted cause-specific HR: 1.33; 95% CI: 0.78-2.30]) (Table 5).
Figure 3.
Survival Curves for the Composite Outcome and Death
(A) Cumulative incidence function curve of the occurrence to first composite outcome (recurrent venous thromboembolism or major bleeding) on the basis of baseline Eastern Cooperative Oncology Group (ECOG) performance status (0 or 1 vs 2). (B) Kaplan-Meier curve for all-cause death on the basis of baseline ECOG performance status (0 or 1 vs 2).
Table 5.
Multivariable Regression Models for the Composite Outcome of Recurrent VTE or Major Bleeding
| Cause-Specific HR (95% CI) With Censoring at Death | Subdistribution HR (95% CI) Using Fine-Gray Method | |
|---|---|---|
| Baseline covariates | ||
| ECOG status at baseline (0-2) | 1.33 (0.78-2.30) | 1.57 (0.74-3.34) |
| Age ≥65 y (vs <65 y) | 0.90 (0.53-1.53) | 0.96 (0.56-1.66) |
| Previous VTE | 0.97 (0.39-2.43) | 1.03 (0.41-2.63) |
| Regionally advanced cancer or metastatic disease | 1.32 (0.74-2.38) | 1.18 (0.65-2.15) |
| Prior surgery for cancer | 0.89 (0.52-1.54) | 0.97 (0.55-1.68) |
| Dalteparin (vs edoxaban) | 1.24 (0.52-2.94) | 1.32 (0.62-2.84) |
| Unsuspected PE (vs symptomatic PE) | 2.90 (1.06-7.91) | 2.34 (0.96-5.75) |
| Time-dependent covariates | ||
| ECOG status at follow-up (0-5) | 2.13 (1.24-3.67) | 1.64 (0.99-2.73) |
| Discontinuation of anticoagulation | 0.89 (0.34-2.32) | 0.84 (0.32-2.17) |
| Interaction terms | ||
| ECOG at follow-up (0-5)× randomization arm (dalteparin vs edoxaban) | 0.88 (0.48-1.62) | 0.81 (0.49-1.34) |
| ECOG at follow-up (0-5) × index PE event (unsuspected vs symptomatic) | 0.59 (0.33-1.06) | 0.72 (0.43-1.19) |
ECOG = Eastern Cooperative Oncology Group; PE = pulmonary embolism; VTE = venous thromboembolism.
With regard to the components of the primary outcome, the cumulative rates of VTE recurrence (8.5% [95% CI: 4.9%-14.6%] vs 6.4% [95% CI: 4.5%-8.9%]) and major bleeding (9.6% [95% CI: 5.8%-15.8%] vs 4.9% [95% CI: 3.3%-7.4%]) at 12 months were higher in patients with baseline ECOG status 2 than in those with baseline ECOG status 0 or 1. Furthermore, the point estimates for HRs on the basis of time-dependent ECOG changes suggested that patients with worsening ECOG status over the follow-up period had an increased risk both for VTE recurrence (adjusted HR: 1.25) and for major bleeding (adjusted HR: 1.56), after adjusting for age, randomized anticoagulation arm, and bleeding risk factors; however, the results did not reach statistical significance (95% CIs: 0.74-2.10 and 0.91-2.67, respectively).
Patients with incidental PE were more likely to experience the composite primary outcome than patients with symptomatic PE (adjusted cause-specific HR: 2.90; 95% CI: 1.06-7.91) (Table 5). Time-dependent ECOG changes were associated with the composite outcome in the subgroup of symptomatic PE patients (adjusted HR: 2.09; 95% CI: 1.19-3.68) but not in the incidental PE subgroup (adjusted HR: 1.11; 95% CI: 0.63-1.95), although the interaction was not significant (P = 0.07). No interaction was found regarding treatment arm (edoxaban vs dalteparin).
Overall, 245 of 648 patients (37.8%) died during the course of the study (4 patients were lost to follow-up). Death was cancer related in 89%, VTE related in 2.3%, and bleeding related in 0.4%. When death not related to VTE was modeled using the Fine-Gray method, the association of ECOG status with the composite outcome did not reach statistical significance, although the lower boundary of the CI was very close to 1 (adjusted subdistribution HR: 1.64; 95% CI: 0.99-2.73; P = 0.06).
The crude rates of clinical events in the baseline categories of ECOG values (0 or 1 and 2) and stratified by treatment arm are shown in Supplemental Table 3.
Discussion
In this study, we report a significant association between poor performance status and long-term VTE-related clinical outcomes such as VTE recurrence and major bleeding, in patients with cancer-associated PE. In particular, changes in ECOG status during follow-up were a stronger predictor of VTE recurrence or major bleeding than ECOG performance status at baseline. In parallel to being more likely to experience VTE-related outcomes, patients with worsening ECOG status had a greater risk for anticoagulant discontinuation, even after adjustment for bleeding risk factors and incident bleeding events (Central Illustration).
Central Illustration.
Performance Status and Long-Term Outcomes in Patients With Cancer-Associated Pulmonary Embolism
In patients with cancer-associated pulmonary embolism, worse performance status over follow-up is associated with anticoagulation discontinuation, recurrent venous thrombosis or major bleeding.
Our results suggest that performance status measured with the ECOG scale, which is a traditional metric in oncology and is known to be associated with overall survival, may also predict potentially preventable clinical outcomes such as VTE recurrence and major bleeding. A previous study showed that in patients with cancer and symptomatic PE, an ECOG value ≥2 at the time of PE diagnosis had a discriminative risk stratification ability equal to that of the Pulmonary Embolism Mortality and Computerized Registry of Patients With Venous Thromboembolism scores and of the dichotomized clinical decision rule comprising 6 clinical criteria.7 Two further studies, conducted at a single and multiple centers, respectively, investigated the prognostic value of baseline parameters in incidental cancer-associated PE and concluded that the patients’ performance status at baseline was, along with patient-reported symptoms prior to the index PE event, a strong predictor of both early and long-term mortality.12,13 The results of our analysis now propose an association of ECOG status not only with the overall risk for death but also with VTE-specific outcomes. The availability of follow-up data from a large phase III clinical trial allowed us to incorporate the serial evaluation of performance status in the model and explore the notion that ECOG status deterioration during follow-up is a major determinant of discontinuation of anticoagulant treatment over time and may predict a greater risk for VTE recurrence or major bleeding.
The connection among cancer progression, VTE, and mortality is well established. Indeed, the Khorana clinical score for the prediction of risk for VTE in patients receiving chemotherapy can be also used to predict early mortality and cancer progression.16 In our analysis, when death not related to VTE was treated as a competing event, the subdistribution hazard related to the changes in ECOG status marginally failed to reach statistical significance with regard to the composite outcome. This might be expected, as patients with worse ECOG baseline and follow-up values were more likely to die than to experience a further VTE-related outcome. Although it is known that a worsening of ECOG status reflects a worsening of the underlying cancer and increases the risk for death, the finding that cancer-associated risk for thrombosis and bleeding may increase concomitantly is novel and, in our view, clinically relevant. Our results are supported by recent data that show a disproportionally high incidence of thrombosis recurrence in patients with stage IV cancer, even in the era of immunotherapy.17,18 Conversely, patients with earlier stage cancer are less likely to develop VTE recurrence after an episode of cancer-associated VTE.19 Hence, the deterioration of a patient’s performance status during follow-up after an episode of acute cancer-associated PE should be of concern not only for the treating oncologist but also for the thrombosis specialist because it indicates an increased risk for thromboembolic complications, potentially life-threatening and detrimental to the patient’s quality of life.20 In particular, upon worsening of performance status, the treating oncologist may consider referral to a thrombosis specialist for reassessment of the risk/benefit ratio of anticoagulation, review of the patient’s perspective on quality of life and the burden new complications would impose, and discussion of the merits of alternative management choices. Of note, in recent years, the importance of patient-reported outcomes, treatment-related complications, and long-term consequences of disease beyond “classical” VTE complications has been increasingly emphasized.21 In this perspective, each treatment, including anticoagulation, should be interpreted in the context of these factors and accounting for the course of performance and functional status.
In the present study, patients with worse performance status over follow-up were more likely to discontinue anticoagulant therapy. Interaction analysis showed that the association of higher ECOG status with discontinuation was more pronounced in patients randomized to edoxaban (as opposed to dalteparin) or, alternatively, that discontinuation in dalteparin (but not in edoxaban) occurred at lower ECOG already. Among the reasons provided for discontinuation of anticoagulation, an adverse event or clinical outcome was more often mentioned in those with higher baseline ECOG who discontinued edoxaban, “other reasons” (including patient preference or physician decision) in those with lower baseline ECOG who discontinued dalteparin. Although the interaction may suggest that a patient with cancer might have been more likely to discontinue an injected drug (such as dalteparin) when feeling better, and more likely to discontinue an oral anticoagulant agent when feeling worse or experiencing adverse events, the difference was small, and our observational data cannot conclusively support such causal interpretation. Evidence collected since has led the most recent guidelines to recommend direct oral agents over low–molecular weight heparin in cancer-associated VTE, in the absence of elevated bleeding risk from particular sites.22,23
Current guidelines recommend indefinite anticoagulant therapy in cancer-associated PE, until the cancer is cured; however, this scenario was infrequent in the present study.2 The decision whether to continue anticoagulation in patients with cancer after the first 6 months from the event is complex and based on cancer-specific characteristics (such as location and activity of disease), concomitant therapies, and patient preferences.24 A recent meta-analysis showed that in patients with cancer, fatality from VTE recurrence is higher than from major bleeding.25 Our results suggest that patients with worse performance status at baseline are more prone to anticoagulation discontinuation, even when adjusting for the occurrence of bleeding or other criteria for anticoagulation dose reduction or interruption such as fall in platelet count, hemoglobin, or creatinine clearance. The presence of bleeding risk factors at baseline, as well as the incidence of bleeding events at follow-up, was also significantly associated with discontinuation of anticoagulation for any reason other than death, as expected, when death was treated as a competing event. It must be acknowledged, though, that extended anticoagulation in cancer-associated PE has not been extensively studied. The ongoing API-CAT (Apixaban Cancer Associated Thrombosis) study focusing on extended anticoagulant treatment with reduced-dose vs full-dose apixaban in cancer-associated VTE could offer insights regarding this clinical conundrum.26
An interaction between incidental or symptomatic PE and performance status for the primary outcome was not found in this analysis, but incidental PE was associated with greater risk for the primary outcome. In a previous analysis of the overall VTE population of the Hokusai-VTE Cancer trial, there was no statistical difference in terms of the primary outcome.27 However, a recent predefined analysis of the Caravaggio trial showed that statistically significant lower risk for recurrent VTE and no difference in risk for major bleeding in patients with incidental compared with symptomatic VTE.28
Study limitations
The present analysis included a diverse population of patients with cancer with extended baseline high-quality data, minimal rates of loss to follow-up, and adjudication of all events by an independent blinded committee. However, there were some limitations. In the Hokusai-VTE Cancer study, patients were not randomized according to the ECOG functional status, and our results should thus be interpreted as hypothesis generating. Also, patients received either dalteparin or edoxaban; therefore, the findings may not necessarily apply to patients treated with other oral or parenteral anticoagulant agents. Moreover, patients with ECOG status 3 or 4 at baseline were excluded from the trial, as they are expected to have very limited median survival times.29 Although this is usual in trials conducted in oncological populations, our conclusions cannot be generalized to more severely ill patients with cancer-associated PE. We included only patients with PE in our analysis in light of their specific characteristics30; therefore a future analysis encompassing patients with DVT alone may be of additional worth. Last, the number of patients in the different subgroups of cancer types was small; consequently, differences in prognostic outcomes across cancer types were not studied, as analysis power was limited.
Conclusions
In patients with cancer-associated PE, ECOG performance status may be a useful and reliable tool for the prediction of VTE-related outcomes, such as VTE recurrence or major bleeding. The assessment of performance status may support oncological follow-up by aiding in the interdisciplinary decision making regarding anticoagulation beyond overall prognosis.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Worse performance status over follow-up of cancer-associated PE is associated with anticoagulation discontinuation, recurrent venous thrombosis or major bleeding. Worsening of performance status appears to reflect worsening of underlying cancer and to increase the risk for death; risk for thrombosis and bleeding may increase concomitantly. Specialists following patients after episodes of cancer-associated PE may include performance status when making decisions on anticoagulation management.
TRANSLATIONAL OUTLOOK: Future prospective studies should examine the significance of the performance status assessment in clinical decision-making pathways regarding VTE-related outcomes.
Funding Support and Author Disclosures
This study was supported by an unrestricted grant from Daiichi Sankyo (grant DSE-DE-CV-20001). Dr Barco has received lecture and consulting fees from Bayer HealthCare, Concept Medical, BTG Pharmaceuticals, Inari Medical, Boston Scientific, and LeoPharma; has received institutional grants from Boston Scientific, Bentley, Bayer HealthCare, Inari Medical, Medtronic, Concept Medical, Bard, and Sanofi; and has received financial support for travel and congress costs from Daiichi Sankyo, BTG Pharmaceuticals, and Bayer HealthCare, outside the submitted work. Dr Konstantinides has received institutional grants and personal lecture and advisory fees from Bayer, Daiichi Sankyo, and Boston Scientific; has received institutional grants from Inari Medical; and has received personal lecture and advisory fees from Merck Sharpe & Dohme and Bristol Myers Squibb/Pfizer. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
This publication is based on research using data from data contributor Daiichi Sankyo that has been made available through Vivli, Inc. Vivli has not contributed to or approved, and is not in any way responsible for, the contents of this publication. The authors thank Daiichi Sankyo, the steering committee of the Hokusai-VTE Cancer study, and the Hokusai-VTE Cancer investigators use of the data. In particular, the authors thank Prof Harry R. Büller and Prof Gary Raskob for their support.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For exclusion criteria and definitions as well as supplemental figures and tables, please see the online version of this paper.
Appendix
References
- 1.Barco S., Valerio L., Ageno W., et al. Age-sex specific pulmonary embolism-related mortality in the USA and Canada, 2000-18: an analysis of the WHO Mortality Database and of the CDC Multiple Cause of Death database. Lancet Respir Med. 2021;9:33–42. doi: 10.1016/S2213-2600(20)30417-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Konstantinides S.V., Meyer G., Becattini C., et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS) Eur Heart J. 2020;41:543–603. doi: 10.1093/eurheartj/ehz405. [DOI] [PubMed] [Google Scholar]
- 3.Carmona-Bayonas A., Jimenez-Fonseca P., Font C., et al. Predicting serious complications in patients with cancer and pulmonary embolism using decision tree modelling: the EPIPHANY index. Br J Cancer. 2017;116:994–1001. doi: 10.1038/bjc.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Font C., Carmona-Bayonas A., Beato C., et al. Clinical features and short-term outcomes of cancer patients with suspected and unsuspected pulmonary embolism: the EPIPHANY study. Eur Respir J. 2017;49 doi: 10.1183/13993003.00282-2016. [DOI] [PubMed] [Google Scholar]
- 5.Kline J.A., Roy P.M., Than M.P., et al. Derivation and validation of a multivariate model to predict mortality from pulmonary embolism with cancer: the POMPE-C tool. Thromb Res. 2012;129:e194–e199. doi: 10.1016/j.thromres.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.den Exter P.L., Gomez V., Jimenez D., et al. A clinical prognostic model for the identification of low-risk patients with acute symptomatic pulmonary embolism and active cancer. Chest. 2013;143:138–145. doi: 10.1378/chest.12-0964. [DOI] [PubMed] [Google Scholar]
- 7.Carmona-Bayonas A., Font C., Jimenez-Fonseca P., et al. On the necessity of new decision-making methods for cancer-associated, symptomatic, pulmonary embolism. Thromb Res. 2016;143:76–85. doi: 10.1016/j.thromres.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Delluc A., Miranda S., Exter P.D., et al. Accuracy of the Ottawa score in risk stratification of recurrent venous thromboembolism in patients with cancer-associated venous thromboembolism: a systematic review and meta-analysis. Haematologica. 2020;105:1436–1442. doi: 10.3324/haematol.2019.222828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roila F., Lupattelli M., Sassi M., et al. Intra and interobserver variability in cancer patients’ performance status assessed according to Karnofsky and ECOG scales. Ann Oncol. 1991;2:437–439. doi: 10.1093/oxfordjournals.annonc.a057981. [DOI] [PubMed] [Google Scholar]
- 10.Kuderer N.M., Poniewierski M.S., Culakova E., et al. Predictors of venous thromboembolism and early mortality in lung cancer: results from a global prospective study (CANTARISK) Oncologist. 2018;23:247–255. doi: 10.1634/theoncologist.2017-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guven D.C., Aksun M.S., Sahin T.K., et al. Poorer baseline performance status is associated with increased thromboembolism risk in metastatic cancer patients treated with immunotherapy. Support Care Cancer. 2021;29:5417–5423. doi: 10.1007/s00520-021-06139-3. [DOI] [PubMed] [Google Scholar]
- 12.Bozas G., Jeffery N., Ramanujam-Venkatachala D., et al. Prognostic assessment for patients with cancer and incidental pulmonary embolism. Thromb J. 2018;16:8. doi: 10.1186/s12959-017-0157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maraveyas A., Kraaijpoel N., Bozas G., et al. The prognostic value of respiratory symptoms and performance status in ambulatory cancer patients and unsuspected pulmonary embolism; analysis of an international, prospective, observational cohort study. J Thromb Haemost. 2021;19:2791–2800. doi: 10.1111/jth.15489. [DOI] [PubMed] [Google Scholar]
- 14.Raskob G.E., van Es N., Verhamme P., et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378:615–624. doi: 10.1056/NEJMoa1711948. [DOI] [PubMed] [Google Scholar]
- 15.van Es N., Di Nisio M., Bleker S.M., et al. Edoxaban for treatment of venous thromboembolism in patients with cancer. Rationale and design of the Hokusai VTE-Cancer study. Thromb Haemost. 2015;114:1268–1276. doi: 10.1160/TH15-06-0452. [DOI] [PubMed] [Google Scholar]
- 16.Kuderer N.M., Culakova E., Lyman G.H., Francis C., Falanga A., Khorana A.A. A validated risk score for venous thromboembolism is predictive of cancer progression and mortality. Oncologist. 2016;21:861–867. doi: 10.1634/theoncologist.2015-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carmona-Bayonas A., Gomez D., Martinez de Castro E., et al. A snapshot of cancer-associated thromboembolic disease in 2018-2019: first data from the TESEO prospective registry. Eur J Intern Med. 2020;78:41–49. doi: 10.1016/j.ejim.2020.05.031. [DOI] [PubMed] [Google Scholar]
- 18.Moik F., Chan W.E., Wiedemann S., et al. Incidence, risk factors, and outcomes of venous and arterial thromboembolism in immune checkpoint inhibitor therapy. Blood. 2021;137:1669–1678. doi: 10.1182/blood.2020007878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louzada M.L., Carrier M., Lazo-Langner A., et al. Development of a clinical prediction rule for risk stratification of recurrent venous thromboembolism in patients with cancer-associated venous thromboembolism. Circulation. 2012;126:448–454. doi: 10.1161/CIRCULATIONAHA.111.051920. [DOI] [PubMed] [Google Scholar]
- 20.Valerio L., Barco S., Jankowski M., et al. Quality of life 3 and 12 months following acute pulmonary embolism: analysis from a prospective multicenter cohort study. Chest. 2021;159:2428–2438. doi: 10.1016/j.chest.2021.01.071. [DOI] [PubMed] [Google Scholar]
- 21.International Consortium for Health Outcomes Measurement Patient-centered outcome measures: venous thromboembolism. https://connect.ichom.org/patient-centered-outcome-measures/venous-thromboembolism/
- 22.Lyman G.H., Carrier M., Ay C., et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer [published correction appears in Blood Adv. 2021;5:1953] Blood Adv. 2021;5(4):927–974. doi: 10.1182/bloodadvances.2020003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens S.M., Woller S.C., Kreuziger L.B., et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545–e608. doi: 10.1016/j.chest.2021.07.055. [DOI] [PubMed] [Google Scholar]
- 24.Englisch C., Moik F., Ay C. Risk assessment for recurrent venous thromboembolism in patients with cancer. Thrombosis Update. 2021;5 [Google Scholar]
- 25.Abdulla A., Davis W.M., Ratnaweera N., Szefer E., Ballantyne Scott B., Lee A.Y.Y. A meta-analysis of case fatality rates of recurrent venous thromboembolism and major bleeding in patients with cancer. Thromb Haemost. 2020;120:702–713. doi: 10.1055/s-0040-1708481. [DOI] [PubMed] [Google Scholar]
- 26.Mahe I., Agnelli G., Ay C., et al. Extended anticoagulant treatment with full- or reduced-dose apixaban in patients with cancer-associated venous thromboembolism: rationale and design of the API-CAT study. Thromb Haemost. 2022;122(4):646–656. doi: 10.1055/a-1647-9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulder F.I., Di Nisio M., Ay C., et al. Clinical implications of incidental venous thromboembolism in cancer patients. Eur Respir J. 2020;55 doi: 10.1183/13993003.01697-2019. [DOI] [PubMed] [Google Scholar]
- 28.Giustozzi M., Connors J.M., Ruperez Blanco A.B., et al. Clinical characteristics and outcomes of incidental venous thromboembolism in cancer patients: insights from the Caravaggio study. J Thromb Haemost. 2021;19:2751–2759. doi: 10.1111/jth.15461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jang R.W., Caraiscos V.B., Swami N., et al. Simple prognostic model for patients with advanced cancer based on performance status. J Oncol Pract. 2014;10:e335–e341. doi: 10.1200/JOP.2014.001457. [DOI] [PubMed] [Google Scholar]
- 30.Wenger N., Sebastian T., Engelberger R.P., Kucher N., Spirk D. Pulmonary embolism and deep vein thrombosis: similar but different. Thromb Res. 2021;206:88–98. doi: 10.1016/j.thromres.2021.08.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.