Abstract
Background
Low QRS voltages (LQRSVs) are a common electrocardiographic feature in patients with light chain amyloidosis (AL) and transthyretin amyloidosis (ATTR) cardiac amyloidosis (CA).
Objectives
The aim of this study was to identify clinical and echocardiographic correlates of LQRSV and to investigate their prognostic significance in patients with CA.
Methods
This was a multicenter, retrospective study performed in 6 CA referral centers including consecutive patients with AL and ATTR CA. LQRSVs were defined as a QRS amplitude ≤5 mm (0.5 mV) in all peripheral leads. The study outcome was cardiovascular (CV) mortality.
Results
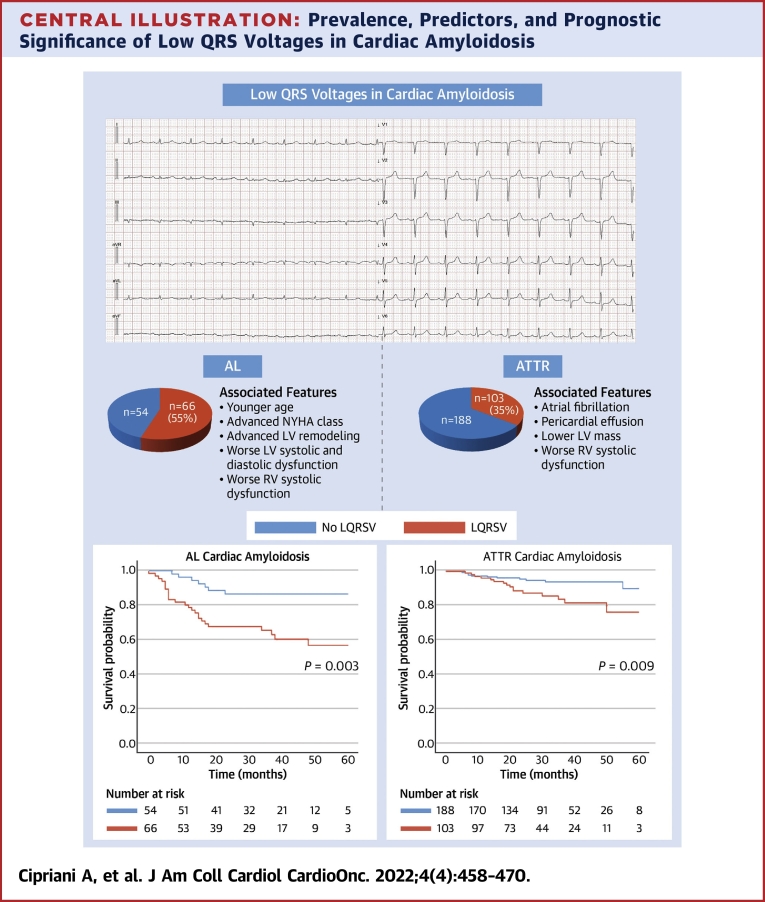
Overall, 411 (AL CA: n = 120, ATTR CA: n = 291) patients were included. LQRSVs were present in 66 (55%) patients with AL CA and 103 (35%) with ATTR CA (P < 0.001). In AL CA, LQRSVs were independently associated with younger age (P = 0.015), higher New York Heart Association functional class (P = 0.016), and natriuretic peptides (P = 0.041); in ATTR CA, LQRSVs were independently associated with pericardial effusion (P = 0.008) and lower tricuspid annulus peak systolic excursion (P = 0.038). During a median follow-up of 33 months (Q1-Q3: 21-46), LQRSVs independently predicted CV death in both AL CA (HR: 1.76; 95% CI: 2.41-10.18; P = 0.031) and ATTR CA (HR: 2.64; 95% CI: 1.82-20.17; P = 0.005). Together with the National Amyloidosis Centre (NAC) staging, LQRSVs provided incremental prognostic value in ATTR CA (AUC for NAC model: 0.83 [95% CI: 0.77-0.89]; AUC for NAC + LQRSV model: 0.87 [95% CI: 0.81-0.93]; P = 0.040).
Conclusions
LQRSVs are common but not ubiquitous in CA; they are more frequent in AL CA than in ATTR CA. LQRSVs reflect an advanced disease stage and independently predict CV death. In ATTR CA, LQRSVs can provide incremental prognostic accuracy over the NAC staging system in patients with intermediate risk.
Key Words: cardiac amyloidosis, echocardiography, electrocardiography, low QRS voltages, prognostic significance, risk stratification
Abbreviations and Acronyms: AL, light chain amyloidosis; ATTR, transthyretin amyloidosis; ATTR-wt, wild-type transthyretin; ATTR-v, variant transthyretin; AUC, area under the curve; BNP, B-type natriuretic peptide; CA, cardiac amyloidosis; CMR, cardiac magnetic resonance; CV, cardiovascular; ECG, electrocardiogram; LQRSV, low QRS voltages; LV, left ventricle; LVEDD, left ventricular end-diastolic diameter; NAC, National Amyloidosis Centre; NT-proBNP, N-terminal pro–B-type natriuretic peptide; NYHA, New York Heart Association; ROC, receiver-operating characteristic; RV, right ventricular; TAPSE, tricuspid annulus peak systolic excursion
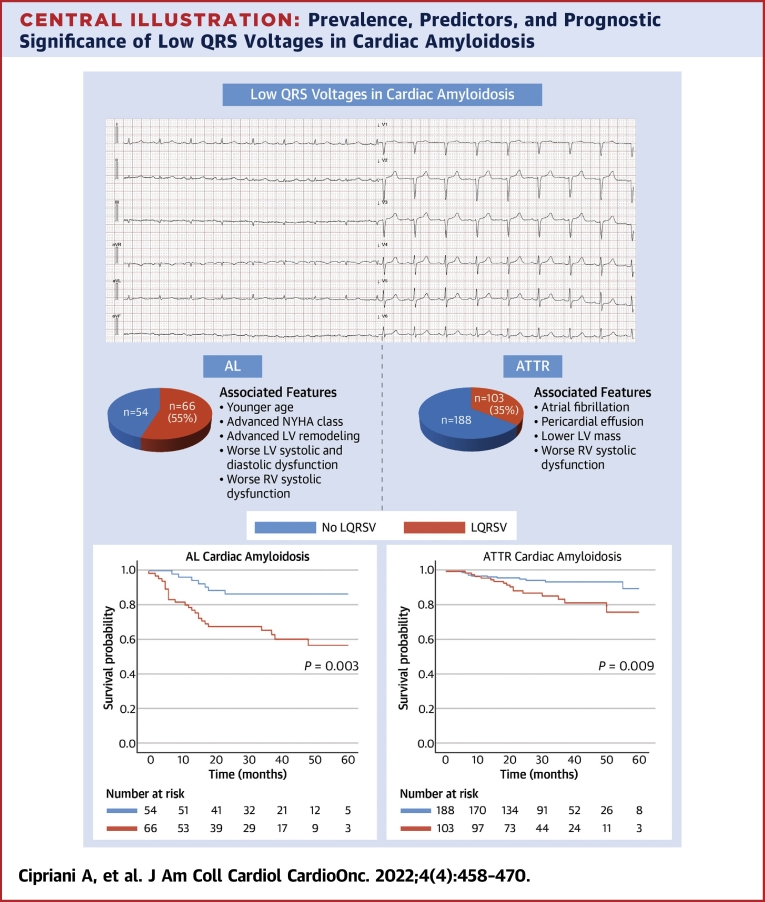
Central Illustration
Cardiac amyloidosis (CA) is an increasingly recognized cause of cardiovascular morbidity and mortality.1, 2, 3 It is characterized by extracellular myocardial infiltration of amyloid fibrils, mostly derived from either wild-type (ATTR-wt) or variant forms of the transthyretin (ATTR-v) protein or from monoclonal immunoglobulin light chain amyloidosis (AL).4 Two main mechanisms of tissue and organ damage are known: chronic infiltration and acute proteotoxic effect of circulating precursors and nonamyloid aggregates. The latter is particularly evident and documented in AL but has also been shown in ATTR-v and ATTR-wt.5 Despite a common cardiomyopathy phenotype, characterized by increased left ventricular (LV) mass and stiffness and restrictive hemodynamics, AL- and transthyretin amyloidosis (ATTR)-CA have different clinical profiles, courses, and outcomes, which are usually more severe in AL CA.6
The diagnosis of CA relies on invasive or noninvasive criteria aimed at demonstrating amyloid fibrils within the myocardium.7 Although not included in these criteria, a 12-lead electrocardiogram (ECG) is an essential test in the diagnostic evaluation of patients with suspected CA. In particular, ECG can raise or support the clinical suspicion of CA by revealing various red flags, such as atrioventricular conduction disturbances, pseudoinfarction patterns, and low QRS voltages (LQRSV).7, 8, 9 The most striking electrocardiographic abnormality in patients with CA is the reduction of QRS voltages, particularly in the limb leads, and the disproportion between QRS voltages and left ventricular (LV) thickness at echocardiography.10 LQRSVs, which are described also in multiple noncardiac (obesity and emphysema) and cardiac disorders (pericardial effusion and arrhythmogenic cardiomyopathy),11 can be observed in up to 60% of patients with CA, more frequently in AL,6 and may hypothetically reflect the burden of amyloid infiltration in the heart. Nevertheless, the underlying structural correlates of LQRSV in both AL- and ATTR CA remains to be clearly defined as well as their prognostic meaning. Thus, the present study was designed to assess the prevalence of LQRSVs in the different forms of CA, to identify their clinical and echocardiographic correlates, and to investigate their prognostic significance in a large multicenter study of patients with CA.
Methods
This is a multicenter, retrospective, observational study performed in 6 referral centers for CA: Padua (Padua University Hospital), Trieste (Cattinara Hospital), Rome (Sant’Andrea Hospital), Florence (Careggi Hospital), Genoa (San Martino Hospital), and Messina (Messina University Hospital). The local regional Institutional Review Board approved the study, and the participating centers obtained local Institutional Review Board approvals for the collection of anonymous data. The study was conducted according to the Declaration of Helsinki, and informed consent was obtained according to the local review board policies. Details on the study population and methods have been published previously.12
Study design and study population
Consecutive patients with AL- and ATTR CA diagnosed at participating centers between January 1, 2017, and December 31, 2020, were included in the analysis. The diagnosis of AL and ATTR CA was confirmed by tissue biopsy or through established noninvasive criteria according to the latest European Society of Cardiology diagnostic guidelines.8 Genetic testing was performed in all patients with ATTR CA to identify mutations in the TTR gene. Patients with AL CA were treated with chemotherapy and/or autologous stem cell transplant according to specific guidelines.13 Patients were systematically followed up from the date of the first cardiologic evaluation at participating referral centers (baseline) until the end of the study period (ie, October 31, 2021). The clinical data recorded within ±1 month from a baseline visit included all of the following: 1) clinical examination including ongoing therapy; 2) ECG; and 3) echocardiography. The same data were collected from the last visit available for each patient during the study period.
Patients with characteristics likely to alter electrocardiographic voltage measurements such as severe pericardial effusion (>20 mm), chronic obstructive lung disease with emphysema, body mass index >35 kg/m2, and paced rhythm were excluded from this analysis.
Electrocardiography
Twelve-lead ECGs were acquired in the supine position with the limb leads placed at the wrists and ankles at standard speed (25 mm/s) and amplification (10 mm/mV). We reviewed them collecting the following data: electrocardiographic machine filter setting; rhythm; PR interval; QRS voltages and duration; depolarization and repolarization abnormalities. Specifically, first-degree atrioventricular block was defined as a PR interval >200 milliseconds; left anterior hemiblock, right bundle branch block, and left bundle branch block as previously defined14; and T-wave inversion as ≥0.1 mV in depth in ≥2 contiguous leads in the absence of right bundle branch block/left bundle branch block. The broadest definition for LQRSV was applied (ie, QRS amplitude ≤5 mm [0.5 mV] in all peripheral leads, including both negative and positive components). We applied this definition throughout this work given its previous consistent use,6,7,15, 16, 17, 18, 19, 20 high specificity,15 and practical applicability in a multicentric project to ensure high reliability and reproducibility between centers. Limb, precordial, and total QRS scores were also calculated by the sum of the Q, R, and S height, each taken as the absolute value in millimeters (1 mm = 0.1 mV), in all 6 limb and precordial leads.6,15
Echocardiography
Echocardiographic images stored on the electronic databases of the participating centers were systematically reviewed and postprocessed for this analysis. All echocardiographic parameters were measured according to the most recent American Society of Echocardiography/European Association of Cardiovascular Imaging guidelines.21 LV mass and LV mass indexed to body surface area were calculated using the Devereux formula, relative wall thickness as 2 × posterior wall thickness in diastole/LV end-diastolic diameter (2 × posterior wall thickness in diastole/left ventricular end-diastolic diameter [LVEDD]), and myocardial volume as LV mass/1.05. Left ventricular ejection fraction was calculated with the biplane Simpson method from volumes acquired in both the 4- and 2-chamber views. Tricuspid annulus peak systolic excursion (TAPSE) was assessed with M-mode in the 4-chamber view, and systolic pulmonary artery pressure was estimated based on tricuspid regurgitation velocity and estimated right atrial pressure. The LV filling pattern (E-wave, A-wave, and deceleration time) was evaluated with pulsed Doppler in the 4-chamber view. The lateral mitral annulus velocity (e′ wave) was assessed with tissue Doppler in the 4-chamber view; the ratio between LV early diastolic filling and lateral mitral annulus velocity (E/e′) was calculated.22 The voltage-to-mass ratio was calculated as the limb or total QRS score divided by LV mass indexed to body surface area.
Nuclear medicine
Cardiac scintigraphy was performed with different bone tracers (99mTc-3,3-diphosphono- 1,2-propanodicarboxylic acid and 99mTc-hydroxymethylene diphosphonate) according to each center’s local practice. A semiquantitative score for the left ventricle was obtained based on the results of planar images as previously described by Perugini et al.23
Biomarkers
Levels of N-terminal pro–B-type natriuretic peptide (NT-proBNP) and B-type natriuretic peptide (BNP) were measured according to local laboratories. High BNP levels were defined as NT-proBNP >1,800 ng/L or BNP >400 ng/L for AL24 and NT-proBNP >3,000 ng/L or BNP >250 ng/L for ATTR.25,26 The National Amyloidosis Centre (NAC) ATTR staging from Gillmore et al25 was measured in ATTR patients, and 3 classes were identified using biomarker cutoffs of 45 mL/min/1.73 m2 for estimated glomerular filtration rate (standard Modified Diet in Renal Disease formula) and 3,000 ng/L for NT-proBNP (or 250 ng/L for BNP).
Outcomes
Survival analysis was calculated with day 1 being the first day of evaluation and electrocardiographic recording at the referral center rather than the day of diagnosis in order to avoid any time referral bias. We collected data regarding overall mortality and cardiovascular (CV) mortality over the study period. However, because a non-negligible proportion of patients died because of severe acute respiratory syndrome coronavirus 2 infection, we decided to perform our survival analysis including only CV mortality as an outcome in order to avoid biases related to the coronavirus disease-2019 pandemic.
Statistical analysis
Baseline characteristics are expressed as mean ± SD when normally distributed (as assessed by the Shapiro-Wilk test) or as median with 25th and 75th percentiles (Q1-Q3) when non-normally distributed. Variables with normal distribution and equal variances were compared with the Student’s t-test, and variables with unequal variances were compared with Welch’s t-test. Variables with non-normal distribution were compared using the Wilcoxon rank sum test. Categoric variables were expressed as absolute numbers and percentages and were compared using the chi-square test or Fisher exact test when appropriate.
Because of the potential high collinearity among covariates and the low ratio between the number of observations and covariates, the multivariable analysis has been based on a penalized generalized linear model (GLM). This class of models includes the lasso or elastic net penalty at a grid of values for the regularization parameter lambda.27 The optimal lambda value was derived via 20-fold cross-validation. LQRSV status was modeled using a logit link in the binomial family of GLM, whereas CV mortality was modeled using a Cox proportional hazard specification of the penalized regression.28 The approximate P values and CIs for the penalized regression were derived.28 Model results were presented as ORs or HRs and the corresponding 95% CIs.
The receiver-operating characteristic (ROC) curves were used to evaluate the accuracy of survival prediction between multiple measures of QRS voltages, including the peripheral QRS score, precordial QRS score, total QRS score, and limb and total voltage-to-mass ratios. The maximum value of the Youden index was then used as the best cutoff to calculate sensitivity and specificity.
The log-rank test and Kaplan-Meier survival analysis were used to explore survival in groups with and without LQRSV. As a sensitivity analysis, competing risk between CV and all-cause mortality was explored using the Fine and Gray model,29 and the cause-specific cumulative incidence functions were estimated.
The possible incremental prognostic role of LQRSV in patients with ATTR CA, when combined with NAC ATTR staging, was first explored by means of the log-rank test and Kaplan-Meier survival analysis and the time-dependent area under the curve (AUC) of the corresponding ROCs.30 Then, a formal evaluation on the incremental value of LQRSV to NAC was conducted by estimating the net reclassification improvement index.31 We defined a P value <0.05 as statistically significant. All statistical analyses were performed using R System (R Foundation for Statistical Computing) and the IBM SPSS Statistics 27.0 package.
Results
Of the 455 patients diagnosed with CA in our centers during the study period, 411 (AL: n = 120, ATTR: n = 291) fulfilled the inclusion criteria and were included in this analysis (Supplemental Figure 1). Among ATTR, 57 patients were diagnosed with ATTR-v (further description of TTR variants is presented in the Supplemental Results).
At the baseline visit, overall 74% of patients were in sinus rhythm, 26% were in atrial fibrillation or atrial flutter, and 6% had a backup pacing cardiac device (2% implantable cardioverter-defibrillator and 4% pacemaker). Most patients were in New York Heart Association (NYHA) functional class I or II (65% in the AL amyloidosis group and 78% in the ATTR amyloidosis group). Among ATTR CA, 21 of 291 (7%) patients, all hereditary forms, were on antiamyloid therapy because of familial amyloid polyneuropathy.
The electrocardiographic machines’ filter setting was deemed appropriate given that in 91% patients low-pass filters of 150 Hz were used. Low QRS voltages at ECG were detected in 169 patients with CA (41%), 66 (55%) with AL CA and 103 (35%) patients with ATTR CA (P < 0.001). The limb and total voltage-to-mass ratios were lower in ATTR CA than AL CA, although they were not significantly different (0.19 vs 0.21 [P = 0.24] and 0.64 vs 0.70 [P = 0.058], respectively). LQRSVs were more common in younger patients (72 ± 11 vs 75 ± 10; P < 0.001) with NYHA class III and higher NT-proBNP/BNP values; more frequent pericardial effusions; and decreased LVEDD, myocardial volume, and TAPSE. Further analysis and description about the differences of patients with AL-CA versus ATTR CA and with LQRSV versus without LQRSV are presented in Supplemental Table 1.
Clinical predictors of LQRSVs in cardiac amyloidosis
Differences in clinical and echocardiographic features of patients with AL CA and ATTR CA according to the presence of LQRSV are reported in Table 1. In both groups, the presence of LQRSV was not associated with body mass index. In patients with AL CA, LQRSV were more frequently observed in those with younger age (P = 0.046), NYHA functional class III (P < 0.001), anterior pseudoinfarction QRS pattern (P < 0.001), high NT-proBNP/BNP values (P = 0.009), greater posterior wall thickness in diastole (P = 0.036), diastolic interventricular septum thickness (P = 0.019), relative wall thickness (P < 0.001), and lower LVEDD (P < 0.001) and TAPSE (P = 0.037). On the other hand, in patients with ATTR CA, LQRSV were less common in those with NAC staging = 1 (P = 0.048) and more common in those with atrial fibrillation (P = 0.013), anterior pseudoinfarction QRS pattern (P = 0.015), lower LV mass (P = 0.029), pericardial effusion (P = 0.005), and lower TAPSE (P = 0.005). Using a penalized GLM analysis, LQRSVs were independently associated with younger age, higher NYHA functional class and natriuretic peptides in AL CA, and pericardial effusion and lower TAPSE in ATTR CA (Table 2).
Table 1.
Baseline Characteristics of the Overall Population Based on the CA Subtype and the Presence of LQRSV
| AL CA |
ATTR CA |
|||||||
|---|---|---|---|---|---|---|---|---|
| Overall (N = 120) | No LQRSV (n = 54) | LQRSV (n = 66) | P Value | Overall (N = 291) | No LQRSV (n = 188) | LQRSV (n = 103) | P Value | |
| Sex, % | M: 78 (65) F: 42 (35) |
M: 37 (69) F: 17 (31) |
M: 41 (62) F: 25 (38) |
0.50 | M: 263 (90) F: 28 (10) |
M: 168 (89) F: 20 (11) |
M: 95 (92) F: 8 (8) |
0.43 |
| Age, y | 66 ± 10 | 68 ± 10 | 64 ± 10 | 0.046 | 77 ± 9 | 77 ± 10 | 76 ± 8 | 0.61 |
| BMI, kg/m2 | 24 ± 3 | 25 ± 4 | 24 ± 3 | 0.39 | 26 ± 3 | 26 ± 3 | 26 ± 3 | 0.85 |
| PM/ICD | 6 (5) | 2 (4) | 4 (6) | 0.57 | 19 (7) | 13 (7) | 6 (6) | 0.72 |
| NYHA functional classa | ||||||||
| II | 55 (47) | 32 (59) | 23 (37) | 0.014 | 176 (62) | 112 (61) | 64 (64) | 0.60 |
| III | 36 (31) | 7 (13) | 29 (46) | <0.001 | 60 (21) | 41 (22) | 19 (19) | 0.55 |
| IV | 5 (4) | 1 (2) | 4 (6) | 0.37 | 2 (0.7) | 1 (1) | 1 (1) | 1.00 |
| GFR, mL/min | 61 ± 30 | 59 ± 30 | 64 ± 31 | 0.52 | 65 ± 20 | 67 ± 20 | 63 ± 20 | 0.15 |
| NT-proBNP >3,000/BNP >250b | — | — | — | 118 (41) | 71 (39) | 47 (48) | 0.17 | |
| NT-proBNP >1,800/BNP >400b | 74 (62) | 27 (50) | 47 (73) | 0.009 | - | - | - | |
| NAC ATTR staging | ||||||||
| 1 | — | — | — | 162 (56) | 113 (60) | 49 (48) | 0.048 | |
| 2 | — | — | — | 108 (37) | 63 (34) | 45 (44) | 0.14 | |
| 3 | — | — | — | 20 (7) | 12 (6) | 8 (8) | 0.88 | |
| TTR variant | — | — | — | 57 (20) | 35 (19) | 22 (21) | 0.57 | |
| Perugini gradec | ||||||||
| 1 | — | — | — | 14 (6) | 11 (6) | 3 (3) | 0.26 | |
| 2 | — | — | — | 79 (32) | 53 (33) | 26 (30) | 0.58 | |
| 3 | — | — | — | 151(61) | 95 (60) | 56 (64) | 0.48 | |
| Electrocardiography | ||||||||
| Atrial fibrillation | 17 (14) | 8 (15) | 9 (14) | 0.83 | 92 (32) | 50 (27) | 42 (41) | 0.013 |
| First-degree AV block | 23/103 (22) | 14/46 (30) | 9/57 (16) | 0.076 | 94/197 (48) | 62/137 (45) | 32/60 (53) | 0.30 |
| LBBB | 3 (3) | 2 (4) | 1 (2) | 0.59 | 38 (13) | 33 (18) | 5 (5) | 0.003 |
| LAH | 49 (41) | 28 (53) | 21 (32) | 0.017 | 101 (35) | 84 (46) | 17 (17) | <0.001 |
| RBBB | 16 (13) | 13 (24) | 3 (5) | 0.002 | 59 (20) | 45 (24) | 14 (14) | 0.039 |
| TWI | 39 (33) | 18 (33) | 21 (32) | 0.86 | 43 (15) | 26 (14) | 17 (17) | 0.52 |
| Anterior pseudoinfarction | 47 (39) | 11 (20) | 36 (55) | <0.001 | 75 (26) | 40 (21) | 35 (34) | 0.015 |
| Limb QRS score | 33 ± 17 | 47 ± 16 | 22 ± 6 | <0.001 | 36 ± 15 | 44 ± 13 | 22 ± 5 | <0.001 |
| Precordial QRS score | 75 ± 25 | 85 ± 26 | 66 ± 22 | <0.001 | 79 ± 24 | 84 ± 25 | 71 ± 20 | <0.001 |
| Total QRS score | 106 ± 40 | 130 ± 38 | 85 ± 28 | <0.001 | 113 ± 33 | 126 ± 31 | 91 ± 23 | <0.001 |
| Echocardiography | ||||||||
| LA diameter, mm | 44 ± 8 | 45 ± 9 | 44 ± 7 | 0.66 | 46 ± 7 | 46 ± 7 | 46 ± 7 | 0.69 |
| IVSd, mm | 16 ± 3 | 15 ± 3 | 16 ± 3 | 0.019 | 18 ± 3 | 18 ± 4 | 17 ± 3 | 0.11 |
| PWTd, mm | 14 ± 3 | 13 ± 3 | 14 ± 3 | 0.036 | 15 ± 3 | 16 ± 3 | 15 ± 3 | 0.087 |
| LVEDD, mm | 44 ± 6 | 47 ± 6 | 42 ± 5 | <0.001 | 46 ± 6 | 46 ± 6 | 46 ± 6 | 0.89 |
| RWT | 0.60 (0.50-0.74) | 0.55 (0.45-0.67) | 0.67 (0.57-0.78) | <0.001 | 0.68 (0.57-0.80) | 0.68 (0.56-0.83) | 0.67 (0.58-0.76) | 0.21 |
| LVMi, g/m2 | 142 (116-168) | 144 (118-165) | 140 (115-167) | 0.64 | 169 (141-209) | 170 (144-216) | 166 (133-195) | 0.029 |
| Limb voltage-to-mass ratio | 0.21 (0.15-0.31) | 0.32 (0.22-0.46) | 0.16 (0.11-0.21) | <0.001 | 0.20 (0.14-0.27) | 0.24 (0.18-0.33) | 0.12 (0.10-0.17) | <0.001 |
| Total voltage-to-mass ratio | 0.70 (0.53-0.99) | 0.88 (0.68-1.22) | 0.60 (0.46-0.73) | <0.001 | 0.64 (0.52-0.83) | 0.70 (0.55-0.87) | 0.55 (0.45-0.68) | <0.001 |
| Myocardial volume | 135 (112-160) | 137 (113-157) | 133 (109-159) | 0.89 | 161 (134-199) | 162 (138-205) | 157 (127-182) | 0.063 |
| Pericardial effusion | 33 (28) | 11 (20) | 22 (33) | 0.15 | 40 (14) | 18 (10) | 22 (22) | 0.005 |
| Maximum size, mm | 5 (4-7) | 6 (4-10) | 5 (4 - 6) | 0.37 | 5 (4-9) | 5 (4-10) | 6 (4-9) | 0.74 |
| LVEF, % | 56 ± 10 | 57 ± 11 | 55 ± 8 | 0.22 | 54 ± 10 | 54 ± 11 | 54 ± 9 | 0.54 |
| E/e’ | 18.3 ± 8.8 | 16.9 ± 9.3 | 19.4 ± 8.3 | 0.18 | 17.2 ± 7.8 | 17.2 ± 8.1 | 16.8 ± 7.1 | 0.53 |
| TAPSE, mm | 19 ± 5 | 20 ± 4 | 18 ± 5 | 0.037 | 19 ± 4 | 19 ± 4 | 17 ± 4 | 0.033 |
| sPAP, mm Hg | 34 ± 11 | 32 ± 10 | 36 ± 11 | 0.078 | 37 ± 12 | 37 ± 12 | 39 ± 12 | 0.17 |
Values are n (%), mean ± SD , or median (Q1-Q3).
AL = light chain amyloidosis; ATTR = transthyretin amyloidosis; AV = atrioventricular; BMI = body mass index; BNP = B-type natriuretic peptide; CA = cardiac amyloidosis; F = female; GFR = glomerular filtration rate; ICD = implantable cardioverter-defibrillator; IVSd = interventricular septum in diastole; LA = left atrium; LAH = left anterior hemiblock; LBBB = left bundle branch block; LQRSV = low QRS voltage; LVEF = left ventricular ejection fraction; LVEDD = left ventricular end-diastolic diameter; LVMi = left ventricular mass index; M = male; NAC = National Amyloidosis Centre; NT-proBNP = N-terminal pro–B-type natriuretic peptide; NYHA = New York Heart Association; PM = pacemaker; PWTd = posterior wall thickness in diastole; RBBB = right bundle branch block; RWT = relative wall thickness; sPAP = systolic pulmonary artery pressure; TAPSE = tricuspid annular peak systolic excursion; TWI = T-wave inversion.
Missing data in AL = 3 and in ATTR = 7.
NT-proBNP and BNP in ng/L; missing data in AL n = 2 and ATTR n = 10.
Bone scintigraphy done in n = 246 ATTR.
Table 2.
Clinical and Echocardiographic Predictors of LQRSV on 12-Lead ECG Based on Multivariable Penalized Regression Models
| AL CA |
ATTR CA |
|||||
|---|---|---|---|---|---|---|
| OR | CI | P Value | OR | CI | P Value | |
| Age, y | 0.942 | 0.907-0.984 | 0.015 | |||
| NT-proBNP >3,000/BNP >250a | 1.477 | 0.997-1.887 | 0.18 | |||
| NT-proBNP >1,800/BNP >400a | 2.992 | 1.067-6.74 | 0.041 | |||
| LVEF, % | 1.012 | 0.979-1.031 | 0.40 | |||
| LVMi, g/m2 | 0.987 | 0.971-1.017 | 0.235 | 0.994 | 0.91-1.091 | 0.097 |
| Pericardial effusion | 2.812 | 1.929-4.100 | 0.008 | |||
| TAPSE, mm | 0.935 | 0.908-0.991 | 0.038 | |||
| NYHA class | 2.33 | 1.242-4.826 | 0.016 | 0.802 | 0.674-1.318 | 0.38 |
| Atrial fibrillation | 1.876 | 0.757-2.268 | 0.28 | |||
| IVSd, mm | 1.215 | 0.80-1.57 | 0.216 | |||
| LVEDD, mm | 0.925 | 0.824-1.047 | 0.138 | |||
Variables with nonzero coefficients in the penalized generalized linear (binomial, logit link) model.
Abbreviations as in Table 1.
NT-proBNP and BNP in ng/L.
Prognosis
During a median follow-up of 33 months (IQR: 21-46), 61 (15%) of 411 patients died of CV causes, 33 (28%, 25 patients with LQRSV) in the AL group and 28 (10%, 16 patients with LQRSV) in the ATTR group (crude percentages). No significant differences in the CV mortality rate were found between ATTR-v and ATTR-wt patients (log-rank P = 0.728) (Supplemental Figure 2).
ROC curve analysis showing the capability and best cutoff of multiple measures of QRS voltages and voltage-to-mass ratios in predicting CV mortality are shown in Supplemental Table 2 and Supplemental Figure 3.
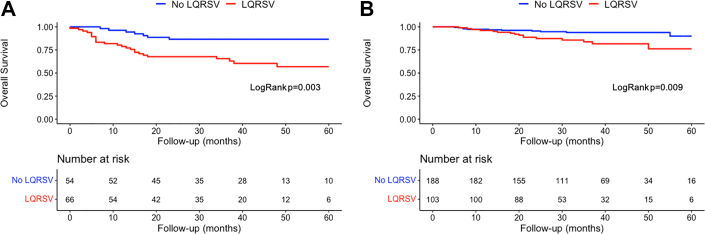
In AL amyloidosis, survival curves indicated that at 40 months there was an approximately 90% chance of survival in the non-LQRSV group compared with 60% in the LQRSV group (log-rank P = 0.003) (Figure 1A). These findings were confirmed also using a competing risk model including CV and all-cause mortality (Fine and Gray HR: 3.14; 95% CI: 1.46-6.75; P = 0.003) (Supplemental Figure 4A). Cox univariable regression analysis showed that LQRSVs were significantly associated with CV mortality (HR: 3.14; 95% CI: 1.41-6.98; P = 0.005) and remained so (HR: 1.76; 95% CI: 2.41-10.18; P = 0.031), also in a penalized Cox proportional hazards survival model including all variables with nonzero coefficients (Table 3).
Figure 1.
Survival Curves Stratified by the Presence or Absence of LQRSV
Survival curves based on Kaplan-Meier survival analysis in patients with (A) light chain cardiac amyloidosis (AL CA) and (B) transthyretin cardiac amyloidosis (ATTR CA) stratified by the presence (red line) or absence (blue line) of low QRS voltages (LQRSV) at baseline 12-lead electrocardiography. Patients with LQRSV, both with AL CA and ATTR CA, manifested a shorter survival compared with those without LQRSV.
Table 3.
Analysis of Predictors of CV Mortality in AL CA and ATTR CA
| Univariable Analysis |
Multivariable Analysis |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| AL CA | ||||||
| Age, y | 1.028 | 0.991-1.067 | 0.13 | 1.055 | 0.995-1.16 | 0.16 |
| NYHA functional class | 6.291 | 2.881-13.735 | <0.001 | 3.216 | 3.564-30.938 | 0.013 |
| GFR, mL/min | 1.004 | 0.990-1.018 | 0.59 | |||
| NT-proBNP >1,800/BNP >400a | 6.875 | 2.088-22.639 | 0.002 | |||
| Peripheral low QRS voltages | 3.143 | 1.414-6.982 | 0.005 | 1.759 | 2.411-10.176 | 0.031 |
| Anterior pseudoinfarction | 1.545 | 0.780-3.061 | 0.21 | |||
| LA diameter, mm | 1.007 | 0.967-1.047 | 0.75 | |||
| IVSd, mm | 1.237 | 1.082-1.413 | 0.002 | 1.35 | 1.284-2.382 | 0.040 |
| PWTd, mm | 1.098 | 0.978-1.234 | 0.11 | 0.78 | 0.355-0.765 | 0.045 |
| LVEDD, mm | 0.938 | 0.879-1.001 | 0.052 | |||
| RWT | 8.823 | 1.244-62.581 | 0.029 | |||
| LVMi, g/m2 | 1.006 | 0.999-1.013 | 0.11 | 1.006 | 0.971-1.021 | 0.47 |
| Total voltage-to-mass ratio | 0.027 | 0.001-0.987 | 0.049 | |||
| Pericardial effusion | 1.433 | 0.690-2.974 | 0.33 | |||
| LVEF, % | 0.970 | 0.941-1.000 | 0.051 | |||
| E/e’ | 1.051 | 1.013-1.091 | 0.008 | |||
| TAPSE, mm | 0.900 | 0.818-0.989 | 0.029 | |||
| sPAP, mm Hg | 1.042 | 1.009-1.076 | 0.013 | |||
| ATTR CA | ||||||
| Age, y | 1.003 | 0.964-1.044 | 0.88 | 0.965 | 0.941-1.194 | 0.63 |
| NYHA functional class | 3.709 | 1.929-7.129 | <0.001 | 1.65 | 2.401-294.712 | 0.038 |
| GFR, mL/min | 0.988 | 0.965-1.012 | 0.34 | |||
| NT-proBNP >3,000/BNP >250a | 6.539 | 2.460-17.376 | <0.001 | |||
| NAC ATTR staging | 4.150 | 2.441-7.054 | <0.001 | 6.945 | 5.145-150.918 | 0.006 |
| Peripheral low QRS voltages | 2.605 | 1.230-5.516 | 0.012 | 2.643 | 1.815-20.166 | 0.005 |
| Anterior pseudonecrosis | 0.965 | 0.410-2.272 | 0.94 | 0.414 | 0.001-2.377 | 0.21 |
| LA diameter, mm | 1.059 | 1.004-1.118 | 0.041 | 0.969 | 0.941-1.038 | 0.34 |
| IVSd, mm | 1.000 | 0.901-1.109 | 0.99 | 0.968 | 0.006-0.694 | 0.08 |
| PWTd, mm | 1.030 | 0.915-1.159 | 0.62 | |||
| LVEDD, mm | 1.010 | 0.948-1.077 | 0.75 | |||
| RWT | 1.399 | 0.194-10.077 | 0.74 | |||
| LVMi, g/m2 | 1.002 | 0.996-1.007 | 0.61 | |||
| Total voltage-to-mass ratio | 0.010 | 0.001-1.977 | 0.088 | |||
| Pericardial effusion | 1.951 | 0.861-4.423 | 0.11 | |||
| LVEF, % | 0.960 | 0.930-0.992 | 0.01 | |||
| E/e’ | 1.031 | 0.986-1.078 | 0.17 | |||
| TAPSE, mm | 0.905 | 0.824-0.994 | 0.04 | |||
| sPAP, mm Hg | 1.016 | 0.987-1.047 | 0.28 | |||
Variables with nonzero coefficients in the penalized Cox proportional hazard model used to estimate CIs for the multivariable model.
Abbreviations as in Table 1.
NT-proBNP and BNP in ng/L.
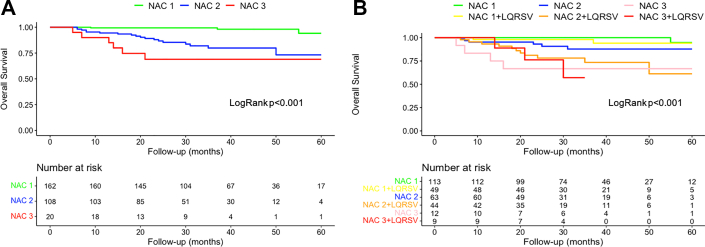
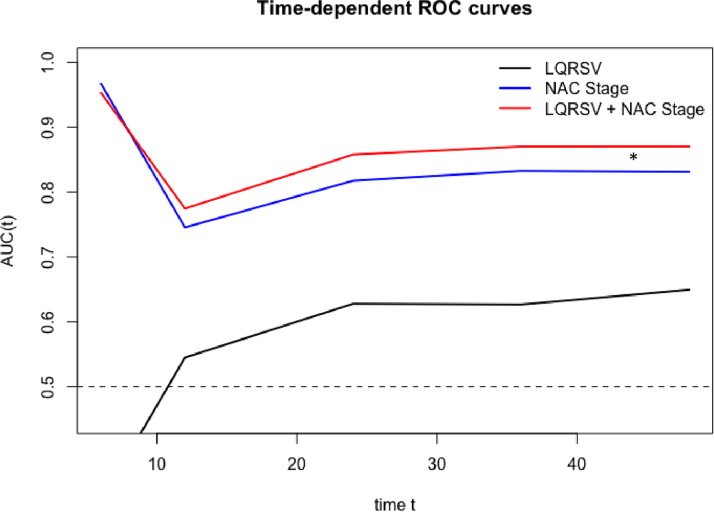
In ATTR amyloidosis, at 40 months there was an approximately 95% chance of survival for the non-LQRSV group compared with 80% for the LQRSV group (log-rank P = 0.009) (Figure 1B). These findings were confirmed also using a competing risk model including CV and all-cause mortality (Fine and Gray HR: 2.66; 95% CI: 1.27-5.56; P = 0.010) (Supplemental Figure 4B). Cox univariable regression analysis showed that LQRSV were significantly associated with CV mortality (HR: 2.61; 95% CI: 1.23-5.52; P = 0.012) and remained so (HR: 2.64; 95% CI: 1.82-20.17; P = 0.005), also in a penalized Cox proportional hazards survival model including all variables with nonzero coefficients (Table 3). Survival probabilities by Kaplan-Meier analysis stratified by NAC stage for the ATTR cohort are shown in Figure 2A. At 36 months, stage I patients had a 99% chance of survival, stage II patients 85%, and stage III patients 67% (P < 0.001). For this model, the time-dependent AUC was 0.83 (95% CI: 0.77-0.89). The competing risk model including CV and all-cause mortality is shown in Supplemental Figure 5A. By adding LQRSV to the NAC staging system (Figure 2B), significant differences in survival probability were found among stage II versus stage II + LQRSV (P = 0.032) but not for stage I versus stage I + LQRSV (P = 0.15) and stage III versus stage III + LQRSV (P = 0.62). The competing risk model including CV and all-cause mortality is shown in Supplemental Figure 5B. For this model, the time-dependent AUC was 0.87 (95% CI: 0.81-0.93), resulting in a further incremental prognostic accuracy for CV mortality with respect to NAC staging (adjusted Blanche’s P < 0.001; net reclassification improvement = 0.24) (Figure 3).
Figure 2.
Survival in ATTR CA Based on NAC Staging System and LQRSV
Survival curves performed with Kaplan-Meier analysis for patients with ATTR CA based on (A) National Amyloidosis Center (NAC) staging system alone and (B) including LQRSV. By adding LQRSV to the NAC staging system, significant differences in survival probability were found among NAC stage II versus NAC stage II + LQRSV (P = 0.032) but not for NAC stage I versus NAC stage I + LQRSV and NAC stage III versus NAC stage III + LQRSV. Abbreviations as in Figure 1.
Figure 3.
Time-Dependent ROC Analysis
The area under the curve (AUC) improves significantly after the first 6 months (adjusted Blanche’s P value <0.001) when LQRSV information is added to NAC stratification. Net reclassification improvement = 0.24 (95% CI: −0.098 to 0.338). ∗P < 0.05 between AUCs up to 36 months of follow-up. ROC = receiver-operating characteristic; other abbreviations as in Figures 1 and 2.
Discussion
This study was designed to assess the prevalence and clinical predictors of electrocardiographic LQRSV in a large population of patients with CA and investigate their prognostic significance in AL- and ATTR CA subtypes. We reported several important findings from this multicenter study (Central Illustration). First, LQRSV were overall a common feature (41%) and were significantly more frequent in AL CA (55%) than in ATTR CA (35%). Second, LQRSV were more commonly identified in younger patients with AL CA with increased LV thickness and right ventricular (RV) dysfunction or in patients with ATTR CA with atrial fibrillation, pericardial effusion, lower LV mass, and RV dysfunction. Third, LQRSV were a significant risk factor and independent predictor for CV mortality in both AL- and ATTR CA. Finally, in patients with ATTR CA, LQRSV, when combined with the NAC staging system, provided incremental prognostic accuracy for CV mortality, in particular in those with NAC stage II.
Central Illustration.
Prevalence, Predictors, and Prognostic Significance of Low QRS Voltages in Cardiac Amyloidosis
Low QRS voltages (LQRSV) are more frequent in light chain cardiac amyloidosis (AL CA) than in transthyretin cardiac amyloidosis (ATTR CA) and are more commonly identified in younger patients with AL CA with a higher New York Heart Association functional class, natriuretic peptides, advanced left ventricular (LV) remodeling and systolic right ventricular (RV) and LV dysfunction or in patients with ATTR CA with atrial fibrillation, pericardial effusion, lower LV mass, and systolic RV dysfunction. Moreover, LQRSVs are a significant risk factor for cardiovascular mortality in both AL- and ATTR CA. NYHA = New York Heart Association; RV = right ventricular; AL = light chain amyloidosis; ATTR = transthyretin amyloidosis; LQRSV = low QRS voltages.
LQRSV in CA
Electrocardiographic LQRSV have been consistently reported as a typical finding of CA.6,7,15, 16, 17, 18, 19, 20,32 Their presence, particularly when combined with a disproportionately increased LV thickness on cardiac imaging, represents 1 of the classic hallmarks of CA, and it is mentioned among the diagnostic red flags for CA according to a recent European consensus document.8 In previously published series, LQRSV were recorded in 25% to 40% of ATTR CA and in up to 68% of AL CA.6,32 Our data from a large multicenter database confirmed these earlier observations (ie, LQRSV are common in CA but not ubiquitous and are more prevalent in AL- than ATTR CA subtype despite both conditions sharing a similar pathogenesis and mechanism of amyloid fibril myocardial infiltration). The (mild) discrepancies in LQRSV prevalence in our study compared with previous studies may be explained by the differences of the method used for the definition of LQRSV, as pointed out by Mussinelli et al.15 In the present study, we used a nadir-to-peak QRS amplitude <5 mm in all limb leads, which is 1 of the most practical and widely used in the clinical setting and has previously demonstrated high values of sensitivity and specificity for CA.15,32
Predictors of LQRSV in CA
Electrocardiographic QRS voltage amplitudes can be altered by multiple conditions that impair either the cardiac generation of electrical signals or their transmission from the heart to body surface.11,33 In CA, both mechanisms may be implicated in QRS voltage attenuation because chronic amyloid deposition may lead to myocyte death, thereby reducing the “electrically active” myocardium, or act as an electrical insulator, increasing the electrical resistance both within and outside the heart.19 Nonetheless, amyloid infiltration does not seem to be the only determinant for LQRSV. Indeed, patients with ATTR CA usually present greater LV thickness and LV mass compared with patients with AL CA,6,34 and this can be hypothesized as being caused by a higher amyloid infiltration burden in ATTR CA. Nevertheless, LQRSV are more common in AL patients, so other pathogenetic mechanisms must be involved. Myocardial inflammation and edema have been recently demonstrated in CA using cardiac magnetic resonance (CMR) imaging,35 and this finding seems more prominent in untreated AL patients given the well-established direct toxicity of the circulating immunoglobulin ALs to the myocardium.36,37 Moreover, cardiac inflammation characterizes other conditions such as acute myocarditis and takotsubo syndrome in which electrocardiographic LQRSV are frequently observed.38 Therefore, edema and inflammation may play a role in QRS voltage reduction in AL CA and may be responsible for the more advanced disease stage of our patients with AL CA with LQRSV, characterized as typical by younger age, worse NYHA functional class, higher natriuretic peptide levels, greater LV thickness, and worse RV dysfunction.
Conversely, pathologic mechanisms underlying LQRSV in ATTR seem more complex. Our ATTR patients with LQRSV compared with those without were characterized by similar NYHA functional class, natriuretic peptide levels, and LV systolic and diastolic function but lower LV mass, lower TAPSE, and more frequent atrial fibrillation and pericardial effusion. Some possible explanations can be speculated. Given that patients with ATTR CA are older and more frequently affected by age-related comorbidities such as long-standing arterial hypertension, aortic stenosis, and metabolic disorders, QRS voltages may likely reflect the hypertrophic remodeling of the heart occurring in these conditions. Alternatively, during the initial phases of amyloid deposition (most of our ATTR patients were in NYHA functional class I or II), hypertrophic compensatory mechanisms in the myocardium able to increase both LV mass and QRS voltages may occur to preserve cardiac performance. On the other hand, LQRSV may be the consequence of a significant loss of “electrically active” LV myocardium caused by amyloid infiltration-related cell death/apoptosis occurring in the more advanced stages of the disease (Figure 4). However, CMR and autopsy studies are needed to confirm this hypothesis. Regarding the relationship between LQRSV and TAPSE, a recent CMR study39 showed that abnormal TAPSE was among the parameters reflecting the highest burden of cardiac amyloid infiltration (extracellular volume >70%). Therefore, the consistent association between LQRSV and worse TAPSE in our cohort may reflect an advanced disease stage represented by severe infiltration and cardiomyocyte loss. Finally, pericardial effusion, a well-known marker of advanced disease,40 played a role as an independent predictor of LQRSV in ATTR, possibly through an electric signal attenuation effect. This finding is in keeping with that of Murtagh et al.20
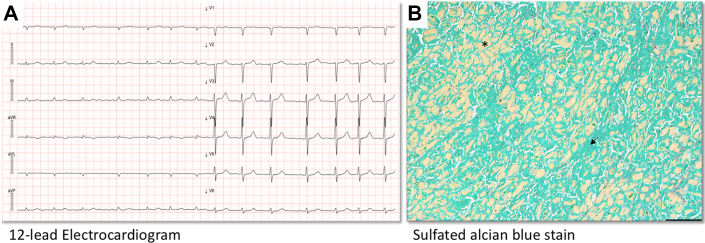
Figure 4.
Electrocardiographic and Histologic Features of a Patient With ATTR CA
Electrocardiographic and histologic features of a 74-year-old patient with ATTR CA who died from refractory heart failure. (A) Basal 12-lead electrocardiogram showing LQRSV. (B) An autopsy specimen of the myocardium showing diffuse and massive amyloid interstitial deposition (black arrow) together with rarefied and atrophic cardiomyocytes (black asterisk) (sulfated Alcian blue stain, scale bar = 200 μm). Abbreviations as in Figure 1.
Prognostic role of LQRSV in CA
In the present study, patients with LQRSV, both with AL CA and ATTR CA, manifested a shorter survival compared with those without LQRSV. Previous work from Kristen et al32 reported that LQRSV were associated with a reduced survival in a smaller cohort of patients with AL CA and in a pooled analysis including AL CA and ATTR CA. Sperry et al19 demonstrated in a U.S.-based study that LQRSV, defined with both limb and Sokolow voltage criteria (S-wave in V1 plus R-wave in V5 or V6 ≤15 mm), predicted all-cause mortality after adjusting for amyloid subtype and other classic predictors of electrocardiographic voltage. Cyrille et al18 reported that a Sokolow index ≤15 mm was independently associated with adverse outcomes in a cohort of U.S. patients with CA including both AL CA and ATTR CA. Our data add important information to this large cohort that allowed for separate analysis of survival in the 2 subsets of patients. We demonstrated that LQRSV were independent predictors of CV mortality in the 2 CA subsets analyzed separately. Interestingly, in ATTR CA, the integration of LQRSV in the NAC ATTR staging system further increased the prognostic performance, particularly in patients classified as NAC stage II. We believe that the lack of an adjunctive prognostic discriminative value of LQRSV even in stage III is most probably related to the small number of patients classified in this subgroup; further studies with larger cohorts might highlight the role of LQRSV in more advanced stages of the disease. With the emerging treatment options available for patients with ATTR CA,41, 42, 43 a precise prognostic stratification, in particular for the intermediate-risk patients such as those in NAC stage II group, becomes of crucial importance. Our study results showed that a cost-effective and noninvasive tool like a 12-lead ECG was able to improve risk stratification overall and specifically in these intermediate-risk patients; if validated, our results could help clinicians to better risk stratify patients with ATTR CA and properly select patients who may benefit from new therapeutic strategies.
Study limitations
The major limitations include the retrospective nature of the study and a possible referral bias because of the recruitment of study patients from tertiary centers in Italy. The limited sample size of patients with ATTR-v and the variability in TTR variants did not allow further analysis of LQRSV significance in this specific population. Sokolov-Lyon index data have not been collected; however, diagnostic performance of this index was demonstrated to be comparable with the peripheral QRS score.15 Electrocardiographic machines and filters were not rigorously standardized between centers, even though low-pass filters (which are those potentially affecting the QRS amplitude the most44) were set at 150 Hz in most centers. Among AL patients, an exact estimation of patients who have already started chemotherapy before our first visit was not available. Nevertheless, to date, no case studies demonstrating QRS voltage modifications after therapy in patients with AL CA are reported in the literature. CMR data were not available or standardized enough in most patients, so they were not included in this analysis. Although data regarding cardiac troponin levels have been collected, significant heterogeneity between centers emerged caused by poor standardization and high variability in assays (regular sensitivity or high sensitivity) and troponin type (T or I) use over time, so these data were omitted from the analysis. As a consequence, we could not apply the Mayo Clinic staging system45 to better characterize the disease severity of our AL patients. Possible relationships between LQRSV occurrence and symptom onset have not been investigated. The relatively low number of adverse events both in AL-CA and ATTR CA may limit the statistical power of the study to rigorously address the prognostic value of LQRSV, even though this is 1 of the largest cohorts presented to date to address this topic.
Conclusions
LQRSV were a common but not ubiquitous electrocardiographic pattern in patients with CA and were more prevalent in the AL- than the ATTR CA subtype, thus suggesting distinct pathophysiologic mechanisms. In both AL- and ATTR CA, LQRSV reflected an advanced disease stage and represented an independent risk factor for CV mortality. In patients with ATTR CA, LQRSV provided an incremental prognostic value over the NAC staging system toward CV mortality in patients with intermediate risk.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: LQRSV are a common feature in CA, particularly in AL CA, and they are associated with a more advanced disease stage. Importantly, LQRSV were associated with a worse CV survival in both conditions and were independent predictors of CV death in ATTR CA. In ATTR CA, they provided an incremental prognostic value to the NAC staging system, especially in intermediate-risk patients.
TRANSLATIONAL OUTLOOK: Further studies on the hypothesized LQRSV mechanisms, including CMR studies and pathology studies, are needed to further investigate this important electrocardiographic feature. Moreover, larger studies with a standardized inclusion of cardiac biomarkers are necessary to understand the significance of LQRSV when compared and integrated with markers of myocardial injury.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgment
This paper was presented at the International Symposium on Amyloidosis, September 7, 2022.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For an expanded Results section and supplemental tables and figures, please see the online version of this paper.
Appendix
References
- 1.Wechalekar A.D., Gillmore J.D., Hawkins P.N. Systemic amyloidosis. Lancet. 2016;387:2641–2654. doi: 10.1016/S0140-6736(15)01274-X. [DOI] [PubMed] [Google Scholar]
- 2.Westin O., Butt J.H., Gustafsson F., et al. Two decades of cardiac amyloidosis: a Danish nationwide study. J Am Coll Cardiol CardioOnc. 2021;3:522–533. doi: 10.1016/j.jaccao.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nativi-Nicolau J., Siu A., Dispenzieri A., et al. Temporal trends of wild-type transthyretin amyloid cardiomyopathy in the Transthyretin Amyloidosis Outcomes Survey. J Am Coll Cardiol CardioOnc. 2021;3:537–546. doi: 10.1016/j.jaccao.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muchtar E., Dispenzieri A., Magen H., et al. Systemic amyloidosis from A (AA) to T (ATTR): a review. J Intern Med. 2021;289:268–292. doi: 10.1111/joim.13169. [DOI] [PubMed] [Google Scholar]
- 5.Griffin J.M., Rosenblum H., Maurer M.S., et al. Pathophysiology and therapeutic approaches to cardiac amyloidosis. Circ Res. 2021;128:1554–1575. doi: 10.1161/CIRCRESAHA.121.318187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rapezzi C., Merlini G., Quarta C.C., et al. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009;120:1203–1212. doi: 10.1161/CIRCULATIONAHA.108.843334. [DOI] [PubMed] [Google Scholar]
- 7.Rahman J.E., Helou E.F., Gelzer-Bell R., et al. Noninvasive diagnosis of biopsy-proven cardiac amyloidosis. J Am Coll Cardiol. 2004;43:410–415. doi: 10.1016/j.jacc.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Pavia P., Rapezzi C., Adler Y., et al. Diagnosis and treatment of cardiac amyloidosis: a position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2021;42:1554–1568. doi: 10.1093/eurheartj/ehab072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rapezzi C., Lorenzini M., Longhi S., et al. Cardiac amyloidosis: the great pretender. Heart Fail Rev. 2015;20:117–124. doi: 10.1007/s10741-015-9480-0. [DOI] [PubMed] [Google Scholar]
- 10.Quarta C.C., Perlini S., Longhi S., et al. A simple voltage/mass index improves diagnosis of cardiac amyloidosis: an electrocardiographic and echocardiographic study of 570 patients with left ventricular hypertrophy. J Am Coll Cardiol. 2012;59:E1586. [Google Scholar]
- 11.Valentini F., Anselmi F., Metra M., et al. Diagnostic and prognostic value of low QRS voltages in cardiomyopathies: old but gold. Eur J Prev Cardiol. 2022;29:1177–1187. doi: 10.1093/eurjpc/zwaa027. [DOI] [PubMed] [Google Scholar]
- 12.Porcari A., Rossi M., Cappelli F., et al. Incidence and risk factors for pacemaker implantation in light-chain and transthyretin cardiac amyloidosis. Eur J Heart Fail. 2022;24:1227–1236. doi: 10.1002/ejhf.2533. [DOI] [PubMed] [Google Scholar]
- 13.Palladini G., Milani P., Merlini G. Management of AL amyloidosis in 2020. Blood. 2020;136:2620–2627. doi: 10.1182/blood.2020006913. [DOI] [PubMed] [Google Scholar]
- 14.Kligfield P., Gettes L.S., Bailey J.J., et al. Recommendations for the standardization and interpretation of the electrocardiogram: part I: the electrocardiogram and its technology: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2007;115:1306–1324. doi: 10.1161/CIRCULATIONAHA.106.180200. [DOI] [PubMed] [Google Scholar]
- 15.Mussinelli R., Salinaro F., Alogna A., et al. Diagnostic and prognostic value of low QRS voltages in cardiac AL amyloidosis. Ann Noninvasive Electrocardiol. 2013;18:271–280. doi: 10.1111/anec.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng Z., Kang L., Tian Z., et al. Utility of combined indexes of electrocardiography and echocardiography in the diagnosis of biopsy proven primary cardiac amyloidosis. Ann Noninvasive Electrocardiol. 2011;16:25–29. doi: 10.1111/j.1542-474X.2010.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubrey S.W., Bilazarian S., LaValley M., et al. Signal averaged electrocardiography in patients with AL (primary) amyloidosis. Am Heart J. 1997;134:994–1001. doi: 10.1016/s0002-8703(97)70017-6. [DOI] [PubMed] [Google Scholar]
- 18.Cyrille N.B., Goldsmith J., Alvarez J., Maurer M.S. Prevalence and prognostic significance of low QRS voltage among the three main types of cardiac amyloidosis. Am J Cardiol. 2014;114:1089–1093. doi: 10.1016/j.amjcard.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 19.Sperry B.W., Vranian M.N., Hachamovitch R., et al. Are classic predictors of voltage valid in cardiac amyloidosis? A contemporary analysis of electrocardiographic findings. Int J Cardiol. 2016;214:477–481. doi: 10.1016/j.ijcard.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 20.Murtagh B., Hammill S.C., Gertz M.A., Kyle R.A., Tajik A.J., Grogan M. Electrocardiographic findings in primary systemic amyloidosis and biopsy-proven cardiac involvement. Am J Cardiol. 2005;95:535–537. doi: 10.1016/j.amjcard.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 21.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. e14. [DOI] [PubMed] [Google Scholar]
- 22.Nagueh S.F., Smiseth O.A., Appleton C.P., et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–1360. doi: 10.1093/ehjci/jew082. [DOI] [PubMed] [Google Scholar]
- 23.Perugini E., Guidalotti P.L., Salvi F., et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol. 2005;46:1076–1084. doi: 10.1016/j.jacc.2005.05.073. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S., Dispenzieri A., Lacy M.Q., et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30:989–995. doi: 10.1200/JCO.2011.38.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gillmore J.D., Damy T., Fontana M., et al. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. 2018;39:2799–2806. doi: 10.1093/eurheartj/ehx589. [DOI] [PubMed] [Google Scholar]
- 26.Nakashima N., Takashio S., Morioka M., et al. A simple staging system using biomarkers for wild-type transthyretin amyloid cardiomyopathy in Japan. ESC Heart Fail. 2022;9:1731–1739. doi: 10.1002/ehf2.13847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedman J., Hastie T., Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- 28.Simon N., Friedman J., Hastie T., Tibshirani R. Regularization paths for Cox’s proportional hazards model via coordinate descent. J Stat Softw. 2011;39:1–13. doi: 10.18637/jss.v039.i05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 30.Blanche P., Dartigues J.F., Jacqmin-Gadda H. Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Stat Med. 2013;32:5381–5397. doi: 10.1002/sim.5958. [DOI] [PubMed] [Google Scholar]
- 31.Pencina M.J., D’Agostino R.B., Sr., Steyerberg E.W. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kristen A.V., Perz J.B., Schonland S.O., et al. Non-invasive predictors of survival in cardiac amyloidosis. Eur J Heart Fail. 2007;9:617–624. doi: 10.1016/j.ejheart.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Maanja M., Wieslander B., Schlegel T.T., et al. Diffuse myocardial fibrosis reduces electrocardiographic voltage measures of left ventricular hypertrophy independent of left ventricular mass. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.003795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quarta C.C., Solomon S.D., Uraizee I., et al. Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation. 2014;129:1840–1849. doi: 10.1161/CIRCULATIONAHA.113.006242. [DOI] [PubMed] [Google Scholar]
- 35.Kotecha T., Martinez-Naharro A., Treibel T.A., et al. Myocardial edema and prognosis in amyloidosis. J Am Coll Cardiol. 2018;71:2919–2931. doi: 10.1016/j.jacc.2018.03.536. [DOI] [PubMed] [Google Scholar]
- 36.Brenner D.A., Jain M., Pimentel D.R., et al. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ Res. 2004;94:1008–1010. doi: 10.1161/01.RES.0000126569.75419.74. [DOI] [PubMed] [Google Scholar]
- 37.Guan J., Mishra S., Qiu Y., et al. Lysosomal dysfunction and impaired autophagy underlie the pathogenesis of amyloidogenic light chain-mediated cardiotoxicity. EMBO Mol Med. 2014;6:1493–1507. doi: 10.15252/emmm.201404190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perazzolo Marra M., Zorzi A., Corbetti F., et al. Apicobasal gradient of left ventricular myocardial edema underlies transient T-wave inversion and QT interval prolongation (Wellens’ ECG pattern) in Tako-Tsubo cardiomyopathy. Heart Rhythm. 2013;10:70–77. doi: 10.1016/j.hrthm.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Boldrini M., Cappelli F., Chacko L., et al. Multiparametric echocardiography scores for the diagnosis of cardiac amyloidosis. J Am Coll Cardiol Img. 2020;13:909–920. doi: 10.1016/j.jcmg.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 40.Damy T., Jaccard A., Guellich A., et al. Identification of prognostic markers in transthyretin and AL cardiac amyloidosis. Amyloid. 2016;23:194–202. doi: 10.1080/13506129.2016.1221815. [DOI] [PubMed] [Google Scholar]
- 41.Griffin J.M., Rosenthal J.L., Grodin J.L., Maurer M.S., Grogan M., Cheng R.K. ATTR amyloidosis: current and emerging management strategies: JACC: CardioOncology state-of-the-art review. J Am Coll Cardiol CardioOnc. 2021;3:488–505. doi: 10.1016/j.jaccao.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Emdin M., Aimo A., Rapezzi C., et al. Treatment of cardiac transthyretin amyloidosis: an update. Eur Heart J. 2019;40:3699–3706. doi: 10.1093/eurheartj/ehz298. [DOI] [PubMed] [Google Scholar]
- 43.Porcari A, Fontana M, Gillmore JD. Transthyretin cardiac amyloidosis. Cardiovasc Res. Published online August 5, 2022. https://doi.org/10.1093/cvr/cvac119. [DOI] [PMC free article] [PubMed]
- 44.Kligfield P., Okin P.M. Prevalence and clinical implications of improper filter settings in routine electrocardiography. Am J Cardiol. 2007;99:711–713. doi: 10.1016/j.amjcard.2006.09.123. [DOI] [PubMed] [Google Scholar]
- 45.Bianchi G., Zhang Y., Comenzo R.L. AL amyloidosis: current chemotherapy and immune therapy treatment strategies: JACC: CardioOncology state-of-the-art review. J Am Coll Cardiol CardioOnc. 2021;3:467–487. doi: 10.1016/j.jaccao.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.