Abstract
Amyloid light chain (AL) amyloidosis is a rare, debilitating, often fatal disease. Symptoms of cardiomyopathy are common presenting features, and patients often are referred to cardiologists. Cardiac amyloid infiltration is the leading predictor of death. However, the variable presentation and perceived rarity of the disease frequently lead to delay in suspecting amyloidosis as a cause of heart failure, leading to misdiagnoses and a marked delay in diagnosis, with devastating consequences for the patient. A median time from symptom onset to correct diagnosis of about 2 years is often too long when median survival from diagnosis for patients with AL amyloidosis and cardiomyopathy is 4 months to 2 years. The authors highlight the challenges to diagnosis, identify gaps in the current knowledge, and summarize novel treatments on the horizon to raise awareness about the critical need for early recognition of symptoms and diagnosis of AL amyloidosis aimed at accelerating treatment and improving outcomes for patients.
Key Words: AL amyloidosis, awareness, diagnosis, future therapies
Abbreviations and Acronyms: AL, amyloid light chain; ASCT, autologous stem cell transplantation; ATTR, transthyretin; CMR, cardiac magnetic resonance imaging; CR, complete response; CyBorD, cyclophosphamide-bortezomib-dexamethasone; FLC, free light chain; Ig, immunoglobulin; LGE, late gadolinium enhancement; NT-proBNP, N-terminal pro–brain natriuretic peptide; PCD, plasma cell dyscrasia; QoL, quality of life; VGPR, very good partial response
Central Illustration
Highlights
-
•
There is an acute need for increased awareness of AL amyloidosis.
-
•
Early diagnosis and correct treatment selection are critical in AL management.
-
•
Combined targeted amyloid removal with current therapies may improve outcomes.
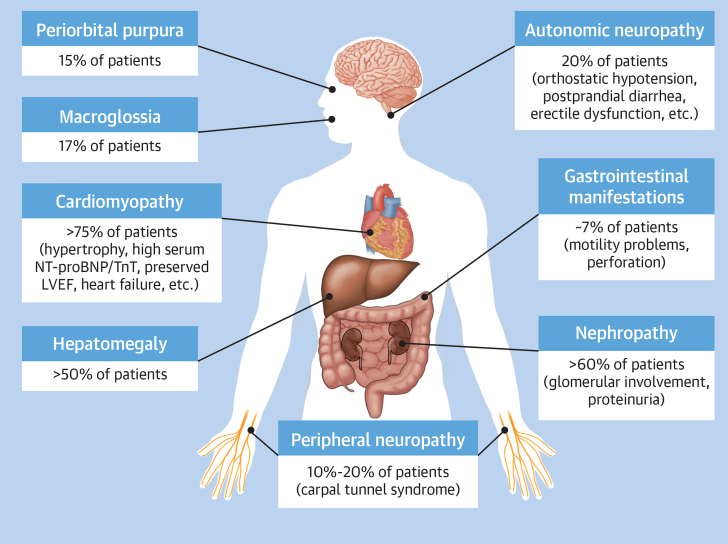
Amyloid light chain (AL) amyloidosis is a rare, severe, progressive, debilitating, systemic disease that is slightly more common in men, with a median age at diagnosis of about 65 years.1, 2, 3, 4 Median survival depends on the cardiac stage of the disease at diagnosis.5 The median survival is nearly 5 years in contemporary cohorts. However, up to 43% of patients may die in the first year.6 The median survival of patients with very advanced cardiac disease is only 4 months and is about 2 years among patients with less severe cardiac involvement.7 The introduction of new therapeutic agents and regimens and earlier diagnosis have improved overall survival of patients.8, 9, 10, 11 The underlying etiology of AL amyloidosis is clonal plasma cell expansion (plasma cell dyscrasia [PCD]), producing amyloidogenic immunoglobulin (Ig) light chains that aggregate to form insoluble fibrils, which deposit in tissues and cause organ dysfunction.1, 2, 3, 4,12,13 In 80% of cases, the amyloidogenic light chain is the λ subtype.13 Preclinical studies demonstrated the proteotoxicity of the amyloidogenic light chains, especially in cardiac tissue.14,15 The heart is the most commonly involved organ in AL amyloidosis, with >75% of patients exhibiting symptoms of cardiac amyloid infiltration at diagnosis.16,17 As shown in Figure 1, other common presentations at diagnosis among patients with AL amyloidosis include nephropathy (>60% of patients), hepatomegaly (>50% of patients), neuropathy (10%-20% of patients), macroglossia (17% of patients), and periorbital purpura (15% of patients).2,13,17,18
Figure 1.
Common Pathologies Among Patients With Amyloid Light Chain Amyloidosis
This figure illustrates the systemic nature of amyloid light chain amyloidosis, the number of organs and tissues that are affected, the percentage of patients in whom these organs are affected at diagnosis, and some commonly observed symptoms for the affected organ or system. LVEF = left ventricular ejection fraction; NT-proBNP = N-terminal pro–brain natriuretic peptide; TnT = troponin T.
Cardiac amyloid infiltration is the leading cause of death, accounting for >61% of fatalities and the primary determinant of survival in patients with AL amyloidosis.13,19 Cardiovascular events leading to death include progressive heart failure or arrhythmias, although the exact cause is often unclear.19 Common cardiovascular complications include atrial or ventricular (less common, but often fatal) arrhythmia, heart failure, embolism, stroke, and conduction delays, including advanced degrees of atrioventricular block. Patients with cardiac involvement experience the greatest impact on all aspects of quality of life (QoL), including physical health, work productivity, and emotional health, which includes depression and role limitation, compared with patients with involvement of other organs.20,21
Cardiac amyloidosis is often listed as a cause of heart failure with preserved ejection fraction, but this does not fully characterize the functional phenotype that typically shows both systolic and diastolic impairment.13,19 In patients with AL amyloidosis, amyloid infiltration results in symmetrical biventricular wall thickening with nondilated or small ventricles. Ejection fraction is typically normal until late disease stage.22 The reduction in systolic function disproportionally affects the longitudinal rather than the radial axes.22 This is much more marked than other causes of thick-walled heart failure, and the reduction in longitudinal strain typically spares the apex, giving the characteristic relatively apical sparing picture (“bull’s-eye pattern”) on parametric longitudinal strain polar maps.23 Initial infiltration in the left ventricle is characterized by impaired relaxation, which then invariably progresses to typical restrictive physiology. Nonspecific extensively described findings include thickening of the valves and the interatrial septum and a speckled appearance of the myocardium. Pericardial and pleural effusions are relatively common findings.22
AL amyloidosis is difficult to diagnose because many of the presenting symptoms are not specific to this disease.2,12,24,25 Delays in diagnosis and misdiagnoses are common and result in increased symptom burden, cumulative organ damage, poorer prognosis, and higher early mortality.25, 26, 27, 28 Rapid accurate diagnosis within 6 months of symptom onset correlated positively with survival, whereas diagnostic delay (>6 months) correlated with poor survival.10 Worsening organ damage due to diagnostic delay limits treatment options, such as making patients ineligible for autologous stem cell transplantation (ASCT) or having poorer tolerance to standard-of-care chemotherapy. As many patients present with cardiovascular symptoms or complications, they are often referred to cardiologists by their primary care physicians.26,29 A survey of patients and caregivers indicated that about 44% of patients initially were misdiagnosed and treated as having unrelated cardiomyopathy, with 3 of 4 cardiologists surveyed reporting that lack of disease awareness was the most common cause of misdiagnosis.26 Delays in diagnosis can increase the risk for death 3- to 5-fold.30 Thus, it is critical to increase awareness among health care providers to ensure timely diagnosis and improved outcomes.
Previously published state-of-the art reviews focused on the utility of supportive therapy for cardiac involvement, chemotherapy, or immunotherapy.31,32 This review, focusing on cardiac aspects of AL amyloidosis, builds on that foundation, with the aim of improving awareness about suspicion, diagnosis, gaps in treatment and response evaluations, and recent advances in the management of this disease (Central Illustration).
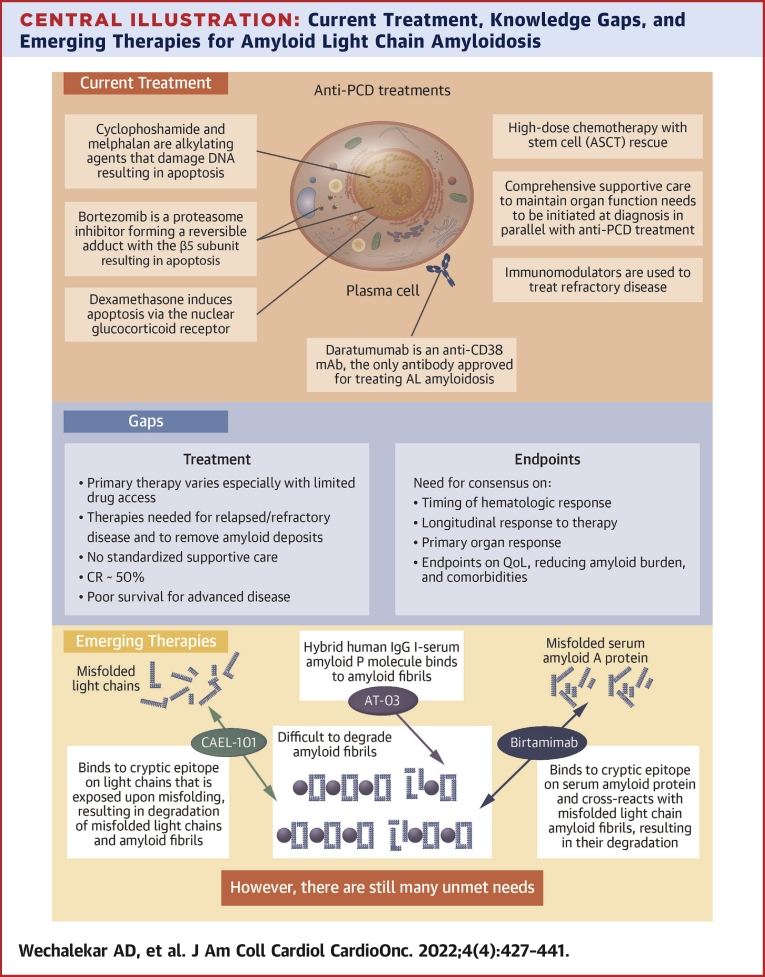
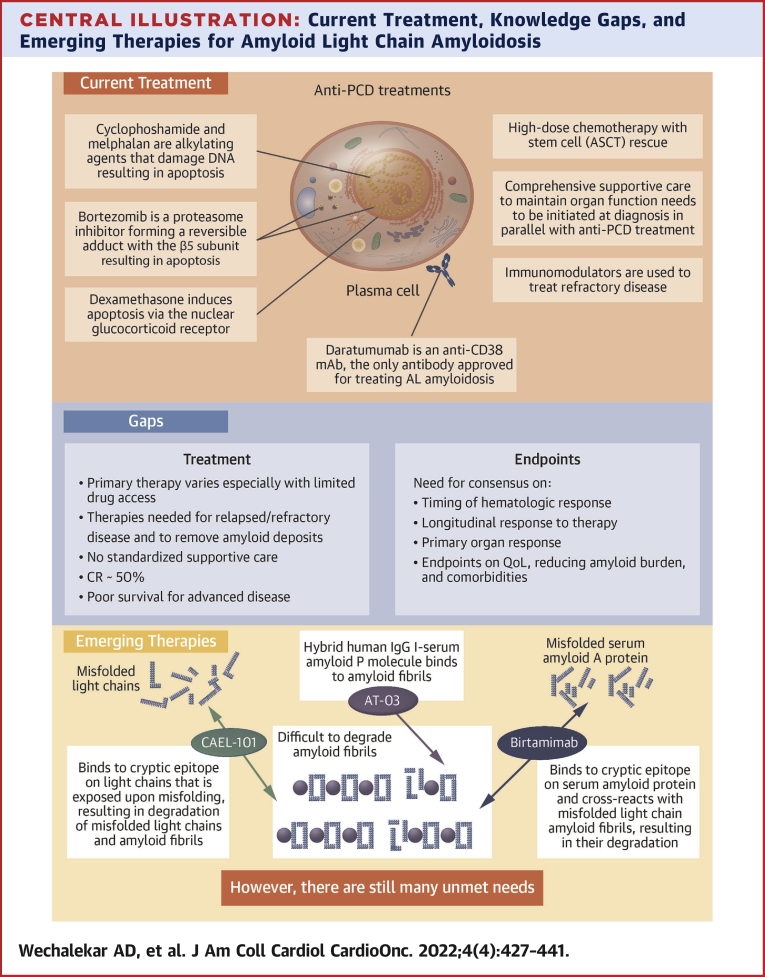
Central Illustration.
Current Treatment, Knowledge Gaps, and Emerging Therapies for Amyloid Light Chain Amyloidosis
This figure summarizes (A) the current treatment paradigm, (B) the gaps that still exist in treatment and in relevant endpoints, and (C) emerging therapies that target removal of existing amyloid fibril deposits from organs and tissues. AL = amyloid light chain; ASCT = autologous stem cell transplantation; CR = complete response; IgG = immunoglobulin G; PCD = plasma cell dyscrasia; QoL = quality of life.
Diagnosis
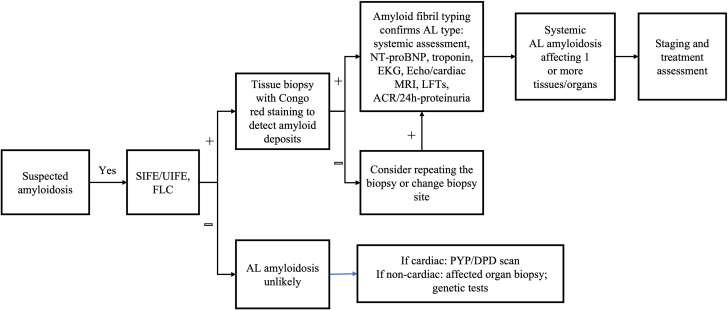
Most of the symptoms and clinical manifestations of AL amyloidosis are nonspecific.33,34 Diagnosis of AL amyloidosis is a multistep process (Figure 2).25,35 The first step is suspicion of amyloidosis, which initiates specific diagnostic tests to verify the diagnosis.25,35 This is followed by tests to identify the amyloid fibril type, which is critical to differentiate AL amyloidosis from other subtypes.25,35 The final step of diagnosis is to identify the organs involved and the extent to which they are affected by the deposition of amyloid fibrils.25,35 This forms the basis of accurate diagnosis and a risk-adapted treatment plan for patients with AL amyloidosis.
Figure 2.
Algorithm for Diagnosing AL Amyloidosis
This figure illustrates the current systematic, stepwise process for diagnosing amyloid light chain (AL) amyloidosis and differentiating it from other cardiomyopathies. It identifies each type of test that is essential for suspicion of AL amyloidosis, diagnosing the disease and typing of the amyloidogenic free light chains (FLCs). ACR = albumin/creatinine ratio; DPD = 99mTc-3-diphosphono-1,2-propanodicarboxylic acid; Echo = echocardiography; EKG = electrocardiography; LFT = liver function test; MRI = magnetic resonance imaging; NT-proBNP = N-terminal pro–brain natriuretic peptide; PYP = 99mTc-pyrophosphate; SIFE = serum immunofixation electrophoresis; UIFE = urine immunofixation electrophoresis.
Step 1: suspicion of amyloidosis
Suspicion of amyloidosis should be very high when a patient presents with heart failure combined with a constellation of unexplained extracardiac symptoms such as neuropathy, bleeding, carpal tunnel syndrome, nephrotic syndrome, proteinuria, diarrhea, hepatomegaly, peripheral and autonomic neuropathy, macroglossia, and periorbital purpura.2,35, 36, 37 Another key cardiovascular presentation that should trigger suspicion of amyloidosis is an increase in left ventricular mass or wall thickness in the absence of hypertension or the presence of hypotension, especially when associated with increased right ventricular mass.35,36,38 Cardiovascular findings that suggest amyloidosis include restrictive cardiomyopathy with disproportionately impaired longitudinal function compared with radial contraction (apical sparing on longitudinal strain imaging) and low-voltage electrocardiogram or pseudoinfarct pattern.35, 36, 37, 38 Elevated serum cardiac troponin T and/or N-terminal pro-brain natriuretic peptide (NT-proBNP) are common in patients with cardiac AL amyloidosis.2,35,37 Although macroglossia and periorbital purpura are considered pathognomonic for AL amyloidosis, they occur in only about 15% of patients.2 Although no single symptom is specific for AL amyloidosis, it should be suspected when there is a combination of cardiomyopathy and/or other aforementioned nonspecific symptoms.
Step 2: diagnosis of amyloidosis
Once amyloidosis is suspected, there are several tests that can be undertaken to verify the diagnosis, detect the presence of an underlying PCD, and determine which organs are involved (Figure 3). Free light chain (FLC) assays can detect abnormal levels of Ig light chain in serum.39, 40, 41 Serum and urine immunofixation electrophoresis assays are used to quantify and determine the type of abnormal Ig light chains or monoclonal protein.40, 41, 42 Positive results in these assays would indicate the presence of a monoclonal gammopathy of undetermined significance, multiple myeloma, indolent B cell lymphoma, Waldenström macroglobulinemia, or AL amyloidosis.40, 41, 42, 43 The combination of these assays can be very effective in detecting amyloidogenic light chain in most patients with AL amyloidosis.43 The absence of a detectable monoclonal protein in the serum and urine by immunofixation electrophoresis and serum FLC assays makes AL amyloidosis less likely. Congo red staining of a tissue biopsy sample is required for confirmation of the diagnosis. Abdominal fat pad biopsies are a fast and safe option to detect amyloid deposits.44 Although the presence of amyloid deposits by Congo red staining in fat pad confirms the diagnosis of amyloidosis, their absence does not rule out disease.44 Bone marrow examination is an essential component of AL amyloidosis diagnosis. Combination of abdominal fat and bone marrow biopsies will identify 85% of patients with AL amyloidosis.45 In case of a negative biopsy result, alternative sites (labial salivary glands, gut) or a biopsy of the affected organ (eg, heart, kidney) should be considered.4 There are structural and functional differences between cardiac AL amyloidosis and other hypertrophic cardiomyopathies, but significant overlap exists.46 Echocardiography can provide an assessment of the likelihood of cardiac amyloid infiltration vs other hypertrophic cardiomyopathies and can assess the severity of cardiac involvement.23,47, 48, 49 Although echocardiography is useful in patients with proven amyloidosis for both diagnosis and monitoring of heart involvement, the overlap of echocardiographic features among multiple diseases makes echocardiography poorly suited to rule out the diagnosis definitively.23 Cardiac magnetic resonance imaging (CMR) offers accurate information regarding the heart’s structure and function with precision advantages over echocardiography; the key is its unique ability to give information about the tissue composition by “myocardial tissue characterization,” making it a highly sensitive and specific imaging modality to distinguish cardiac amyloidosis from other hypertrophic phenocopies.38,50, 51, 52, 53, 54, 55, 56, 57, 58, 59 CMR with late gadolinium enhancement (LGE) can visualize the continuum of cardiac infiltration, from subendocardial LGE to increasing transmurality as the disease progresses.52,60,61 When combined with T1 mapping, CMR with LGE can be used to characterize and measure the degree of cardiac amyloid infiltration with 70% to 80% accuracy in patients with known systemic AL amyloidosis.62, 63, 64, 65, 66, 67, 68, 69 It should be mentioned that although CMR is sensitive for distinguishing AL from other cardiomyopathies, it may not be suggestive in patients with early involvement of the heart by AL amyloidosis (ie, the absence of LGE on CMR does not rule out early cardiac involvement). Extracellular volume mapping should be considered in the CMR protocol to exclude early cardiac amyloid infiltration.
Figure 3.
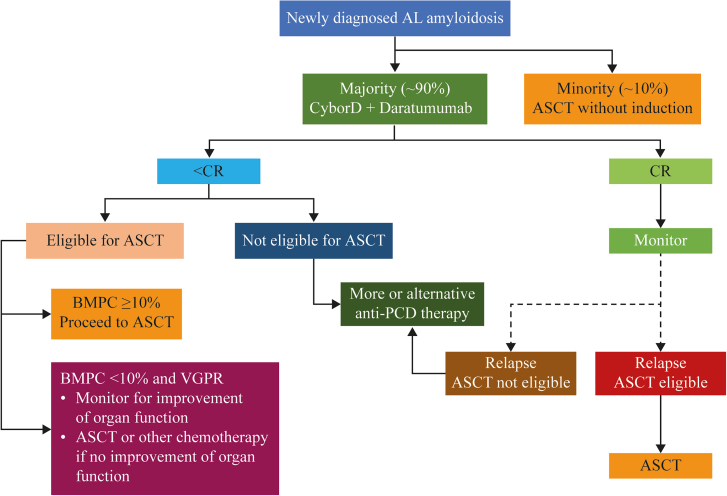
Algorithm for Treating Patients With AL Amyloidosis
This figure illustrates the current approaches to treatment after diagnosis of amyloid light chain (AL) amyloidosis. It includes the decision process in determining which first-line treatment to administer and what to do on the basis of the outcomes of that treatment regimen. ASCT = autologous stem cell transplantation; BMPC = bone marrow plasmacytosis; CR = complete response; CyBorD. = cyclophosphamide-bortezomib-dexamethasone; PCD = plasma cell dyscrasia; VGPR = very good partial response.
Step 3: typing and confirming diagnosis of AL amyloidosis
It is critical to identify the type of amyloid fibril to avoid misdiagnosis and initiation of incorrect or inappropriate treatment, which could have disastrous consequences for a patient. This has become even more important with increasing recognition of cardiac amyloidosis in older patients (differential diagnosis between wild-type transthyretin [ATTR] and AL amyloidosis). The most common methods of amyloid fibril typing include immunohistochemistry or laser capture, followed by mass spectrometry to identify the amyloid subtype.70,71 As the accuracy of immunohistochemistry is dependent on the expertise of the laboratory and needs an extensive panel of antibodies for accurate reporting, laser capture with mass spectrometry has become the method of choice for amyloid fibril typing.70
Biopsy has been an essential step in diagnosis of amyloidosis until recent advances, showing accurate diagnosis of cardiac ATTR amyloidosis using 99mTc-labeled bone scintigraphy tracers, a noninvasive procedure.72 Specifically, in the absence of a detectable monoclonal protein in blood or urine by immunofixation and normal serum FLCs, grade 2 or greater uptake on 99mTc-pyrophosphate or 99mTc-3-diphosphono-1,2-propanodicarboxylic acid scintigraphy is considered diagnostic of cardiac ATTR amyloidosis.72 Conversely, among patients who present with abnormal results on serum protein electrophoresis or FLC assays, no cardiac uptake on 99mTc-pyrophosphate or 99mTc-3-diphosphono-1,2-propanodicarboxylic acid scan can only exclude cardiac ATTR amyloidosis but crucially does not rule out cardiac AL amyloidosis.73,74 Indeed, in AL amyloidosis, a full nonbiopsy path to diagnosis does not exist.
For cardiac AL amyloidosis, different centers may have different approaches as it pertains to cardiac biopsy, given the associated risks of cardiac biopsy and given that noninvasive cardiac imaging confirming cardiac involvement along with Congo red staining and typing of a noncardiac biopsy can diagnose AL amyloidosis with high sensitivity.58,75 However, if there is high clinical suspicion of cardiac AL amyloidosis, cardiac biopsy should be performed if biopsy of noncardiac sites is negative.76
Current Standard of Care
The current standard of care is based on observations of improved overall survival and organ function when synthesis of amyloidogenic light chain is suppressed or stopped, along with supportive care to address specific organ dysfunction.42 The response to treatment requires dual assessment: 1) primary assessment of hematologic response of the underlying clone to chemotherapy, which can manifest within a few weeks of treatment initiation; and 2) assessment of change in organ function for organ response or progression.77,78 Improved organ function is usually delayed by several months (median 10.4 months), and only 25% to 50% of all patients treated with current chemotherapy regimens achieve an organ response.79 The criteria for evaluating hematologic and organ responses are given in Table 1.80,81
Table 1.
Criteria for Treatment Response
| Category | Response | Criteria |
|---|---|---|
| Hematologic76 | Complete response | Absence of amyloidogenic light chains (either free and/or as part of a complete immunoglobulin) defined by negative IFE of both serum and urine AND Either an FLC ratio within the reference range or uninvolved FLC concentration greater than involved FLC concentration with or without an abnormal FLC ratio |
| Very good partial response | dFLC <40 mg/L | |
| Partial response | dFLC decrease >50% from baseline | |
| No response | All other patients | |
| Cardiac75 | Improvement | Decrease in NT-proBNP by >30% AND >300 pg/mL decrease (if baseline NT-proBNP >650 pg/mL)a OR ≥2-point decrease in NYHA functional class (if baseline NYHA functional class III or IV) |
| Renal75,78 | Improvement | Proteinuria decreases ≥30% or a drop in proteinuria to <0.5 g/24 h in patients without renal progressionb (>25% decrease in eGFR) |
| Hepatic75,79 | Improvement | Decrease in abnormal alkaline phosphatase levels by ≥50% OR Decrease in radiographic liver size by ≥2 cm |
dFLC = difference between involved and uninvolved free light chain; eGFR = estimated glomerular filtration rate; FLC = free light chain; IFE = immunofixation electrophoresis; NT-proBNP = N-terminal pro–brain natriuretic peptide; NYHA = New York Heart Association.
Patients with baseline NT-proBNP ≤ 650 pg/mL are not considered to have cardiac involvement.
Renal progression is defined as a >25% decrease in eGFR.
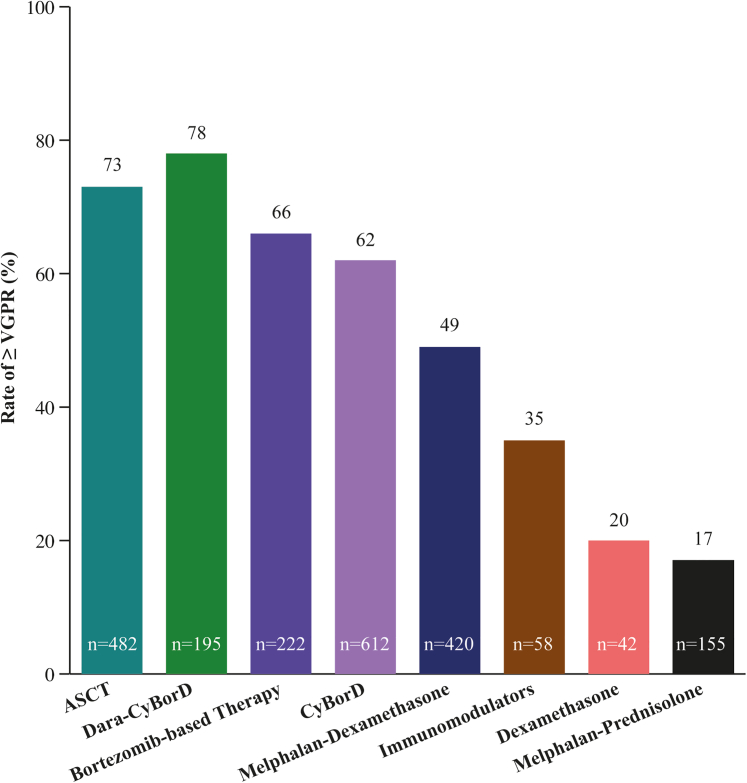
At diagnosis, patients are first evaluated to determine whether they are eligible to receive ASCT (∼20% of patients) or will be receiving combination chemotherapy (∼80% of patients) (Figure 3).82,83 The criteria for ASCT are very strict to minimize treatment-related mortality, and many patients opt to not undergo the procedure even when eligible.84 Data suggest better outcomes for patients who undergo induction chemotherapy prior to ASCT, with the caveat that a small proportion of patients may become ASCT ineligible during induction treatment.85 Among patients receiving ASCT as first-line therapy to treat AL amyloidosis, 73% exhibited complete response (CR) or very good partial response (VGPR) as determined by hematologic response criteria (Figure 4).9,86 Patients not eligible for ASCT (∼80%) and those who have opted not to receive it are treated with one of several options of anti-PCD therapy.77,87 Current guidelines recommend a combination of cyclophosphamide, bortezomib, and dexamethasone (CyBorD) and daratumumab as the preferred first-line anti-PCD therapy, with CyBorD and bortezomib-melphalan-dexamethasone as alternatives when there is limited access to daratumumab.87 Efficacy of the most commonly used first-line treatments with respect to hematologic response are shown in Figure 4. The hematologic efficacy of daratumumab-CyBorD is very high, with 78% of patients achieving VGPR or better.88, 89, 90, 91 More than 20% of patients discontinue at least 1 therapy, and >30% of patients reduce treatments because of adverse events.92 Patients who present with significant neuropathy can be treated with regimens that avoid bortezomib and use daratumumab monotherapy. Ixazomib may be useful in patients with relapsed disease who have not been previously exposed to a proteasome inhibitor.87,93, 94, 95, 96
Figure 4.
Hematologic Response of Patients With Amyloid Light Chain Amyloidosis to First-Line Therapies
This figure compares the efficacies of different first-line treatments on the basis of hematologic response criteria of very good partial response (VGPR) or better and using autologous stem cell transplantation (ASCT) as the benchmark. The numbers in parentheses indicate the number of patients on which these data are based. CyBorD = cyclophosphamide-bortezomib-dexamethasone; Dara = daratumumab.
Orthostatic hypotension, a highly debilitating symptom experienced by patients with AL amyloidosis as a consequence of autonomic neuropathy, can be managed by adjunct supportive treatment, including wearing compression garments and medications such as midodrine or droxidopa.2,12,97 Although fludrocortisone may help some patients, it often causes fluid retention and is often not well tolerated.2
Antifibril Treatments in Development
At the present time, 2 antibodies, birtamimab and CAEL-101, are being investigated as antifibril agents.98, 99, 100, 101, 102, 103, 104, 105, 106 Dezamizumab, another such agent, is no longer being investigated. Antifibril antibodies have the potential to remove existing amyloid fibrils from organs by activating immune cells to cause chemical and enzymatic degradation of existing amyloid fibrils and inducing antibody-dependent phagocytosis of the fibrils.107,108 In addition, although this utility has not yet been adequately investigated, antifibril antibodies have the potential to be used as diagnostic agents to image amyloid deposits and for amyloid typing on tissue biopsies.
Birtamimab
Birtamimab (NEOD0001) is a fully humanized monoclonal antibody that targets a cryptic epitope on serum amyloid A protein that is revealed when misfolded.109 It cross-reacts with Ig light chain amyloid fibrils and reportedly activates macrophage-mediated degradation and clearance of light chain fibrils. A first-in-human phase 1/2 study (NCT01707264) of intravenous birtamimab given every 4 weeks was conducted in 27 patients who had experienced at least a partial hematologic response to prior light chain–suppressive chemotherapy but had persistent organ dysfunction. The treatment was well tolerated, with no dose-limiting toxicities or discontinuations due to drug-related adverse events. Organ responses were observed in 57% of cardiac-evaluable patients and 60% of renal-evaluable patients.110 Birtamimab, given as an intravenous infusion every 28 days, was well tolerated up to 24 mg/kg and demonstrated cardiac or renal response in the majority of patients, with the remainder having stable disease.110 In some cases, organ response was delayed by several months relative to hematologic response.111 On the basis of these promising results, the phase 3 VITAL study (NCT02312206) randomized 260 patients with newly diagnosed AL amyloidosis with cardiac involvement to receive standard-of-care chemotherapy plus either birtamimab or placebo. A futility analysis performed after 103 primary endpoint events (time to all-cause mortality or cardiac hospitalization) showed no advantage for the birtamimab arm, and the study was therefore terminated. However, a post hoc analysis identified a benefit for both primary endpoints in patients with Mayo stage IV cardiac disease, with median survival of 8.3 months in the placebo group compared with not reached (>12 months) in the birtamimab group, leading to the initiation of AFFIRM-AL (A Study to Evaluate the Efficacy and Safety of Birtamimab in Mayo Stage IV Patients With AL Amyloidosis; NCT04973137), a new phase 3 trial in patients with advanced cardiac disease.
CAEL-101
CAEL-101 is a chimeric monoclonal antibody that targets a cryptic epitope on Ig light chains that is exposed when the light chains are misfolded.107,112 It binds to misfolded free Ig light chains as well as amyloid fibrils deposited in organs.107,113,114 It is hypothesized that CAEL-101 opsonizes the amyloid fibrils and misfolded light chains, thereby attracting and activating macrophages that degrade the complex by phagocytosis and/or enzymatic and chemical proteolysis.107 Preclinical studies further demonstrated that treatment with CAEL-101 resulted in regression of transplanted human amyloidomas in a mouse model.113,115,116 Phase 1 studies with CAEL-101 (NCT00807872 and NCT02245867) demonstrated that it recognized amyloid fibrils deposited in tissues. Furthermore, CAEL-101 had a dose-proportional pharmacokinetic profile without any dose-limiting toxicities.114,117,118 Preliminary results also showed that there were reductions in biomarkers of cardiomyopathy and nephropathy.117,118 The phase 2 study (NCT04304144) confirmed that there were no dose-limiting toxicities up to 1,000 mg/m2 and that it could be administered with CyBorD or CyBorD plus daratumumab.100,101 Furthermore, the exposure to CAEL-101 was not affected by adding daratumumab to the anti-PCD treatment regimen. Initial evaluations also confirmed the improvements in biomarkers of cardiomyopathy and nephropathy.100 On the basis of these promising results, 2 randomized, double-blind phase 3 studies are currently enrolling patients at more than 70 sites in several countries to evaluate the efficacy and safety of coadministering CAEL-101 with standard-of-care anti-PCD therapy in patients with AL amyloidosis and severe cardiomyopathy in stages IIIa (NCT04512235) and IIIb (NCT04504825). These are the first time-to-event studies focusing exclusively on patients with severe cardiomyopathy.
Gaps
Treatment gaps
At present, although treatment guidelines exist, there is no consensus on which regimen should be used as first-line treatment.87 The anti-PCD treatment recommended in current National Comprehensive Cancer Network guidelines is CyBorD with daratumumab.87 The components of this treatment, namely, bortezomib and dexamethasone, can increase NT-proBNP levels and even risk for death, especially among patients with advanced cardiomyopathy.119,120 In addition, these drugs are not always available or accessible, especially in developing countries, where other regimen do not appear to be inferior with respect hematologic response and overall survival.121 However, the reasons for this equivalence in response is unclear. Despite advances, the rate of overall CR is about 50% under the best circumstances for any of the currently available treatments, indicating that the disease may continue to progress for more than one-half of patients.77 Furthermore, ASCT does not appear to have an advantage over chemotherapy, especially among patients receiving daratumumab.122 Furthermore, none of the currently available treatments substantially improves survival rates among patients who are diagnosed with stage IIIb AL amyloidosis, although a small number of patients who achieve a rapid deep hematologic response appear to have a survival advantage.79,123
All currently available treatment options, including ASCT, are adapted from treatments and regimens developed for multiple myeloma.89,124, 125, 126 The goal of the current treatments is to eliminate the source of the amyloidogenic protein production, and none targets removal of amyloid deposits.24,38,124,127,128 Thus, current therapies prevent further amyloid formation and deposition, with no treatment for the organ damage that has already occurred prior to treatment initiation or the damage that can continue to be caused by these preexisting deposits.24,38,124,127,128 Reducing or eliminating amyloid burden by partial or complete removal of preexisting amyloid deposits to repair and/or restore organ function, especially that of the heart, remains a huge treatment gap.
Immunomodulatory agents, such as lenalidomide and pomalidomide, usually in combination with dexamethasone, are commonly used to treat relapsed or refractory AL amyloidosis.129,130 However, this combination has a low hematologic response rate and can result in hematological toxicity, increased NT-proBNP, and sometimes nephrotoxicity.129,130 Most of the current chemotherapy regimens include dexamethasone (or other corticosteroids) as a key component. However, it is poorly tolerated, often causing worsening of heart failure, fatigue, increased skin fragility, and risk for sudden death in patients with advanced cardiac AL amyloidosis.119,120,130 Careful monitoring for complications and using the lowest doses possible are crucial. Results from a retrospective analysis show that venetoclax may be effective among patients with AL amyloidosis with a t(11:14) translocation and refractory to standard anti-PCD treatment, suggesting that genetics may play a role in response.131 Thus, there is a need to better understand resistance to treatment and more effective and safer treatment options for patients with relapsed or refractory AL amyloidosis. This has become especially important with use of daratumumab as a frontline agent in treatment of AL amyloidosis.
Because AL amyloidosis is very heterogeneous in its presentation, supportive care to help manage the many symptoms experienced by patients is critical.132 However, the heterogeneous nature of AL amyloidosis also makes standardization of supportive care very difficult. At present, there are no consensus standards for supportive care. Diuretic agents, β-blockers, and angiotensin-converting enzyme inhibitors are the principal drugs used to prevent cardiac failure among patients with nonamyloid cardiomyopathy.2,132,133 However, in patients with AL amyloidosis with advanced cardiomyopathy, hypotension is common, and β-blockers and angiotensin-converting enzyme inhibitors are poorly tolerated and can be dangerous.2,132,133 These drugs should not be used for this group of patients except in very particular circumstances, determined on an individual case basis.133 Bradyarrhythmia may require implantation of pacemakers, but the role of cardiac resynchronization devices remains unclear. Hypotension can also severely limit hemodialysis, which is essential for patients with renal involvement.132,133 Management of autonomic neuropathy remains challenging. In addition, there is a huge unmet need for supportive care among patients with comorbid gastrointestinal symptoms, for whom the current drugs and dietary modifications are not sufficient.132
Gaps in relevant endpoints
The primary endpoint preferred by regulatory agencies is overall survival.134 However, as many patients, especially those with minimal or no cardiomyopathy, can live for 5 years or longer after diagnosis, determining median overall survival requires very long trials, which proves difficult with a rare disease because of the paucity of patients available and delaying trial readouts. Better understanding from regulators is needed to accept surrogate endpoints such as the established criteria for hematologic response and NT-proBNP for cardiac response.134 However, outcomes that reduce comorbidities, reduce amyloid burden, improve organ function, or improve patient QoL are less likely to be accepted as primary endpoints for trials, even if they are clinically relevant. It is essential that this gap be addressed, and preferably eliminated, to ensure that the best treatments are available to patients.
The goal for hematologic response has been for patients to achieve VGPR or better (Table 1).16,77,79 Recently, the goal changed to achieving CR because it correlated better with improved patient survival and QoL. However, the current criteria for CR do not include measurement of the difference between involved and uninvolved FLC (<10 mg/dL) or involved FLC (<20 mg/dL) in the serum, both of which are significant predictors of survival and organ response.124 There also is no consensus on the timing of hematologic response assessment. Hematologic response is variously reported 1, 3, or 6 months after treatment initiation, making it difficult to objectively evaluate the relative benefits of different treatment regimens as also is the lack of validated hematologic progression criteria. Time to retreatment often is used as a surrogate endpoint. These limitations in current hematologic response and progression criteria highlight the need for better means to measure initial and longitudinal response to treatment.
Organ response is typically determined only for a few organs, mainly the heart, kidneys, and liver, as these are considered the most relevant for survival.26,122 There are no established criteria for response to treatment for other organs, such as the gastrointestinal system, or for the neuropathy often experienced by patients. Although the hematologic and cardiac response criteria may reflect the level of cardiomyopathy, they do not inform the patients or those treating them about the disease state of other organs and tissues. Furthermore, neither the hematologic nor the organ response criteria measure the amyloid burden remaining in the body and continuing to have an adverse impact. Typically, organ response is observed much later than hematologic response, sometimes being delayed by months.79,135 Although achieving organ response within 1 year is predictive of longer survival, there are no standardized criteria on when organ response should be reported.79
Functional changes are often measured in clinical trials using the 6-minute walk test and various QoL instruments.21,136, 137, 138 The 6-minute walk test is a simple method to determine improvements in physical function and requires only an even flat surface at least 30 m in length and a timer.136 It is an easy test to implement and can reflect a patient’s level of cardiac impairment and the ability to perform an activity of daily living.136 The most commonly used instruments to measure QoL among patients with AL amyloidosis are the Short Form-36 (SF-36) and the Kansas City Cardiomyopathy Questionnaire.21,68,137, 138, 139 Both are measures of patient-reported outcomes. While the Short Form 36 is a robust, generic instrument that provides a reasonable snapshot of the physical and mental health of the patient, the Kansas City Cardiomyopathy Questionnaire is more disease specific and provides an estimation of the health of the heart of the patient at the time the test is administered. Both instruments have been validated for use with patients with AL amyloidosis.21,137, 138, 139 Although it is understandable that QoL instruments are focused on general and cardiac health because survival is most closely associated with degree of cardiomyopathy, there are gaps in understanding the health of other organs or systems from a patient’s perspective, despite instruments’ being available to determine the degree of nephropathy or hepatopathy.140,141
Structural improvements in the heart and decrease in myocardial amyloid deposits can be determined with CMR.50 Functional improvements of the heart can be determined with echocardiography and global longitudinal strain measurements, which can help with predicting survival.142, 143, 144, 145 There are also several studies under way to validate positron emission tomography–based scintigraphy as a relatively noninvasive method to determine health of and the amount of amyloid deposited in the heart.47,106,146, 147, 148, 149, 150, 151
Given the spectrum of endpoints used to evaluate response to treatment, there is no consensus on what defines a clinically meaningful response that translates to patient benefit in survival and QoL. Thus, there is a lack of consensus on what defines disease progression and, consequently, how to define progression-free survival.
It must be noted that the endpoints measured and the criteria for response to treatment were developed exclusively on the basis of the effectiveness of anti-PCD therapy, including ASCT. There are no generally accepted endpoints for reduction in amyloid burden or functional changes in organs other than the heart. Thus, there is a huge informational gap that needs to be addressed.
Conclusions
AL amyloidosis is a very devastating disease with continuing high early mortality. A significant majority of patients present with cardiomyopathy and are usually referred to cardiologists for diagnosis. However, for a substantial number of these patients, accurate diagnosis is either delayed or completely missed. These delays lead to poor prognosis and survival. It is therefore critical to increase awareness, knowledge, and understanding of AL amyloidosis among all health care providers, cardiologists in particular. In this review, we have attempted to provide a concise overview of this very complex rare disease. Furthermore, we have summarized the promising novel interventions under investigation to address removal of amyloid fibrils already deposited in the organs, an important treatment gap that currently exists. This review is but one step toward improving the diagnostic and treatment odysseys experienced by patients with AL amyloidosis.
Funding Support and Author Disclosures
Support for the development of this paper was provided by Alexion, AstraZeneca Rare Disease. Drs Wechalekar, Fontana, and Liedtke have received trial support from Alexion, AstraZeneca Rare Disease. Dr Quarta is an employee of Alexion and AstraZeneca Rare Disease, and has stock in the company.
Acknowledgment
Medical writing support was provided by Mukund Nori, PhD, MBA, CMPP, of rareLife solutions and funded by Alexion.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Blank M., Campbell M., Clarke J.O., et al. The amyloidosis forum: a public private partnership to advance drug development in AL amyloidosis. Orphanet J Rare Dis. 2020;15:268. doi: 10.1186/s13023-020-01525-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desport E., Bridoux F., Sirac C., et al. AL amyloidosis. Orphanet J Rare Dis. 2012;7:54. doi: 10.1186/1750-1172-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quock T.P., Yan T., Chang E., Guthrie S., Broder M.S. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2018;2:1046–1053. doi: 10.1182/bloodadvances.2018016402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaxman I., Gertz M. Recent advances in the diagnosis, risk stratification, and management of systemic light-chain amyloidosis. Acta Haematol. 2019;141:93–106. doi: 10.1159/000495455. [DOI] [PubMed] [Google Scholar]
- 5.Kumar S., Dispenzieri A., Lacy M.Q., et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30:989–995. doi: 10.1200/JCO.2011.38.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar S.K., Gertz M.A., Lacy M.Q., et al. Recent improvements in survival in primary systemic amyloidosis and the importance of an early mortality risk score. Mayo Clin Proc. 2011;86:12–18. doi: 10.4065/mcp.2010.0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manwani R., Cohen O., Sharpley F., et al. A prospective observational study of 915 patients with systemic AL amyloidosis treated with upfront bortezomib. Blood. 2019;134:2271–2280. doi: 10.1182/blood.2019000834. [DOI] [PubMed] [Google Scholar]
- 8.Barrett C.D., Dobos K., Liedtke M., et al. A changing landscape of mortality for systemic light chain amyloidosis. J Am Coll Cardiol HF. 2019;7:958–966. doi: 10.1016/j.jchf.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Muchtar E., Gertz M.A., Kumar S.K., et al. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood. 2017;129:2111–2119. doi: 10.1182/blood-2016-11-751628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulman A., Connors L.H., Weinberg J., et al. Patient outcomes in light chain (AL) amyloidosis: the clock is ticking from symptoms to diagnosis. Eur J Haematol. 2020;105:495–501. doi: 10.1111/ejh.13472. [DOI] [PubMed] [Google Scholar]
- 11.Vaxman I., Kumar S.K., Buadi F., et al. Outcomes among newly diagnosed AL amyloidosis patients with a very high NT-proBNP: implications for trial design. Leukemia. 2021;35:3604–3607. doi: 10.1038/s41375-021-01297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker K.R., Rice L. The amyloidoses: clinical features, diagnosis and treatment. Methodist Debakey Cardiovasc J. 2012;8:3–7. doi: 10.14797/mdcj-8-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merlini G. AL amyloidosis: from molecular mechanisms to targeted therapies. Hematology Am Soc Hematol Educ Program. 2017;2017:1–12. doi: 10.1182/asheducation-2017.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imperlini E., Gnecchi M., Rognoni P., et al. Proteotoxicity in cardiac amyloidosis: amyloidogenic light chains affect the levels of intracellular proteins in human heart cells. Sci Rep. 2017;7 doi: 10.1038/s41598-017-15424-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lavatelli F., Imperiini E., Orrù S., et al. Novel mitochondrial protein interactors of immunoglobulin light chains causing heart amyloidosis. FASEB J. 2015;29:4614–4628. doi: 10.1096/fj.15-272179. [DOI] [PubMed] [Google Scholar]
- 16.Gertz M.A., Dispenzieri A. Systemic amyloidosis recognition, prognosis, and therapy: a systematic review. JAMA. 2020;324:79–89. doi: 10.1001/jama.2020.5493. [DOI] [PubMed] [Google Scholar]
- 17.Gillmore J.D., Hawkins P.N. Pathophysiology and treatment of systemic amyloidosis. Nat Rev Nephrol. 2013;9:574–586. doi: 10.1038/nrneph.2013.171. [DOI] [PubMed] [Google Scholar]
- 18.Merlini G., Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583–596. doi: 10.1056/NEJMra023144. [DOI] [PubMed] [Google Scholar]
- 19.Escher F., Senoner M., Doerler J., et al. When and how do patients with cardiac amyloidosis die? Clin Res Cardiol. 2020;109:78–88. doi: 10.1007/s00392-019-01490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shu J., Lo S., Phillips M., et al. Depression and anxiety in patients with AL amyloidosis as assessed by the SF-36 questionnaire: experience in 1226 patients. Amyloid. 2016;23:188–193. doi: 10.1080/13506129.2016.1208081. [DOI] [PubMed] [Google Scholar]
- 21.Bayliss M., McCausland K.L., Guthrie S.D., White M.K. The burden of amyloid light chain amyloidosis on health-related quality of life. Orphanet J Rare Dis. 2017;12:15. doi: 10.1186/s13023-016-0564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falk R.H. Diagnosis and management of the cardiac amyloidoses. Circulation. 2005;112:2047–2060. doi: 10.1161/CIRCULATIONAHA.104.489187. [DOI] [PubMed] [Google Scholar]
- 23.Boldrini M., Cappelli F., Chacko L., et al. Multiparametric echocardiography scores for the diagnosis of cardiac amyloidosis. J Am Coll Cardiol Img. 2020;13:909–920. doi: 10.1016/j.jcmg.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Lin H.M., Gao X., Cooke C.E., et al. Disease burden of systemic light-chain amyloidosis: a systematic literature review. Curr Med Res Opin. 2017;33:1017–1031. doi: 10.1080/03007995.2017.1297930. [DOI] [PubMed] [Google Scholar]
- 25.McCausland K.L., White M.K., Guthrie S.D., et al. Light chain (AL) amyloidosis: the journey to diagnosis. Patient. 2018;11:207–216. doi: 10.1007/s40271-017-0273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lousada I., Comenzo R.L., Landau H., Guthrie S., Merlini G. Light chain amyloidosis: patient experience survey from the amyloidosis research consortium. Adv Ther. 2015;32:920–928. doi: 10.1007/s12325-015-0250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fotiou D., Dimopoulos M.A., Kastritis E. Systemic AL amyloidosis: current approaches to diagnosis and management. HemaSphere. 2020;4:e454. doi: 10.1097/HS9.0000000000000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muchtar E., Buadi F.K., Dispenzieri A., Gertz M.A. Immunoglobulin light-chain amyloidosis: from basics to new developments in diagnosis, prognosis and therapy. Acta Haematologica. 2016;135:172–190. doi: 10.1159/000443200. [DOI] [PubMed] [Google Scholar]
- 29.Grogan M., Dispenzieri A., Gertz M.A. Light-chain cardiac amyloidosis: strategies to promote early diagnosis and cardiac response. Heart. 2017;103:1065–1072. doi: 10.1136/heartjnl-2016-310704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oubari S., Naser E., Papathanasiou M., et al. Impact of time to diagnosis on Mayo stages, treatment outcome, and survival in patients with AL amyloidosis and cardiac involvement. Eur J Haematol. 2021;107:449–457. doi: 10.1111/ejh.13681. [DOI] [PubMed] [Google Scholar]
- 31.Witteles R.M., Liedtke M. AL amyloidosis for the cardiologist and oncologist. J Am Coll Cardiol CardioOnc. 2019;1:117–130. doi: 10.1016/j.jaccao.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bianchi G., Zhang Y., Comenzo R.L. AL amyloidosis: current chemotherapy and immune therapy treatment strategies. J Am Coll Cardiol CardioOnc. 2021;3:467–487. doi: 10.1016/j.jaccao.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bishop E., Brown E.E., Fajardo J., Barouch L.A., Judge D.P., Halushka M.K. Seven factors predict a delayed diagnosis of cardiac amyloidosis. Amyloid. 2018;25:174–179. doi: 10.1080/13506129.2018.1498782. [DOI] [PubMed] [Google Scholar]
- 34.Zumbo G., Sadeghi-Alavijeh O., Hawkins P.N., Fontana M. New and developing therapies for AL amyloidosis. Expert Opin Pharmacother. 2017;18:139–149. doi: 10.1080/14656566.2016.1274971. [DOI] [PubMed] [Google Scholar]
- 35.Bhutani D., Lentzsch S. Diagnosis and management of systemic light chain AL amyloidosis. Pharmacol Ther. 2020;214 doi: 10.1016/j.pharmthera.2020.107612. [DOI] [PubMed] [Google Scholar]
- 36.Ash S., Shorer E., Ramgobin D., et al. Cardiac amyloidosis-a review of current literature for the practicing physician. Clin Cardiol. 2021;44:322–331. doi: 10.1002/clc.23572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griffin J.M., Rosenblum H., Maurer M.S. Pathophysiology and therapeutic approaches to cardiac amyloidosis. Circ Res. 2021;128:1554–1575. doi: 10.1161/CIRCRESAHA.121.318187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fikrle M., Paleček T., Kuchynka P., et al. Cardiac amyloidosis: a comprehensive review. Cor Vasa. 2013;55:e60–e75. [Google Scholar]
- 39.Kumar S., Dispenzieri A., Katzmann J.A., et al. Serum immunoglobulin free light-chain measurement in primary amyloidosis: prognostic value and correlations with clinical features. Blood. 2010;116:5126–5129. doi: 10.1182/blood-2010-06-290668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim H.-S., Kim H.S., Shin K.-S., et al. Clinical comparisons of two free light chain assays to immunofixation electrophoresis for detecting monoclonal gammopathy. Biomed Res Int. 2014;2014 doi: 10.1155/2014/647238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubinstein S.M., Stockerl-Goldstein K. How to screen for monoclonal gammopathy in patients with a suspected amyloidosis. J Am Coll Cardiol CardioOnc. 2021;3:590–593. doi: 10.1016/j.jaccao.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanchorawala V. Light-chain (AL) amyloidosis: diagnosis and treatment. Clin J Am Soc Nephrol. 2006;1:1331–1341. doi: 10.2215/CJN.02740806. [DOI] [PubMed] [Google Scholar]
- 43.Palladini G., Russo P., Bosoni T., et al. Identification of amyloidogenic light chains requires the combination of serum-free light chain assay with immunofixation of serum and urine. Clin Chem. 2009;55:499–504. doi: 10.1373/clinchem.2008.117143. [DOI] [PubMed] [Google Scholar]
- 44.Wisniowski B., Wechalekar A. Confirming the diagnosis of amyloidosis. Acta Haematologica. 2020;143:312–321. doi: 10.1159/000508022. [DOI] [PubMed] [Google Scholar]
- 45.Gertz M.A. Immunoglobulin light chain amyloidosis: 2016 update on diagnosis, prognosis, and treatment. Am J Hematol. 2016;91:947–956. doi: 10.1002/ajh.24433. [DOI] [PubMed] [Google Scholar]
- 46.Sankaranarayanan R., Fleming E.J., Garratt C.J. Mimics of hypertrophic cardiomyopathy—diagnostic clues to aid early identification of phenocopies. Arrhythm Electrophysiol Rev. 2013;2:36–40. doi: 10.15420/aer.2013.2.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manwani R., Page J., Lane T., et al. A pilot study demonstrating cardiac uptake with 18F-florbetapir PET in AL amyloidosis patients with cardiac involvement. Amyloid. 2018;25:247–252. doi: 10.1080/13506129.2018.1552852. [DOI] [PubMed] [Google Scholar]
- 48.Quarta C.C., Kruger J.L., Falk R.H. Cardiac amyloidosis. Circulation. 2012;126:e178–e182. doi: 10.1161/CIRCULATIONAHA.111.069195. [DOI] [PubMed] [Google Scholar]
- 49.Yang H., Wright L., Negishi T., Negishi K., Liu J., Marwick T.H. Research to practice. J Am Coll Cardiol Img. 2018;11:1196–1201. doi: 10.1016/j.jcmg.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Banypersad S.M. The evolving role of cardiovascular magnetic resonance imaging in the evaluation of systemic amyloidosis. Magn Reson Insights. 2019;12 doi: 10.1177/1178623X19843519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Banypersad S.M., Sado D.M., Flett A.S., et al. Quantification of myocardial extracellular volume fraction in systemic AL amyloidosis: an equilibrium contrast cardiovascular magnetic resonance study. Circ Cardiovasc Imaging. 2013;6:34–39. doi: 10.1161/CIRCIMAGING.112.978627. [DOI] [PubMed] [Google Scholar]
- 52.Austin B.A., Tang W.H., Rodriguez E.R., et al. Delayed hyper-enhancement magnetic resonance imaging provides incremental diagnostic and prognostic utility in suspected cardiac amyloidosis. J Am Coll Cardiol Img. 2009;2:1369–1377. doi: 10.1016/j.jcmg.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 53.Di Bella G., Pizzino F., Minutoli F., et al. The mosaic of the cardiac amyloidosis diagnosis: role of imaging in subtypes and stages of the disease. Eur Heart J Cardiovasc Imaging. 2014;15:1307–1315. doi: 10.1093/ehjci/jeu158. [DOI] [PubMed] [Google Scholar]
- 54.Ruberg F.L., Appelbaum E., Davidoff R., et al. Diagnostic and prognostic utility of cardiovascular magnetic resonance imaging in light-chain cardiac amyloidosis. Am J Cardiol. 2009;103:544–549. doi: 10.1016/j.amjcard.2008.09.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Syed I.S., Glockner J.F., Feng D., et al. Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. J Am Coll Cardiol Img. 2010;3:155–164. doi: 10.1016/j.jcmg.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 56.Vogelsberg H., Mahrholdt H., Deluigi C.C., et al. Cardiovascular magnetic resonance in clinically suspected cardiac amyloidosis: noninvasive imaging compared to endomyocardial biopsy. J Am Coll Cardiol. 2008;51:1022–1030. doi: 10.1016/j.jacc.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 57.Fontana M., Banypersad S.M., Treibel T.A., et al. Differential myocyte responses in patients with cardiac transthyretin amyloidosis and light-chain amyloidosis: a cardiac MR imaging study. Radiology. 2015;277:388–397. doi: 10.1148/radiol.2015141744. [DOI] [PubMed] [Google Scholar]
- 58.Fontana M., Chung R., Hawkins P.N., Moon J.C. Cardiovascular magnetic resonance for amyloidosis. Heart Fail Rev. 2015;20:133–144. doi: 10.1007/s10741-014-9470-7. [DOI] [PubMed] [Google Scholar]
- 59.Treibel T.A., Bandula S., Fontana M., et al. Extracellular volume quantification by dynamic equilibrium cardiac computed tomography in cardiac amyloidosis. J Cardiovasc Comput Tomogr. 2015;9:585–592. doi: 10.1016/j.jcct.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aquaro G.D., Pugliese N.R., Perfetto F., et al. Myocardial signal intensity decay after gadolinium injection: a fast and effective method for the diagnosis of cardiac amyloidosis. Int J Cardiovasc Imaging. 2014;30:1105–1115. doi: 10.1007/s10554-014-0436-6. [DOI] [PubMed] [Google Scholar]
- 61.Fontana M., Pica S., Reant P., et al. Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2015;132:1570–1579. doi: 10.1161/CIRCULATIONAHA.115.016567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Banypersad S.M., Fontana M., Maestrini V., et al. T1 mapping and survival in systemic light-chain amyloidosis. Eur Heart J. 2015;36:244–251. doi: 10.1093/eurheartj/ehu444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fontana M., Banypersad S.M., Treibel T.A., et al. Native T1 mapping in transthyretin amyloidosis. J Am Coll Cardiol Img. 2014;7:157–165. doi: 10.1016/j.jcmg.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 64.Karamitsos T.D., Piechnik S.K., Banypersad S.M., et al. Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. J Am Coll Cardiol Img. 2013;6:488–497. doi: 10.1016/j.jcmg.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 65.White J.A., Kim H.W., Shah D., et al. CMR imaging with rapid visual T1 assessment predicts mortality in patients suspected of cardiac amyloidosis. J Am Coll Cardiol Img. 2014;7:143–156. doi: 10.1016/j.jcmg.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khalique Z., Ferreira P.F., Scott A.D., et al. Diffusion tensor cardiovascular magnetic resonance in cardiac amyloidosis. Circ Cardiovasc Imaging. 2020;13 doi: 10.1161/CIRCIMAGING.119.009901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Orini M., Graham A.J., Martinez-Naharro A., et al. Noninvasive mapping of the electrophysiological substrate in cardiac amyloidosis and its relationship to structural abnormalities. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Green C.P., Porter C.B., Bresnahan D.R., Spertus J.A. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 69.Muchtar E., Dispenzieri A., Magen H., et al. Systemic amyloidosis from A (AA) to T (ATTR): a review. J Intern Med. 2021;289:268–292. doi: 10.1111/joim.13169. [DOI] [PubMed] [Google Scholar]
- 70.Schönland S.O., Hegenbart U., Bochtler T., et al. Immunohistochemistry in the classification of systemic forms of amyloidosis: a systematic investigation of 117 patients. Blood. 2012;119:488–493. doi: 10.1182/blood-2011-06-358507. [DOI] [PubMed] [Google Scholar]
- 71.Abildgaard N., Rojek A.M., Møller H.E.H., et al. Immunoelectron microscopy and mass spectrometry for classification of amyloid deposits. Amyloid. 2020;27:59–66. doi: 10.1080/13506129.2019.1688289. [DOI] [PubMed] [Google Scholar]
- 72.Treglia G., Glaudemans A., Bertagna F., et al. Diagnostic accuracy of bone scintigraphy in the assessment of cardiac transthyretin-related amyloidosis: a bivariate meta-analysis. Eur J Nucl Med Mol Imaging. 2018;45:1945–1955. doi: 10.1007/s00259-018-4013-4. [DOI] [PubMed] [Google Scholar]
- 73.Tsutsui Y., Kubota T., Kato S., et al. Utility of 99 mTc-pyrophosphate scintigraphy in diagnosing transthyretin cardiac amyloidosis in real-world practice. Circ Rep. 2019;1:277–285. doi: 10.1253/circrep.CR-19-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bokhari S., Castaño A., Pozniakoff T., Deslisle S., Latif F., Maurer M.S. 99mTc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging. 2013;6:195–201. doi: 10.1161/CIRCIMAGING.112.000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brownrigg J., Lorenzini M., Lumley M., Elliott P. Diagnostic performance of imaging investigations in detecting and differentiating cardiac amyloidosis: a systematic review and meta-analysis. ESC Heart Failure. 2019;6:1041–1051. doi: 10.1002/ehf2.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cooper L.T., Baughman K.L., Feldman A.M., et al. The role of endomyocardial biopsy in the management of cardiovascular disease. Circulation. 2007;116:2216–2233. doi: 10.1161/CIRCULATIONAHA.107.186093. [DOI] [PubMed] [Google Scholar]
- 77.Dispenzieri A., Buadi F., Kumar S.K., et al. Treatment of immunoglobulin light chain amyloidosis: Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus statement. Mayo Clin Proc. 2015;90:1054–1081. doi: 10.1016/j.mayocp.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 78.Palladini G., Schönland S.O., Sanchorawala V., et al. Clarification on the definition of complete haematologic response in light-chain (AL) amyloidosis. Amyloid. 2021;28:1–2. doi: 10.1080/13506129.2020.1868810. [DOI] [PubMed] [Google Scholar]
- 79.Kaufman G.P., Dispenzieri A., Gertz M.A., et al. Kinetics of organ response and survival following normalization of the serum free light chain ratio in AL amyloidosis. Am J Hematol. 2015;90:181–186. doi: 10.1002/ajh.23898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Palladini G., Hegenbart U., Milani P., et al. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood. 2014;124:2325–2332. doi: 10.1182/blood-2014-04-570010. [DOI] [PubMed] [Google Scholar]
- 81.Gertz M.A., Comenzo R., Falk R.H., et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18-22 April 2004. Am J Hematol. 2005;79:319–328. doi: 10.1002/ajh.20381. [DOI] [PubMed] [Google Scholar]
- 82.Gertz M.A. Immunoglobulin light chain amyloidosis: 2018 update on diagnosis, prognosis, and treatment. Am J Hematol. 2018;93:1169–1180. doi: 10.1002/ajh.25149. [DOI] [PubMed] [Google Scholar]
- 83.Gertz M.A. Immunoglobulin light chain amyloidosis diagnosis and treatment algorithm 2018. Blood Cancer J. 2018;8:44. doi: 10.1038/s41408-018-0080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roy V. Autologous stem cell transplant for AL amyloidosis. Bone Marrow Res. 2012;2012 doi: 10.1155/2012/238961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hwa Y.L., Kumar S.K., Gertz M.A., et al. Induction therapy pre-autologous stem cell transplantation in immunoglobulin light chain amyloidosis: a retrospective evaluation. Am J Hematol. 2016;91:984–988. doi: 10.1002/ajh.24453. [DOI] [PubMed] [Google Scholar]
- 86.Dispenzieri A., Seenithamby K., Lacy M.Q., et al. Patients with immunoglobulin light chain amyloidosis undergoing autologous stem cell transplantation have superior outcomes compared with patients with multiple myeloma: a retrospective review from a tertiary referral center. Bone Marrow Transplant. 2013;48:1302–1307. doi: 10.1038/bmt.2013.53. [DOI] [PubMed] [Google Scholar]
- 87.National Comprehensive Cancer Network Systemic light chain amyloidosis: NCCN Evidence Blocks™ v2. https://www.nccn.org/professionals/physician_gls/pdf/amyloidosis_blocks.pdf
- 88.Kastritis E., Palladini G., Minnema M.C., et al. Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med. 2021;385:46–58. doi: 10.1056/NEJMoa2028631. [DOI] [PubMed] [Google Scholar]
- 89.Palladini G., Kastritis E., Maurer M.S., et al. Daratumumab plus CyBorD for patients with newly diagnosed AL amyloidosis: safety run-in results of andromeda. Blood. 2020;136:71–80. doi: 10.1182/blood.2019004460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sidiqi M.H., Gertz M.A. Daratumumab for the treatment of AL amyloidosis. Leuk Lymphoma. 2019;60:295–301. doi: 10.1080/10428194.2018.1485914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Janssen Biotech Inc. Daralex Faspro® (daratumumab and hyaluronidase-fihj) https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/DARZALEX+Faspro-pi.pdf
- 92.Rizio A.A., White M.K., McCausland K.L., et al. Treatment tolerability in patients with immunoglobulin light-chain amyloidosis. Am Health Drug Benefits. 2018;11:430–437. [PMC free article] [PubMed] [Google Scholar]
- 93.Chakraborty R., Lentzsch S. Emerging drugs for the treatment of light chain amyloidosis. Expert Opin Emerg Drugs. 2020;25:299–317. doi: 10.1080/14728214.2020.1803829. [DOI] [PubMed] [Google Scholar]
- 94.Cohen O.C., Sharpley F., Gillmore J.D., et al. Use of ixazomib, lenalidomide and dexamethasone in patients with relapsed amyloid light-chain amyloidosis. Br J Haematol. 2020;189:643–649. doi: 10.1111/bjh.16401. [DOI] [PubMed] [Google Scholar]
- 95.Lentzsch S., Lagos G.G., Comenzo R.L., et al. Bendamustine with dexamethasone in relapsed/refractory systemic light-chain amyloidosis: results of a phase II study. J Clin Oncol. 2020;38:1455–1462. doi: 10.1200/JCO.19.01721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dispenzieri A., Kastritis E., Wechalekar A.D., et al. A randomized phase 3 study of ixazomib-dexamethasone versus physician’s choice in relapsed or refractory AL amyloidosis. Leukemia. 2022;36:225–235. doi: 10.1038/s41375-021-01317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ho A.H., Kinter C.W., Wight J., Neelam A.R., Krakow D. Droxidopa as an effective treatment for refractory neurogenic orthostatic hypotension and reflex bradycardia in amyloid light-chain amyloidosis: a case report. J Med Case Rep. 2020;14:73. doi: 10.1186/s13256-020-02405-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ehsan H., Wahab A., Sana M.K., et al. Updates on emerging therapies in cardiac light chain (AL) amyloidosis. Blood. 2020;136:39–41. [Google Scholar]
- 99.Kaufman G.P., Cerchione C. Beyond andromeda: improving therapy for light chain amyloidosis. Front Oncol. 2021;10 doi: 10.3389/fonc.2020.624573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Khouri J., Anwer F., Samaras C.J., et al. Safety, tolerability and efficacy of CAEL-101 in AL amyloidosis patients treated on a phase 2, open-label, dose selection study to evaluate the safety and tolerability of CAEL-101 in patients with AL amyloidosis. Blood. 2020;136:21. [Google Scholar]
- 101.Valent J., Silowsky J., Kurman M.R., et al. CAEL-101 is well-tolerated in AL amyloidosis patients receiving concomitant cyclophosphamide-bortezomib-dexamethasone (CyBorD): a phase 2 dose-finding study ( NCT04304144) Blood. 2020;136:26–27. [Google Scholar]
- 102.Palladini G., Milani P., Merlini G. Management of AL amyloidosis in 2020. Hematology. 2020;2020:363–371. doi: 10.1182/hematology.2020006913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Richards D.B., Cookson L.M., Barton S.V., et al. Repeat doses of antibody to serum amyloid P component clear amyloid deposits in patients with systemic amyloidosis. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aan3128. [DOI] [PubMed] [Google Scholar]
- 104.Hasib Sidiqi M., Gertz M.A. Immunoglobulin light chain amyloidosis diagnosis and treatment algorithm 2021. Blood Cancer J. 2021;11:90. doi: 10.1038/s41408-021-00483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Itzhaki Ben Zadok O., Kornowski R. Cardiac care of patients with cardiac amyloidosis. Acta Haematol. 2020;143:343–351. doi: 10.1159/000506919. [DOI] [PubMed] [Google Scholar]
- 106.Cuddy S.A.M., Bravo P.E., Falk R.H., et al. Improved quantification of cardiac amyloid burden in systemic light chain amyloidosis: redefining early disease? J Am Coll Cardiol Img. 2020;13:1325–1336. [Google Scholar]
- 107.Solomon A., Weiss D.T., Wall J.S. Immunotherapy in systemic primary (AL) amyloidosis using amyloid-reactive monoclonal antibodies. Cancer Biother Radiopharm. 2003;18:853–860. doi: 10.1089/108497803322702824. [DOI] [PubMed] [Google Scholar]
- 108.Popkova T., Hajek R., Jelinek T. Monoclonal antibodies in the treatment of AL amyloidosis: co-targetting the plasma cell clone and amyloid deposits. Br J Haematol. 2020;189:228–238. doi: 10.1111/bjh.16436. [DOI] [PubMed] [Google Scholar]
- 109.Wall J.S., Kennel S.J., Williams A., et al. AL amyloid imaging and therapy with a monoclonal antibody to a cryptic epitope on amyloid fibrils. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0052686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gertz M.A., Landau H., Comenzo R.L., et al. First-in-human phase I/II study of NEOD001 in patients with light chain amyloidosis and persistent organ dysfunction. J Clin Oncol. 2016;34:1097–1103. doi: 10.1200/JCO.2015.63.6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gertz M.A., Landau H.J., Weiss B.M. Organ response in patients with AL amyloidosis treated with NEOD001, an amyloid-directed monoclonal antibody. Am J Hematol. 2016;91:E506–E508. doi: 10.1002/ajh.24563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.O’Nuallain B., Allen A., Kennel S.J., Weiss D.T., Solomon A., Wall J.S. Localization of a conformational epitope common to non-native and fibrillar immunoglobulin light chains. Biochemistry. 2007;46:1240–1247. doi: 10.1021/bi0616605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Solomon A., Weiss D.T., Wall J.S. Therapeutic potential of chimeric amyloid-reactive monoclonal antibody 11-1f4. Clin Cancer Res. 2003;9:3831s–3838s. [PubMed] [Google Scholar]
- 114.Wall J.S., Kennel S.J., Stuckey A.C., et al. Radioimmunodetection of amyloid deposits in patients with AL amyloidosis. Blood. 2010;116:2241–2244. doi: 10.1182/blood-2010-03-273797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hrncic R., Wall J., Wolfenbarger D.A., et al. Antibody-mediated resolution of light chain-associated amyloid deposits. Am J Pathol. 2000;157:1239–1246. doi: 10.1016/S0002-9440(10)64639-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wall J.S., Kennel S.J., Paulus M., et al. Radioimaging of light chain amyloid with a fibril-reactive monoclonal antibody. J Nucl Med. 2006;47:2016–2024. [PMC free article] [PubMed] [Google Scholar]
- 117.Edwards C.V., Bhutani D., Mapara M., et al. One year follow up analysis of the phase 1a/b study of chimeric fibril-reactive monoclonal antibody 11-1f4 in patients with AL amyloidosis. Amyloid. 2019;26:115–116. doi: 10.1080/13506129.2019.1584892. [DOI] [PubMed] [Google Scholar]
- 118.Edwards C.V., Gould J., Langer A.L., et al. Interim analysis of the phase 1a/b study of chimeric fibril-reactive monoclonal antibody 11-1f4 in patients with AL amyloidosis. Amyloid. 2017;24:58–59. doi: 10.1080/13506129.2017.1292900. [DOI] [PubMed] [Google Scholar]
- 119.Bézard M., Oghina S., Vitiello D., et al. Dexamethasone is associated with early deaths in light chain amyloidosis patients with severe cardiac involvement. PLoS ONE. 2021;16 doi: 10.1371/journal.pone.0257189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Le Bras F., Molinier-Frenkel V., Guellich A., et al. Sequential cyclophosphamide-bortezomib-dexamethasone unmasks the harmful cardiac effect of dexamethasone in primary light-chain cardiac amyloidosis. Eur J Cancer. 2017;76:183–187. doi: 10.1016/j.ejca.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 121.Liu B., Wang Y., Bai M., et al. Cyclophosphamide + thalidomide + dexamethasone versus melphalan + dexamethasone for the treatment of amyloid light-chain amyloidosis with kidney involvement: a retrospective study in Chinese patients. Clin Ther. 2019;41:1186–1198. doi: 10.1016/j.clinthera.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 122.Sharpley F.A., Manwani R., Petrie A., et al. Autologous stem cell transplantation vs bortezomib based chemotherapy for the first-line treatment of systemic light chain amyloidosis in the UK. Eur J Haematol. 2021;106:537–545. doi: 10.1111/ejh.13582. [DOI] [PubMed] [Google Scholar]
- 123.Ravichandran S., Cohen O.C., Law S., et al. Impact of early response on outcomes in AL amyloidosis following treatment with frontline bortezomib. Blood Cancer J. 2021;11:118. doi: 10.1038/s41408-021-00510-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cohen O.C., Wechalekar A.D. Systemic amyloidosis: moving into the spotlight. Leukemia. 2020;34:1215–1228. doi: 10.1038/s41375-020-0802-4. [DOI] [PubMed] [Google Scholar]
- 125.Chung A., Kaufman G.P., Sidana S., et al. Organ responses with daratumumab therapy in previously treated AL amyloidosis. Blood Adv. 2020;4:458–466. doi: 10.1182/bloodadvances.2019000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sanchorawala V., Sarosiek S., Schulman A., et al. Safety, tolerability, and response rates of daratumumab in relapsed AL amyloidosis: results of a phase 2 study. Blood. 2020;135:1541–1547. doi: 10.1182/blood.2019004436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cohen A.D., Comenzo R.L. Systemic light-chain amyloidosis: advances in diagnosis, prognosis, and therapy. Hematology Am Soc Hematol Educ Program. 2010;2010:287–294. doi: 10.1182/asheducation-2010.1.287. [DOI] [PubMed] [Google Scholar]
- 128.Kastritis E., Dimopoulos M.A. Recent advances in the management of AL amyloidosis. Br J Haematol. 2016;172:170–186. doi: 10.1111/bjh.13805. [DOI] [PubMed] [Google Scholar]
- 129.Basset M., Kimmich C.R., Schreck N., et al. Lenalidomide and dexamethasone in relapsed/refractory immunoglobulin light chain (AL) amyloidosis: results from a large cohort of patients with long follow-up. Br J Haematol. 2021;195:230–243. doi: 10.1111/bjh.17685. [DOI] [PubMed] [Google Scholar]
- 130.Milani P., Palladini G. Conventional therapy for amyloid light-chain amyloidosis. Acta Haematologica. 2020;143:365–372. doi: 10.1159/000507072. [DOI] [PubMed] [Google Scholar]
- 131.Premkumar V.J., Lentzsch S., Pan S., et al. Venetoclax induces deep hematologic remissions in t(11;14) relapsed/refractory AL amyloidosis. Blood Cancer J. 2021;11:10. doi: 10.1038/s41408-020-00397-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cibeira M.T., Ortiz-Pérez J.T., Quintana L.F., Fernádez de Larrea C., Tovar N., Bladé J. Supportive care in AL amyloidosis. Acta Haematologica. 2020;143:335–342. doi: 10.1159/000506760. [DOI] [PubMed] [Google Scholar]
- 133.Wong S.W., Fogaren T. Supportive care for patients with systemic light chain amyloidosis. Hematol Oncol Clin North Am. 2020;34:1177–1191. doi: 10.1016/j.hoc.2020.08.007. [DOI] [PubMed] [Google Scholar]
- 134.Amyloidosis Research Consortium Guidance for industry: AL amyloidosis—developing drugs for treatment. https://www.arci.org/wp-content/uploads/2018/04/Guidance-for-Industry-AL-Amyloidosis-Developing-Drugs-for-Treatment-12_16.pdf
- 135.Muchtar E., Dispenzieri A., Leung N., et al. Depth of organ response in AL amyloidosis is associated with improved survival: grading the organ response criteria. Leukemia. 2018;32:2240–2249. doi: 10.1038/s41375-018-0060-x. [DOI] [PubMed] [Google Scholar]
- 136.Giannitsi S., Bougiakli M., Bechlioulis A., Kotsia A., Michalis L.K., Naka K.K. 6-Minute walking test: a useful tool in the management of heart failure patients. Ther Adv Cardiovasc Dis. 2019;13 doi: 10.1177/1753944719870084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bayliss M., Quock T.P., Guthrie S.D., White M.K., McCausland K.L. Longitudinal associations between health-related quality of life and healthcare utilization in AL amyloidosis. Haematologica. 2017;102:599–600. [Google Scholar]
- 138.Sanchorawala V., Palladini G., Minnema M.C., et al. Health-related quality of life in patients with AL amyloidosis treated with daratumumab, bortezomib, cyclophosphamide, and dexamethasone: results from the phase 3 Andromeda study. Blood. 2020;136:37–40. [Google Scholar]
- 139.McCausland K.L., Quock T.P., Rizio A.A., et al. Cardiac biomarkers and health-related quality of life in patients with light chain (AL) amyloidosis. Br J Haematol. 2019;185:998–1001. doi: 10.1111/bjh.15693. [DOI] [PubMed] [Google Scholar]
- 140.Jay C.L., Butt Z., Ladner D.P., Skaro A.I., Abecassis M.M. A review of quality of life instruments used in liver transplantation. J Hepatol. 2009;51:949–959. doi: 10.1016/j.jhep.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zazzeroni L., Pasquinelli G., Nanni E., Cremonini V., Rubbi I. Comparison of quality of life in patients undergoing hemodialysis and peritoneal dialysis: a systematic review and meta-analysis. Kidney Blood Press Res. 2017;42:717–727. doi: 10.1159/000484115. [DOI] [PubMed] [Google Scholar]
- 142.Hwang I.C., Koh Y., Park J.B., et al. Time trajectory of cardiac function and its relation with survival in patients with light-chain cardiac amyloidosis. Eur Heart J Cardiovasc Imaging. 2021;22:459–469. doi: 10.1093/ehjci/jeaa146. [DOI] [PubMed] [Google Scholar]
- 143.Lei C., Zhu X., Hsi D.H., et al. Predictors of cardiac involvement and survival in patients with primary systemic light-chain amyloidosis: roles of the clinical, chemical, and 3-D speckle tracking echocardiography parameters. BMC Cardiovasc Disord. 2021;21:43. doi: 10.1186/s12872-021-01856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nahhas O., Chuy K.L., Yang J.C., et al. Incremental value of GLS for classification of prognosis in patients with advanced light chain (AL) amyloidosis. J Am Coll Cardiol. 2020;75:1006. [Google Scholar]
- 145.Chuy K.L., Drill E., Yang J.C., et al. Incremental value of global longitudinal strain for predicting survival in patients with advanced AL amyloidosis. J Am Coll Cardiol CardioOnc. 2020;2:223–231. doi: 10.1016/j.jaccao.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ehman E.C., El-Sady M.S., Kijewski M.F., et al. Early detection of multiorgan light-chain amyloidosis by whole-body 18F-florbetapir PET/CT. J Nucl Med. 2019;60:1234–1239. doi: 10.2967/jnumed.118.221770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Khor Y.M., Cuddy S., Harms H.J., et al. Quantitative [18F]florbetapir PET/CT may identify lung involvement in patients with systemic AL amyloidosis. Eur J Nucl Med Mol Imaging. 2020;47:1998–2009. doi: 10.1007/s00259-019-04627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Santarelli M.F., Genovesi D., Positano V., et al. Deep-learning-based cardiac amyloidosis classification from early acquired PET images. Int J Cardiovasc Imaging. 2021;37:2327–2335. doi: 10.1007/s10554-021-02190-7. [DOI] [PubMed] [Google Scholar]
- 149.Santarelli M.F., Genovesi D., Scipioni M., et al. Cardiac amyloidosis characterization by kinetic model fitting on [18F]florbetaben PET images. J Nucl Cardiol. 2021;29:1919–1932. doi: 10.1007/s12350-021-02608-8. [DOI] [PubMed] [Google Scholar]
- 150.Santarelli M.F., Scipioni M., Genovesi D., Giorgetti A., Marzullo P., Landini L. Imaging techniques as an aid in the early detection of cardiac amyloidosis. Curr Pharm Des. 2021;27:1878–1889. doi: 10.2174/1381612826666200813133557. [DOI] [PubMed] [Google Scholar]
- 151.Seo M., Cha H.J., Kim M., et al. Clinical utility of 18F-florbetaben PET for detecting amyloidosis associated with multiple myeloma: a prospective case-control study. Clin Nucl Med. 2019;44:e503–e509. doi: 10.1097/RLU.0000000000002699. [DOI] [PubMed] [Google Scholar]