Abstract
Background
Despite the widely acknowledged benefit of exercise for patients with cancer, little evidence on the optimal timing of exercise on adverse effects of cancer treatment is available.
Objectives
The aim of this study was to determine whether an exercise intervention initiated during chemotherapy is superior to an intervention initiated after chemotherapy for improving long-term cardiorespiratory fitness (peak oxygen uptake [VO2peak]).
Methods
In this prospective, randomized clinical trial, patients scheduled to receive curative chemotherapy were randomized to a 24-week exercise intervention, initiated either during chemotherapy (group A) or afterward (group B). The primary endpoint was VO2peak 1 year postintervention. Secondary endpoints were VO2peak postintervention, muscle strength, health-related quality of life (HRQoL), fatigue, physical activity, and self-efficacy. Between-group differences were calculated using intention-to-treat linear mixed-models analyses.
Results
A total of 266 patients with breast (n = 139), testicular (n = 95), and colon cancer (n = 30) as well as lymphoma (n = 2) were included. VO2peak immediately postintervention and 1 year postintervention did not differ between the 2 groups. Immediately postchemotherapy, patients in group A exhibited significantly lower decreases in VO2peak (3.1 mL/kg/min; 95% CI: 2.2-4.0 mL/kg/min), HRQoL, and muscle strength and reported less fatigue and more physical activity than those in group B.
Conclusions
Exercise can be safely performed during chemotherapy and prevents fatigue and decreases in VO2peak, muscle strength, and HRQoL, in addition to hastening the return of function after chemotherapy. Also, if exercise cannot be performed during chemotherapy, a program afterward can enable patients to regain the same level of function, measured 1 year after completion of the intervention. (Optimal Timing of Physical Activity in Cancer Treatment [ACT]; NCT01642680)
Key Words: cardiorespiratory fitness, chemotherapy, fatigue, muscle strength, physical exercise, quality of life
Abbreviations and Acronyms: HIIT, high-intensity interval training; HRQoL, health-related quality of life; MET-h, the amount of kcal burnt per kilogram body weight per hour; PASE, Physical Activity Scale for the Elderly; SAE, serious adverse event(s); UMCG, University Medical Center Groningen; VO2peak, peak oxygen uptake
Central Illustration
Cancer treatment frequently leads to adverse effects, such as reduced cardiorespiratory fitness, increased fatigue, and cardiovascular morbidity.1,2 These effects substantially impair health-related quality of life (HRQoL) and may even reduce survival. Substantial evidence indicates that physical exercise intervention during and after cancer treatment is beneficial to mitigate adverse effects of cancer treatment and is safe.3,4
As reflected by peak oxygen uptake (VO2peak), cardiorespiratory fitness is considered one of the most important independent predictors of cardiovascular health.5,6 VO2peak declines up to 25% during cancer treatment.7 A recent meta-analysis by Scott et al,3 including 48 randomized controlled trials performed between 2000 and 2018 representing 3,632 patients who were allocated to either exercise therapy or control or usual-care groups, showed that exercise therapy was associated with a significant increase in cardiorespiratory fitness (+2.80 mL/kg/min) compared with no change (+0.02 mL/kg/min) in the control group (P = 0.001). Other meta-analyses of patients with breast, colorectal, and testicular cancer and cancer survivors also showed that a physical exercise intervention during and after cancer treatment prevents physical deterioration and reduces the decline in VO2peak.4,8 Improvement in physical activity and VO2peak is associated with significant decreases in cardiovascular morbidity, overall mortality, and cancer mortality.9,10 Therefore, preventing the chemotherapy-induced decrease in VO2peak might lower cardiovascular risk and mortality in cancer survivors.
Evidence on the optimal timing and dose of a physical exercise intervention to prevent treatment-induced toxicity such as VO2peak is sparse.11 We hypothesized that the optimal timing for an exercise intervention to mitigate chemotherapy-induced adverse effects is during, not after, chemotherapy. In a randomized trial, we investigated whether a physical exercise intervention initiated during chemotherapy is superior to one initiated after chemotherapy for improving long-term VO2peak. The primary endpoint was the difference in VO2peak 1 year after completing the intervention. Secondary endpoints were VO2peak after completion of chemotherapy and directly after the intervention, muscle strength, and the patient-reported outcomes HRQoL, fatigue, physical activity, and self-efficacy at all time points.
Methods
Study design and participants
Between February 2013 and November 2018, eligible patients were prospectively included in a multicenter, randomized trial, ACT (Optimal Timing of a Tailored Physical Activity Program During Chemotherapeutic Cancer Treatment to Reduce Long-Term Cardiovascular Morbidity), at the University Medical Center Groningen (UMCG), Martini Hospital (Groningen), and Ommelander Hospital (Scheemda) in the Netherlands. Adult patients recently diagnosed with breast cancer, colon cancer, testicular cancer, or B-cell non-Hodgkin lymphoma scheduled to receive curative chemotherapy were eligible. Inclusion criteria were normal blood counts, regulated blood pressure, respiratory rate < 20 breaths/min, resting heart rate 50 to 100 beats/min, no fever, and left ventricular ejection fraction ≥ 50%. Exclusion criteria were infections requiring antibiotics, signs of ongoing bleeding, critical organ impairment or uncontrolled symptoms due to malignancy, no recovery from earlier surgical intervention, inability to travel independently to the rehabilitation center, a recent cardiovascular event (<6 months), and a cognitive disorder or emotional instability. The medical ethics committee of UMCG approved the trial, and all patients gave written informed consent (NCT01642680). Patients were randomized in a 1:1 ratio to an exercise intervention initiated during (group A) or after (group B) chemotherapy. Randomization was stratified by hospital and cancer diagnosis. The randomization was performed by an independent research assistant using a table of random numbers generated by a computer. The sequence of allocation was concealed from the investigators.
Procedures
The intervention consisted of 12 weeks of supervised exercise followed by 12 weeks of home-based unsupervised exercise (both 36 sessions). Group A initiated the 12-week supervised exercise intervention during chemotherapy and continued with the 12-week unsupervised home-based exercise after completing chemotherapy. Given the different treatment regimens for the various cancer types, all patients randomized in group A initiated the supervised exercise intervention 12 weeks before the end of chemotherapy. Patients treated with chemotherapy for 12 weeks started the supervised exercise intervention immediately at the start of chemotherapy. Patients who underwent chemotherapy for 18 or 24 weeks started the supervised exercise intervention 6 or 12 weeks after beginning chemotherapy, respectively. Patients in group B initiated the supervised exercise intervention approximately 3 weeks after the administration of the final dose of chemotherapy. The supervised exercise intervention consisted of 18.95 MET-h/wk, using the 2011 Compendium of Physical Activities: bicycle stationery (moderate to vigorous effort), 6.8 METs × 1.5 hours = 10.2 MET-h; resistance training (weightlifting, free weight), 6 METs × 1 hour = 6 MET-h; and badminton, 5.5 METs × 0.5 hours = 2.75 MET-h. More details on the exercise intervention are described in the Supplemental Appendix.
Patients visited the outpatient clinic for assessments before the start of chemotherapy, postchemotherapy, postintervention, and 1 year postintervention (Figure 1). Patient characteristics were derived from medical records, including tumor characteristics; type of surgery; chemotherapy; radiotherapy; comorbidities at the start of chemotherapy; smoking habit; alcohol consumption; and use of cholesterol-lowering medications, anticoagulant medications, and antihypertensive medication.
Figure 1.
Design of the ACT Trial
Patients were randomized in a 1:1 ratio to an exercise intervention initiated during (group A) or after (group B) chemotherapy. Randomization was stratified by hospital and cancer diagnosis. The 24-week physical exercise intervention consisted of 2 components: 12 weeks of supervised exercise followed by 12 weeks of home-based unsupervised exercise. ACT = Optimal Timing of a Tailored Physical Activity Program During Chemotherapeutic Cancer Treatment to Reduce Long-Term Cardiovascular Morbidity; VO2peak = peak oxygen uptake; X months = duration varied from 0 to 12 weeks depending on chemotherapy regimen.
Outcomes
The primary outcome, VO2peak, was determined by cardiopulmonary exercise testing on a stationary bicycle ergometer (Jaeger Oxycon Pro/Vyntus CPX, CareFusion). Muscle strength was assessed by maximal voluntary isometric muscle force of quadriceps, hamstrings, biceps, and triceps, which was measured using a handheld dynamometer (Force Evaluating & Testing [microFET2], Hoggan Health Industries).12 Details of cardiopulmonary exercise testing and muscle strength measurements are described in Supplemental Tables 1 to 4 and Supplemental Figure 1. Body weight was determined without wearing shoes to the nearest 0.1 kg on an electronic scale. Height was measured to the nearest 0.5 cm using a stadiometer. Adherence to the exercise intervention was expressed as the number of attended exercise sessions divided by the number of prescribed exercise sessions. Serious adverse events (SAE) were monitored until the final measurement. HRQoL was assessed using the validated Dutch version of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (version 3.0).13 Fatigue was determined using the Multidimensional Fatigue Inventory.14 Physical activity was assessed using the sum score of the Physical Activity Scale for the Elderly (PASE) questionnaire.15 Self-efficacy, measuring patients’ expectations of their general capacities, was evaluated using the Dutch version of the General Self-Efficacy Scale, Algemene Competentie Schaal.16
Sample size and statistical analysis
To detect a between-group difference of 2.5 mL/kg/min in VO2peak (SD = 6.38 mL/kg/min) at 1 year postintervention, with power of 0.80 and a 2-sided alpha value of 0.05, we calculated that 103 patients per group were needed. To account for a potential 30% dropout rate, 266 patients were included. The SD was derived from a pilot study including 31 patients that estimated the intervention’s effect size, conducted at UMCG.
Descriptive data are presented as mean ± SD, median (IQR), or median (range) for continuous variables, and count (percentage) for categorical variables per relevant subgroup (patients with breast cancer, testicular cancer, and colon cancer).
Intention-to-treat linear mixed-models analyses were performed to calculate within-group and between-group differences at 3 time points—postchemotherapy, postintervention, and 1 year postintervention—in VO2peak, muscle strength, and patient-reported outcomes. An unstructured covariance structure was used. These models were adjusted for baseline values and the fixed effects of cancer type, hospital, group, time, and time-by-group interaction. Normality of the residuals was checked using a normal probability plot. Exploratory post hoc subgroup analyses of within-group and between-group differences in VO2peak per cancer type were performed. Two-sided P values <0.05 were considered to indicate statistical significance. Statistical analyses were performed using SPSS Statistics version 22.0 (IBM).
Results
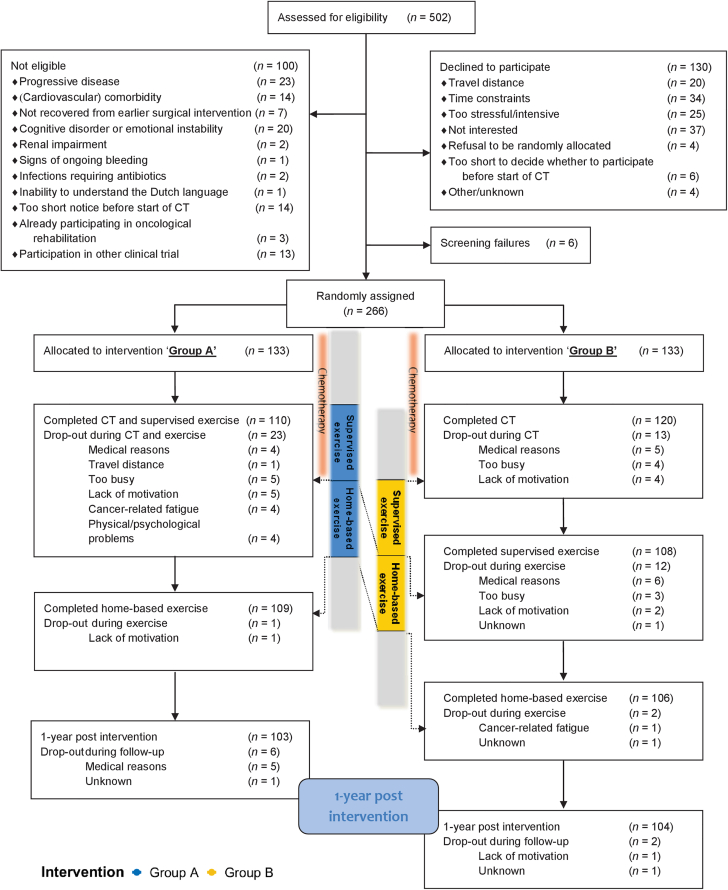
From February 2013 until November 2018, 502 patients were screened for eligibility, of whom 100 were deemed ineligible, 130 declined to participate, and 6 could not be screened (Figure 2). A total of 266 patients were included and randomized to group A (n = 133) or group B (n = 133). In total, 139 patients with breast cancer, 95 patients with testicular cancer, 30 patients with colon cancer, and 2 patients with B-cell non-Hodgkin lymphoma were included. After randomization, 30 patients dropped out of group A, and 29 patients dropped out of group B. Patient characteristics are depicted in Table 1.
Figure 2.
Consort Diagram of the ACT Trial
In total, 502 patients were screened for eligibility, of whom 100 were deemed ineligible, 130 declined to participate, and 6 could not be screened. A total of 266 patients were included and randomized to group A or group B. In total, 139 patients with breast cancer, 95 patients with testicular cancer, 30 patients with colon cancer, and 2 patients with B-cell non-Hodgkin lymphoma were included. After randomization, 30 patients dropped out of group A, and 29 patients dropped out of group B. CT = chemotherapy.
Table 1.
Baseline Characteristics
| Group A | Group B | |
|---|---|---|
| Baseline characteristics of all patients | ||
| Number of patients | 133 | 133 |
| Age, y, mean (range) | 45.8 (20-74) | 48.3 (21-76) |
| Gender | ||
| Male | 57 (43) | 56 (42) |
| Female | 76 (57) | 77 (58) |
| BMI, kg/m2, mean (range) | 25.4 (17.8-41.4) | 26.3 (17.2-41.3) |
| Smoking | ||
| Current | 22 (17) | 15 (11) |
| Previous | 56 (42) | 53 (40) |
| Never | 55 (41) | 65 (49) |
| Baseline characteristics of patients with breast cancer | ||
| Number of patients | 70 | 69 |
| Age, y, mean (range) | 51 (30-71) | 54 (32-72) |
| Postmenopausal | ||
| Yes | 28 (40) | 33 (49) |
| No | 38 (54) | 34 (48) |
| Unknown/male patient | 4 (6) | 2 (3) |
| Tumor type | ||
| Ductal | 56 (80) | 59 (86) |
| Lobular | 9 (13) | 7 (10) |
| Other | 4 (6) | 3 (4) |
| Unknown | 1 (1) | 0 |
| Side | ||
| Left | 37 (53) | 29 (42) |
| Right | 32 (46) | 37 (54) |
| Left and right | 1 (1) | 3 (4) |
| ER status (positive) | 62 (89) | 52 (75) |
| HER2/neu status (positive) | 16 (23) | 17 (25) |
| Type of surgery | ||
| Mastectomy | 33 (53) | 32 (46) |
| Lumpectomy | 37 (47) | 37 (54) |
| Radiotherapy | ||
| No | 17 (24) | 14 (20) |
| Before chemotherapy | 25 (36) | 24 (35) |
| After chemotherapy | 28 (40) | 31 (45) |
| Dose of radiotherapy, whole breast, Gy, median (IQR) | 46.0 (43.0-46.0) | 46.0 (43.0-46.0) |
| Number of fractions of radiotherapy, median (IQR) | 21 (16-21) | 21 (16-21) |
| Type of chemotherapy | ||
| With anthracyclines | 54 (77) | 58 (84) |
| No anthracyclines | 15 (22) | 11 (16) |
| No chemotherapya | 1 (1) | — |
| Duration of chemotherapy | ||
| 12 wk | 13 (19) | 10 (15) |
| 18-24 wk | 57 (81) | 59 (85) |
| Trastuzumab (yes) | 17 (24) | 16 (23) |
| Hormonal therapy (yes) | 63 (90) | 52 (75) |
| Surgery after completion of chemotherapy | ||
| Breast reconstruction | 10 (14) | 8 (12) |
| Other | 8 (11) | 3 (4) |
| Baseline characteristics of patients with colon cancer | ||
| Number of patients | 14 | 16 |
| Age, y, mean (range) | 63 (46-74) | 62 (44-76) |
| Gender | ||
| Male | 7 (50) | 8 (50) |
| Female | 7 (50) | 8 (50) |
| Dukes stage | ||
| III | 12 (86) | 16 (100) |
| IV | 1 (7) | 0 |
| Unknown | 1 (7) | 0 |
| Type surgery | ||
| Laparoscopic | 5 (36) | 12 (75) |
| Laparotomic | 9 (64) | 4 (25) |
| Type of chemotherapy | ||
| FOLFOX (12 courses) | 10 (71) | 12 (75) |
| CAPOX (4 or 8 courses) | 3 (21) | 1 (6) |
| FOLFOX and CAPOX | 1 (7) | 3 (19) |
| Baseline characteristics of patients with testicular cancer | ||
| Number of patients | 48 | 47 |
| Age, y, mean (range) | 33 (20-48) | 35 (21-62) |
| Diagnosis | ||
| Seminoma | 19 (40) | 14 (30) |
| Nonseminoma | 29 (60) | 31 (66) |
| Other | — | 2 (4)a |
| Royal Marsden stage | ||
| I | 1 (2) | — |
| II | 37 (77) | 33 (70) |
| III | 3 (6) | 4 (9) |
| IV | 7 (15) | 8 (17) |
| Other | — | 2 (4) |
| IGCCCG prognosis group | ||
| Good | 46 (98) | 41 (87) |
| Intermediate | 1 (2) | 3 (6) |
| Poor | — | 1 (2) |
| Other | — | 2 (4) |
| Pulmonary metastases (yes) | 9 (19) | 8 (17) |
| Chemotherapy regime | ||
| BEP/EP | 45 (94) | 43 (91) |
| Other | 2 (4) | 4 (9) |
| No chemotherapyb | 1 (2) | — |
| Courses of chemotherapy | ||
| 3 | 35 (73) | 32 (68) |
| 4 | 12 (25) | 14 (30) |
| Other | 1 (2) | 1 (2) |
| Cumulative dose of bleomycin, USP, median (range) | 270 (30-330) | 270 (60-360) |
| Cumulative dose of cisplatin, mg/m2, median (range) | 300 (300-700c) | 300 (100-700c) |
| RPLND after completion of chemotherapy | 7 (15) | 2 (4) |
Values are n (%) unless indicated otherwise. In group A, exercise intervention was initiated during chemotherapy; in group B, exercise intervention was initiated after chemotherapy.
BEP = bleomycin, etoposide, and cisplatin combination chemotherapy; BMI = body mass index; CAPOX = capecitabine and oxaliplatin combination chemotherapy; EP = etoposide and cisplatin combination chemotherapy; ER = estrogen receptor; FOLFOX = 5-fluorouracil and oxaliplatin combination chemotherapy; HER2 = human epidermal growth factor receptor 2; IGCCCG = International Germ Cell Cancer Collaborative Group; RPLND = retroperitoneal lymph node dissection; USP = United States Pharmacopeia.
In one patient, abnormalities on electrocardiography during the cardiorespiratory exercise test were found. This patient was considered a dropout at time 0.
In one patient, tumor markers normalized without treatment. This patient was considered a dropout at time 0.
Two patients received additional paclitaxel, ifosfamide, and cisplatin chemotherapy. Therefore, the cumulative dose of cisplatin was 700 mg/m2.
The median adherence rate to the supervised exercise of patients in group A was 75.0% (range: 3%-103%). In group B, the median adherence rate was 83.3% (range: 0%-100%). Adherence to the supervised exercise intervention was not statistically significantly different between the groups (P = 0.11). Training logs were kept by 141 of the 215 patients (65.6%) who completed the home-based exercise intervention with data on adherence to this component. Adherence to the home-based exercise intervention was 82% in group A (range: 0%-133%; n = 70 patients) and 83% in group B (range: 0%-144%; n = 71 patients).
No between-group differences were found postintervention or 1 year postintervention in VO2peak. From baseline to postchemotherapy, VO2peak declined significantly in both groups A and B, with within-group differences of −2.8 mL/kg/min (95% CI: −3.5 to −2.0 mL/kg/min) and −5.8 mL/kg/min (95% CI: −6.6 to −5.1 mL/kg/min), respectively (means and within-group differences are depicted in Table 2 and between-group differences in Table 3 and Central Illustration A). This decline was less in group A than in group B (adjusted between-group difference 3.1 mL/kg/min; 95% CI: 2.2 to 4.0; P < 0.001). In the exploratory analysis of the separate diagnosis groups (breast cancer, colon cancer, and testicular cancer), patients in both groups regained baseline levels of VO2peak immediately postintervention and remained stable at 1 year postintervention. Immediately postchemotherapy, the adjusted between-group differences in patients with testicular cancer, breast cancer and colon cancer were 4.4 mL/kg/min (95% CI: 2.7-6.1 mL/kg/min; P < 0.001), 2.2 mL/kg/min (95% CI: 1.1-3.3 mL/kg/min; P < 0.001), and 3.5 mL/kg/min (95% CI: 1.2-5.7 mL/kg/min; P = 0.004), respectively (Table 2). Significant between-group differences in both patients with breast cancer who were treated with chest radiotherapy (n = 107) and who did not receive chest radiotherapy (n = 32) were found in favor of the exercise groups (1.6 mL/kg/min [95% CI: 0.6-2.7 mL/kg/min; P = 0.004] and 3.8 mL/kg/min [95% CI: 0.9-6.7 mL/kg/min; P = 0.011], respectively).
Table 2.
Peak Oxygen Uptake Postchemotherapy, Postintervention, and 1-Year Postintervention
| Baseline |
Postchemotherapy |
Postintervention |
1 Year Postintervention |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | n | Mean ± SD | n | Within-Group Difference vs Baseline (95% CI) | Mean ± SD | n | Within-Group Difference vs Baseline (95% CI) | Mean ± SD | n | Within- Group Difference vs Baseline (95% CI) | |
| All | |||||||||||
| Group A | 30.4 ± 8.2 | 132a | 28.3 ± 6.7 | 108 | −2.8 (−3.5 to −2.0) | 31.5 ± 7.9 | 106 | +0.2 (−0.5 to 1.0) | 32.0 ± 8.4 | 102 | +0.5 (−0.4 to 1.3) |
| Group B | 29.7 ± 8.4 | 132b | 24.1 ± 7.2 | 118 | −5.8 (−6.6 to −5.1) | 30.9 ± 9.2 | 99 | +0.6 (−0.1 to 1.4) | 30.5 ± 9.5 | 103 | +0.2 (−0.7 to 1.0) |
| Testicular cancer | |||||||||||
| Group A | 36.3 ± 7.9 | 48 | 32.5 ± 6.8 | 40 | −4.4 (−5.8 to −3.1) | 36.8 ± 7.8 | 40 | −0.2 (−1.6 to 1.2) | 38.2 ± 7.9 | 40 | +1.2 (−0.4 to 2.8) |
| Group B | 37.6 ± 7.6 | 47 | 28.8 ± 7.6 | 42 | −9.1 (−10.4 to −7.8) | 38.9 ± 8.4 | 35 | +0.9 (−0.6 to 2.4) | 39.0 ± 8.4 | 36 | +0.5 (−1.2 to 2.1) |
| Breast cancer | |||||||||||
| Group A | 28.0 ± 6.2 | 69a | 25.9 ± 5.6 | 59 | −2.6 (−3.4 to −1.9) | 28.6 ± 6.1 | 56 | −0.1 (−1.0 to 0.7) | 28.6 ± 5.8 | 54 | −0.3 (−1.3 to 0.7) |
| Group B | 25.3 ± 5.1 | 68b | 21.1 ± 5.4 | 63 | −4.5 (−5.3 to −3.8) | 26.5 ± 6.3 | 54 | +0.2 (−0.7 to 1.0) | 25.6 ± 6.4 | 56 | −0.5 (−1.4 to 0.5) |
| Colon cancer | |||||||||||
| Group A | 22.3 ± 3.2 | 14 | 25.4 ± 2.5 | 9 | +1.8 (0.1 to 3.6) | 25.9 ± 5.5 | 10 | +3.1 (0.7 to 5.5) | 24.6 ± 5.5 | 8 | +1.3 (−1.2 to 3.8) |
| Group B | 25.4 ± 5.4 | 16 | 23.6 ± 5.7 | 13 | −1.6 (−3.1 to −0.1) | 26.1 ± 5.6 | 10 | +1.8 (−0.6 to 4.1) | 27.4 ± 5.7 | 11 | +2.4 (0.2 to 4.6) |
The shown within-group differences are estimates extracted from the linear mixed-effects models. In group A, exercise intervention was initiated during chemotherapy; in group B, exercise intervention was initiated after chemotherapy.
1 baseline measurement of peak oxygen uptake had to be stopped early because the patient did not tolerate the mouthpiece. This patient was considered a dropout at time 0.
1 baseline measurement was not measured correctly because of technical difficulties.
Table 3.
Linear-Mixed Effects Model Results of VO2peak Postchemotherapy, Postintervention, and 1 Year Postintervention
| Postchemotherapy |
Postintervention |
1-Year Postintervention |
Between-Group Difference Postchemotherapy |
Between-Group Difference Postintervention |
Between-Group Difference 1-Year Postintervention |
||||
|---|---|---|---|---|---|---|---|---|---|
| LSM ± SE | LSM ± SE | LSM ± SE | LSM Difference (95% CI) | P Value | LSM difference (95% CI) | P Value | LSM difference (95% CI) | P Value | |
| All | |||||||||
| Group A | 26.3 ± 1.4 | 29.3 ± 1.5 | 29.5 ± 1.5 | 3.1 (2.2 to 4.0) | <0.001 | −0.3 (−1.3 to 0.8) | 0.64 | 0.5 (−0.7 to 1.7) | 0.43 |
| Group B | 22.7 ± 1.4 | 29.1 ± 1.5 | 28.7 ± 1.5 | ||||||
| Testicular | |||||||||
| Group A | 31.9 ± 1.0 | 36.2 ± 1.2 | 37.5 ± 1.2 | 4.4 (2.7 to 6.1) | <0.001 | −1.3 (−3.3 to 0.7) | 0.19 | 0.5 (−1.8 to 2.7) | 0.68 |
| Group B | 28.4 ± 1.0 | 38.5 ± 1.2 | 38.0 ± 1.2 | ||||||
| Breast | |||||||||
| Group A | 25.0 ± 0.9 | 27.6 ± 1.0 | 27.3 ± 1.0 | 2.2 (1.1 to 3.3) | <0.001 | 0.0 (−1.2 to 1.2) | 1.00 | 0.5 (−0.9 to 1.8) | 0.52 |
| Group B | 20.5 ± 0.9 | 25.2 ± 1.0 | 24.5 ± 1.0 | ||||||
| Colon | |||||||||
| Group A | 23.4 ± 1.7 | 24.7 ± 1.9 | 22.9 ± 1.8 | 3.5 (1.2 to 5.7) | 0.004 | 1.4 (−2.0 to 4.8) | 0.40 | −1.1 (−4.6 to 2.5) | 0.53 |
| Group B | 23.4 ± 1.3 | 26.7 ± 1.6 | 27.4 ± 1.5 | ||||||
P value for mixed-model between-group measures comparing changes in groups A and B from baseline to postchemotherapy, postintervention, and 1 year postintervention, adjusted for baseline values, diagnosis, and center (adjusted baseline values: all patients, 30.8; testicular cancer, 37.6; breast cancer, 27.4; colon cancer, 24.0). The LSMs are estimates extracted from the linear mixed-effects models, which means that these are adjusted for the baseline value, center and diagnosis. In group A, exercise intervention was initiated during chemotherapy; in group B, exercise intervention was initiated after chemotherapy.
LSM = least squares mean.
Central Illustration.
Effects of Exercise on Peak Oxygen Uptake, Health-Related Quality of Life, and Fatigue
This randomized clinical trial in patients with breast, testicular, and colon cancer examined effects of physical exercise initiated during or after chemotherapy. In the first weeks during chemotherapy, groups A and B declined similarly in terms of (A) peak oxygen uptake, (B) health-related quality of life, and (C) general fatigue. However, at the completion of chemotherapy, these parameters in group A had increased, whereas the values in group B continued to decline. Three months after completion of chemotherapy and 1 year after the exercise intervention, the values were similar in both groups. HRQoL = health-related quality of life; VO2peak = peak oxygen uptake.
At 1 year postintervention and postintervention, the strength of all muscle groups regained baseline values, without between-group differences. Immediately postchemotherapy, muscle strength of the quadriceps, biceps, and triceps declined less in group A compared with group B, with the following between-group differences for these respective muscles: 17.9 N (95% CI: 3.9-32.0 N; P = 0.012), 11.0 N (95% CI: 2.9-19.1 N; P = 0.008), and 6.3 N (95% CI: 0.5-12.1 N; P = 0.033) (Supplemental Table 1).
At 1 year postintervention, HRQoL was higher in both group A and group B compared with baseline (within-group differences 8.2 [95% CI: 4.5 to 11.8] and 4.4 [95% CI: 0.9 to 7.9], respectively). At 1 year postintervention and postintervention, no between-group differences were found in HRQoL. Immediately postchemotherapy, HRQoL (Central Illustration B) and the physical functioning subscale declined less in group A than in group B (adjusted between-group differences 6.1 [95% CI: 1.0 to 11.2; P = 0.027] and 5.5 [95% CI: 0.6 to 10.4; P = 0.027]). The dyspnea and appetite loss subscales favored group A; adjusted between-group differences were −8.4 (95% CI: −16.0 to −0.7; P = 0.032) and −6.6 (95% CI: −12.7 to −0.6; P = 0.033) (Supplemental Table 2, Figure 3).
Figure 3.
Between-Group Changes From Baseline: HRQoL, Functioning, and Cancer-Related Symptoms
Between-group changes from baseline were calculated using intention-to-treat linear mixed-models analyses, adjusted for baseline values, cancer type, and hospital. The dotted lines denote the threshold for a clinical meaningful difference. An adjusted mean difference that is positive suggests that the measure was greater in group A. After chemotherapy, HRQoL and the physical functioning subscale declined significantly less in group A than in group B. The dyspnea and appetite loss subscales favored group A. HRQoL = health-related quality of life.
At 1 year postintervention and postintervention, no between-group differences were found in general fatigue and physical fatigue. Immediately postchemotherapy, patients in group A experienced less general fatigue and physical fatigue and scored higher on the reduced activity subscale than patients in group B. Adjusted between-group differences were −2.1 (95% CI: −3.3 to −0.8; P = 0.001) for general fatigue, −2.9 (95% CI: −4.3 to −1.5; P < 0.001) for physical fatigue, and −1.5 (95% CI: −2.9 to −0.1; P = 0.030) for reduced activity (Central Illustration C, Supplemental Table 3).
In both groups A and B, self-reported physical activity was similar at baseline compared with the measurement directly after chemotherapy (the mean ± SE PASE sum scores of group A were 132 ± 9 at baseline and 136 ± 9 immediately postchemotherapy; in group B, these scores were 119 ± 9 and 108 ± 8, respectively). Immediately postchemotherapy and 1 year postchemotherapy, the PASE sum score had increased in both groups (group A, 173 ± 10 and 167 ± 10; group B, 148 ± 10 and 154 ± 10, respectively).
No significant between-group differences were found in the PASE sum score at 1 year postintervention between groups A and B. Immediately postintervention and postchemotherapy, the PASE sum score was higher in group A compared with group B (adjusted between-group differences 27.8 [95% CI: 7.1-48.4; P = 0.009] and 28.0 [95% CI: 11.2-44.8; P = 0.001], respectively) (Supplemental Table 4).
Self-efficacy did not differ between the groups at 1 year postintervention, postintervention, and postchemotherapy (Supplemental Table 4).
No differences between groups A and B were found in the number of patients with breast cancer or testicular cancer who received reduced doses of chemotherapy. In patients with breast cancer, 35 of 69 patients in group A (50.7%) and 34 of 69 patients in group B (49.3%) received reduced chemotherapy doses (P = 0.87). In patients with testicular cancer, 8 of 47 patients in group A (17.0%) and 9 of 47 patients in group B (19.1%) received reduced chemotherapy doses (P = 0.79). Because of low patient numbers, we did not calculate difference in the received dose of chemotherapy in patients with colon cancer.
A total of 53 SAE occurred in 49 patients, 27 SAE in group A and 26 SAE in group B. One of the 53 SAE was probably related to the intervention (vasovagal syncope, group A), and 1 was related (biceps tendon rupture, group B) (Supplemental Appendix).
Discussion
The randomized multicenter clinical ACT trial showed that patients with breast cancer, testicular cancer, or colon cancer who engaged in an exercise program regained their baseline cardiorespiratory fitness 1 year after completing the exercise intervention, irrespective of timing. This suggests that physical fitness remains at a stable level once patients resume participation in daily life and the associated physical activities. Indeed, we found that the self-reported physical activity in both groups was significantly higher after completion of the exercise intervention and at 1-year follow-up compared with baseline. Additionally, the passage of time also contributes to the recovery of patients. These findings are in line with previous studies examining the effects of exercise during chemotherapy.17, 18, 19
In the first weeks during chemotherapy, patients in groups A and B declined similarly in terms of VO2peak, muscle strength, HRQoL, and general fatigue. However, at the completion of chemotherapy, after group A had been exercising for 3 months, these parameters for group A had increased, whereas the values for group B continued to decline. Three months after completion of chemotherapy (after completion of supervised exercise in group B and after completion of home-based exercise in group A), the values were similar in both groups. The additional home exercise in group B seemed not to increase these values. One year after stopping exercise, the values were similar in both groups and compared with the baseline values. These findings suggest that the optimal timing of physical exercise is during chemotherapy. However, initiating a physical exercise program after chemotherapy is a viable alternative when exercising during chemotherapy is not possible. Our study has thus provided more data on the timing of initiating physical exercise therapy as part of anticancer treatment.
We found that the level of VO2peak 1 year after completion of the exercise program recovered in both groups to baseline values. The level of self-reported physical activity before the start of chemotherapy is lower compared with postintervention and at 1 year postintervention, measured using the PASE questionnaire. After chemotherapy, the level of physical activity is comparable with baseline. In previous studies in which patients did not attend an exercise intervention during or after adjuvant treatment (chemotherapy, radiotherapy, or hormonal therapy), a decline of up to 25% in VO2peak was found compared with healthy, sedentary women, which frequently did not recover.7,20 VO2peak and physical activity levels are strongly associated with cardiovascular risk.9,10 The level of physical activity can be expressed as METs, defined as the number of kilocalories burned per kilogram body weight per hour (MET-h).21 In 2 population-based cohort studies of 2,973 patients with nonmetastatic breast cancer, it was found that engaging in 11 to 24.5 MET-h/wk and >24.5 MET-h/wk resulted in risk reductions of 21% (HR: 0.79; 95% CI: 0.66-0.96) and 35% (HR: 0.65; 95% CI: 0.53-0.80) for developing cardiovascular events.22 Therefore, regaining VO2peak and the level of physical activity to baseline values might lower the risk for cardiovascular events in cancer survivors.
Regarding specific outcomes, fatigue is considered one of the most distressing adverse effects of cancer therapy and occurs in up to 80% of patients treated with chemotherapy.23 Fatigue can negatively affect reintegration, social relationships, and participation in daily activities.23 In this trial, we found a clinically significant difference in general and physical fatigue between the groups in favor of the group that exercised during treatment, measured directly after chemotherapy, which might accelerate the return to everyday life.24,25 This is in line with previous research demonstrating less fatigue in patients attending an exercise intervention during cancer treatment.26
In our study, we found that exercise during and after chemotherapy is safe. Our study’s supervised physical exercise intervention consisted of personalized aerobic and strength training, gradually increasing in intensity during the program. This intervention differs from a recent study in which patients with testicular cancer were randomized to 2 supervised high-intensity interval training (HIIT) sessions (85%-95% of peak heart rate) per week during chemotherapy or control group patients, who were asked not to initiate HIIT and to maintain their baseline exercise levels.27 Three of the first 9 patients in the intervention group developed severe thromboembolic events during chemotherapy. Possibly, HIIT increases vascular shear stress and causes relative dehydration, inducing higher blood viscosity. In healthy, sedentary men who performed high-intensity physical exercise, thrombus formation, and coagulation activity were increased, whereas moderate-intensity exercise did not affect thrombus formation.28,29
Including a nonexercise control group would have been informative to compare the effects of exercise during and after chemotherapy to no exercise at all. However, in the period when the study protocol was written (2012), it was already known that VO2peak declines during chemotherapy, that physical exercise during and after treatment with chemotherapy has evident beneficial effects on various chemotherapy-related adverse effects, and that physical training was beneficial on several outcomes such as physical fitness, fatigue, and quality of life.30, 31, 32 In addition, exercise programs appeared to be safe. However, knowledge of the timing of the physical exercise training was lacking. Therefore, we considered it unethical to include a nonexercise control group.
Study limitations
The strengths of our trial are the multicenter randomized design, the relatively large sample size, the high adherence rate, and the personalized and supervised training schedule followed by a home-based physical exercise plan. Although the inclusion of understudied cancer types was a strength of the study, the study sample allowed only explorative analyses by cancer type. Explorative subgroup analyses showed comparable beneficial effect sizes for VO2peak immediately postchemotherapy in patients with breast, colon, and testicular cancer who exercised compared with no exercise during chemotherapy. This effect was largest in patients with testicular cancer, who were on average younger and all men compared with patients with breast or colon cancer. As no adjustment for type 1 error was performed, these results should be interpreted with caution. In line with our findings, a large meta-analysis showed that the exercise effect on VO2peak in patients without severe chronic diseases is largest in men and individuals younger than 50 years (regardless of sex).33 Another meta-analysis investigated moderators of demographic characteristics on the effects of exercise on physical functioning in cancer patients and did not identify age and sex as moderators.34 However, this meta-analysis did not include patients with testicular cancer, and physical function was measured using a questionnaire instead of VO2peak.
Data on physical activity in this trial were measured using the self-reported PASE questionnaire. In future studies, it would be interesting to more objectively measure physical activity with, for example, accelerometers or smart phone applications that more accurately measure daily physical activity in the period after the intervention for maintenance of physical activity. Another limitation concerns patients’ unexpectedly high dropout rate (29% instead of 15%) and the long inclusion period. To account for this, we amended the protocol, which resulted in a larger sample size, and patients with B-cell non-Hodgkin lymphoma were included as an additional patient group; unfortunately, only 2 patients with B-cell non-Hodgkin lymphoma were included. The patients with B-cell non Hodgkin lymphoma were included in the analysis, but no conclusions from the analysis were drawn from these patients. This high dropout rate may have been due to the frequent and intensive exercise sessions and study measurements. We experienced a significant challenge for patients participating in a supervised exercise program during and after chemotherapy during the trial. This was mainly because of logistic hurdles, such as time restrictions, returning to work, childcare commitments, and even in highly motivated patients. It should be taken into account that it is not self-evident to integrate an exercise program as standard care during and after cancer treatment. Finally, we included patients interested in a healthy lifestyle, making the data less generalizable for less motivated patients.
The findings of our study contribute to the evidence of the benefits of exercise therapy as part of anticancer treatment and should motivate health care providers to inform and guide patients to engage in physical exercise programs during the period of anticancer treatment.
Conclusions
Exercise can be performed during chemotherapy, prevents fatigue and decreases in VO2peak, muscle strength, and HRQoL, and hastens the return of function after chemotherapy. Also, if exercise cannot be performed during chemotherapy, a program afterward can allow patients to regain the same level of function, measured 1 year after completion of the intervention.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: If possible, initiating a physical exercise program during instead of after chemotherapy would be preferred to reduce fatigue and decreases in VO2peak, muscle strength, and HRQoL in the period of active treatment. Initiating an exercise program after completion of chemotherapy is a viable alternative, as patients in both groups regained the same level of function measured 1 year after completion of the intervention.
TRANSLATIONAL OUTLOOK: Prospective randomized studies per cancer diagnosis are needed to further investigate the effects of timing of an exercise program on VO2peak and long-term adverse effects of chemotherapy.
Funding Support and Author Disclosures
This work was supported by the Dutch Cancer Society, Alpe d’HuZes (grant DCS 2011-5265). The funder had no role in the design, data collection, management, analysis, interpretation, report writing, and decision to submit the manuscript. Dr Hummel has patents, royalties, and other intellectual properties with Us2.ai (institutional). Dr van der Meer has received research funding from Vifor Pharma (institutional), AstraZeneca (institutional), Ionis (institutional), and Pfizer (institutional); and has received payments or honoraria from Vifor Pharma (institutional), Novartis (institutional), and Pharmacosmos (institutional). Dr Nijland has received research funding from Takeda (institutional) and Roche (institutional); and has performed consulting in an advisory role for Genmab (institutional). Dr de Vries has received research funding from Amgen (institutional), AstraZeneca (institutional), Bayer (institutional), Chugai Pharma (institutional), Crescendo (institutional), CytomX (institutional), Therapeutics (institutional), G1 Therapeutics (institutional), Genentech (institutional), Nordic Nanovector (institutional), Radius Health (institutional), Regeneron, Roche (institutional), Servier (institutional), and Synthon (institutional); and has performed consulting in an advisory role for Daiichi Sankyo (institutional), the National Surgical Adjuvant Breast and Bowel Project (institutional), and Sanofi (institutional). Dr Gietema has received research funding from Abbvie (institutional), Roche (institutional), and Siemens (institutional). Dr Walenkamp has received a grant from the Dutch Cancer Society (institutional); has received research funding from Abbvie (institutional), Bristol Myers Squibb (institutional), Genzyme (institutional), Karyopharm Therapeutics (institutional), and Roche (institutional); and has performed consulting in an advisory role for Polyphor (institutional), Ipsen (institutional), Karyopharm (institutional), and Novartis (institutional). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank all participating patients, oncologists, research staff members, and physical therapists. Also, the authors thank the Dutch Cancer Society, Alpe d’HuZes (grant DCS 2011-5265) and the MD-PhD program provided by the Junior Scientific Master class at UMCG for making this clinical trial possible.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental methods, tables, and figures, please see the online version of this paper.
Appendix
References
- 1.Jones L.W., Courneya K.S., Mackey J.R., et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30(20):2530–2537. doi: 10.1200/JCO.2011.39.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azim H.A.J., de Azambuja E., Colozza M., Bines J., Piccart M.J. Long-term toxic effects of adjuvant chemotherapy in breast cancer. Ann Oncol. 2011;22(9):1939–1947. doi: 10.1093/annonc/mdq683. [DOI] [PubMed] [Google Scholar]
- 3.Scott J.M., Zabor E.C., Schwitzer E., et al. Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta-analysis. J Clin Oncol. 2018;36(22):2297–2304. doi: 10.1200/JCO.2017.77.5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maginador G., Lixandrão M.E., Bortolozo H.I., et al. Aerobic exercise-induced changes in cardiorespiratory fitness in breast cancer patients receiving chemotherapy: a systematic review and meta-analysis. Cancers (Basel) 2020;12(8):1–14. doi: 10.3390/cancers12082240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaminsky L.A., Arena R., Ellingsen Ø., et al. Cardiorespiratory fitness and cardiovascular disease—the past, present, and future. Prog Cardiovasc Dis. 2019;62(2):86–93. doi: 10.1016/j.pcad.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Nauman J., Nes B.M., Lavie C.J., et al. Prediction of cardiovascular mortality by estimated cardiorespiratory fitness independent of traditional risk factors: the HUNT study. Mayo Clin Proc. 2017;92(2):218–227. doi: 10.1016/j.mayocp.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Peel A.B., Thomas S.M., Dittus K., Jones L.W., Lakoski S.G. Cardiorespiratory fitness in breast cancer patients: a call for normative values. J Am Heart Assoc. 2014;3(1) doi: 10.1161/JAHA.113.000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallen M.P., Hennessy D., Brown S., et al. High-intensity interval training improves cardiorespiratory fitness in cancer patients and survivors: a meta-analysis. Eur J Cancer Care. 2020;29(4):1–21. doi: 10.1111/ecc.13267. [DOI] [PubMed] [Google Scholar]
- 9.Ross R., Blair S.N., Arena R., et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134(24):e653–e699. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 10.Groarke J.D., Payne D.L., Claggett B., et al. Association of post-diagnosis cardiorespiratory fitness with cause-specific mortality in cancer. Eur Hear Journal Qual Care Clin Outcomes. 2020;6(4):315–322. doi: 10.1093/ehjqcco/qcaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iyengar N.M., Jones L.W., Kettering S. Development of exercise as interception therapy for cancer: a review. JAMA Oncol. 2019;5:1620–1627. doi: 10.1001/jamaoncol.2019.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stark T., Walker B., Phillips J.K., Fejer R., Beck R. Hand-held dynamometry correlation with the gold standard isokinetic dynamometry: a systematic review. PM R. 2011;3(5):472–479. doi: 10.1016/j.pmrj.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 13.Koller M., Aaronson N.K., Blazeby J., et al. Translation procedures for standardised quality of life questionnaires: the European Organisation for Research and Treatment of Cancer (EORTC) approach. Eur J Cancer. 2007;43(12):1810–1820. doi: 10.1016/j.ejca.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 14.Smets E.M., Garssen B., Bonke B., De Haes J.C. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39(3):315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 15.Washburn R.A., McAuley E., Katula J., Mihalko S.L., Boileau R.A. The Physical Activity Scale for the Elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52(7):643–651. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 16.Bosscher R.J., Smit J.H. Confirmatory factor analysis of the General Self-Efficacy Scale. Behav Res Ther. 1998;36(3):339–343. doi: 10.1016/s0005-7967(98)00025-4. [DOI] [PubMed] [Google Scholar]
- 17.Travier N., Velthuis M.J., Steins Bisschop C.N., et al. Effects of an 18-week exercise programme started early during breast cancer treatment: a randomised controlled trial. BMC Med. 2015;13(1):1–11. doi: 10.1186/s12916-015-0362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mijwel S., Jervaeus A., Bolam K.A., et al. High-intensity exercise during chemotherapy induces beneficial effects 12 months into breast cancer survivorship. J Cancer Surviv. 2019;13(2):244–256. doi: 10.1007/s11764-019-00747-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolam K.A., Mijwel S., Rundqvist H., Wengström Y. Two-year follow-up of the OptiTrain randomised controlled exercise trial. Breast Cancer Res Treat. 2019;175(3):637–648. doi: 10.1007/s10549-019-05204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lakoski S.G., Barlow C.E., Koelwyn G.J., et al. The influence of adjuvant therapy on cardiorespiratory fitness in early-stage breast cancer seven years after diagnosis: the Cooper Center Longitudinal Study. Breast Cancer Res Treat. 2013;138(3):909–916. doi: 10.1007/s10549-013-2478-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ainsworth B.E., Haskell W.L., Herrmann S.D., et al. 2011 compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 22.Jones L.W., Habel L.A., Weltzien E., et al. Exercise and risk of cardiovascular events in women with nonmetastatic breast cancer. J Clin Oncol. 2016;34(23):2743–2749. doi: 10.1200/JCO.2015.65.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofman M., Ryan J.L., Figueroa-Moseley C.D., Jean-Pierre P., Morrow G.R. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(suppl 1):4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- 24.Wolvers M.D.J., Leensen M.C.J., Groeneveld I.F., Frings-Dresen M.H.W., De Boer A.G.E.M. Predictors for earlier return to work of cancer patients. J Cancer Surviv. 2018;12(2):169–177. doi: 10.1007/s11764-017-0655-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leensen M.C.J., Groeneveld I.F., Heide I Van Der, et al. Return to work of cancer patients after a multidisciplinary intervention including occupational counselling and physical exercise in cancer patients: a prospective study in the Netherlands. BMJ Open. 2017;7(6):1–10. doi: 10.1136/bmjopen-2016-014746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Vulpen J.K., Peeters P.H.M., Velthuis M.J., Van Der Wall E., May A.M. Effects of physical exercise during adjuvant breast cancer treatment on physical and psychosocial dimensions of cancer-related fatigue: a meta-analysis. Maturitas. 2016;85:104–111. doi: 10.1016/j.maturitas.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Thorsen L., Fossa S.D., Haugnes H.S., et al. Thromboembolic events after high-intensity training during cisplatin-based chemotherapy for testicular cancer. J Clin Oncol. 2017;35(15_suppl):4551. doi: 10.1002/ijc.33151. [DOI] [PubMed] [Google Scholar]
- 28.Menzel K., Hilberg T. Blood coagulation and fibrinolysis in healthy, untrained subjects: effects of different exercise intensities controlled by individual anaerobic threshold. Eur J Appl Physiol. 2011;111(2):253–260. doi: 10.1007/s00421-010-1640-2. [DOI] [PubMed] [Google Scholar]
- 29.Cadroy Y., Pillard F., Sakariassen K.S., Thalamas C., Boneu B., Riviere D. Strenuous but not moderate exercise increases the thrombotic tendency in healthy sedentary male volunteers. J Appl Physiol. 2002;93(3):829–833. doi: 10.1152/japplphysiol.00206.2002. [DOI] [PubMed] [Google Scholar]
- 30.Jones L.W., Liang Y., Pituskin E.N., et al. Effect of exercise training on peak oxygen consumption in patients with cancer: a meta-analysis. Oncologist. 2011;16(1):112–120. doi: 10.1634/theoncologist.2010-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown J.C., Huedo-Medina T.B., Pescatello L.S., et al. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20(1):123–133. doi: 10.1158/1055-9965.EPI-10-0988. [DOI] [PubMed] [Google Scholar]
- 32.Lin X., Zhang X., Guo J., et al. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2015;4(7):1–28. doi: 10.1161/JAHA.115.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buffart L.M., Kalter J., Sweegers M.G., et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: an individual patient data meta-analysis of 34 RCTs. Cancer Treat Rev. 2017;52:91–104. doi: 10.1016/j.ctrv.2016.11.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.