Abstract
Experiments to isolate and characterize rhesus macaque myeloid α-defensins (RMADs) were conducted. Seven RMAD peptides were isolated and sequenced, and the cDNAs encoding six of these peptides and one other α-defensin from bone marrow were also characterized. Four of the RMADs were found to be highly similar to human neutrophil α-defensins HNP-1 to HNP-3, while the remaining four peptides were much more similar to human enteric α-defensin HD-5. Two α-defensin pairs differed only by the presence or absence of an additional arginine at the amino termini of their mature peptides, indicative of alternate posttranslational processing. The primary translation products of RMAD-1 to -8 are 94- and 96-amino-acid prepropeptides that are highly similar to those of human α-defensins. Immunolocalization experiments revealed a granular cytoplasmic pattern in the cytoplasms of neutrophils, indistinguishable from the pattern observed after immunostaining of human myeloid α-defensins in polymorphonuclear leukocytes. Each of the purified peptides was tested for its in vitro activities against Staphylococcus aureus 502a, Listeria monocytogenes EGD, Escherichia coli ML35, and Cryptococcus neoformans 271A. Several of the peptides were microbicidal for the gram-positive bacteria and C. neoformans at defensin concentrations in the range of 2 to 5 μM. All of the peptides were bacteriostatic against E. coli, but none were bactericidal for this organism. This study is the first to characterize the sequences and activities of α-defensins from nonhuman primates, data that should aid in delineating the role of these peptides in rhesus macaque host defense.
Antimicrobial peptides and proteins of leukocytes contribute to the intra- and extracellular destruction of invading microorganisms (5, 11, 17, 27). Among the microbicidal peptides characterized to date are the α- and β-defensins, members of conformationally similar peptide families that are distinguished by different tridisulfide motifs (10, 21, 26, 30). α-Defensins are prominently expressed in neutrophils and intestinal Paneth cells of many mammals (22), in rabbit kidney (3, 28), and in female reproductive epithelium (18). Many defensins exert potent activities against a broad spectrum of targets, including gram-positive and gram-negative bacteria, fungi, spirochetes, mycobacteria, protozoans, and enveloped viruses (reviewed in reference 13).
As a step toward the further elucidation of primate defensin function in vivo, we conducted experiments to characterize rhesus macaque myeloid α-defensins (RMADs). Seven RMADs were isolated and characterized, and the cDNAs encoding six of these peptides and one other α-defensin from bone marrow were also characterized. Immunolocalization experiments were performed to identify cells that expressed peptides that cross-reacted with purified human neutrophil α-defensins 1 to 3 (HNP-1 to -3). Each of the purified peptides was tested for its in vitro antimicrobial properties under conditions allowing for the differentiation of microbistatic and microbicidal activities.
MATERIALS AND METHODS
Leukocyte isolation.
Anticoagulated whole blood was obtained from healthy adult rhesus macaques housed at the California Regional Primate Research Center. After sedimentation of erythrocytes with 3% dextran sulfate, leukocytes were recovered from the plasma phase by centrifugation at 300 × g. In initial experiments, leukocytes were further purified by fractionation over Ficoll, which produced neutrophil preparations of >98% purity that were snap frozen and stored at −80°C until they were extracted (see below). In later experiments, leukocytes were harvested and frozen after dextran sedimentation of erythrocytes but without further purification.
Peptide extraction and purification.
We used a two-step method for peptide extraction in which leukocyte pellets containing 107 cells were first suspended in 0.9 ml of 80:10 methanol-acetic acid, stirred for 18 h at 8°C, clarified by centrifugation, and then dried in a Speed Vac centrifugal evaporator. The dried residue was suspended in 1.0 ml of 80:20 methanol-water, stirred for 6 h at 8°C, clarified as before, and lyophilized. The lyophilate was dissolved in 0.1 ml of 5% acetic acid and clarified, and the supernatant was subjected to high-performance liquid chromatography (HPLC) purification as described below.
Chromatography.
Reversed-phase (RP) HPLC was performed with a 4.6- by 250-mm Vydac C18 column equilibrated in 0.1% trifluoroacetic acid (TFA)–water. Conditions for gradient elution with acetonitrile containing 0.1% TFA are described in the legend to Fig. 1. Peptides were purified to homogeneity by sequential rounds of RP-HPLC. Stock solutions of each peptide, prepared in 0.01% acetic acid, were quantified by amino acid analysis (6).
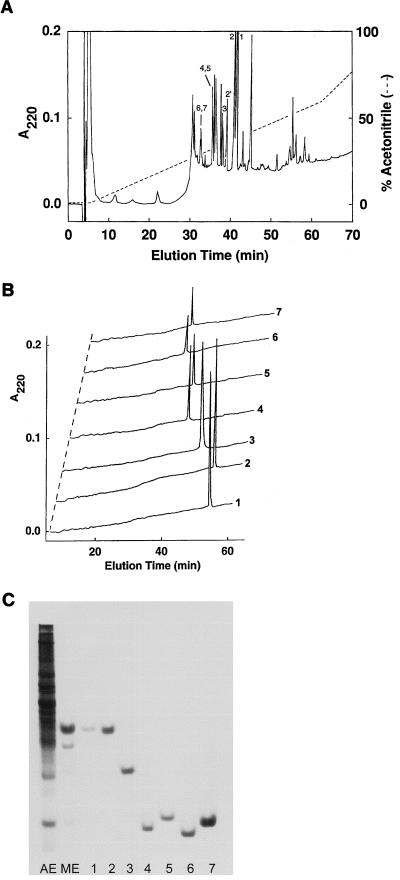
FIG. 1.
Purification of RMADs. (A) Methanol-acid extracts of neutrophil-enriched leukocytes were chromatographed on a 4.6- by 250-mm Vydac C18 column equilibrated in 0.1% TFA–H2O (solvent A) at a flow rate of 1.0 ml/min. A linear gradient of acetonitrile containing 0.1% TFA (solvent B) was applied as indicated by the dashed line. Fractions 1, 2, 3, 4, 5, 6, and 7 contained RMAD-1, -2, -3, -4, -5, -6, and -7, respectively. Fraction 2′ contained a methionine-oxidized form of RMAD-2. (B) Analytical RP-HPLC of ca. 1 μg of each purified RMAD was performed by rechromatographing samples as described for panel A but with a linear 0-to-40% gradient of acetonitrile developed over 60 min. (C) A 12.5% acrylamide acid–urea gel was loaded with 5% acetic acid extract (AE) of 3 × 106 buffy coat cells, a methanol-acetic acid–methanol-water extract (ME) of 7 × 106 buffy coat cells, and 0.5 to 1.5 μg each of RMAD-1 to -7. The gel was stained with formalin-Coomassie blue.
Sequence determinations.
RMAD-1 to -7 were characterized by amino acid analysis (6), matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectroscopy (MS), and automated Edman sequence analysis. Prior to being sequenced, peptides were reduced with 2-mercaptoethanol and alkylated with 4-vinylpyridine as described previously (25).
Reverse transcriptase PCR.
Total RNA was isolated from monkey bone marrow with RNA STAT-60 (TEL-TEST). Reverse transcription was performed with SuperScript II reverse transcriptase (GIBCO BRL) followed by PCR amplification with Taq polymerase (Qiagen) for 35 cycles at 94°C for 1 min, 57°C for 1 min, and 72°C for 1 min. PCR primers were chosen according to published cDNA sequences of HNP-1 and human enteric α-defensin 5 (HD-5) and were as follows: RMAD-1s (5′-GTCTGCCCTCTCTGGTCAC-3′) and RMAD-1a (5′-CAAGCTCAGCAGCAGAATGC-3′) for RMAD-1, -3, and -8 and RMAD-1s and HD-5a (5′-TCTAGAAGCTCAGCGACAGC-3′) for RMAD-4 to -7. PCR products were gel purified, cloned into pCR 2.1 (Invitrogen), and sequenced.
Immunohistochemistry.
Anti-HNP-1 to -3 antibody was prepared by immunization of a New Zealand White rabbit with a glutaraldehyde-mediated, keyhole limpet hemocyanin conjugate of an equimolar mixture of HNP-1, -2, and -3 (9). Dot blot analysis with 40 to 60 ng each of RMAD-1 to -7 and 50 ng of HNP-2 (as a positive control) demonstrated that anti-HNP-1 to -3 antibody recognized RMAD-1, -2, and -3 only. Cytospin preparations of rhesus macaque buffy coat cells were incubated with a 1:100 dilution of immune serum or preimmune serum for 1 h at room temperature. Immunoreactivity was visualized with a 1:200 dilution of biotinylated goat anti-rabbit immunoglobulin G and developed with the avidin-biotin-glucose oxidase system described previously (29).
Antimicrobial assays.
Escherichia coli ML35, Staphylococcus aureus 502A, Listeria monocytogenes EGD, and Cryptococcus neoformans 271A were utilized as target organisms in microbistatic (14) or microbicidal (16) assays. Organisms were grown to mid-log phase in Trypticase soy broth (bacteria) or Sabouraud dextrose broth (C. neoformans), diluted to 105 cells per ml in 10 ml of warm 1% agarose containing 3 mg of glucose and 106 cells, and poured into 9-cm2 plastic petri plates. Samples of peptide were loaded into 5-μl wells in the agarose, incubated for 2 h at 37°C and then overlaid with nutrient agar and incubated for 24 to 48 h. The zone of clearing around each well was measured to quantify antimicrobial activity. In microbicidal assays, incubation mixtures contained 2 × 106 organisms per ml in 50 μl of 10 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid), pH 7.4], 5 mM glucose, and peptide at concentrations ranging from 0 to 40 μg/ml. Incubation was carried out at 37°C for 2 h. Serial dilutions of the incubation mixture were plated on nutrient agar and CFU were counted to quantitate microbicidal activity.
Nucleotide sequence accession numbers.
The cDNA sequences for the following RMADs have been submitted to GenBank and assigned the numbers indicated: RMAD-1, AF188268; RMAD-3, AF188269; RMAD-8, AF188270; RMAD-4 and -5, AF188271; and RMAD-6 and -7, AF188272.
RESULTS
Purification of RMADs.
Preliminary experiments revealed that purified samples of previously isolated human and rabbit neutrophil defensins were soluble in neat methanol but that the majority of leukocyte proteins were methanol insoluble (data not shown). Exploiting these solubility characteristics, we subjected rhesus macaque neutrophil pellets to extraction with methanol-acetic acid, and this process was followed by a methanol-water extraction step as described in Materials and Methods. The resulting extract was highly enriched for defensins and contained relatively small amounts of slower-migrating proteins (Fig. 1C), allowing for further RP-HPLC purification of low-molecular-weight proteins without the intermediate gel filtration step used in previous defensin purification protocols (9, 20). Fractions obtained by RP-HPLC purification of the extract (Fig. 1A) were tested for antibacterial activity in the agar diffusion assay described in Materials and Methods. Peptides in peaks labeled 1 to 7, corresponding to RMAD-1 to -7, respectively, were active against E. coli and S. aureus and had apparent molecular masses of ca. 4 kDa; the RMADs were further purified by RP-HPLC under modified elution conditions to obtain pure preparations (Fig. 1B). The relative abundance of the RMADs isolated from several pools of rhesus macaque neutrophils was determined (RMAD-1 ≈ RMAD-2 > RMAD-4 ≈ RMAD-5 > RMAD-6 ≈ RMAD-7 > RMAD-3), and the combined yield of RMADs was ca. 900 μg per 109 cells. There was batch-to-batch variability in the quantities of individual RMADs obtained, and some samples lacked one or more of RMAD-3 to -7. This variability may reflect differing levels of expression in individual animals and/or allelic heterogeneity.
Sequence analysis of RMAD-1 to -8.
The amino acid sequences of RMAD-1 to -7 were determined by automated Edman sequencing (Fig. 2). The close agreement between the calculated masses and those determined by MALDI-TOF MS analysis confirmed that the sequences obtained were complete. The sequence of the peptide from peak 2′ (Fig. 1A) was the same as that of RMAD-2, but the mass of the former was 17.9 atomic mass units more than that of the latter, suggesting that the 2′ peptide is the Met-21 sulfoxide derivative of RMAD-2. cDNAs corresponding to the coding regions of six of the seven isolated peptides and that of an additional peptide not isolated, RMAD-8, were isolated and sequenced; all of the DNA sequences were in agreement with the sequences obtained by Edman degradation.
FIG. 2.
Amino acid sequences of RMAD-1 to -8. Complete amino acid sequences of RMAD-1 to -7 were determined by Edman sequencing and confirmed by MALDI-TOF MS. The sequence of RMAD-8 was deduced from its cDNA. The two peptide subfamilies are aligned manually with HNP-1 or HD-5. Amino acids that vary among members of the subfamilies are shaded. The 11 highly conserved residues found in nearly all myeloid α-defensins are denoted by asterisks. The net charge listed for each peptide was calculated at pH 7. MW, molecular weight; Exptal, experimental.
As shown in Fig. 2, the eight RMADs comprise two α-defensin subfamilies. Those included in the first subgroup, RMAD-1 to -3 and -8, differ from each other only by substitutions at positions 22 and/or 23 and differ in sequence from HNP-1 at four or five residue positions. The RMAD-4 to -7 subgroup showed a high degree of identity among its members, the only differences being an additional amino-terminal arginine in RMAD-4 and -6 and serine-for-phenylalanine substitutions 6 residues from the carboxyl terminus of each peptide. However, the subgroups containing RMAD-1 to -3 and -8 and RMAD-4 to -7 were remarkably dissimilar, having ≤40% identity between them. The two subgroups also differed in net charge, as the overall cationicity of RMAD-4 to -7 (+7 or +8) was significantly greater than that of RMAD-1 to -3 and -8 (+3 to +5). A BLAST P similarity search (2) revealed that RMAD-4 to -7 are most similar to human enteric α-defensin 5 (HD-5) (60.6 to 62.5% identity), a peptide expressed in human intestinal Paneth cells (12) and female reproductive epithelium (18) but not in human leukocytes.
RMAD precursors.
cDNAs containing the complete coding regions for all RMADs except RMAD-2 were isolated and sequenced (Fig. 3). The initiation methionine in each cDNA is preceded by the same Kozak box sequence, CCAGCC. The RMAD-1, -3, and -8 cDNAs are >99% identical, and the only nucleotide substitutions in the coding region result in amino acid substitutions at residues 22 and 23 of the mature peptides, as noted above. Like the HNP-1 precursor (94 amino acids [aa]), the RMAD-1, -3, and -8 precursors (96 aa) are prepropeptides and are 85% identical to HNP-1 at the amino acid level (Fig. 4).
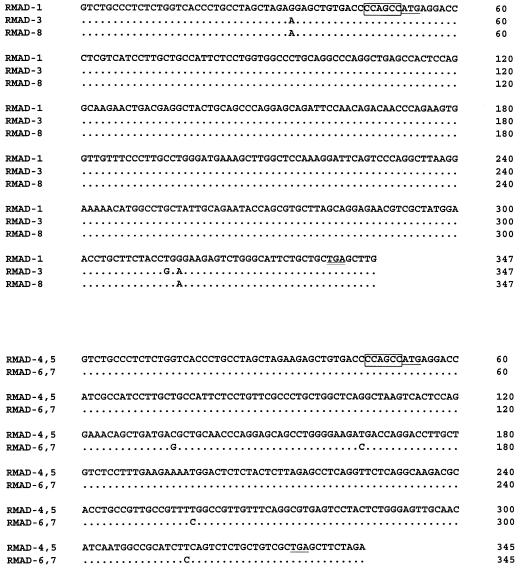
FIG. 3.
cDNA sequences of RMADs. cDNA sequences corresponding to the coding regions of the two RMAD subfamilies are separately aligned. Kozak boxes are outlined, and the initiation methionines are singly underlined. The TGA stop codons are doubly underlined. Dots denote nucleotide identities.
FIG. 4.
RMAD precursors. Deduced amino acid sequences of members of the two RMAD subfamilies are aligned with those of HNP-1 and HD-5. Dots denote amino acid identities. Sequence gaps are shown as dashes. The signal, prosegment, and mature peptide sequences are indicated. Underlined arginines in the RMAD-4 and -5 and RMAD-6 and -7 precursors identify the single residue that distinguishes the mature peptides in each case (Fig. 2).
Analysis of cDNAs encoding the 94-aa precursors of RMAD-4 to -7 revealed that RMAD-4 and -5 and RMAD-6 and -7 derive from alternative processing of their respective prepropeptides (Fig. 4), consistent with the occurrence of an additional amino-terminal arginine in RMAD-4 and -6 (Fig. 2). The RMAD-4 and -5 and the RMAD-6 and -7 cDNAs are 98.8% identical, and the corresponding prepropeptide precursors are 97.9% identical at the amino acid level. When compared to HD-5, RMAD-4 and -5 and RMAD-6 and -7 precursors were 72 to 73% identical to the human enteric defensin at the protein level and 83% identical at the nucleotide level (Fig. 3 and 4).
Immunohistochemical experiments.
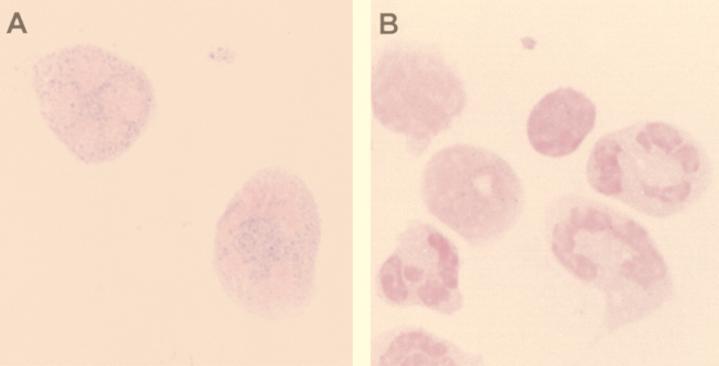
Dot blot analyses demonstrated that anti-HNP-1 to -3 antibody recognized RMAD-1 to -3 but not RMAD-4 to -7. To identify the cells that were RMAD-1 to -3 positive, rhesus buffy coat cytospin preparations were immunostained with anti-HNP-1 antibody. Punctate cytoplasmic immunoreactivity was observed in neutrophils (Fig. 5) but in no other leukocytes, an immunostaining pattern identical to that demonstrated for HNP-1 to -3 (9), peptides that are present in a subpopulation of azurophil granules of human neutrophils.
FIG. 5.
Immunohistochemical staining of RMADs in neutrophils. Buffy coat cytospin preparations were stained with anti-HNP-1 to -3 antiserum (A) or preimmune serum (B) as described in Materials and Methods. The granular pattern of cytoplasmic staining of the two neutrophils (A) is identical to that observed in the staining of HNP-1 to -3 in human polymorphonuclear leukocytes.
Antimicrobial activities.
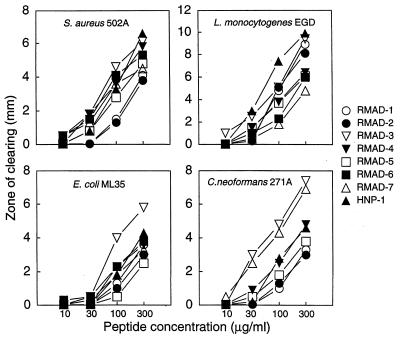
The antimicrobial activities of the purified RMADs were evaluated with E. coli ML35, S. aureus 502A, L. monocytogenes EGD, and C. neoformans 271A as target organisms. An agar diffusion assay was first used to assess the microbistatic or microbicidal activity of each peptide. As shown in Fig. 6, all peptides produced dose-dependent zones of clearing, but the relative potencies of the peptides varied rather markedly depending on the target organism. RMAD-1 and -2 were the least active peptides against S. aureus but were among the most active against L. monocytogenes. Similarly, RMAD-7 was highly effective against C. neoformans but was the least active peptide against L. monocytogenes. RMAD-3 was the α-defensin with the greatest overall potency. Based on inhibition zone size, E. coli ML35 was the least susceptible of the organisms tested while L. monocytogenes was the most sensitive.
FIG. 6.
Antimicrobial activities of RMADs. RMAD-1 to -7 were tested for their activities against the indicated organisms in an agar diffusion assay. Five-microliter samples of each peptide, dissolved at the indicated concentrations in 0.01% acetic acid, were applied to wells in agarose seeded with 106 CFU of each organism per plate. Zones of clearing were measured after incubation for 24 to 48 h.
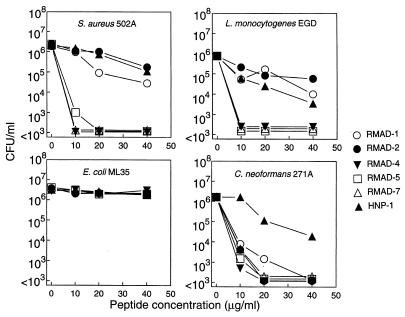
The microbicidal activities of RMAD-1, -2, -4, -5, and -7 were analyzed by incubating the four test organisms with 0 to 40 μg of peptide per ml for 2 h at 37°C (Fig. 7). The HD-5-like peptides, RMAD-4, -5, and -7, were substantially more effective than the HNP-1-like peptides, RMAD-1 and -2, in their killing of S. aureus and L. monocytogenes. At 10 μg/ml, RMAD-4, -5, and -7 killed >3 log units of both gram-positive organisms whereas RMAD-1 and -2 and HNP-1 killed ≤1 log unit of either bacterium. None of the peptides killed E. coli ML35 under these conditions, indicating that the activities against this organism observed in the agar diffusion assays (Fig. 6) were due to bacteriostasis. In contrast, all peptides tested were fungicidal for C. neoformans and the activities were approximately equal. Interestingly, the anticryptococcal activity of HNP-1 was substantially less than that of RMAD-1 and -2, even though it differs from the two rhesus defensins by only four amino acids (Fig. 2).
FIG. 7.
Microbicidal activities of RMADs. Mixtures containing 2 × 106 CFU of each organism per ml were incubated with purified RMADs at the indicated peptide concentrations for 2 h at 37°C in 10 mM PIPES (pH 7.4)–5 mM glucose. CFU were determined after the plates were incubated at 37°C for 24 to 48 h.
DISCUSSION
In this study we isolated and characterized seven rhesus macaque leukocyte defensins and identified one other by sequencing the cDNA corresponding to its coding region. Our data demonstrate that leukocytes of this Old World monkey contain two subfamilies of defensins that are highly similar to either human myeloid (HNP-1 to -3) or enteric (HD-5) defensins at the protein and DNA levels. Antibody that recognized RMAD-1 to -3 immunostained neutrophils in a granular pattern similar to that observed with human cells. Immunolocalization of RMAD-4 to -7 must await production of appropriate antibody reagents, but the fact that these peptides were isolated from highly purified neutrophil preparations indicates that it is likely that they are also granule constituents of polymorphonuclear leukocytes.
In previous studies we noted a general relationship between higher defensin cationicity and greater microbicidal potency (23, 24). Since RMAD-4 to -7 are more cationic (+7 or +8) than any of the human myeloid α-defensins or the HNP-1-like RMADs (Fig. 2), we predicted that the more basic RMADs would be more potent than those defensins with less charge. The predicted correlation held for killing of S. aureus and L. monocytogenes, where the microbicidal potencies of RMAD-4, -5, and -7 were markedly superior to those of RMAD-1 and -2 and HNP-1. However, relative cationicity did not appear to be a factor in the killing of E. coli or C. neoformans, as none of the peptides killed E. coli and all possessed significant and nearly equal fungicidal activities (Fig. 7). These results are consistent with those of previous studies demonstrating that the microbicidal activities of α-defensins are not merely a function of their net charge (7, 24). The data also suggest that certain molecular features may have evolved to enable different peptides, some of which differ by a single amino acid, to interact selectively with one or more microbial targets, a feature of defensin structure-activity relationships noted previously (1, 9, 15, 19). An understanding of how these subtle structural alterations confer antimicrobial selectivity might provide valuable insights into the molecular mode of action of α-defensins.
The number of myeloid α-defensin genes expressed in rhesus macaques is not known. The sequences of the RMAD-4 and -5 and RMAD-6 and -7 cDNAs indicate that the corresponding peptide isoforms derive from differential processing at the amino termini of the mature peptides (Fig. 2 and 4). A similar relationship exists between HNP-2, which lacks an amino-terminal residue present in HNP-1 or HNP-3 and is produced by amino-terminal processing (8). Aside from sequence relationships resulting from differential processing, the high levels of cDNA and amino acid sequence similarity among peptides within each RMAD subgroup may represent allelic diversity. The fact that certain peptides were lacking in samples prepared from different animals suggests that certain alleles (e.g., those in RMAD-4 to -7) may be less frequent in the monkey colony that was studied. However, resolution of these issues must await additional population studies as well as genomic cloning and mapping of the rhesus macaque α-defensin locus.
Based on their genetic analyses of human myeloid and enteric defensin genes, Bevins and coworkers have postulated that human myeloid defensin genes are the hybrid products of unequal crossover events between ancestral genes encoding HD-5 and HD-6, the modern enteric defensins (4). In that scheme, the ancestral myeloid defensin had to acquire its hematopoietic promoter for expression in myeloid cells. Our results indicate that, in addition to HNP-1-like peptides (RMAD-1 to -3 and -8), HD-5 orthologs (RMAD-4 to -7) that are expressed in subhuman primates possess the required myeloid promoters. This result suggests that the evolution of myeloid and enteric defensins in humans differed from that in Old World monkeys or that a different process led to the differentiation of human hematopoietic and epithelial α-defensins. Further characterization of rhesus macaque α-defensin genes may provide insights on this evolutionary question.
ACKNOWLEDGMENTS
This study was supported in part by NIH grant AI22931 and Biosource Technologies, Inc. (M.E.S.), and NIH grant RR00169 (C.J.M.).
We thank Chi Kim, Timothy Cole, Patti Tran, Ding Lu, and Yichuan Wang for expert technical assistance and Agnes Henschen, Director of the UCI Microchemical Core Facility.
REFERENCES
- 1.Aley S B, Zimmerman M, Hetsko M, Selsted M E, Gillin F D. Killing of Giardia lamblia by cryptdins and cationic neutrophil peptides. Infect Immun. 1994;62:5397–5403. doi: 10.1128/iai.62.12.5397-5403.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bateman A, MacLeod R J, Lembessis P, Hu J, Esch F, Solomon S. The isolation and characterization of a novel corticostatin/defensin-like peptide from the kidney. J Biol Chem. 1996;271:10654–10659. doi: 10.1074/jbc.271.18.10654. [DOI] [PubMed] [Google Scholar]
- 4.Bevins C L, Jones D E, Dutra A, Schaffzin J, Muenke M. Human enteric defensin genes: chromosomal map position and a model for possible evolutionary relationships. Genomics. 1996;31:95–106. doi: 10.1006/geno.1996.0014. [DOI] [PubMed] [Google Scholar]
- 5.Boman H G. Gene encoded peptide antibiotics and the concept of innate immunity: an update review. Scand J Immunol. 1998;48:15–25. doi: 10.1046/j.1365-3083.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 6.Cohen S A, Michaud D P. Synthesis of a fluorescent derivatizing reagent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal Biochem. 1993;211:279–287. doi: 10.1006/abio.1993.1270. [DOI] [PubMed] [Google Scholar]
- 7.Cullor J S, Wood S, Smith W, Panico L, Selsted M E. Bactericidal potency and mechanistic specificity of neutrophil defensins against bovine mastitis pathogens. Vet Microbiol. 1991;29:49–58. doi: 10.1016/0378-1135(91)90109-s. [DOI] [PubMed] [Google Scholar]
- 8.Daher K A, Lehrer R I, Ganz T, Kronenberg M. Isolation and characterization of human defensin cDNA clones. Proc Natl Acad Sci USA. 1988;85:7327–7331. doi: 10.1073/pnas.85.19.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganz T, Selsted M E, Szklarek D, Harwig S S L, Daher K, Lehrer R. Defensins: natural peptide antibiotics of human neutrophils. J Clin Investig. 1985;76:1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganz T, Lehrer R I. Antimicrobial peptides of vertebrates. Curr Opin Immunol. 1998;10:41–44. doi: 10.1016/s0952-7915(98)80029-0. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann J A, Kafatos F C, Janeway C A, Jr, Ezekowitz R A B. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 12.Jones D E, Bevins C L. Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem. 1992;267:23216–23225. [PubMed] [Google Scholar]
- 13.Kagan B L, Ganz T, Lehrer R I. Defensins: a family of antimicrobial and cytotoxic peptides. Toxicology. 1994;87:113–149. doi: 10.1016/0300-483x(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 14.Lehrer R I, Rosenman M, Harwig S S L, Jackson R, Eisenhauer P. Ultrasensitive assays for endogenous antimicrobial polypeptides. J Immunol Methods. 1991;137:167–173. doi: 10.1016/0022-1759(91)90021-7. [DOI] [PubMed] [Google Scholar]
- 15.Ouellette A J, Hsieh M M, Nosek M T, Cano-Gauci D F, Huttner K M, Buick R N, Selsted M E. Mouse Paneth cell defensins: primary structures and antibacterial activities of numerous cryptdin isoforms. Infect Immun. 1994;62:5040–5047. doi: 10.1128/iai.62.11.5040-5047.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouellette A J, Hsieh M M, Selsted M E. Structure and function of cryptdins isolated from mouse small intestinal lumen. Gastroenterology. 1996;110(Suppl.):A985. . (Abstract.) [Google Scholar]
- 17.Ouellette A J, Selsted M E. Paneth cell defensins: endogenous peptide components of intestinal host defense. FASEB J. 1996;10:1280–1289. doi: 10.1096/fasebj.10.11.8836041. [DOI] [PubMed] [Google Scholar]
- 18.Quayle A J, Porter E M, Nussbaum A A, Wang Y M, Brabec C, Yip K-P, Mok S C. Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract. Am J Pathol. 1998;152:1247–1258. [PMC free article] [PubMed] [Google Scholar]
- 19.Sawyer J G, Martin N L, Hancock R E W. Interaction of macrophage cationic proteins with the outer membrane of Pseudomonas aeruginosa. Infect Immun. 1988;56:693–698. doi: 10.1128/iai.56.3.693-698.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selsted M E, Harwig S S L. Purification, primary structure, and antimicrobial activities of a guinea pig neutrophil defensin. Infect Immun. 1987;55:2281–2286. doi: 10.1128/iai.55.9.2281-2286.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selsted M E, Harwig S S L. Determination of the disulfide array in the human defensin HNP-2. A covalently cyclized peptide. J Biol Chem. 1989;264:4003–4007. [PubMed] [Google Scholar]
- 22.Selsted M E, Ouellette A J. Defensins in granules of phagocytic and non-phagocytic cells. Trends Cell Biol. 1995;5:114–119. doi: 10.1016/s0962-8924(00)88961-8. [DOI] [PubMed] [Google Scholar]
- 23.Selsted M E, Szklarek D, Lehrer R I. Purification and antibacterial activity of antimicrobial peptides of rabbit granulocytes. Infect Immun. 1984;45:150–154. doi: 10.1128/iai.45.1.150-154.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selsted M E, Szklarek D, Ganz T, Lehrer R I. Activity of rabbit leukocyte peptides against Candida albicans. Infect Immun. 1985;49:202–206. doi: 10.1128/iai.49.1.202-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selsted M E, Tang Y-Q, Morris W L, McGuire P A, Novotny M J, Smith W. Purification, primary structures, and antibacterial activities of beta-defensins, a new family of antimicrobial peptides from bovine neutrophils. J Biol Chem. 1993;268:6641–6648. [PubMed] [Google Scholar]
- 26.Tang Y-Q, Selsted M E. Characterization of the disulfide motif in BNBD-12, an antimicrobial β-defensin peptide from bovine neutrophils. J Biol Chem. 1993;268:6649–6653. [PubMed] [Google Scholar]
- 27.Weinberg A, Krisanaprakornkit S, Dale B A. Epithelial antimicrobial peptides: review and significance for oral applications. Crit Rev Oral Biol Med. 1998;9:399–414. doi: 10.1177/10454411980090040201. [DOI] [PubMed] [Google Scholar]
- 28.Wu E R, Daniel R, Bateman A. RK-2: a novel rabbit kidney defensin and its implications for renal host defense. Peptides. 1998;19:793–799. doi: 10.1016/s0196-9781(98)00016-3. [DOI] [PubMed] [Google Scholar]
- 29.Yount N Y, Wang M-S C, Yuan J, Banaiee N, Ouellette A J, Selsted M E. Rat neutrophil defensins. Precursor structures and expression during neutrophilic myelopoiesis. J Immunol. 1995;155:4476–4484. [PubMed] [Google Scholar]
- 30.Zimmermann G R, Legault P, Selsted M E, Pardi A. Solution structure of bovine neutrophil β-defensin-12: the peptide fold of the β-defensins is identical to that of the classical defensins. Biochemistry. 1995;34:13663–13671. doi: 10.1021/bi00041a048. [DOI] [PubMed] [Google Scholar]