Abstract
We investigated the effects of astaxanthin supplementation on the egg quality, antioxidant capacity, and ovarian aging of aged laying hens. Six groups of 68-wk-old Hy-line brown laying hens with six replications each, fifteen chickens in each replicate were fed for 12 wk. The control group was fed a basal diet, the positive control group was fed the basal diet supplemented with 100 mg/kg vitamin E, and the experimental groups were fed the basal diet supplemented with 15 mg/kg, 30 mg/kg, 45 mg/kg, or 60 mg/kg astaxanthin (Ax15, Ax30, Ax45, and Ax60, respectively). The results showed that astaxanthin accumulated in the egg yolks and improved egg yolk color (P < 0.01) and Haugh unit (P < 0.05). Compared with the control group, the experimental groups a higher number of follicles in the ovary and a lower rate of atresia (P < 0.01). Astaxanthin increased the expression of nuclear factor e2-related factor 2 (NRF2) in the ovary (P < 0.05), enhanced the antioxidant capacity of aged laying hens (P < 0.05), and reduced cellular apoptosis (P < 0.05). In addition, astaxanthin improved serum reproductive hormone levels (follicle-stimulating hormone, luteinizing hormone, and progesterone) (P < 0.05) with a maximum value observed in Ax60. However, astaxanthin had no effects on estrogen level (P > 0.05). The expression of FSHR and CYP11A1 increased in the follicular granulosa cells (P < 0.05). Therefore, astaxanthin prevented ovarian aging by improving the antioxidant capacity of laying hens and promoting the production of reproductive hormones. The declining reproductive performance of laying hens in the late laying period may be improved with astaxanthin supplementation.
Key words: astaxanthin, aged laying hen, antioxidant, reproductive hormone
INTRODUCTION
The female reproductive capacity of mammals is negatively correlated with age (Stoop et al., 2014). Like mammals, laying hens experience a rapid decline in fecundity with age. Egg production of laying hens declines rapidly after 480 d (Liu et al., 2018a). A reduction in egg quality and an increase in the proportion of soft-break eggs pose problems in the production of aged laying hens. These phenomena are linked to ovary aging and related dysfunctions, especially the reduction of follicle number and follicular atresia (Hao et al., 2020). Therefore, alleviating ovarian aging may improve the production of aged laying hens.
An important feature of aged laying hen ovary is the decline in the number and quality of oocytes in the follicles (Ben-Meir et al., 2015). In addition, increased oxidative stress, reproductive hormone imbalance, and paracrine environmental changes are also some important factors involved in ovarian aging (Gosden and Faddy, 1994; Lim et al., 2015; Colella et al., 2021). Egg-laying performance in chickens is regulated by variety of reproductive hormones (Du et al., 2020). Follicle-stimulating hormone (FSH) promotes follicle growth, development and maturation, and granulosa cell proliferation by binding to FSHR on the gonadal target cells. The elevated expression of FSHR mRNA in follicle tissue is an important indicator of granulosa cell differentiation, selection of dominant follicles, and entry into grade development (Johnson P.A. et al., 2015). Progesterone (PROG) and estrogen (E2) indirectly promote the proliferation and differentiation of granulosa cells by regulating the production of steroid hormones. Additionally, these hormones participate in the recruitment of dominant follicles ( Fiedler et al., 2008; Zhao et al., 2017). Previous studies have shown that hormonal levels change during the later stages of poultry production, with plasma FSH and LH levels significantly lower in 60-wk-old broiler breeders than in 30-wk-old broiler breeders (Ciccone et al., 2005). Oxidative stress is an another important factor leading to ovarian aging. In laying hens, increased ovarian oxidative stress during aging can lead to decreased fertility (Surai et al., 2019).
Ovarian aging is a high risk factor for decreased ovarian function. Therefore, exploring anti-aging strategies is crucial for delaying ovarian aging. Several natural extracts such as lycopene and resveratrol have been used to reduce oxidative stress in ovarian tissue to maintain normal ovarian function (Liu et al., 2013; Liu Xingting et al., 2018b). Astaxanthin is a natural carotenoid commonly extracted from Haematococcus pluvialis (Kishimoto et al., 2016). Astaxanthin imparts a rich pink color to aquatic species, including salmon and crustaceans (Sztretye et al., 2019). Astaxanthin is widely used in industries such as food, feed, and aquaculture (Kumar et al., 2021). It has strong antioxidant activity and has clinical applications for the prevention and treatment of cancer, chronic inflammation, atherosclerosis, aging, eye-related disorders, and cardiovascular diseases (Farruggia et al., 2018; Eren et al., 2019; Sztretye et al., 2019). In poultry, astaxanthin improves male sperm quality by modulating antioxidant enzyme activity (Gao et al., 2021). In vitro studies have revealed that astaxanthin improves bovine oocyte maturation and promotes progesterone production in luteal cells (Li et al., 2015; Kamada et al., 2017). Aging leads to increased oxidative stress, and the antioxidant function of astaxanthin has potential to improve aged reproduction.
Previous studies on laying hens and astaxanthin have mainly focused on egg yolk astaxanthin deposition, nutrient enrichment (Walker et al., 2012; Shevchenko et al., 2021), and oxidative stress reduction. However, few studies have evaluated the effects of astaxanthin on ovaries in aged laying hens. The purpose of this study was to investigate the effects of supplemental Haematococcus pluvialis astaxanthin on the ovaries of aged laying hens. We expected astaxanthin to alleviate ovarian aging in aged laying hens, thereby increasing the yield of aged laying hens.
MATERIALS AND METHODS
Animals and Diets
The Animal Care and Use Committee of the Beijing Institute of Animal Husbandry and Veterinary Medicine, Chinese Academy of Agricultural Sciences approved our study.
We selected and randomly divided five hundred forty 68-wk-old Hy-line brown laying hens of similar body weight and healthy constitution into 6 groups with 6 replicates per group and 15 laying hens in each replicate. The control group (Control) was fed a basal diet, and the positive control group was fed the basal diet supplemented with 100 mg/kg vitamin E (E100). The experimental groups were fed the basal diet supplemented with 15, 30, 45, or 60 mg/kg astaxanthin (Ax15, Ax30, Ax45, and Ax60, respectively). Haematococcus pluvialis astaxanthin (Kunming Gull Microalgae Technology Ltd., Kunming, China) had an astaxanthin content of 2.0%. Table 1 shows the basal diet composition and nutritional level. The prefeeding period lasted 1 wk, and the complete trial lasted 12 wk.
Table 1.
Composition and nutrient levels of the basal diet (air-dry basis).
| Items | Content (%) | Items | Content |
|---|---|---|---|
| Corn | 64.60 | Nutrient level2 | |
| Soybean meal | 24.35 | Crude Protein (%) | 15.70 |
| Limestone | 9.44 | Metabolisable energy (MJ/kg) | 11.19 |
| CaHPO4 | 0.90 | Calcium (%) | 3.52 |
| Premix1 | 0.65 | Available phosphorus (%) | 0.32 |
| DL-Met | 0.06 | Lysine (%) | 0.81 |
| Total | 100.00 | Methionine (%) | 0.32 |
| Methionine + Cystine (%) | 0.61 |
Premix provided per kg of diet: vitamin A (vitamin A acetate), 10,000 IU; vitamin D3, 3,200 IU; vitamin E (DL-α-Tocopherol acetate), 8 IU; vitamin K, 2.2 mg; vitamin B1, 1 mg; vitamin B2, 4 mg; vitamin B6, 2.2 mg; vitamin B12, 0.01 mg; phytases, 300 mg; NaCl, 3,000 mg; choline chloride (50%), 1,000 mg; D-pantothenic acid, 6.6 mg; niacin, 20 mg; biotin, 0.12 mg; folic acid, 0.6 mg; Cu (copper sulfate), 12 mg; I (calcium iodate), 0.5 mg; Fe (ferrous sulfate), 95 mg; Mn (manganese sulfate), 100 mg; Zn (zinc sulfate), 50 mg.
Metabolizable energy, phosphorus, and nutrient levels are calculated values.
The hens had a light/dark cycle of 16 h/8 h with a light intensity of 15 lx. The house was maintained at 18 ± 2°C with a relative humidity of 45% to 60%. The hens were raised in three layer cages, with three animals per cage having free access to water. We fed the hens 3 times a day and collected the eggs once a day. The house was sterilized once every 3 d, and the hens were routinely immunized during the trial.
Production Performance of Laying Hens
During the trial, we monitored total egg weight, number of laying hens, broken eggs, and abnormal eggs every day. Feed consumption was calculated weekly. Egg production (EP), average egg weight (EW), daily egg mass (DEM), average daily feed intake (ADFI), and feed conversion ratio (FCR) were determined.
Tissue Collection
At the end of the 12-wk trial, 2 hens were selected from each repetition. Following the collection of 5 mL of blood from the wing vein, we centrifuged the sample at 4,000 rpm for 10 min at 4°C and collected the supernatant to determine the contents of 4 reproductive hormones (FSH, LH, PROG, and E2). After slaughter, some ovarian tissues were harvested and fixed in formaldehyde solution (4%). The remaining tissue was frozen in nitrogen and stored at −80°C. We used the method by Gilbert et al. (1977) to separate the follicular granulosa cells of laying hens. The small yellow follicles (SYF, diameter: 6–8 mm) were separated from the ovaries. Briefly, we lifted one end of the follicle, gently pierced the membrane layer with an ophthalmology camera, and carefully peeled off the membrane layer completely. The yolk automatically flows out and is separated from the granulosa cell layer. The separated granular cell layer was stored in liquid nitrogen.
Egg Physical Quality Analysis and Astaxanthin Concentration
At the end of trial, we removed 3 eggs from each replicate for egg quality determination. Albumen height, Haugh unit, and yolk color (Roche colorimetric unit) were determined using an automatic egg quality analyzer (ORKA Food Technology Ltd., Ramat HaSharon, Israel). We measured eggshell strength using an eggshell strength tester (ORKA Food Technology Ltd., Ramat HaSharon, Israel) and CIELAB values (L*, a*, b*) of yolk color using a CR-400 (Konica Minolta Inc., Chiyoda, Japan) chromatograph. An egg separator was used to separate the yolk from the egg white. We weighed the egg yolk and calculated the percentage of egg yolk. We used an HPLC (liquid chromatography LC-20A Shimadzu Corp., Kyoto Japan) to measure the concentration of astaxanthin (Dansou et al., 2021a).
Antioxidant Index
We homogenized ovarian tissue in phosphate-buffered saline (PBS) and centrifuged the mixture at 3,500 rpm for 20 min at 4°C to obtain a 10% tissue homogenate. We measured total protein concentration and oxidative parameters in the supernatant. Total protein concentration was measured using a bicinchoninic acid (BCA) kit (Thermo Fisher Scientific Ltd., Waltham, MA). Total superoxide dismutase (T-SOD) activity, malondialdehyde (MDA) content, and catalase (CAT) activity were measured using commercial kits (Nanjing Jiancheng Bioengineering Institute Ltd., Nanjing, China) according to the manufacturer's instructions.
Reproductive Hormone Assay
We measured hormone concentrations (FSH, LH, PROG, and E2) using commercial kits (Shanghai Enzyme Link Biotechnology Ltd., Shanghai, China) according to the manufacturer's instructions.
Number of Ovarian Follicles
We examined tissue morphology under a light microscope (100 ×) and analyzed primary and secondary follicles according to the number of granulosa cell layers (single layer was primary follicle cells, and multiple layers were secondary follicular cells, and only secondary follicle cells with a nucleus diameter of less than 1 mm were recorded). According to Abercrombie (1946) formula: P = A × M/(M+L), P is the average number of nuclear points per section, A is the crude count of number of nuclei seen in the section, M is the thickness of the section, and L is the average length of the nuclei. The total number of primary follicles, the total number of secondary follicles and the number of atretic secondary follicles on each ovary were obtained, and the secondary follicle atresia rate was calculated. Depending on the cell diameter, the tissue morphology was divided into small white follicles (SWF, diameter: ≤4 mm), large white follicles (LWF, diameter: 4–6 mm) and SYF (diameter: 6–8 mm).
The qRT-PCR
We extracted total RNA from ovarian tissue and SYF granulosa cells using commercial kits (TaKaRa, Dalian, China) according to the manufacturer's protocol. We synthesized (Beijing New Times Zhonghe Technology Ltd., Beijing, China) and reverse-transcribed cDNA (RR047A kit, TaKaRa, Shiga Prefecture, Japan). Real-time fluorescence quantification was performed using an RR820A kit (TaKaRa, Shiga, Japan), and qRT-PCR was performed using a Y062 real-time PCR detection system (Thermo Fishier Scientific Ltd.). Expression levels were determined using the 2−∆∆CT method normalized to β-actin. Table 2 shows the sequences of primers.
Table 2.
Primer sequences used in qRT-PCR.
| Gene | Primer sequence | Fragment size (bp) | Accession number |
|---|---|---|---|
| NRF2 | F:CAAGGTATGAGGAAGAAGGTGCT R:GCAAGGCAGATCTCTTCCAAC |
124 | NM_205117.1 |
| BCL-2 | F:ACAAAGGCATCCCATCCTCC R:ACAAAGGCATCCCATCCTCC |
75 | NM_205339.2 |
| Caspase3 | F:TGGTGGAGGTGGAGGAGC R:TCCAGAGTCCACAGACTTGC |
284 | NM_204725.1 |
| BAX | F:CTACTTGGTGCTGGTCTG R:AAGATGGTGACGATGATGG |
183 | XM_015290060.3 |
| FSHR | F:ATGTCTCCGGCAAAGCAAGA R:CAAATTCGTTCTCCACAGGGC |
121 | NM_205079.1 |
| LHR | F:GCAACGAATCGCTGACACTC R:CTCTCAGGGCATCGTTGTGT |
141 | NM_204936.1 |
| CYP11A1 | F:AGCACTTCAAGGGACTGAGC R:ACTTGGTCCCAACTTCCACC |
147 | NM_001001756.1 |
| HSD3B1 | F:GTTTGTTTAGCACTGAGGCAA R:GTTTGCTCCTCTTTGGCAGG |
78 | NM_205118.1 |
| CYP17A1 | F:GTGCACCACGATGAGAAGGA R:GGACACTCGAGCGTGAATCT |
209 | NM_001001901.2 |
| HSD17B1 | F:GTGTACTGCGCCAGCAAGTT R:GCGTCATGTGGATGTTGAAGG |
85 | NM_204837.1 |
| CYP19A1 | F:GCAGATCTGAACAGAACTTTGAGC R:GCTGGTGAAGTAGTTCAGTGGA |
149 | NM_001001761.3 |
| β-actin | F:CATTGTCCACCGCAAATGCT R:AGCCATGCCAATCTCGTCTT |
108 | NM_205518.1 |
TUNEL Analysis of Apoptosis
To assess apoptosis, we used the TUNEL bright green apoptosis detection kit (Vazyme Biotech Ltd., Nanjing, China) according to the manufacturer's protocol. Tissue sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) for 5 min. Three available fields were randomly selected for each group, and the number of TUNEL-positive cells (green) were counted. Apoptotic rate was calculated as the percentage of green-labeled cells relative to the total number of ovarian cells.
Western Blot
We homogenized 100 mg of ovarian tissue in Tissue Protein Extraction Reagent (Huaxingbio Ltd., Beijing, China) followed by centrifugation at 12,000 rpm for 10 min at 4°C. The homogenized supernatant was loaded onto a sodium dodecyl sulfate-polyacrylamide gel (12%), transferred to a polyvinylidene fluoride membrane after electrophoresis, and incubated with appropriate phosphorylated nuclear factor E2-related factor 2 (p-NRF2) and apoptosis antibodies. The densitometric value and relative quantification of the target band were determined using the software ImageJ, and GAPDH protein was used as the internal reference standard.
Statistical Analysis
We used SPSS 23.0 (SPSS 23.0 for Windows, SPSS Inc., Chicago) for statistical analysis and compared differences among groups using one-way ANOVA. When the differences were significant, Duncan's method was used for multiple comparisons. P < 0.05 was statistically significant. Data were expressed as mean and standard error of mean.
RESULTS
Production Performance of Laying Hens
Table 3 shows the production performance of the laying hens. There was no difference in EP, DEM, ADFI, or FCR among the groups (P > 0.05). Compared with the control group, the Ax60 group had a higher EW (P < 0.05).
Table 3.
Effects of supplemental astaxanthin on performance of laying hens (12 weeks).1
| Item | Control2 | E1002 | Ax152 | Ax302 | Ax452 | Ax602 | SEM3 | P value |
|---|---|---|---|---|---|---|---|---|
| EP/(%)4 | 90.81 | 89.25 | 88.87 | 90.43 | 90.69 | 90.01 | 0.45 | 0.796 |
| DEM/(g/hen/d)5 | 57.60 | 56.69 | 56.03 | 57.39 | 57.73 | 58.05 | 0.34 | 0.492 |
| EW/(g)6 | 63.42a | 63.50a | 64.08ab | 63.48a | 63.58a | 64.91b | 0.15 | 0.020 |
| ADFI/(g/hen/d)7 | 120.79 | 120.60 | 122.43 | 121.18 | 120.50 | 121.22 | 0.25 | 0.150 |
| FCR8 | 2.08 | 2.09 | 2.18 | 2.09 | 2.07 | 2.11 | 0.01 | 0.466 |
Different superscript letters indicate significant differences between groups (P < 0.05).
Means were obtained from 6 replicates of 15 birds each.
Control, E100, Ax15, Ax30, Ax45, and Ax60 correspond to groups of laying hens fed a basal diet supplemented with astaxanthin at 0 mg/kg (control), vitamin E at 100 mg/kg, and astaxanthin at 15 mg/kg, 30 mg/kg, 45 mg/kg, and 60 mg/kg, respectively.
SEM: standard error of mean.
EP: egg production.
DEM: daily egg mass.
EW: average egg weight.
ADFI: average daily feed intake.
FCR: feed conversion ratio.
Egg Physical Quality
Table 4 shows the egg physical quality and astaxanthin concentration. Astaxanthin was not detected in the egg yolks of both the control and E100 groups. Astaxanthin supplementation increased the concentration of astaxanthin in egg yolk (P < 0.01). The changes in egg yolk color were similar to the changes in astaxanthin content. Egg yolk color improved in the astaxanthin group (P < 0.01). CIELAB showed that the color of egg yolk was obtained by decreasing the L* and b* values of the yolk and increasing the a* value (P < 0.01). There was no difference in albumen height, egg yolk ratio, or eggshell strength (P > 0.05). Haugh unit improved in the experimental and E100 groups (P < 0.05). Among the groups, Ax15 had the highest Haugh unit. E100 and Ax15 had higher (+6.44% and +10.99%, respectively) Haugh unit than the control group.
Table 4.
Effects of supplemental astaxanthin on egg quality and astaxanthin concentration in egg yolks (week 12).1
| Item | Control | E100 | Ax15 | Ax30 | Ax45 | Ax60 | SEM | P value |
|---|---|---|---|---|---|---|---|---|
| Albumen height/(mm) | 6.14 | 6.63 | 6.53 | 6.66 | 6.51 | 6.44 | 0.11 | 0.786 |
| Haugh unit | 75.73a | 80.61b | 84.05b | 81.80b | 81.24b | 81.06b | 0.70 | 0.020 |
| Eggshell strength/(kg/cm2) | 3.31 | 3.61 | 3.68 | 3.48 | 3.71 | 3.82 | 0.05 | 0.093 |
| Yolk color | 4.97a | 5.06a | 11.42b | 11.94b | 12.89c | 14.08d | 0.62 | <0.001 |
| Yolk percent/% | 26.98 | 28.04 | 26.40 | 27.01 | 26.95 | 27.69 | 0.01 | 0.347 |
| L* | 59.47c | 56.56b | 54.31b | 53.98b | 50.84a | 49.44a | 0.68 | <0.001 |
| a* | −1.77a | −1.35a | 10.71b | 15.77c | 21.55d | 24.28e | 1.84 | <0.001 |
| b* | 44.34c | 41.79bc | 41.12bc | 41.06bc | 38.66b | 35.42a | 0.62 | 0.005 |
| Astaxanthin concentration /(μg/g)2 | ND3 | ND3 | 15.85a | 31.90b | 38.22c | 64.34d | 4.34 | <0.001 |
Different superscript letters indicate significant differences between groups (P < 0.05).
Egg quality determination from six replicates (3 eggs per replicate) per treatment (n = 14). We mixed three eggs per replicate and determined astaxanthin concentration (n = 4).
Astaxanthin concentration = all-trans + 9-cis + 13-cis.
ND: not detected.
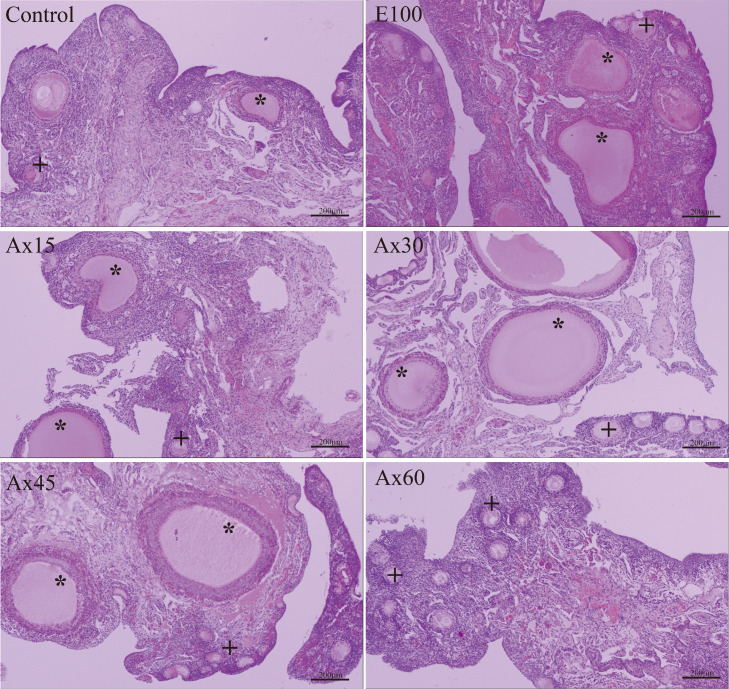
Number of Ovarian Follicles
Figure 1 shows representative images of HE-stained sections of the ovary, and Table 5 shows the number of ovarian follicles in each group. Supplementation with astaxanthin and vitamin E increased the number of primary and secondary follicles (P < 0.01). Ax60 had the highest number of follicles. E100 and Ax60 had a higher number of primary (+31.18% and +33.92%, respectively) and secondary (+36.01% and +38.98%, respectively) follicles than the control group. Supplementation with astaxanthin and vitamin E decreased the atresia rate of secondary follicles (P < 0.01), and Ax60 had the lowest atresia rate. Supplementation with astaxanthin and vitamin E increased the number of SWF, LWY, and SYF (P < 0.01). Ax60 had the highest number of follicles but there was no significant difference as compared to E100.
Figure 1.
Representative images of HE-stained sections of the ovary (week 12). Scale bar = 200 μm.*: primary follicle, +: secondary follicle. Control, E100, Ax15, Ax30, Ax45, and Ax60 correspond to groups of laying hens fed a basal diet supplemented with astaxanthin at 0 mg/kg (control), vitamin E at 100 mg/kg, and astaxanthin at 15 mg/kg, 30 mg/kg, 45 mg/kg, and 60 mg/kg, respectively.
Table 5.
Effects of supplemental astaxanthin on follicle number of laying hens (week 12).1
| Item | Control | E100 | Ax15 | Ax30 | Ax45 | Ax60 | SEM | P value |
|---|---|---|---|---|---|---|---|---|
| Primary follicle number | 16493.00a | 23965.94cd | 18561.2ab | 20942.13bc | 22080.28cd | 24960.68d | 664.22 | <0.001 |
| Secondary follicle number | 1436.75a | 2245.37cd | 1666.00ab | 1922.15bc | 2040.80cd | 2354.57d | 71.88 | <0.001 |
| Secondary follicle shutting rate/% | 8.80c | 5.12a | 7.48bc | 6.47ab | 5.78a | 4.92a | 0.31 | <0.001 |
| Small white follicle number | 58.27a | 69.23bc | 62.85ab | 67.50b | 69.85bc | 75.07c | 1.28 | <0.001 |
| Large white follicle number | 9.58a | 14.99cd | 11.12ab | 12.82bc | 13.60cd | 15.70d | 0.48 | <0.001 |
| Small yellow follicle number | 3.88a | 5.51bc | 4.63ab | 5.20bc | 5.58c | 6.05c | 0.17 | <0.001 |
Different superscript letters indicate significant differences between groups (P < 0.05).
Data are presented as mean and standard error of mean (SEM) (n = 5, one laying hen was randomly selected for each replicate).
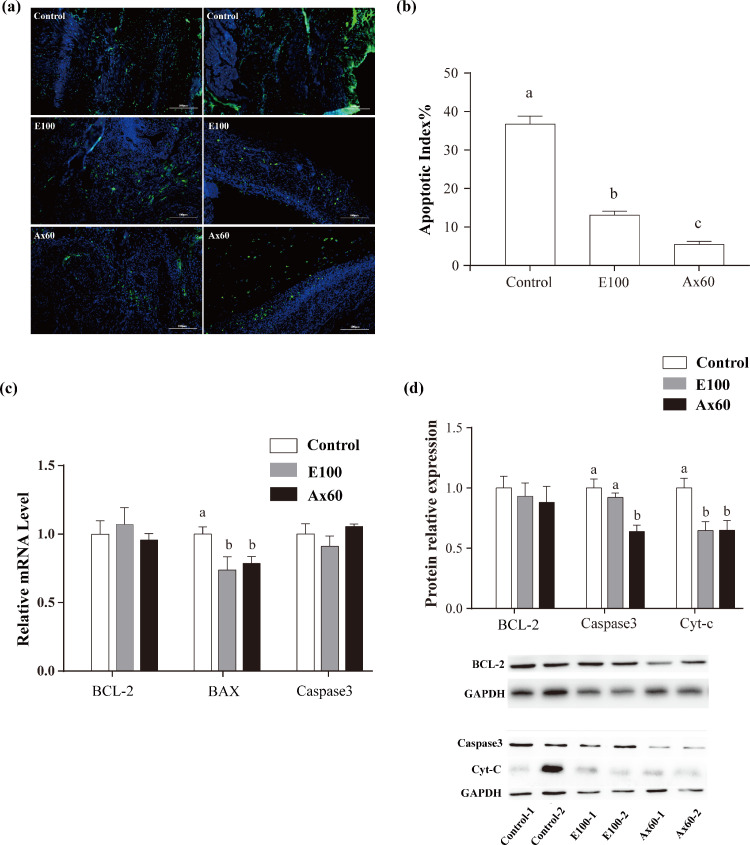
TUNEL Analysis and Apoptosis
The rate of positive cells in the ovarian tissue was lower in E100 and experimental groups (P < 0.05) than in the control group (Figure 2). Ax60 had the lowest proportion of apoptotic cells. Supplementation with vitamin E and astaxanthin reduced the expression of BAX, but did not affect the expression of Caspase3 or BCL-2 (P > 0.05). Furthermore, there were no significant changes in BCL-2 protein levels following supplementation with vitamin E or astaxanthin. The apoptotic proteins Caspase3 and Cyt-C significantly decreased following astaxanthin supplementation.
Figure 2.
TUNEL analysis in the ovary (week 12). We tested the control, E100, and Ax60 groups. Values are expressed as mean ± SEM (gene and protein expression from six replicates of two laying hens per treatment, n = 9, TUNEL data are from ovarian HE slices, n = 4). Scaleplate:100 μm. (a) TUNEL-staining of ovarian tissue, positive expression in green light. (b) TUNEL-staining for apoptosis index. (c) The qRT-PCR expression of BCL-2, BAX, and Caspase3 in ovary. (d) Expression of BCL-2, Caspase3, and Cyt-C proteins in ovary. a−c: different superscript letters within a test indicate significant differences between groups (P < 0.05).
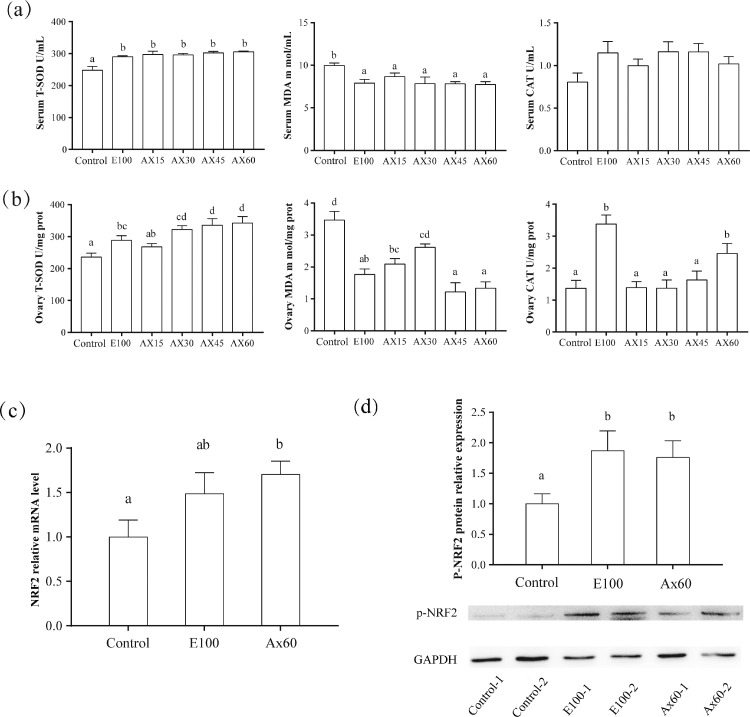
Antioxidant Indices and NRF2 Expression in Ovary
Figure 3 shows the ovarian antioxidant indexes and expression of NRF2 in the ovary. Supplementation with vitamin E and astaxanthin increased T-SOD activity in serum and ovary (P < 0.05) and decreased MDA content (P < 0.05). In the ovary, CAT activity was higher in E100 and Ax60 (P < 0.05), but the difference in serum was not significant (P > 0.05). Compared with the control group, the Ax60 group had a higher relative expression of NRF2 and p-NRF2 in the ovary (P < 0.05). However, there was no difference in the expression of NRF2 and p-NRF2 between the Ax60 and E100 groups (P > 0.05).
Figure 3.
Antioxidant index of laying hens and expression of NRF2 in ovary (week 12). Values are expressed as mean ± SEM (n = 8). Control, E100, Ax15, Ax30, Ax45, and Ax60 correspond to groups of laying hens fed a basal diet supplemented with astaxanthin at 0 mg/kg (control), vitamin E at 100 mg/kg, and astaxanthin at 15 mg/kg, 30 mg/kg, 45 mg/kg, and 60 mg/kg, respectively. (a) Serum antioxidant index: T-SOD, MDA, and CAT. (b) Antioxidant index in ovary: T-SOD, MDA, and CAT. (c) mRNA relative expression level of NRF2 in ovary. (d) Protein relative expression of p-NRF2 in ovary. a−d: different superscript letters within a test indicate significant differences between groups (P < 0.05). T-SOD, total superoxide dismutase; MDA, malondialdehyde; CAT, catalase.
Reproductive Hormones of Laying Hens
Supplementation with vitamin E and astaxanthin increased the levels of FSH, LH, and PROG in serum (P < 0.05; Table 6). The Ax60 group had the highest levels of reproductive hormones. However, supplementation with vitamin E and astaxanthin had no effects on E2 (P > 0.05).
Table 6.
Reproductive hormone levels in serum (week 12).1
| Item | Control | E100 | Ax15 | Ax30 | Ax45 | Ax60 | SEM | P value |
|---|---|---|---|---|---|---|---|---|
| FSH (mIU/mL)2 | 13.76a | 14.27ab | 14.43ab | 14.15a | 14.87ab | 15.35b | 0.15 | 0.032 |
| LH (mIU/mL)3 | 14.79a | 15.81bc | 15.36ab | 15.86bc | 16.67cd | 17.20d | 0.16 | <0.001 |
| E2 (pg/mL)4 | 290.04 | 288.62 | 294.16 | 299.11 | 298.75 | 313.60 | 2.90 | 0.130 |
| PROG (pmol/L)5 | 1276.74a | 1248.74a | 1326.75ab | 1378.90b | 1300.71ab | 1381.25b | 13.31 | 0.031 |
Different superscript letters indicate significant differences between groups (P < 0.05).
Data are presented as mean and standard error of mean (SEM) (n = 10).
FSH: follicle-stimulating hormone.
LH: luteinizing hormone.
E2: estrogen.
PROG: progesterone.
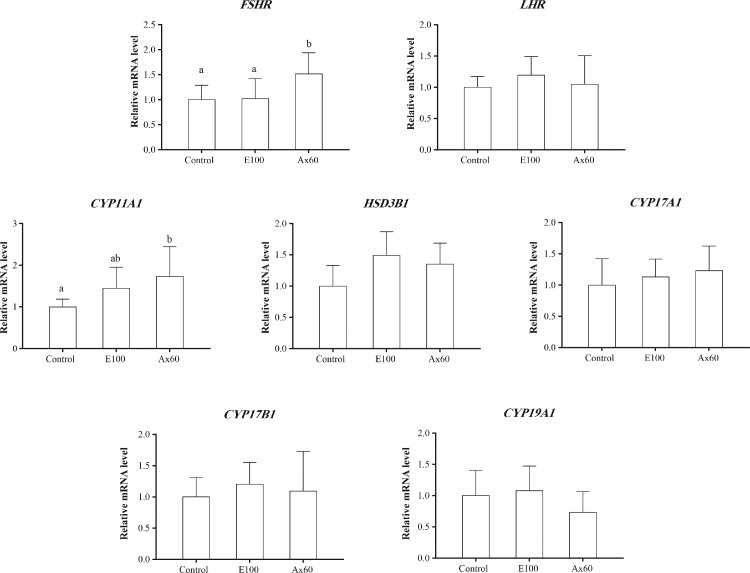
Expression of Steroid Synthesis Genes
The expression of steroid synthesis genes is shown in Figure 4. The results of qRT-PCR analysis showed that supplementation with astaxanthin increased the expression of granulosa cell hormone receptor FSHR in SYF (P < 0.05). However, the expression of the hormone receptor LHR did not change (P > 0.05). Supplementation with astaxanthin increased mRNA expression in the initial stage of steroid synthesis (SYF). Compared with the control group, the experimental groups had a higher (+73%) expression of CYP11A1 (P < 0.05). Supplementation with astaxanthin had no effect on mRNA expression in subsequent stages of steroid synthesis. Therefore, there was no difference in the expression of HSD3B1, CYP17A1, HSD17B1, or CYP19A1 (P > 0.05).
Figure 4.
Expression of steroid synthesis genes in the ovary (wk 12). We tested the control, E100, and Ax60 groups. Values are expressed as mean ± SEM (n = 9). a−b: different superscript letters within a test indicate significant differences between groups (P < 0.05).
DISCUSSION
Poultry meat and eggs are rich sources of animal protein. The production of most laying hens declines rapidly after the peak of egg production. A reduction in egg quality inevitably affects the commercial value of eggs. Therefore, it is important to alleviate the aging of reproductive organs of laying hens. A study reported that astaxanthin supplementation did not significantly improve EP, FCR, or ADFI of laying hens (Heng et al., 2021). This is consistent with our findings, with no significant changes in production performance except for EW. Albumen height and Haugh unit are important indicators of egg freshness (Wang et al., 2019). Egg Haugh unit is significantly reduced when astaxanthin is excluded from the diet (Yang et al., 2006). Our results showed that eggs Haugh unit increased in E100 and astaxanthin groups. Yet, there was no significant difference in egg Haugh unit between the E100 and astaxanthin groups, indicating that supplementation with antioxidants can improve Haugh unit of eggs. Astaxanthin is a natural pigment that accumulates in egg yolks (Dansou et al., 2021b). In this study, astaxanthin changed the color of egg yolks.
Ovulation occurs by a mechanism similar to an inflammatory reaction and is accompanied by oxidative stress (Miyamoto et al., 2010). Excessive apoptotic rates may lead to organ dysfunction. Astaxanthin reduces granulosa cell apoptosis (Ebrahimi et al., 2021). In our study, dietary supplementation with astaxanthin resulted in the lowest proportion of TUNEL-positive cells compared to the control and E100 groups. As laying hens shift from the peak to the late laying period, the number of follicles decreases, and the rate of follicular atresia increases, leading to a sharp decline in egg production (Lillpers and Wilhelmson, 1993; Grossman et al., 2000). Vitamin E (tocotrienol) has been shown to display senescence delaying activity in primary cells and rejuvenating effects in senescent cells (Malavolta et al., 2016). In our study, astaxanthin and vitamin E supplementation decreased the atresia rate of secondary follicles so that the number of follicles increased. Our results showed that 60 mg/kg of astaxanthin was more effective than 100 mg/kg of vitamin E. Apoptosis is regulated by the caspase and BCL-2 families (Fan et al., 2005; Ow et al., 2008). The BCL-2 family triggers the release of Cyt-C into the cytoplasm and activates the caspase apoptotic factor (Edlich, 2018). Liu et al. (2009) have reported that astaxanthin inhibits the apoptosis of neuronal cells by reducing ROS and Cyt-C release. In our study, BAX, caspase-3, and Cyt-C decreased in the astaxanthin groups, meanwhile BAX and Cyt-C decreased in the E100 group. Therefore, compared with the control group and E100 group, astaxanthin may reduce the expression of caspase-3 apoptotic factor by inhibiting the pro-apoptotic gene BAX and reducing the release of Cyt-C into the cytoplasm.
Oxidative stress contributes to the development of ovarian aging-related etiologies, such as apoptosis, mitochondrial dysfunction, and inflammation (Yang et al., 2020). Antioxidant enzymes convert pro-oxidants into stable molecules, thereby maintaining redox homeostasis and protecting against oxidative damage (Zhang et al., 2015). Antioxidant supplementation is an effective strategy to reduce ovarian oxidative stress (Banu et al., 2016; Zhang et al., 2016). Astaxanthin and vitamin E have therapeutic and preventive effects on oxidative stress, inflammation, and infectious diseases (Jiang, 2014; Nabi et al., 2020). In our study, antioxidant enzyme activity increased and MDA decreased. These results suggested that astaxanthin and vitamin E modulated the enzymatic system in ovaries by increasing the levels of antioxidant enzymes and reducing ovarian cell apoptosis by regulating the enzymatic system in the ovaries. In ovary, astaxanthin 60 mg/kg supplementation has a better antioxidant and anti-apoptotic capacity than vitamin E. NRF2 repairs and degrades damaged macromolecules as a result of oxidative stress by upregulating the expression of antioxidant enzymes ( Shaw and Chattopadhyay, 2020). p-NRF2 is an important indicator of NRF2 function (Bellezza et al., 2018). Previous studies have shown that astaxanthin enhanced antioxidant capacity and improves semen quality in aged layer breeders through the MAPK/NRF2 pathway (Gao et al., 2021). In our study, the expression of NRF2 and p-NRF2 in the ovary was significantly up-regulated in the experimental groups, indicating that the oxidative stress level in laying hens in the late laying period was alleviated by NRF2.
Reproductive hormones play a vital role in follicle development and fertility (Bosch et al., 2021). FSH is the main hormone involved in the development and maturation of small follicles. LH promotes the secretion of PROG in F3 and F1 follicles (Palermo, 2007). Previous studies have shown that carotenoids alleviated acrylamide-induced testicular damage and increased FSH and LH levels (Gül et al., 2021). Astaxanthin protects hydrogen peroxide-induced mouse Leydig cells and restores PROG levels (Wang et al., 2015). In our study, serum reproductive hormone levels increased in the experimental groups; therefore, astaxanthin supplementation had positive effects on reproductive hormones. High doses of astaxanthin have shown better effects than vitamin E. As the last developmental stage of pregrade follicles, when SYF completes the selection, it can enter the stage of grade follicle development and eventually become an egg. The granulosa cells of the developing follicles had different responsiveness to FSH. Even though the pregrade follicles have the expression of FSHR, they cannot accept the stimulation of FSH. When the follicles are selected, the expression of FSHR increases and promotes the synthesis of steroids (Kim et al., 2013). Therefore, elevated FSHR and cAMP levels in granulosa cells are generally considered markers of follicle selection (Johnson, 2015). Lin et al. (2021) reported that astaxanthin binds to several key nodes in steroid hormone biosynthesis (SRD5A2, STS, AKR1C2, HSD11B1, and CYP17A1). Astaxanthin significantly increased the expression of FSHR in granulosa cells in our experiments, thereby promoting the selection process and synthesis of steroid hormones. In addition, the relative expression of CYP11A1 was significantly increased in the experimental groups. CYP11A1 encodes and regulates the synthesis of cholesterol side-chain cleavage enzyme (P450scc), and the cholesterol transport involved in P450scc is the rate-limiting step in steroid synthesis (Franks et al., 1998). Astaxanthin may accelerate this rate-limiting reaction, allowing more substrates to participate in steroidogenesis. HSD3B1, CYP17A1, HSD17B1, and CYP19A1 regulate the synthesis of PROG and E2 (Luu-The, 2013). Even though previous studies have shown that astaxanthin increased the production of granulosa cell E2 in bovine oocytes cultured in vitro (Abdel-Ghani et al., 2019), there was no significant change in our experiment.
CONCLUSION
We investigated the effect of astaxanthin on ovarian aging in laying hens during the late stage of laying. Astaxanthin improved the average egg weight, egg yolk astaxanthin concentration, egg yolk color, and Haugh unit. Additionally, astaxanthin improved the antioxidant indexes in the ovary, promoted reproductive hormones, and decreased the rate of follicular atresia by reducing the apoptosis of ovarian tissue cells. Altogether, these factors may delay ovarian decline and increase the number of follicles. Therefore, astaxanthin alleviates ovarian aging in aged laying hens and may potentially improve the yield and egg quality of aged laying hens.
ACKNOWLEDGMENTS
This work was supported by The China Agriculture Research Systems (CARS-40-K11) and The Chinese Academy of Agricultural Science and Technology Innovation Project (ASTIP-IAS-12).
DISCLOSURES
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
REFERENCES
- Abdel-Ghani M.A., Yanagawa Y., Balboula A.Z., Sakaguchi K., Kanno C., Katagiri S., Takahashi M., Nagano M. Astaxanthin improves the developmental competence of in vitro-grown oocytes and modifies the steroidogenesis of granulosa cells derived from bovine early antral follicles. Reprod. Fertil. Dev. 2019;31:272–281. doi: 10.1071/RD17527. [DOI] [PubMed] [Google Scholar]
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat. Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Banu S.K., Stanley J.A., Sivakumar K.K., Arosh J.A., Burghardt R.C. Resveratrol protects the ovary against chromium-toxicity by enhancing endogenous antioxidant enzymes and inhibiting metabolic clearance of estradiol. Toxicol. Appl. Pharmacol. 2016;303:65–78. doi: 10.1016/j.taap.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellezza I., Giambanco I., Minelli A., Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018;1865:721–733. doi: 10.1016/j.bbamcr.2018.02.010. [DOI] [PubMed] [Google Scholar]
- Ben-Meir A., Burstein E., Borrego-Alvarez A., Chong J., Wong E., Yavorska T., Naranian T., Chi M., Wang Y., Bentov Y., Alexis J., Meriano J., Sung H.-K., Gasser D.L., Moley K.H., Hekimi S., Casper R.F., Jurisicova A. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell. 2015;14:887–895. doi: 10.1111/acel.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch E., Alviggi C., Lispi M., Conforti A., Hanyaloglu A.C., Chuderland D., Simoni M., Raine-Fenning N., Crépieux P., Kol S., Rochira V., D'Hooghe T., Humaidan P. Reduced FSH and LH action: implications for medically assisted reproduction. Hum. Reprod. 2021;36:1469–1480. doi: 10.1093/humrep/deab065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccone N.A., Sharp P.J., Wilson P.W., Dunn I.C. Changes in reproductive neuroendocrine mRNAs with decreasing ovarian function in ageing hens. Gen. Comp. Endocrinol. 2005;144:20–27. doi: 10.1016/j.ygcen.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Colella M., Cuomo D., Peluso T., Falanga I., Mallardo M., De Felice M., Ambrosino C. Ovarian aging: role of pituitary-ovarian axis hormones and ncRNAs in regulating ovarian mitochondrial activity. Front. Endocrinol. (Lausanne). 2021;12 doi: 10.3389/fendo.2021.791071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansou D.M., Wang H., Nugroho R.D., He W., Zhao Q., Tang C., Zhang H., Zhang J. Effects of duration and supplementation dose with astaxanthin on egg fortification. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansou D.M., Wang H., Nugroho R.D., He W., Zhao Q., Zhang J. Assessment of response to moderate and high dose supplementation of astaxanthin in laying hens. Animals (Basel). 2021;11:1138. doi: 10.3390/ani11041138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Liu L., He Y., Dou T., Jia J., Ge C. Endocrine and genetic factors affecting egg laying performance in chickens: a review. Br. Poult. Sci. 2020;61:538–549. doi: 10.1080/00071668.2020.1758299. [DOI] [PubMed] [Google Scholar]
- Ebrahimi F., Rostami S., Nekoonam S., Rashidi Z., Sobhani A., Amidi F. The effect of astaxanthin and metformin on oxidative stress in granulosa cells of BALB C mouse model of polycystic ovary syndrome. Reprod. Sci. 2021;28:2807–2815. doi: 10.1007/s43032-021-00577-4. [DOI] [PubMed] [Google Scholar]
- Edlich F. BCL-2 proteins and apoptosis: recent insights and unknowns. Biochem. Biophys. Res. Commun. 2018;500:26–34. doi: 10.1016/j.bbrc.2017.06.190. [DOI] [PubMed] [Google Scholar]
- Eren B., Tuncay Tanrıverdi S., Aydın Köse F., Özer Ö. Antioxidant properties evaluation of topical astaxanthin formulations as anti-aging products. J. Cosmet. Dermatol. 2019;18:242–250. doi: 10.1111/jocd.12665. [DOI] [PubMed] [Google Scholar]
- Fan T.-J., Han L.-H., Cong R.-S., Liang J. Caspase family proteases and apoptosis. Acta. Biochim. Biophys. Sin. (Shanghai). 2005;37:719–727. doi: 10.1111/j.1745-7270.2005.00108.x. [DOI] [PubMed] [Google Scholar]
- Farruggia C., Kim M.-B., Bae M., Lee Y., Pham T.X., Yang Y., Han M.J., Park Y.-K., Lee J.-Y. Astaxanthin exerts anti-inflammatory and antioxidant effects in macrophages in NRF2-dependent and independent manners. J. Nutr. Biochem. 2018;62:202–209. doi: 10.1016/j.jnutbio.2018.09.005. [DOI] [PubMed] [Google Scholar]
- Fiedler S.D., Carletti M.Z., Hong X., Christenson L.K. Hormonal regulation of microRNA expression in periovulatory mouse mural granulosa cells. Biol. Reprod. 2008;79:1030–1037. doi: 10.1095/biolreprod.108.069690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks S., Gharani N., Waterworth D., Batty S., White D., Williamson R., McCarthy M. Genetics of polycystic ovary syndrome. Mol. Cell. Endocrinol. 1998;145:123–128. doi: 10.1016/s0303-7207(98)00178-6. [DOI] [PubMed] [Google Scholar]
- Gao S., Heng N., Liu F., Guo Y., Chen Y., Wang L., Ni H., Sheng X., Wang X., Xing K., Xiao L., Qi X. Natural astaxanthin enhanced antioxidant capacity and improved semen quality through the MAPK/Nrf2 pathway in aging layer breeder roosters. J. Anim. Sci. Biotechnol. 2021;12:112. doi: 10.1186/s40104-021-00633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert A.B., Evans A.J., Perry M.M., Davidson M.H. A method for separating the granulosa cells, the basal lamina and the theca of the preovulatory ovarian follicle of the domestic fowl (Gallus domesticus) J. Reprod. Fertil. 1977;50:179–181. doi: 10.1530/jrf.0.0500179. [DOI] [PubMed] [Google Scholar]
- Gosden R.G., Faddy M.J. Ovarian aging, follicular depletion, and steroidogenesis. Exp. Gerontol. 1994;29:265–274. doi: 10.1016/0531-5565(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Grossman M., Gossman T.N., Koops W.J. A model for persistency of egg production. Poultry Sci. 2000;79:1715–1724. doi: 10.1093/ps/79.12.1715. [DOI] [PubMed] [Google Scholar]
- Gül M., Kayhan Kuştepe E., Erdemli M.E., Altınöz E., Gözükara Bağ H.G., Gül S., Göktürk N. Protective effects of crocin on acrylamide-induced testis damage. Andrologia. 2021;53:e14176. doi: 10.1111/and.14176. [DOI] [PubMed] [Google Scholar]
- Hao E.-Y., Chen H., Wang D.-H., Huang C.-X., Tong Y.-G., Chen Y.-F., Zhou R.-Y., Huang R.-L. Melatonin regulates the ovarian function and enhances follicle growth in aging laying hens via activating the mammalian target of rapamycin pathway. Poult. Sci. 2020;99:2185–2195. doi: 10.1016/j.psj.2019.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng N., Gao S., Chen Y., Wang L., Li Z., Guo Y., Sheng X., Wang X., Xing K., Xiao L., Ni H., Qi X. Dietary supplementation with natural astaxanthin from Haematococcus pluvialis improves antioxidant enzyme activity, free radical scavenging ability, and gene expression of antioxidant enzymes in laying hens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014;72:76–90. doi: 10.1016/j.freeradbiomed.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A.L. Ovarian follicle selection and granulosa cell differentiation. Poult. Sci. 2015;94:781–785. doi: 10.3382/ps/peu008. [DOI] [PubMed] [Google Scholar]
- Johnson P.A., Stephens C.S., Giles J.R. The domestic chicken: causes and consequences of an egg a day. Poult. Sci. 2015;94:816–820. doi: 10.3382/ps/peu083. [DOI] [PubMed] [Google Scholar]
- Kamada H., Akagi S., Watanabe S. Astaxanthin increases progesterone production in cultured bovine luteal cells. J. Vet. Med. Sci. 2017;79:1103–1109. doi: 10.1292/jvms.17-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Ocón-Grove O., Johnson A.L. Bone morphogenetic protein 4 supports the initial differentiation of hen (Gallus gallus) granulosa cells. Biol. Reprod. 2013;88:161. doi: 10.1095/biolreprod.113.109694. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y., Yoshida H., Kondo K. Potential anti-atherosclerotic properties of astaxanthin. Mar Drugs. 2016;14:35. doi: 10.3390/md14020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Kumar R., Kumari A., Panwar A. Astaxanthin: a super antioxidant from microalgae and its therapeutic potential. J. Basic Microbiol. 2021;62:1064–1082. doi: 10.1002/jobm.202100391. [DOI] [PubMed] [Google Scholar]
- Li R., Wu H., Zhuo W.W., Mao Q.F., Lan H., Zhang Y., Hua S. Astaxanthin normalizes epigenetic modifications of bovine somatic cell cloned embryos and decreases the generation of lipid peroxidation. Reprod. Domest. Anim. 2015;50:793–799. doi: 10.1111/rda.12589. [DOI] [PubMed] [Google Scholar]
- Lillpers K., Wilhelmson M. Age-dependent changes in oviposition pattern and egg production traits in the domestic hen. Poultry Science. 1993;72:2005–2011. doi: 10.3382/ps.0722005. [DOI] [PubMed] [Google Scholar]
- Lim J., Nakamura B.N., Mohar I., Kavanagh T.J., Luderer U. Glutamate cysteine ligase modifier subunit (Gclm) null mice have increased ovarian oxidative stress and accelerated age-related ovarian failure. Endocrinology. 2015;156:3329–3343. doi: 10.1210/en.2015-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z., Li F., Zhang Y., Tan X., Luo P., Liu H. Analysis of astaxanthin molecular targets based on network pharmacological strategies. J. Food Biochem. 2021;45:e13717. doi: 10.1111/jfbc.13717. [DOI] [PubMed] [Google Scholar]
- Liu M., Yin Y., Ye X., Zeng M., Zhao Q., Keefe D.L., Liu L. Resveratrol protects against age-associated infertility in mice. Hum. Reprod. 2013;28:707–717. doi: 10.1093/humrep/des437. [DOI] [PubMed] [Google Scholar]
- Liu X., Lin X., Mi Y., Li J., Zhang C. Grape seed proanthocyanidin extract prevents ovarian aging by inhibiting oxidative stress in the hens. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/9390810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Lin X., Zhang S., Guo C., Li J., Mi Y., Zhang C. Lycopene ameliorates oxidative stress in the aging chicken ovary via activation of Nrf2/HO-1 pathway. Aging (Albany NY). 2018;10:2016–2036. doi: 10.18632/aging.101526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Shibata T., Hisaka S., Osawa T. Astaxanthin inhibits reactive oxygen species-mediated cellular toxicity in dopaminergic SH-SY5Y cells via mitochondria-targeted protective mechanism. Brain Res. 2009;1254:18–27. doi: 10.1016/j.brainres.2008.11.076. [DOI] [PubMed] [Google Scholar]
- Luu-The V. Assessment of steroidogenesis and steroidogenic enzyme functions. J. Steroid Biochem. Mol. Biol. 2013;137:176–182. doi: 10.1016/j.jsbmb.2013.05.017. [DOI] [PubMed] [Google Scholar]
- Malavolta M., Pierpaoli E., Giacconi R., Costarelli L., Piacenza F., Basso A., Cardelli M., Provinciali M. Pleiotropic Effects of Tocotrienols and Quercetin on Cellular Senescence: Introducing the Perspective of Senolytic Effects of Phytochemicals. Curr. Drug Targets. 2016;17:447–459. doi: 10.2174/1389450116666150907105104. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Sato E.F., Kasahara E., Jikumaru M., Hiramoto K., Tabata H., Katsuragi M., Odo S., Utsumi K., Inoue M. Effect of oxidative stress during repeated ovulation on the structure and functions of the ovary, oocytes, and their mitochondria. Free Radic. Biol. Med. 2010;49:674–681. doi: 10.1016/j.freeradbiomed.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Nabi F., Arain M.A., Rajput N., Alagawany M., Soomro J., Umer M., Soomro F., Wang Z., Ye R., Liu J. Health benefits of carotenoids and potential application in poultry industry: a review. J. Anim. Physiol. Anim. Nutr. (Berl). 2020;104:1809–1818. doi: 10.1111/jpn.13375. [DOI] [PubMed] [Google Scholar]
- Ow Y.-L.P., Green D.R., Hao Z., Mak T.W. Cytochrome c: functions beyond respiration. Nat. Rev. Mol. Cell. Biol. 2008;9:532–542. doi: 10.1038/nrm2434. [DOI] [PubMed] [Google Scholar]
- Palermo R. Differential actions of FSH and LH during folliculogenesis. Reprod. Biomed. Online. 2007;15:326–337. doi: 10.1016/s1472-6483(10)60347-1. [DOI] [PubMed] [Google Scholar]
- Shaw P., Chattopadhyay A. Nrf2-ARE signaling in cellular protection: Mechanism of action and the regulatory mechanisms. J. Cell. Physiol. 2020;235:3119–3130. doi: 10.1002/jcp.29219. [DOI] [PubMed] [Google Scholar]
- Shevchenko L.V., Iakubchak O.M., Davydovych V.A., Honchar V.V., Ciorga M., Hartung J., Kołacz R. Influence of lycopene and astaxanthin in feed on metabolic parameters of laying hens, yolk color of eggs and their content of carotenoids and vitamin A when stored under refrigerated conditions. Pol. J. Vet. Sci. 2021;24:525–535. doi: 10.24425/pjvs.2021.139977. [DOI] [PubMed] [Google Scholar]
- Stoop D., Cobo A., Silber S. Fertility preservation for age-related fertility decline. Lancet. 2014;384:1311–1319. doi: 10.1016/S0140-6736(14)61261-7. [DOI] [PubMed] [Google Scholar]
- Surai P.F., Kochish I.I., Romanov M.N., Griffin D.K. Nutritional modulation of the antioxidant capacities in poultry: the case of vitamin E. Poult. Sci. 2019;98:4030–4041. doi: 10.3382/ps/pez072. [DOI] [PubMed] [Google Scholar]
- Sztretye M., Dienes B., Gönczi M., Czirják T., Csernoch L., Dux L., Szentesi P., Keller-Pintér A. Astaxanthin: a potential mitochondrial-targeted antioxidant treatment in diseases and with aging. Oxid. Med. Cell Longev. 2019;2019 doi: 10.1155/2019/3849692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L.A., Wang T., Xin H., Dolde D. Supplementation of laying-hen feed with palm tocos and algae astaxanthin for egg yolk nutrient enrichment. J. Agric. Food Chem. 2012;60:1989–1999. doi: 10.1021/jf204763f. [DOI] [PubMed] [Google Scholar]
- Wang J.-Y., Lee Y.-J., Chou M.-C., Chang R., Chiu C.-H., Liang Y.-J., Wu L.-S. Astaxanthin protects steroidogenesis from hydrogen peroxide-induced oxidative stress in mouse Leydig cells. Mar. Drugs. 2015;13:1375–1388. doi: 10.3390/md13031375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang Z., Shan Y. Assessment of the relationship between ovomucin and albumen quality of shell eggs during storage. Poult. Sci. 2019;98:473–479. doi: 10.3382/ps/pey349. [DOI] [PubMed] [Google Scholar]
- Yang L., Chen Y., Liu Y., Xing Y., Miao C., Zhao Y., Chang X., Zhang Q. The role of oxidative stress and natural antioxidants in ovarian aging. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.617843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.X., Kim Y.J., Jin Z., Lohakare J.D., Kim C.H., Ohh S.H., Lee S.H., Choi J.Y., Chae B.J. Effects of dietary supplementation of astaxanthin on production performance, egg quality in layers and meat quality in finishing pigs. Asian-Australas. J. Anim. Sci. 2006;19:1019–1025. [Google Scholar]
- Zhang H., Davies K.J.A., Forman H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015;88:314–336. doi: 10.1016/j.freeradbiomed.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.-Q., Gao B.-W., Wang J., Ren Q.-L., Chen J.-F., Ma Q., Zhang Z.-J., Xing B.-S. Critical role of FoxO1 in granulosa cell apoptosis caused by oxidative stress and protective effects of grape seed procyanidin B2. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/6147345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D., Lv C., Liu G., Mi Y., Zhang C., et al. Effect of estrogen on chick primordial follicle development and activation. Cell Biology International. 2017;41:630–638. doi: 10.1002/cbin.10766. [DOI] [PubMed] [Google Scholar]