Abstract
OBJECTIVE
This study aimed to estimate the proportion of health facilities without the capability to remove contraceptive implants and those that have the capability to insert them and to understand facility-level barriers to implant removal across 6 countries in sub-Saharan Africa.
STUDY DESIGN
Using facility data from the Performance Monitoring for Action in Burkina Faso, the Democratic Republic of Congo, Ethiopia, Kenya, Nigeria, and Uganda from 2020, we examined the extent to which implant-providing facilities (1) lacked necessary supplies to remove implants, (2) did not have a provider trained to remove implants onsite, (3) could not remove deeply placed implants onsite, and (4) reported any of the above barriers to implant removal. We calculated the proportion of facilities that report each barrier, stratifying by facility type.
RESULTS
Between 31% and 58% of implant-providing facilities reported at least 1 barrier to implant removal in each country (6 sub-Saharan African countries). Lack of trained providers was the least common barrier to implant removal (0%–17% of facilities), whereas lack of supplies (17%–44% of facilities) and the inability to remove a deeply placed implant (16%–42%) represented more common obstacles to removal. Blades and forceps were commonly missing supplies across all 6 countries. Barriers to implant removal were less commonly reported at hospitals than at lower-level facilities in all countries except Burkina Faso.
CONCLUSION
This multicountry analysis showed that facility-level barriers to contraceptive implant removal are widespread among facilities that offer implant insertion. By preventing users from being able to discontinue their implants on request, these barriers pose a threat to contraceptive autonomy and reproductive health.
Key words: Facility-readiness, reproductive health, women's health, contraceptive implants, LARC removal, health systems, family planning
AJOG Global Reports at a Glance.
Why was this study conducted?
A growing body of in-depth qualitative literature indicates that women face barriers to the removal of subdermal implants, but there is little evidence of the scope of these barriers at the national or regional levels.
Key findings
Between 31% and 58% of facilities that offer implant insertion reported at least 1 barrier to implant removal.
What does this add to what is known?
Facility-level barriers to contraceptive implant removal are widespread in these six African countries.
Introduction
Enthusiasm for subdermal contraceptive implants has intensified in recent years among global reproductive health practitioners. As part of a broader movement to promote the use of long-acting reversible contraceptive (LARC) methods, scholars and advocates have been eager to expand access to and use of the contraceptive implant, citing the implant's 5-year duration, high effectiveness, and low levels of user error.1, 2, 3, 4 Recent work on contraceptive implants has described the past few years as a period of “liftoff” and “blossoming” for implants in sub-Saharan Africa in particular, with population-based survey data showing considerable growth in implant use across an array of sociodemographic groups in that region.3 In Kenya, for example, implant use grew from 1.7% of married women in 2003 to 18.1% in 2016.3
However, as implant insertion has risen throughout sub-Saharan Africa, concerns about implant removal have risen as well. Many scholars have expressed apprehension that the rapid rise in implant insertions may not be accompanied by a commensurate rise in the skills, supplies, or services to remove implants.5, 6, 7 For example, a 2016 article projected that the need for implant removals would more than double between 2015 and 2018 across the 69 focus countries of the Family Planning 2020 initiative.6 As the contraceptive implant is a provider-dependent method, a skilled provider and an array of medical supplies are required to safely remove the implant's rods from the arm. Implant removal is widely considered to be a more difficult procedure than insertion, and the difficulty of removal can be heightened when the implant is deeply placed or when it migrates from its initial place of insertion.8, 9, 10 Family planning advocates have published a series of commentaries to call attention to the underexamined issue of implant removal services, expressing “serious concern about lack of quality removal services.”6,11
In recent years, an emerging body of in-depth qualitative research has shed light on the types of challenges that women can face when accessing removal services in sub-Saharan Africa. Studies from Ethiopia, Kenya, Ghana, and an anonymized setting showed that barriers include providers refusing to remove implants on user request, treating the labeled duration of efficacy as the minimum duration of use, telling users that “early” removal (before the end of the labeled duration of use) costs more, and refusing to remove the implant even when women express a desire to become pregnant.7,12, 13, 14, 15 The only peer-reviewed quantitative study on implant removal from sub-Saharan Africa—conducted in Ghana in 2018 among a sample of approximately 2200 implant users—found that more than one-third of those who sought to discontinue an implant were unable to do so on their first attempt.7 Although localized studies like this provide important depth and a formative understanding of the challenges to implant removal, there is little systematic evidence of the scope of these issues at national or regional levels in the peer-reviewed literature.
Here, we began to fill this gap, using national data from facility-based surveys conducted as part of the Performance Monitoring for Action (PMA) project, to improve the understanding of facility-level barriers to implant removal across sub-Saharan Africa. Our goal was to understand the extent to which there may be asymmetry in the availability of implant provision and removal services. Among health facilities that are equipped to provide clients with contraceptive implants, we examined the proportion that lacked the commensurate ability to remove implants. Moreover, exploring facility readiness to remove contraceptive implants, we documented the nature of the barriers to removal services by country and facility type.
Methods
Data
We used data collected as part of the PMA project, which uses mobile technology to conduct annual rapid national and regional surveys on a range of reproductive health topics, including family planning services, in sub-Saharan Africa and South Asia. The PMA collects facility-level data via samples of public and private service delivery points (herein referred to as “facilities”) that offer primary and/or reproductive health services to a community in each context. Facility types range from small pharmacies or drug shops to tertiary-level hospitals. Trained interviewers conduct the facility survey using a structured questionnaire with the facility or departmental manager to record facility characteristics and the scope of health services provided. For family planning services, an observation-based facility audit is conducted to assess the availability of contraceptive commodities and other key supplies and equipment. We included all countries in sub-Saharan Africa that had data on facility readiness for implant removal in publicly available datasets as of 2020: Burkina Faso, the Democratic Republic of the Congo (DRC), Ethiopia, Kenya, Nigeria, and Uganda.
The PMA selected facilities following a multistage sampling process to generate a sample that is reflective of the health facility environment of women surveyed in the PMA's nationally or regionally representative household sample. In the first stage of sampling, designed to generate a representative population of women of reproductive age, a series of enumeration areas (EAs) were drawn in each country or region; EAs were used as sampling units from which to identify a probability sample of households and female survey participants. The selection of health facilities was generated by identifying the lowest level public health facility (equivalent to a health post or clinic), the second lowest public facility (generally a health center), and the third lowest public facility (generally a primary-level hospital) whose catchment area included the selected EA. All private facilities that offered generalized or primary health services or specialized obstetrics and gynecology services or had the capacity to distribute contraceptives, including pharmacies and drug shops, within the EA were listed, and up to 3 facilities were randomly selected. This resulted in a total of 4 to 6 facilities per EA across the service delivery point (SDP) data. Although the women-level data that PMA collects are nationally representative in most settings, the SDP data used in this analysis were not nationally or regionally representative. PMA's sampling strategy has been described in greater detail in the studies of Zimmerman et al.16,17 Data from this analysis were collected between November 2020 and January 2021. Additional information about this data source can be found at www.pmadata.org.
Analytical sample
A total of 2792 facilities were surveyed across geographies, of which 2582 (92%) offered family planning services. Among these facilities, we restricted our analytical sample to the 2031 facilities that reported providing implant services to clients. This resulted in an analytical sample of 203 facilities in Burkina Faso, 157 facilities in the Democratic Republic of Congo, 494 facilities in Ethiopia, 829 facilities in Kenya, 117 facilities from 2 states in Nigeria (Kano in the North and Lagos in the South), and 231 facilities in Uganda.
Variables
We examined 3 primary binary outcomes, reflecting whether each facility (1) lacked any of the necessary supplies to remove implants, (2) did not have a provider trained to remove implants onsite, and (3) could not remove deeply placed implants onsite. A facility was classified as lacking necessary supplies to remove implants if a facility representative reported that one or more of the following supplies was unavailable on the day of the interview: antiseptic, sterile gauze, anesthetic, scalpels, forceps, or clean gloves. In Ethiopia, data on clean gloves were not collected because of a skip pattern error; therefore, clean gloves were not included in the supplies necessary to remove implants in Ethiopia. A facility was classified as lacking a provider trained in implant removal if the representative answered “no” to the question, “On days when you offer family planning services, are there providers trained to remove implants?” A facility was classified as being unable to remove deeply placed implants if the representative responded “no” when asked, “Could implant removal (when deeply inserted) onsite be provided to a woman today?” Finally, we developed a fourth outcome reflecting whether the facility reported any of the 3 barriers to implant removal (yes or no).
Analytical strategy
We first described facility characteristics, including facility type, management (public or private), and availability of key infrastructure, stratified by country. We calculated for each country the proportion of implant-providing facilities that lack necessary supplies to remove implants, do not have a provider trained in implant removal, or are not able to remove a deeply placed implant onsite and the composite outcome of facilities experiencing any barrier to implant removal. Furthermore, we calculated the proportion of facilities that were missing each of the necessary supplies for implant removal. We stratified by facility type, calculating the proportion of hospitals and all other facility types (including clinics, health posts, dispensaries, health centers, surgery centers, and pharmacies) in each country that have a barrier to implant removal. Following PMA standard practice for the facility survey, we did not report results for any cells where the disaggregation results in <10 facilities.
Ethics approval
Ethical approval for PMA data collection efforts has been granted by the relevant ethics boards in each country (n=6) presented in this analysis and for the case of PMA Ethiopia, by the Johns Hopkins University Bloomberg School of Public Health Institutional Review Board (FWA00000287). Facility data used in this analysis were exempted as nonhuman subjects research.
Role of funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Publicly managed facilities constituted most of our samples across contexts because of the sampling approach, although this proportion was considerably lower in the DRC (52%) than in other countries (89%–94%) (Table 1). However, we noted that the distinction between public and privately managed facilities is highly context specific. Furthermore, the proportion of facilities that were hospitals varied substantially by country, from 10% in Burkina Faso to 33% in Nigeria. In all countries, more than half of the facilities had electricity and running water.
Table 1.
Characteristics of included facilities that offer implant removal in 6 countries, 2020
| Characteristic | Burkina Faso, n (%) | Democratic Republic of Congo, n (%) | Ethiopia, n (%) | Kenya, n (%) | Nigeria, n (%) | Uganda, n (%) |
|---|---|---|---|---|---|---|
| n | 203 | 157 | 494 | 829 | 117 | 231 |
| Hospital | 21 (10) | 45 (29) | 140 (28) | 98 (12) | 39 (33) | 45 (19) |
| Government funded | 188 (93) | 81 (52) | 442 (89) | 770 (93) | 107 (91) | 218 (94) |
| Facility has electricity | 195 (96) | 83 (53) | 406 (82) | 677 (82) | 75 (64) | 200 (87) |
| Facility has running water | 164 (81) | 91 (58) | 481 (97) | 605 (73) | 88 (75) | 173 (75) |
Senderowicz. Facility readiness to remove contraceptive implants. Am J Obstet Gynecol Glob Rep 2022.
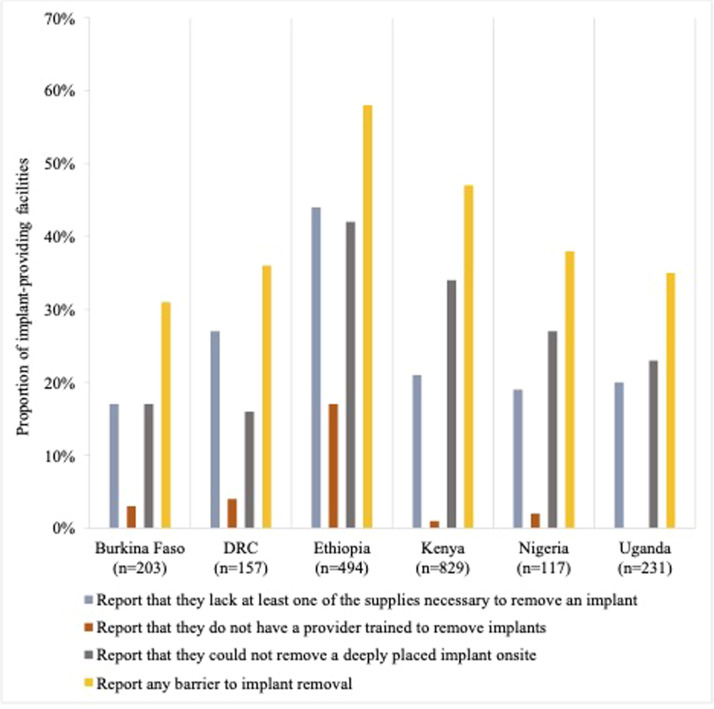
The proportion of facilities that reported that they lacked at least some of the supplies necessary for implant removal ranged from 17% in Burkina Faso to 44% in Ethiopia (Figure 1). The proportion of facilities that reported that they lacked a provider trained to remove implants ranged from 1% in Kenya to 17% in Ethiopia. Between 16% and 42% of facilities reported that they could not remove a deeply placed implant. The proportion of facilities that reported at least 1 of these barriers to implant removal ranged from 31% in Burkina Faso to 58% in Ethiopia. Lack of at least 1 of the supplies necessary for implant removal was the most common facility-level barrier to removal among implant-providing facilities in Burkina Faso (where 17% lacked supplies and 17% were unable to remove a deeply placed implant), the DRC, and Ethiopia and was reported by at least 15% of facilities in each country. The inability to remove a deeply placed implant onsite was the leading barrier in Kenya, Nigeria, and Uganda and was reported by at least 16% of facilities in each country.
Figure 1.
Service delivery barriers to implant removal
DRC, Democratic Republic of the Congo.
Senderowicz. Facility readiness to remove contraceptive implants. Am J Obstet Gynecol Glob Rep 2022.
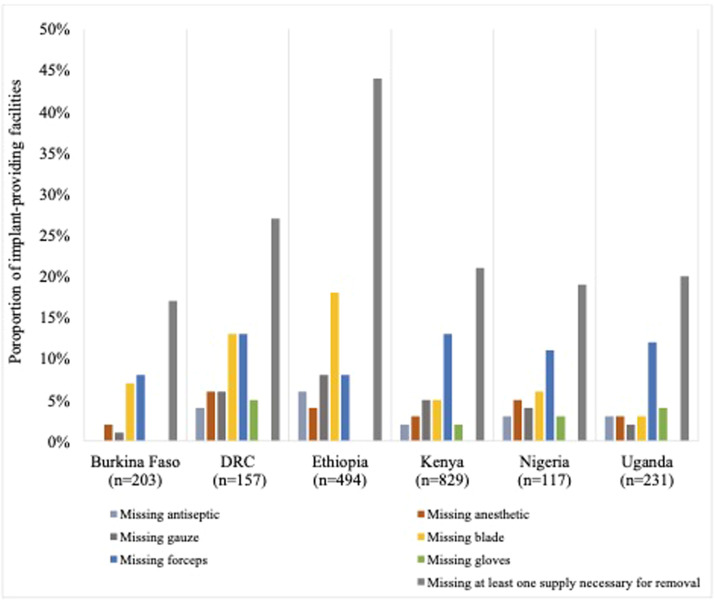
Focusing on the lack of supplies in Figure 2, we showed the proportion of implant-providing facilities that lacked each medical supply necessary for the routine removal of contraceptive implants. Forceps were the most commonly missing supply in all countries except Ethiopia, with 8% to 13% of implant-providing facilities reporting they were unavailable. In Ethiopia, blades were most commonly lacking (18% of implant-providing facilities). The proportion of facilities missing antiseptic ranged from 0% in Burkina Faso to 6% in Ethiopia. Missing anesthetic ranged from 2% of facilities in Burkina Faso to 6% of facilities in the DRC. Missing gauze ranged from 1% of facilities in Burkina Faso to 8% of facilities in Ethiopia. The proportion of facilities missing gloves ranged from 0% in Burkina Faso to 5% in the DRC.1
Figure 2.
Proportion of implant-inserting facilities missing supplies necessary for removal
DRC, Democratic Republic of the Congo.
Senderowicz. Facility readiness to remove contraceptive implants. Am J Obstet Gynecol Glob Rep 2022.
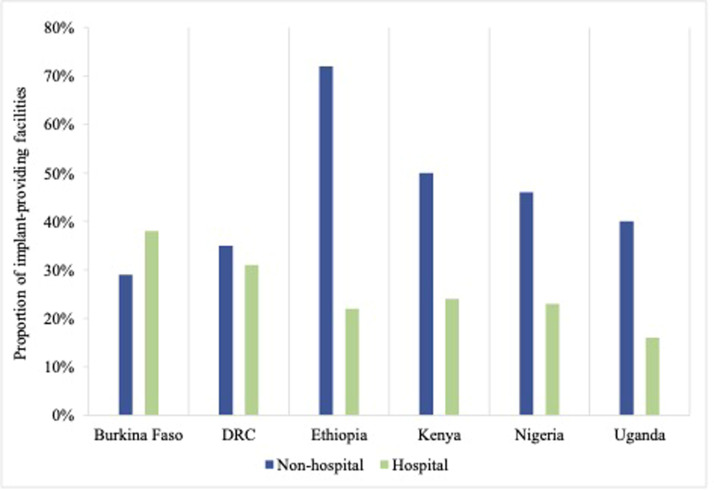
In Table 2, we disaggregated facility-level barriers to implant removal by facility type in our sample, comparing hospitals to nonhospital lower-level facilities.2 Although there was variation by barrier and country, we observed a broad association between the level of care provided at the facility and the ability to remove implants, with lower-level clinics in our sample reporting greater barriers to providing removal services than hospitals (Figure 3). For example, in Ethiopia, 53% of nonhospital facilities reported lacking at least 1 supply necessary for implant removal, compared with 21% of hospitals. However, higher-level hospital facilities still reported substantial barriers to implant removal, including 29% of hospitals in the DRC that lacked at least 1 necessary supply and 19% of hospitals in Burkina Faso and Kenya that stated that they could not remove a deeply placed implant onsite. The only setting where lower-level health facilities had fewer barriers to implant removal was Burkina Faso, where 38% of hospitals reported at least 1 barrier, compared with 29% of nonhospitals.
Table 2.
Facility-level barriers to implant removal, by country and facility type
| Variable | Burkina Faso (n=203) |
Democratic Republic of Congo (n=157) | Ethiopia (n=494) | Kenya (n=829) | Nigeria (n=117) | Uganda (n=231) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hospitals | Nonhospitals | Hospitals | Nonhospitals | Hospitals | Nonhospitals | Hospitals | Nonhospitals | Hospitals | Nonhospitals | Hospitals | Nonhospitals | |
| n | 21 | 182 | 45 | 112 | 140 | 354 | 98 | 731 | 39 | 78 | 45 | 186 |
| Report they lack at least some of the supplies necessary to remove an implanta | 24% | 16% | 29% | 27% | 21% | 53% | 6% | 24% | 18% | 19% | 16% | 22% |
| Report they do not have a provider trained to remove implants | 0% | 3% | 0% | 5% | 2% | 23% | 0% | 1% | 3% | 1% | 0% | 0% |
| Report they could not remove a deeply placed implant onsite | 19% | 16% | 7% | 16% | 4% | 57% | 19% | 36% | 10% | 36% | 0% | 28% |
| Report at least 1 of the above barriers | 38% | 29% | 31% | 35% | 22% | 72% | 24% | 50% | 23% | 46% | 16% | 40% |
aNecessary supplies include antiseptic, sterile gauze, anesthetic, scalpel blade, forceps, and clean gloves. In Ethiopia, data on clean gloves were missing, so clean gloves were excluded from the list of necessary supplies.
Senderowicz. Facility readiness to remove contraceptive implants. Am J Obstet Gynecol Glob Rep 2022.
Figure 3.
Facilities that report any barrier to removal services
DRC, Democratic Republic of the Congo.
Senderowicz. Facility readiness to remove contraceptive implants. Am J Obstet Gynecol Glob Rep 2022.
Discussion
This multicountry analysis found that barriers to contraceptive implant removal are widespread among facilities that provide the implant. Among our sample of 2031 health facilities across 6 sub-Saharan African countries, we found that nearly half of the health facilities (45%) had at least 1 barrier to implant removal. More than one-quarter of implant-providing facilities lacked the supplies needed to remove the implant. Blades and forceps were the most commonly missing supplies across all 6 countries. Of note, 5% of facilities lack a provider trained to remove the implant, and nearly one-third of the facilities could not remove a deeply placed implant onsite.
Although the medical imaging equipment needed in select cases to locate migrated implants may be expensive or challenging to operate, the supplies necessary for routine implant removal that we examined (such as gloves and scalpels) are low-tech staples of health service provision. Ensuring that these supplies are universally available in all implant-providing facilities is 1 key way to reduce barriers to removal for women who wish to discontinue the use of the implant. Our results suggested that lack of trained providers is a less common impediment to implant removal at health facilities than lack of supplies and the ability to remove implants that are deeply placed. That lack of trained providers was a relatively infrequent contributor to barriers to implant removal relative to lack of supplies suggested that increased provider training may be insufficient to address the totality of facility-level barriers.
Across 5 of 6 countries, barriers to implant removal were more commonly reported at clinics, health centers, and other first-line facilities than they were at hospitals. These results were in line with the expectation that hospitals, as larger, tertiary-level facilities, would benefit from more highly trained personnel and more advanced equipment (such as imaging devices to locate implants that have migrated) and would suffer from fewer stockouts of basic supplies than their lower-level counterparts. However, this did not seem to be the case in Burkina Faso, where hospitals were more likely than nonhospitals to report that they could not remove a deeply placed implant onsite and that they lacked at least 1 of the necessary supplies. More research is required to understand why this may be the case in that setting. However, we noted that across all 6 countries, we observed high proportions of barriers to implant removal even at the hospital level. That nearly one-fifth of higher-level hospitals surveyed in Burkina Faso and Kenya reported that they could not remove a deeply placed implant onsite raises concerns about a lack of recourse for women facing difficult removals in those settings.
The limitations of these data and this analysis are important to note. The facility audits captured data from only 1 discrete time point, which may not always be representative of the facility's typical care capabilities at different points throughout the year. Especially when facilities have a notification that a data collector is coming, they may make efforts to obtain stock in advance of the visit or otherwise prepare for the evaluation.18 More generally, SDP data are not nationally or regionally representative. In several cases, reports of facility readiness were verbal attestations by a health worker or facility administrator rather than visual inspection of supplies or provider training certificates. Respondents may have provided a socially desirable response that the facility has the needed supplies when it does not. These methodological limitations may potentially lead to the underestimation of the true scope of facility barriers to implant removal in these settings. Furthermore, the presence of additional equipment necessary to remove deeply placed removal (such as medical imaging devices) was not measured.
Perhaps more importantly, the exclusive focus of these data on facility readiness leaves us with little understanding of the crucial issues of provider confidence and willingness to provide removal services. Much of the formative research on barriers to implant removal has identified issues of provider reluctance as a key barrier to discontinuation from users’ perspectives, and we were unable to measure this here.13, 14, 15 As women may encounter provider hesitancy to remove implants even at a facility that has the right supplies and training, the estimates that we presented were likely an underestimation of the true barriers to removal that implant users face in these contexts. Some previous research studies, although limited, have identified that high percentages of providers have struggled to remove implants or received removal training that did not include practice on actual patients.19 Limitations in training and infrequent opportunities to apply skills may contribute to provider hesitancy to remove implants.20 However, to the best of our knowledge, neither provider confidence nor provider willingness to remove methods on request is currently measured by any publicly available survey. Researchers need data that capture both facility readiness and provider ability or willingness to support contraceptive users in their efforts to discontinue LARC methods when they choose.21 Accurately measuring provider willingness and ability to remove LARC methods is necessary to develop health system–level indicators of contraceptive autonomy.22
Conclusion
Respect for persons is a fundamental principle of biomedical ethics, and the ability to choose what contraceptive method to use, for how long, and when to discontinue it is an essential reproductive right.23,24 Scaling up the availability of contraceptive implants and other provider-dependent methods is important to expand access to a broad contraceptive method mix that meets user preferences. However, a focus on method provision without a commensurate emphasis on removal has resulted in substantial barriers to free contraceptive choice for women who wish to discontinue implant use. The facility-level barriers to implant removal reported in our study can result in “structural contraceptive coercion,” in which women have no choice but to keep a method they wish to discontinue, even in the absence of any ill will or intent to coerce the part of providers, health facilities, or family planning programs.14 Programs that insert provider-dependent contraceptive methods, such as implants and intrauterine devices, should ensure that method removal is as easily available to clients as insertion and is performed on request. Such a person-centered orientation can safeguard the principles of contraceptive autonomy and reproductive justice as the cornerstone of contraceptive services worldwide.
Footnotes
The following members of the Performance Monitoring for Action (PMA) Principal Investigators Group contributed to the data collection and development of this manuscript: Georges Guiella, University Joseph Ki-Zerbo, Ouagadougou, Burkina Faso; Solomon Shiferaw and Assefa Seme, Addis Ababa University, Addis Ababa, Ethiopia; Peter Gichangi, Technical University of Mombasa, Mombasa, Kenya, and the International Center for Reproductive Health Kenya (ICRH-K), Mombasa, Kenya, and Mary Thiongo, the ICRH-K, Mombasa, Kenya; Elizabeth Omoluabi, Statistics and Population Studies Department, University of the Western Cape, Cape Town, South Africa; and Fredrick Edward Makumbi and Simon Kibera, Makerere University School of Public Health, Kampala, Uganda.
The data used in this analysis are publicly available at www.pmadata.org.
All PMA participants completed an informed consent process, as outlined by the ethical review boards in each geography.
The authors report no conflict of interest.
The PMA project relies on the work of many individuals, both in the United States and in survey countries. The project team is grateful for support from the Bill & Melinda Gates Foundation and would like to thank the country teams and resident enumerators who are ultimately responsible for the success of the PMA project.
L.S.’s contribution was supported by a Ruth L Kirschstein National Research Service Award (grant number T32 HD049302) and a Population Research Infrastructure grant (grant number P2C HD047873). B.W.B.’s contribution was supported by a National Research Service Award (grant number T32HD52468) and a Population Infrastructure grant (grant number P2CHD050924). K.T.’s contribution was supported by an infrastructure grant for population research (grant number P2C HD047879) to the Carolina Population Center at the University of North Carolina at Chapel Hill. The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) of the National Institutes of Health (NIH) awarded these grants. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH/NICHD.
Cite this article as: Senderowicz L, Karp C, Bullington BW, et al. Facility readiness to remove subdermal contraceptive implants in 6 sub-Saharan African countries. Am J Obstet Gynecol Glob Rep 2022;XX:x.ex–x.ex.
These data were not available for Ethiopia.
Cells for which the number of facilities is less than 10 were left intentionally blank.
References
- 1.Stoddard A, McNicholas C, Peipert JF. Efficacy and safety of long-acting reversible contraception. Drugs. 2011;71:969–980. doi: 10.2165/11591290-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ouedraogo L, Habonimana D, Nkurunziza T, et al. Towards achieving the family planning targets in the African region: a rapid review of task sharing policies. Reprod Health. 2021;18:22. doi: 10.1186/s12978-020-01038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobstein R. Liftoff: the blossoming of contraceptive implant use in Africa. Glob Health Sci Pract. 2018;6:17–39. doi: 10.9745/GHSP-D-17-00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun R, Grever A. Scaling up access to implants: a summative evaluation of the Implants Access Program. Glob Health Sci Pract. 2020;8:205–219. doi: 10.9745/GHSP-D-19-00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendrixson A. Population control in the troubled present: the ‘120 by 20’ target and implant access program: population control in the troubled present. Dev Change. 2018;50:786–804. [Google Scholar]
- 6.Christofield M, Lacoste M. Accessible contraceptive implant removal services: an essential element of quality service delivery and scale-up. Glob Health Sci Pract. 2016;4:366–372. doi: 10.9745/GHSP-D-16-00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callahan R, Lebetkin E, Brennan C, et al. What goes in must come out: a mixed-method study of access to contraceptive implant removal services in Ghana. Glob Health Sci Pract. 2020;8:220–238. doi: 10.9745/GHSP-D-20-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reproductive Health Access Project. Contraceptive pearl: implant removal: pop out technique. 2016. Available at: https://www.reproductiveaccess.org/resource/contraceptive-pearl-implant-removal-pop-out-technique/. Accessed Feb 28 2022.
- 9.Pillai M, Gazet AC, Griffiths M. Continuing need for and provision of a service for non-standard implant removal. J Fam Plann Reprod Health Care. 2014;40:126–132. doi: 10.1136/jfprhc-2013-100619. [DOI] [PubMed] [Google Scholar]
- 10.Odom EB, Eisenberg DL, Fox IK. Difficult removal of subdermal contraceptive implants: a multidisciplinary approach involving a peripheral nerve expert. Contraception. 2017;96:89–95. doi: 10.1016/j.contraception.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howett R, Gertz AM, Kgaswanyane T, et al. Closing the gap: ensuring access to and quality of contraceptive implant removal services is essential to rights-based contraceptive care. Afr J Reprod Health. 2019;23:19–26. doi: 10.29063/ajrh2019/v23i4.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Utaile MM, Debere MK, Nida ET, Boneya DJ, Ergano AT. A qualitative study on reasons for early removal of Implanon among users in Arba Minch town, Gamo Goffa zone, South Ethiopia: a phenomenological approach. BMC Womens Health. 2020;20:2. doi: 10.1186/s12905-019-0876-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Britton LE, Williams CR, Onyango D, Wambua D, Tumlinson K. When it comes to time of removal, nothing is straightforward”: a qualitative study of experiences with barriers to removal of long-acting reversible contraception in Western Kenya. Contracept X. 2021;3 doi: 10.1016/j.conx.2021.100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Senderowicz L. I was obligated to accept”: a qualitative exploration of contraceptive coercion. Soc Sci Med. 2019;239 doi: 10.1016/j.socscimed.2019.112531. [DOI] [PubMed] [Google Scholar]
- 15.Yirgu R, Wood SN, Karp C, Tsui A, Moreau C. You better use the safer one… leave this one”: the role of health providers in women's pursuit of their preferred family planning methods. BMC Womens Health. 2020;20:170. doi: 10.1186/s12905-020-01034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimmerman L, Olson H, PMA2020 Principal Investigators Group. Tsui A, Radloff S. PMA2020: rapid turn-around survey data to monitor family Planning Service and practice in ten countries. Stud Fam Plann. 2017;48:293–303. doi: 10.1111/sifp.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmerman L, Desta S, Yihdego M, et al. Protocol for PMA-Ethiopia: a new data source for cross-sectional and longitudinal data of reproductive, maternal, and newborn health. Gates Open Res. 2020;4:126. doi: 10.12688/gatesopenres.13161.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tumlinson K, Speizer IS, Curtis SL, Pence BW. Accuracy of standard measures of family planning service quality: findings from the simulated client method. Stud Fam Plann. 2014;45:443–470. doi: 10.1111/j.1728-4465.2014.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christofield MM, Breithaupt L, Muthamia MM, et al. Contraceptive implant removal: service availability and readiness assessments in Uganda and Kenya. Contraception. 2017;96:295. [Google Scholar]
- 20.Charyeva Z, Oguntunde O, Orobaton N, et al. Task shifting provision of contraceptive implants to community health extension workers: results of operations research in northern Nigeria. Glob Health Sci Pract. 2015;3:382–394. doi: 10.9745/GHSP-D-15-00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howett R, Krogstad EA, Badubi O, et al. Experiences of accessing and providing contraceptive implant removal services in Gaborone, Botswana: a qualitative study among implant users and healthcare providers. Front Glob Womens Health. 2021;2 doi: 10.3389/fgwh.2021.684694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senderowicz L. Contraceptive autonomy: conceptions and measurement of a novel family planning indicator. Stud Fam Plann. 2020;51:161–176. doi: 10.1111/sifp.12114. [DOI] [PubMed] [Google Scholar]
- 23.National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research (NIH OHSR). The Belmont report: ethical principles and guidelines for the protection of human subjects of research [internet]; 1978. Clearinghouse. Available at: http://ohsr.od.nih.gov/guidelines/belmont.html#top. Bethesda: Educational Resources Information Center.
- 24.Starrs AM, Ezeh AC, Barker G, et al. Accelerate progress-sexual and reproductive health and rights for all: report of the Guttmacher-Lancet Commission. Lancet. 2018;391:2642–2692. doi: 10.1016/S0140-6736(18)30293-9. [DOI] [PubMed] [Google Scholar]