Abstract
Background:
Active surveillance (AS) is an alternative to thyroidectomy for the management of low-risk papillary thyroid microcarcinoma (PTMC). However, prospective AS data collected from diverse populations are needed.
Methods:
This multicenter prospective cohort study enrolled patients from three referral hospitals in Korea. The participants were self-assigned into two groups, AS or immediate surgery. All patients underwent neck ultrasound every 6–12 months to monitor for disease progression. Progression under AS was evaluated by a criterion of tumor size increment by 3 mm in one dimension (3 mm), 2 mm in two dimensions (2 × 2 mm), new extrathyroidal extension (ETE), or new lymph node metastasis (LNM), and a composite outcome was defined using all four criteria.
Results:
A total of 1177 eligible patients with PTMC (919 female, 78.1%) with a median age of 48 years (range 19–87) were enrolled; 755 (64.1%) patients chose AS and 422 (35.9%) underwent surgery. Among 755 patients under AS, 706 (female 537, 76.1%) underwent at least two ultrasound examinations and were analyzed. Over a follow-up period of 41.4 months (standard deviation, 16.0), 163 AS patients (23.1%) underwent surgery. Progression defined by the composite outcome was observed in 9.6% (68/706) of patients, and the 2- and 5-year progression estimates were 5.3% and 14.2%, respectively. The observed progression rates were 5.8% (41/706) and 5.4% (38/706) as defined by tumor size enlargement by 3 mm and 2 × 2 mm, respectively, and 1.3% (9/706) and 0.4% (3/706) for new LNM and ETE, respectively. No distant metastases developed during AS. In multivariate logistic regression analysis examining variables associated with progression under AS, age at diagnosis <30 years (odds ratio [OR], 2.86; 95% confidence interval [CI], 1.10 − 7.45), male sex (OR, 2.48; 95% CI, 1.47 − 4.20), and tumor size ≥6 mm (OR, 1.89; 95% CI, 1.09 − 3.27) were independently significant.
Conclusions:
The progression of low-risk PTMC during AS in the Korean population was low, but slightly higher than previously reported in other populations. Risk factors for disease progression under AS include younger age, male sex, and larger tumor size.
Clinical trial registration:
Keywords: active surveillance, immediate surgery, microcarcinoma, papillary, thyroid cancer, thyroidectomy, watchful waiting
Introduction
The prevalence of thyroid cancer has increased rapidly over the past few decades and papillary thyroid microcarcinomas (PTMCs) measuring ≤1 cm account for a significant proportion of thyroid cancer incidence.1 This high detection rate of thyroid cancer has resulted in a significant socioeconomic burden in Korea.2 Key reasons for the high observed thyroid cancer incidence rate in Korea are the easy access to medical care and the widespread use of ultrasound devices in hospitals.3 Although the increase in the detection rate of small-sized thyroid cancer has slowed recently,4 it remains the most common cancer diagnosed among Koreans.1,5 Active surveillance (AS) is an alternative to immediate surgery for the management of low-risk PTMC according to the guidelines published in Japan,6 the United States,7 and Korea.8,9 Although previous results for AS are available for a Korean cohort, these are retrospective,10,11 and prospectively collected data are needed.
Disease progression during AS is defined as tumor size enlargement or development of metastasis,6 ranging from 5% to 25% per 5 years.12–18 Variables associated with disease progression have been studied, and the only risk factor identified to date is age <40 years.12,19 This contradicts the classical concept that old age is a poor prognostic factor for thyroid cancer,7 although the underlying pathophysiology remains unclear. It is controversial whether tumor size,13,15 presence of tumor calcification,20 the coexistence of Graves' disease or benign nodules,6 multiplicity,21,22 and family history12 are associated with disease progression under AS. Prospective data collected from diverse populations are needed to inform understanding of AS outcomes for PTMC.
We designed the Multicenter Prospective Cohort Study of Active Surveillance on Papillary Thyroid Microcarcinoma (MAeSTro) in 2016 to compare the oncological outcomes of AS for low-risk PTMC with immediate surgery (iOP) in Korea.23 We planned to examine the five-year progression rate in patients with PTMC under AS using various definitions. We also planned to explore factors associated with disease progression. In this study, we reported interim results of natural courses of low-risk PTMCs after 3.5 years of follow-up of AS and clinical factors associated with disease progression.
Materials and Methods
Study design and participants
The MAeSTro was a multicenter prospective cohort study performed in three referral hospitals in Korea (Clinicaltrials.gov identifier: NCT02938702). Patients who were diagnosed with low-risk PTMC were screened and self-assigned into two groups; iOP and AS. The detailed protocol including eligibility criteria and outcome measures was described previously.23 In brief, patients with PTMC aged ≥18 years, diagnosed by fine-needle aspiration (FNA) or core needle biopsy, between 2016 and 2020, were included. Participants were excluded if they had apparent extrathyroidal extension (ETE), regional lymph node metastasis (LNM), or distant metastasis. To evaluate cervical LNM, FNA was performed from suspicious LNs with a short diameter >3–5 mm.24 If deemed necessary, contrast-enhanced computed tomography (CT) scans were obtained following the thyroid-specific protocol that early (arterial)-phase scans (25–40 second delay) depicted early strong enhancement of metastatic LNs.24 Pregnancy was not an exclusion criterion.
The study protocol was reviewed and approved by the Ethics Committee of Seoul National University Hospital (IRB number 1603-044-747), the Seoul National University Bundang Hospital (IRB number B-1605-348-402), and the National Cancer Center (IRB number NCC2016-0183). All patients provided informed consent and were informed that they could withdraw from the study or change their decision about AS or surgery at any time.
Procedures
At enrollment, demographic and clinical data such as age and sex, results of ultrasound and/or CT scans, and cytological information were collected. Patients in both groups were scheduled for follow-up visits every 6 months during the first 2 years and yearly thereafter. During the visits, a physical examination and neck ultrasound were performed. If progression was suspected in the AS group, surgery was recommended. If a patient declined surgery despite progression, AS was continued without any changes. If patients or physicians wanted to perform the evaluation more often or change their decision on the surgery, the interval of evaluation and the strategy were changed.
Definitions of disease progression
We evaluated the incidence of disease progression and the rate of treatment during the follow-up period until December 31, 2021. Disease progression under AS was defined by a composite outcome as follows: a size increase of ≥3 mm in at least one dimension (3 mm) or ≥2 mm in at least two dimensions (2 × 2 mm); new ETE; suspicion on neck ultrasound of adjacent organ involvement (e.g., trachea, esophagus, nerve, vessel, or muscle); cytologically confirmed LNM; or radiologically or pathologically confirmed distant metastases. Any suspicious LN with a short diameter >3–5 mm was aspirated to confirm LNM according to the current guidelines.24 These criteria of disease progression were established by referring to the previous studies6,12,13,21,25 and agreed upon by the MAeSTro steering committee while designing the study.23
Statistical methods
Demographic data and participants' descriptive variables are presented as means and standard deviations or medians and ranges, as appropriate. Continuous variables were compared using the Student's t-test or the Mann–Whitney U test. Categorical data are presented as rates and percentages in each category and were compared using chi-square tests or Fisher's exact test. A multivariate logistic regression analysis was performed to explore variables associated with progression under AS. Time-to-progression curves and survival estimates were based on the Kaplan–Meier method, and the log-rank test was used for comparing the time-to-event endpoints (progression, metastasis, and mortality rate). The Cox proportional hazard model was used for modeling risk factors that influenced the events adjusting with clinical risk factors. All tests of statistical significance were carried out at the 0.05 level. All statistical analyses were performed using SPSS (version 28.0; Chicago, Illinois, USA).
Results
Baseline characteristics of the study population
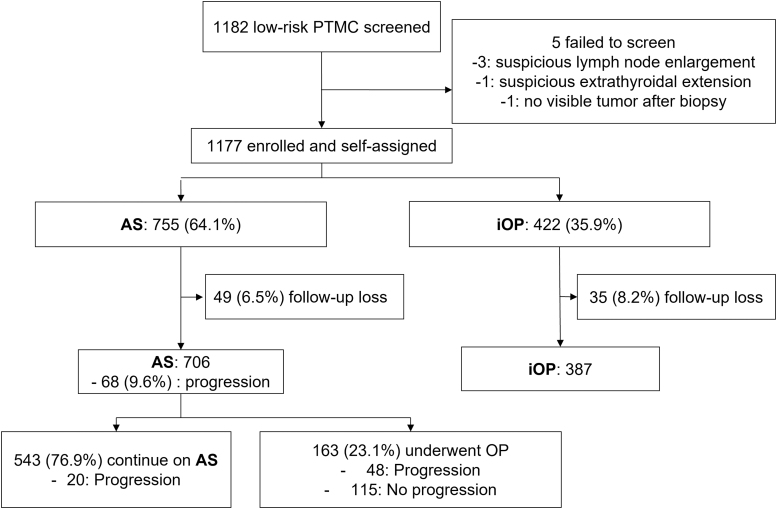
We screened 1182 patients with low-risk PTMC after obtaining informed consent. After excluding five patients (three with suspicious LN enlargement, one with suspicious ETE, and one with no visible tumor after biopsy), 1177 were enrolled. Among the included patients, 755 (64.1%) patients and 422 (35.9%) patients chose to undergo AS (AS group) and immediate surgery (iOP group), respectively (Fig. 1). Patients who chose AS were older (p < 0.001) and with smaller tumor sizes (p < 0.001) compared with those in the iOP group (Table 1).
FIG. 1.
Study population and flowchart. A total of 1177 patients of newly diagnosed low-risk PTMC were enrolled and assigned to two groups of AS and iOP. After excluding those who were not followed up after the decision or evaluated less than twice with ultrasound until December 2021, 706 patients in the AS group were analyzed. AS, active surveillance; iOP, immediate surgery; PTMC, papillary thyroid microcarcinoma.
Table 1.
Baseline Characteristics of the MAeSTro Cohort
| Initial iOP | Initial AS | p-Value | |
|---|---|---|---|
| Number, n (%) | 422 (35.9) | 755 (64.1) | |
| Age at diagnosis | |||
| Age (years) | 46.3 ± 10.5 | 49.3 ± 11.8 | <0.001 |
| <30 years, n (%) | 22 (40.7) | 32 (59.3) | 0.443 |
| ≥30 years, n (%) | 400 (35.6) | 723 (64.4) | |
| Sex | |||
| Female, n (%) | 341 (37.1) | 578 (62.9) | 0.106 |
| Male, n (%) | 81 (31.4) | 177 (68.6) | |
| Hospital | |||
| A, n (%) | 122 (31.6) | 264 (68.4) | <0.001 |
| B, n (%) | 45 (11.7) | 338 (88.3) | |
| C, n (%) | 255 (62.5) | 153 (37.5) | |
| Initial tumor size (largest diameter) | |||
| Size (mm) | 6.8 ± 1.7 | 6.2 ± 1.6 | <0.001 |
| <6 mm, n (%) | 157 (29.6) | 373 (70.4) | 0.344 |
| 6≤, ≤10 mm, n (%) | 182 (32.3) | 382 (67.7) | |
| Duration of follow-up (months) | 40.5 ± 18.4 | 38.8 ± 18.4 | 0.129 |
| Year of enrollment | |||
| 2016 Jan–2017 Dec, n (%) | 317 (39.6) | 484 (60.4) | 0.0001 |
| 2018 Jan–2020 Jan, n (%) | 105 (27.9) | 271 (72.1) | |
Data are presented with mean ± standard deviation or number (%).
AS, active surveillance; iOP, immediate surgery; MAeSTro, Multicenter Prospective Cohort Study of Active Surveillance on Papillary Thyroid Microcarcinoma.
Natural course of PTMC during AS
Of the 755 patients in the AS group, 49 (6.5%) were lost to follow-up and were excluded from the final outcome analysis. Among these 49 patients, 40 patients were lost to follow-up after the first examination, and 9 visited again after changing their decision to surgery but were lost to follow-up without further sonographic evaluation. A total of 706 AS patients were finally analyzed, all of whom underwent two or more ultrasound examinations till December 2021 (Fig. 1).
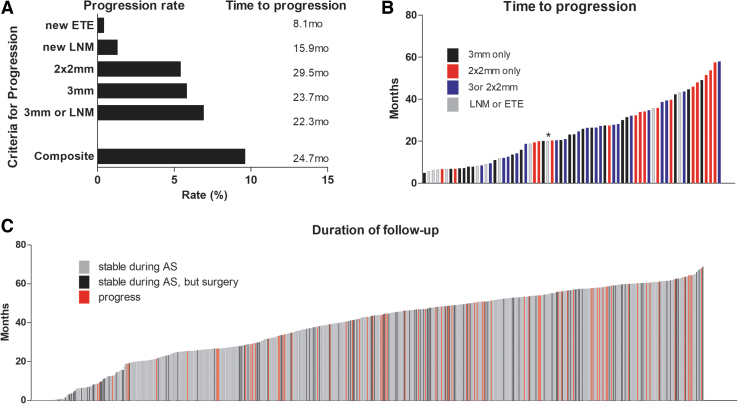
During a mean AS duration of 41.4 ± 16.0 months (median of 44.9 months), progression was confirmed through an increase in tumor size of 3 mm (41, 5.8%) or 2 × 2 mm (38, 5.4%), new LNM (9, 1.3%), and ETE (3, 0.4%) with overlap allowed (Table 2 and Fig. 2A). There was no difference in progression according to the participating institution or period of enrollment (Supplementary Table S1).
Table 2.
Observed and Estimated Progression Rates of Tumors During Active Surveillance
| Progression rate |
Progression observed during the study periods |
Progression estimated rate, % (95% CI) |
||
|---|---|---|---|---|
| Criteria of progression | Observed rate, n (%) | Mean time to progression (months) | 2 years | 5 years |
| ≥3 mm | 41 (5.8) | 23.7 ± 12.9 | 3.2 (1.9–4.6) | 8.3 (5.5 − 11.2) |
| ≥2 × 2 mm | 38 (5.4) | 29.5 ± 14.0 | 2.0 (0.9–3.1) | 9.4 (6.0 − 12.8) |
| ≥3 mm or ≥2 × 2 mm | 57 (8.1) | 26.9 ± 14.1 | 3.8 (2.4–5.3) | 12.7 (9.0 − 16.3) |
| New LNM | 9 (1.3) | 15.9 ± 12.3 | 1.2 (0.4–2.1) | 1.5 (0.5 − 2.5) |
| New ETE | 3 (0.4) | 8.1 ± 1.1 | 0.4 (−0.1 to 0.9) | 0.4 (−0.1 to 0.9) |
| ≥3 mm or new LNM | 49 (6.9) | 22.3 ± 13.2 | 4.3 (2.7–5.8) | 9.6 (6.6 − 12.5) |
| Composite outcomea | 68 (9.6) | 24.7 ± 14.6 | 5.3 (3.6 − 7.0) | 14.2 (10.5 − 17.9) |
Data are presented with the number (%), mean ± standard deviation, or % with a 95% CI.
Composite outcome was defined as any progression by tumor growth of 3 mm in one dimension, 2 mm in two dimensions, new ETE, or new LNM.
3 mm, an increment of tumor size by 3 mm at least in one dimension; 2 × 2 mm, an increment of tumor size by 2 mm at least in two dimensions; CI, confidence interval; ETE, extrathyroidal extension; LNM, lymph node metastasis.
FIG. 2.
Progression in AS group. (A) Progression rates and a mean time to progress by each criterion. The composite outcome was defined by any progression of tumor growth of 3 mm in one dimension, 2 mm in two dimensions, new ETE, or new LNM. (B) The time to progress that each patient showed a disease progression (*one case who developed tumor growth more than 3 mm and new LNM simultaneously). (C) Follow-up duration of each AS patient until progression, surgery, or last follow-up. ETE, extrathyroidal extension; LNM, lymph node metastasis.
Of the 57 patients who showed a tumor size increment, 22 patients met both the criteria of 3 mm and 2 × 2 mm simultaneously, while 19 met only the criterion of 3 mm and 16 met only the criterion of 2 × 2 mm. The time to progression in those meeting the 3 mm criterion was earlier than those with 2 × 2 mm increment even when analyzed in the subjects who exclusively met only one criterion (p = 0.007) or both criteria simultaneously (p = 0.064) (Supplementary Table S2 and Fig. 2B). The simultaneous occurrence of tumor growth and new LNM was observed in only one patient. All three patients who presented with new ETE did not show tumor size increment or new LNM, and one patient had tumor extension posteriorly; however, she did not present with voice change or vocal cord palsy.
Progression meeting any of the composite outcome criteria was observed in 68 of 706 (9.6%) patients, and the 2- and 5-year progression estimates were 5.3% and 14.2%, respectively (Table 2). Using the criterion of 3 mm or LNM, which is commonly used in other studies,10,13,15,21 the observed progression rate was 6.9% (49/706), and the 2- and 5-year progression estimates were 4.3% and 9.6%, respectively. New LNM was found in 1.3%, and its estimated rates were 1.2% at 2 years and 1.5% at 5 years. Although lead-time bias was not adjusted, progression was observed throughout the follow-up period (Fig. 2C). Distant metastases did not develop during AS.
Of the 68 patients with disease progression during AS, 20 (29.4%) patients declined surgery and maintained AS despite the physicians' recommendations. During a median of 19.4 (range 12.1–57.4) months of follow-up after progression, most (8 out of 10 followed) patients remained stable; however, one showed decreased tumor size from 7.3 × 8.5 × 9.2 mm to 5.9 × 6.8 × 5.7 mm after 26 months, while the other one showed further increment of its size from 6.0 × 4.0 × 5.0 mm to 7.6 × 7.3 × 6.0 mm after 12 months.
A new thyroid cancer was diagnosed by FNA cytology in 11 patients after a median AS of 14.1 months (3.3–35.8). Among them, 10 were preexisting benign-looking nodules that had transformed into sonographically suspicious ones. Only one case was a new PTC, diagnosed 6 months later.
In contrast, 39 patients (5.5%) had a reduction in tumor size compared with baseline; 29 (4.1%) by 3 mm and 10 (1.4%) by 2 × 2 mm, without overlap of these groups. However, despite the tumor size reduction, one patient presented with progression with new LNM after a total of 43.2 months in follow-up.
Clinical factors associated with disease progression under AS
Baseline clinical characteristics of patients who progressed according to the respective criteria for tumor progression are summarized in Supplementary Table S3. There was no significant difference among the patients according to the type of disease progression, except for a larger tumor size observed in those with new ETE (p < 0.001). New LNMs were observed in patients whose tumor growth during AS was minimal, compared with those who progressed by other criteria.
Then we compared the clinical characteristics of the patients who progressed with those who did not. From the finding of the Kaplan–Meier curve analysis for the composite outcome of progression (Supplementary Fig. S1), we used the cutoff level of age <30 years and size ≥6 mm in further analysis. It was observed that disease progression for a composite outcome was associated with age <30 years, male sex, tumor size ≥6 mm, and the duration of follow-up (Table 3). The average age at the time of diagnosis, the subcapsular location of the index tumor, the registered hospital, and the year of enrollment were not associated with disease progression (Table 3 and Supplementary Fig. S2). Tumor size ≥6 mm was significantly associated with the tumor growth defined by 3 mm and by 2 × 2 mm in multivariate regression analysis (Supplementary Table S4).
Table 3.
Comparison of Clinical Variables in Patients Without or With Progression During Active Surveillance
| No progression | Progression (composite outcomea) | p-Value | Progression (3 mm or new LNM) | p-Valueb | |
|---|---|---|---|---|---|
| Number of patients, n (%) | 638 (90.4) | 68 (9.6) | 49 (6.9) | ||
| Age at diagnosis | |||||
| Age (years) | 49.6 ± 11.7 | 48.7 ± 12.1 | 0.539 | 49.9 ± 13.3 | 0.876 |
| <30 years old, n (%) | 23 (80.0) | 6 (20.0) | 0.051 | 5 (17.9) | 0.024 |
| ≥30 years old, n (%) | 615 (90.8) | 62 (9.2) | 44 (6.7) | ||
| Sex | |||||
| Female, n (%) | 497 (92.6) | 40 (7.4) | <0.001 | 27 (5.2) | <0.001 |
| Male, n (%) | 141 (83.5) | 28 (16.5) | 22 (13.5) | ||
| Hospital | |||||
| A, n (%) | 209 (88.2) | 28 (11.8) | 0.375 | 22 (9.5) | 0.215 |
| B, n (%) | 297 (91.4) | 28 (8.6) | 18 (5.7) | ||
| C, n (%) | 132 (91.7) | 12 (8.3) | 9 (6.4) | ||
| Initial tumor size (largest diameter) | |||||
| Size (mm) | 6.2 ± 1.6 | 6.5 ± 1.4 | 0.051 | 6.7 ± 1.4 | 0.045 |
| <6 mm, n (%) | 289 (92.9) | 22 (7.1) | 0.010 | 13 (4.3) | 0.011 |
| 6≤, ≤10 mm, n (%) | 349 (88.4) | 46 (11.6) | 36 (9.4) | ||
| Subcapsular location of index tumor, n (%) | 285 (45.2) | 35 (51.5) | 0.425 | 24 (49.0) | 0.648 |
| Last tumor size (mm) | 6.1 ± 1.7 | 8.6 ± 2.0 | <0.001 | 8.7 ± 2.0 | <0.001 |
| Duration of follow-up (months) | 40.9 ± 16.1 | 45.9 ± 14.4 | 0.015 | 48.2 ± 13.8 | <0.001 |
Data are presented with mean ± standard deviation or number (%).
Composite outcome was defined as any progression by tumor growth of 3 mm in one dimension, 2 mm in two dimensions, new ETE, or new LNM.
Compared the subjects with progression defined by 3 mm in one dimension or new LNM and those with no progression.
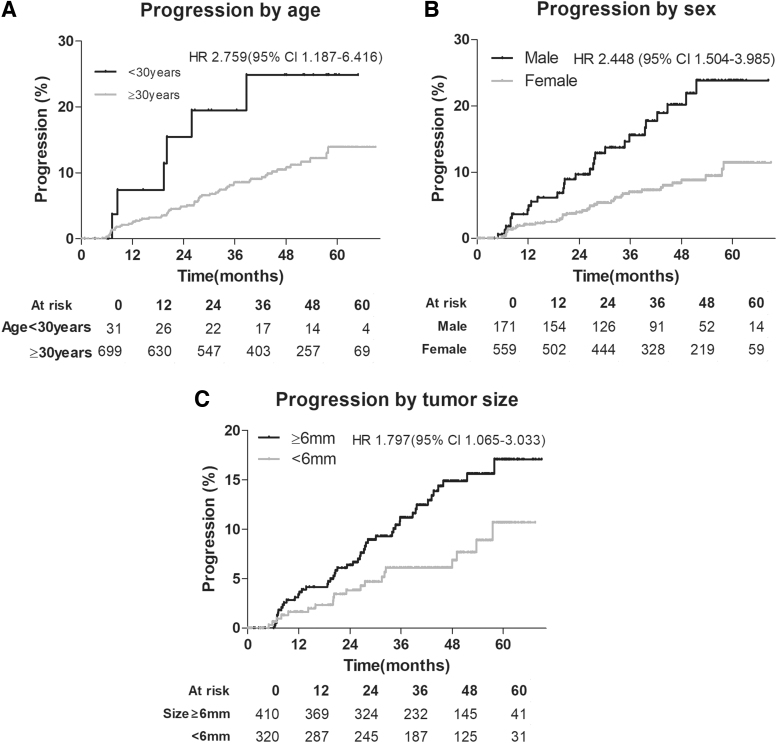
Multivariate logistic regression analysis for predicting a composite outcome showed that age at diagnosis <30 years (odds ratio [OR], 2.86; 95% confidence interval [CI], 1.10 − 7.45), male sex (OR, 2.48; 95% CI, 1.47 − 4.20), and tumor size ≥6 mm (OR, 1.89; 95% CI, 1.09 − 3.27) were significantly independently associated with disease progression under AS (Table 4). Survival and multivariate Cox proportional hazard model analyses according to age (hazard ratio [HR] of age <30 years, 3.2670; 95% CI, 1.280–8.306), sex (HR of male, 2.928; 95% CI, 1.657–5.174), and tumor size groups (HR of tumor size ≥6 mm, 2.068; 95% CI, 1.095–3.907) demonstrated similar results even after adjusting other prognostic factors (Fig. 3 and Supplementary Fig. S3).
Table 4.
Logistic Regression Analysis of the Risk of Tumor Progression
| Progression | Univariate analysis |
Multivariate analysis |
||||||
|---|---|---|---|---|---|---|---|---|
| Composite outcomea |
3 mm or new LNM |
Composite outcomea |
3 mm or new LNM |
|||||
|
n = 68 |
n = 49 |
n = 68 |
n = 49 |
|||||
| B | OR (95% CI) | B | OR (95% CI) | B | OR (95% CI) | B | OR (95% CI) | |
| Age <30 years | 0.95 | 2.59 (1.02 − 6.60) | 1.11 | 3.04 (1.10 − 8.38) | 1.05 | 2.86 (1.10 − 7.45) | 1.25 | 3.48 (1.22 − 9.87) |
| Male | 0.90 | 2.47 (1.47 − 4.14) | 1.01 | 2.87 (1.59 − 5.20) | 0.91 | 2.48 (1.47 − 4.20) | 1.07 | 2.91 (1.59 − 5.33) |
| Size ≥6 mm | 0.70 | 2.01 (1.17 − 3.47) | 0.84 | 2.32 (1.21 − 4.46) | 0.63 | 1.89 (1.09 − 3.27) | 0.77 | 2.16 (1.12 − 4.18) |
Composite outcome was defined as any progression by tumor growth of 3 mm in one dimension, 2 mm in two dimensions, new ETE, or new LNM.
n, number; OR, odds ratio.
FIG. 3.
Survival analysis for progression defined by the composite outcome according to age (A), sex (B), and tumor size (C) groups of PTMC during AS. Cox proportional HRs were adjusted with age at diagnosis, sex, tumor size, or enrolled hospital. A composite outcome was defined by any progression of tumor growth of 3 mm in one dimension, 2 mm in two dimensions, new ETE, or new LNM. CI, confidence interval; HR, hazard ratio.
Reasons for patients under AS undergoing conversion surgery
Of the 706 patients in the AS group, 163 (23.1%) patients underwent conversion surgery after a mean of 20.8 ± 15.3 months of AS follow-up (Fig. 1). Excluding patients who did not disclose their reasons for conversion surgery, the most common reason was significant tumor progression (48 patients, 29.4%) after a median AS duration of 19.1 (range 3.2–56.8) months. The second-most common reason was a patient's fear of progression (90 patients, 55.2%), and the change was made after a median of 10 (range 0–55.7) months of AS. Of them, 18 patients (11.0%) changed their decision followed by subclinical progression (tumor size increment <3 mm in one dimension or <2 mm in two dimensions) after a median of 13.5 (range 4.8–37.7) months.
Other reasons included other comorbidities, such as incidentally finding tumors in other organs, follicular neoplasm in the contralateral lobe of the thyroid, moving abroad, and hyperthyroidism (7 patients, 4.3%), and these patients changed their decision after a median of 6.7 (range 1.4–24.1) months.
Discussion
This study reported the outcomes of 706 patients undergoing AS for low-risk PTMC in Korea. We observed that older patients with smaller tumors preferred AS to surgery as a treatment strategy for low-risk PTMC. During an average follow-up duration of 41.4 ± 16.0 months, the progression rates were 9.6% for our composite disease progression outcome and 6.8% with the criteria (size increment of 3 mm by one dimension or new LNM) of previous studies (Supplementary Table S5). Baseline variables associated with the risk for disease progression under AS were age <30 years, male sex, and tumor size of ≥6 mm.
In research on AS of PTMC from Japan, Ito et al reported that the progression rate was 7.1% with a mean follow-up duration of 60 months where progression was defined by a 3 mm increment or new LNM.12 They reported that younger age and pregnancy were associated with disease progression, whereas older age was not. Tuttle et al reported that in a U.S. study of AS of tumors ≤1.5 cm, the progression rate was 3.8% within 25 months of observation where surgery was recommended if the primary tumor increased 3 mm or more in greatest dimension over baseline or if there was evidence for ETE or nodal or distant metastases.13
In our study, the incidence rate of new LNM was 1.3% with a mean follow-up duration of 41 months, whereas it was 1.2% at 5 years in the report of Ito et al12 and not reported during 25 months by Tuttle et al.13 The estimated 2-year (4.3%) or 5-year (9.6%) progression rates defined by 3 mm or new LNM in this study were relatively higher than that reported by of Tuttle et al (3.8%), even though our patients had smaller tumor sizes at enrollment, or Ito et al (7.1%), respectively. Longer term follow-up is needed to clarify these findings.
In this study, we used both 3 mm and 2 × 2 mm tumor size increment as definitions of tumor progression under AS. Since most thyroid nodule clinical practice guidelines use the criterion of 2 × 2 mm increase in nodule follow-up,7,9 we believe that the two-dimensional measurement may be applied in the follow-up of PTMC. A more sensitive detection of progression conflicts with the goal of AS, which aims at minimal intervention in patients with indolent cancers.21
An observational study adopting the “less sensitive” criterion of 3 mm reported that there was no difference in the pathological prognostic parameters between the immediate and the delayed surgery groups.21 We observed that the progression defined by 3 mm appeared earlier and more frequently than that by 2 × 2 mm, suggesting the latter could be the less sensitive criterion for size increment. Although the intra- and interobserver variation can be larger in the measurement of 2 mm than 3 mm, the high resolution of the ultrasound currently used made the measurement errors smaller than before, and the measurement in this study was performed by the same radiologists based on prospective criteria to minimize the concerns. More studies are needed to determine optimal tumor size enlargement criteria for progression under AS.
With the establishment of AS as an applicable option for low-risk PTMC, shared decision-making based on the individual risks with disease progression is one of the most important issues. In our cohort, a significant association with progression in age <30 years at diagnosis, men, and tumor size of 6 mm or more was observed. Age is considered a selection variable for AS in patients with low-risk PTMC,26 and younger age was suggested as a predictive marker for early progression,12 which was also consistent with our study showing 2.76 times higher progression in patients with age <30 years. Male sex was also reported to be associated with adverse outcomes of papillary thyroid cancer,27 and this was confirmed in our results with a 2.45 times higher progression rate than females.
Regarding tumor size, our results demonstrated that size ≥6 mm was a predictive factor for progression, but the growth activity was maximal in tumors sized 6–9 mm. Also, most (8 of 10) patients who continued AS after progression were stable. An observational study of 824 patients with PTMC on AS, with a median duration of 6.04 years, suggested that most PTMCs demonstrate a significant decrease in growth activity after a 3 mm enlargement in maximal tumor size or 50% in tumor volume.28 Therefore, our findings might provide supportive evidence for the hypothesis that performing surgery immediately after the point of enlargement may be premature.12 A recent recommendation also defined tumor diameter reaching up to 13 mm as one of the key indications for surgery after AS.6 The underlying mechanism remains unknown, and further studies to understand the tumor biology and kinetics are crucial.
The tumor location, especially on the dorsal side, was associated with the risk of invading vital organs including recurrent laryngeal nerve or trachea.29 However, a recent study showed that invasion of the recurrent laryngeal nerve was not observed for tumors <9 mm in diameter, regardless of tumor location,6 and this is consistent with our observations. In this study, in two of three patients who developed ETE during AS, the tumor location was not on the dorsal side, and in the case with a tumor on the dorsal side, voice change or vocal cord palsy was not observed. The progression was not influenced by the subcapsular location of the index tumor in this study. Therefore, the risk assessment of new ETE based on the tumor location should be investigated further.
This study is the first multicenter AS study performed prospectively based on the consensus of the multidisciplinary steering committee to elucidate the progression rate of PTMC. However, there are some limitations to this study. First, this was not a randomized study that baseline features between the AS and iOP groups showed some differences. Second, the criteria for performing FNA for suspicious LNs or defining ETE were at the discretion of the investigator. Nonetheless, to obtain objective validity to exclude LN metastases, we evaluated the status of cervical LNs at the time of diagnosis with a CT scan, which provides a higher diagnostic value and more objective evidence than ultrasound for detecting new LNs in low-risk PTMC.30 Third, unavoidable discrepancies could exist between observers in ultrasound examination. To reduce the variations, two (hospital A) or one (hospitals B and C) experienced radiologists performed the ultrasounds.
Before the initiation, this study protocol was discussed by the four radiologists. If there were adverse events, the radiologists discussed the case, and discrepancies in observations during follow-up were resolved in several consensus meetings. It is important to acknowledge that progression under AS is not the final outcome of low-risk PTMC, but a surrogate indicator for surgery. Also, the paucity of new LNM in our study precluded meaningful analysis of risk factors for that outcome. A longer study follow-up period is needed.
In conclusion, the progression of PTMC during AS in our cohort was low, but slightly higher than that of other populations, confirming the reliability of AS as an alternative treatment strategy in the Korean population. Caution is required when making future medical decisions as the risk of progression under AS may be higher in younger or male patients.
Supplementary Material
Acknowledgments
The authors thank the members of the MAeSTro Study Group for the provision of the MAeSTro Cohort data and Dong-Eun Lee, Biostatistics Collaboration Team, National Cancer Center, Goyang, Korea, for assistance during the design of this study. These findings were presented at the 90th Annual Meeting of the American Thyroid Association (Highlighted Poster 72) on October 2, 2021.
Authors' Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Author Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Information
This study was supported by the Seoul National University Hospital (Research Grant 25-2016-0010) and the National Cancer Center (Research Grant 1810151-3 and 2210521-1).
Supplementary Material
References
- 1. Li M, Dal Maso L, Vaccarella S. Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol 2020;8(6):468–470; doi: 10.1016/S2213-8587(20)30115-7 [DOI] [PubMed] [Google Scholar]
- 2. Ahn HS, Kim HJ, Welch HG. Korea's thyroid-cancer “epidemic”—screening and overdiagnosis. N Engl J Med 2014;371(19):1765–1767; doi: 10.1056/NEJMp1409841 [DOI] [PubMed] [Google Scholar]
- 3. Brito JP, Kim HJ, Han SJ, et al. Geographic distribution and evolution of thyroid cancer epidemic in South Korea. Thyroid 2016;26(6):864–865; doi: 10.1089/thy.2016.0057 [DOI] [PubMed] [Google Scholar]
- 4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70(1):7–30; doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 5. Kang MJ, Won YJ, Lee JJ, et al. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2019. Cancer Res Treat 2022;54(2):330–344; doi: 10.4143/crt.2022.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sugitani I, Ito Y, Takeuchi D, et al. Indications and strategy for active surveillance of adult low-risk papillary thyroid microcarcinoma: Consensus statements from the Japan Association of Endocrine Surgery Task Force on management for papillary thyroid microcarcinoma. Thyroid 2021;31(2):183–192; doi: 10.1089/thy.2020.0330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016;26(1):1–133; doi: 10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yi KH. The Revised 2016 Korean Thyroid Association Guidelines for thyroid nodules and cancers: Differences from the 2015 American Thyroid Association Guidelines. Endocrinol Metab (Seoul) 2016;31(3):373–378; doi: 10.3803/EnM.2016.31.3.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yi KH, Lee EK, Kang H-C, et al. 2016 Revised Korean Thyroid Association Management Guidelines for patients with thyroid nodules and thyroid cancer. Int J Thyroidol 2016;9(2):59–126; doi: 10.11106/ijt.2016.9.2.59 [DOI] [Google Scholar]
- 10. Oh HS, Ha J, Kim HI, et al. Active surveillance of low-risk papillary thyroid microcarcinoma: A multi-center cohort study in Korea. Thyroid 2018;28(12):1587–1594; doi: 10.1089/thy.2018.0263 [DOI] [PubMed] [Google Scholar]
- 11. Kwon H, Oh HS, Kim M, et al. Active surveillance for patients with papillary thyroid microcarcinoma: A single center's experience in Korea. J Clin Endocrinol Metab 2017;102(6):1917–1925; doi: 10.1210/jc.2016-4026 [DOI] [PubMed] [Google Scholar]
- 12. Ito Y, Miyauchi A, Kihara M, et al. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid 2014;24(1):27–34; doi: 10.1089/thy.2013.0367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tuttle RM, Fagin JA, Minkowitz G, et al. Natural history and tumor volume kinetics of papillary thyroid cancers during active surveillance. JAMA Otolaryngol Head Neck Surg 2017;143(10):1015–1020; doi: 10.1001/jamaoto.2017.1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosario PW, Mourao GF, Calsolari MR. Active surveillance in adults with low-risk papillary thyroid microcarcinomas: A prospective study. Horm Metab Res 2019;51(11):703–708; doi: 10.1055/a-1015-6684 [DOI] [PubMed] [Google Scholar]
- 15. Sakai T, Sugitani I, Ebina A, et al. Active surveillance for T1bN0M0 papillary thyroid carcinoma. Thyroid 2019;29(1):59–63; doi: 10.1089/thy.2018.0462 [DOI] [PubMed] [Google Scholar]
- 16. Sanabria A. Active surveillance in thyroid microcarcinoma in a Latin-American cohort. JAMA Otolaryngol Head Neck Surg 2018;144(10):947–948; doi: 10.1001/jamaoto.2018.1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Molinaro E, Campopiano MC, Pieruzzi L, et al. Active surveillance in papillary thyroid microcarcinomas is feasible and safe: Experience at a single Italian center. J Clin Endocrinol Metab 2020;105(3):e172–e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chou R, Dana T, Haymart M, et al. Active surveillance versus thyroid surgery for differentiated thyroid cancer: A systematic review. Thyroid 2022;32(4):351–367; doi: 10.1089/thy.2021.0539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koshkina A, Fazelzad R, Sugitani I, et al. Association of patient age with progression of low-risk papillary thyroid carcinoma under active surveillance: A systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg 2020;146(6):552–560; doi: 10.1001/jamaoto.2020.0368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fukuoka O, Sugitani I, Ebina A, et al. Natural history of asymptomatic papillary thyroid microcarcinoma: Time-dependent changes in calcification and vascularity during active surveillance. World J Surg 2016;40(3):529–537; doi: 10.1007/s00268-015-3349-1 [DOI] [PubMed] [Google Scholar]
- 21. Ito Y, Miyauchi A, Inoue H, et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg 2010;34(1):28–35; doi: 10.1007/s00268-009-0303-0 [DOI] [PubMed] [Google Scholar]
- 22. Nagaoka R, Ebina A, Toda K, et al. Multifocality and progression of papillary thyroid microcarcinoma during active surveillance. World J Surg 2021;45(9):2769–2776; doi: 10.1007/s00268-021-06185-2 [DOI] [PubMed] [Google Scholar]
- 23. Moon JH, Kim JH, Lee EK, et al. Study protocol of multicenter prospective cohort study of active surveillance on papillary thyroid microcarcinoma (MAeSTro). Endocrinol Metab (Seoul) 2018;33(2):278–286; doi: 10.3803/EnM.2018.33.2.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ha EJ, Chung SR, Na DG, et al. 2021 Korean thyroid imaging reporting and data system and imaging-based management of thyroid nodules: Korean society of thyroid radiology consensus statement and recommendations. Korean J Radiol 2021;22(12):2094–2123; doi: 10.3348/kjr.2021.0713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zanocco KA, Hershman JM, Leung AM. Active surveillance of low-risk thyroid cancer. JAMA 2019;321(20):2020–2021; doi: 10.1001/jama.2019.5350 [DOI] [PubMed] [Google Scholar]
- 26. Brito JP, Ito Y, Miyauchi A, et al. A clinical framework to facilitate risk stratification when considering an active surveillance alternative to immediate biopsy and surgery in papillary microcarcinoma. Thyroid 2016;26(1):144–149; doi: 10.1089/thy.2015.0178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shobab L, Burman KD, Wartofsky L. Sex differences in differentiated thyroid cancer. Thyroid 2022;32(3):224–235; doi: 10.1089/thy.2021.0361 [DOI] [PubMed] [Google Scholar]
- 28. Ito Y, Miyauchi A, Kudo T, et al. Kinetic analysis of growth activity in enlarging papillary thyroid microcarcinomas. Thyroid 2019;29(12):1765–1773; doi: 10.1089/thy.2019.0396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Newman SK, Harries V, Wang L, et al. Invasion of a recurrent laryngeal nerve from small well-differentiated papillary thyroid cancers: Patient selection implications for active surveillance. Thyroid 2022;32(2):164–169; doi: 10.1089/thy.2021.0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee DH, Kim YK, Yu HW, et al. Computed tomography for detecting cervical lymph node metastasis in patients who have papillary thyroid microcarcinoma with tumor characteristics appropriate for active surveillance. Thyroid 2019;29(11):1653–1659; doi: 10.1089/thy.2019.0100 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.