Abstract
Background:
The change in size of the papillary thyroid cancer (PTC) nodule during active surveillance has traditionally been characterized as either stable, increasing, or decreasing based on changes in maximal tumor diameter or tumor volume. More recently, it has been observed that the changes in tumor size observed during observation are more complex with tumor volume kinetic patterns that can be characterized either as stable (Pattern I), early increase in volume (Pattern II), later increase in volume (Pattern III), early increase in volume followed by stability (Pattern IV), stability followed by an increase in volume (Pattern V), or a decrease in tumor volume (Pattern VI).
Methods:
The frequency, time course, and clinical correlates of these six tumor volume kinetic patterns were analyzed in a cohort of 483 patients with low-risk PTC up to 1.5 cm in maximal diameter followed with active surveillance at our center for a median of 3.7 years.
Results:
The cumulative incidence of an increase in tumor volume for the entire cohort was 15.9% [confidence interval (CI) 11.8–20.0] at 5 years. At 5 years, most tumors demonstrated stability (78.8%, Pattern I) with 10.0% showing early growth (Pattern II), 4.1% late growth (Pattern III), 1.9% growth then stability (Pattern IV), 0.6% stability then growth (Pattern V), and 5.6% with a decrease in tumor volume (Pattern VI). Tumor volume doubling time during exponential growth significantly differed across the kinetic patterns, with median values of 2.4, 7.1, and 3.3 years for Patterns II, III, and IV, respectively (p < 0.01). Similarly, the time to a change in tumor volume was significantly different across the kinetic patterns, with median values of 1.5, 3, 1.6, 4.7, and 4.1 years for Patterns II, III, IV, V, and VI, respectively (analysis of variance, p < 0.01). Clinical correlates at baseline were not associated with tumor volume kinetic pattern.
Conclusions:
These six kinetic tumor volume patterns provide a comprehensive description of the changes in PTC tumor volume observed during the first 5 years of active surveillance.
Keywords: active surveillance, papillary thyroid cancer, tumor kinetic volume, tumor progression
Introduction
Active surveillance is considered a safe and effective management option for properly selected patients with low-risk papillary thyroid cancer (PTC).1–10 These studies have demonstrated that a ≥ 3 mm increase in maximal diameter and/or ≥50–100% increase in tumor volume can be used as clinically relevant indicators of tumor growth.1,4,5,8,10–13 However, because of the small nodule size, poorly defined margins, and irregular shapes often encountered in papillary thyroid microcarcinoma, Chung et al found that differences up to ±72% in tumor volume or ±24% in maximum diameter were within the serial measurement error on ultrasound further refining the parameters necessary to determine if a nodule is stable, decreasing, or increasing in size during observation.14
Prior publications have described three common tumor volume kinetic patterns in low-risk PTC cases on active surveillance: (1) a steady exponential increase in tumor volume, (2) a quiescent phase of apparently stable tumor volumes not varying by more than the expected degree of measurement variation, and (3) a decrease over time.13,15–19 More recent publications have differentiated exponential patterns as short versus long tumor volume doubling times (TVDT),12,15,16,19 with a TVDT of <5 years associated with a higher probability of disease progression and lymph node metastasis.15,19 Ito et al20 identified a subset of patients whose tumor volume doubling rates declined after tumors had increased by ≥3 mm in diameter or ≥50% in volume, suggesting that continued exponential increase in tumor volume may not be inevitable.
To better conceptualize how tumor volume kinetic patterns should guide clinical management of low-risk PTC, we developed a classification system for six common tumor volume kinetic patterns seen in our center's active surveillance program (Table 1) and assessed the frequency and time course of these patterns.
Table 1.
Six Most Common Tumor Volume Kinetic Growth Patterns Observed in Patients on Active Surveillance for Papillary Thyroid Cancer or Suspicious for Papillary Thyroid Cancer
| Tumor volume kinetic growth pattern | Description | Definition | Implications for clinical management |
|---|---|---|---|
| I | Stable | Tumor volume measurements on observation remain stable (up to ±72% change in tumor volume from baseline) | Continue active surveillance |
| II | Early increase in tumor volume | Steady exponential growth from time of diagnosis, with a TVDT of <5 years | Consider (1) transition to a therapeutic intervention or (2) continue observation depending on tumor size, location, rate of change, and patient preference |
| III | Later increase in tumor volume | Steady exponential growth from the time of diagnosis, with a TVDT of ≥5 years | Consider (1) transition to a therapeutic intervention or (2) continue observation depending on tumor size, location, rate of change, and patient preference |
| IV | Early increase in tumor volume followed by stability | Steady exponential growth from the time of diagnosis followed by transition to stability (tumor volume remains up to ±72% of size after exponential growth window) | Continue active surveillance |
| V | Stability followed by increase in tumor volume | Initial stability (tumor volume remains up to ±72% of baseline) with a subsequent transition to steady exponential growth | Transition to therapeutic intervention |
| VI | Decrease in tumor volume | Tumor volume decreases >72% from baseline | Continue active surveillance |
TVDT, tumor volume doubling time.
Materials and Methods
Study design and population
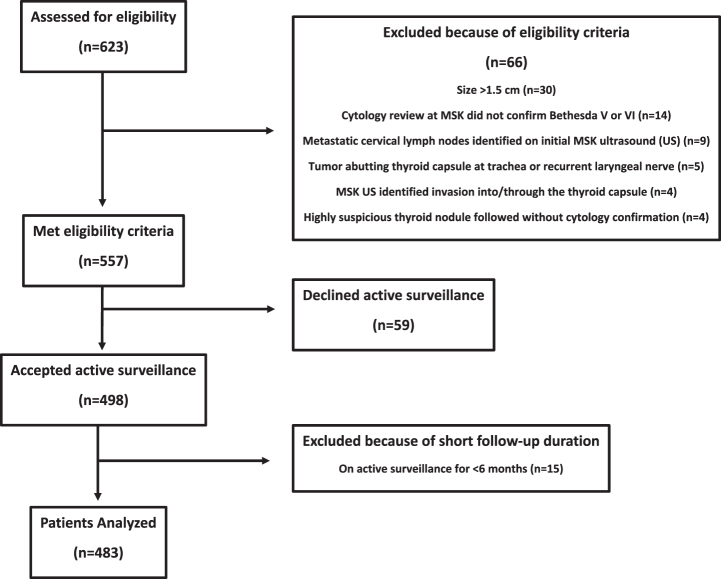
We assessed a cohort of 623 patients with PTC followed on active surveillance at Memorial Sloan Kettering Cancer Center (MSK) for eligibility. Ultimately, based on inclusion criteria, a cohort of 483 patients with low-risk PTC was analyzed for this study (see Fig. 1). Inclusion criteria for active surveillance at MSK have been previously described: a diagnosis of PTC or suspicious for PTC (based on cytology, molecular, and ultrasonographic findings confirmed at MSK) with a maximal tumor diameter at baseline of ≤1.5 cm and no clinical or radiographic evidence of extrathyroidal extension, invasion of local structures, or regional/distant metastases.13
FIG. 1.
Flow diagram of participant enrollment.
The cohort consisted of patients enrolled onto a prospective data collection protocol (n = 394 patients) and patients included on the basis of retrospective data collection (n = 89 patients) from the medical records in patients that met the same inclusion, exclusion, and follow-up criteria. The protocol was approved by the MSK Institutional Review Board (IRB) and Privacy Board (PB)-B and the study was performed in accordance with the Declaration of Helsinki as revised in 2013 (see Methods Supplementary S1). All ultrasound evaluations were performed by academic radiologists at MSK.
Additional criteria for inclusion included patients who were (1) followed by an endocrinologist and/or head and neck surgeon at MSK for ≥6 months, and (2) had neck ultrasonography performed at 6-month intervals for the first 2 years, yearly for the next 3 years, and then approximately every 2 years for ongoing follow-up. Levothyroxine was prescribed to patients as needed to maintain a thyrotropin (TSH) level <3 mIU/L while avoiding TSH suppression. Methods for measurement of tumor volume kinetics are described in the Methods Supplementary S1.
Statistical analysis
Continuous data are presented as mean and standard deviation or median and interquartile range, as appropriate for each variable. Tumor growth rates were calculated by fitting a least squares regression model to log-transformed tumor volume measurements and plotted on semi-log curves. The volume (mL) of the tumor was calculated using the ellipsoid volume formula: π/6 × length × width × height. Percentage change in volume and maximal tumor diameter was calculated relative to the baseline before the initial biopsy by fine needle aspiration (FNA). A meaningful change in tumor volume was defined as a >72% increase or decrease in tumor volume from baseline.14
The cumulative incidence of lymph node metastasis, volume growth, and tumor kinetic patterns was calculated using the Kaplan–Meier method, and statistical testing performed using the log-rank test or univariate Cox regression analysis. Categorical variables were compared using chi-squared analysis and continuous variables compared using either unpaired t-test or analysis of variance (ANOVA) as appropriate. In all analyses, p-values <0.05 were considered statistically significant. Analyses were performed using SPSS Statistics version 27.0 (IBM Corporation, Armonk, NY, USA).
Results
Study cohort
The cohort comprised 483 patients followed on active surveillance for a median of 3.7 years (interquartile range [IQR] 2.2–5.6 years, range 0.5–17 years) (Table 2). With a median age at diagnosis of 52 years, the majority were female (372/483 [77%]), with a definitive diagnosis of PTC (386/483 [80%]) and tumor nodules ≤1 cm in maximal diameter (361/483 [75%]).
Table 2.
Clinicopathologic Features of the Study Cohort
| Variable | Value (%) (participant N = 483) |
|---|---|
| Age at diagnosis (years) | |
| Mean, SD | 52, ±15 |
| Median | 52 |
| Range | 20–89 |
| Tumor size | |
| ≤1 cm | 361 (75) |
| 1.1–1.5 cm | 122 (25) |
| Sex | |
| Female | 372 (77) |
| Male | 111 (23) |
| Diagnosis | |
| PTC | 386 (80) |
| Suspicious for PTC | 97 (20) |
| Duration of active surveillance (years) | |
| Mean, SD | 4, ±2.3 |
| Median | 3.7 |
| Range | 0.5–17 |
PTC, papillary thyroid cancer; SD, standard deviation.
Outcomes
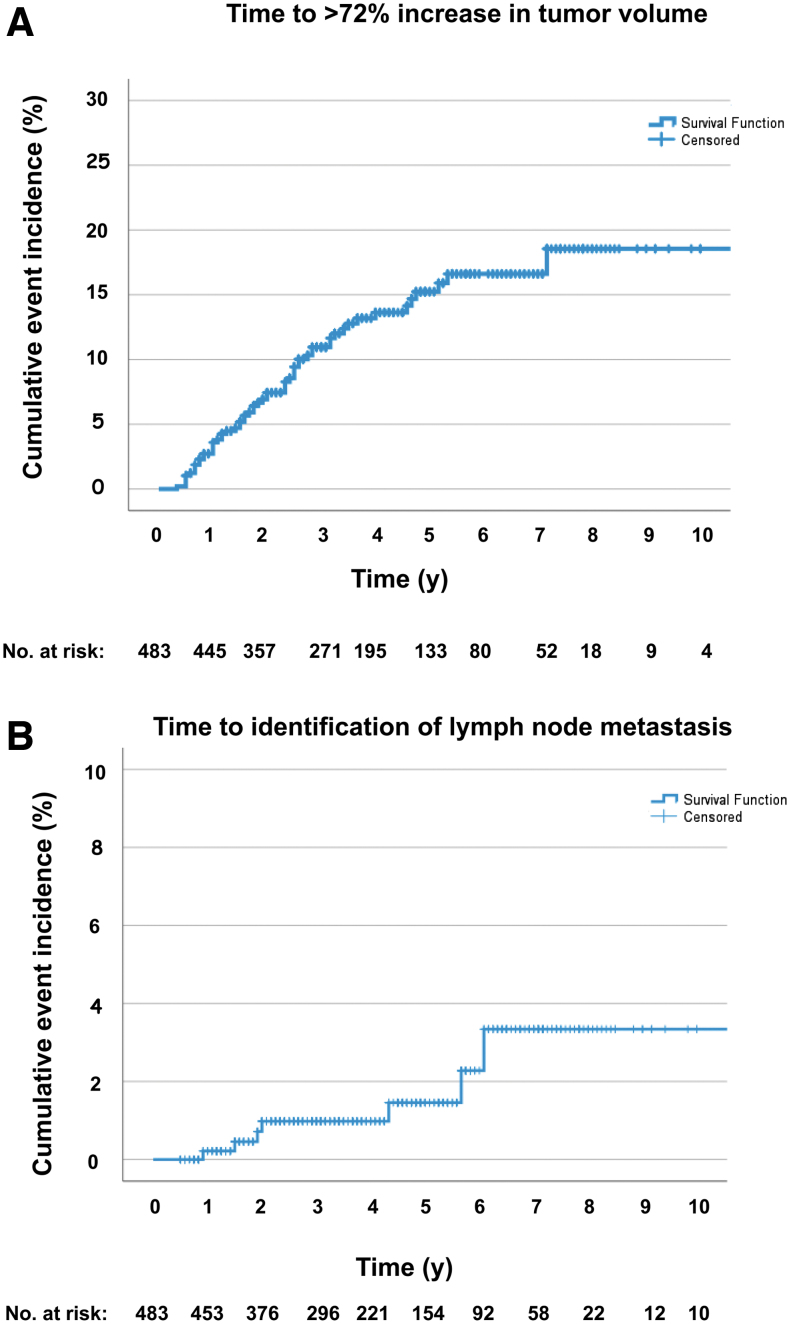
The cumulative incidence of tumor volume increase of >72% was 15.9% at 5 years [CI 11.8–20.0]. Seven of 483 patients (1.4%) developed newly identified cervical lymph node metastases while on observation. The cumulative incidence of lymph node metastasis was 1.5% at 5 years [CI 0.1–2.9] (Fig. 2).
FIG. 2.
Kaplan–Meier curves showing (A) cumulative event rates for increase in tumor volume of >72% and (B) identification of lymph node metastases during active surveillance.
At last follow-up, 82.2% (397/483) of the cohort continued on active surveillance following their individualized management plans while 9.3% (45/483) transitioned to surgical intervention. There were 1.7% (8/483) of patients lost to follow-up, 2.5% (12/483) died of unrelated causes, and 4.3% (21/483) transferred their care to community clinicians after a median observation period at MSK of 2.6 years (IQR 1.8–3.2 years). None of the cases showed clinical or radiological evidence of distant metastasis or locoregionally advanced disease. There were no deaths related to thyroid cancer.
Tumor volume kinetics
Baseline and changes on active surveillance in maximal tumor diameter and volume are presented in Table 3. Tumors were classified as increased, stable, or decreased in size at similar rates when cutoff values defining stable tumors were ±3 mm change in maximal tumor diameter, ±24% change in maximal tumor diameter, or ±72% change in tumor volume. In tumors with increased volume, analysis of kinetic curves demonstrated a classic exponential growth pattern with a median TVDT of 3.1 years (IQR 1.6–5.1 years, r = 0.79) (TVDT for each of the patterns are presented in Table 4).
Table 3.
Tumor Volume Kinetics of Study Cohort
| Variable | Value (%) (N = 483) |
|---|---|
| Maximal diameter at baseline (mm) | |
| Mean, SD | 8.5, ±2.7 |
| Median | 8 |
| Range | 3–15 |
| Maximal diameter at final follow-up (mm) | |
| Mean, SD | 8.6, ±3.3 |
| Median | 8 |
| Range | 2–24 |
| Tumor volume nodule at baseline (mm3) | |
| Mean, SD | 271, ±252 |
| Median | 178 |
| Range | 6–1521 |
| Tumor volume nodule at final follow-up (mm3) | |
| Mean, SD | 294, ±337 |
| Median | 168 |
| Range | 4–2389 |
| Change in tumor volume >72% from baseline | |
| Increase | 59 (12) |
| Stable | 401 (83) |
| Decrease | 23 (5) |
| Change in maximal diameter >3 mm from baseline | |
| Increase | 42 (9) |
| Stable | 407 (84) |
| Decrease | 34 (7) |
| Change in maximal diameter >24% from baseline | |
| Increase | 64 (13) |
| Stable | 362 (75) |
| Decrease | 57 (12) |
| TVDT for enlarging tumors (years) | 53 (11) |
| Mean, SD | 4.3, ±5.4 |
| Median | 3.1 |
| Range | 1–37 |
Table 4.
Cumulative Incidence and Time Course of the Six Tumor Kinetic Patterns in Study Cohort
| Pattern I: Stable (N = 401) | Pattern II: Early increase in tumor volume (N = 40) | Pattern III: Later increase in tumor volume (N = 12) | Pattern IV: Early increase in tumor volume followed by stability (N = 6) | Pattern V: Stability followed by increase in tumor volume (N = 1) | Pattern VI: Decrease in tumor volume (N = 23) | |
|---|---|---|---|---|---|---|
| Age at diagnosis (years) | ||||||
| Median | 53 | 47 | 45 | 50 | 50 | 55 |
| Mean, SD | 53, ±15 | 49, ±15 | 46, ±8 | 54, ±20 | NA | 53, ±16 |
| Range | 21–87 | 20–89 | 31–59 | 31–77 | NA | 27–82 |
| 5-Year cumulative incidence % [CI] | 78.8 [74.1–83.5] | 10.0 [6.9–13.1] | 4.1 [1.14–6.8] | 1.9 [0.3–3.5] | 0.6 [0.0–1.8] | 5.6 [2.7–8.5] |
| Duration of active surveillance (years)a | ||||||
| Median | 3.4 | 3.5 | 6.4 | 5.6 | 5.4 | 4.9 |
| Mean, SD | 3.9, ±2.3 | 3.9, ±2.5 | 6.8, ±1.7 | 5.0, ±2 | NA | 5.0, ±3.3 |
| Range | 0.5–14.3 | 0.8–14.0 | 4.8–11.0 | 1.7–7.1 | NA | 1.2–17.0 |
| Time to increase or decrease in tumor volume (years)a | ||||||
| Median | NA | 1.5 | 3.0 | 1.6 | 4.7 | 4.1 |
| Mean, SD | NA | 1.7, ±1.1 | 3.3, ±1.8 | 2.1, ±1.1 | NA | 3.8, ±1.8 |
| Range | NA | (0.3–5.3) | (0.5–7.2) | (1–3.4) | NA | (1.2–6.4) |
| TVDT during exponential growth phase (years) | ||||||
| Median | NA | 2.4 | 7.1 | 3.3 | 0.7 | NA |
| Mean, SD | NA | 2.4, ±1.2 | 10.1, ±9 | 3.2, ±1.4 | NA | NA |
| Range | NA | 1–5 | 5.2–37 | 1.6–4.5 | NA | NA |
ANOVA, p < 0.01.
ANOVA, analysis of variance; CI, confidence interval; NA, not applicable.
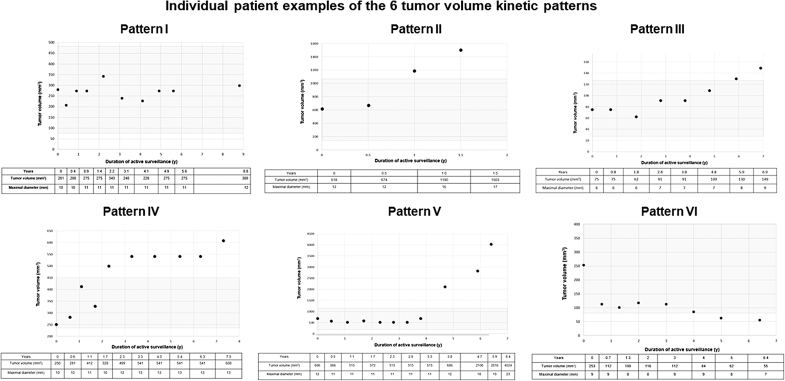
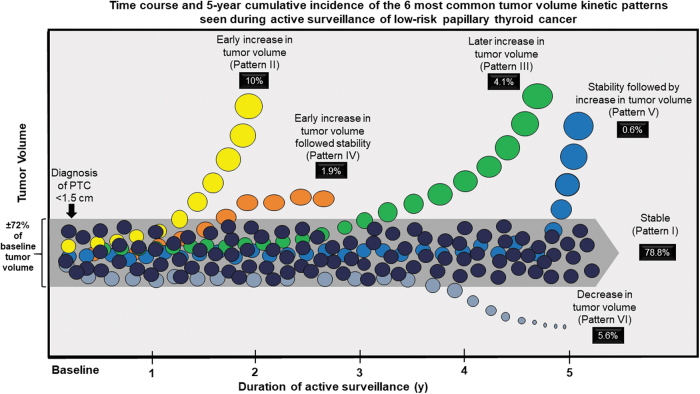
Individual patient examples of each tumor kinetic pattern with corresponding tumor diameters and tumor volumes are shown in Figure 3. Table 4 and Figure 4 present the cumulative incidence rates, duration of active surveillance, time to increase in tumor volume, and TVDT for each tumor volume kinetic pattern. Stability (Pattern I) was the most common outcome at 5 years of follow-up (78.8%) while an early increase in tumor volume (Pattern II) was seen in 10%, a later increase in tumor volume was seen in 4.1% (Pattern III), an early increase in volume followed by stability in 1.9% (Pattern IV), stability followed by increase in 0.6% (Pattern V), and a decrease in volume (pattern VI) was seen in 5.6%.
FIG. 3.
Individual patient examples of each of the six tumor volume kinetic patterns. The gray area represents ±72% of baseline tumor volume.
FIG. 4.
Graphic representation of the time course and cumulative incidence of the six tumor volume kinetic patterns.
Initial stability followed by increased tumor volume was an uncommon event seen in a 50-year-old male (<1% of patients), who required surgery for a BRAF V600E mutated unifocal poorly differentiated PTC with oncocytic features without angioinvasion or extrathyroidal extension.
Identification of lymph node metastasis while on active surveillance was not related to patients' tumor kinetic pattern. Of the seven patients with newly identified lymph node metastases during active surveillance, five demonstrated Pattern I, 1 had Pattern II, and 1 had Pattern III.
Baseline clinicopathologic characteristics were not predictive of the tumor kinetic patterns (I–VI) or increase in tumor volume of >72%. Baseline clinicopathologic characteristics comprised the covariates of age at diagnosis, sex, tumor size category (≤1 cm vs. 1.1–1.5 cm), baseline tumor volume/maximal tumor diameter, presence/absence of lymph node metastasis on observation, cytology (PTC vs. suspicious for PTC), family history of thyroid cancer, personal history of another malignancy or radiation exposure, autoimmune thyroid disease, pregnancy during active surveillance, fluorodeoxyglucose avidity, or multifocality (data not shown).
As shown in Table 4, the time to detection of a change in tumor volume varied from a median of 1.5 years in Pattern II, to 3.0 years in Pattern III, and 4.1 years in Pattern VI. Of interest, the TVDT during exponential growth of 2.4 years in Pattern II and 3.3 years in Pattern IV allowed the majority of these cases to be recognized and properly categorized in the early years of follow-up, while the TVDT of 7.1 years in Pattern III means that slower rates of tumor progression may not be recognized until these patients are observed for longer periods of time. The duration of active surveillance was statistically different across the patterns, with shorter durations seen in Patterns I–II as compared with the longer follow-up durations demonstrated in Patterns III–VI (ANOVA, p < 0.01).
Discussion
We describe the time course and cumulative incidence of the six most common tumor volume kinetic growth patterns observed in 483 patients with low-risk PTC undergoing active surveillance for tumors of <1.5 cm for a median follow-up of 3.7 years. As proposed in the implications for clinical management in Table 1, this description of the frequency and time course of these tumor growth patterns can inform and enhance the shared decision-making process by physicians and patients, which Davies et al describe as analogous to a journey in which a guide (clinician) advises a traveler (patient) on options, advocating for specific paths, supporting previous decisions, or recommending alternatives as conditions change.21
While Table 1 describes the implications for clinical management based only on the tumor volume kinetic patterns, it is also important to understand that other clinical factors, such as the identification of metastatic cervical lymph nodes, evidence of direct invasion into the thyroid capsule, identification of other PTC foci within the thyroid gland, alterations in the angle of the tumor with the thyroid capsule, or change in patient preference could also lead to recommendations to transition from active surveillance to therapeutic intervention.
Using three definitions of tumor growth (>3 mm increase in maximal diameter, >24% increase in maximal diameter, and >72% increase in tumor volume), we report a similar percentage of thyroid cancer nodules (9–13%) as increasing during observation. These findings support the use of either the well-established >3 mm increase in size, or the recently validated reproducibility data specific to papillary microcarcinoma nodules (>24% increase in maximal diameter or >72% increase in tumor volume) as clinically applicable practical definitions of tumor growth with equivalent effectiveness.
We found that most PTCs do not demonstrate an increase in tumor volume at 5 years (84%). Interestingly, a comparison of the 10-year cumulative incidence shows that the likelihood of decreased tumor volume (14.2%) was nearly as high as the likelihood of increased tumor volume (19%). Furthermore, while on active surveillance, no patients developed distant metastases or locoregionally unresectable disease. Only 7 of 483 patients (1.4%; 10-year cumulative incidence of 3.3%) had cervical lymph node metastasis identified during observation, comparable with the 3–5% probability of lymph node metastasis after thyroidectomy in patients with papillary microcarcinoma.22
These data from a United States cancer center are similar to findings from other centers globally and confirm that patients with relatively small intrathyroidal PTCs can be safely offered active surveillance as an alternative to immediate surgery, without significant risk of clinically problematic local, regional, or distant progression.2 We also expand upon prior reports of active surveillance for microcarcinoma by including tumors up to 1.5 cm in maximal diameter.
Ongoing follow-up is required for patients on active surveillance as the observed growth patterns need to be re-evaluated at each follow-up using a dynamic risk assessment approach to risk stratification. For example, tumors that appear to be stable (Pattern I; variation in tumor volume ≤72%) in the first few years of follow-up could be very slowly growing (with prolonged TVDTs) and could transition to Pattern III (later increase in tumor volume) after longer observation. These data are also consistent with the findings by Miyauchi et al16 and Kasahara et al23 indicating that tumors classified as stable and suitable for active surveillance may have had a previously unobserved exponential growth pattern before entering the quiescent phase before diagnosis.
Interestingly, Ito et al20 observed that tumor growth rates sometimes decrease after tumors enlarge >3 mm, consistent with a transition from an early increase in tumor volume to stability (Pattern IV). Thus, the distribution of kinetic growth patterns observed during active surveillance will vary based on both the starting point and duration of active surveillance with respect to the natural history of PTC, indicating that a dynamic risk assessment approach to ongoing management will be required.
An increase in tumor volume (Pattern II and Pattern III) should not always be considered an immediate indication for surgery. While some patients elect to transition from active surveillance to therapeutic intervention as soon as the nodule is documented to increase >72% in volume, in our experience, others prefer to delay intervention if possible. We consider continued active surveillance to be a reasonable management option for tumors with a documented increase in volume provided the tumor growth rate is slow (TVDT >2–3 years), the tumor is not in a worrisome location, and delayed intervention is acceptable to the clinician and to the patient.8,10,12
After two years of follow-up, most patients transition to yearly ultrasound examinations and follow-up. However, the interval between ultrasound evaluations is shortened to every six months if there is any question that a nodule may be exhibiting an increase in size. Similarly, to minimize the interobserver variation as much as possible, patients who transitioned to outside follow-up return to receive an ultrasound at MSK if the outside imaging suggests an increase in tumor volume.
Fortunately, the pattern of transition from prolonged periods of apparently stable disease to exponential increase in tumor volume (Pattern V) appears to be uncommon during the initial observation period, occurring in only 1 of 483 patients. While uncommon, this is not unexpected as rapid growth after stability can be seen in patients with known lymph node or distant metastasis. While not fully understood, this unexpected sudden transition may be associated with changes within the thyroid cancer cell, in the surrounding microenvironment, or in the host immune response.
Most active surveillance studies report a decrease in tumor volume or tumor diameter in 5–10% of patients with PTC being followed on observation. Often there is an early decrease in tumor volume of 25–50% in the first year after biopsy, followed by a slower decline in volume for many years, reaching the 72% decreased volume threshold at a median of 4.1 years of observation (Fig. 4). While the cause of decreased tumor volume with time is not known, anecdotally, it is interesting that many patients attribute the stability and/or decrease in tumor size to the major lifestyle, nutrition, exercise, and health food supplement changes often made after diagnosis; it is also possible that the FNA biopsy itself disrupts tumor vasculature or immune system recognition.
Unlike previous studies,15,19 we found no association between rapid TVDT and lymph node metastases or age at diagnosis. However, the small number of events (7/483 patients with lymph node metastases identified during follow-up) limits the generalizability of our findings, which should be further evaluated with larger studies and longer follow-up. We also found no clinical covariates associated with tumor volume kinetic patterns. While previous studies have demonstrated an inverse relationship between age and increase in tumor size, the relatively small numbers of patients in the various groups we studied may have hampered our ability to detect a statistical difference in age at diagnosis across the patterns. Since these patterns may change over time within an individual and could differ depending on the observed timepoint during the natural history of the thyroid cancer, it is perhaps not surprising that baseline clinical characteristics did not reliably predict tumor volume changes while patients were on active surveillance.
As with any clinical study, we recognize that our findings may be influenced by significant limitations. While we believe that these volume changes and patterns can be utilized across a wide variety of clinical settings, it is important to acknowledge that the patients included in this study had ultrasound evaluations performed by radiologist working in an academic medical center. In addition, the nodule size determinations were obtained from the clinical radiology report and not independently reverified by a single investigator.
Data were not available to evaluate other potentially predictive ultrasonographic features (e.g., rim calcification, other patterns of calcification, echogenicity, margins, or nodule location). Unlike many thyroid centers, MSK does not routinely use the TIRADS system for thyroid nodule descriptions and thus we were unable to analyze our data based on TIRADS criteria.
In conclusion, rather than simply describing the natural history of low-risk PTCs on active surveillance as either stable, increasing, or decreasing in size, the kinetic tumor volume patterns in our classification system provide a more clinically relevant description of the frequency and time course of the changes in tumor volume that are seen during observation. Integration of these six tumor volume kinetic patterns into a clinical framework that also considers tumor size, tumor location, and patient preference can inform individualized management decisions by differentiating identifiable from actionable findings.24
Acknowledgments
Editorial assistance was provided by Katharine Olla, MA, and Hannah Rice, BA, ELS.
Authors' Contributions
Conceptualization (equal), methodology (equal), data curation (lead), formal analysis (equal), funding acquisition (equal), supervision, validation (equal), visualization (equal), and writing—original draft (lead) by R.M.T. Conceptualization (equal), funding acquisition (equal), review, and editing (equal) by J.F. Investigation (equal) and writing—review and editing (equal) by G.M., D.L., A.B., J.G., O.L., J.-M.C., R.G., and G.M. Writing—review and editing (equal) by R.W., S.P., I.G., A.S., J.S., M.C., J.C., M.S., L.B., and S.F. Methodology (equal) and writing—review and editing (equal) by B.R. and B.U. Conceptualization (equal), methodology (equal), formal analysis (equal), validation (equal), visualization (equal), and writing—review and editing (equal) by L.M.
Author Disclosure Statement
R.M.T.: THANC Foundation, Inc. (provision of services). J.F.: Endocrine Society, Journal of the Endocrine Society, Loxo Oncology (provision of services); International Thyroid Oncology Group (provision of services [uncompensated]); Kura Oncology (intellectual property rights). R.W., B.R., I.G., A.S., D.L., A.B., J.G., J.-M.C., J.C., R.G., M.S., L.B., and S.F.: have no disclosures. S.P.: ColdSteel Laser, LLC (intellectual property rights, ownership/equity interests, provision of services [uncompensated]); Elucida Oncology (intellectual property rights); Summit Biomedical Imaging, LLC (fiduciary role/position, intellectual property rights, ownership/equity interests). B.U.: Kura Oncology (intellectual property rights). J.S.: Sechenov University (provision of services). O.L.: Hologic, Janssen Research & Development, LLC (provision of services). M.C.: UroGen Pharma, Inc. (provision of services). L.M.: Personal Genome Diagnostics (intellectual property rights).
Disclaimer
The authors will honor any reasonable request for materials, methods, or data necessary to reproduce or validate the research findings during peer review unless it violates the privacy or confidentiality of human subjects' research.
Funding Information
Funding was provided by the NIH/NCI Specialized Program of Research Excellence (SPORE) in Thyroid Cancer at Memorial Sloan Kettering Cancer Center (PIs: R.M.T. and M.B.) Project 1: Genomic predictors of papillary microcarcinoma disease progression (P50 CA172012-01A1). Further institutional support was provided at Memorial Sloan Kettering Cancer Center by the NIH/NCI Cancer Center Support Grant P30 CA008748. L.M.: NIH R01 DE027738, the Jayme and Peter Flowers Fund. Remaining authors have no funding to report.
Supplementary Material
Methods Supplementary S1
References
- 1. Cho SJ, Suh CH, Baek JH, et al. Active surveillance for small papillary thyroid cancer: A systematic review and meta-analysis. Thyroid 2019;29(10):1399–1408; doi: 10.1089/thy.2019.0159 [DOI] [PubMed] [Google Scholar]
- 2. Chou R, Dana T, Haymart MR, et al. Active surveillance versus thyroid surgery for differentiated thyroid cancer: A systematic review. Thyroid 2022;32(4):351–367; doi: 10.1089/thy.2021.0539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016;26(1):1–133; doi: 10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Horiguchi K, Yoshida Y, Iwaku K, et al. Position paper from the Japan Thyroid Association task force on the management of low-risk papillary thyroid microcarcinoma (T1aN0M0) in adults. Endocr J 2021;68(7):763–780; doi: 10.1507/endocrj.EJ20-0692 [DOI] [PubMed] [Google Scholar]
- 5. Ito Y, Miyauchi A.. Active surveillance as first-line management of papillary microcarcinoma. Annu Rev Med 2019;70:369–379; doi: 10.1146/annurev-med-051517-125510 [DOI] [PubMed] [Google Scholar]
- 6. Ito Y, Miyauchi A, Oda H. Low-risk papillary microcarcinoma of the thyroid: A review of active surveillance trials. Eur J Surg Oncol 2018;44(3):307–315; doi: 10.1016/j.ejso.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 7. Ma T, Semsarian CR, Barratt A, et al. Rethinking low-risk papillary thyroid cancers <1cm (papillary microcarcinomas): An evidence review for recalibrating diagnostic thresholds and/or alternative labels. Thyroid 2021;31(11):1626–1638; doi: 10.1089/thy.2021.0274 [DOI] [PubMed] [Google Scholar]
- 8. Molinaro E, Campopiano MC, Elisei R. Management of endocrine disease: Papillary thyroid microcarcinoma: Toward an active surveillance strategy. Eur J Endocrinol 2021;185(1):R23–R34; doi: 10.1530/EJE-21-0256 [DOI] [PubMed] [Google Scholar]
- 9. Ramundo V, Sponziello M, Falcone R, et al. Low-risk papillary thyroid microcarcinoma: Optimal management toward a more conservative approach. J Surg Oncol 2020;121(6):958–963; doi: 10.1002/jso.25848 [DOI] [PubMed] [Google Scholar]
- 10. Sugitani I, Ito Y, Takeuchi D, et al. Indications and strategy for active surveillance of adult low-risk papillary thyroid microcarcinoma: Consensus Statements from the Japan Association of Endocrine Surgery Task Force on management for papillary thyroid microcarcinoma. Thyroid 2021;31(2):183–192; doi: 10.1089/thy.2020.0330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brauer VF, Eder P, Miehle K, et al. Interobserver variation for ultrasound determination of thyroid nodule volumes. Thyroid 2005;15(10):1169–1175; doi: 10.1089/thy.2005.15.1169 [DOI] [PubMed] [Google Scholar]
- 12. Campopiano MC, Matrone A, Rago T, et al. Assessing mPTC progression during active surveillance: Volume or diameter increase? J Clin Med 2021;10(18):4068; doi: 10.3390/jcm10184068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tuttle RM, Fagin JA, Minkowitz G, et al. Natural history and tumor volume kinetics of papillary thyroid cancers during active surveillance. JAMA Otolaryngol Head Neck Surg 2017;143(10):1015–1020; doi: 10.1001/jamaoto.2017.1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chung SR, Choi YJ, Lee SS, et al. Interobserver reproducibility in sonographic measurement of diameter and volume of papillary thyroid microcarcinoma. Thyroid 2021;31(3):452–458; doi: 10.1089/thy.2020.0317 [DOI] [PubMed] [Google Scholar]
- 15. Jin M, Kim HI, Ha J, et al. Tumor volume doubling time in active surveillance of papillary thyroid microcarcinoma: A multicenter cohort study in Korea. Thyroid 2021;31(10):1494–1501; doi: 10.1089/thy.2021.0094 [DOI] [PubMed] [Google Scholar]
- 16. Miyauchi A, Kudo T, Ito Y, et al. Natural history of papillary thyroid microcarcinoma: Kinetic analyses on tumor volume during active surveillance and before presentation. Surgery 2019;165(1):25–30; doi: 10.1016/j.surg.2018.07.045 [DOI] [PubMed] [Google Scholar]
- 17. Nagaoka R, Ebina A, Toda K, et al. Multifocality and progression of papillary thyroid microcarcinoma during active surveillance. World J Surg 2021;45(9):2769–2776; doi: 10.1007/s00268-021-06185-2 [DOI] [PubMed] [Google Scholar]
- 18. Oh HS, Ha J, Kim HI, et al. Active surveillance of low-risk papillary thyroid microcarcinoma: A multi-center cohort study in Korea. Thyroid 2018;28(12):1587–1594; doi: 10.1089/thy.2018.0263 [DOI] [PubMed] [Google Scholar]
- 19. Oh HS, Kwon H, Song E, et al. Tumor volume doubling time in active surveillance of papillary thyroid carcinoma. Thyroid 2019;29(5):642–649; doi: 10.1089/thy.2018.0609 [DOI] [PubMed] [Google Scholar]
- 20. Ito Y, Miyauchi A, Kudo T, et al. Kinetic analysis of growth activity in enlarging papillary thyroid microcarcinomas. Thyroid 2019;29(12):1765–1773; doi: 10.1089/thy.2019.0396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Davies L, Chang CH, Sirovich B, et al. Thyroid cancer active surveillance program retention and adherence in Japan. JAMA Otolaryngol Head Neck Surg 2021;147(1):77–84; doi: 10.1001/jamaoto.2020.4200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mazzaferri EL. Management of low-risk differentiated thyroid cancer. Endocr Pract 2007;13(5):498–512; doi: 10.4158/EP.13.5.498 [DOI] [PubMed] [Google Scholar]
- 23. Kasahara T, Miyauchi A, Ito Y, et al. Tumor volume kinetic analyses might explain excellent prognoses in young patients with papillary thyroid carcinoma. J Thyroid Res 2020;2020:4652767; doi: 10.1155/2020/4652767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tuttle RM, Alzahrani AS. Risk stratification in differentiated thyroid cancer: From detection to final follow-up. J Clin Endocrinol Metab 2019;104(9):4087–4100; doi: 10.1210/jc.2019-00177 [DOI] [PMC free article] [PubMed] [Google Scholar]