Abstract
Background: Mucopolysaccharidoses (MPS) are rare, metabolic lysosomal storage disorders caused by the deficiency of enzymes required for the stepwise breakdown of glycosaminoglycans (GAGs). We report a case that was discovered to be Mucopolysaccharidosis Type II and was presented to the hospital with signs and symptoms of congenital diaphragmatic hernia. The hernia was repaired, and the patient was discharged on BiPAP and High-flow Oxygen.
Case Presentation: A vaginally delivered male child was presented to the hospital with a fever, cough, and shortness of breath. He has a history of recurrent chest infections treated as pneumonia. The child was born to a mother with a pregnancy complicated with gestational diabetes and the presence of polyhydramnios. Screening tests during the pregnancy reported no congenital anomalies. The patient was admitted, and a chest X-ray was performed and revealed cardiomegaly, pulmonary infiltrates consistent with pneumonia and bowel herniation noted through the right hemidiaphragm. A Computed Tomography (CT) scan of the thorax and Barium swallow was done, and Pneumonia and Congenital diaphragmatic hernia was confirmed. Genetic testing was done because the patient has coarse facial features, developmental delay, hypotonia and skeletal abnormalities. Iduronate-2-sulfatase enzyme levels were significantly low, all the other enzymes were normal in range, and the diagnosis of Type II Mucopolysaccharidosis was established. The patient was stabilized and operated on for diaphragmatic hernia repair. No enzyme-replacement therapy (ERT) was given because it is not available in Jordan. He was discharged on high-flow Oxygen and bilevel positive airway pressure (BiPAP).
Conclusion: Depending on the typical presentation to suspect Mucopolysaccharidosis is not always the key to diagnose it. MPS is a multi-organ disorder and rare presentations should not be disregarded.
Keywords: Mucopolysaccharidosis, Inherited Diseases, Congenital Diaphragmatic Hernia, Hunter Syndrome, Lysosomal Storage Diseases
Introduction
↑What is “already known” in this topic:
Common presentations of Type II Mucopolysaccharidosis include inguinal hernia, pulmonary dysfunction, and dysmorphic facial features.
→What this article adds:
Congenital diaphragmatic hernia is never reported in the literature as a presentation of MPS Type II. This will give an idea of how to approach such patients.
Mucopolysaccharidosis (MPS) is a group of rare genetic lysosomal storage disorders caused by either deficiency or abnormality of one of the 11 enzymes required to break down glycosaminog lycan (GAG) (1).
MPS Type II (Hunter syndrome) is the only member of the MPS group which inherited as X-linked recessive occurs due to mutations in the iduronate 2-sulfatase (IDS) gene. The IDS gene encodes a lysosomal enzyme, iduronate 2-sulfatase, an essential enzyme for the catabolism of glycosaminoglycans (GAGs) (2).
Impairment of GAGs metabolism results in Systemic pathological GAGs accumulation within lysosomes and then damage of cell tissue organs. There are four primary groups of GAGs based on their core disaccharide units and include heparan which accumulates in the nervous tissue and results in impairment of neurological function. Keratan, which accumulates in the skeletal system and then causes skeletal abnormalities. Dermatan, which accumulates in the skin, lungs and heart valves, results in skin changes, mitral valve damage, lung diseases, and finally, hyaluronic acid (3,4).
Clinical symptoms in MPS Type II fall within a wide range of differences between patients, as two-thirds of patients are classified into the severe (neuropathic) phenotype. In this type, symptoms start from childhood with growth retardation shown as difficulty in walking and speaking, and then a decrease in activity, so they need assistance in all life matters and respiratory assistance. The patient often dies at the age of 20 as a result of respiratory and cardiovascular symptoms if they are not treated. On the other hand, the mild severity (non-neuropathic) type appears with relatively few symptoms, such as joint stiffness and contractures. This makes it undiagnosed in some patients until adulthood (5).
We report this case for its diversity, rarity, and its difference from the typical presentation as no cases in the literature reported MPS Type II associated with diaphragmatic hernia. This report aims to highlight a hidden aspect of MPS Type II.
Case Presentation
A 3-year-old male child presented to the hospital with a history of fever, cough and shortness of breath. The child was born to a mother with a pregnancy complicated with gestational diabetes and the presence of polyhydramnios. Prenatal screening tests for congenital anomalies were negative. The child was delivered vaginally at 39 weeks gestation and was admitted to the Neonatal Intensive Care Unit (NICU) for 7 days due to respiratory distress. The doctors told the parents that their baby needed genetic counseling because of abnormal facial features but the parents refused. Upon taking a history from the parents, they stated that by the age of 6 months, the child was diagnosed with Hip Dysplasia (Fig. 1) and was managed by Pavlik Harness. Also, he suffered from recurrent chest infections for the last 2 years that were treated as pneumonia without proper investigations. Additionally, they reported that their child could not walk or sit by himself and rolled to his parents if they called him. He also cannot speak or say understandable words. The father and the mother were relatives ( positive consanguinity). The baby was on oxygen therapy and inhalers (salbutamol and fluticasone propionate) at home.
Fig. 1.

Pelvic X-ray showing hip dysplasia
On examination, the patient looked ill, distressed (had tachypnea, subcostal, and intercostal retractions without grunting) and well hydrated (no dry mouth, no sunken eyes and normal capillary refill). He was tachycardic with a pulse rate of 150 beats per minute (bpm), tachypneic with a respiratory rate of 33 /min and desaturated with oxygen saturation (O2 sat) of 67 %. His weight was 7 kilograms (Kg) (below 5th centile). Head circumference was 42.5 centimeters (cm) (below 3rd centile). Height was 72 cm (below 5th centile).
Maxillofacial examination revealed retrognathia, gum hypertrophy, and a low arched palate (Fig. 2). Ear examination was normal.
Fig. 2.

Child with Mucopolysaccharidosis showing short stature, acrocephalic head, coarse facial features, and depressed nasal bridge.
Cardiovascular examination revealed an early diastolic murmur heard over the left upper sternal border with a normal S1 and S2.
Lung examination revealed crackles and wheezes on the right side of the chest. Bowel sounds also were heard on the right side.
The abdomen was soft, lax, not tender, and had no hepatosplenomegaly and no hernia.
Central nervous system (CNS) examination revealed hypotonia with a muscle power of 3/5 in the upper and lower extremities. Head lag was present. Vertical suspension test was weak.
Skin and back examinations were normal. Genitalia examination revealed normal external genitalia. No lymphadenopathy was present
ICU care and further workup
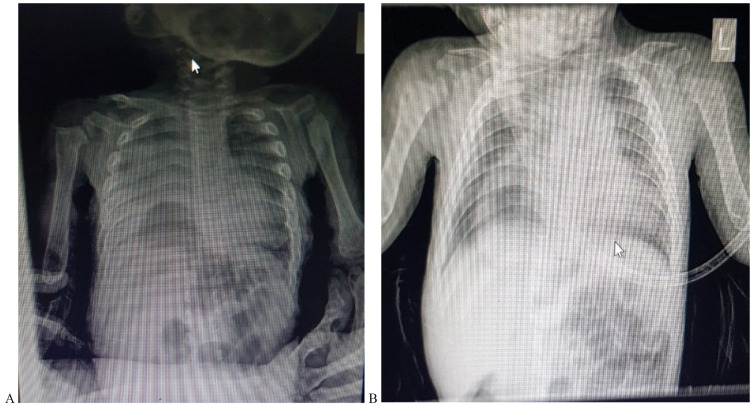
The child was admitted to the Intensive Care Unit (ICU) as a case of suspected pneumonia, management was started, and further investigations were done. A chest X-ray was done and revealed an increase in cardiothoracic ratio with pulmonary infiltrates consistent with pneumonia and bowel herniation noted through the right hemidiaphragm (Fig. 3A and 3B). He was put on High-flow Oxygen using a nasal cannula, intravenous antibiotics (ceftriaxone) were started, and nebulizers of salbutamol were given frequently. After 5 days of intensive therapy, the child was stable. No fever was documented. O2 saturation was 98% on 1/2-liter O2 delivered via nasal cannula and 89% on room air.
Fig. 3.
Anteroposterior Chest X-ray showing right pulmonary infiltrated in the upper and middle lobes as well as herniated bowel in the right hemidiaphragm.
Further investigations were done. Barium swallow confirmed the diagnosis of congenital diaphragmatic hernia (Fig. 4). Abdominal Ultrasound showed the following findings: The liver measures 10 cm, appearing normal in shape and echogenicity. The spleen measures 6 cm, appearing normal in shape and echogenicity. The right kidney measures 6 cm and the left kidney 7 cm. Both kidneys appear normal in shape and echogenicity. No obstructions. No free fluid in the abdomen or pelvis.
Fig. 4.

Barium swallow showing diaphragmatic Hernia
Pediatric cardiology consultation was ordered because of the cardiomegaly and the murmur that was heard. Echocardiography was done and showed: a mildly dilated right ventricle with normal systolic function, normal left ventricular volume with normal systolic function, mild pulmonary regurgitation, mild-moderate tricuspid regurgitation, estimated mean pulmonary artery pressure of 41 millimeters of mercury (mmHg), confirming the diagnosis of moderate pulmonary hypertension.
Fluorescence in situ hybridization (FISH) analysis was negative for William's Syndrome.
Chromosomal analysis was consistent with the male genotype, and karyotyping was 46, XY.
MPS enzymes assay was ordered. Iduronate-2-sulfatase enzyme levels were significantly low. All the other enzymes were normal in range. MPS Type II was confirmed.
Unfortunately, we could not measure any levels of GAG, dermatan, and heparan sulfates, in his urine because the kits needed were not available in our laboratories.
Whole-Exome Sequencing could not be performed because it is not available in Jordan.
Diaphragmatic Hernia Repair
After a week of recovery, the hernia was repaired. Surgery was done under general anesthesia. A right subcostal incision was made. Herniated bowel was carefully pushed back into its normal place in the abdominal cavity. The defect was repaired using a Gore-Tex patch. The incision was closed using dissolvable sutures. Three days after the surgery, the patient was discharged from the ICU. One week later, the patient was discharged home.
Home therapy after discharge
The patient was discharged home on long-term medications prescribed for him, which are Salbutamol 4-5 times a day and Fluticasone Propionate nasal spray every day, along with his oxygen therapy. Additionally, BiPAP was prescribed for him to be used along with oxygen while sleeping.
The child’s growth will be monitored because the patch may be torn or pulled away while he is growing.
Written informed consent was obtained from the parents of the patient for publication of this case report and any accompanying images.
Discussion
In our case, the taken history along with the clinical findings such as coarse facial features, developmental delay, retrognathia, gum hypertrophy, low arched palate, hypotonia, hip dysplasia, and recurrent chest infections were all raising the suspicion of MPS Type II.
MPS Type II (Hunter syndrome) is a disease inherited as X-linked recessive that results from an iduronate-2-sulfatase (I2S) lysosomal enzyme deficiency due to a mutation in the I2S gene (I2S). Like other mucopolysaccharides, a deficiency of the enzyme in MPS II will lead to the accumulation of glycosaminoglycans (GAGs) (6).
Hunter syndrome is one of the most common MPS disorders, with a prevalence of one in 170,000 male live births (7).
The most important aspect of the disease is the involvement of the CNS, which usually comes in the form of progressive cognitive degeneration, and depending on it. The disease is divided into attenuated and severe phenotypes. Patients with the attenuated type have minimal involvement. In contrast, the severe phenotype is characterized by CNS involvement. Progressive cardiac and airway problems, in addition to cognitive degeneration in severe cases, usually result in death during the first or second decade of life, whereas patients with the attenuated phenotype may survive into adulthood (8).
At birth, infants with MPS Type II do not show any features related to their condition. However, by the age of 2-4, they start to develop coarse facial features, developmental delays, and vocal cord enlargement, which can lead to narrowing of the airways, resulting in recurrent airway infections, breathing difficulties and sleep apnea (9).
MPS Type II usually presents with macrocephaly, hepatosplenomegaly, inguinal hernia, and carpal tunnel syndrome. Other complications of the disease include valvular heart diseases, spinal stenosis and retinal problems. In addition, Joint and skeletal deformities are common findings in patients with MPS Type II (9).
Heart diseases and airway obstruction are known to be the major causes of death in patients with MPS Type II (9).
The diagnosis of MPS Type II using clinical recognition of signs and symptoms may not be straightforward; due to their specificity. However, the typical facial feature could raise suspicion, especially in severe cases (2).
When the suspicion of MPS Type II is established, measurement of GAG levels in a 24-hour urine sample is needed, followed by electrophoresis or mass spectrometry to identify GAG species accumulation. once the urinary GAG levels are abnormal, enzymatic activity measurements are essential to detect IDS and sulphatase activity in order to rule out multiple sulphatase deficiencies. Also, molecular genetic testing allows the recognition of the causative gene variants. The molecular analysis is carried out using Polymerase Chain Reaction (PCR) (2).
Back to our case, we performed an MPS Enzymes assay to identify which enzyme is deficient so we can confirm the type of MPS we are dealing with. Unfortunately, we could not perform 24-hour urinary GAGs levels because of the lack of the kits needed in our laboratories.
In the era between 1970-1990, the management of MPS Type II focused on symptomatic and palliative treatment. But with medical community efforts, Hematopoietic stem cell transplantation (HSCT) and Enzymatic-replacement therapy (ERT) were introduced to the clinical practice in 1980 and 2006, respectively (2).
ERT aims to replace the deficient enzyme by using functional recombinant versions. It is available in two different recombinant enzymes for MPS Type II, which are idursulfase and idursulfase beta. Both are similar in their efficacy in decreasing GAG levels. However, idursulfase beta shows faster cellular uptake and lower resistance. Moreover, Clinical trials for ERT showed a significant reduction in urinary GAGs as well as a reduction in spleen and liver volume by using idursulfase at the dose of 0.5mg/kg weekly for 1 year (2).
HSCT application as part of MPS Type II remains controversial. It is used as a therapeutic option in some countries such as Japan, Brazil, and China while it is discouraged and rarely used in most western countries (2).
Our patient did not receive any form of ERT because it is not available in Jordan. He is now dependent on Oxygen therapy at home and a BiPAP device prior to sleeping.
Conclusion
MPS is a disease that affects various body systems. This increases the chance of rare and unusual symptoms. Proper approaching whenever suspicion occurs is crucial as early diagnosis and treatment can result in a better outcome. Our case calls for more investigations to understand the relationship between MPS type II and diaphragmatic hernia and the possibility of its occurrence in upcoming cases. Also, it will help in determining the most appropriate medical and surgical approach for such cases that may be more difficult than our reported case.
Conflict of Interests
The authors declare that they have no competing interests.
Cite this article as: Al-Mashakbeh Y, Heissat N, Al-Shaibei A, Heissat N, Al-Faqeeh A, Al-Jeady A, Al Katatbeh M, Khasawneh L. Congenital Diaphragmatic Hernia as a Presentation of Mucopolysaccharidosis in a 3-year-old child: A Case Report. Med J Islam Repub Iran. 2022 (24 Oct);36:123. https://doi.org/10.47176/mjiri.36.123
References
- 1. Shah G, Mahal T, Sharma S. Atypical clinical presentation of mucopolysaccharidosis type II (Hunter syndrome): a case report. J Med Case Rep 2010;4(1). [DOI] [PMC free article] [PubMed]
- 2.D’Avanzo F, Rigon L, Zanetti A, Tomanin R. Mucopolysaccharidosis Type II: One Hundred Years of Research, Diagnosis, and Treatment. Int J Mol Sci. 2020;21(4):1258. doi: 10.3390/ijms21041258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Casale J, Jonathan S. Crane. Biochemistry, Glycosaminoglycans. StatPearls Publishing LLC; 2019.. [PubMed]
- 4.Coutinho M, Lacerda L, Alves S. Glycosaminoglycan Storage Disorders: A Review. Biochem Res Int. 2012;2012:1–16. doi: 10.1155/2012/471325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomita K, Okamoto S, Seto T, Hamazaki T, So S, Yamamoto T, et al. Divergent developmental trajectories in two siblings with neuropathic mucopolysaccharidosis type II (Hunter syndrome) receiving conventional and novel enzyme replacement therapies: A case report. JIMD Rep. 2021;62(1):9–14. doi: 10.1002/jmd2.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scarpa M, Almássy Z, Beck M, Bodamer O, Bruce I, De Meirleir L, et al. Mucopolysaccharidosis type II: European recommendations for the diagnosis and multidisciplinary management of a rare disease. Orphanet J Rare Dis 2011;6(1). [DOI] [PMC free article] [PubMed]
- 7.Ramalingam K, Bhadrashetty D, Gajula P. A rare case of mucopolysaccharidosis: Hunter syndrome. J Nat Sci Biol Med. 2012;3(1):97. doi: 10.4103/0976-9668.95984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomatsu S, Patel P, Suzuki Y, Orii T. Growth charts for patients with Hunter syndrome. Mol Genet Metab. 2014;111(2):S104. doi: 10.1016/j.ymgmr.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Conditions G. Mucopolysaccharidosis type II: MedlinePlus Genetics [Internet]. Medlineplus.gov. 2022 [cited 2 January 2022]. https://medlineplus.gov/genetics/condition/mucopolysaccharidosis-type-ii/#resources.