Abstract
Introduction
Myosin II has been investigated with optical trapping, but single motor-filament assay arrangements are not reflective of the complex cellular environment. To understand how myosin interactions propagate up in scale to accomplish system force generation, we devised a novel actomyosin ensemble optical trapping assay that reflects the hierarchy and compliancy of a physiological environment and is modular for interrogating force effectors.
Methods
Hierarchical actomyosin bundles were formed in vitro. Fluorescent template and cargo actin filaments (AF) were assembled in a flow cell and bundled by myosin. Beads were added in the presence of ATP to bind the cargo AF and activate myosin force generation to be measured by optical tweezers.
Results
Three force profiles resulted across a range of myosin concentrations: high force with a ramp-plateau, moderate force with sawtooth movement, and baseline. The three force profiles, as well as high force output, were recovered even at low solution concentration, suggesting that myosins self-optimize within AFs. Individual myosin steps were detected in the ensemble traces, indicating motors are taking one step at a time while others remain engaged in order to sustain productive force generation.
Conclusions
Motor communication and system compliancy are significant contributors to force output. Environmental conditions, motors taking individual steps to sustain force, the ability to backslip, and non-linear concentration dependence of force indicate that the actomyosin system contains a force-feedback mechanism that senses the local cytoskeletal environment and communicates to the individual motors whether to be in a high or low duty ratio mode.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12195-022-00731-1.
Keywords: Myosin, Actin, Optical trapping, Motor ensemble
Introduction
Myosins are motor proteins that convert chemical energy into mechanical work to step along actin filaments (AFs).24,55 The dynamics of myosin–actin networks facilitate the movement and reorganization necessary for essential large-scale cellular tasks such as cell motility and the contractile ring during cytokinesis.1,24,45,55 Skeletal myosin II is responsible for muscle contraction through working as an ensemble to carry out the relative sliding of AFs within a sarcomere.29,54 Myosins within a thick filament form crossbridges between AFs and use conformational changes due to its mechanochemical cycle to collectively move within the aligned filaments to promote overall contraction.29,31,54,55
Skeletal myosin II has been studied extensively at the single molecule (SM) level and at the muscle level to better understand the underlying mechanics of muscle contraction.12,16,17,34,44,51,66 SM studies, such as those using optical trapping (OT), have been essential in evaluating the mechanistic behavior of molecular motors, including myosin, determining properties such as SM force generation and step sizes that can be overshadowed in bulk experiments.17,61 A distinct property of skeletal myosin II is its low duty ratio or non-processive nature as a single molecule, or it does not stay engaged with its AF track for the majority of its mechanochemical cycle.17,28,42 Thus, myosin II has been classified as a “rower” type motor, or a motor that must work in large arrays in order to facilitate productive movement, instead of a “porter” type motor like conventional kinesin-1 that can tote cargo as a SM while remaining engaged with its microtubule track for a considerable distance.2,28,36
A seminal paper by Finer et al. analyzed the force generation properties of single myosin II motor constructs using OT through development of the “three-bead” or “dumbbell” assay.16,17 As muscle myosin II works in teams to contract AFs and is non-processive as a single molecule, the OT assay orientation needed to be rearranged from the classic motor-bound bead approach with the filament adhered to the coverslip.2 Their alternative was to utilize two laser traps to suspend an AF bound on each end to the trapped beads (forming the “dumbbell”) over a myosin motor bound to a bead attached to a coverslip so that upon AF release, myosin would not be able to diffuse away and thus have the ability to obtain multiple force and step measurements.16,17
However, a prevalent challenge still present in the SM biophysics field is reconstituting a motor-filament environment that better reflects physiological function.13 Many optical trapping motor studies use isolated, reductionist geometries, such as a single motor interacting with a single filament, which does not reflect the structural hierarchy in which some motors, such as myosin II, function.2,13 More complex in vitro structures that contain multiple myosin motors are necessary to better understand the synergy of myosin and AFs networks.2 The dumbbell assay orientation has been used to probe small motor ensemble force generation by having multiple myosins attached to a third bead or using a myosin thick filament attached to a surface and allowing the motors to interact with the suspended AF.10,32,33,64 Kaya et al. evaluated single myosin mechanics within a myofilament attached to a coverslip and with the AF suspended above, finding that low system stiffness minimizes drag of negatively strained myosins during loaded conditions.32 In addition, myosin’s elastic portion is stretched during active force generation, reducing step size with increasing load even though the working stroke remains approximately constant, and step sizes of single myosin heads varied from 7 to 4 nm in a load dependent manner.32 In a subsequent paper using the same assay orientation, Kaya et al. observed ~ 4 nm stepwise actin displacements beyond a load of 30 pN, suggesting that steps cannot be driven exclusively by single myosins but instead by potentially coordinated force generations among multiple myosins, and the probability of coordinated force generation can be enhanced against high loads by using strain-dependent kinetics between force states, multiple power strokes, and high ATP concentration.33 Walcott et al. observed that mechanical coupling between myosins causes differences between SM and ensemble through employing the three-bead assay orientation but having multiple myosin motors attached to the coverslip-bound bead to form a small myosin ensemble, measuring smooth increase in force as opposed to individual binding events.64 They also employed an AF gliding assay to observe AF length-dependent motility and that the myosin ensembles glide unloaded AFs faster than predictions from SM measurements would indicate.64 Using a similar AF gliding assay, Hilbert et al. measured three distinct myosin group size-dependent motility regimes where above a critical AF length, increasing the number of myosins attached to the AF leads to a further increase in gliding velocity, and at lower myosin concentration, the group effect becomes lost, suggesting that a minimal myosin concentration is needed to achieve an inter-myosin communication effect also observed by Stachowiak et al. where myosin II self-organizes within reconstituted actomyosin bundles.25 Recently, Stewart et al. utilized the AF gliding assay to find that velocity and ATPase activity are both strain-dependent, and gliding velocity maximizes with the saturation of myosin binding sites on actin, which challenges the conventional independent force model of muscle contraction that assumes AF sliding is limited by detachment of individual myosins from actin.59 Similarly, Wagoner et al. purported that the amount of force felt by an individual motor will depend on the forces exerted by the other motors, giving rise to a force-mediated motor cooperativity that affects the number of motors bound but also the order in which each motor performs its transitions; further, myosins have evolved to reduced filament backsliding to increase the speed and efficiency of muscle contraction.63 Sung et al. demonstrated that human β-cardiac S1 myosin subfragment ADP release rate depends exponentially on applied load by using harmonic force spectroscopy, and values are in agreement with a previous study by Greenberg et al. who investigated the load-dependent ADP release of porcine β-cardiac myosin at saturating ATP.21,60 In a subsequent study, Liu et al. determined that load-dependent cardiac S1 myosin detachment rates, and thus ensemble duty ratio and force generation, can be altered by cardiomyopathy-causing mutations and small molecule activators and inhibitors of cardiac myosin, suggesting that large-scale cardiac contractility can be controlled by tuning molecular level load-dependent kinetics.37
Altogether, there is overwhelming evidence that myosin motor behavior deviates between the SM level and when working teams, and these motor ensembles are not a sum of the individual parts, which has been attributed to myosin’s inherent need to adapt to multiple types of environments and external loads.10,42,51,63,64 Additionally, the feedback loop from the environment to the motor ensemble, such as network stiffness, motor compliancy, and detecting number of bound motors, will also dictate force output through altering motor communication and coordination in the form of force generation and duty ratio.3,14,15,25,33,57,59,65 However, in ensemble studies to this point, the myosins are bound to a rigid surface, such as a bead or coverslip, and utilize one AF. In these cases, the motors are not able to move or communicate freely with each other, nor does having myosins rigidly bound reflect the physiological compliancy and hierarchical environment in which the motors would work together.2 As this critical parameter is necessary for force readout, there is a need to develop an optical trapping assay that fosters and captures motor coordination and system compliancy to paint a more realistic picture of the mechanistic underpinnings of myosin II ensemble force generation. Here, we formulate an approach that combines the precision of OT with in vitro active assembly of cytoskeletal hierarchy and measure ensemble myosin II force generation between two actin filaments using optical tweezers to capture elements of motor communication and cooperativity suggested by previous simulation and AF gliding experiment studies.3,15,57,59,63,64
Methods
Actin Polymerization
Actin polymerization was performed as described previously.5,9 Non-labeled rabbit skeletal muscle actin (Cytoskeleton) was reconstituted by adding 100 µL of reverse osmosis (RO) water to 1 mg of lyophilized actin. The contents were mixed by gently pipetting up and down, aliquoted, and stored at − 80 °C with final actin concentration of 10 mg/mL. To polymerize non-labeled actin into filaments, 5 µL of 10 mg/mL actin were mixed with 100 µL General Actin Buffer (GAB: 5 mM Tris–HCl, 0.2 mM CaCl2, 0.5 mM DTT, 0.2 mM ATP). The mixture was kept on ice and allowed to incubate for one hour. Actin was then polymerized into filaments by adding 5.5 µL of Actin Polymerizing Buffer (APB: 50 mM Tris–HCl, 500 mM KCl, 2 mM MgCl2, 2 mM CaCl2 2 mM DTT, 5 mM ATP) to the actin mixture, mixed well by gently pipetting up and down, and allowed to incubate for 20 min on ice. Actin filaments were stabilized and fluorescently labeled by adding 5 µL of rhodamine-labeled phalloidin (Cytoskeleton). The vial was wrapped in aluminum foil to block light and allowed to incubate on ice for one hour. The mixture was stored at 4 °C to be used for preparing actin myosin bundles for up to one week.
Biotinylated skeletal muscle actin (Cytoskeleton) was reconstituted by adding 20 µL of RO water to 20 μg of lyophilized actin. The contents were mixed well by gently pipetting up and down, aliquoted, and stored at − 80 °C with final biotinylated actin concentration of 1 mg/mL. Biotinylated actin was formed so that actin and biotinylated actin were in a 10:1 ratio by mixing 5 µL of 10 mg/mL actin and 5 µL of 1 mg/mL biotinylated actin. This solution was mixed with 100 µL GAB and allowed to incubate for one hour on ice. To polymerize the biotinylated actin filaments, 11 µL of APB was added to the actin mixture, mixed well by gently pipetting up and down, and allowed to incubate for 20 min in ice. To stabilize and fluorescently label the biotinylated actin filaments, 5 µL of Alexa Fluor 488 labeled phalloidin (ThermoFisher) were added to the biotinylated actin. The vial was wrapped in aluminum foil to block light and allowed to incubate on ice for one hour. The mixture was stored at 4 °C to be used for preparing actin myosin bundles for up to one week.
Optical Trapping Actomyosin Bundle Assay Preparation
Full length rabbit skeletal muscle myosin II (Cytoskeleton) was reconstituted to 10 mg/mL by adding 100 µL of RO water containing 1 mM DTT. Stock myosin was diluted 10 × by adding 10 µL of 10 mg/mL myosin to 90 µL of 1 mM DTT in RO water, snap frozen, and stored at -80 °C.
1 µm streptavidin-coated beads (Spherotech) were cleaned by diluting 20 µL of 1 µm streptavidin beads into 80 µL RO water and washing 4 times by spinning down at 10,000 rpm and reconstituting in 100 µL RO water. The beads were sonicated for 2 min at 40% and stored on a rotator at 4 °C.
Etched coverslips were soaked in poly-l-lysine solution (PLL: 30 mL of 100% ethanol, 200 µL of 0.1% w/v poly-l-lysine (SigmaAldrich)) for 15 min to allow coating the surface to facilitate actin filament binding. The coverslip was removed from the PLL solution with tweezers, taking care to only touch the edge of the coverslip, and dried with a filtered airline until there was no ethanol left and no residue on the coverslip.
A standard 10–15 μL flow cell was formed using a microscope slide, the PLL coverslip, and double-sided sticky tape. On the microscope slide, two pieces of double-sided sticky tape were applied to the middle of the slide and separated by 3–4 mm. The PLL coated coverslip was then added to the top of the tape perpendicular to the long axis of the microscope slide to form a channel where the liquid will be added. The coverslip was compressed onto the tape and microscope slide thoroughly using a small tube until the tape was transparent.
For formation of actomyosin bundles, both rhodamine and biotinylated 488-labeled actin filament solutions were diluted 600 × in APB as this dilution was sufficient for multiple bundles to form per slide but were spread out enough to ensure isolated measurements. To ensure robust labeling of filaments, 5 µL of their respective labeled phalloidin were added to each tube and incubated on ice in the dark for 15 min.
To begin assembling the actomyosin bundles, 15 µL of the diluted rhodamine actin were introduced to the PLL flow cell and allowed to incubate for 10 min in a humidity chamber. During this incubation in a separate tube, 15 µL of the diluted biotinylated actin were mixed with an oxygen scavenging system of 1 µL beta-d-glucose at 500 mg/mL, 1 µL glucose oxidase at 25 mg/mL, and 1 µL catalase at 500 units/mL to stabilize the filaments and reduce photobleaching during fluorescence imaging.9,49 In addition, 1 µL of 100 mM ATP and 1 µL of 10 × diluted cleaned streptavidin beads were added to the mixture. The solution was gently mixed by pipetting up and down, and the mixture was put in a rotator at 4 °C while the rest of the actomyosin bundle was being assembled.
A 1 mg/mL casein solution (Blotting Grade Blocker, Biorad) was made in APB. After the rhodamine-labeled actin incubation, 15 µL of 1 mg/mL casein was added to the flow cell to prevent non-specific binding of subsequent components and incubated for 5 min in humidity chamber.4,9,11,18,40,49,50 Before adding the biotinylated actin mixture to the flow cell, 1 µL of the desired concentration of myosin (10, 1, 0.1, 0.01, 0.002, or 0.0001 μM) was added to the solution, mixed by gently pipetting up and down, immediately added to the flow cell, and allowed to incubate for 20 min. The ends of the flow cell were sealed with nail polish to prevent evaporation during imaging and optical trapping experiments.
Optical Trapping Measurements and Analysis
The flow cell was loaded onto the optical trapping instrument (NT2 Nanotracker2 from JPK/BrukerNano)48 which contains a single trapping laser and is combined with brightfield, differential interference contrast, and epifluorescence imaging to simultaneously image and measure force generated by actomyosin bundles. Epifluorescence imaging of filaments was achieved by excitation with an ultra-stable metal-halide light source (Photofluor LM-75, 89North) and 488 and 532 nm excitation filter cubes. Before force measurement, bead position and trap stiffness were calibrated by trapping a bead in solution above the coverslip surface and running the power spectrum calibration routine within the JPK NT2 software. Bundle formation was investigated by verifying colocalization of single rhodamine and biotinylated 488-labeled actin filaments bundled by myosin motors through fluorescence imaging (Fig. S1). After verification, the bead bound to the colocalized filament bundle was trapped. Resistance of bead movement from the trap center due to bundle filament sliding was measured as change in bead position and force generation vs. time. Custom MATLAB codes were used to visualize traces and perform position, force, and stepping/dwell analysis.49,50 The step/dwell finding algorithm is based on a Student’s t-test to determine the edge of each step so that a dwell is defined in between.6,49,50 One-way ANOVA was performed on overall step size and detachment time averages, as well as step sizes and detachment times within force profile categories. p values are provided in figure captions and indications of non-significance (n.s.).
Results
Development of the Actomyosin Bundle Assay
Optical tweezers combined with fluorescence microscopy were employed to probe the mechanics of full-length myosin II motor ensembles interacting with actin filaments. Actomyosin bundles assays were developed with the goal of formulating an assay that incorporates AF structural hierarchy and compliancy, multiple myosin motors, and modular assay conditions to probe how myosin II motors work together to achieve force generation (Fig. 1). The bundle assay was constructed by first introducing rhodamine phalloidin actin to a flow cell made with a poly-l-lysine coated coverslip. After subsequent incubation with casein to prevent non-specific binding, myosin II, biotinylated Alexa Fluor 488 phalloidin AF, and streptavidin beads were added to the flow cell in the presence of ATP. Isolated bundles were identified through fluorescence imaging of each actin filament within the bundle, and force generation by the confirmed bundle was measured using the optical trap.
Figure 1.
Assay schematic. Actin filament–myosin bundles are formed in vitro, and force generation by the motor ensemble is probed using optical tweezers. Actin filaments within the bundle are labeled with rhodamine and Alexa Fluor 488 phalloidin to differentiate between filaments and confirm bundle formation. The cargo actin filament is also biotinylated to bind streptavidin beads for optical trapping experiments.
Actomyosin Bundle Measurements Reveal Three Force Profiles
Understanding factors that affect communication between myosin motors within actomyosin ensembles is important for deducing overall mechanisms of force generation at the molecular level. We investigated how the change in myosin concentration affects the interactions between myosin II motors and therefore dynamics of actin-myosin bundles, force generation, and motor stepping. Six myosin II solution concentrations were used to develop actin-myosin bundle assays (10, 1, 0.1, 0.01, 0.002, or 0.0001 μM). For myosin concentrations 10, 1, 0.1, 0.01, and 0.002 μM, measuring ensemble force generation by the optical trap yielded three similar force profiles at each concentration: a smooth force ramp followed by a plateau, a sawtooth-like pattern with antagonistic force generation, and low force generation close to baseline (Fig. 2). At 0.0001 μM myosin solution concentration, bundles did not form nor was force generation measured. Force profiles were categorized depending on the number of sequential steps taken in one direction and the overall net force generated. Traces with no net force generation and no consecutive steps taken were classified as baseline traces. Traces with overall low force generation and multiple back and forth sequential 4–5 nm steps were classified as sawtooth. Force ramp traces were characterized as having a steady increase in force in a primary direction, having multiple sequential steps in the same direction, ultimately plateauing after a substantial period of time (at least > 1 min), and having higher overall net force generation. Each solution concentration yielding three similar force profiles suggests that myosins have the ability to self-optimize within their bundle environment based on the local concentration of myosin, and thus number of occupied binding sites, within the bundle. Interestingly, for the ramping and sawtooth traces at each concentration, patterns that resembled stepping were observed (Fig. 2B) at intervals of approximately 4–5 nm, which is similar to previously recorded measurements of single myosin II step sizes.31–33 Further, across the full concentration range, 51% of the traces were categorized as ramp/plateau, 30% were sawtooth-like, and 19% were baseline, suggesting that the favored orientation during self-assembly is one that permits a substantial level of movement and force generation.
Figure 2.
Myosin ensemble force profiles. Representative traces of the force profiles measured using the actomyosin bundle assay: (a) smooth force ramp followed by a plateau, (b) expanded box from (a) to emphasize single motor stepping with a 4 nm axis spacing and detachment times between steps, (c) sawtooth-like pattern with antagonistic force generation, and (d) low force generation close to baseline.
Myosin Step Size and Detachment Time Within Ensembles Depends on Local Motor Concentration
The individual interactions between motors within the ensemble and the AF bundle during force generation were quantified using a step/dwell finding algorithm to analyze the dependence of step size and time between steps on motor concentration and force profile. As multiple motors are working in each of the measured traces, it is possible that the measured time between steps, or dwells, are due to multiple motor activity and may not be true single molecule dwells; thus, we will refer to the dwell times as detachment times between detected steps. Step sizes and detachment times between detected steps were measured at each myosin solution concentration for the ramp and sawtooth-like traces (Figs. 3a–3c 10 μM, Figs. 3d–3f 1 μM, Figs. 3g–3i 0.1 μM, Figs. 3j–3k 0.01 μM, and Figs. 3l–3m 0.002 μM). Forward and backward step sizes were measured and fit to Gaussian functions (Figs. 3a, 3d, 3g, 3j, 3l). Forward (Fig. 3b, 3e, 3h, 3k, 3m) and backward (Figs. 3c, 3f, 3i) detachment times were averaged using Gaussian fitting, and decay constants were found through fitting single exponential functions. Figure 3n shows that step sizes ranged from ~ 4 to 9 nm across the range of concentrations. At higher myosin solution concentrations, step size decreased and plateaued at ~ 4 nm, suggesting that these myosin bundles may have their actin binding sites saturated. Also, most of the steps for higher myosin concentrations were forward steps, but backward steps that did not significantly differ in size were also detected, which also supports AF saturation as the step sizes in both directions appear to be restricted. In lower myosin concentrations, the spread of myosin step size increased and no backwards steps were detected. Corresponding forward detachment times for the higher myosin concentrations were very similar, and the detected backward detachment times were shorter than the forward times. At lower myosin concentration, the detachment times between detected steps had a larger spread, were on average longer than for higher concentrations, and significantly different, indicating the start of possible communication breakdown between the motors, especially as at even lower motor concentration (0.0001 μM solution concentration), bundles could not reliably form or generate force.
Figure 3.
Dependence of step size and detachment time on ensemble concentration. Step sizes and detachment times between detected steps at a range of myosin solution concentrations: (a–c) at 10 μM, (d–f) at 1 μM, (g–i) at 0.1 μM, (j–k) at 0.01 μM, and (l–m) at 0.002 μM. (a), (d), (g), (j), and (l) Step size distributions are fit to single Gaussians, and forward (b, e, h, k, m) and backward (c, f, i) detachment times between detected steps are fit to single exponentials. (a) Average forward step size at 10 μM myosin is 4.7 ± 0.8 nm (N = 145), and average backward step size is 3.8 ± 0.7 nm (N = 43). (b–c) Forward and backward detachment times average 5.8 ± 0.6 s and 0.8 ± 0.2 s, respectively, and decay constants of 3.8 s and 0.5 s, respectively. (d) Average forward step size at 1 μM is 4.3 ± 0.2 nm (N = 79), and average backward step size is 4.0 ± 0.2 nm (N = 49). (e–f) Forward and backward detachment times average 3.2 ± 0.7 s and 0.6 ± 0.05 s, respectively, and decay constants of 1.4 s and 0.8 s, respectively. (g) Average forward step size at 0.1 μM is 3.8 ± 0.2 nm (N = 101), and average backward step size is 4.2 ± 0.3 nm (N = 64). (h–i) Forward and backward detachment times average 3.0 ± 0.6 s and 1.6 ± 0.3 s, respectively, and decay constants of 1.3 s and 0.9 s, respectively. (j) Average forward step size at 0.01 μM is 5.4 ± 0.5 nm (N = 59) with no detected backward steps. (k) Forward detachment times average 13.6 ± 1.5 s and decay constant of 17.6 s. (l) Average forward step size at 0.002 μM is 8.9 ± 1.4 nm (N = 18) with no detected backward steps. (m) Forward detachment times average 12.6 ± 1.4 s and decay constant of 15.7 s. (n) Overall trend of increasing step size and detachment time with decreasing myosin concentration. One-way ANOVA analysis revealed that the forward step sizes and detachment times across the concentration range are significantly different (p < 0.0001 for both) while the backward steps and detachment times are not (p = 0.11 and p = 0.15, respectively). Error bars, SEM.
Step Size, Detachment Time, and Force Dependence on Trace Profile
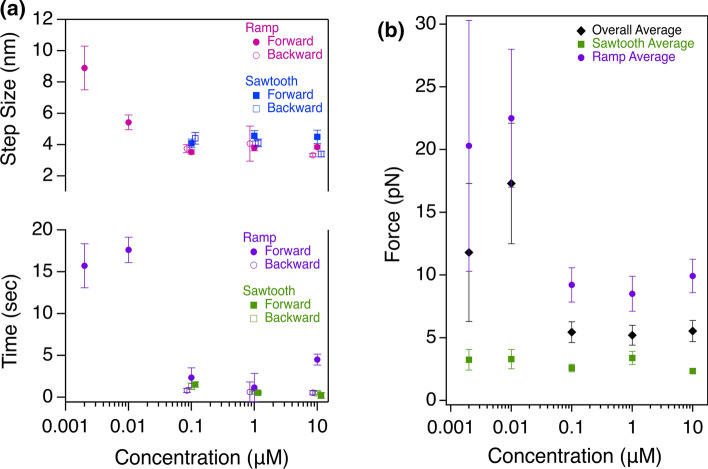
As the myosin II concentrations investigated were solution concentrations and the three force profiles were observed for each concentration, this suggests that the motors have the ability to self-optimize within their AF bundle based on local concentration of myosin and number of occupied filament binding sites accordingly. As such, we asked whether step size, detachment time, or maximum force generated depended on force profile. In Fig. 4a, step size and detachment time are divided into categories of ramp vs. sawtooth-like traces. As observed from Fig. 3, backward step sizes and detachment times were not detected at more dilute motor concentrations. However, the significantly larger step sizes and detachment times at these more dilute concentrations were found only in force ramping traces. Forward and backward steps from the sawtooth-like traces did not significantly differ between concentrations but the detachment times were slightly different. Figure 4b reveals that maximum force generation at each concentration was significantly higher in the ramp/plateau traces than the sawtooth-like traces. Interestingly, the maximum force of the sawtooth traces remained essentially the same regardless of myosin solution concentration, but at more dilute concentrations, the maximum force of the ramp/plateau traces increased on average and had a larger variation. If the AF binding sites are indeed saturated by myosins in the higher concentration cases, then it is possible that having a concentration slightly less restrictive than saturated can yield higher force generation. However, this trend drops off sharply as no force generation was measured for bundles at a myosin concentration an order of magnitude lower than what is plotted.
Figure 4.
Step size, detachment time, and force dependence on trace profile. (a) Step size (top) and detachment decay constant (bottom) dependence trends on concentration separated by trace profile. (A-top) Magenta closed and open circles are average step forward and backward step sizes within force ramping traces compared to blue closed and open squares that are average forward and backward step sizes within sawtooth-like traces. (A-bottom) Purple closed and open circles are decay constants for forward and backward transitions within force ramping traces compared to green closed and open squares that are decay constants within sawtooth-like traces. One-way ANOVA analysis revealed that the forward step sizes and detachment times for force ramp traces are significantly different (p < 0.0001 for both). Backward steps for force ramp traces are not significantly (n.s.) different (p = 0.5) while backward detachment times are different (p = 0.02). Differences in forward and backward step sizes across the indicated concentration range for sawtooth-like traces are n.s. (p = 0.5 and p = 0.4, respectively), while the forward and backward detachment times are significantly different (p = 0.002 and p = 0.0008, respectively). (b) Average maximum force generation at each myosin bundle concentration (black diamonds: 10 μM 5.5 ± 0.8 pN (N = 59), 1 μM 5.2 ± 0.8 pN (N = 49), 0.1 μM 5.4 ± 0.8 pN (N = 66), 0.01 μM 17.3 ± 4.8 pN (N = 11), 0.002 μM 11.8 ± 5.5 pN (N = 14)), and average maximum force generation divided by trace profile. Ramp (purple circles): 10 μM 9.9 ± 1.3 pN (N = 28), 1 μM 8.5 ± 1.4 pN (N = 22), 0.1 μM 9.2 ± 1.4 pN (N = 33), 0.01 μM 22.5 ± 5.5 pN (N = 8), 0.002 μM 20.3 ± 10.0 pN (N = 7). Sawtooth-like (green squares): 10 μM 2.3 ± 0.2 pN (N = 16), 1 μM 3.4 ± 0.5 pN (N = 17), 0.1 μM 2.6 ± 0.3 pN (N = 17), 0.01 μM 3.3 ± 0.8 pN (N = 3), 0.002 μM 3.2 ± 0.8 pN (N = 7). One-way ANOVA analysis revealed that maximum forces across the concentration range for the ramping traces are significantly different (p = 0.011) while the maximum forces across all tested concentrations for the sawtooth-like traces are n.s. (p = 0.31). Error bars, SEM.
Discussion
We have integrated actomyosin bundles consisting of multiple AFs and myosin motors within an optical trapping assay in order to capture myosin ensemble mechanics that have been shown previously to depend on system compliancy and motor communication. Unlike previous studies that used heavy meromyosin (HMM) or the further truncated S1 construct, full length myosin motors were used here in order to understand the mechanical implications of the full protein and its role in force coordination, net force generation, and motor stepping. By incorporating AF-myosin-AF bundles into the assay, this allows the myosin motors to self-optimize within their more native multi-AF hierarchical environment instead of being rigidly attached to a bead or coverslip surface and allow for more elastic communications to occur between motors directly, as well as through the AFs to more distal motors within the bundle environment.20,38,50 We probed a range of myosin concentrations, and bundles at each concentration yielded three force profiles of low to no force generation, middle range force generation with a sawtooth-like pattern, and higher force generation with a smoother force ramp followed by a plateau. The finding of three force regimes aligns with the results presented by Hilbert et al. with the exception that their study used an unloaded gliding filament assay.25
Using our loaded OT bundle assay, we observed patterns that resembled individual motor steps, especially in the sawtooth-like and ramp/plateau patterns. Thus, we used a step/dwell finding algorithm to analyze the steps in each force profile. We found step sizes that ranged from 4 to 9 nm, which is consistent with previously measured step sizes of individual myosin II motors and suggests that even though the myosins are in an ensemble, they are taking steps one at a time in order to build and sustain force generation. Further, the step size increases as likely fewer actin binding sites are occupied with myosin motors within the bundles, which resembles the findings of Kaya et al. where in myofilaments, step sizes decreased from 7 to 4 nm with increasing load on the system.33 As myosin concentration within the AF bundles decreases, so does the number of occupied AF binding sites; thus, the stiffness of the overall architecture decreases as well, which would likely not facilitate efficient motor communication or movement.3,15,19,52 A smaller percentage of backward steps were also detected. At higher concentrations, the backward steps were similar in size to the forward steps, but at more dilute concentrations, backsteps were not detected by the algorithm. Stewart et al. suggest that AF binding sites can become saturated and yield a maximum AF gliding velocity.59 If AF binding site saturation is the case in our more concentrated AF bundles, then having backsteps that are essentially the same size as the forward steps makes sense. If a forward step occurred in a saturated environment, it is possible that a cascade of force/tension signals from the end of the AF bundle let the motor know that it has the ability to move forward so that energy is not wasted in a diffusional search for the next binding site. However, the motor could still slip and fall backward a maximum of one step size. This also aligns with the study from Wagoner et al. who suggests that the amount of force felt by an individual motor will depend on the forces exerted by surrounding motors and affect the order in which each motor performs its transitions.63 Further, backstepping or backsliding is not detected in more dilute myosin ensembles, suggesting that motor communication begins to break down with fewer motors present. In conjunction, the step size increases with decreased myosin concentration as the bundle environment is not as crowded, giving the motors more freedom to move a larger distance than when the AF binding sites are saturated.
Using the same algorithm, we analyzed the dwell times between detected steps. However, as there are multiple motors at play and there are different degrees of motor ensemble coordination due to changes in concentration, these could also be thought of as detachment times or the time between one motor taking a detected step forward or backward and the subsequent step. At higher concentrations, the forward detachment time decreases slightly with decreasing concentration, and the backward detachment time increases with decreasing concentration. At more dilute concentrations, the forward dwell times increase significantly. In these cases, the bundle is not saturated or as crowded and thus it is likely that communication takes longer across longer distances to make sure that space is available to move the longer step size. This also suggests a lower duty ratio state than in the higher concentration bundles. There is more time between each step or time motors are not trying to actively engage with the AF. If the duty ratio decreases and approaches a more single molecule type state, there will be more time spent detached from the AF and therefore when a motor does release, there is not the propensity for surrounding motors to rapidly reattach and sustain previously built-up force. Change in duty ratio of myosin between single molecules and ensembles has been previously suggested and modeled computationally,3,15,33,57,63 and here, we are able to observe the transition of increased duty ratio when moving between smaller and larger concentration ensembles through changes in stepping and force output as facilitated by using a compliant multi-AF architecture.
The interdependence of motor ensemble size and effective force generation has been suggested previously,10,25,57,59,63–65 but here we also find that under load, ensemble size likely dictates the force generation trace profile of whether there is a sawtooth-like, almost antagonistic back-and-forth tug-of-war or the motors work together to produce a smooth force ramp. Interestingly, these force profiles are similar to the computational results of Erdmann et al. who investigated the mechanics of small myosin ensembles using a parallel cluster model.3,15 They also found sawtooth and ramping force generation profiles that depend on external force, system stiffness, and ensemble size.3,15 In myosin ensembles, they purport that there are two types of mechano-sensitive processes taking place: catch bonding of post-power stroke state motors that directly depends on load, and the transition from the post-power stroke state to the weakly-bound state provides another type of catch bonding due to differing unbinding times that reduces reverse rates.3,15 Additionally, post-power stroke to weakly-bound transitions only occur in elastic environments and suggest that both internal and external mechanics need to be considered when evaluating myosin ensemble function.3,15 If the dwell or detachment times presented here are analogous to the post-power stroke to weakly-bound transitions and facilitate the second type of catch bonding behavior, it makes sense that there is such a stark difference in stepping/dwell behavior between the more concentrated and more dilute bundles. At higher concentrations, there are more steps and shorter dwell times which matches with a reduction in reverse rates. Further, if these transitions only occur in elastic environments, our assay setup allowed us to observe and measure these changes in stepping behavior.
Interestingly, as solution myosin concentration decreases, we observe a significant increase in maximum force measured in each trace. By separating the force profiles into ramp vs. sawtooth-like, we observe that the substantially higher average force generation occurs in the smoother force ramp traces as well as an increase in step size from 4 to almost 9 nm. This is intriguing because previous studies suggest that saturation of AFs by myosins yields maximum sliding velocity, but these are also referring to unloaded gliding filament assays.59 However, it is possible that when the amount of motors available to bind into actin binding sites within the bundle is slightly lower than saturation, the system yields a bit more flexibility in a mechanical sense but also for self-optimization within the bundle. This has been suggested to occur on larger system scale by Stachowiak et al. and aligns with our observation of an essentially doubled step size.56 The lower maximal force and lack of detection of backslips in the sawtooth-like traces then suggests that there is a lower local concentration of myosin that formed those particular bundle assemblies than in the force ramp cases.
Overall, these results suggest that the local environment of myosin II, including concentration and system stiffness, influence the level of motor cooperativity, communication, and thus force regulation, within AF bundles. Our proposed working mechanism includes when myosin binding sites within the AF bundles are saturated (Fig. 5—top), step sizes, both forward and backward, are restricted to the single molecule step size due to the surrounding occupied sites. In order to make forward progress, the saturated bundle has to communicate systematically to know when to move each individual motor efficiently, establishing a method to proceed one step at a time and sustain the already generated force by the system, as in a competitive tug-of-war game. This communication could occur locally between adjacent myosin motors through their individual stiffness that change throughout the mechanochemical system but also within the AF system.19,52,62 Having the filaments saturated with bound motors will increase the overall stiffness, allowing for larger scale communication, and as discussed previously, yields maximal unloaded velocity.59 Thus, it is possible that to achieve the maximal unloaded velocity observed by others, the motors use system stiffness to relay a metachronal-like effect, which has been suggested previously for contractile systems using heavy meromyosin.22,41,67 However, being restricted to ~ 4 nm steps may not yield the highest force generation potential. Once the actomyosin system becomes slightly less saturated (Fig. 5—middle), the inherent increased flexibility of the system due to fewer bound motors allows for self-organization within the bundle, and a slightly less stiff system facilitates communication to increase step sizes and foster higher force generation potential.
Figure 5.
Force Dependence on Ensemble Environmental Conditions. Proposed mechanism comparison for how motor number, environment, and system compliancy dictate force-feedback within actomyosin bundles. The top bundle is saturated with only a few available binding sites open (red rectangles), thus the system can only proceed one step at time. In addition, having many motors bound stiffens the filaments and may act as a system-scale force sensor to determine motor ensemble speed and force generation. In the middle bundle, there are more binding sites available, thus myosins within the system have the opportunity to self-organize into an optimal arrangement that facilitates larger step sizes and higher force generation than the saturated case because the system is not as limited by availability of the next open AF binding site. Fewer motors bound result in a more compliant AF system that may communicate to the myosins’ catch bonding ability and thus regulate motor organization within the bundle. However, motility and force generation efficiency drop off when motor number reduces further, as in the bottom bundle. Lack of nearby motors and increased AF compliancy signal back to the motors to reduce their duty ratio and act more similarly to single molecule behavior where myosins remain detached from AFs for the majority of their mechanochemical cycle.
Lowering myosin concentration beyond this “sweet spot” begins a communication breakdown due to lack of nearby motors and changes in flexural rigidity due to fewer motors. Motors begin to act more as single molecules with an inherently low duty ratio and rapid detachment rate that does not facilitate productive ensemble force generation (Fig. 5—bottom). Another possible reason for baseline force trace profiles is that myosin “dead heads” may be present and contribute to filament bundles becoming stuck. While we cannot completely rule this out, we believe that dead heads are an issue more inherent to the design of the in vitro motility assay, or gliding filament assay, which we are not using here. In gliding filament assays, myosins (typically HMM or S1) are added to a coverslip surface and can bind in an uncontrolled variety of orientations, including some that are not conducive for stepping or gliding and thus enter a rigor state.39,43 Also, myosin tail interactions with actin have been shown to cause significant drag in such assays and may contribute to further sliding degradation if myosin motors are bound to the glass surface in a “tail up” orientation.23 Other studies have demonstrated that dead head purification (removal of “dead” myosin motors) had very little to no effect on improving actin motility26,30 or even had a detrimental effect on activity of the remaining myosins.46 However, as we are building actomyosin bundles with a casein-blocked surface, the opportunity to bind anything but the actin filaments is minimized, as are the artifacts that can result from in vitro motility assay preparations. Thus, we believe this is a less likely scenario.
In conjunction with local concentration and system stiffness, other environmental and structural factors may also be at play in regulating myosin ensemble dynamics. Myosin is known to form the interacting heads motif (IHM) where the heads fold back on the myosin tail, as well as the autoinhibited, super-relaxed (SRX) state with slow ATP turnover.27,47,58 Recent studies have demonstrated a direct correlation between the respective structural and biochemical states.47 As the IHM and SRX states are a form of muscle regulation important for energy conservation and are sensitive to force, these combined actions would certainly downregulate system contractility due to fewer AF-engaged heads.27,47 However, instead of SRX engagement being a strict “on” and “off” type of regulation, these states provide a more gradual recruitment or withdrawal of myosin heads and force production, providing a downstream, metachronal-like communication effect of increasingly higher or lower filament stiffness and thus telling more heads to enter such a state, which aligns with our hypothesis above.47,53
Another factor to consider is the structure of the actin filament bundle, including overlap length and filament polarity. Size of the overlap length between two filaments would determine the amount of intrabundle space and AF binding sites available for myosins to step and generate collective force. Previous studies have demonstrated that a critical AF length is needed to increase velocity in a gliding filament assay, and myosin II has the ability to self-organize within reconstituted actomyosin bundles where myosin spontaneously reorganized into discrete clusters during contraction.25,56 Other studies have indicated that diffusible motors and filament crosslinkers exhibit entropic behavior in the self-assembly of large cytoskeletal structures like the mitotic spindle and contractile ring partly through maximizing the overlap length of overlapping filaments.7,8,35 Perhaps there is a level of entropic contribution to the self-optimization observed previously and in this study due to myosin’s ability to exhibit both diffusional and processive properties depending on its local environment. Further, as the time to reach plateau in the force ramping traces were all similar, it is possible that finding the optimal position within the bundle for system force generation is found first before substantial collective work is performed by the motor ensemble to ensure that energy is not wasted in a non-ideal environment. This would likely put actin filament overlap lengths at a similar starting geometry; however, further study should be pursued to clarify the mechanism.
Then what drives actomyosin bundles to enter a force ramp vs. sawtooth-like force generation state? Perhaps actin filament polarity contributes. As depicted in Fig. S2, myosins step toward the barbed or plus end of the AF, so it makes sense that track directionality would affect overall force generation. We assume that myosins in our experiments are not aggregated into thick filaments due to the ionic strength of the buffer. In addition, myosin tail interactions with AFs have shown to induce significant drag in gliding filament assays.23 Thus, in an anti-parallel oriented bundle (plus and minus ends of each filament are opposite of each other), a motor with heads stepping along the bottom filament and tail interacting with the top filament would work in concert with a motor stepping on the top filament in the opposite direction to collectively move the top AF in the same direction. In a parallel bundle, where the plus and minus ends of both filaments are aligned, there could be a degree of antagonization from tail drag and could lead to a ramp or sawtooth-like force generation state depending on the number of tail interactions vs. bound/stepping motors. So, collective force generation could occur in either orientation. However, going back to Fig. 5, if a bundle is saturated with motors, it is likely that weaker tail interactions will not have the opportunity to interact with the AF due to steric hinderance. This then leads to the metachronal-like communication wave of myosin force generation that experiences feedback due to the stiffness of the AF environment. When fewer motors are present and bound, there are more opportunities for tail interactions that may also entropically drive motors to separate and create the larger steps sizes observed above. The nature of these interactions would be of interest in future study. Taken together, we propose that main contributors for force generation in self-optimized actomyosin bundles are local motor concentration within the bundle and concomitantly how many motors are bound, leading to a force-feedback mechanism driven by system stiffness and compliancy.
Conclusions
By using a novel actomyosin assay design consisting of multiple myosin motors within an AF bundle, we were able to capture elements such as structural hierarchy, system compliancy, and motor self-optimization that have been previously alluded to be critical to elucidate how myosin behavior evolves from the single molecule to ensemble level but have not been investigated collectively using optical tweezers. The results above indicate that motor number, environment, and system stiffness are likely significant contributors to dictating motor duty ratio and overall force output in small myosin ensembles, as suggested by the changes in motor ensemble motility and force generation between saturated and less concentrated actomyosin bundles. Experimental and mechanistic details of the force-feedback mechanism between neighboring myosin motors through high resolution motor imaging within these environments, changes in system compliancy, entropic contributions, and cues from the local cytoskeletal environment are interesting subjects for further investigation in order to better understand how force propagates throughout motor-filament systems and specifically the molecular basis of mechanosensation in actomyosin systems that facilitate larger-scale muscle contraction.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
This work is supported in part by the University of Mississippi Graduate Student Council Research Fellowship (OA), University of Mississippi Sally McDonnell-Barksdale Honors College (JCW, JER), the Mississippi Space Grant Consortium under Grant Number NNX15AH78H (JCW, DNR), and the American Heart Association under Grant Number 848586 (DNR).
Author Contributions
OA was involved in all aspects of the work, including assay development, performing experiments, data analysis, and manuscript preparation. OA, JCW, JED, JER, and DNR aided in assay development, data acquisition, and analysis. OA and DNR designed the experiments, analyzed data, and prepared the manuscript.
Conflict of interest
Omayma M. Al Azzam, Janie C. Watts, Justin E. Reynolds, Juliana E. Davis, and Dana N. Reinemann declare that they have no conflict of interest.
Research Involving Human Rights
No human studies were carried out by the authors for this article.
Research Involving Animal Rights
No animal studies were carried out by the authors for this article.
Abbreviations
- AF
Actin filament
- OT
Optical tweezers, optical trapping
- SM
Single molecule
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Akhshi TK, Wernike D, Piekny A. Microtubules and actin crosstalk in cell migration and division. Cytoskeleton. 2014;71:1–23. doi: 10.1002/cm.21150. [DOI] [PubMed] [Google Scholar]
- 2.Al Azzam O, Trussell CL, Reinemann DN. Measuring force generation within reconstituted microtubule bundle assemblies using optical tweezers. Cytoskeleton. 2021;78:111–125. doi: 10.1002/cm.21678. [DOI] [PubMed] [Google Scholar]
- 3.Albert PJ, Erdmann T, Schwarz US. Stochastic dynamics and mechanosensitivity of myosin II minifilaments. New J. Phys. 2014;16:093019. doi: 10.1088/1367-2630/16/9/093019. [DOI] [Google Scholar]
- 4.Appleyard DC, Vandermeulen KY, Lee H, Lang MJ. Optical trapping for undergraduates. Am. J. Phys. 2007;75:5–14. doi: 10.1119/1.2366734. [DOI] [Google Scholar]
- 5.Balikov DA, et al. The nesprin-cytoskeleton interface probed directly on single nuclei is a mechanically rich system. Nucleus. 2017;1034:1–14. doi: 10.1080/19491034.2017.1322237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brady SK, Sreelatha S, Feng Y, Chundawat SPS, Lang MJ. Cellobiohydrolase 1 from Trichoderma reesei degrades cellulose in single cellobiose steps. Nat. Commun. 2015;6:1–9. doi: 10.1038/ncomms10149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun M, Lansky Z, Fink G, Ruhnow F, Diez S, Janson ME. Adaptive braking by Ase1 prevents overlapping microtubules from sliding completely apart. Nat. Cell Biol. 2011;13:1259–1264. doi: 10.1038/ncb2323. [DOI] [PubMed] [Google Scholar]
- 8.Braun M, Lansky Z, Hilitski F, Dogic Z, Diez S. Entropic forces drive contraction in cytoskeletal networks. BioEssays. 2016;38:474–481. doi: 10.1002/bies.201500183. [DOI] [PubMed] [Google Scholar]
- 9.Cordova JC, et al. Bioconjugated core-shell microparticles for high-force optical trapping. Part. Part. Syst. Charact. 2018;35:1–8. doi: 10.1002/ppsc.201700448. [DOI] [Google Scholar]
- 10.Debold EP, Walcott S, Woodward M, Turner MA. Direct observation of phosphate inhibiting the force-generating capacity of a miniensemble of myosin molecules. Biophys. J. 2013;105:2374–2384. doi: 10.1016/j.bpj.2013.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong J, Castro CE, Boyce MC, Lang MJ, Lindquist S. Optical trapping with high forces reveals unexpected behaviors of prion fibrils. Nat. Struct. Mol. Biol. 2010;17:1422–1430. doi: 10.1038/nsmb.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duke TAJ. Molecular model of muscle contraction. Proc. Natl. Acad. Sci. U.S.A. 1999;96:2770–2775. doi: 10.1073/pnas.96.6.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elting MW, Spudich JA. Future challenges in single-molecule fluorescence and laser trap approaches to studies of molecular motors. Dev. Cell. 2012;23:1084–1091. doi: 10.1016/j.devcel.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ennomani H, et al. Architecture and connectivity govern actin network contractility. Curr. Biol. 2016;26:616–626. doi: 10.1016/j.cub.2015.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erdmann T, Schwarz US. Stochastic force generation by small ensembles of myosin II motors. Phys. Rev. Lett. 2012;108:1–5. doi: 10.1103/PhysRevLett.108.188101. [DOI] [PubMed] [Google Scholar]
- 16.Finer JT, et al. Characterization of single actin-myosin interactions. Biophys. J. 1995;68:291–296. [PMC free article] [PubMed] [Google Scholar]
- 17.Finer JT, Simmons RM, Spudich JA. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature. 1994;368:113–119. doi: 10.1038/368113a0. [DOI] [PubMed] [Google Scholar]
- 18.Fordyce PM, Valentine MT, Block SM. Advances in surface-based assays for single molecules. Single-Mol. Tech. A. 2008;17:431–460. [Google Scholar]
- 19.Galkin VE, Orlova A, Egelman EH. Actin filaments as tension sensors. Curr. Biol. 2012;22:R96–R101. doi: 10.1016/j.cub.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gittes F, Meyhöfer E, Baek S, Howard J. Directional loading of the kinesin motor molecule as it buckles a microtubule. Biophys. J. 1996;70:418–429. doi: 10.1016/S0006-3495(96)79585-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenberg MJ, Moore JR. The molecular basis of frictional loads in the in vitro motility assay with applications to the study of the loaded mechanochemistry of molecular motors. Cytoskeleton. 2010;67:273–285. doi: 10.1002/cm.20441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guérin T, Prost J, Martin P, Joanny JF. Coordination and collective properties of molecular motors: theory. Curr. Opin. Cell Biol. 2010;22:14–20. doi: 10.1016/j.ceb.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Guo B, Guilford WH. The tail of myosin reduces actin filament velocity in the in vitro motility assay. Cell Motil. Cytoskeleton. 2004;59:264–272. doi: 10.1002/cm.20040. [DOI] [PubMed] [Google Scholar]
- 24.Hartman MA, Spudich JA. The myosin superfamily at a glance. J. Cell Sci. 2012;125:1627–1632. doi: 10.1242/jcs.094300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hilbert L, Cumarasamy S, Zitouni NB, Mackey MC, Lauzon AM. The kinetics of mechanically coupled myosins exhibit group size-dependent regimes. Biophys. J. 2013;105:1466–1474. doi: 10.1016/j.bpj.2013.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hooft AM, Maki EJ, Cox KK, Baker JE. An accelerated state of myosin-based actin motility. Biochemistry. 2007;46:3513–3520. doi: 10.1021/bi0614840. [DOI] [PubMed] [Google Scholar]
- 27.Hooijman P, Stewart MA, Cooke R. A new state of cardiac myosin with very slow ATP turnover: a potential cardioprotective mechanism in the heart. Biophys. J. 2011;100:1969–1976. doi: 10.1016/j.bpj.2011.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howard J. Mechanics of Motor Proteins and the Cytosksleton. Appl. Mech. Rev. 2001;55(2):B39–B39. doi: 10.1115/1.1451234. [DOI] [Google Scholar]
- 29.Huxley HE. Fifty years of muscle and the sliding filament hypothesis. Eur. J. Biochem. 2004;271:1403–1415. doi: 10.1111/j.1432-1033.2004.04044.x. [DOI] [PubMed] [Google Scholar]
- 30.Jackson DR, Baker JE. The energetics of allosteric regulation of ADP release from myosin heads. Phys. Chem. Chem. Phys. 2009;11:4808–4814. doi: 10.1039/b900998a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kad NM, Kim S, Warshaw DM, VanBuren P, Baker JE. Single-myosin crossbridge interactions with actin filaments regulated by troponin-tropomyosin. Proc. Natl. Acad. Sci. U.S.A. 2005;102:16990–16995. doi: 10.1073/pnas.0506326102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaya M, Higuchi H. Nonlinear elasticity and an 8-nm working stroke of single myosin molecules in myofilaments. Science (80-) 2010;329:686–689. doi: 10.1126/science.1191484. [DOI] [PubMed] [Google Scholar]
- 33.Kaya M, Tani Y, Washio T, Hisada T, Higuchi H. Coordinated force generation of skeletal myosins in myofilaments through motor coupling. Nat. Commun. 2017;8:1–13. doi: 10.1038/ncomms16036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kron SJ, Uyeda TQP, Warrick HM, Spudich JA. An approach to reconstituting motility of single myosin molecules. J. Cell Sci. 1991;98:129–133. doi: 10.1242/jcs.1991.Supplement_14.26. [DOI] [PubMed] [Google Scholar]
- 35.Lansky Z, et al. Diffusible crosslinkers generate directed forces in microtubule networks. Cell. 2015;160:1159–1168. doi: 10.1016/j.cell.2015.01.051. [DOI] [PubMed] [Google Scholar]
- 36.Leibler S, Huse DA. Porters versus rowers: a unified stochastic model of motor proteins. J. Cell Biol. 1993;121:1357–1368. doi: 10.1083/jcb.121.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu C, Kawana M, Song D, Ruppel KM, Spudich JA. Controlling load-dependent kinetics of β-cardiac myosin at the single-molecule level. Nat. Struct. Mol. Biol. 2018;25:505–514. doi: 10.1038/s41594-018-0069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lüdecke A, Seidel A, Braun M, Diez S. Diffusive tail anchorage determines velocity and force produced by kinesin-14 between crosslinked microtubules. Nat. Commun. 2018;9:2214. doi: 10.1038/s41467-018-04656-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mansoon A, Balaz M, Albet-Torres N, Rosengren KJ. In vitro assays of molecular motors—impact of motor-surface interactions. Front. Biosci. 2008;13:5732–5754. doi: 10.2741/3112. [DOI] [PubMed] [Google Scholar]
- 40.Miller-Jaster KN, Petrie Aronin CE, Guilford WH. A quantitative comparison of blocking agents in the in vitro motility assay. Cell. Mol. Bioeng. 2012;5:44–51. doi: 10.1007/s12195-011-0202-y. [DOI] [Google Scholar]
- 41.Mitsuka M, Yamada T, Shimizu H. On the contraction of myosin-extracted skinned single fibers with active myosin fragments. J. Biochem. 1979;85:559–565. doi: 10.1093/oxfordjournals.jbchem.a132364. [DOI] [PubMed] [Google Scholar]
- 42.O’Connell CB, Tyska MJ, Mooseker MS. Myosin at work: Motor adaptations for a variety of cellular functions. Biochim. Biophys. Acta - Mol. Cell Res. 2007;1773:615–630. doi: 10.1016/j.bbamcr.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 43.Persson M, et al. Heavy meromyosin molecules extending more than 50 nm above adsorbing electronegative surfaces. Langmuir. 2010;26:9927–9936. doi: 10.1021/la100395a. [DOI] [PubMed] [Google Scholar]
- 44.Piazzesi G, et al. Skeletal muscle performance determined by modulation of number of myosin motors rather than motor force or stroke size. Cell. 2007;131:784–795. doi: 10.1016/j.cell.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 45.Pollard TD. Mechanics of cytokinesis in eukaryotes. Curr. Opin. Cell Biol. 2010;22:50–56. doi: 10.1016/j.ceb.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rahman MA, Salhotra A, Månsson A. Comparative analysis of widely used methods to remove nonfunctional myosin heads for the in vitro motility assay. J. Muscle Res. Cell Motil. 2018;39:175–187. doi: 10.1007/s10974-019-09505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rasicci DV, et al. Dilated cardiomyopathy mutation E525K in human beta-cardiac myosin stabilizes the interacting heads motif and super-relaxed state of myosin. BioRxiv. 2022;10:1465. doi: 10.7554/eLife.77415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rauch P, Jähnke T. Optical tweezers for quantitative force measurements and live cell experiments. Micros. Today. 2014;22:24–31. doi: 10.1017/S1551929514000741. [DOI] [Google Scholar]
- 49.Reinemann DN, et al. Collective force regulation in anti-parallel microtubule gliding by dimeric Kif15 kinesin motors. Curr. Biol. 2017;27:2810–2820.e6. doi: 10.1016/j.cub.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reinemann DN, Norris SR, Ohi R, Lang MJ. Processive kinesin-14 HSET exhibits directional flexibility depending on motor traffic. Curr. Biol. 2018;28:2356–2362.e5. doi: 10.1016/j.cub.2018.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruegg C, Veigel C, Molloy JE, Schmitz S, Sparrow JC, Fink RHA. Molecular motors: Force and movement generated by single myosin II molecules. Physiology. 2002;17:213–218. doi: 10.1152/nips.01389.2002. [DOI] [PubMed] [Google Scholar]
- 52.Santos A, Shauchuk Y, Cichoń U, Vavra KC. How actin tracks affect myosin motors. In: Coluccio LM, editor. Myosins. Cham: Springer; 2020. pp. 183–197. [DOI] [PubMed] [Google Scholar]
- 53.Schmid M, Toepfer CN. Cardiac myosin super relaxation (SRX): a perspective on fundamental biology, human disease and therapeutics. Biol. Open. 2021;10:1–11. doi: 10.1242/bio.057646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spudich JA. The myosin swinging cross-bridge model. Nat. Rev. Mol. Cell Biol. 2001;2:387–392. doi: 10.1038/35073086. [DOI] [PubMed] [Google Scholar]
- 55.Spudich JA, Finer J, Simmons B, Ruppel K, Patterson B, Uyeda T. Myosin structure and function. Cold Spring Harb. Symp. Quant. Biol. 1995;LX:783–791. doi: 10.1101/SQB.1995.060.01.084. [DOI] [PubMed] [Google Scholar]
- 56.Stachowiak MR, et al. Self-organization of myosin II in reconstituted actomyosin bundles. Biophys. J. 2012;103:1265–1274. doi: 10.1016/j.bpj.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stam S, Alberts J, Gardel ML, Munro E. Isoforms confer characteristic force generation and mechanosensation by myosin II filaments. Biophys. J. 2015;108:1997–2006. doi: 10.1016/j.bpj.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stewart MA, Franks-Skiba K, Chen S, Cooke R. Myosin ATP turnover rate is a mechanism involved in thermogenesis in resting skeletal muscle fibers. Proc. Natl. Acad. Sci. U.S.A. 2010;107:430–435. doi: 10.1073/pnas.0909468107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stewart TJ, Murthy V, Dugan SP, Baker JE. Velocity of myosin-based actin sliding depends on attachment and detachment kinetics and reaches a maximum when myosin-binding sites on actin saturate. J. Biol. Chem. 2021;297:101178. doi: 10.1016/j.jbc.2021.101178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sung J, et al. Harmonic force spectroscopy measures load-dependent kinetics of individual human β-cardiac myosin molecules. Nat. Commun. 2015;6:1–9. doi: 10.1038/ncomms8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Svoboda K, Block SM. Force and velocity measured for single kinesin molecules. Cell. 1994;77:773–784. doi: 10.1016/0092-8674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 62.Uyeda TQP, Iwadate Y, Umeki N, Nagasaki A, Yumura S. Stretching actin filaments within cells enhances their affinity for the myosin ii motor domain. PLoS ONE. 2011;6:e26200. doi: 10.1371/journal.pone.0026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wagoner JA, Dill KA. Evolution of mechanical cooperativity among myosin II motors. Proc. Natl. Acad. Sci. U.S.A. 2021;118:20. doi: 10.1073/pnas.2101871118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walcott S, Warshaw DM, Debold EP. Mechanical coupling between myosin molecules causes differences between ensemble and single-molecule measurements. Biophys. J. 2012;103:501–510. doi: 10.1016/j.bpj.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weirich KL, Stam S, Munro E, Gardel ML. Actin bundle architecture and mechanics regulate myosin II force generation. Biophys. J. 2021;120:1957–1970. doi: 10.1016/j.bpj.2021.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yanagida T, et al. Single-motor mechanics and models of the myosin motor. Philos. Trans. R. Soc. B. 2000;355:441–447. doi: 10.1098/rstb.2000.0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yasuda K, Shindo Y, Ishiwata S. Synchronous behavior of spontaneous oscillations of sarcomeres in skeletal myofibrils under isotonic conditions. Biophys. J. 1996;70:1823–1829. doi: 10.1016/S0006-3495(96)79747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.