Abstract
Background
The optimal radiotherapy dose for locally advanced esophageal squamous cell carcinoma in endemic areas treated with neoadjuvant concurrent chemoradiotherapy is unclear.
Methods
Eligible patients diagnosed between 2010 and 2019 were identified via the Taiwan Cancer Registry. We used propensity score (PS) weighting to balance observable potential confounders. The hazard ratio (HR) of death was compared between high dose (50–50.4 Gy) and low dose (40–41.4 Gy) radiotherapy. We also evaluated other outcomes and performed supplementary analyses via an alternative approach.
Results
Our study population consisted of 644 patients. The PS weight-adjusted HR of death was 0.92 (95% confidence interval: 0.7–1.19, p = 0.51). There were no statistically significant differences for other outcomes or supplementary analyses.
Conclusions
In this population-based study from an endemic area, we found no significant difference in overall survival between high vs. low radiotherapy doses.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-022-00594-y.
Keywords: Esophageal squamous cell carcinoma, Neoadjuvant concurrent chemoradiotherapy, Radiotherapy dose
Background
Esophageal cancer is a common cause of cancer mortality around the world [1]. The common histological types are squamous cell carcinoma in the East and adenocarcinoma in the West [1, 2]. For locally advanced esophageal squamous cell carcinoma (LA-ESCC) patients, neoadjuvant concurrent chemoradiotherapy (nCCRT) is one of the most commonly recommended approaches in the literature [1, 3–5].
However, the optimal radiotherapy (RT) dose of nCCRT for ESCC is unclear. In the current national comprehensive cancer network (NCCN) guidelines, a wide range of doses (41.4–50.4 Gy) are recommended [3]. Relatively low doses (40–41.4 Gy) were used in two landmark randomized controlled trials (RCTs) [6–8]. One may speculate whether a higher dose may lead to better outcomes due to the commonly believed radiotherapy dose response [9]. However, a systematic review (SR) published in 2021 reported that a low radiotherapy dose using modern techniques might provide the optimal therapeutic ratio for esophageal cancer patients treated with nCCRT [10]. Similar results were obtained in ESCC subgroup analyses [10]. However, only three non-RCTs included in this SR [10] directly compared high vs. low doses [11–13]. This systematic review [10] (including studies not limited to RCTs) also commented on results from three population-based studies [14–16] and stated that a dose of 40.0–41.4 Gy did not negatively impact overall survival (OS) compared to a higher dose. Another systematic review (including only RCTs comparing nCCRT vs. surgery) published in 2019 reported no difference in OS between low-dose and high-dose radiotherapy [17].
All of the abovementioned studies [11–16] that directly compared high vs. low radiotherapy doses were non-RCTs conducted in North America or Europe rather than an endemic area of ESCC [1, 2]. Due to an insufficiency of RT dose-relevant studies, as mentioned above, and since the benefit of nCCRT is possibly larger for ESCC than for adenocarcinoma, as reported in the long-term results of the CROSS study [18], we aimed to directly compare high vs. low RT doses in nCCRT for LA-ESCC patients from Taiwan, an endemic area of ESCC [2].
Materials and methods
Data source
In this retrospective cohort study, we used the Taiwan Cancer Registry (TCR) with personal identifiers removed for our data analysis. The TCR database provides comprehensive information (such as patient, disease, and treatment characteristics) and has been reported to be one of the highest quality cancer registries in the world [19, 20].
Study design, study population, and intervention
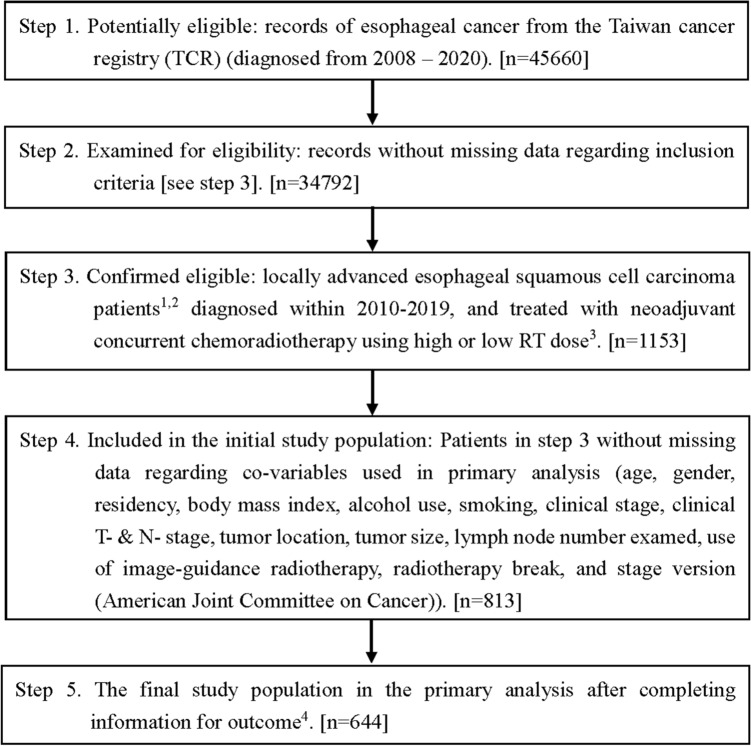
The study flowchart as suggested in the STROBE statement [21] is depicted in Fig. 1. We identified LA-ESCC adult patients diagnosed from 2010–2019 and treated with nCCRT followed by esophagectomy within 4–12 weeks after RT. We did not include patients diagnosed before 2010 because the T/N/M staging in the American Joint Committee on Cancer (AJCC) cancer staging manual 7th edition (used since 2010) was different from the AJCC 6th edition but the same as the AJCC 8th edition [22, 23]. Locally advanced stage was defined as clinical stage cT2-4aN0M0 or cT1-4aN1-3M0 by the AJCC 7th or 8th edition [22, 23]. We included adult patients aged 18–75 years who were treated with modern (3D conformal or intensity modulated) radiotherapy techniques according to the literature [10] and excluded those with multiple treatment records or prior cancer(s) to ensure data quality. We further selected patients treated with nCCRT using a high external beam radiotherapy dose (50 Gy/25 fractions or 50.4 Gy/28 fractions) or a low RT dose (40 Gy/20 fractions or 41.4 Gy/23 fractions) according to the literature [3, 6, 10, 18].
Fig. 1.
STROBE study flowchart and the number of individuals at each stage of the study. 1: We only included those treated (class 1–2) to ensure data consistency. 2: Clinical stage cT2-4aN0M0 or cT1-4aN1-3M0 from the 7th or 8th American Joint Committee on Cancer staging manual. 3: High RT dose (50 Gy/25 fractions or 50.4 Gy/28 fractions), low RT dose (40 Gy/20 fractions or 41.4 Gy/23 fractions). 4: Without missing information in the TCR and death registry regarding survival status and cause of death
Our explanatory variable of interest (high vs. low RT dose), primary outcome of interest (OS) and other supplementary outcomes (incidence of esophageal cancer mortality (IECM), pathological complete response (pCR) and R0 resection (no invasive cancer at surgical margin)) were determined via recordings in the TCR or death registry. We also defined the date of diagnosis as the index date and calculated the OS or IECM from the index date to the date of death or to Dec 31, 2020 (the censoring date of the death registry).
Covariates
We collected covariates to adjust for potential nonrandomized treatment selection. These covariates were modified from recent relevant studies and our clinical and research experience [6, 7, 24, 25]. Patient demographics (age, gender, residency), patient characteristics (body mass index (BMI), alcohol use, smoking), disease characteristics (clinical stage and version, clinical T- and N- stage, tumor location, tumor size, number of lymph nodes (LNs) examined), and treatment characteristics (use of image-guided radiotherapy (IGRT), radiotherapy break) were defined as follows. The patient residency region was classified as northern Taiwan or nonnorth. Smoking, alcohol use and the use of IGRT were classified as yes or no. The clinical stage was classified as 1–2 or 3–4A. The clinical T-stage was classified as 1–2 or 3–4. The clinical N-stage was classified as 0 or 1–3. Tumor location was classified as cervical vs. noncervical. Tumor size was classified by a diameter < = 5 cm or > 5 cm. Those with radiotherapy prolongation of more than one week were classified as yes for a radiotherapy break, whereas those without were classified as no. Cancer staging manual version was classified according to the AJCC 7th or 8th edition. The other covariates (age, BMI, number of LNs examined) were defined as continuous variables.
Statistical analysis
As advocated in the literature [26–29], we adopted the propensity score (PS) approach to balance the measured potential confounders and used PS weighting (PSW) as the primary framework for analysis [30].
In the primary analysis (PA), we evaluated the probability of receiving a high RT dose (vs. a low RT dose) as the PS via a logistic regression model based on the above covariates and then assessed the balance in covariates between groups after PSW using the overlap weight [30] via the standardized difference (SDif). During the entire follow-up period, we compared the hazard ratio (HR) of death between groups via the Cox proportional hazards model in the weighted sample for point estimation and used the bootstrap method to estimate the 95% confidence interval (95% CI) [31–33]. We used the E-value to assess the robustness of our findings regarding potential unmeasured confounder(s), as suggested in the literature [34]. We also evaluated the IECM between groups using the competing risk approach [35] and compared pCR and R0 resection between groups in the weighted sample [36].
In the supplementary analysis (SA), we performed seven analyses during revision to clarify the robustness of our findings. In SA-1, we used an alternative analytic approach (PS matching, PSM) for the primary study population by constructing a subgroup (1:1 PS matched cohort without replacement) and then compared the HR of death (via a robust variance estimator [31]) and other outcomes [37, 38] between groups. In SA-2, we explored the impact of the radiotherapy dose on additional outcomes (disease-free survival (DFS), local regional recurrence free survival (LRRFS), distant metastatic free survival (DMFS), and peri-operative mortality (POM)) among patients without missing information in the TCR. We used postoperative 30-day mortality as POM per the literature [39]. In SA-3 ~ SA-6, we performed subgroup analyses for patients with cT1-2, cT3-4, cN0, and cN1-3 disease. In SA-7, we performed the analyses among patients with additional information regarding surgical method (with or without minimally invasive esophagectomy, MIE) available in the TCR.
The statistical analyses were performed using SAS 9.4 software (SAS Institute, Cary, NC) and R version 4.2.0 (R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria).
Results
Study population
As shown in Fig. 1, the study population consisted of 644 eligible LA-ESCC adult patients who received a high RT dose (430 patients) or a low RT dose (214 patients) from 2010 to 2019. The patient characteristics are described in Table 1. Some covariates (age, residency, number of LNs examined, clinical stage, clinical T stage, RT break, and staging manual version) were imbalanced before PS weighting [40], but all covariates achieved balance (standardized differences ≈ 0) after PS weighting via the overlap weights.
Table 1.
Patient characteristics of the study population in the primary analysis
| High RT dose (n = 430) |
Low RT dose (n = 214) |
Standardized differencea | ||||
|---|---|---|---|---|---|---|
| Number or mean (SD)a |
(%)a | Number or mean (SD)a |
(%)a | Before PSW |
After PSW |
|
| Age | 54.98 (7.98) | 57.25 (8.45) | 0.276 | ≈ 0 | ||
| Gender | ||||||
| Female | 26 | (6) | 13 | (6) | 0.001 | ≈ 0 |
| Male | 404 | (94) | 201 | (94) | ||
| Residency | ||||||
| Nonnorth | 303 | (70) | 58 | (27) | 0.963 | ≈ 0 |
| North | 127 | (30) | 156 | (73) | ||
| BMI | 22.30 (3.61) | 22.43 (3.38) | 0.037 | ≈ 0 | ||
| Alcohol use | ||||||
| No | 60 | (14) | 39 | (18) | 0.116 | ≈ 0 |
| Yes | 370 | (86) | 175 | (82) | ||
| Smoking | ||||||
| No | 55 | (13) | 29 | (14) | 0.022 | ≈ 0 |
| Yes | 375 | (87) | 185 | (86) | ||
| Clinical stage | ||||||
| 1–2 | 84 | (20) | 19 | (9) | 0.309 | ≈ 0 |
| 3–4A | 346 | (80) | 195 | (91) | ||
| Clinical T-stage | ||||||
| T1–T2 | 64 | (15) | 10 | (5) | 0.349 | ≈ 0 |
| T3–T4 | 366 | (85) | 204 | (95) | ||
| Clinical N-stage | ||||||
| N0 | 53 | (12) | 14 | (7) | 0.199 | ≈ 0 |
| N1–N3 | 377 | (88) | 200 | (93) | ||
| Tumor location | ||||||
| Noncervical | 428 | (99) | 211 | (99) | 0.098 | ≈ 0 |
| Cervical | 2 | (1) | 3 | (1) | ||
| Tumor size | ||||||
| ≤ 5 cm | 202 | (47) | 101 | (47) | 0.004 | ≈ 0 |
| > 5 cm | 228 | (53) | 113 | (53) | ||
| Number of LNs examined | 22.44 (12.65) | 35.67 (17.61) | 0.863 | ≈ 0 | ||
| Use of IGRT | ||||||
| No | 338 | (79) | 155 | (72) | 0.144 | ≈ 0 |
| Yes | 92 | (21) | 59 | (28) | ||
| RT break | ||||||
| ≤ 1 week | 401 | (93) | 211 | (99) | 0.273 | ≈ 0 |
| > 1 week | 29 | (7) | 3 | (1) | ||
| AJCC staging manual version | ||||||
| 7th edition | 301 | (70) | 181 | (85) | 0.353 | ≈ 0 |
| 8th edition | 129 | (30) | 33 | (15) | ||
AJCC American Joint Committee on Cancer, BMI body mass index, IGRT image-guided radiotherapy, LN lymph node, PSW propensity score weighting, RT radiotherapy, SD standard deviation
aRounded
Primary analysis
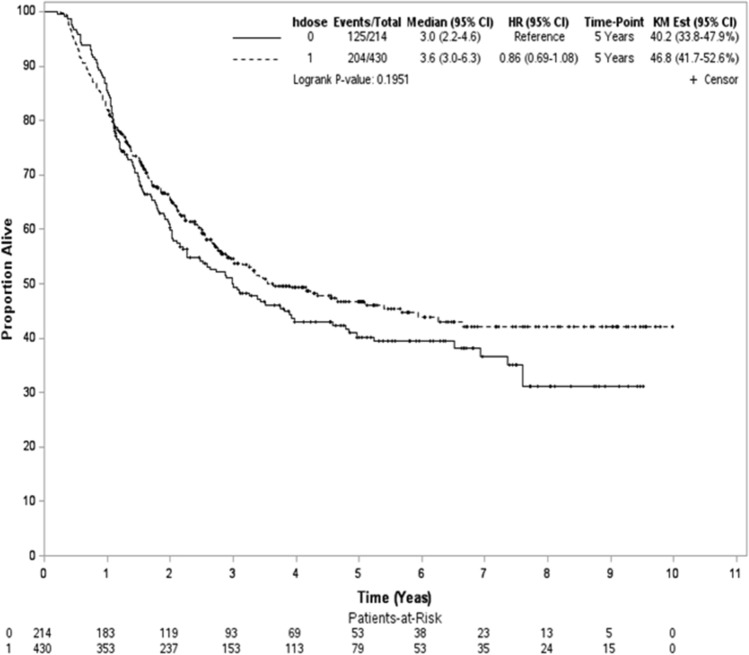
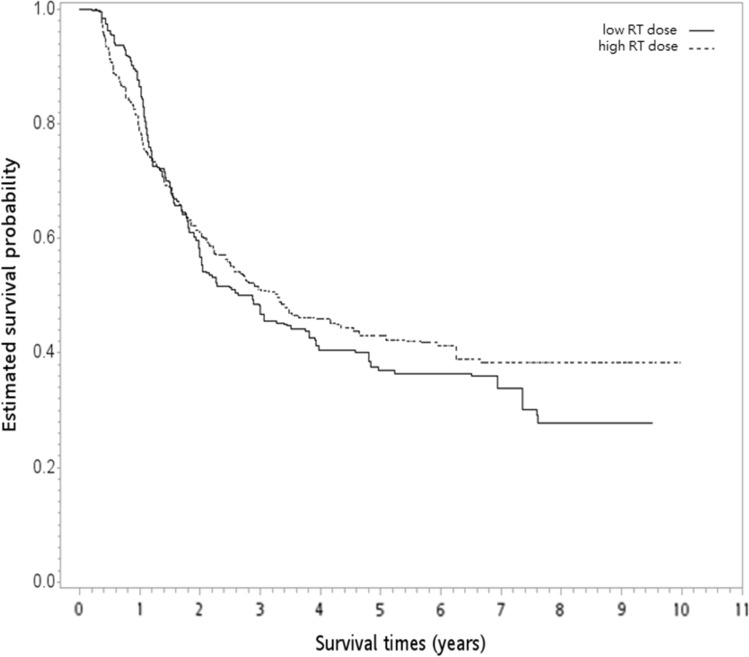
After a median follow-up of 27 months (range 3–119 months), 329 deaths were observed (204 and 125 patients for the high and low RT dose groups, respectively). The median follow-up was 49 months (range 12–119 months) for survivors. In the unadjusted analysis, the 5-year OS rates were 47% and 40% for the high and low RT dose groups, respectively (log-rank test, P = 0.2; Fig. 2). The overlap weight-adjusted OS curve is shown in Fig. 3. The 5-year PSW-adjusted OS rates for the two groups were 43% (high RT dose) and 37% (low RT dose). The PSW-adjusted HR of death was 0.92 (95% CI 0.70–1.19, P = 0.51) when a high RT dose was compared to a standard RT dose. The observed HR of 0.92 for OS could be explained by an unmeasured confounder associated with the selection of treatment (high or low RT dose) and survival by a risk ratio of 1.31 (E-value)-fold each, but weaker confounding factors could not. The result was not significantly different for the IECM (HR = 0.98, P = 0.93). We also found that the rates of pCR and R0 resection were not significantly different between the groups. The PSW-adjusted relative risks were 1.11 (95% CI 0.90–1.33) for pCR and 1 (95% CI 0.96–1.04) for R0 resection.
Fig. 2.
Kaplan–Meier unadjusted overall survival curve (in years) in the primary analysis. hdose high dose
Fig. 3.
The overlap weight-adjusted overall survival curve (in years) in the primary analysis. RT radiotherapy
Supplementary analysis
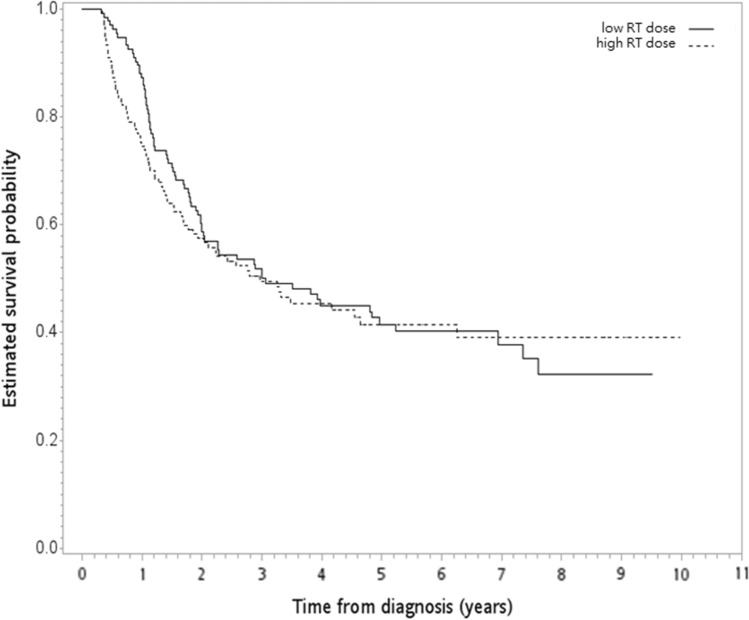
The constructed PS-matched subgroup (n = 268) for SA-1 is shown in Table 2, and all covariates were balanced after PSM [40]. The Kaplan–Meier OS curve is shown in Fig. 4. The 5-year OS rates were 42% (high RT dose) and 42% (low RT dose), and there was no statistically significant difference between the groups (HR = 1.07, 95% CI 0.77–1.49, P = 0.68). The results were not significant for the IECM (HR = 1.05; 95% CI = 0.73–1.52, p = 0.79). We also found that the rates of pCR and R0 resection were not significantly different between the groups (P = 1 for both pCR and R0). In SA-2, there were 600 patients without missing information in the TCR regarding recurrence with a good balance of covariates after PSW (Table S1). We found no significant differences in DFS (HR = 0.96, P = 0.77), LRRFS (HR = 0.77, P = 0.14), or DMFS (HR = 1.08, P = 0.33). The rate of POM was also similar between the two groups, with a PSW-adjusted relative risk of 0.56 (P = 0.21). In SA-3, among patients with cT1-2 disease, we found that the distribution of covariates between the groups was balanced after PSW (Table S2), and the PSW-adjusted HR of death was 1.64 (P = 0.47) when a high RT dose was compared to a standard RT dose. In SA-4, SA-5 and SA-6, the distribution of covariates between the groups was also balanced after PSW (Tables S3, S4 and S5), and the corresponding results were 0.89 (P = 0.42), 0.44 (P = 0.64), and 0.99 (P = 0.94) for patients with cT3-4, cN0, and cN1-3 disease, respectively. In SA-7, among patients with additional information regarding the surgical method, the distribution of covariates (including the surgical method) between the groups was also balanced after PSW (see Table S6), and the PSW-adjusted HR of death was 1.10 (P = 0.28) when a high RT dose was compared to a standard RT dose.
Table 2.
Patient characteristics of the PS-matched subgroup
| High RT dose (n = 134) |
Low RT dose (n = 134) |
||||
|---|---|---|---|---|---|
| Number or mean (SD)a |
(%)a | Number or mean (SD)a |
(%)a | Standardized differencea | |
| Age | 56.54 (8.55) | 56.30 (8.36) | 0.028 | ||
| Gender | |||||
| Female | 9 | (7) | 6 | (4) | 0.098 |
| Male | 125 | (93) | 128 | (96) | |
| Residency | |||||
| Nonnorth | 63 | (47) | 55 | (41) | 0.120 |
| North | 71 | (53) | 79 | (59) | |
| BMI | 21.89 (3.74) | 22.49 (3.41) | 0.169 | ||
| Alcohol use | |||||
| No | 24 | (18) | 24 | (18) | 0 |
| Yes | 110 | (82) | 110 | (82) | |
| Smoking | |||||
| No | 20 | (15) | 19 | (14) | 0.021 |
| Yes | 114 | (85) | 115 | (86) | |
| Clinical stage | |||||
| 1–2 | 20 | (15) | 15 | (11) | 0.111 |
| 3–4A | 114 | (85) | 119 | (89) | |
| Clinical T-stage | |||||
| T1–T2 | 10 | (7) | 9 | (7) | 0.029 |
| T3–T4 | 124 | (93) | 125 | (93) | |
| Clinical N-stage | |||||
| N0 | 14 | (10) | 11 | (8) | 0.077 |
| N1–N3 | 120 | (90) | 123 | (92) | |
| Tumor location | |||||
| Noncervical | 134 | (100) | 134 | (100) | |
| Cervical | 0 | (0) | 0 | (0) | |
| Tumor size | |||||
| ≤ 5 cm | 62 | (46) | 60 | (45) | 0.030 |
| > 5 cm | 72 | (54) | 74 | (55) | |
| Number of LNs examined | 28.13 (14.92) | 27.53 (13.80) | 0.042 | ||
| Use of IGRT | |||||
| No | 100 | (75) | 101 | (75) | 0.017 |
| Yes | 34 | (25) | 33 | (25) | |
| RT break | |||||
| ≤ 1 week | 129 | (96) | 131 | (98) | 0.088 |
| > 1 week | 5 | (4) | 3 | (2) | |
| AJCC staging manual version | |||||
| 7th edition | 109 | (81) | 107 | (80) | 0.038 |
| 8th edition | 25 | (19) | 27 | (20) | |
AJCC American Joint Committee on Cancer, BMI body mass index, IGRT image-guided radiotherapy, LN lymph node, PSW propensity score weighting, RT radiotherapy, SD standard deviation
aRounded
Fig. 4.
Kaplan‒Meier overall survival curve (in years) in supplementary analysis 1. RT radiotherapy
Discussion
In this population-based study, we found no significant difference in OS between high vs. low RT doses, and there were no significant differences between other endpoints. To our knowledge, this was the first population-based study from an endemic area of ESCC.
The results of our study were in line with the results from a previous systematic review published in 2019 [17]. The lack of an obvious radiotherapy dose response in this dose range may be in line with the lack of a radiotherapy dose response within 50–60 Gy in definitive concurrent chemoradiotherapy for esophageal cancer [41–43]. Another possibility was that although there was a radiotherapy dose response, the clinical benefit was diminished after subsequent esophagectomy. A similar possibility has been reported in rectal cancer patients treated with neoadjuvant therapies [44]. Therefore, the use of a low dose in current clinical practice seems reasonable for patients deemed to receive esophagectomy. However, the optimal dose may be unclear in the era of adjuvant immunotherapy [45, 46].
There were several limitations of the current study. First, potential unobservable covariates are always possible in nonrandomized studies, although we used the propensity score method to adjust for observable covariates. Therefore, some factors, such as performance status, chemotherapy regimen and definition of radiotherapy volume, which are not the same for every patient, were not included in our analyses due to data limitations. However, we reported the E-value to evaluate the robustness of our findings to potential unobservable covariates, as suggested in the literature [34]. Second, the use of postrecurrence therapy (such as immunotherapy [47]) may have impacted our primary endpoint (OS) but could not be evaluated due to data limitations in the TCR. Finally, other endpoints, such as quality of life and peri-operative or late complications (all were likely to be exacerbated by high-dose radiotherapy), in addition to our primary endpoint (OS), might also be relevant, but these were not investigated due to concerns regarding data availability.
Conclusions
In this population-based study from an endemic area, we found no significant difference in OS between high vs. low radiotherapy doses for locally advanced esophageal squamous cell carcinoma patients treated with neoadjuvant concurrent chemoradiotherapy followed by esophagectomy. Further studies are needed to clarify our findings.
Supplementary Information
Acknowledgements
This work was funded by the Health Promotion Administration, Ministry of Health and Welfare (Grant No. A1101117) and the Tobacco Health and Welfare Taxation. The content of this research may not represent the opinion of the Health Promotion Administration, Ministry of Health and Welfare. The authors thank American Journal Experts for their editorial contributions.
Abbreviations
- 95% CI
95% Confidence interval
- AJCC
American Joint Committee on Cancer
- BMI
Body mass index
- HR
Hazard ratio
- IECM
Incidence of esophageal cancer mortality
- IGRT
Image-guided radiotherapy
- LA-ESCC
Locally advanced esophageal squamous cell carcinoma
- LN
Lymph node
- nCCRT
Neoadjuvant concurrent chemoradiotherapy
- OS
Overall survival
- pCR
Pathological complete response
- PA
Primary analysis
- PS
Propensity score
- PSM
PS matching
- PSW
PS weighting
- RCT
Randomized controlled trial
- RT
Radiotherapy
- SA
Supplementary analysis
- SDif
Standardized difference
- SR
Systematic review
- TCR
Taiwan Cancer Registry
Author contributions
CCR participated in the concept and design, analysis and interpretation of data, and drafting of the manuscript. CCY, CYH, HCJ, KHY, and LYC participated in the concept and design, interpretation of data, and drafting of the manuscript. LCC participated in the concept and design, analysis of data and drafting of the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Data availability
The data that support the findings of this study are available from the Taiwan Cancer Registry Center, but restrictions apply to the availability of these data, which were used under license for the current study and are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of the Taiwan Cancer Registry Center.
Declarations
Ethics approval and consent to participate
This study was approved by the National Health Research Institute review committee (EC1090502-E). This was a retrospective study performed in accordance with the Declaration of Helsinki. Informed consent was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chia-Chin Li, Chih-Yi Chen, Ying-Hsiang Chou, Chih-Jen Huang, Hsiu-Ying Ku, and Ying-Chun Lin are contributed equally
References
- 1.Waters JK, Reznik SI. Update on management of squamous cell esophageal cancer. Curr Oncol Rep. 2022;24(3):375–385. doi: 10.1007/s11912-021-01153-4. [DOI] [PubMed] [Google Scholar]
- 2.Chien CR, Lin CY, Chen CY. Re: Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2009;101(20):1428. doi: 10.1093/jnci/djp304. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network Guidelines for Esophageal and Esophagogastric Junction Cancers, version 2.2022. https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf. Accessed 18 Jun 2022
- 4.Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO clinical practice guidelines. https://www.esmo.org/guidelines/guidelines-by-topic/gastrointestinal-cancers/oesophageal-cancer. Accessed 18 Jun 2022. [DOI] [PubMed]
- 5.Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: part 2. Esophagus. 2019;16(1):25–43. doi: 10.1007/s10388-018-0642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang H, Liu H, Chen Y, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized open-label clinical trial. J Clin Oncol. 2018;36(27):2796–2803. doi: 10.1200/JCO.2018.79.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 8.van Heijl M, van Lanschot JJ, Koppert LB, et al. Neoadjuvant chemoradiation followed by surgery versus surgery alone for patients with adenocarcinoma or squamous cell carcinoma of the esophagus (CROSS) BMC Surg. 2008;8:21. doi: 10.1186/1471-2482-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moiseenko V, Marks LB, Grimm J, et al. A primer on dose-response data modeling in radiation therapy. Int J Radiat Oncol Biol Phys. 2021;110(1):11–20. doi: 10.1016/j.ijrobp.2020.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Liu H, Sun C, et al. Comparison of clinical efficacy of neoadjuvant chemoradiation therapy between lower and higher radiation doses for carcinoma of the esophagus and gastroesophageal junction: a systematic review. Int J Radiat Oncol Biol Phys. 2021;111(2):405–416. doi: 10.1016/j.ijrobp.2021.04.031. [DOI] [PubMed] [Google Scholar]
- 11.Fakhrian K, Ordu AD, Lordick F, et al. Long-term outcomes of trimodality treatment for squamous cell carcinoma of the esophagus with cisplatin and/or 5-FU: more than 20 years' experience at a single institution. Strahlenther Onkol. 2014;190(12):1133–1140. doi: 10.1007/s00066-014-0711-4. [DOI] [PubMed] [Google Scholar]
- 12.Münch S, Pigorsch SU, Feith M, et al. Comparison of neoadjuvant chemoradiation with carboplatin/ paclitaxel or cisplatin/ 5-fluoruracil in patients with squamous cell carcinoma of the esophagus. Radiat Oncol. 2017;12(1):182. doi: 10.1186/s13014-017-0904-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blom RLGM, Sosef MN, Nap M, et al. Comparison of two neoadjuvant chemoradiotherapy regimens in patients with potentially curable esophageal carcinoma. Dis Esophagus. 2014;27(4):380–387. doi: 10.1111/dote.12110. [DOI] [PubMed] [Google Scholar]
- 14.Ji KSY, Thomas SM, Roman SA, et al. Low- vs. high-dose neoadjuvant radiation in trimodality treatment of locally advanced esophageal cancer. J Gastrointest Surg. 2019;23(5):885–894. doi: 10.1007/s11605-018-4007-3. [DOI] [PubMed] [Google Scholar]
- 15.Ising MS, Marino K, Trivedi JR, et al. Influence of neoadjuvant radiation dose on patients undergoing esophagectomy and survival in locally advanced esophageal cancer. J Gastrointest Surg. 2019;23(4):670–678. doi: 10.1007/s11605-019-04141-z. [DOI] [PubMed] [Google Scholar]
- 16.Haque W, Verma V, Butler EB, et al. Radiation dose in neoadjuvant chemoradiation therapy for esophageal cancer: patterns of care and outcomes from the National Cancer Data Base. J Gastrointest Oncol. 2018;9(1):80–89. doi: 10.21037/jgo.2017.09.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engel S, Awerbuch A, Kwon D, et al. Optimal radiation dosing in concurrent neoadjuvant chemoradiation for resectable esophageal cancer: a meta-analysis. J Gastrointest Oncol. 2019;10(3):391–399. doi: 10.21037/jgo.2019.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eyck BM, van Lanschot JJB, Hulshof MCCM, et al. Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial. J Clin Oncol. 2021;39(18):1995–2004. doi: 10.1200/JCO.20.03614. [DOI] [PubMed] [Google Scholar]
- 19.Chiang CJ, You SL, Chen CJ, et al. Quality assessment and improvement of nationwide cancer registration system in Taiwan: a review. Jpn J Clin Oncol. 2015;45(3):291–296. doi: 10.1093/jjco/hyu211. [DOI] [PubMed] [Google Scholar]
- 20.Chiang CJ, Wang YW, Lee WC. Taiwan's nationwide cancer registry system of 40 years: past, present, and future. J Formos Med Assoc. 2019;118(5):856–858. doi: 10.1016/j.jfma.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 21.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 22.Edge SB, Byrd DR, Compton CC, et al. AJCC cancer staging manual. 7. New York, NY: Springer; 2010. [Google Scholar]
- 23.Amin MB, Gress DM, Meyer Vega LR, et al. AJCC cancer staging manual. 8. New York, NY: Springer; 2017. [Google Scholar]
- 24.Kuo YH, Chien YW, Chen PR, et al. Impact of the interval between neoadjuvant concurrent chemoradiotherapy and esophagectomy in the modern era: a population-based propensity-score-matched retrospective cohort study in Asia. World J Surg Oncol. 2019;17(1):222. doi: 10.1186/s12957-019-1712-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li CC, Chen CY, Chou YH, et al. Optimal radiotherapy dose in cervical esophageal squamous cell carcinoma patients treated with definitive concurrent chemoradiotherapy: a population based study. Thorac Cancer. 2021;12(14):2065–2071. doi: 10.1111/1759-7714.14009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao XI, Wang X, Speicher PJ, et al. Reporting and guidelines in propensity score analysis: a systematic review of cancer and cancer surgical studies. J Natl Cancer Inst. 2017;109(8):djw323. doi: 10.1093/jnci/djw323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fundamentals of Causal Inference with R. Babette A. Brumback. Chapman & Hall/CRC; 2022. ISBN: 978-0-3677-0505-3.
- 28.Webster-Clark M, Stürmer T, Wang T, et al. Using propensity scores to estimate effects of treatment initiation decisions: state of the science. Stat Med. 2021;40(7):1718–1735. doi: 10.1002/sim.8866. [DOI] [PubMed] [Google Scholar]
- 29.Goetghebeur E, Cessie S, De Stavola B, et al. Formulating causal questions and principled statistical answers. Stat Med. 2020;39(30):4922–4948. doi: 10.1002/sim.8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas LE, Li F, Pencina MJ. Overlap weighting: a propensity score method that mimics attributes of a randomized clinical trial. JAMA. 2020;323(23):2417–2418. doi: 10.1001/jama.2020.7819. [DOI] [PubMed] [Google Scholar]
- 31.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33(7):1242–1258. doi: 10.1002/sim.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75(1):45–49. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med. 2016;35(30):5642–5655. doi: 10.1002/sim.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haneuse S, VanderWeele TJ, Arterburn D. Using the E-Value to assess the potential effect of unmeasured confounding in observational studies. JAMA. 2019;321(6):602–603. doi: 10.1001/jama.2018.21554. [DOI] [PubMed] [Google Scholar]
- 35.Bolch CA, Chu H, Jarosek S, et al. Inverse probability of treatment-weighted competing risks analysis: an application on long-term risk of urinary adverse events after prostate cancer treatments. BMC Med Res Methodol. 2017;17(1):93. doi: 10.1186/s12874-017-0367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mao H, Li L, Greene T. Propensity score weighting analysis and treatment effect discovery. Stat Methods Med Res. 2019;28(8):2439–2454. doi: 10.1177/0962280218781171. [DOI] [PubMed] [Google Scholar]
- 37.Austin PC, Fine JP. Propensity-score matching with competing risks in survival analysis. Stat Med. 2019;38(5):751–777. doi: 10.1002/sim.8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Austin PC, Chiu M, Ko DT, et al. et al. Propensity score matching for estimating treatment effect. In: Faries DE, Leon AC, Haro JM, et al.et al., editors. Analysis of observational health care data using SAS. Cary, NC: SAS Institute; 2010. pp. 51–84. [Google Scholar]
- 39.van Kooten RT, Voeten DM, Steyerberg EW, et al. Patient-related prognostic factors for anastomotic leakage, major complications, and short-term mortality following esophagectomy for cancer: a systematic review and meta-analyses. Ann Surg Oncol. 2022;29(2):1358–1373. doi: 10.1245/s10434-021-10734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garrido MM, Kelley AS, Paris J, et al. Methods for constructing and assessing propensity scores. Health Serv Res. 2014;49(5):1701–1720. doi: 10.1111/1475-6773.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hulshof MCCM, Geijsen ED, Rozema T, et al. Randomized study on dose escalation in definitive chemoradiation for patients with locally advanced esophageal cancer (ARTDECO Study) J Clin Oncol. 2021;39(25):2816–2824. doi: 10.1200/JCO.20.03697. [DOI] [PubMed] [Google Scholar]
- 42.Xu Y, Dong B, Zhu W, et al. A phase III multicenter randomized clinical trial of 60 Gy versus 50 Gy radiation dose in concurrent chemoradiotherapy for inoperable esophageal squamous cell carcinoma. Clin Cancer Res. 2022;28(9):1792–1799. doi: 10.1158/1078-0432.CCR-21-3843. [DOI] [PubMed] [Google Scholar]
- 43.Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94–05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20(5):1167–1174. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 44.Deng Y, Chi P, Lan P, et al. Neoadjuvant modified FOLFOX6 with or without radiation versus fluorouracil plus radiation for locally advanced rectal cancer: final results of the Chinese FOWARC trial. J Clin Oncol. 2019;37(34):3223–3233. doi: 10.1200/JCO.18.02309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. 2021;384(13):1191–1203. doi: 10.1056/NEJMoa2032125. [DOI] [PubMed] [Google Scholar]
- 46.Shah MA, Hofstetter WL, Kennedy EB, et al. Immunotherapy in patients with locally advanced esophageal carcinoma: ASCO treatment of locally advanced esophageal carcinoma guideline rapid recommendation update. J Clin Oncol. 2021;39(28):3182–3184. doi: 10.1200/JCO.21.01831. [DOI] [PubMed] [Google Scholar]
- 47.Kojima T, Shah MA, Muro K, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38(35):4138–4148. doi: 10.1200/JCO.20.01888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the Taiwan Cancer Registry Center, but restrictions apply to the availability of these data, which were used under license for the current study and are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of the Taiwan Cancer Registry Center.