Abstract
Introduction
Extracellular vesicles (EVs) are promising carriers for the delivery of biotherapeutic cargo such as RNA and proteins. We have previously demonstrated that the innate EV mitochondria in microvesicles (MVs), but not exosomes (EXOs) can be transferred to recipient BECs and mouse brain slice neurons. Here, we sought to determine if the innate EV mitochondrial load can be further increased via increasing mitochondrial biogenesis in the donor cells. We hypothesized that mitochondria-enriched EVs (“mito-EVs”) may increase the recipient BEC ATP levels to a greater extent than naïve MVs.
Methods
We treated NIH/3T3, a fibroblast cell line and hCMEC/D3, a human brain endothelial cell (BEC) line using resveratrol to activate peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α), the central mediator of mitochondrial biogenesis. Naïve EVs and mito-EVs isolated from the non-activated and activated donor cells were characterized using transmission electron microscopy, dynamic light scattering and nanoparticle tracking analysis. The effect of mito-EVs on resulting ATP levels in the recipient BECs were determined using Cell Titer Glo ATP assay. The uptake of Mitotracker Red-stained EVs into recipient BECs and their colocalization with recipient BEC mitochondria were studied using flow cytometry and fluorescence microscopy.
Results
Resveratrol treatment increased PGC-1α expression in the donor cells. Mito-MVs but not mito-EXOs showed increased expression of mitochondrial markers ATP5A and TOMM20 compared to naïve MVs. TEM images showed that a greater number of mito-MVs contained mitochondria compared to naïve MVs. Mito-MVs but not mito-EXOs showed a larger particle diameter compared to their naïve EV counterparts from the non-activated cells suggesting increased mitochondria incorporation. Mito-EVs were generated at higher particle concentrations compared to naïve EVs from non-activated cells. Mito-EVs increased the cellular ATP levels and transferred their mitochondrial load into the recipient BECs. Mito-MV mitochondria also colocalized with recipient BEC mitochondria.
Conclusions
Our results suggest that the pharmacological modulation of mitochondrial biogenesis in the donor cells can change the mitochondrial load in the secreted MVs. Outcomes of physicochemical characterization studies and biological assays confirmed the superior effects of mito-MVs compared to naïve MVs—suggesting their potential to improve mitochondrial function in neurovascular and neurodegenerative diseases.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12195-022-00738-8.
Keywords: Exosomes, Microvesicles, EVs, Mitochondria, PGC-1α, ATP, Brain endothelial cells, Metabolic function
Introduction
Extracellular vesicles (EVs) are naturally secreted by cells and play a role in intercellular communication.45,51,60 The innate ability of EVs to transfer their cargo, including nucleic acids, lipids, and proteins, makes them attractive drug delivery systems.4,14,19,20,27,33,41,42,61,63,70 EVs are being developed as carriers for drugs such as RNA, proteins, and small-molecule drugs. EVs can be classified based on their size into small EVs and medium-to-large EVs that are also referred to as exosomes (EXOs) and ectosomes/microvesicles (MVs), respectively.45 EXOs are reported to contain mitochondrial DNA and mitochondrial proteins, and MVs incorporate mitochondria during their biogenesis.26,29,40,48,49,53,56–58,71 We collectively refer to EXOs and MVs as EVs wherever applicable but will also distinguish their effects as fit. While the drug delivery field primarily focuses on developing EVs as drug carriers, we focused on their capability to deliver their innate mitochondria38—the overlords that control cellular bioenergetics among other functions. Our prior work demonstrated that MVs contain polarized mitochondria and they transfer their mitochondrial load to recipient brain endothelial cells (BECs) and neurons in mouse brain slices.17 While EVs (EXOs and MVs) increased ATP levels, we specifically demonstrated that MVs but not EXOs increase mitochondrial function via increasing oxygen consumption and glycolytic rates in the recipient BECs.17 We also showed that MVs colocalized with the recipient cell’s mitochondrial network, likely suggesting mitochondrial fusion.11 The MV-mediated increase in mitochondrial function suggested that the transfer of mitochondria, but not mitochondrial proteins and mitochondrial DNA, is critical for functional mitochondrial delivery. Collectively, our results suggested that MVs can be developed as delivery carriers for functional mitochondria.38
The current work aims to test the hypothesis that increasing mitochondrial biogenesis in the parent/donor cells will consequently increase the mitochondrial load in the secreted EVs—mito-EVs. Testing this hypothesis also broadly addresses whether manipulating the donor cell’s content will subsequently affect the vesicular content in the secreted EVs. We chose to answer this question by focusing on activating the mitochondrial biogenesis in the donor cells, as our past work has demonstrated the feasibility of harnessing the innate EV mitochondrial load as a therapeutic cargo.9,11,12,17,38 Peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α) is a transcriptional coactivator and a central regulator of cellular mitochondrial biogenesis.21,54,55,62,68,69 In this proof-of-concept study, we used resveratrol to activate PGC-1α in the donor cells. Resveratrol is a natural inflammatory compound with a favorable safety profile.6,7,50,72 Resveratrol increased mitochondrial mass and mitochondrial DNA by inducing mitochondrial biogenesis factors such as PGC-1α, nuclear respiratory factor-1 and mitochondrial transcription factor A in human coronary arterial endothelial cells.7 We hypothesized that activation of the PGC-1α pathway via resveratrol will increase the mitochondrial biogenesis in the donor BECs, subsequently resulting in increased MV mitochondrial load (“mito-MV”) that may increase the recipient BEC ATP levels to a greater extent compared to the naïve MVs (from non-activated cells). As a result, mito-MV mediated increase in mitochondrial function and BEC ATP levels under ischemic conditions may improve the post-stroke outcomes.
Ikeda et al. reported that mitochondria-containing EVs isolated from stem cell-derived cardiomyocytes contained mitochondrial biogenesis-related RNAs, including PGC-1α. They also noted that PGC-1α activated mitochondrial biogenesis in the recipient human-induced pluripotent stem-cell-derived cardiomyocytes. The isolated EVs were smaller than the expected size of eukaryotic cell mitochondria (0.5–3.0 µm), and the authors suggested that mitochondria fragmented during mitochondrial fission were likely entrapped in the EVs. However, the EV-mitochondria retained its structure and function as previously demonstrated by us and others,11,17,56 indicating its therapeutic potential. We hypothesized that mitochondria-enriched EVs (mito-EVs) generated from the activated cells may contain a greater mitochondrial load compared to naïve EVs generated from control, untreated cells. EVs transfer their mitochondrial load to the recipient cells leading to increased bioenergetics.11,17,26,29,40,48,49,53,56–58,71 Therefore, we hypothesize that the mito-EVs may also transfer their mitochondrial load to recipient cells.
Mitochondrial dysfunction plays a causal role in a variety of acute and chronic diseases such as type II diabetes, drug/toxin-induced liver injury and nephrotoxicity, cardiovascular and neurodegenerative diseases.2,8,18,22,32,36,39,64,66,69 In addition to targeting drugs to the mitochondria,65 we propose that transfer of mitochondria via EVs may be a promising approach to rescue mitochondrial function in the damaged cells and tissues.38 For example, protection of mitochondrial integrity and function is a potent strategy to limit stroke-induced damage.1,30,37 Stroke-induced mitochondrial damage in BECs results in endothelial and neural cell death and contributes to impaired blood–brain barrier integrity.16 Therefore, restoring mitochondrial function in BECs via exogenous delivery of mitochondria is a promising approach to protect the neurovascular unit in ischemic stroke and also to reverse mitochondrial dysfunction in multiple neurodegenerative diseases.38
Damaged mitochondria in BECs increases BBB permeability and worsens post-stroke outcomes.16,52,67 Importantly, BECs have a greater reliance on their mitochondrial load compared to peripheral/non-BBB endothelial cells—allowing the orchestrated maintenance of its structural integrity and metabolic function.34,46 Our laboratory has championed the paradigm that supplementation of healthy mitochondria to BECs lining the damaged/ischemic BBB will protect its structural and function and limit post-stroke damage.9 Therefore, we use a cell line model of human BECs to isolate EVs allowing us to harness its unique membrane signature41 to increase uptake into recipient BECs.
In this work, we used two cell models: hCMEC/D3, a human BEC cell line and NIH/3T3 mouse fibroblasts as donor cells to activate PGC-1α via resveratrol treatment. The rationale behind choosing two unrelated cell line models was to avoid any unintentional bias in studying the effects of resveratrol. Secondly, our prior work showed that naïve EVs derived from donor BECs show increased uptake into the recipient BECs compared to EVs from a non-homologous, macrophage cell line.17 We wanted to determine if the BEC-derived mito-EVs retain the inherent targeting capabilities by comparing mito-EVs from fibroblasts vs. BECs. In this proof-of-concept study, we report physicochemical characteristics of mito-EVs studied using transmission electron microscopy, dynamic light scattering and nanoparticle tracking analysis. Additionally, we demonstrated that mito-MV mitochondria colocalized with recipient BEC mitochondria. Mito-MVs significantly increased recipient BEC ATP levels compared to naïve MVs under conditions of ischemia/oxygen–glucose deprivation.
Materials
Resveratrol was purchased from Tokyo Chemical Industry (Portland, OR). Branched polyethyleneimine (PEI, 25 kDa), L-ascorbic acid, hydrocortisone, and human basic fibroblast growth factor, rotenone, and oligomycin A were purchased from Sigma-Aldrich (Saint Louis, MO). Cell Titer Glo 2.0 reagent for ATP assay was purchased from Promega (Madison, WI) and Pierce BCA protein assay kit and MitoTracker Deep Red FM, MitoTracker green, and calcein-AM were procured from Thermo Scientific (Rockford, IL). Type I Collagen was purchased from Corning (Discovery Labware Inc, Bedford, MA), and endothelial cell basal medium-2 (EBM-2) was procured from Lonza (Walkersville, MD). Penicillin–Streptomycin solution and Chemically Defined Lipid Concentrate were procured from Invitrogen (Carlsbad, CA). Heat-inactivated fetal bovine serum was bought from Hyclone Laboratories (Logan, UT). RIPA buffer was purchased from Alfa Aesar (Ward Hill, MA) and aprotinin was purchased from Fisher Bioreagents (Fair Lawn, NJ). Nitrocellulose membrane was purchased from GE Healthcare Life Sciences (Germany), and Intercept blocking buffer was purchased from LI-COR Inc. (Lincoln, NE). Polycarbonate centrifuge tubes were purchased from Beckman Coulter, Inc. (Brea, CA). Mouse monoclonal antibodies against ATP5A, TOMM20, GAPDH, and PGC1α were purchased from Abcam (Waltham, MA). Alexa Fluor 790-conjugated donkey anti-mouse and anti-rabbit IgG were received from Jackson ImmunoResearch Lab Inc (West Grove, PA).
Cell Lines
We used the human cerebral microvascular endothelial cell line (hCMEC/D3, Cedarlane Laboratories, Burlington, Ontario) between passage numbers (P) 25 and P35 as a brain endothelial cell model in all experiments.12,17 hCMEC/D3 cells were cultured in tissue culture flasks and well plates pre-coated with rat collagen I (0.15 mg/mL) in complete growth medium containing endothelial cell basal medium (EBM-2) supplemented with fetal bovine serum (5% FBS), penicillin (100 units/mL)-streptomycin (100 μg/mL) (pen/strep), hydrocortisone (1.4 µM), ascorbic acid (5 µg/mL), chemically defined lipid concentrate (0.01%), 10 mM HEPES (pH 7.4), and basic fibroblast growth factor (1 ng/mL). The cells were maintained in a humidified 5% CO2 incubator at 37 ± 0.5 °C (Isotemp, Thermo Fisher Scientific) and replenished with complete growth medium every 48 h until they formed confluent monolayers. For sub-culturing and plating, hCMEC/D3 cells were washed using 1× phosphate buffer saline (PBS), detached with 1× TrypLE express (gibco, Denmark) followed by neutralization with complete growth medium. Cell suspensions stained with trypan blue (1:1 v/v ratio) were counted to calculate % live cells using a hemocytometer before passaging at 1:3 to 1:5 v/v ratios or plating in well plates at defined cell densities.
Mouse embryo fibroblast NIH/3T3 cell line (ATCC, Manassas, VA) was a kind gift from Dr. Ellen S. Gawalt (Duquesne University, Pittsburgh, PA). Cells between P10 and P20 were used in all experiments. NIH/3T3 cells were grown in complete growth medium composed of modified DMEM/F12 supplemented with 10% FBS and 1% pen-strep solution. Culture medium was replaced with pre-warmed fresh medium every 48 h until cells reached 80–90% confluency. The cells were washed with 1× PBS, detached with TrypLE express, followed by neutralization with complete growth medium prior to passaging or plating.
Activation of hCMEC/D3 Brain Endothelial Cells and NIH/3T3 Fibroblasts with Resveratrol and Preparation of Cell Lysate for Western Blotting
hCMEC/D3 brain endothelial cells (BECs) and NIH/3T3 fibroblasts were seeded in a 24-well plate at 100,000 cells/well-seeding density in complete growth medium and cultured for 48 h in a humidified incubator at 37 °C. The cells were treated with different concentrations of resveratrol ranging from 0.1 to 50 µM in complete growth medium for 24 h. Untreated cells were incubated with complete growth medium and used as the control for the treatment groups. Post-treatment, the cells were washed with 1× PBS, and the cell suspension was collected in centrifuge tubes. The cell suspension was centrifuged at 300×g for 10 min at 4 °C, washed with 1× PBS, and the cell pellet was lysed with 1× RIPA buffer containing 3 μg/mL aprotinin. The total protein concentration in cell lysates was measured using a BCA assay.
Isolation of Naïve EVs and Mito-EVs from hCMEC/D3 and NIH/3T3 Cells
EXOs and MVs were isolated from the EV-conditioned medium of hCMEC/D3 BECs and NIH/3T3 fibroblasts using a differential ultracentrifugation method we have previously reported.11,12,17 Briefly, hCMEC/D3 BECs and NIH/3T3 fibroblasts were cultured in 175 cm2 tissue culture flasks (T175) until about 90% cell confluency. For naïve EVs, confluent cells were washed with pre-warmed 1× PBS and incubated with serum-free medium for 48 h in a humidified 5% CO2 incubator at 37 ± 0.5 °C. For isolating mito-EVs, the confluent cells were incubated with 10 µM resveratrol in serum-free medium for 48 h. Post-incubation, the EV-conditioned medium was collected in centrifuge tubes and spun at 300×g for 11 min and 2000×g for 22 min at 4 °C to pellet down apoptotic bodies and cell debris using an Eppendorf 5810 R 15 amp version centrifuge (Eppendorf, Germany). The supernatants were transferred into polycarbonate tubes to isolate naïve and mito-MVs via centrifugation at 20,000×g for 45 min at 4 °C using a Sorvall MX 120+ micro-ultracentrifuge (Thermo Scientific, Santa Clara, CA). Next, the supernatant was filtered through 0.22 µm PES syringe filters, and the filtrate was centrifuged at 120,000×g for 70 min at 4 °C to collect naïve or mito-EXOs. Lastly, EXO and MV pellets were washed with 1× PBS and suspended in either 1× PBS for particle diameter measurements and in vitro experiments or in 10 mM HEPES buffer pH 7.4 for zeta potential measurements. The total protein content in isolated EXOs and MVs were measured using a MicroBCA assay kit. Briefly, EXOs and MVs were lysed using 1× RIPA buffer at a 1:15 volume ratio. A 150 µL volume of EV lysates was transferred to a 96-well plate along with bovine serum albumin protein standards (0.5 to 200 µg/mL). An equal volume of the MicroBCA working reagent (reagent A: reagent B: reagent C at 25:24:1 volume ratio) was added to each well and the plate was incubated at 37 °C for 2 h. The absorbance was measured at 562 nm using a SYNERGY HTX multi-mode reader (BioTek Instruments Inc., Winooski, VT).
Transmission Electron Microscopy Analysis of EVs
EVs were loaded on Formvar/carbon-coated grids, negatively stained with 1% uranyl acetate and examined with a JEM-1400 Plus transmission electron microscope (JEOL, Peabody, MA, USA) fitted with an AMT digital camera (Danvers, MA, USA).56 Suspensions of EVs were pelleted at 100,000×g in a Beckman airfuge for 20 min and the pellets were fixed in 2.5% glutaraldehyde in PBS overnight. The supernatant was removed and the cell pellets were washed 3× in PBS and post-fixed in 1% OsO4, 1% K3Fe(CN)6 for 1 h. Following three additional PBS washes, the pellet was dehydrated through a graded series of 30–100% ethanol. After several changes of 100% resin over 24 h, the pellet was embedded in a final change of resin, cured at 37 °C overnight, followed by additional hardening at 65 °C for two more days. Ultrathin (70 nm) sections were collected on 200 mesh copper grids, stained with 2% uranyl acetate in 50% methanol for 10 min, followed by 1% lead citrate for 7 min. Sections were imaged using a JEOL JEM 1400 Plus transmission electron microscope (Peabody, MA) at 80 kV fitted with a side mount AMT 2k digital camera (Advanced Microscopy Techniques, Danvers, MA).
Dynamic Light Scattering (DLS)
The particle diameter, dispersity indices, and surface charge of EVs were measured using dynamic light scattering. EV samples were diluted to a final concentration of 0.1 mg EV protein/mL in 1× PBS for particle diameter and in 10 mM HEPES buffer pH 7.4 for zeta potential measurements. The samples were analyzed using a Malvern Zetasizer Pro (Worcestershire, UK). All samples were run in triplicate. Average particle diameter, dispersity index, and zeta potential values were reported as mean ± standard deviation.
Nanoparticle Tracking Analysis
EXOs and MVs were diluted at 1:100 and 1:200 ratios in 1× PBS and analyzed using multiple-laser Zetaview f-NTA Nanoparticle Tracking Analyzer (Particle Metrix Inc., Mebane, NC). Prior to measurement, ZetaView was calibrated with 100 nm polystyrene beads and EVs were analyzed at 520 nm to measure particle concentration. Average EV concentrations were reported as mean ± standard deviation (n = 6).
Western Blot Analysis for Cellular PGC-1α Expression and EV Protein Markers
For detecting PGC-1α protein expression in BECs and NIH/3T3 fibroblasts, a 40 µg cell lysate of untreated and resveratrol-treated hCMEC/D3 BECs and NIH/3T3 fibroblasts were mixed with 4× laemmli buffer and distilled water. Characteristic EV protein markers were detected using fifty µg EV lysates and hCMEC/D3 cell lysates mixed with laemmli sample buffer. The samples were incubated at 95 °C for 5 min. The samples and premixed molecular weight markers (ladder) were separated on a 4–10% SDS–polyacrylamide gel at 120 V for two hours using a PowerPac Basic setup (BioRad Laboratories Inc.).11,12,17 The proteins were transferred onto a 0.45 µm nitrocellulose membrane at 75 V for 90 min using a transfer assembly (BioRad Laboratories Inc.). The membrane was washed with 0.1% Tween-20-containing tris-buffered saline (T-TBS) and blocked with Intercept blocking solution (Intercept blocking buffer:T-TBS:: 1:1) for an hour at room temperature. For PGC-1α detection, the membrane was incubated with rabbit polyclonal anti-PGC1α antibody (1 µg/mL), and mouse monoclonal anti-GAPDH antibody (1 µg/mL) solutions in blocking buffer at 4 °C overnight. For EV markers, the membrane was incubated with mouse anti-CD9 (0.2 µg/mL), mouse anti-ATP5A (1 µg/mL), rabbit anti-TOMM20 (1 µg/mL), and mouse anti-GAPDH (1 µg/mL) primary antibodies in blocking solution for overnight at 4 °C. The membrane was washed with T-TBS and incubated with anti-mouse AF790 (0.05 µg/mL), and anti-rabbit AF790 (0.05 µg/mL) secondary antibodies in a blocking solution for an hour at room temperature. The membrane was washed and scanned under 800 and 700-nm near infrared channels using an Odyssey imager (LI-COR Inc., Lincoln, NE) at intensity setting 5.
Uptake of Mitotracker-Labeled Naïve and Mito-EVs into the Recipient hCMEC/D3 Cells
Isolation of Mitochondria-Labeled Naïve and Mito-EVs
hCMEC/D3 BECs and NIH/3T3 fibroblasts were cultured in T175 flasks to confluency. The complete growth medium was removed and cells were washed with 1× PBS. Cells were treated with 100 nM MitoTracker deep red (MitoT-red) diluted in serum-free medium for 30 min in a 37 °C humidified incubator, and following that, the cells were washed with 1× PBS. For naïve EVs, cells were incubated with serum-free medium for 48 h, whereas for mito-EVs, cells were incubated with serum-free medium containing 10 µM resveratrol for 48 h. Post-incubation, the conditioned medium was collected into polystyrene centrifuge tubes. MitoT-red-stained EXO (MitoT-red-EXO) and MV (MitoT-red-MV) were isolated from EV-conditioned media using the differential ultracentrifugation method described in “Isolation of Naïve EVs and Mito-EVs from hCMEC/D3 and NIH/3T3 Cells” section. The EV protein content in MitoT-red-EXOs and MitoT-red-MVs were determined using MicroBCA assay, and the samples were stored at − 80 °C until further use.
Uptake of hCMEC/D3 BEC- and NIH/3T3 Fibroblast-Derived MitoT-red-EVs into Recipient hCMEC/D3 Cells Using Fluorescence Microscopy
hCMEC/D3 BECs (P30) were seeded in a 24-well plate at 100,000 cells/well-seeding density in complete growth medium and cultured for 48 h in a humidified incubator at 37 °C. The cells were treated with BEC-derived naïve and mito-MitoT-red-EXOs and MitoT-red-MVs at 5, 10, and 25 µg EV protein/well in complete growth medium for 48 h in a humidified incubator. In addition, the cells were also treated with fibroblast-derived naïve and mito-MitoT-red-EXOs and MitoT-red-MVs at 5 and 25 µg EV protein/well in complete growth medium for 48 h. Post-treatment, the cells were washed with 1× PBS, and incubated in phenol-red free growth medium. The cells were observed under an Olympus IX 73 epifluorescent microscope (Olympus, Pittsburgh, PA) using the Cyanine-5 (Cy5, excitation 651 nm, and emission 670 nm) and bright-field channels at 20× magnification. Images were acquired using CellSens Dimension software (Olympus, USA).
Colocalization of MitoT-red MVs with Recipient BEC Mitochondria
hCMEC/D3 cells were cultured in a 24-well plate at 50,000 cells/well and incubated in a humidified incubator at 37 °C. Post-confluency, the cells were treated with MitoT-red MV, and mito-MV at 50 µg EV protein/well in complete growth medium for 48 h under normoxic conditions. Post-incubation, the treatment mixture was removed, and cells were washed with 1× PBS. The cells were treated with Mitotracker green (MitoT-green) at 100 nM in complete growth medium for 30 min. Cells treated with free MitoT-green dye was used as a control. Post-incubation, the treatment mixture was removed and cells were washed with 1× PBS. Cells were incubated with phenol-red free, serum-containing medium. The cells were then observed under an Olympus IX 73 epifluorescent inverted microscope (Olympus, Pittsburgh, PA) using Cyanine-5 channel (Cy5, excitation 651 nm, and emission 670 nm) to detect MitoT-red-EV signals and GFP channel to detect MitoT-green signals. Images were acquired using CellSens Dimension software (Olympus, USA) at 40× magnification. Pearson's correlation coefficient was obtained from the overlay images of Cy5 and GFP channels at constant signal intensities for both channels.
Uptake of hCMEC/D3 BEC- and NIH/3T3 Fibroblast-Derived MitoT-red-EVs into Recipient hCMEC/D3 Cells Using Flow Cytometry
hCMEC/D3 BECs (P31) were seeded in a 48-well plate at a seeding density of 50,000 cells/well in complete growth medium and cultured for 48 h in a humidified incubator at 37 °C. Unstained and untreated cells were used as a control, whereas cells stained with 100 nM MitoT-red for 30 min in complete growth medium were used as a positive control. The cells were treated with BEC-derived naïve and mito-MitoT-red-EXOs and MitoT-red-MVs at 5, 10, and 25 µg EV protein/well in complete growth medium for 48 h in a humidified incubator. Post-treatment, the cells were washed with 1× PBS, dissociated using TrypLE Express, diluted with PBS, and collected into centrifuge tubes. For each sample, an aliquot of a 100 µL cell suspension was analyzed on an Attune NxT Flow cytometer and 10,000 events were recorded in FSC vs. SSC plots. The MitoT-red fluorescent signals were detected at 670/10-nm and percentage signal intensities were presented in histogram plots generated using Attune software version 3.2.3. Any MitoT-red background signals were gated using the control, untreated cells.
Isolation of Rotenone-EV (RTN-EV) and Oligomycin A-EV (OGM-EV) from BECs
For isolating RTN-EVs and OGM-EVs, the confluent cells were incubated with 0.5, 1, and 10 µM rotenone (RTN) or 1 µM oligomycin A (OGM) in a complete growth medium for 4 h. Post-incubation, the treatment mixture was replaced with serum-free medium and incubated for 48 h in a humidified incubator. Post-incubation, EV-containing conditioned medium was collected in centrifuge tubes and spun at 300×g for 11 min and 2000×g for 22 min at 4 °C using an Eppendorf 5810 R 15 amp version centrifuge (Eppendorf, Germany). The supernatants were transferred into polycarbonate tubes to isolate naïve, RTN-MV, or OGM-MV via centrifugation at 20,000×g for 45 min at 4 °C using a Sorvall MX 120 + micro-ultracentrifuge (Thermo Scientific, Santa Clara, CA). Next, the supernatant was filtered through 0.22 µm PES syringe filters, and the filtrate was centrifuged at 120,000×g for 70 min at 4 °C to collect naïve, RTN-EXO or OGM-EXO. Naïve EV, RTN-EV, and OGM-EV pellets were resuspended in 1× PBS for further use.
Effect of EVs on Relative ATP Levels in the Recipient hCMEC/D3 BECs
We compared the effects of naïve vs. mito-EVs at the same “volume” to account for the donor cell activation-related changes in EV particle concentrations between the naive and mito-EVs (Fig. 1g). hCMEC/D3 BECs (P30-P33) were seeded in 96-well plates at a seeding density of 16,500 cells/well in complete growth medium and cultured for 48 h in a humidified incubator at 37 °C. Recipient BECs were treated with naïve EXO, mito-EXO, naïve MV, and mito-MV at 10, 30, and 50 µL EV volume/well for 24 h under oxygen–glucose deprivation (OGD) (defined as 120 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgCl2, 25 mM Tris–HCl, pH 7.4) for 24 h in a chamber (Billups Rothenberg, Del Mar, CA) pre-flushed with 5% carbon dioxide, 5% hydrogen and 90% nitrogen at 37 ± 0.5 °C simulating hypoxic conditions in vitro.10 Untreated cells were incubated with either OGD medium (for OGD control) or in complete growth medium (for normoxic control). For normoxic conditions, the cells were cultured in complete growth medium in a 37 °C humidified incubator. To evaluate the effects of RTN-EV and OGM-EV on BEC ATP levels under OGD conditions, hCMEC/D3 cells were treated with BEC-derived naïve and RTN-EV and OGM-EV at 25 µg EV protein/well for 24 h in OGD conditions. For intracellular ATP measurements, the treatment mixture was removed, cells were washed, and cells were incubated with a 1:1 v/v mixture of complete growth medium and Cell titer glo 2.0 reagent for 15 min at room temperature in the dark. Post-incubation, the cell lysates were transferred into a white opaque plate and relative luminescence units were measured at 1 s integration time using a SYNERGY HTX multi-mode reader (BioTek Instruments Inc., Winooski, VT). The relative ATP levels were measured using Eq. (1).
| 1 |
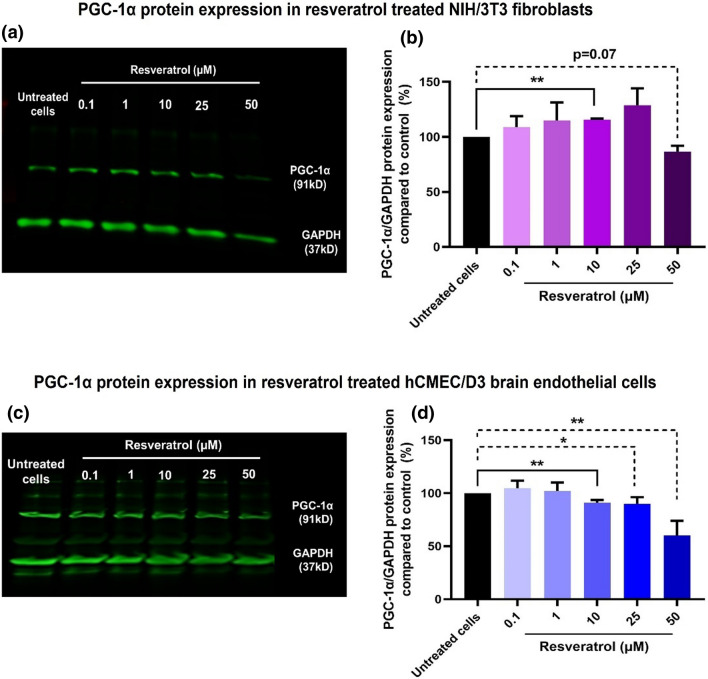
Figure 1.
PGC-1α protein expression in resveratrol-treated NIH/3T3 fibroblasts and hCMEC/D3 BECs. Forty µg total protein was electrophoresed in 4–10% gradient SDS-PAGE. The proteins were transferred to a nitrocellulose membrane prior to blocking non-specific proteins using a blocking buffer. The membrane was incubated with anti-PGC-1α and GAPDH primary antibodies followed by Alexa Fluor 790-conjugated secondary antibodies. The membrane was scanned under 700 nm (red) and 800 nm (green) channels using an Odyssey infrared scanner at intensity setting 5. PGC-1α was detected at its characteristic molecular mass of 91 kD, whereas GAPDH was detected at 37 kD. PGC-1α and GAPDH protein expression in NIH/3T3 and hCMEC/D3 cells treated with 0.1 to 50 µM resveratrol (a and c). Densitometry analysis: PGC-1α expression was normalized with GAPDH, and relative PGC-1α/GAPDH levels were compared to control, untreated cells (b and d). The blots are representative of three independent experiments. The PGC-1α/GAPDH densitometry analysis represents mean ± standard deviation (SD) (n = 3). A two-tail unpaired t-test was performed using GraphPad Prism to determine statistically significant differences between resveratrol-treated vs. untreated cells. *p < 0.05, **p < 0.01.
Statistical Analysis
Statistical significance among the mean of controls and treatment groups or within treatment groups were analyzed using Students’s t test, one-way analysis of variance (ANOVA) or two-way ANOVA at 95% confidence intervals using GraphPad Prism 9 (GraphPad Software, LLC). The notations for the different levels of significance are indicated as follows: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Results and Discussion
Mitochondria are the primary source of cellular ATP and regulate important events such as ATP synthesis, cell differentiation, immune activation, and induction of apoptosis and mitophagy.23 Mitochondrial dysfunction is one of the major causes of cell death in various neurodegenerative disorders and brain injuries, including ischemic stroke.3,44 Cells cannot remain metabolically active without functional mitochondria and therefore, exogenous delivery of mitochondria or mitochondrial components is a promising approach to revitalize the damaged brain endothelial and neural cells in various CNS disorders. Our previous report demonstrated using transmission electron microscopy that in situ endothelial cellular buds (MVs) contain mitochondria.11,38 In contrast, mitochondrial proteins such as ATP5A (a subunit of ATP synthase present in the inner mitochondrial membrane) and TOMM20 (translocase of the outer mitochondrial membrane receptor) were present at greater amounts in MVs compared to EXOs.11,12,17
Inspired by our previous findings, we wanted to understand if the innate EV mitochondrial load can be modulated by the levels of mitochondria in the donor cells. The goal, as stated earlier, is to engineer EVs with a greater mitochondrial load (mito-EVs) to further harness the benefits of MVs. Numerous in vitro and in vivo studies demonstrated the potential of polyphenols in increasing mitochondrial biogenesis in the recipient cells.7,13,47,73 For instance, Davinalli et al. showed that human vascular endothelial cells co-incubated with polyphenols such as resveratrol and equol increased cellular mitochondrial mass, mtDNA, and mitochondrial biogenesis factors such as PGC-1α, mitochondrial transcription factor, and nuclear respiratory factor.13 Mechanistically, polyphenols activate sirtuin1 (NAD+-dependent protein deacetylase), which is a critical factor for the regulation of PGC-1α and mitochondrial functions.13 A recent study also confirmed resveratrol-mediated PGC-1α activation that resulted in increased mitochondrial biogenesis in a rat model of hemorrhagic brain injury.73 Given the known effects of resveratrol in increasing mitochondrial biogenesis, we used resveratrol to activate PGC-1α in the donor cells and subsequently isolated mito-EXOs and mito-MVs.
We hypothesized that mito-EVs may increase the mitochondrial transfer and resulting ATP levels in recipient BECs compared to naïve EVs isolated from control, non-activated cells. We (1) first optimized the resveratrol concentration to increase PGC-1α expression in hCMEC/D3 BECs and NIH/3T3 fibroblasts, (2) studied physicochemical characteristics and compared the mitochondrial content in mito-EVs vs. naïve EVs, (3) compared the effects of naïve vs. mito-EVs in transferring their mitochondria into recipient BECs using fluorescence microscopy and flow cytometry and (4) determined the efficacy of mito-EVs vs. naïve EVs by measuring relative ATP levels of EV-treated BECs under OGD conditions.
Resveratrol Treatment Increased PGC-1α Protein Expression in NIH/3T3 Cells, but not in hCMEC/D3 Cells
Resveratrol-mediated modulation of PGC-1α protein expression in NIH/3T3 and hCMEC/D3 cells was determined using western blot analysis (Fig. 1). NIH/3T3 and hCMEC/D3 cells was treated with resveratrol at concentrations ranging from 0.1 to 50 µM for 24 h in complete culture medium to identify the optimal [resveratrol] that increases PGC-1α expression. In NIH/3T3 cells, resveratrol treatment showed a concentration-dependent gradual increase in PGC-1α band densities, whereas band densities of GAPDH remained unchanged up to 25 µM. However, there was a considerable decrease in PGC-1α and GAPDH expression at 50 µM resveratrol compared to untreated cells (Fig. 1a). The densitometry analysis of three independent western blots demonstrated that resveratrol induced a concentration-dependent increase in PGC-1α expression, where 10 µM resveratrol showed a statistically significant (p < 0.01) increase in normalized PGC-1α expression compared to untreated cells (Fig. 1b). However, resveratrol at 50 µM showed a considerable (p = 0.07) decrease in PGC-1α expression, likely suggesting that the 50 µM concentration resulted in cellular toxicity.
On the other hand, hCMEC/D3 cells treated with resveratrol at 0.1 and 1 µM for 24 h showed a slight increase in PGC-1α protein band densities compared to untreated cells (Fig. 1c). However, resveratrol concentrations from 10 µM to 50 µM showed a gradual and considerable decrease in PGC-1α and GAPDH protein expression (Fig. 1c). The densitometry analysis of three independent western blots demonstrated that resveratrol treatment up to 1 µM showed a modest 5% increase in normalized PGC-1α protein expression compared to untreated cells (Fig. 1d).
In conclusion, NIH/3T3 cells treated with 10 µM resveratrol showed a significant increase in PGC-1α protein expression, a key activator of mitochondrial biogenesis. Resveratrol-mediated increase in PGC-1α protein expression was in agreement with a published report.73 We speculate that the observed modest changes in PGC-1α protein expression in the resveratrol-treated cells13 may not be reflective of the actual changes in the PGC-1α mRNA levels. In fact, the half-life of PGC-1α is about 2–3 h21,35 and therefore, the observed relative levels of PGC-1α expression in the activated cells may not be representative of the actual levels present in the cells. Moreover, BECs contain a greater mitochondrial load compared to non-brain endothelial and other cell types and as a result, the kinetics of PGC-1α metabolism may occur more rapidly in the BECs. This may explain the apparent lack of changes in PGC-1α protein expression in BECs as opposed to the NIH/3T3 fibroblasts. We are currently optimizing a qPCR setup in-house to measure changes in the PGC-1α mRNA levels in the resveratrol-treated cells. Mitochondria-containing EVs are known to contain PGC-1α RNA,28 and our further studies will characterize this aspect as well. Therefore, for the subsequent studies, despite the lack of dramatic changes in PGC-1α protein levels, we treated NIH/3T3 and hCMEC/D3 cells with 10 µM resveratrol prior to the isolation of mito-EVs from the EV-conditioned medium.
Transmission Electron Microscopy Analysis of EVs
We acquired transmission electron microscope (TEM) images of the naïve EXOs and MVs-isolated from control, non-activated hCMEC/D3 cells and those of mito-EXOs, and mito-MVs-derived from resveratrol (10 µM) pretreated hCMEC/D3 cells (Fig. 2).
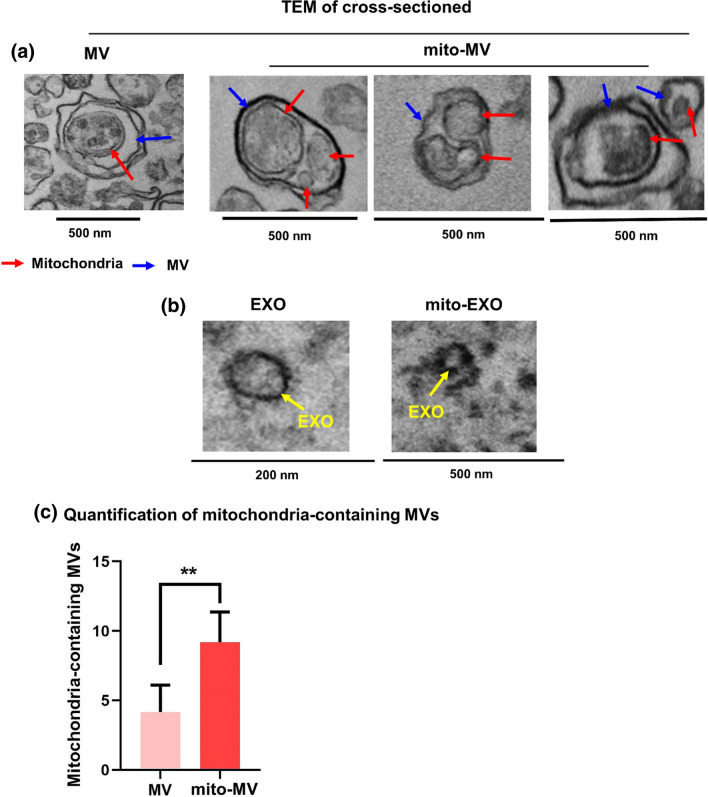
Figure 2.
TEM images of EV cross-sections. (a) Representative TEM images of mitochondria-containing naïve MV and mito-MVs. (b) Representative TEM images of naïve EXOs and mito-EXOs. (c) TEM images were manually counted to quantify mitochondria-containing MVs. Quantitative analysis of mitochondria-containing MVs in naïve MV (n = 4) vs. mito-MV images (n = 6). Data are represented as mean ± SD and statistical significance was determined using the Student’s unpaired t-test at 95% confidence intervals using GraphPad Prism 9. **p < 0.01.
Cross-sections of naïve MVs (blue arrow, Fig. 2a) showed the presence of mitochondria (red arrow, Fig. 2a) in MV lumen. Notably, the cross-sections of mito-MVs typically showed more numbers of mitochondria (Fig. 2a), suggesting that resveratrol-mediated activation increased mitochondrial biogenesis in the donor cells that may have resulted in the additional mitochondria in the secreted mito-MVs. We counted the total number of mitochondria-containing vesicles in the TEM images of naïve MV and mito-MV sections. The number of mitochondria-containing MVs was significantly (p < 0.01) higher in mito-MVs compared to naïve MVs (Figs. 2a and 2c). We wish to emphasize that prior publications25,26,28,48 showed EV mitochondria with similar structures and comparable resolution. In contrast, images of EXO cross-sections that looked empty did not contain electron-dense structures in the lumen (yellow arrow, Fig. 2b), suggesting the absence of mitochondria in EXOs. Similarly, mito-EXOs too did not show any mitochondrial structures (Fig. 2b).
Resveratrol is a polyphenol compound that interacts with lipid rafts and regulates the formation of lipid microdomains on the plasma membrane. Neves et al. demonstrated that resveratrol promoted the formation of tightly ordered membrane domains that induced phase separation between cholesterol and sphingomyelin in lipid rafts of the cell membrane.43 Resveratrol accumulates into lipid rafts and rapidly activates (a) various kinases, (b) intracellular transduction cascades, (c) actin polymerization, and (d) assembly of signaling complexes.15 These signaling events promote raft migration to the signaling sites and facilitate the fusion of microdomains to form macrodomains.15 Formation of macrodomains triggers the integrin-signaling complexes that can induce a signaling transduction cascade through the cytoplasmic domain, which then interacts with the cytoskeleton.15 Thus, resveratrol-mediated reorientation of lipid rafts, formation of macrodomains, and cytoskeletal rearrangements facilitate the incorporation of signaling molecules into the plasma membrane buds (or “microvesicles/MVs”) for intercellular signal transduction.
On the other hand, numerous in vitro and in vivo studies demonstrated the potential of polyphenols in increasing mitochondrial biogenesis in the recipient cells.7,13,47,73 For instance, Davinalli et al. showed that human vascular endothelial cells co-incubated with polyphenols such as resveratrol and equol increased cellular mitochondrial mass, mtDNA, and mitochondrial biogenesis factors such as PGC-1α, mitochondrial transcription factor, and nuclear respiratory factor.13 A recent study also confirmed that resveratrol-mediated PGC-1α activation and resulted in increased mitochondrial biogenesis in a rat model of hemorrhagic brain injury.73
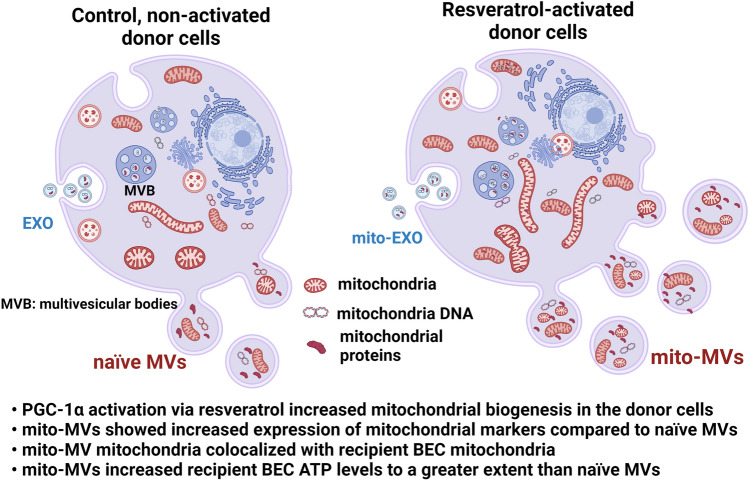
In our studies, MVs isolated from resveratrol-treated BECs (mito-MVs) showed increased mitochondrial load and higher numbers of mitochondria-containing MVs compared to naïve MVs isolated from control, non-activated BECs. The resveratrol-mediated increase in mitochondrial biogenesis and changes in plasma membrane lipid domains may likely explain the resveratrol-mediated increase in secretion of a greater number of mitochondria-containing MVs compared to non-activated cells. Resveratrol treatment likely increased the mitochondrial mass in BECs that may have led to increased interactions between mitochondria and actin cytoskeleton. Mitochondria-cytoskeletal elements likely migrate towards the lipid raft macrodomains formed at the plasma membrane surface resulting in more mitochondria being incorporated in MVs and subsequently released into the extracellular spaces. The above-described effects of resveratrol on BECs and more specifically, on the formation of mito-MVs is only speculative at this point and our future studies will explore these mechanistic aspects of mito-MV secretion.
Physicochemical Characteristics of Naïve EVs and Mito-EVs Using Dynamic Light Scattering and Nanoparticle Tracking Analysis
We used dynamic light scattering to determine EV physicochemical characteristics such as particle diameters, dispersity indices, and surface charge. We compared these parameters in naïve- (from control, non-activated cells) and mito-EVs-derived from resveratrol treated NIH/3T3 and hCMEC/D3 cells (Fig. 3).
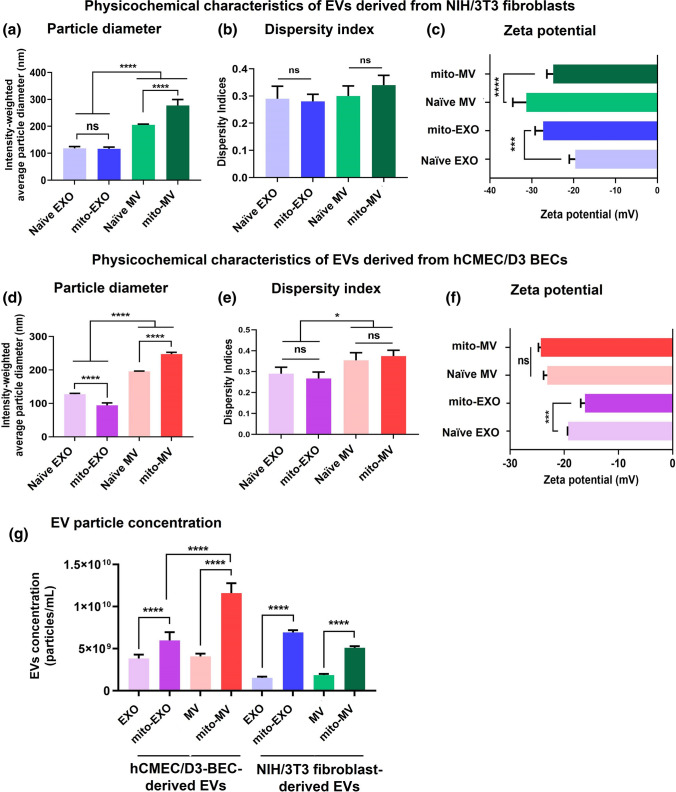
Figure 3.
Physicochemical characterization of EVs derived from NIH/3T3 fibroblasts and hCMEC/D3 BECs. Naïve and mito-EVs were suspended in 1× PBS at a 0.1 mg EV protein/mL for particle diameter and dispersity indices measurements (a, b, d, e) and in 10 mM HEPES buffer pH 7.4 for zeta potential measurements (c, f) on a Malvern Pro DLS. EVs were diluted at 1:100 or 1:200 in 1× PBS and analyzed at 520 nm using a Zetaview f-NTA Nanoparticle Tracking Analyzer (g). Data represent mean ± SD (n = 6). One-way analysis of variance (ANOVA) followed by Tukey's multiple comparison statistical analysis was performed using GraphPad Prism to determine the statistical differences between naïve and mito-EVs. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns: not significant.
Naïve EXOs derived from NIH/3T3 showed an average particle diameter of 115 nm (Fig. 3a). In contrast, naïve MVs showed an average size of 205 nm, confirming that MVs are significantly (p < 0.0001) larger than EXOs (Fig. 3a).11,12,17 Importantly, mito-EXOs (116 nm) did not show a statistically significant change in particle diameter compared to naïve EXOs (115 nm, Fig. 3a). In contrast, mito-MVs (280 nm) showed a statistically significant (p < 0.0001) increase in particle diameter compared to naïve MVs (205 nm, Fig. 3a). The data likely suggest that resveratrol increases mitochondrial biogenesis due to PGC-1α activation as a result, the secreted mito-MVs have a greater mitochondrial load compared to naïve MVs. In addition, there were no statistical differences in the dispersity indices between naïve and mito-EXOs as well as naïve and mito-MVs (Fig. 3b). Naïve EXOs and MVs showed zeta potentials ranging from − 20 to − 25 mV (Fig. 3c). Interestingly, while mito-EXOs showed a significantly lower zeta potential, mito-MVs showed a significantly greater zeta potential compared to their naïve EV counterparts (Fig. 3c).
Consistent with the NIH/3T3-derived EVs, hCMEC/D3 derived naïve EXOs (125 nm) showed a significantly lower particle diameter compared to naïve MVs (average 195 nm, Fig. 3d), confirming our prior observations.11,12,17 Mito-EXOs showed a lower particle diameter (95 nm, p < 0.0001) compared to naïve EXOs (Fig. 3d). Importantly, hCMEC/D3-derived mito-MVs (250 nm) showed a significantly (p < 0.0001) greater particle size compared to naïve MVs (195 nm) (Fig. 3d). Noteworthy, the increased particle diameters of mito-MVs compared to the naïve MVs were conserved regardless of the donor cell source: NIH/3T3 fibroblasts vs. hCMEC/D3 brain endothelial cells. The dispersity indices of naïve EVs and mito-EVs remained similar (Fig. 3e). hCMEC/D3-derived EXOs showed significantly lower dispersity indices than MVs (Fig. 3e). Lastly, hCMEC/D3-derived naïve EXOs and MVs showed zeta potential ranging from − 20 to − 23 mV (Fig. 3f). hCMEC/D3-derived mito-EXO showed a significantly (p < 0.001) greater zeta potential compared to naïve EXOs, while no changes were noted between naïve and mito-MVs (Fig. 3f).
We used nanoparticle tracking analysis (NTA) to determine if PGC-1α activation in the donor cells and the subsequent changes in the mitochondrial load affect the secretion of EVs as a function of its particle concentration. We measured the concentration of mito-EVs and naïve EVs using NTA. NTA measures the particle diameter and concentration based on the diffusion coefficient of individual particles captured in the optical videos.31 The particle concentration of BEC-derived naïve EXOs and MVs was about 4.0 × 109 particles/mL, which was significantly higher than the fibroblast-derived naïve EXOs and MVs (1.65 × 109 particles/mL, Fig. 3g). The data suggest that hCMEC/D3 BECs produced significantly (p < 0.0001) more EVs compared to NIH/3T3 fibroblasts.
Importantly, resveratrol treated hCMEC/D3 BECs released significantly (p < 0.0001) increased mito-EXOs (6.0 × 109 particles/mL) and mito-MVs (1.2 × 1010 particles/mL) compared to non-treated BECs (Fig. 3g). Notably, mito-MV concentration was significantly (p < 0.0001) greater than mito-EXO, suggesting that resveratrol-mediated activation of PGC-1α increases the mitochondrial load and as a consequence, the activated cells produce more EVs (Fig. 3g). It is likely that PGC-1α-mediated increase in cellular mitochondrial biogenesis accelerates mitochondria packaging into MVs, which are subsequently released at a greater rate compared to naïve MVs from non-activated donor cells. A similar trend was observed in resveratrol-treated NIH/3T3 fibroblast-derived mito-EVs. The concentrations of mito-EXOs and mito-MVs were significantly (p < 0.0001) greater than the naïve EV counterparts (Fig. 3g). Notably, the concentration of fibroblast-derived mito-EXOs was comparable to BEC-derived mito-EXOs, whereas the concentration of BEC-derived mito-MVs was significantly higher than the fibroblast-derived mito-MVs (Fig. 3g). Overall, resveratrol-mediated PGC-1α activation significantly increased EXO and MV concentrations regardless of the donor cell type, and the most increases were noted in the case of mito-MVs. It is likely that the PGC-1α-mediated mitochondrial biogenesis increases the content of mitochondrial DNA/proteins as well as mitochondria numbers in the cells that are eventually released into the EXOs53,59 and MVs,26,29,40,48,57,58 respectively.
Characterization of EVs and Mito-EVs Using Western Blotting
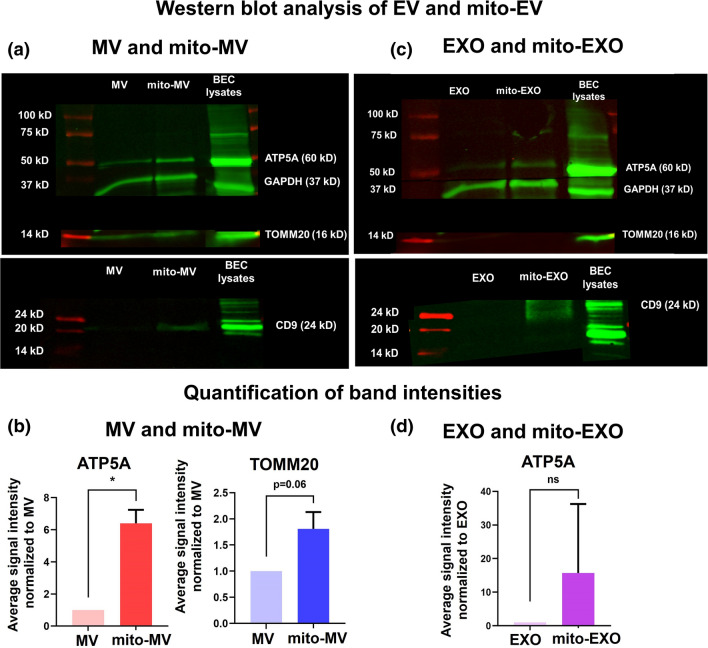
We performed western blotting to detect EXO and MV-specific biomarkers (Figs. 4a and 4c). Naïve and mito-MVs were analyzed to detect the following proteins: ATP5A (a subunit of mitochondrial ATP synthase), TOMM20 (Translocase of Outer Mitochondrial Membrane 20), CD9 (EXO marker), and GAPDH (glyceraldehyde-3-phosphate dehydrogenase, cytosolic marker). ATP5A and TOMM20 were enriched in naïve MVs (Fig. 4a), in contrast, EXOs showed low expression of ATP5A and did not show the presence of TOMM20—a mitochondrial membrane protein (Fig. 4c). This data suggested that although both EXOs and MVs contain mitochondrial proteins, only MVs contain mitochondria. The selective presence of mitochondria structural markers (TOMM20) agreed with the TEM images showing the presence of mitochondria in MVs (Fig. 2a). Mito-MV showed significantly (p < 0.05) higher levels of ATP5A and TOMM20 expression compared to naïve MVs (Figs. 4a and 4b), suggesting the increased mitochondrial load in mito-MVs compared to naïve MVs. Mito-EXOs showed higher ATP5A expression than naïve EXOs (Fig. 4c), however, the difference in ATP5A expression levels was not statistically significant (p > 0.05, Fig. 4d). Moreover, mito-EXOs showed the presence of CD9, whereas the expression levels of CD9 in naïve EXOs were below the limit of detection. We use GAPDH as an additional control to demonstrate the existence of cytosolic components in naïve and mito-EVs (Figs. 4a and 4c).
Figure 4.
Western blot analysis of naïve and mito-EVs to detect their characteristic markers. (a, c) Naïve and mito-EVs were isolated from control, non-activated, and resveratrol pretreated hCMEC/D3 BECs. The protein content of cell lysates and EVs was analyzed using a MicroBCA assay. A 50 µg EV protein/sample was incubated with Laemmli sample buffer at 95 °C for 5 min. The samples were electrophoresed in a 4–10% sodium dodecyl sulfate–polyacrylamide gel. The proteins were transferred to nitrocellulose membranes following which the membranes were washed and blocked with a blocking buffer. The blot was incubated with primary antibodies for ATP5A, GAPDH, TOMM20, and CD9 overnight at 4 °C. The blot was washed and incubated with AF790 secondary antibodies at room temperature. The blot was imaged on the 800 nm channel using Odyssey imager (LI-COR Inc. Lincoln, NE) at intensity setting 5. (b, d) The band densities were quantified using ImageStudio 5.2 software. Data represent mean ± SD (n = 2). *p < 0.05, ns: non-significant.
Mito-EVs Show Significantly Greater Mitochondria Transfer Compared to Naïve EVs
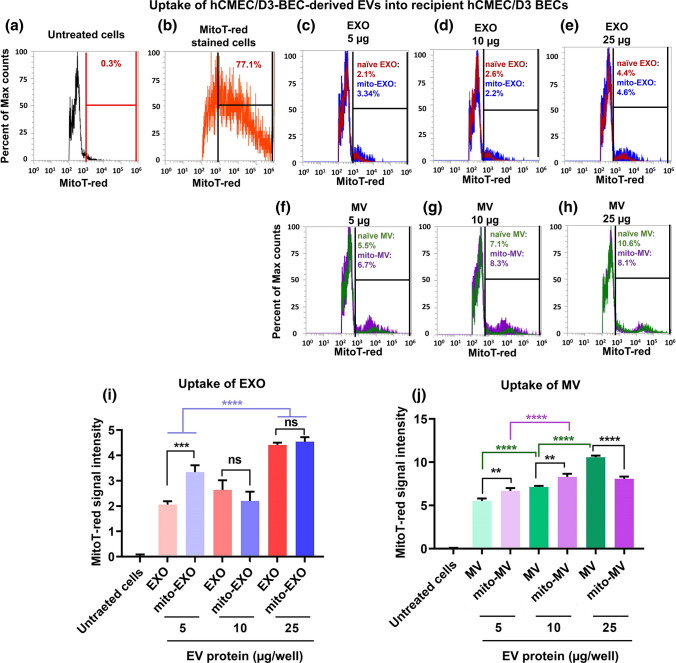
EVs contain mitochondrial components, including mitochondrial DNA, mitochondrial proteins and entire mitochondria.26,29,40,48,49,53,56–58,71 We compared the uptake of mito-EVs with naïve EVs in the recipient BECs to determine if the increased mitochondrial load in mito-EVs translated to a greater extent of uptake. Mito-EVs or naïve EVs were generated from cells pre-stained with MitoTracker Red that selectively stains polarized, functional mitochondria in addition to mitochondrial proteins.5 These labeled samples are referred to as MitoT-red-EXOs or MitoT-red-MVs. Recipient hCMEC/D3 BECs were treated with naïve and mito-EVs at 5, 10, and 25 µg doses for 48 h, and EV-mediated mitochondrial transfer was interpreted as purple MitoT puncta under a fluorescence microscope.
Untreated cells did not show any MitoT-red associated signals indicating that only MitoT-red specific signals were detected under the Cy5 channel (Fig. 5). BECs treated with free MitoT-red dye at 100 nM for 30 min showed intense signals in BECs, indicating the presence of polarized mitochondria in BECs. Naïve EXOs isolated from MitoT-red-prestained BECs at 5, 10, and 25 µg doses did not show positive signals in the recipient BECs for 48 h. However, mito-EXO at 25 µg dose showed faint intracellular Cy5 signals suggesting low levels of EV mitochondria in the recipient BECs (Fig. 5a). The quantitative analysis showed that there was no statistical difference between naïve and mito-EXO signals at 5 and 10 µg doses, however, mito-EXOs at 25 µg dose led to significantly (p < 0.05) greater MitoT-red signals than naïve EXOs (Fig. 5b). MitoT-red-MV at a dose as low as 5 µg showed intense intercellular purple puncta (Fig. 5a), suggesting efficient MV uptake into the recipient BECs. The levels of MV uptake increased significantly (p < 0.05) as the dose of MitoT-red-MV increased from 5 to 25 µg (Fig. 5b). MV-mitochondria signals at 25 µg dose were significantly (p < 0.05) higher than EXO, confirming our prior findings.11,17 Mito-MVs showed significantly (p < 0.05) greater signals in the recipient BECs cells at 5 and 25 µg EV doses (Fig. 5b), likely due to a greater enrichment of functional mitochondria in mito-MVs compared to naïve MVs. BECs treated with unlabeled naïve and mito-EVs did not show any MitoT-red signals in recipient BECs (Fig. 5a), suggesting that the MitoT-red signals are associated with MV mitochondria.
Figure 5.
Uptake of Mitotracker-red labeled BEC-derived EVs into recipient hCMEC/D3 BECs using fluorescence microscopy. (a) BECs were treated with unlabeled and MitoT-red labeled EVs. Confluent monolayers of hCMEC/D3 BECs were incubated with the indicated samples diluted in complete growth medium for 48 h. Post-incubation, the cells were washed and incubated with phenol-red-free growth medium. Intracellular MitoT-red-EVs signals were observed under an Olympus IX 73 epifluorescent microscope using Cy5 channel (purple puncta) at 20× magnification. Scale bar: 50 µm. (b) Quantification of EV mitochondria in the recipient BECs. BECs were treated with the indicated samples for 48 h. At least three images were acquired from each control and treatment group, and the total sum of grayscale signal intensities in the Cy5 channel was quantified using Olympus CellSens software. Data represent mean ± SD.
We also studied the transfer of fibroblast-derived EVs into the recipient BECs (Fig. S1). Recipient BECs were treated with fibroblast-derived EVs at 5 and 25 µg doses for 48 h. Consistent with the BEC-derived EVs, fibroblast-derived MVs but not EXOs showed a greater mitochondrial load and consequent transfer into the recipient BECs (Fig. S1). Importantly, mito-EXOs and mito-MVs showed a considerably greater mitochondrial load and subsequent transfer into recipient BECs.
We also performed flow cytometry analysis to quantify EV-mediated mitochondrial transfer into the recipient BECs using MitoT-red-stained EVs. Recipient BECs were treated with BEC-derived naïve and mito-EVs at 5, 10, and 25 µg EV doses for 48 h, and the intensity of MitoT-red-EVs were analyzed using histogram plots (Fig. 6). Untreated hCMEC/D3 cells were used as control and were gated for data analysis (Fig. 6a). Cells pre-stained with MitoT-red were used as a positive control and were about 77% MitoT + ve (Fig. 6b), suggesting the sensitivity to detect MitoT-red-stained polarized mitochondria and mitochondrial proteins. Naïve and mito-EXOs showed about 2 to 5% MitoT + ve cells (Figs. 6c–6e). Importantly, 5 µg mito-EXO showed a significantly (p < 0.001) greater mitochondrial transfer compared to naïve EXO, whereas no statistical differences were observed at higher doses of EXO (Fig. 6i). Cells treated with MVs at a low dose of 5 µg showed about 5% MitoT-positive signals, that increased by twofold at the 25 g EV dose suggesting efficient mitochondrial transfer into the recipient BECs (Figs. 6f–6h). Importantly, mito-MV showed significantly (p < 0.01) greater mitochondrial transfer at 5 and 10 µg EV doses compared to their naïve MV counterparts (Fig. 6j). However, at a higher amount (25 µg EV protein), mito-MV-mediated mitochondrial transfer was lower than naïve MV (Fig. 6j).
Figure 6.
Uptake of Mitotracker-red labeled BEC-derived EVs into recipient hCMEC/D3 BECs using flow cytometry. Confluent monolayers of hCMEC/D3 BECs were incubated with the indicated samples diluted in complete growth medium for 48 h. Post-incubation, the cells were washed, collected, and analyzed using an Attune NxT flow cytometer. The histograms showing MitoT-red events were acquired using a 674/10-nm side scatter filter. Untreated and unstained cells were used to gate the histograms for estimating percentage MitoT-red-positive counts (a). MitoT-red dye-stained BECs were used as the positive control (b). Overlay histograms of naïve and mito-EXOs (c-e). Overlay histograms of naïve and mito-MVs (f–h). Quantification of % MitoT-red signal intensities in BECs treated with varying doses of EXOs and MVs (i, j). Data represent mean ± SD of n = 3. One-way ANOVA followed by Tukey's multiple comparison statistical analysis was performed using GraphPad Prism to determine the statistical differences. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns: not significant.
Overall, mito-EXOs and mito-MVs showed significantly greater mitochondrial transfer at lower EV doses compared to naïve EXOs and MVs. We speculate that the lack of difference between naïve and mito-EVs at the higher doses could be due to saturation of particle uptake into the recipient cells.
Colocalization of MitoT-red MVs with Recipient BEC Mitochondria
We performed studies to confirm the colocalization of naïve and mito-EV mitochondria with the mitochondria of recipient BECs. MitoT-red-MVs showed Cy5 signals in BECs, demonstrating that MVs contain mitochondria (Fig. 7a). The overlay images showed that recipient BEC mitochondria colocalized with MV mitochondria in the MV-treated cells (Fig. 7a). Moreover, mito-MV showed more intense signals in BECs compared to naïve MVs. PCC of naïve MVs and mito-MVs suggested that mito-MV showed a significantly (p < 0.01) greater colocalization than naïve MVs (Fig. 7b). In conclusion, naïve and mito-MVs transferred mitochondria into BECs, that subsequently colocalized with the mitochondrial network of the recipient cells. Mito-MVs showed significantly greater colocalization than their naïve MV counterparts.
Figure 7.
Colocalization of naïve and mito-MV mitochondria with BEC mitochondria. (a) Recipient hCMEC/D3 BECs were treated with MitoT-red naïve MV, and mito-MV at 50 µg EV protein/well in complete growth medium for 48 h. Post-incubation, the treatment mixture was removed, and cells were washed with PBS. Next, cells were treated with 100 nM MitoT-green in complete growth medium for 30 min. Post-incubation, the cells were washed with PBS and incubated with phenol-red-free and serum-containing DMEM/high glucose medium. The Mitotracker green staining in recipient BECs were acquired using the GFP channel, whereas the purple fluorescence associated with EV mitochondria was captured using Cy5 channel in an Olympus IX 73 epifluorescent inverted microscope. Colocalization of the mitochondrial signals was confirmed by the presence of yellow signals in the overlay images. Scale bar: 50 µm. (b) Pearson’s correlation coefficient was obtained from the overlay images of Cy5 and GFP channels at constant signal intensities for both channels using cellSens software. Data are presented as mean ± SD (n = 3 images per treatment group). **p < 0.01, ***p < 0.001.
The absence of GFP and Cy5 signals in the untreated cells suggested the lack of nonspecific signals at the respective channel settings (Fig. 7a). The cells prestained with MitoT-green alone showed green cytosolic signals associated with the recipient BEC mitochondria (Fig. 7a). Unlabeled mito-MVs did not show any purple signals in BECs (Fig. 7a), suggesting that fluorescence signals in MitoT-red-MV treated BECs were specific to MV mitochondria.
We performed additional microscopic studies to determine whether MV mitochondria were indeed associated with MVs during uptake into BECs. We used calcein-AM as a general EV label24 to assess the association of EV and EV mitochondria once internalized into recipient BECs. Calcein-AM, an acetoxymethyl derivative of the fluorescent molecule calcein, is membrane-permeant and non-fluorescent until activated by intravesicular esterases.12,24 Calcein-AM is hydrolyzed by EV intraluminal esterases and converted into a membrane impermeant green fluorescent calcein.12,24 Gray et al. utilized calcein-AM to determine the integrity of EVs and EV transfer into the recipient cells.24
MitoT-red-EVs were incubated with calcein-AM (10 µM) for 30 min at room temperature. BECs were incubated with the double-labeled EVs at 25 µg EV protein dose for 48 h in a humidified incubator. BECs treated with calcein-AM labeled MitoT-red-EXOs showed the presence of calcein signals but not MitoT-red signals (Fig. S2). These data suggested that BECs internalized EXOs and mito-EXOs. However, due to the lack of mitochondrial content in EXOs and mito-EXOs, we did not notice MitoT-red signals in BECs (Fig. S2). BECs treated with calcein-AM labeled MitoT-red-MV and mito-MVs showed the presence of both calcein and MitoT-red signals in BECs (Fig. S2). The overlay image showed that calcein and MitoT-red signals were colocalized in BECs (Fig. S2). These data suggested that EV mitochondria transfer is associated with mitochondria-containing MVs and mito-MVs.
The Effects of Mito-EVs on Relative ATP Levels in the Recipient Hypoxic Endothelial Cells
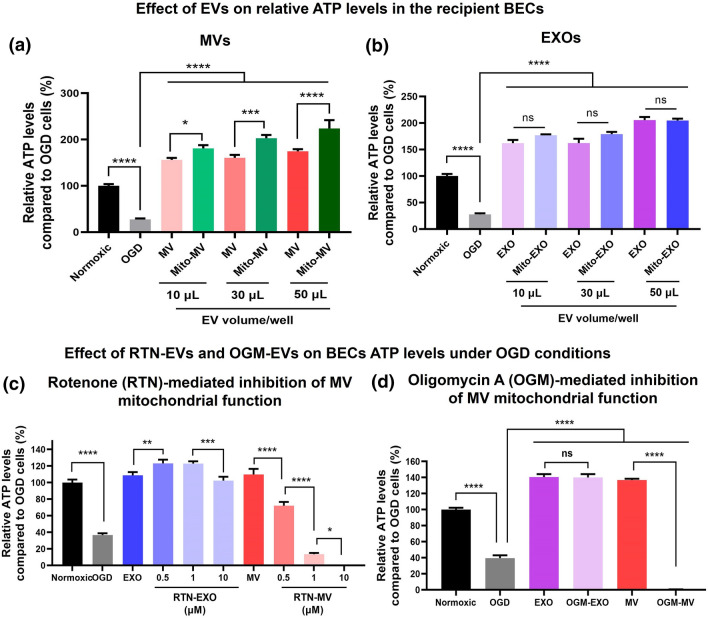
One of the main functions of mitochondria is ATP synthesis via oxidative phosphorylation. We measured the resulting relative ATP levels in the recipient BECs treated with naïve and mito-EVs-derived from hCMEC/D3 BECs. The effects of naïve and mito-EV exposure on relative ATP levels in the hypoxic recipient hCMEC/D3 (Fig. 5) were measured using the Cell titer Glo ATP assay.
Recipient BECs were treated with the indicated samples at 10, 30, and 50 µL EV volume/well for 24 h under OGD conditions. Untreated cells were incubated with either OGD medium (for OGD control) or in complete growth medium (for normoxic control). OGD control showed about 65% reduction in hCMEC/D3 BEC ATP levels suggesting that OGD conditions significantly (p < 0.0001) reduced cell viability (Fig. 8a). Recipient BECs treated with naïve and mito-MVs at 10–50 µL volume/well showed a significant (p < 0.0001) increase in BEC ATP levels compared to OGD control. Importantly, BECs treated with mito-MV at 10, 30, and 50 µL EV volume showed significantly (at least p < 0.05) higher ATP levels than their naïve MV counterparts. BECs treated with 10 µL naïve EXO showed a significant (p < 0.0001) increase in BEC ATP levels compared to OGD control (Fig. 8b). Increasing EXO treatment volumes from 10 to 50 µL showed a gradual rise in BEC ATP levels. Mito-EXO also significantly (p < 0.0001) increased BEC ATP levels compared to OGD control (Fig. 8b). However, mito-EXO-mediated increase in ATP levels was not substantially greater than naïve EXO at the same treatment volume. These data suggest that mito-MVs isolated from resveratrol-activated BECs show superior effects of MV mitochondria compared to naïve MVs. The observed increase in ATP levels of recipient BECs is likely due to MV-mediated mitochondrial transfer into recipient cells that subsequently colocalized with the cell’s mitochondrial network and participated in oxidative phosphorylation.
Figure 8.
Effect of naïve and mito-EVs-derived from hCMEC/D3 BECs on relative ATP levels in the recipient BECs. (a,b) Recipient hCMEC/D3 cells were treated with BEC-derived naïve and mito-MVs (a) and naïve and mito-EXOs (b) at 10, 30, and 50 µL EV volume/well for 24 h in OGD medium. Untreated cells incubated in the OGD medium were used as OGD control, whereas untreated cells incubated in complete growth medium were used as the normoxic control. (c-d) Effects of RTN-EVs and OGM-EVs on the resulting ATP levels in recipient BECs under OGD conditions. Recipient BECs were treated with hCMEC/D3 BEC-derived naïve, RTN-EVs (c), and OGM-EVs (d) at 25 µg EV protein/well for 24 h in OGD medium. Untreated cells incubated in OGD medium were used as OGD control, whereas untreated cells incubated in complete growth medium were used as the normoxic control. Post-incubation, CellTiter-Glo 2.0 reagent was added to an equal volume of cell culture medium in each well. Luminescence was measured at 1 s integration time using a SYNERGY HTX multi-mode plate reader. The % relative ATP levels of the treated cells were calculated as follows = (relative luminescence unit (RLU) of treated cells/RLU of untreated OGD cells) × 100. The differences in ATP levels of the treated groups were compared against control using one-way ANOVA using GraphPad Prism software. *p < 0.05, ***p < 0.001, ****p < 0.0001, ns: non-significant.
We performed additional ATP assays to determine if the MV-mediated increases in the recipient BEC ATP levels are associated with MV mitochondria. We first inhibited the mitochondrial electron transport chain in the donor BECs by blocking complex I using rotenone (RTN) and ATP5A synthase complex using oligomycin A (OGM). We hypothesized that inhibiting mitochondrial function in the donor BECs would affect the loading of functionally active mitochondria into the MVs and, consequently, affect the resulting ATP levels in the recipient BECs.
Recipient hCMEC/D3 BECs were treated with naïve and RTN-EXO and MV at 25 µg EV protein/well for 24 h in OGD conditions. Untreated cells were incubated in either OGD medium (for OGD control) or in complete growth medium (for normoxic control). OGD control showed about 60% reduction in ATP levels suggesting that OGD conditions significantly (p < 0.0001) reduced cell viability (Fig. 8c). BECs treated with naïve EXO showed a significant (p < 0.0001) increase in ATP levels compared to OGD control. RTN-EXO derived from donor cells treated with 0.5, 1, and 10 µM RTN showed a significant (p < 0.0001) increase in ATP levels (Fig. 8c). Notably, RTN-EXO did not show any reduction in ATP levels compared to naïve EXO (Fig. 8c), suggesting that RTN-mediated mitochondrial complex I inhibition in donor cells did not affect EXO functionality.
BECs treated with naïve MVs showed a significant (p < 0.0001), 2.5-fold increase in ATP levels. Notably, RTN-MVs showed RTN-concentration dependent significant (p < 0.0001) decrease in ATP levels (Fig. 8c). RTN-MVs from donor BECs treated with 0.5 µM RTN showed about a 30% reduction while RTN-MVs from donor cells treated with 1 µM RTN showed about a 90% reduction in ATP levels compared to naïve MVs (Fig. 8c). Notably, RTN-MVs from donor cells treated with 10 µM RTN completely abolished the BEC ATP levels compared to naive MVs (Fig. 8c). These data suggest that mitochondrial complex I inhibition in the donor BECs inhibits the mitochondrial function in MVs. Based on this data, we conclude that the MV-mediated increase in BECs ATP levels is a function of MV mitochondria.
Similarly, OGM-EXO did not affect BEC ATP levels compared to naïve EXOs (Fig. 8d). Consistent with RTN-MVs, OGM-MVs from donor BECs treated at 1 µM OGM showed complete loss of BEC ATP levels (Fig. 8d). Collectively, these data confirm that MVs isolated from RTN and OGM-exposed BECs showed a loss of RTN-MV and OGM-MV mitochondrial functionality to a much greater extent than RTN-EXO and OGM-EXO, respectively. This further confirms that MVs but not EXOs contain functional mitochondria. RTN and OGM-mediated mitochondrial damage in BECs likely activates mitophagy leading to the incorporation of damaged mitochondria in the MVs.60 Therefore, RTN-MVs and OGM-MVs fail to show any functional increases in recipient BEC ATP levels.
In summary, while mito-EXOs did not show any changes in recipient BEC ATP levels compared to naïve EXOs, mito-MVs showed a significant, dose-dependent increase in ATP levels compared to naïve MVs. Overall, our collective findings from western blotting, DLS, TEM, fluorescence microscopy, and ATP measurement studies support our hypothesis that resveratrol-mediated increase in mitochondrial biogenesis in donor cells enhance mitochondrial load in secreted MVs and mito-MVs outperform naïve MVs to increase ischemic BEC ATP levels (Scheme 1).
Scheme 1.
Generation of mito-MVs and highlights of key findings.
Conclusions
Despite the lack of major changes in PGC-1α protein expression in the resveratrol-treated donor cells, we noticed consistent and significant changes in MV mitochondrial load, particle diameters and EV particle concentrations. Strikingly, mito-MVs showed a greater extent of mitochondrial transfer and resulting ATP levels in the recipient BECs. Our further studies will involve detailed characterization of the mitochondrial load in mito-MVs and optimization of the mito-MV platform for delivery of mitochondria to the blood–brain barrier to treat ischemic stroke.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Funding
Maura Farinelli and Abigail Sullivan’s NURE fellowship were supported through a grant from the National Institute of Neurological Disorders and Stroke (5R25NS100118-04) and this study was supported via start-up funds to Devika S. Manickam (Duquesne University).
Conflict of interest
The authors declare no conflicts of interest.
Devika S. Manickam
received her Ph.D. in Pharmaceutical Sciences from Wayne State University (Detroit, MI). She started her independent career in 2016 as an Assistant Professor of Pharmaceutics at the School of Pharmacy, Duquesne University (Pittsburgh, PA). Her laboratory is identifying novel therapeutic strategies for delivery “to” the blood–brain barrier—an under-explored target for brain drug delivery. Her laboratory develops lipid nanoparticle- and extracellular vesicle (EV)-based systems for the delivery of small molecule, nucleic acid and protein drugs. Importantly, her laboratory has championed the idea of harnessing the innate EV mitochondrial load as a therapeutic cargo, in addition to engineering EVs for the delivery of exogenous drugs. In this work, for the first time, her group has demonstrated the potential to increase the innate EV mitochondrial load via pharmacological activation of the donor cells. Prof. Manickam has published papers in leading drug delivery journals including Journal of Controlled Release, Biomaterials and Advanced Drug Delivery Reviews. She also serves as an Assistant Editor at the Journal of Controlled Release.
Footnotes
This article is part of the 2022 CMBE Young Innovators special issue.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Maura N. Farinelli and Abigail Sullivan have contributed equally to this study.
References
- 1.Ahluwalia M, Kumar M, Ahluwalia P, Rahimi S, Vender JR, Raju RP, et al. Rescuing mitochondria in traumatic brain injury and intracerebral hemorrhages—a potential therapeutic approach. Neurochem. Int. 2021;150:105192. doi: 10.1016/j.neuint.2021.105192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreux PA, Houtkooper RH, Auwerx J. Pharmacological approaches to restore mitochondrial function. Nat. Rev. Drug Discov. 2013;12(6):465–483. doi: 10.1038/nrd4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernardo-Castro S, Sousa JA, Brás A, Cecília C, Rodrigues B, Almendra L, et al. Pathophysiology of blood–brain barrier permeability throughout the different stages of ischemic stroke and its implication on hemorrhagic transformation and recovery. Front. Neurol. 2020;11:1605. doi: 10.3389/fneur.2020.594672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busatto S, Pham A, Suh A, Shapiro S, Wolfram J. Organotropic drug delivery: synthetic nanoparticles and extracellular vesicles. Biomed. Microdevices. 2019;21(2):46. doi: 10.1007/s10544-019-0396-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chazotte B. Labeling mitochondria with MitoTracker dyes. Cold Spring Harb. Protoc. 2011;2011(8):990–992. doi: 10.1101/pdb.prot5648. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Fan Q, Li A, Liao D, Ge J, Laties AM, et al. Dynamic mobilization of PGC-1α mediates mitochondrial biogenesis for the protection of RGC-5 cells by resveratrol during serum deprivation. Apoptosis. 2013;18(7):786–799. doi: 10.1007/s10495-013-0837-3. [DOI] [PubMed] [Google Scholar]
- 7.Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2009;297(1):H13–H20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Currais A. Ageing and inflammation—a central role for mitochondria in brain health and disease. Ageing Res. Rev. 2015;21:30–42. doi: 10.1016/j.arr.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 9.D’Souza A, Dave KM, Stetler RA, Manickam DS. Targeting the blood–brain barrier for the delivery of stroke therapies. Adv. Drug Deliv. Rev. 2021;171:332–351. doi: 10.1016/j.addr.2021.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Dai X, Chen J, Xu F, Zhao J, Cai W, Sun Z, et al. TGFalpha preserves oligodendrocyte lineage cells and improves white matter integrity after cerebral ischemia. J. Cereb. Blood Flow Metab. 2020;40(3):639–655. doi: 10.1177/0271678X19830791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dave K, Reynolds MJ, Stolz D, Babidhan R, Dobbins D, Yankello H, et al. Extracellular vesicles deliver mitochondria and HSP27 protein to protect the blood–brain barrier. bioRxiv. 2022 doi: 10.1101/2021.10.29.466491. [DOI] [Google Scholar]
- 12.Dave KM, Zhao W, Hoover C, D'Souza A, Manickam DS. Extracellular vesicles derived from a human brain endothelial cell line increase cellular ATP levels. AAPS PharmSciTech. 2021;22(1):18. doi: 10.1208/s12249-020-01892-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davinelli S, Sapere N, Visentin M, Zella D, Scapagnini G. Enhancement of mitochondrial biogenesis with polyphenols: combined effects of resveratrol and equol in human endothelial cells. Immunity Ageing. 2013;10(1):28. doi: 10.1186/1742-4933-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Jong OG, Kooijmans SAA, Murphy DE, Jiang L, Evers MJW, Sluijter JPG, et al. Drug delivery with extracellular vesicles: from imagination to innovation. Acc. Chem. Res. 2019;52(7):1761–1770. doi: 10.1021/acs.accounts.9b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delmas D, Aires V, Colin DJ, Limagne E, Scagliarini A, Cotte AK, et al. Importance of lipid microdomains, rafts, in absorption, delivery, and biological effects of resveratrol. Ann. N. Y. Acad. Sci. 2013;1290(1):90–97. doi: 10.1111/nyas.12177. [DOI] [PubMed] [Google Scholar]
- 16.Doll DN, Hu H, Sun J, Lewis SE, Simpkins JW, Ren X. Mitochondrial crisis in cerebrovascular endothelial cells opens the blood–brain barrier. Stroke. 2015;46(6):1681–1689. doi: 10.1161/STROKEAHA.115.009099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Souza A, Burch A, Dave KM, Sreeram A, Reynolds MJ, Dobbins DX, et al. Microvesicles transfer mitochondria and increase mitochondrial function in brain endothelial cells. J. Control. Release. 2021;338:505–526. doi: 10.1016/j.jconrel.2021.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duchen MR. Mitochondria in health and disease: perspectives on a new mitochondrial biology. Mol. Aspects Med. 2004;25(4):365–451. doi: 10.1016/j.mam.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Elsharkasy OM, Nordin JZ, Hagey DW, de Jong OG, Schiffelers RM, Andaloussi SEL, et al. Extracellular vesicles as drug delivery systems: why and how? Adv. Drug Deliv. Rev. 2020;159:332–343. doi: 10.1016/j.addr.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Evers MJW, van de Wakker SI, de Groot EM, de Jong OG, Gitz-François JJJ, Seinen CS, et al. Functional siRNA delivery by extracellular vesicle-liposome hybrid nanoparticles. Adv. Healthc. Mater. 2022;11(5):e2101202. doi: 10.1002/adhm.202101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 2011;93(4):884S–S890. doi: 10.3945/ajcn.110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiskum G, Murphy AN, Beal MF. Mitochondria in neurodegeneration: acute ischemia and chronic neurodegenerative diseases. J. Cereb. Blood Flow Metab. 1999;19(4):351–369. doi: 10.1097/00004647-199904000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Friedman JR, Nunnari J. Mitochondrial form and function. Nature. 2014;505(7483):335–343. doi: 10.1038/nature12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray WD, Mitchell AJ, Searles CD. An accurate, precise method for general labeling of extracellular vesicles. MethodsX. 2015;2:360–367. doi: 10.1016/j.mex.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayakawa K, Chan SJ, Mandeville ET, Park JH, Bruzzese M, Montaner J, et al. Protective effects of endothelial progenitor cell-derived extracellular mitochondria in brain endothelium. Stem Cells. 2018;36(9):1404–1410. doi: 10.1002/stem.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535(7613):551–555. doi: 10.1038/nature18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021;16(7):748–759. doi: 10.1038/s41565-021-00931-2. [DOI] [PubMed] [Google Scholar]
- 28.Ikeda G, Santoso MR, Tada Y, Li AM, Vaskova E, Jung JH, et al. Mitochondria-rich extracellular vesicles from autologous stem cell-derived cardiomyocytes restore energetics of ischemic myocardium. J. Am. Coll. Cardiol. 2021;77(8):1073–1088. doi: 10.1016/j.jacc.2020.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 2012;18:759. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaushik P, Ali M, Salman M, Tabassum H, Parvez S. Harnessing the mitochondrial integrity for neuroprotection: therapeutic role of piperine against experimental ischemic stroke. Neurochem. Int. 2021;149:105138. doi: 10.1016/j.neuint.2021.105138. [DOI] [PubMed] [Google Scholar]
- 31.Kim A, Ng WB, Bernt W, Cho N-J. Validation of size estimation of nanoparticle tracking analysis on polydisperse macromolecule assembly. Sci. Rep. 2019;9(1):2639. doi: 10.1038/s41598-019-38915-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komen JC, Thorburn DR. Turn up the power—pharmacological activation of mitochondrial biogenesis in mouse models. Br. J. Pharmacol. 2014;171(8):1818–1836. doi: 10.1111/bph.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamichhane TN, Jeyaram A, Patel DB, Parajuli B, Livingston NK, Arumugasaamy N, et al. Oncogene knockdown via active loading of small RNAs into extracellular vesicles by sonication. Cell. Mol. Bioeng. 2016;9(3):315–324. doi: 10.1007/s12195-016-0457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee MJ, Jang Y, Han J, Kim SJ, Ju X, Lee YL, et al. Endothelial-specific Crif1 deletion induces BBB maturation and disruption via the alteration of actin dynamics by impaired mitochondrial respiration. J. Cereb. Blood Flow Metab. 2020;40(7):1546–1561. doi: 10.1177/0271678X19900030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1(6):361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 36.López-Lluch G, Irusta PM, Navas P, de Cabo R. Mitochondrial biogenesis and healthy aging. Exp. Gerontol. 2008;43(9):813–819. doi: 10.1016/j.exger.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madineni A, Alhadidi Q, Shah ZA. Cofilin inhibition restores neuronal cell death in oxygen-glucose deprivation model of ischemia. Mol. Neurobiol. 2016;53(2):867–878. doi: 10.1007/s12035-014-9056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manickam DS. Delivery of mitochondria via extracellular vesicles—a new horizon in drug delivery. J. Control. Release. 2022;343:400–407. doi: 10.1016/j.jconrel.2022.01.045. [DOI] [PubMed] [Google Scholar]
- 39.Moreira PI, Carvalho C, Zhu X, Smith MA, Perry G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim. Biophys. Acta. 2010;1802(1):2–10. doi: 10.1016/j.bbadis.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 40.Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O'Kane CM, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am. J. Respir. Crit. Care Med. 2017;196(10):1275–1286. doi: 10.1164/rccm.201701-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy DE, de Jong OG, Brouwer M, Wood MJ, Lavieu G, Schiffelers RM, et al. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp. Mol. Med. 2019;51(3):1–12. doi: 10.1038/s12276-019-0223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy DE, de Jong OG, Evers MJW, Nurazizah M, Schiffelers RM, Vader P. Natural or synthetic RNA delivery: a stoichiometric comparison of extracellular vesicles and synthetic nanoparticles. Nano Lett. 2021;21(4):1888–1895. doi: 10.1021/acs.nanolett.1c00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neves AR, Nunes C, Reis S. Resveratrol induces ordered domains formation in biomembranes: Implication for its pleiotropic action. Biochim. Biophys. Acta. 2016;1858(1):12–18. doi: 10.1016/j.bbamem.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Nian K, Harding IC, Herman IM, Ebong EE. Blood–brain barrier damage in ischemic stroke and its regulation by endothelial mechanotransduction. Front. Physiol. 2020;11:1681. doi: 10.3389/fphys.2020.605398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Niel G, Carter DRF, Clayton A, Lambert DW, Raposo G, Vader P. Challenges and directions in studying cell–cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2022;23:369–382. doi: 10.1038/s41580-022-00460-3. [DOI] [PubMed] [Google Scholar]
- 46.Oldendorf WH, Cornford ME, Brown WJ. The large apparent work capability of the blood–brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann. Neurol. 1977;1(5):409–417. doi: 10.1002/ana.410010502. [DOI] [PubMed] [Google Scholar]
- 47.Peng K, Tao Y, Zhang J, Wang J, Ye F, Dan G, et al. Resveratrol regulates mitochondrial biogenesis and fission/fusion to attenuate rotenone-induced neurotoxicity. Oxid. Med. Cell Longev. 2016;2016:6705621. doi: 10.1155/2016/6705621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat. Commun. 2015;6(1):8472. doi: 10.1038/ncomms9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Puhm F, Afonyushkin T, Resch U, Obermayer G, Rohde M, Penz T, et al. Mitochondria are a subset of extracellular vesicles released by activated monocytes and induce type I IFN and TNF responses in endothelial cells. Circ. Res. 2019;125(1):43–52. doi: 10.1161/CIRCRESAHA.118.314601. [DOI] [PubMed] [Google Scholar]
- 50.Ragonese F, Monarca L, De Luca A, Mancinelli L, Mariani M, Corbucci C, et al. Resveratrol depolarizes the membrane potential in human granulosa cells and promotes mitochondrial biogenesis. Fertil. Steril. 2021;115(4):1063–1073. doi: 10.1016/j.fertnstert.2020.08.016. [DOI] [PubMed] [Google Scholar]
- 51.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Razmara A, Sunday L, Stirone C, Wang XB, Krause DN, Duckles SP, et al. Mitochondrial effects of estrogen are mediated by estrogen receptor alpha in brain endothelial cells. J. Pharmacol. Exp. Ther. 2008;325(3):782–790. doi: 10.1124/jpet.107.134072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sansone P, Savini C, Kurelac I, Chang Q, Amato LB, Strillacci A, et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc. Natl Acad. Sci. 2017;114(43):E9066. doi: 10.1073/pnas.1704862114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim. Biophys. Acta. 2011;1813(7):1269–78. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scarpulla RC, Vega RB, Kelly DP. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol. Metab. 2012;23(9):459–466. doi: 10.1016/j.tem.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Silva JD, Su Y, Calfee CS, Delucchi KL, Weiss D, McAuley DF, et al. MSC extracellular vesicles rescue mitochondrial dysfunction and improve barrier integrity in clinically relevant models of ARDS. Eur. Respir. J. 2020;58(1):2002978. doi: 10.1183/13993003.02978-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc. Natl Acad. Sci. U.S.A. 2006;103(5):1283. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Torralba D, Baixauli F, Sánchez-Madrid F. Mitochondria know no boundaries: mechanisms and functions of intercellular mitochondrial transfer. Front. Cell Dev. Biol. 2016;4:107. doi: 10.3389/fcell.2016.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Torralba D, Baixauli F, Villarroya-Beltri C, Fernández-Delgado I, Latorre-Pellicer A, Acín-Pérez R, et al. Priming of dendritic cells by DNA-containing extracellular vesicles from activated T cells through antigen-driven contacts. Nat. Commun. 2018;9(1):2658. doi: 10.1038/s41467-018-05077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vader P, Breakefield XO, Wood MJA. Extracellular vesicles: emerging targets for cancer therapy. Trends Mol. Med. 2014;20(7):385–393. doi: 10.1016/j.molmed.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016;106:148–156. doi: 10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 62.Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1α. Cardiovasc. Res. 2008;79(2):208–217. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 63.Walker S, Busatto S, Pham A, Tian M, Suh A, Carson K, et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics. 2019;9(26):8001–8017. doi: 10.7150/thno.37097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weissig V. Drug Development for the therapy of mitochondrial diseases. Trends Mol. Med. 2020;26(1):40–57. doi: 10.1016/j.molmed.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 66.Weissig V, Cheng S-M, D’Souza GGM. Mitochondrial pharmaceutics. Mitochondrion. 2004;3(4):229–244. doi: 10.1016/j.mito.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 67.Wen Y, Li W, Poteet EC, Xie L, Tan C, Yan LJ, et al. Alternative mitochondrial electron transfer as a novel strategy for neuroprotection. J. Biol. Chem. 2011;286(18):16504–16515. doi: 10.1074/jbc.M110.208447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wenz T. Regulation of mitochondrial biogenesis and PGC-1α under cellular stress. Mitochondrion. 2013;13(2):134–142. doi: 10.1016/j.mito.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 69.Whitaker RM, Corum D, Beeson CC, Schnellmann RG. Mitochondrial biogenesis as a pharmacological target: a new approach to acute and chronic diseases. Annu. Rev. Pharmacol. Toxicol. 2016;56:229–249. doi: 10.1146/annurev-pharmtox-010715-103155. [DOI] [PubMed] [Google Scholar]
- 70.Witwer KW, Wolfram J. Extracellular vesicles versus synthetic nanoparticles for drug delivery. Nat. Rev. Mater. 2021;6(2):103–106. doi: 10.1038/s41578-020-00277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Z, Ma Z, Yan C, Pu K, Wu M, Bai J, et al. Muscle-derived autologous mitochondrial transplantation: a novel strategy for treating cerebral ischemic injury. Behav. Brain Res. 2019;356:322–331. doi: 10.1016/j.bbr.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 72.Zhao Q, Tian Z, Zhou G, Niu Q, Chen J, Li P, et al. SIRT1-dependent mitochondrial biogenesis supports therapeutic effects of resveratrol against neurodevelopment damage by fluoride. Theranostics. 2020;10(11):4822–4838. doi: 10.7150/thno.42387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou J, Yang Z, Shen R, Zhong W, Zheng H, Chen Z, et al. Resveratrol improves mitochondrial biogenesis function and activates PGC-1α pathway in a preclinical model of early brain injury following subarachnoid hemorrhage. Front. Mol. Biosci. 2021;8:223. doi: 10.3389/fmolb.2021.620683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.