In individuals of healthy weight, intranasal insulin can enhance cognition and decision-making; these effects are dissociable from insulin’s ability to regulate blood glucose. Furthermore, rising rates of obesity and type II diabetes, diseases characterized by elevated insulin and blunted insulin signaling, highlight the need to understand insulin’s effects on neurophysiology [1]. However, despite expression of insulin receptors throughout the brain, little is known about its effects on neural function and resulting behavior (see ref. [2] for review). For example, in humans and rodents, actions of insulin in the brain decrease food intake, but the mechanisms are undefined; the same is true for insulin’s cognitive-enhancing properties, with or without obesity.
We found that in the nucleus accumbens (NAc) of healthy male rats, insulin receptor activation increases presynaptic glutamate release onto medium spiny neurons [3]. This was due to opioid receptor-mediated disinhibition, suggesting that insulin enhances endogenous opioid tone. Furthermore, insulin’s ability to enhance glutamate release was absent in obesity which was associated with reduced NAc insulin receptor expression. NAc activity is central to many aspects of motivation and decision-making, including urges to seek out and consume food. Indeed, insulin within the NAc influences the formation of associations between caloric value and taste [4], thereby influencing food choice and preference. Furthermore, while intra-NAc insulin is sufficient to reduce food intake and motivation for food, it does not alter motivational responses to cues that predict food availability [5]. These dissociations may arise in part from complex effects of insulin within the NAc.
In addition to effects on glutamate and opioids, insulin enhances local NAc dopamine release by directly increasing cholinergic interneuron firing, and can enhance dopamine transporter function (Fig. 1; see ref. [2] for review). Thus, it’s tempting to speculate that insulin may coordinate activity between NAc dopamine, glutamate, and endogenous opioids, although direct studies are needed. In the VTA, insulin reduced presynaptic glutamate release onto dopamine neurons via a form of endocannabinoid-mediated LTD. In addition, intra-VTA insulin did not alter motivation for sweet foods, but did prevent the expression of food-induced CPP. In contrast to presynaptic effects in NAc and VTA, studies of hippocampus find postsynaptic effects of insulin on AMPA and NMDA receptor trafficking and the induction of LTP. Furthermore, deficits in spatial learning and memory are induced by knock down of hippocampal insulin receptors.
Fig. 1. Summary of effects of insulin receptor activation on synaptic transmission within the nucleus accumbens (striatum) and ventral tegmental area (VTA).
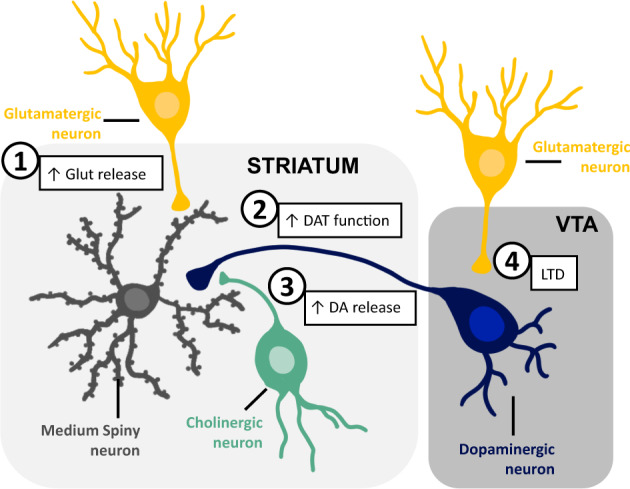
1) Insulin receptor activation increases presynaptic glutamate release onto medium spiny neurons. This is due to opioid receptor-mediated disinhibition. 2) Insulin enhances the function of dopamine transporters (DAT) on presynaptic dopamine terminals. 3) Insulin enhances local presynaptic dopamine (DA) release by increasing cholinergic interneuron firing. 4) Insulin causes a form of endocannabinoid-mediated LTD, reducing presynaptic glutamate release onto VTA dopamine neurons. These effects of insulin are reduced or absent following diet-induced obesity; this may be due in part to a reduction in insulin receptor expression.
There are a number of outstanding questions about insulin’s role in the central nervous system (CNS). First, there is ongoing debate over the source of insulin; recent data showing that the choroid plexus produces and releases insulin into the CNS challenge the traditional view that neural insulin is pancreatic in origin [6]. Second, the physiological range of insulin concentration in the CNS is unclear, particularly in overweight and obesity. Finally, the loss of responsivity to insulin in the brain precedes insulin resistance in peripheral tissues that characterizes Type II Diabetes. Thus, much remains to be examined to gain an understanding of the physiological role of insulin in the brain and how this can be used to alter motivation and cognition.
Acknowledgements
We thank Dr. Victoria Macht for artwork shown in Fig. 1. CRF was supported by NIDDK R01DK130246, R01DK106188, and 1R01DK115526-01; JEF was supported by T32-DK101357.
Author contributions
CRF and JEF both contributed to the writing of this manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kullmann S, Heni M, Hallschmid M, Fritsche A, Preissl H, Haring HU. Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiological Rev. 2016;96:1169–209. doi: 10.1152/physrev.00032.2015. [DOI] [PubMed] [Google Scholar]
- 2.Ferrario CR, Reagan LP. Insulin-mediated synaptic plasticity in the CNS: anatomical, functional and temporal contexts. Neuropharmacology. 2018;136:182–91. doi: 10.1016/j.neuropharm.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fetterly TL, Oginsky MF, Nieto AM, Alonso-Caraballo Y, Santana-Rodriguez Z, Ferrario CR. Insulin bidirectionally alters NAc glutamatergic transmission: interactions between insulin receptor activation, endogenous opioids, and glutamate release. J Neurosci. 2021;41:2360–72. doi: 10.1523/JNEUROSCI.3216-18.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woods CA, Guttman ZR, Huang D, Kolaric RA, Rabinowitsch AI, Jones KT, et al. Insulin receptor activation in the nucleus accumbens reflects nutritive value of a recently ingested meal. Physiol Behav. 2016;159:52–63. doi: 10.1016/j.physbeh.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finnell JE, Ferrario CR. Intra-NAc insulin reduces the motivation for food and food intake without altering cuetriggered food-seeking. Physiol Behav. 2022;254:113892. 10.1016/j.physbeh.2022.113892. Epub 2022 Jun 24. [DOI] [PMC free article] [PubMed]
- 6.Mazucanti CH, Liu QR, Lang D, Huang N, O’Connell JF, Camandola S, et al. Insulin is produced in choroid plexus and its release is regulated by serotonergic signaling. JCI insight. 2019;4:e131682. doi: 10.1172/jci.insight.131682. [DOI] [PMC free article] [PubMed] [Google Scholar]


