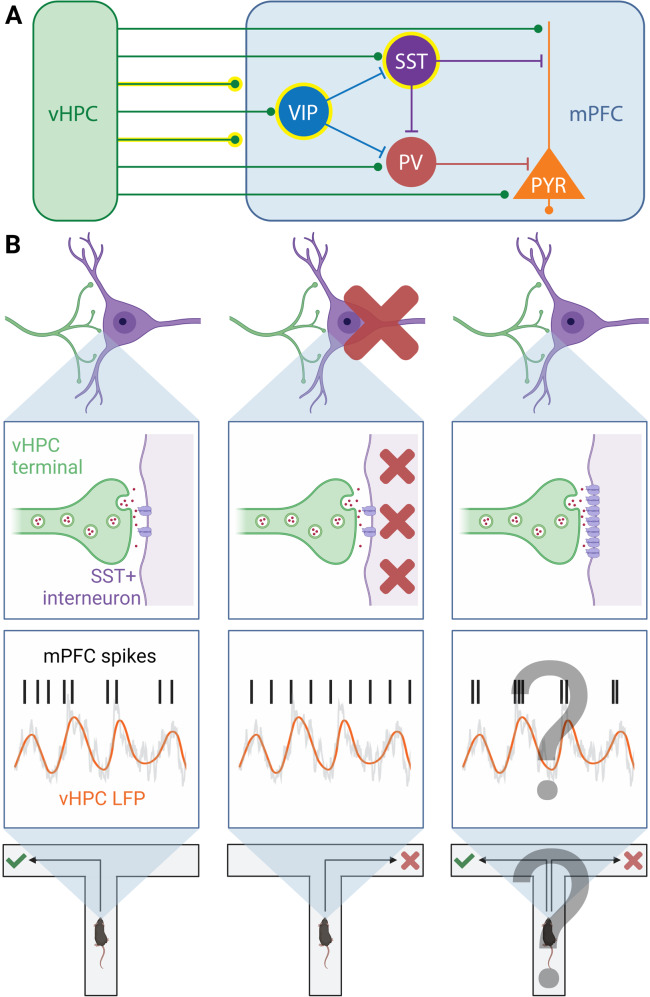
Dynamic communication between prefrontal cortex and its distal network partners underlies cognitive function and dysfunction [1]. Measures of long-range functional connectivity, including oscillatory synchrony, provide the means to quantify and mechanistically dissect this neural dialogue. Prefrontal inhibitory microcircuits, comprised of diverse subclasses of interneurons and their targets, are uniquely positioned to gate long-range functional connectivity (Fig. 1A). For example, during performance of a spatial working memory task, activity in somatostatin-positive interneurons (SST-INs) is essential for theta-frequency oscillatory synchrony between mouse medial prefrontal cortex (mPFC) and ventral hippocampus (vHPC) [2]. Inhibition of SST-INs also disrupts encoding of spatial information in mPFC neurons and working memory accuracy (Fig. 1B). Similarly, mPFC vasoactive intestinal polypeptide-positive interneurons (VIP-INs) support avoidance behavior and mPFC neuronal representations in the elevated plus maze by disinhibiting theta-frequency communication within vHPC-mPFC circuits [3]. How mPFC interneurons dynamically interact with their long-range afferents to mediate contextually tuned network communication, and how genetic and environmental factors bias these interactions, remain important unanswered questions.
Fig. 1. Synaptic plasticity between mPFC interneurons and their long-range afferents is positioned to exert dynamic control of cognition-supporting functional connectivity.
A Heavily simplified schematic of canonical connections between rodent vHPC inputs and mPFC microcircuits. Highlighted in yellow are neuronal elements (non-specific vHPC inputs [1], SST-INs [2] and VIP-INs [3]) that have been implicated in cognition-supporting oscillatory synchrony and neuronal communication between the vHPC and mPFC. B Long-range functional connectivity between the vHPC and mPFC following manipulations of prefrontal SST-IN activity and vHPC input-SST + interneuron synaptic efficacy. Phase locking of mPFC neuron spiking to vHPC theta oscillations, a measure of vHPC-mPFC theta synchrony, during performance of a spatial working memory task (left) [1]. Optogenetic inhibition of mPFC SST-INs impairs vHPC-mPFC theta synchrony and spatial working memory task performance (middle) [2]. How synaptic potentiation at vHPC synapses onto mPFC SST-INs impacts vHPC-mPFC synchrony and spatial working memory task performance is an important question for future research (right). Created with BioRender.com.
Undergirding the study of these dynamic interactions are efforts to map the structural and functional connections between long-range inputs and mPFC interneurons. Indeed, work using trans-synaptic viral tracing, retrograde labelling, optogenetic stimulation, and whole-cell electrophysiology has revealed previously unappreciated specificity in the synaptic targeting and modulation of mPFC interneurons by inputs originating from disparate and intermingled neuronal populations (e.g., [3–6]). In one striking study, Sanchez-Bellot and colleagues [4] showed that a subset of projection neurons in superficial vHPC preferentially synapses onto diverse mPFC interneurons, whereas a subset in deeper vHPC preferentially targets pyramidal neurons and fast-spiking interneurons. This dissociable connectivity seems to underlie functional specificity, as activation of these parallel pathways promoted exploration and avoidance, respectively.
Emerging evidence further reveals that highly specialized input-interneuron connections in mPFC are remodeled by disease-relevant genetic insults and salient experience. In an elegant series of experiments, Joffe and colleagues [6] found that acute restraint stress in mice potentiated excitatory drive of basolateral amygdala inputs onto mPFC SST-INs, likely through a metabotropic glutamate receptor-5 (mGlu5)-dependent form of long-term potentiation. Further, this potentiation preferentially facilitated heterosynaptic inhibition of excitatory transmission at mediodorsal thalamus-derived inputs to mPFC pyramidal neurons. Genetic deletion of mGlu5 in SST-INs also prevented restraint stress-induced impairments in spatial working memory task performance. Collectively, these findings indicate that experience-induced plasticity at SST-INs can bias the routing of circuit-specific, behaviorally relevant information though mPFC.
These and a growing collection of other studies showcase how synaptic plasticity between mPFC interneurons and their afferents is poised to exert dynamic control of cognition-supporting long-range functional connectivity [1] (Fig. 1). Therefore, we propose that characterization of the precise interneuron adaptations that shape translationally relevant long-range functional connectivity with mPFC stands to inform the development of novel pharmacological and pharmacologically assisted brain stimulation therapies. Lastly, we emphasize the critical need for establishing in vivo induction paradigms (e.g., targeted circuit activations) and readouts (e.g., combined optogenetic stimulation and fluorescence imaging) of input-interneuron plasticity to definitively link such plasticity with functional connectivity dynamics in wildtype and disease-relevant animal models.
Author contributions
JAG and DAK conducted a literature review, planned and edited the manuscript. DAK wrote the first draft of the manuscript.
Funding
Support for this work is provided by the NINDS Intramural Research Program (ZIA NS003168-04).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kupferschmidt DA, Gordon JA. The dynamics of disordered dialogue: Prefrontal, hippocampal and thalamic miscommunication underlying working memory deficits in schizophrenia. Brain Neurosci Adv. 2018;2:2398212818771821. doi: 10.1177/2398212818771821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbas AI, Sundiang MJM, Henoch B, Morton MP, Bolkan SS, Park AJ, et al. Somatostatin interneurons facilitate hippocampal-prefrontal synchrony and prefrontal spatial encoding. Neuron. 2018;100:926–39. doi: 10.1016/j.neuron.2018.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee AT, Cunniff MM, See JZ, Wilke SA, Luongo FJ, Ellwood IT, et al. VIP interneurons contribute to avoidance behavior by regulating information flow across hippocampal-prefrontal networks. Neuron. 2019;102:1223–34. doi: 10.1016/j.neuron.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sánchez-Bellot C, AlSubaie R, Mishchanchuk K, Wee RWS, MacAskill AF. Two opposing hippocampus to prefrontal cortex pathways for the control of approach and avoidance behaviour. Nat Commun. 2022;13:339. doi: 10.1038/s41467-022-27977-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tejeda HA, Wang H, Flores RJ, Yarur HE. Dynorphin/kappa-opioid receptor system modulation of cortical circuitry. Handb Exp Pharm. 2022;271:223–53. doi: 10.1007/164_2021_440. [DOI] [PubMed] [Google Scholar]
- 6.Joffe ME, Maksymetz J, Luschinger JR, Dogra S, Ferranti AS, Luessen DJ, et al. Acute restraint stress redirects prefrontal cortex circuit function through mGlu5 receptor plasticity on somatostatin-expressing interneurons. Neuron. 2021;110:1068–83.e5. doi: 10.1016/j.neuron.2021.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]