Abstract
PapG adhesins mediate the binding of uropathogenic Escherichia coli. Although receptors for these adhesins have not been demonstrated in intestinal epithelia, the colonic microflora includes strains of uropathogenic E. coli. We now report that surfactant-like particles secreted by the human intestine contain receptors for PapG adhesins and may provide an intestinal habitat for uropathogenic bacteria.
Adhesion is one mechanism for promoting colonization and infection by pathogenic bacteria. Uropathogenic Escherichia coli isolated from the host's native colonic microflora expresses different types of adhesive organelles, including P pili that bind the α-d-galactopyranosyl-(1-4)-β-d-galactopyranoside [Galα(1-4)Gal] moiety present in the globoseries of glycolipids on cells lining the upper urinary tract (6). Genes involved in the expression of P pili are clustered in an operon structure. About 5 to 10% of all human fecal E. coli isolates contain this operon, but it is present in up to 90% of strains isolated from the urinary tracts of children with acute pyelonephritis (6, 18, 20), supporting the concepts that P pili are involved in recurrent infections and that the organisms expressing these pili could originate in the gastrointestinal tract.
Previously, members of our group identified and purified a membrane particle associated with the apical surfaces of small intestinal and colonic cells. This material, called surfactant-like particles (SLP), manifests surfactant activity and contains multiple surfactant-associated proteins. About 25% of the secreted membrane reaches the apical surface of the enterocyte via tight junctions (2), but its function at that site is obscure. Because one possible role for this material is to interact with luminal contents, including organisms, we sought to determine whether SLP contained a PapG adhesin receptor. The PapG adhesin of the fibrillar tip of P pili is degraded in the periplasm if expressed in the absence of its chaperone, PapD (11). Coexpression of the chaperone stabilizes the adhesin in a PapD-PapG periplasmic complex. The ability of PapG to recognize potential receptors on the surfaces of preparations of microvillous membranes (MVM) or SLP was investigated. The purified PapD-PapG complex bound preferentially to human SLP compared to binding with autologous small intestinal and colonic MVM.
P pili (9, 13) and the PapD-PapG complex (7, 10) were isolated from E. coli HB101 bearing the appropriate plasmid as described previously. The papG gene was subcloned from plasmid pPAP5. This plasmid contains the entire pap operon cloned from the E. coli J96 isolate of serotype O4:K6 (5), derived from a case of human pyelonephritis. The binding specificity of PapGJ96 defines the prototype class I G adhesin (16, 17). PapG− pili were prepared from E. coli HB101 bearing the plasmid pFJ3(G−), which lacks only the papG gene of the wild-type pap operon. The proteins were biotinylated with commercially available reagents (Sigma Chemical Co., St. Louis, Mo.).
MVM from human transplant donors were purified as described previously (14). SLP were isolated from scrapings or washings of the apical surface of enterocytes or colonocytes obtained from transplant donor intestine (1, 14, 15).
An enzyme-linked lectin sorbent assay that had been developed for small intestinal MVM (3) was modified to measure adhesin binding capacity. All experiments were repeated two to five times, with triplicate samples for each point.
Binding curves were constructed from Scatchard plots and computer-derived curves by using least-squares regression analysis, and the affinity constant (Ka) and the binding maximum were calculated. Statistical comparison was performed by analysis of means of unequal variance (two-tailed Student's t test).
Biotinylated PapD-PapG (500 ng/well) was incubated with increasing concentrations of SLP, and binding of PapD-PapG to the particles was quantitated. Maximal binding of purified P pili to small bowel or colonic SLP was found at a membrane protein concentration of 2.5 μg/ml (250 ng/well). Binding to colonic MVM was examined at a membrane protein concentration of 4 μg/ml (400 ng/well), a value similar to the maximal concentration for rat MVM in the lectin sorbent assay (3). These experiments provided the range of concentrations per well for the PapD-PapG complex and pili that were used to analyze binding of these ligands to MVM and SLP adsorbed to multiwell plates.
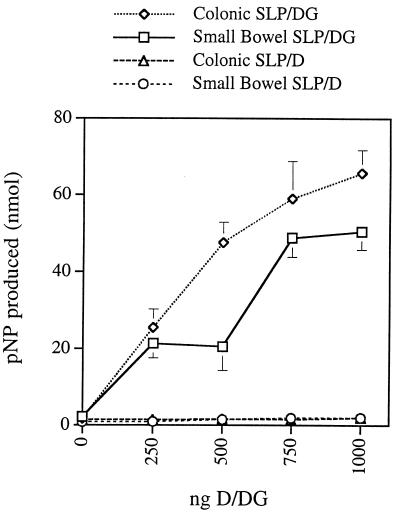
The PapD-PapG complex bound to human small intestinal and colonic SLP (Fig. 1). Binding was dependent on the PapG adhesin, as the purified PapD chaperone (isolated from a papG mutant strain) did not bind to human SLP isolated from either small bowel or colon (Fig. 1). The addition of as little as 0.79 and 7.9 nmol of PapD-PapG produced values for small bowel and colonic SLP that were statistically different (P = 0.0031 and P = 0.027, respectively) from the reading for the control with no complex added. A fivefold excess of purified, unlabeled PapD did not affect the binding of biotinylated PapD-PapG complex to intestinal SLP (data not shown). In contrast to SLP, MVM from small intestine and colon did not demonstrate detectable concentration-dependent binding, even at high PapD-PapG concentrations (data not shown). Binding affinities of the PapD-PapG complex for SLP were calculated by using Scatchard plots. PapD-PapG bound to human small bowel SLP with a Ka of 16 μM and to human colonic SLP with a Ka of 20 μM. The maximum for binding of the PapD-PapG complex to colonic SLP was approximately twice that for small bowel SLP (100 versus 50 nmol of p-nitrophenol). The PapD-PapG complex did not bind to MVM. These findings suggested that SLP contain receptors for the PapD-PapG complex.
FIG. 1.
Binding of purified PapD-PapG complexes and PapD to human SLP. Wells containing either small bowel or colonic SLP were probed with biotinylated PapD-PapG complex (DG) or with biotinylated PapD (D). Each point represents the mean ± 1 standard deviation. pNP, p-nitrophenol.
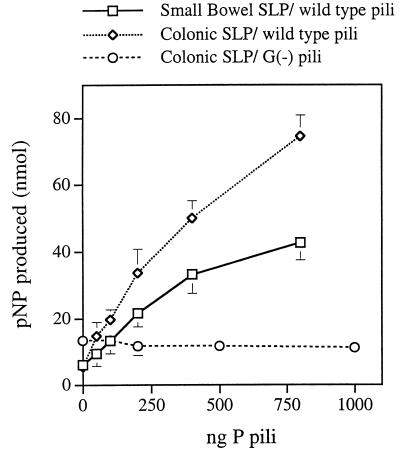
Binding of P pili to SLP showed characteristics similar to those of the isolated PapD-PapG complex. Wild-type pili bound to both small intestinal and colonic SLP (Fig. 2). The addition of pili produced binding significantly different from that of the no-added-adhesin control: as low as 50 ng of pili for colonic SLP (P = 0.012) and 200 ng of pili for small bowel SLP (P = 0.019). Binding was dependent on the PapG adhesin, as pili lacking PapG (purified from a papG mutant, otherwise structurally similar to wild-type pili [10]) did not bind to either human colonic SLP (Fig. 2) or small bowel SLP (data not shown). Binding to small bowel MVM did not occur, and although in a few samples weak binding of wild-type pili to colonic MVM was observed, this binding was inconsistent. The Kas for binding of pili to small intestinal and colonic SLP were 2.0 and 2.1 mg of pilus protein per liter, respectively. The maxima for binding of pili to both SLP preparations were similar.
FIG. 2.
Binding of purified pili from wild-type and PapG−[G(−)] strains of P-piliated E. coli to human small bowel and colonic SLP. Each point represents the mean ± 1 standard deviation. pNP, p-nitrophenol.
Despite the apparent lack of receptors on colonocytes, many uropathogenic E. coli organisms appear to originate in the colon, as determined by serotype analysis (19). The globoseries of pilus-binding glycolipids are readily found in the lumen of the colon (12), but the origin of these glycolipids has been obscure, since the isolated epithelium does not contain large amounts (4, 19). In this study we discovered that the class I PapD-PapG adhesin complex bound to specific receptors present in human small intestinal and colonic SLP. The class I allele of PapG has been found in a group of extraintestinal isolates of E. coli O4:H5, and these represent a virulent clonal group (8).
The finding of PapD-PapG adhesin complex binding to SLP in human tissues makes SLP a candidate for playing a role in E. coli colonization in the gastrointestinal lumen of humans. Such a role is further supported by reports that Galα(1-4)Gal-specific strains of E. coli have been shown to interact with a substance loosely associated (removed by magnetic stirring) with cells isolated from colonic surgical specimens (22). This finding is consistent with binding to SLP. SLP have been isolated from human small intestinal and colonic mucosal surfaces and contain unique proteins not present in either MVM or basolateral membranes. The present study utilized SLP isolated from the small intestine and colon, but the site of the reservoir previously proposed for uropathogenic E. coli is the colon alone (19, 21). Thus, it seems likely that apically located SLP present in the colon (1) could act as mediators of uropathogenic E. coli adhesion. The relative roles for small intestinal and colonic SLP remain to be determined.
The SLP-microbe interaction may represent a delicate balance between symbiosis and pathogenesis. SLP may provide the stratum necessary for colonization of uropathogenic bacteria in the human intestine while at the same time providing a barrier that protects the apical MVM from binding the pathogenic bacteria. On the other hand, when the uropathogen is introduced into the urinary tract, it is associated with a characteristic spectrum of host symptoms and diseases. Further studies are needed to determine whether this difference is due to the absence of an apical SLP-like membrane in the urogenital tract that might provide preferential binding to the epithelium itself or due to different local factors within the lumen of the gastrointestinal and genitourinary tracts. Once the nature and location of this and other membrane bacterial receptors are known, soluble receptor analogues or antagonists could be designed and used to determine the importance of this adhesin in providing the carrier state for uropathogenic E. coli and in developing possible therapeutic and antimicrobial agents.
Acknowledgments
This work was supported in part by grants DK 14038 (D.H.A.) and AI29549 and DK51406 (S.J.H.) from the National Institutes of Health. G.S.G. is supported by grant F32-DK09329 from the National Institutes of Health and by an Astra-Merck/AGA Senior Fellow Award.
REFERENCES
- 1.Eliakim R, Goetz G S, Rubio S, Chailley-Heu B, Shao J-S, Ducroc R, Alpers D H. Isolation and characterization of surfactant-like particles in rat and human colon. Am J Physiol. 1997;272:G425–G434. doi: 10.1152/ajpgi.1997.272.3.G425. [DOI] [PubMed] [Google Scholar]
- 2.Engle M J, Grove M L, Becich M J, Mahmood A, Alpers D H. Appearance of surfactant-like particles in apical medium of Caco-2 cells may occur via tight junctions. Am J Physiol. 1995;268:C1401–C1413. doi: 10.1152/ajpcell.1995.268.6.C1401. [DOI] [PubMed] [Google Scholar]
- 3.Hendriks H G C J M, Koninkx J F J G, Draaijer M, van Kijk J I, Raaijmakers J A M, Mouwen J M V M. Quantitative determination of the lectin binding capacity of small intestinal brush-border membrane. An enzyme linked sorbent assay (ELISA) Biochim Biophys Acta. 1987;905:371–375. doi: 10.1016/0005-2736(87)90465-2. [DOI] [PubMed] [Google Scholar]
- 4.Holgersson J, Stromberg N, Breimer M E. Glycolipids of human large intestine: difference in glucolipid expression related to anatomical localization, epithelial/non-epithelial tissue and the ABO, Le and Se phenotypes of the donors. Biochimie. 1988;70:1565–1574. doi: 10.1016/0300-9084(88)90292-1. [DOI] [PubMed] [Google Scholar]
- 5.Hull R A, Gill R E, Hsu P, Minshew B H, Falkow S. Construction and expression of recombinant plasmids encoding type 1 or d-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981;33:933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hultgren S J, Abraham S N, Caparon M, Falk P, St. Geme III J W, Normark S. Pilus and non-pilus bacterial adhesins: assembly and function in cell recognition. Cell. 1993;73:887–901. doi: 10.1016/0092-8674(93)90269-v. [DOI] [PubMed] [Google Scholar]
- 7.Hultgren S J, Lindberg F, Magnusson G, Kihlberg J, Tennent J M, Normark S. The PapG adhesin of uropathogenic Escherichia coli contains separate regions for receptor binding and for the incorporation into the pilus. Proc Natl Acad Sci USA. 1989;86:4357–4361. doi: 10.1073/pnas.86.12.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson J R, Russo T A, Scheutz F, Brown J J, Zhang L, Palin K, Rode C, Bloch C, Marrs C F, Foxman B. Discovery of disseminated J96-like strains of uropathogenic Escherichia coli O4:H5 containing genes for both PapG(J96) (class I) and PrsG(J96) (class III) Gal(alpha 1-4)Gal binding adhesins. J Infect Dis. 1997;175:983–988. doi: 10.1086/514006. [DOI] [PubMed] [Google Scholar]
- 9.Kuehn M J, Heuser J, Normark S, Hultgren S J. P pili in uropathogenic E. coli are composite fibres with distinct fibrillar adhesive tips. Nature. 1992;356:252–255. doi: 10.1038/356252a0. [DOI] [PubMed] [Google Scholar]
- 10.Kuehn M J, Normark S, Hultgren S J. Immunoglobulin-like PapD chaperone caps and uncaps interactive surfaces of nascently translocated pilus subunits. Proc Natl Acad Sci USA. 1991;88:10586–10590. doi: 10.1073/pnas.88.23.10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuehn M J, Ogg D J, Kihlberg J, Slonim L N, Flemmer K, Bergfors T, Hultgren S J. Structural basis of pilus subunit recognition by the PapD chaperone. Science. 1993;262:1234–1241. doi: 10.1126/science.7901913. [DOI] [PubMed] [Google Scholar]
- 12.Larson G, Falk P, Hynsjo L, Midtvedt A-C, Midtvedt T. Fecal excretion of glycosphingolipids of breast-fed and formula-fed infants. Microb Ecol Health Dis. 1990;3:305–319. [Google Scholar]
- 13.Lund B, Lindberg F P, Marklund B I, Normark S. The PapG protein is the α-d-galactopyranosyl-(1-4)-β-d-galactopyranose-binding adhesin of uropathogenic Escherichia coli. Proc Natl Acad Sci USA. 1987;84:5898–5902. doi: 10.1073/pnas.84.16.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahmood A, Mahmood S, DeSchryver-Kecskemeti K, Alpers D H. Characterization of proteins in rat and human intestinal surfactant-like particles. Arch Biochem Biophys. 1993;300:280–286. doi: 10.1006/abbi.1993.1039. [DOI] [PubMed] [Google Scholar]
- 15.Mahmood A, Yamagishi F, Eliakim R, DeSchryver-Kecskemeti K, Gramlich T L, Alpers D H. A possible role for rat intestinal surfactant-like particles in transepithelial triacylglycerol transport. J Clin Investig. 1994;93:70–80. doi: 10.1172/JCI116986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stromberg N, Marklund B I, Lund B, Ilver D, Hamers A, Gaastra W, Karlsson K A, Normark S. Host-specificity of uropathogenic Escherichia coli depends on differences in binding specificity to Galα(1-4)Gal-containing isoreceptors. EMBO J. 1990;9:2001–2010. doi: 10.1002/j.1460-2075.1990.tb08328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stromberg N, Nyholm P-G, Pascher I, Normark S. Saccharide orientation at the cell surface affects glycolipid receptor function. Proc Natl Acad Sci USA. 1991;88:9340–9344. doi: 10.1073/pnas.88.20.9340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svanborg-Eden C, Eriksson B, Hanson L A, Jodal U, Kaijser B, Lidin-Janson G, Lindberg U, Olling S. Adhesion to normal uroepithelial cells of Escherichia coli from children with various forms of urinary tract infection. J Pediatr. 1978;93:398–403. doi: 10.1016/s0022-3476(78)81145-7. [DOI] [PubMed] [Google Scholar]
- 19.Vosti K L, Goldberg L, Monto A, Rantz L. Host-parasite interaction in patients with infections due to Escherichia coli. I. The serogrouping of Escherichia coli from intestinal and extraintestinal sources. J Clin Investig. 1964;43:2377–2385. doi: 10.1172/JCI105112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westerlund B, Van Die I, Hoekstra W, Virkola R, Korhonen T K. P fimbriae of uropathogenic Escherichia coli as multifunctional adherence organelles. Zentbl Bakteriol. 1993;278:229–237. doi: 10.1016/s0934-8840(11)80840-6. [DOI] [PubMed] [Google Scholar]
- 21.Wold A E, Caugant D A, Lidin-Janson G, de Man P, Svanborg C. Resident colonic Escherichia coli strains frequently display uropathogenic characteristics. J Infect Dis. 1992;165:46–52. doi: 10.1093/infdis/165.1.46. [DOI] [PubMed] [Google Scholar]
- 22.Wold A E, Thorssén M, Hull S, Svanborg Edén C. Attachment of Escherichia coli via mannose- or Galα1→4Galβ-containing receptors to human colonic epithelial cells. Infect Immun. 1988;56:2531–2537. doi: 10.1128/iai.56.10.2531-2537.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]