Abstract
Background
People living with chronic disease, particularly seniors (≥60 years old), made up of most severe symptom and death cases among SARS-CoV-2 infected patients. However, they are lagging behind in the national COVID-19 vaccination campaign in China due to the uncertainty of vaccine safety and effectiveness. Safety and immunogenicity data of COVID-19 vaccines in people with underlying medical conditions are needed to address the vaccine hesitation in this population.
Methods
We included participants (≥40 years old) who received two doses of CoronaVac inactivated vaccines (at a 3–5 week interval) and were healthy or had at least one of 6 common chronic diseases. The incidence of adverse events after vaccination was monitored. Vaccine immunogenicity was studied by determining neutralizing antibodies and SARS-CoV-2-specific T cell responses post vaccination.
Results
Here we show that chronic diseases are associated with a higher rate of mild fatigue following the first dose of CoronaVac. By day 14–28 post vaccination, the neutralizing antibody level shows no significant difference between disease groups and healthy controls, except for people with coronary artery disease (p = 0.0287) and chronic respiratory disease (p = 0.0416), who show moderate reductions. Such differences diminish by day 90 and 180. Most people show detectable SARS-CoV-2-specific T cell responses at day 90 and day 180 without significant differences between disease groups and healthy controls.
Conclusions
Our results highlight the comparable safety, immunogenicity and cellular immunity memory of CoronaVac in seniors and people living with chronic diseases. This data should reduce vaccine hesitancy in this population.
Subject terms: Inactivated vaccines, Phase IV trials
Plain language summary
People living with chronic diseases, particularly those over the age of 60, are more likely to have severe symptoms and die following SARS-CoV-2 infection. However, many have not been vaccinated during the national COVID-19 vaccination campaign in China due to concerns about vaccine safety and effectiveness. Here we show that the inactivated COVID-19 vaccine, CoronaVac, is as safe in older people with chronic diseases as it is for healthy people. Also, only slightly differences are seen in the immune response of people with diseases compared to healthy people. Overall, our results highlight that the CoronaVac vaccine is safe and effective in people living with chronic diseases.
Li et al. evaluate the immunogenicity and safety of the CoronaVac inactivated COVID-19 vaccine in elderly patients with underlying medical conditions in China. Most patients show comparable neutralizing antibody and SARS-CoV-2-specific T cell responses to healthy controls, and no serious adverse effects are seen.
Introduction
The coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to result in large number of deaths and remains to be the greatest challenge for public health. As of March 25, 2022, 475 million COVID-19 cases and more than 6 million deaths were confirmed worldwide1. Vaccine is considered as a safe and effective way to reduce SARS-CoV-2 infection, COVID-19 mortality and morbidity. Yet, recently emerged omicron variant (B.1.1.529 & BA.2), which exhibited strong immune escaping from immunity elicited by natural infection or vaccination, has been rapidly spreading and replaced the delta as the predominant variant worldwide.
The omicron variant has greater transmissibility but causes less severe disease than other latest variant of concern (VOC). Preliminary data suggest that the omicron causes milder symptom and most patients recovered without specific treatment, particularly for young people2,3. This is largely thanks to the immune barrier induced by vaccinations or previous infections4,5. Nevertheless, there are over half of seniors aged 60 years or older remain unvaccinated as of March 20226. Death cases analysis in recent wave of outbreak in Hongkong showed that most fetal cases were senior unvaccinated person with chronic diseases7. Thus, the large number of unvaccinated seniors are at high risk of severe outcome and may contribute to a non-negligible number of morbidity and mortality if infection surge fast in population-dense region in China. In many countries, senior people with medical conditions are prioritized for vaccination due to extra vulnerability8–13. In China, the vaccination among older people, particular those with underlying medical conditions, were lagging behind due to uncertainty of vaccination safety and effectiveness and well-controlled COVID-19 prevalence in the period of the delta prevalence14–16. Significant proportion of this vulnerable population had not yet received one or two doses of COVID-19 vaccine. Lessons from the recent wave of the omicron pandemic indicate increasing coverage of vaccination among seniors ≥60 years of age and people with underlying medical conditions are crucial to reduce COVID-19-associated morbidity and mortality, which in turn contribute to the global pandemic prevention and control.
There have been lots of studies showing comparable safety and effectiveness of SARS-CoV-2 vaccine in special population for various types of vaccines (e.g., diabetes, solid organ transplant, autoimmune disease patients)17–20. However, systematic evaluation of inactivated SARS-CoV-2 vaccine safety and effectiveness in people with common diseases has been rare. Here, we report a multicenter retrospective clinical study comparing immunogenicity and reactogenicity of the most widely deployed inactivated vaccine, CoronaVac, in 6 common chronic diseases: hypertension, diabetes mellitus (DM), coronary artery disease (CAD), chronic respiratory disease (CRD), obesity, and cancer in comparison with healthy control. For people living with chronic disease especially seniors older than 60 years, the CoronaVac vaccines are as safe as in healthy people. Although the immunogenicity is slightly different in subgroup of some diseases compared with that of the healthy population, the overall trend is consistent. People living with medical condition show comparable SARS-CoV-2-specific T cell response with the healthy control. Our findings highlight the clinical data to address vaccine hesitancy for seniors and people living with chronic diseases.
Methods
Study design and participants
We conducted a multicenter retrospective study in four different study sites (Haikou city, Wenchang city, and Qionghai city, Hainan Province; Kunming city, Yunnan Province; China), aiming to evaluate the immunogenicity and safety of CoronaVac vacancies in people with underlying medical conditions in comparison with age matched healthy control.
We recruited participants who have received 2 doses of CoronaVac inactivated vaccine with 3–5 weeks of dose interval and were at the 14th–28th day after the second dose at the time of enrollment. Participants were eligible if they were 40 years of age or older, healthy, or diagnosed with any of the 6 most common chronic diseases: hypertension, diabetes mellitus (DM), coronary artery disease (CAD), chronic respiratory disease (CRD), obesity, and cancer, and were able to understand and complete the questionnaires. The diagnose of six underlying medical conditions in our study were mainly based on the diagnosis of local hospital according to National clinical practice guidelines in China. Specifically, we screened potential eligible participants through the CDC database of chronic diseases and vaccination. We then recruit the volunteers who registered as healthy or with at least one of the diseases we studied by telephone interview. During the telephone visit, we will further narrow down to people who have a proof of chronic diseases diagnosis of interest from a local hospital. At the first visit, we checked their case history and asked about their treatment and disease status. People were considered as having underlying medical conditions if they were once diagnosed as one of the 5 common chronic diseases (hypertension, DM, CAD, CRD and cancer), and the disease was not in the acute phase during the recruitment, and maintained the regular treatment during vaccination. The obesity was diagnosed as BMI ≥ 28.0 kg m−2. Furthermore, they were excluded according to established criteria: had been infected by SARS-CoV-2; had received non-CoronaVac vaccine; with severe mental and neurological diseases; with any other factors unsuitable for clinical observation.
Sex matched healthy participants were recruited as the control group. Since underlying disease condition is common in older adults (≥60 years), participants were grouped into adult (40–59 years old) and senior (≥60 years) subgroups to evaluate the effect of age more accurately on the immunogenicity and safety of the inactivated SARS-CoV-2 vaccine. The disease group was further divided into six subgroups based on the six common diseases we concerned.
Written informed consent was obtained from each participant before enrollment. The study protocol and informed consent form were approved by the Committee on Human Subject Research and Ethics of Yunnan University (CHSRE2021021). This study was conducted in accordance with the requirements of Good Clinical Practice of China and the International Conference on Harmonization. This study is registered with ChiCTR.org.cn, ChiCTR2200058281.
Adverse events collection
Participants who came for serum tests 14–28 days after two-dose vaccination were assessed for adverse events within vaccination period. Adverse events including solicited and non-solicited. Solicited injection-site adverse events containing pain, induration, swelling, redness, rash and itching; Solicited systemic adverse events including acute allergy reaction, skin & mucosa abnormalities, diarrhoea, anorexia, vomiting, nausea, muscle pain, headache, cough, fatigue, and fever. Participant were first screened for grade 3 (daily activity significantly affected, medical attention required, and hospitalization may be necessary) or grade 4 (potential life threat, daily activity severely limited and hospitalization required) solicited and non-solicited adverse events by researcher. Participants were then requested to fill a survey to report grade 1 (short or mild reactions without interfering daily activity) and grade 2 (daily activity mildly interfered, simple treatment or no treatment was necessary) solicited and non-solicited adverse events. The reported adverse events were graded according to the China National Medical Products Administration guidelines (https://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20191231111901460. html).
Venous blood collection
Venous blood was collected for neutralizing antibody response assay at day 14–28, 90 and 180 post two-dose vaccination. For all participants, serum samples were collected by Vacutainer SST tubes (BD, USA). For participants at two sites (Haikou and Kunming), we preserved whole blood using Vacutainer EDTA tubes (BD, USA) at room temperature for no longer than 6 h before isolation. Then we separated peripheral blood mononuclear cells (PBMCs) by density-gradient sedimentation using Ficoll (GE) reagent and cryopreserved at −80 °C until testing.
Assessment of neutralizing antibody responses
The neutralizing antibodies to live SARS-CoV-2 were quantified at the central lab and liver disease research center of the affiliated hospital of Yunnan university, using the gold standard of antibody titration: 50 μL serum was first inactivated at 56 °C for 30 min and serially diluted with cell culture medium in two-fold steps. The diluted serum was then incubated with equal volume of live wild type SARS-CoV-2 virus (virus titers: 6.0 lgCCID 50 mL−1; passage: P5; GenBank number: MT407649.1) for 2 hours at 36.5 °C. Vero cells were then added to the serum-virus mix and incubated at 36.5 °C for 5 days. Neutralizing antibody titer was calculated by the highest dilution number of 50% protective condition21.
Assessment of T-cell responses
PBMCs were tested by activation-induced cell marker (AIM) assay to quantify the T cell responses: SARS-CoV-2 specific mega-pool (MP) consists of commercial peptide pools (GenScript) derived from peptide scan (15 mers with 11 aa overlap) through the entire Spike glycoprotein (Protein ID: P0DTC2), Membrane protein (Protein ID: P0DTC5) and Nucleoprotein (Protein ID: P0DTC9) of the wild type of SARS-CoV-2. Cells were cultured for 24 h in the presence of SARS-CoV-2 specific MP (1 μg mL−1) in 96-wells U bottom plates at 1 × 106 PBMC per well in complete RPMI containing 5% Human AB Serum (Gemini Bioproducts). Negative control was performed by adding an equimolar amount of DMSO, and phytohemagglutinin (Roche, 1 μg mL−1) was included as a positive control. For the surface stain, to remove the cell culture medium, cells were washed with staining buffer (BD). Cells were then resuspended and stained with antibody cocktail (Supplementary Table 1) for 30 min at RT in the dark, washed, and resuspended by staining buffer. All sample flow cytometry data was acquired on a Beckman DxFLEX (The gating strategy was showed in Supplementary Fig. 1).
Outcomes
The primary safety endpoint was the occurrence of adverse events within 14 days after the first dose and the second dose of the vaccination. The primary immunogenic endpoints were the seropositive rate and the geometric mean titers (GMTs) of neutralizing antibodies to live SARS-CoV-2 virus (wild type) 14–28 days, 90 days, and 180 days after two-dose vaccination. The secondary immunogenic endpoint was cellular responses (as measured by AIM assay) post 90 days and 180 days of two-dose vaccination.
Statistical analysis
We assessed the safety endpoints in the safety population, which included all eligible participants. For the immunogenic outcomes, we assessed the primary endpoints in the participants who completed blood collection at day 14–28, 90, and 180 post vaccination, respectively, and has successful antibody measurements. We assessed the secondary immunogenic endpoints in the participants who completed blood collection at day 90 and 180 post vaccination, respectively, and has successful T cell response measurements. All statistical analyses were performed by R scripts.
For participants enrolled and completed the assays in each group, the age, weight, BMI were described by mean and standard deviation; the gender and nationality were described by the ratio among total participants. For immunogenicity evaluation, we used descriptive statistics (geometrical mean and 95% confidence interval [CI]) to summarize antibody levels, and the GMT of post-immunization neutralizing antibody was analyzed after logarithmic conversion, and the least square mean of GMT of post-immunization neutralizing antibody and the ratio between groups and its 95% confidence interval (95%CI) were calculated for comparison. The positive rate of neutralizing antibody after immunization was calculated for the experimental group and the control group, the bilateral 95%CI was calculated by Clopper-Pearson method, and the difference between groups was statistically tested by the Chi-square test/Fisher exact probability method. At the same time, geometric mean and 95%CI were used to statistically describe the GMT of the immune neutralizing antibodies of participants in each cohort, and logarithmic converted group T test was used to statistically compare the difference between groups. For the T-cell response analysis, raw data were processed and exported by FlowJo_v10.8.0. The detected ratio of SARS-CoV-2-specific CD4+ and CD8+ T cells after immunization was calculated for the experimental group and the control group, the bilateral 95%CI was calculated by Clopper-Pearson method, and the difference between groups was statistically evaluated by the Chi-square test/Fisher exact probability method. The fraction of SARS-CoV-2-specific T cells was described by median and interquartile range (IQR), and the difference between groups was statistically tested by linear regression analysis, considering AIM assay experimental batch effect.
For safety evaluation, in accordance with the protocol, systemic adverse events and local adverse events will be classified and counted. In this study, Treatment Emergent Adverse Events (TEAE) occurred after inoculation were statistically analyzed. Adverse events were expressed by counts and frequency. The number and incidence of all adverse events in each group were calculated, and the differences between groups were statistically compared using Fisher’s exact test method. Descriptive statistics were made for the severity, dosage, and occurrence time of adverse events. The adverse events after each dose of inoculation were statistically analyzed. Adverse events for each dose were analyzed based on the safety population of each dose. No serious adverse event has been reported in this study and thus has not been analyzed.
Reporting Summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Study participants
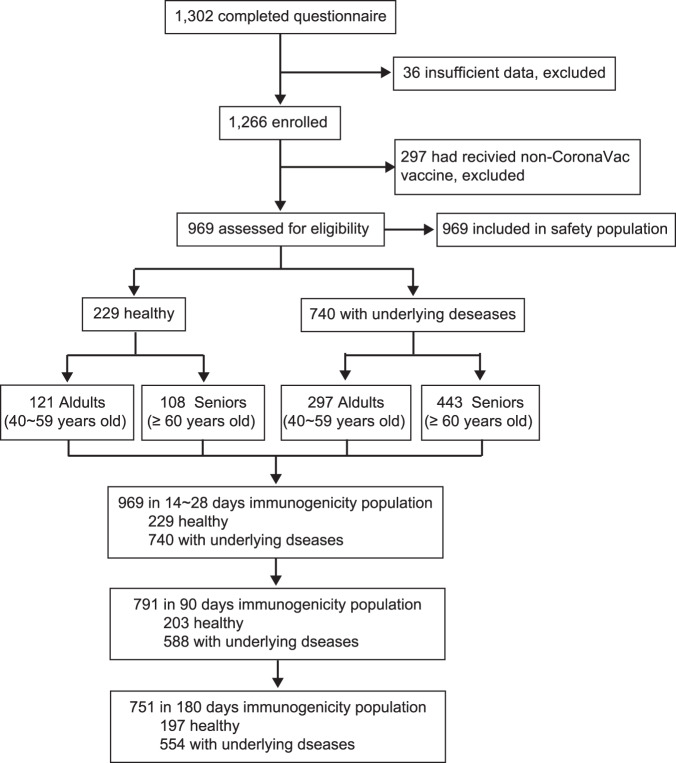
Between 5 Jul and 30 Dec 2021, we recruited 1302 participants at the 14–28th day after the second dose of inactivate SARS-CoV-2 vaccination and collected safety surveys. Among them we enrolled 1266 who had completed all survey questions including demographic information, underlying medical conditions (disease type, duration, severity, and control status) as well as occurrence of adverse events after each dose of vaccination. 297 individuals were excluded from this analysis due to receiving two different products of inactivated vaccines. The remaining 969 participants comprised the analyzed samples: 740 people with underlying medical conditions and 229 people as the healthy control. All of them formed the safety population. For the immunogenicity analysis, 969 participants had completed the blood sampling in the 14–28th day after the second dose of vaccine. 178 participants failed to be on site and were excluded from the 90 days immunity assessment, and 40 participants with same reason were excluded from the 180 days analysis (Fig. 1). Each disease group was further divided into 40–59 years old and ≥60 years old subgroups. Baseline demographic characteristics of the comorbidities group and healthy control were well-balanced (Table 1) across age groups, except for the age in ≥60 years old subgroups.
Fig. 1. Study flow diagram.
Flow chart of recruitment and enrollment (including inclusion/exclusion criteria) for the clinical trial ChiCTR2200058281 to evaluate the immunogenicity and safety of the CoronaVac SARS-CoV-2 vaccine in people with underlying medical conditions.
Table 1.
Demographic and clinical characteristics of the study participants.
| Healthy (N = 229) | Comorbidities (N = 740) | |||
|---|---|---|---|---|
| 40~59 years old | ≥60 years old | 40~59 years old | ≥60 years old | |
| (N = 121) | (N = 108) | (N = 297) | (N = 443) | |
| Age, years | 51.50 (5.55) | 66.89 (5.04) | 53.63 (5.34) | 70.28 (6.54) |
| Height, m | 1.62 (6.98) | 1.61 (9.31) | 1.61 (7.96) | 1.60 (8.53) |
| Weight, kg | 60.20 (9.96) | 61.16 (11.75) | 64.90 (12.62) | 60.95 (10.31) |
| BMI, kg m−2 | 22.83 (3.10) | 23.47 (3.42) | 24.83 (3.97) | 23.71 (3.55) |
| Sex, M/F | 55/66 | 42/66 | 152/141 | 187/256 |
| Han nationality | 121 (100.00%) | 108 (100.00%) | 285 (95.96%) | 435 (98.19%) |
| Comorbidities | ||||
| Coronary artery disease (CAD) | – | – | 31 (10.10%) | 87 (19.86%) |
| Hypertension | – | – | 101 (30.64%) | 131 (31.83%) |
| Diabetes mellitus (DM) | – | – | 71 (22.56%) | 106 (24.83%) |
| Chronic respiratory disease (CRD) | – | – | 31 (9.43%) | 63 (14.90%) |
| Obesity | – | – | 16 (5.39%) | 15 (3.39%) |
| Cancer | – | – | 47 (15.15%) | 41 (9.71%) |
Data are mean (SD) or n (%) unless otherwise specified.
Vaccine safety
150 (20.27%) of 740 comorbidities participants had at least one adverse event, compared with 32 (13.97%) of 229 healthy participants (Supplementary Table 2). Most adverse events were mild (grade 1) in severity and participants recovered within 48 h (Supplementary Table 3). The most frequently reported adverse events in healthy and comorbidities group were injection-site pain (20 [8.73%] of 229 versus 94 [12.70%] of 740), fatigue (9 [3.93%] of 229 versus 48 [6.49%] of 740) and fever (0 [0.00%] of 229 versus 4 [0.54%] of 740). There was no significant difference seen between healthy and comorbidities cohort at overall level (Supplementary Table 2). Although 4 cases of grade 3 adverse events have been reported in 4 individuals in the comorbidities group, including acute allergy, skin & mucosa abnormalities and fever, most events occurred 7 days after vaccination except for a fever that occurred at the 1st day post vaccination. Thus, none was considered to be related to the vaccination except for the fever, which recovered at the 2nd day post vaccination (Supplementary note 1).
When inspecting the adverse events after the first and the second dose of vaccination, respectively, the incidence of adverse events were 16 (6.99%) of 229 in the health group versus 97 (13.11%) of 740 in the comorbidities group after the first dose, 19 (8.30%) of 229 versus 99 (13.37%) of 740 after the second dose (Supplementary Table 2), with significant difference between health and comorbidities group in both doses (P = 0.0129, first dose; P = 0.0487, second dose). We then stratified participants by age group: adults (40–59 years old) and seniors (≥60 years old), to explore if seniors exhibit different response to inactivated SARS-CoV-2 vaccine. In the adults subgroup, 81 (27.27%) of 297 participants in the comorbidities group versus 20 (16.53%) of 121 participants in the healthy control, reported adverse events, with statistically significant difference between groups (P = 0.0231). Moreover, systemic adverse events of the comorbidities group was significantly higher than that of the healthy control (13.47% versus 4.96%, P = 0.0099), while local adverse events showed no significant difference (Table 2). The incidence of adverse events was 54 (18.18%) of 297 in comorbidities people and 9 (7.44%) of 121 in healthy people after the first dose, and 55 (18.52%) of 297 in comorbidities people and 12 (9.92%) of 121 in healthy people after the second dose of vaccine. Both doses showed significant differences between diseases and healthy control (P = 0.0062 and P = 0.0387). In the senior subgroup, comorbidities and healthy group did not show significant difference in the overall (15.58% versus 11.11%, P = 0.2895), the first dose (9.71% versus 6.48%, P = 0.3537) or the second dose (9.93% versus 6.48%, P = 0.3543) vaccination. Thus, the higher incidence of adverse events in the adult comorbidities population was the main driving factor for the difference between the comorbidities cohort and healthy control in the overall incidence of adverse events. More specifically, the major inter-group difference was contributed by the systemic adverse events, mainly fatigue (26 [8.75%] of 297 in comorbidities, 3 [2.48%] of 121 in health, P = 0.0199). Furthermore, the incidence of fatigue in adults group was 20 (6.73%) of 297 in the comorbidities group after the first dose, with significant difference from the health control (1 [0.83%] of 121, P = 0.0115); There was no significant difference between comorbidities and health groups after the second dose with 12 (4.04%) of 297 in the comorbidities group and 2 [1.65%] of 121 in the health control (P = 0.3678).
Table 2.
Incidence of adverse events after vaccination in healthy group and comorbidities group.
| Healthy (N = 229) | Comorbidities (N = 740) | Total (N = 969) | P valuea | |||||
|---|---|---|---|---|---|---|---|---|
| 40~59 years old (N = 121) | ≥60 years old (N = 108) | 40~59 years old (N = 297) | ≥60 years old (N = 443) | 40~59 years old (N = 418) | ≥60 years old (N = 551) | 40~59 years old | ≥60 years old | |
| Total adverse events | 20 (0.17) | 12 (0.11) | 81 (0.27) | 69 (0.16) | 101 (0.24) | 81 (0.15) | 0.0231 | 0.2895 |
| Local reactions | 17 (0.14) | 5 (0.05) | 61 (0.21) | 41 (0.09) | 78 (0.19) | 46 (0.08) | 0.1302 | 0.1722 |
| Pain | 15 (0.12) | 5 (0.05) | 59 (0.20) | 35 (0.08) | 74 (0.18) | 40 (0.07) | 0.0893 | 0.3035 |
| Induration | 0 (0.00) | 0 (0.00) | 5 (0.02) | 3 (0.01) | 5 (0.01) | 3 (0.01) | 0.3275 | 1.0000 |
| Swelling | 3 (0.02) | 0 (0.00) | 5 (0.02) | 5 (0.01) | 8 (0.02) | 5 (0.01) | 0.6956 | 0.5886 |
| Redness | 0 (0.00) | 0 (0.00) | 2 (0.01) | 1 (0.00) | 2 (0.00) | 1 (0.00) | 1.0000 | 1.0000 |
| Rash | 0 (0.00) | 0 (0.00) | 2 (0.01) | 1 (0.00) | 2 (0.00) | 1 (0.00) | 1.0000 | 1.0000 |
| Itching | 0 (0.00) | 0 (0.00) | 3 (0.01) | 6 (0.01) | 3 (0.01) | 6 (0.01) | 0.5599 | 0.6031 |
| Systemic reactions | 6 (0.05) | 7 (0.06) | 40 (0.13) | 33 (0.07) | 46 (0.11) | 40 (0.07) | 0.0099 | 0.8383 |
| Acute allergy | 0 (0.00) | 0 (0.00) | 3 (0.01) | 4 (0.01) | 3 (0.01) | 4 (0.01) | 0.5599 | 1.0000 |
| Skin & mucosa abnormalities | 0 (0.00) | 0 (0.00) | 1 (0.00) | 4 (0.01) | 1 (0.00) | 4 (0.01) | 1.0000 | 1.0000 |
| Diarrhoea | 1 (0.01) | 0 (0.00) | 2 (0.01) | 1 (0.00) | 3 (0.01) | 1 (0.00) | 1.0000 | 1.0000 |
| Anorexia | 0 (0.00) | 1 (0.01) | 4 (0.01) | 1 (0.00) | 4 (0.01) | 2 (0.00) | 0.3284 | 0.3539 |
| Vomiting | 0 (0.00) | 0 (0.00) | 2 (0.01) | 1 (0.00) | 2 (0.00) | 1 (0.00) | 1.0000 | 1.0000 |
| Nausea | 0 (0.00) | 0 (0.00) | 5 (0.02) | 1 (0.00) | 5 (0.01) | 1 (0.00) | 0.3275 | 1.0000 |
| Muscle pain | 1 (0.01) | 0 (0.00) | 12 (0.04) | 6 (0.01) | 13 (0.03) | 6 (0.01) | 0.1203 | 0.6031 |
| Headache | 0 (0.00) | 1 (0.01) | 7 (0.02) | 5 (0.01) | 7 (0.02) | 6 (0.01) | 0.2005 | 1.0000 |
| Cough | 1 (0.01) | 0 (0.00) | 4 (0.01) | 2 (0.00) | 5 (0.01) | 2 (0.00) | 1.0000 | 1.0000 |
| Fatigue | 3 (0.02) | 6 (0.06) | 26 (0.09) | 22 (0.05) | 29 (0.07) | 28 (0.05) | 0.0199 | 0.8075 |
| Fever | 0 (0.00) | 0 (0.00) | 2 (0.01) | 2 (0.00) | 2 (0.00) | 2 (0.00) | 1.0000 | 1.0000 |
aThe p-value was calculated by Fisher’s exact probability method.
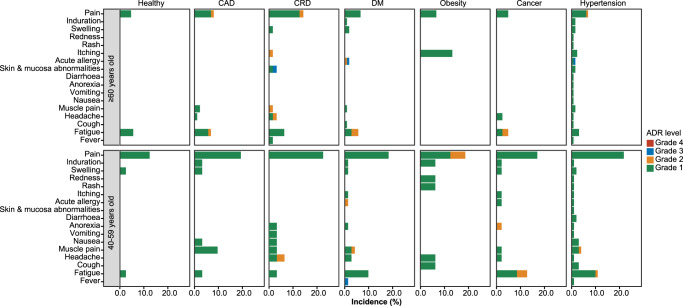
Next, we compared the incidence of adverse events between comorbidities and healthy control stratified by disease types (Supplementary Table 4). The overall incidence of adverse events was 46 (19.83%) of 232 in the hypertension group, 24 (20.34%) of 118 in the CAD group, 34 (19.21%) of 177 in the DM group, 22 (23.40%) of 94 in the CRD group, 19 (21.59%) of 88 in the cancer group, and 5 (6.13%) of 31 in the obesity group versus 32 (13.97%) of 229 in healthy control. The most frequently reported adverse events in six disease groups were same as those in the health control, mainly injection-site pain, and fatigue (Fig. 2). All adverse events showed no significant difference between six disease subgroups and health control. When focusing on seniors, the CRD group showed significantly higher incidence of injection-site pain (9 [14.29%] of 63 vs. 5 [4.63%] of 108 in the health control, P = 0.0404) than that of the healthy control. Regarding adults, fatigue in the hypertension group (11 [10.89%] of 101 versus 3 [2.48%] of 121 in health group, P = 0.0124), DM group (7 [9.86%] of 71, P = 0.0403) and cancer group (6 [12.77%] of 47, P = 0.0152), and headache in the CRD group (2 [6.45%] of 31 versus 0 [0.00%] of 121 in health group, P = 0.0405) showed significant differences compared to the healthy control.
Fig. 2. Incidence of adverse events reported within 14 days post the first dose and the second dose of the vaccination in the safety population, across age groups.
n = 969 study participants. Adverse events post 14 days of first dose and the second dose of the vaccination were collected and graded according to the China National Medical Products Administration guidelines. The histogram shown the incidence of adverse events happened in the 40–60 and ≥60 years old groups. Green, grade 1; orange, grade 2; blue, grade 3; Red, grade 4.
Humoral immunogenicity
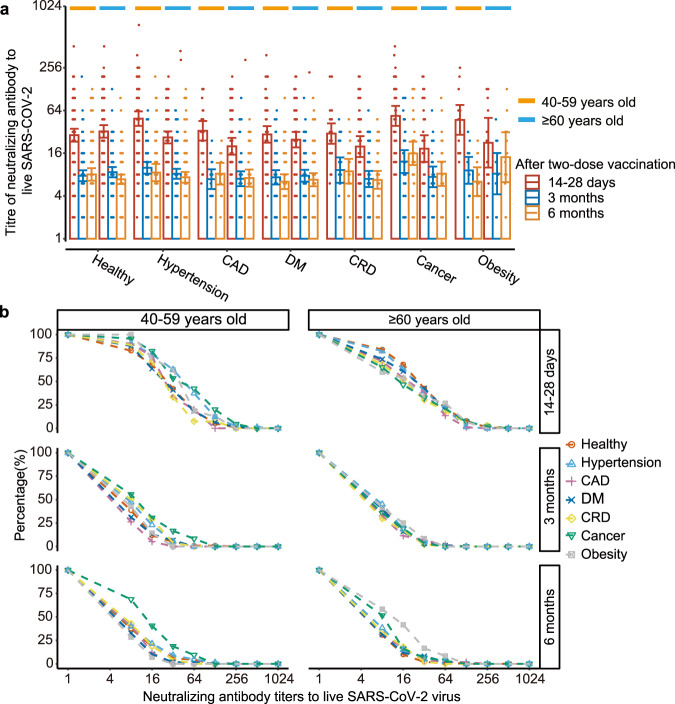
The immunogenicity of people with underlying diseases and healthy control was evaluated at 14–28 days, 3 months, and 6 months after the two-dose vaccination (Table 3). By day 14–28, the seroconversion rates of neutralizing antibodies were 99 (84%) of 118 in the CAD group, 206 (89%) of 232 in the hypertension group, 150 (85%) of 177 in the DM group, 73 (78%) of 94 in the CRD group, 28 (90%) of 31 in the obesity group, and 75 (85%) of 88 in the cancer group versus 204 (89%) of 229 in health group; The neutralizing antibody GMTs were 22.77 (95% CI 18.29–28.35), 33.89 (95% CI 29.08–39.49), 26.45 (95% CI 21.99–31.82), 22.44 (95% CI 17.33–29.06), 32.92 (95% CI 20.89–51.88), 31.96 (95% CI 24.00–42.56) versus 30.50 (95% CI 26.41–35.22), respectively. Most diseases groups showed no difference in seroconversion rates and GMTs from the healthy control, while CAD (P = 0.0287) and CRD group (P = 0.0416) exhibited statistically significant decrease of humoral immune response. By day 90, both the seroconversion rates and GMTs were significantly reduced in all people, with no difference between disease and health groups. By day 180, seroconversion rates and GMTs continued to drop, but declined at a much slower rate. Interestingly, cancer patients showed a significant elevation in the seroconversion rate (68% versus 46%, P = 0.0020) and GMT (11.50 versus 7.42, P = 0.0050) compared to the healthy control (Table 3).
Table 3.
Immunogenicity among participants with underling disease and healthy 14–28 days, 3 months, and 6 months after two-dose vaccination.
| Healthy | CAD | Hypertension | DM | CRD | Obesity | Cancer | |
|---|---|---|---|---|---|---|---|
| Day 14–28 | |||||||
| Seroconversion (%) | 89.00 | 84.00 | 89.00 | 85.00 | 78.00 | 90.00 | 85.00 |
| 95% CIa | (0.84, 0.93) | (0.76, 0.90) | (0.84, 0.93) | (0.79, 0.90) | (0.69, 0.86) | (0.74, 0.98) | (0.76, 0.92) |
| P value | - | 0.1800 | 1.0000 | 0.2900 | 0.0200 | 1.0000 | 0.3400 |
| GMT | 30.50 | 22.77 | 33.89 | 26.45 | 22.44 | 32.92 | 31.96 |
| 95% CIb | (26.41,35.22) | (18.29,28.35) | (29.08,39.49) | (21.99,31.82) | (17.33,29.06) | (20.89,51.88) | (24.00,42.56) |
| P value | - | 0.0300 | 0.3200 | 0.2300 | 0.0400 | 0.7500 | 0.7700 |
| Day 90 | |||||||
| Seroconversion (%) | 55.00 | 52.00 | 58.00 | 52.00 | 51.00 | 65.00 | 61.00 |
| 95% CIa | (0.48, 0.62) | (0.41, 0.64) | (0.50, 0.65) | (0.43, 0.60) | (0.39, 0.63) | (0.44, 0.83) | (0.49, 0.73) |
| P value | - | 0.6900 | 0.6100 | 0.5200 | 0.5800 | 0.4000 | 0.4000 |
| GMT | 8.16 | 6.96 | 8.84 | 7.54 | 7.65 | 8.73 | 9.69 |
| 95% CIb | (7.23,9.20) | (5.73,8.46) | (7.80,10.00) | (6.51,8.73) | (6.15,9.51) | (6.10,12.49) | (7.52,12.49) |
| P value | - | 0.1700 | 0.3600 | 0.4100 | 0.6100 | 0.7200 | 0.2200 |
| Day 180 | |||||||
| Seroconversion (%) | 46.00 | 46.00 | 51.00 | 42.00 | 52.00 | 62.00 | 68.00 |
| 95% CIa | (0.39, 0.53) | (0.35, 0.59) | (0.44, 0.59) | (0.34, 0.50) | (0.40, 0.65) | (0.41, 0.80) | (0.55, 0.79) |
| P value | - | 1.0000 | 0.3100 | 0.5100 | 0.3900 | 0.1500 | 0.0020 |
| GMT | 7.42 | 7.39 | 7.73 | 6.60 | 7.37 | 9.16 | 11.50 |
| 95% CIb | (6.54,8.42) | (5.91,9.24) | (6.66,8.98) | (5.64,7.73) | (5.85,9.28) | (5.87,14.30) | (8.70,15.19) |
| P value | - | 0.9800 | 0.6800 | 0.2500 | 0.9600 | 0.3600 | 0.0050 |
Seroconversion is considered positive when neutralizing antibody titer to live virus is 1:8 or higher.
aEstimated using the Clopper-Pearson method.
bEstimated using the Miettinen-Nurminen method.
In the adult subgroup (40–59 years old) analyses, GMTs of neutralizing antibodies showed elevations in the cancer (53.71 versus 28.71, P = 0.0016) and hypertension (49.02 versus 28.71, P = 0.0009) patients compared to healthy control (Supplementary Table 5). Interestingly, in the senior subgroup (≥60 years old), cancer patients (18.56 versus 32.44, P = 0.0200) showed the opposite trend with a significantly reduced GMT level. Similarly, senior CAD (20.05, P = 0.0052) and CRD (19.85, P = 0.0144) patients also showed the same trend of immunogenicity reduction (Supplementary Table 6). Notably, these inter-group variations were no longer significant at 3- and 6-months post vaccination.
Comparing across age subgroups, we observed distinct patterns for disease and health groups. In healthy participants, the seroconversion and GMT of neutralizing antibodies were slightly higher in seniors (≥60 years old) than their younger counterpart (40–59 years old) on day 14–28 and 90 after the second dose. Conversely, senior people with chronic diseases had a lower neutralizing antibody level compared to their younger counterpart by day 14–28 post vaccination. These differences between age groups in seroconversion rate and GMT of neutralizing antibodies diminished by 3 and 6 months after the second dose (Fig. 3a). The reverse cumulative distribution plot showed that, at each time point, the overall distribution of neutralizing antibody titers was generally close across diseases in the senior and adult subgroups (Fig. 3b).
Fig. 3. Neutralizing antibodies to live SARS-CoV-2 virus (wild type) induced 14–28 days, 90 days, and 180 days after two-dose CoronaVac.
n = 969 study participants. The error bars represent the standard error of the experiment results. a Titers of neutralizing antibodies across age groups. red-, blue- and orange-color refer to 14–28 days, 3 months and 6months after two-dose vaccination, respectively. Orange line refer to 40–59 years old group, and blue line refer to senior group older than 60 years old. b The inverse cumulative distribution of neutralizing antibodies in hypertension (light blue triangle), CAD (pink plus), DM (blue cross), CRD (yellow rhombus), cancer (green triangle), obesity (gray square) subgroups, and healthy control (red circle), across age groups.
Cellular immunogenicity
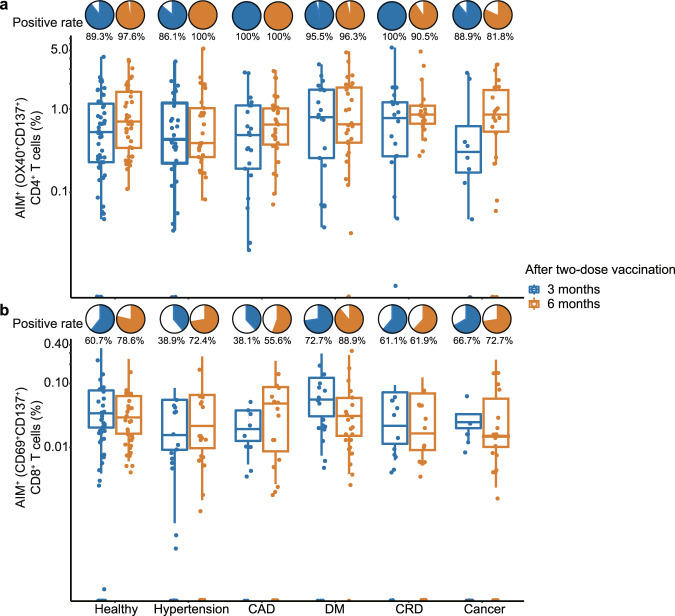
The SARS-CoV-2-specific T-cell response was quantified utilizing an AIM assay at 3 and 6 months post the two-dose vaccination (Fig. 4). By day 90, the SARS-CoV-2-specific CD4+ T cell responses (OX40+ CD137+) were detected in 21 (100%) of 21 in the CAD group, 31 (86.1%) of 36 in the hypertension group, 21 (95.5%) of 22 in the DM group, 18 (100%) of 18 in the CRD group, 8 (88.9%) of 9 in the cancer group versus 50 (89.3%) of 56 in health control (Supplementary Table 7). The median fraction of SARS-CoV-2-specific CD4+ T cells among CD4+ T cells were 0.05% (IQR 0.019%–0.1%), 0.04% (IQR 0.012%–0.084%), 0.08% (IQR 0.02%–0.17%), 0.08% (IQR 0.03%–0.12%), 0.03% (IQR 0.01%–0.12%), and 0.05% (IQR 0.02%–0.11%) in the CAD, hypertension, DM, CRD, cancer, and health group, respectively (Supplementary Table 8). There was no significant difference between disease groups and healthy control on either SARS-CoV-2-specific CD4+ T cell positive rate or fraction. The SARS-CoV-2-specific CD8+ T cell responses (CD69+ CD137+) were detected in 8 (38.1%) of 21 in CAD, 14 (38.9%) of 36 in hypertension, 16 (72.7%) of 22 in DM, 11 (61.1%) of 18 in CRD, 6 (66.7%) of 9 in cancer versus 34 (60.7%) of 56 in the health control (Supplementary Table 7). The median fraction of SARS-CoV-2-specific CD8+ T cell among CD8+ T cell were 0% (IQR 0%–0.01%), 0% (IQR 0%–0.01%), 0.02% (IQR 0%–0.06%), 0.01% (IQR 0%–0.03%), 0.01% (IQR 0%–0.02%), and 0.01% (IQR 0%–0.03%) in the CAD, hypertension, DM, CRD, cancer, and health group, respectively. There were no significant difference between disease groups and healthy control on either SARS-CoV-2-specific CD8+ T cell positive rate or fraction (Supplementary Table 8); By day 180, the detected positive rate and cell fraction of SARS-CoV-2-specific CD4+ and CD8+ T cells were close to that of day 90, with no significant difference between disease groups and healthy control, except for the detected positive rate of the SARS-CoV-2-specific CD4+ T cell in the cancer group at day 180 (81.8% versus 97.6% in health control, P = 0.0440), with significant lower than the health control.
Fig. 4. The SARS-CoV-2-spesific T cell responses after 3 months, and 6 months after two-dose CoronaVac.
n = 229 study participants. Box indicates 25th, 50th, and 75th percentile absolute error, and whiskers indicate 5th and 95th percentile absolute error. Blue color refer to 3 months after two-dose vaccination, while orange color refer to 6 months. The detected positive rate and cell fraction of SARS-CoV-2-specific CD4+ T cell responses (OX40+ CD137+) (a) and the SARS-CoV-2-specific CD8+ T cell responses (CD69+ CD137+) (b) in different chronic diseases subgroups and healthy control, quantified by AIM assays.
Discussion
People living with underlying medical condition, especially seniors, are at high risk for severe COVID-19-related complications and mortality. Considering the risk vs. benefit, vaccination against SARS-CoV-2 should be prioritized for this special population. Guidelines from the USA, the UK, the Korean and the WHO recommend COVID-19 vaccination for patients with comorbidities. However, safety data for the application of inactivated vaccine, the most widely administered vaccine type in China and many countries, in this vulnerable population has been rare. The lack of data on this special population partially contributed to the vaccine hesitancy among people aged 60 or older in China.
To address vaccine hesitation in seniors and with underlying medical conditions22,23, we present the first large cohort study addressing the safety and immunogenicity of CoronaVac, the most widely distributed inactivated vaccine, among population living with underlying medical conditions compared with the healthy control. There were no severe adverse events reported in this study. There were one grade 3 fever related to the vaccination, which resolved in 2 days post vaccination. We didn’t find significant difference in the incidence of adverse events between the disease population and the healthy control in most of the diseases and age subgroups. We observed elevated incidence of adverse events at certain age subgroup in some diseases, in which the differences were mainly contributed by injection-site pain and fatigue. In addition, these adverse events were mostly mild (grade 1) with 1–2 days resolution time without intervention.
For the immunogenicity, there were no significant differences in most of the disease and age subgroups compared with the healthy control. Although a few diseases showed statistically significant lower neutralizing antibody titers than control in certain age subgroups, the differences were <40% and such differences diminished over time. Thus, the immunogenicity of CoronaVac in people with chronic diseases, particularly in people over 60 years of age, was comparable with healthy counterparts. More importantly, the general trend of slightly lower antibody response in seniors emphasized the importance of this population to receive timely booster doses upon completion of primary immunization.
We observed a significant reduction of neutralizing antibody titers (humoral immunity) 3 months after the second dose. The seroconversion rates of neutralizing antibodies were close to 50% by day 90, and mostly lower than 50% by day 180 post the two-dose vaccination. Moreover, neutralizing antibody levels for some seropositive individuals were too low to provide effective protection against infection24. However, in addition to neutralizing antibodies, robust SARS-CoV-2-specific T-cell responses and T-cell memory play a critical role in the long-term immune protection against COVID-19, particularly severe symptoms25. Thus, we quantified the SARS-CoV-2-specific CD4+ and CD8+ T cell responses by day 90 and 180 utilizing the AIM assay, with a stimulation pool containing peptides covering the entire Spike, M, and N protein. Our data indicates that SARS-CoV-2-specific CD4+ T cells and CD8+ T cells were detectable in most individuals up to 6 months post vaccination and showed no significant difference from the healthy control for most diseases groups. Although the rate of individuals with detectable SARS-CoV-2-specific CD4+ T cells were significantly lower in the cancer group than the health control at day 180, the fraction of SARS-CoV-2-specific CD4+ T cells were still higher than people who had no SARS-CoV-2 vaccination or infection history. Thus, most participants had a substantial CD4+ and CD8+ T-cell responses against SARS-CoV-2 and were maintained at a relative stable level to 6 months after vaccination (the latest time point we assayed) despite of the significant reduction in the neutralizing antibody titers 3 month after the vaccination. This is consistent with the test-negative studies that inactivated SARS-CoV-2 vaccines provided poor protection against symptom over time but good protection against severe symptoms26.
For the widely used COVID-19 vaccines, some had recruited participants with underlying medical conditions in the phase 3 clinical trials. For example, the Pfizer COVID-19 mRNA vaccine (BNT162b2) included 20.3% of the sample population reporting one or more underlying diseases. The most common comorbidities were hypertension (24.5%), diabetes mellitus (DM) (7.8%), and chronic lung disease (7.8%). Patients with comorbidities showed similar protection effects and adverse events with the total sample population27,28. In the phase 3 clinical trials (P301) for the mRNA-1273 (Moderna), 22.2% of the sample population had high-risk underlying medical conditions. The most common comorbidities were DM (9.4%), severe obesity with body mass index (BMI) ≥ 40 kg m−2 (6.5%), severe heart disease (4.9%), and CRD (4.8%). The incidence of adverse events was similar in the group with comorbidities and the total sample population with comparable preventive effects27,29. Our analyses for CoronaVac, the most widely administered inactivated SARS-CoV-2 vaccine, demonstrated consistent trend with those analyzed in mRNA vaccine trials. Furthermore, the real-world study in Hongkong showed two doses of CoronaVac vaccine had 69.9% [64.4–74.6] severe disease and death protection effectiveness among adults aged 60 years or older, and three doses of CoronaVac had provided high levels of protection against severe or fatal outcomes (97.9% [97.3–98.4])30. For people with underlying medical conditions, the real-world studies for patient with DM and chronic kidney diseases all showed no increased risk of adverse events and showed high vaccine effectiveness (VE) against severe and mortality of COVID-19 disease post two-dose CoronaVac vaccine31,32. The results of these real-world studies in Hongkong were consistent with our safety and immunogenicity outcomes. Overall, our results for the first time, provided a comprehensive picture of the immunogenicity and safety of COVID-19 inactivated vaccines in people (especially seniors) with underlying medical conditions.
Our study has a few limitations. First, the safety data of this retrospective study was collected 14–28 days after vaccination instead of daily report. The accuracy of adverse event reports could thus be somewhat affected. Second, the immunogenicity data of this study was immune correlate of protection (CoP), which could be influenced by assessment technology platforms and might not accurately translate into clinical protection. Despite of this limitation, our results were consistent with the real-world VE against severe and mortality of COVID-19 disease for CoronaVac in Hongkong. Thus, our CoP results were useful for addressing vaccine hesitancy in people living with studied medical conditions. Third, we only considered the rough classification of the chronic diseases for highly heterogeneous diseases. For example, some subtypes of cancer may have great impact on the immune system, resulting in an abnormal response to the vaccination. However, the assessment for safety and immunogenicity of CoronaVac on HIV positive or autoimmune rheumatic diseases (ARD) patients showed that the immunogenicity of immunosuppressed patients was significantly decreased, but at an acceptable range; and they all showed a good safety profile with the CoronaVac17,33. Therefore, we believe that CoronaVac will be safe in immune-related cancers but likely need further booster doses. Fourth, the health conditions variation we assessed mainly relay on participants reported symptoms, without disease biochemical markers quantitative analysis. As we have followed up the participant’s healthy condition for as long as 6 months after 2 doses of vaccine and received no report on significant alteration of healthy condition. Thus, we thought the CoronaVac vaccine has little influence on the disease progression of stable-period chronic diseases patients. In conclusion, CoronaVac vaccination showed comparable immunogenicity and safety in individuals with and without common chronic diseases, including those ≥60 years of age. Considering that individuals with comorbidities have a significantly higher risk of progression to severe conditions or death when infected with SARS-CoV-2, we strongly recommend people with common chronic diseases get vaccinated when condition permit. Additionally, our results and Hongkong real-world investigations could provide evidence for some chronic diseases to remove from the precautions list of CoronaVac inactivated vaccine.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We thank the Yunnan Provincial Science and Technology Department (202102AA100051 and 202003AC100010, China) and Sinovac Biotech Ltd. (PRO-nCOV-4004) and Spring City Plan: the High-level Talent Promotion and Training Project of Kunming (2022SCP001) for research funding. We thank researchers and clinicians from Haikou, Wenchang and Qionghai CDC for generous support on volunteer recruitment.
Author contributions
Z.Z., C.L., and G.Z. designed and supervised this study. Z.Z., C.L., A.L., and X.Y. wrote the manuscript. J.W., Z.F., Y.T., Y.L., T-C.Z., W.S., Y.L., M.Y., T.S., R.W., W.Q., Z.Z., F.Y., and J.H. processed the clinical samples and information. H.B., A.L., N.W., T.S., H-Y.Z., and Y-T.Z. contributed to the immunogenicity experiments. H.B. and C.L. contributed to the data analysis. T.Z. and Z.Z. contributed to adverse events evaluation.
Peer review
Peer review information
Communications Medicine thanks Longding Liu, Lance Rodewald and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
All data analyzed during this study are included in the Supplementary Data files 1–3. Supplementary Data 1 contains the source data for Fig. 2. Supplementary Data 2 contains the source data for Fig. 3. Supplementary Data 3 contains the source data for Fig. 4. Other data (such as flowcytometry data) are available from the corresponding author upon reasonable request.
Competing interests
Z.Z. declares no competing non-financial interests but the following competing financial interests: this study is sponsored by Sinovac Biotech Ltd. Z.Z. on behalf of Yunnan University as an investigator has received research funding for this study from Sinovac Biotech Ltd. Gang Zeng declares competing non-financial interests as an employee of Sinovac Biotech Ltd. Other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hanfang Bi, Zhenwang Fu.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Chunmei Li, Email: licm89@126.com.
Gang Zeng, Email: zengg@sinovac.com.
Zijie Zhang, Email: zijiezhang@ynu.edu.cn.
the Precise-CoVaccine study group:
Yanli Chen, Wei Yang, Xupu Ma, Xinshuai Zhao, Xinyu Jiang, Qingqin Wu, Yating Yan, Lei Xing, Jingmei Li, Lipei Sun, Hanyi Jiao, Junze Wu, Xueyan Liu, Houze Yu, Muxian Dai, Fengwei Liu, Muhua Feng, Yuemiao Zhang, Ying Wu, Dingyun You, Guo-Dong Wang, Guanghong Yan, Gangxu Xu, Yajing Wang, Lihong Zhang, and Liang Zhang
Supplementary information
The online version contains supplementary material available at 10.1038/s43856-022-00216-2.
References
- 1.WHO. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/ (accessed March 25, 2022) (2022).
- 2.Meo SA, Meo AS, Al-Jassir FF, Klonoff DC. Omicron SARS-CoV-2 new variant: global prevalence and biological and clinical characteristics. Eur. Rev. Med. Pharmacol. Sci. 2021;25:8012–8018. doi: 10.26355/eurrev_202112_27652. [DOI] [PubMed] [Google Scholar]
- 3.Wang, L. et al. Comparison of outcomes from COVID infection in pediatric and adult patients before and after the emergence of Omicron. medRxiv [Preprint] (2022).
- 4.Davies M-A, et al. Outcomes of laboratory-confirmed SARS-CoV-2 infection in the Omicron-driven fourth wave compared with previous waves in the Western Cape Province, South Africa. Trop. Med. Int. Health. 2022;27:564–573. doi: 10.1111/tmi.13752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soriano V, de Mendoza C, Gómez-Gallego F, Corral O, Barreiro P. Third wave of COVID-19 in Madrid, Spain. Int. J. Infect. Dis. 2021;107:212–214. doi: 10.1016/j.ijid.2021.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis D. Will Omicron finally overpower China’s COVID defences? Nature. 2022;604:17–18. doi: 10.1038/d41586-022-00884-z. [DOI] [PubMed] [Google Scholar]
- 7.Centre for Health Protection of the Department of Health. Provisional Analysis of the First 6748 Reported Death Cases in the 5th Wave. https://www.covidvaccine.gov.hk/pdf/ (accessed March 25, 2022) (2022).
- 8.Anderson EJ, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barron E, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8:813–822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, et al. Aging in COVID-19: vulnerability, immunity and intervention. Ageing Res. Rev. 2021;65:101205. doi: 10.1016/j.arr.2020.101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dagan N, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon C, Porteous D, Unsworth J. COVID-19 vaccines and vaccine administration. Br. J. Nurs. 2021;30:344–349. doi: 10.12968/bjon.2021.30.6.344. [DOI] [PubMed] [Google Scholar]
- 13.McGurnaghan SJ, et al. Risks of and risk factors for COVID-19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol. 2021;9:82–93. doi: 10.1016/S2213-8587(20)30405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lazarus JV, et al. A global survey of potential acceptance of a COVID-19 vaccine. Nature Med. 2021;27:225–228. doi: 10.1038/s41591-020-1124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, et al. Acceptance of COVID-19 vaccination during the COVID-19 pandemic in China. Vaccines. 2020;8:482. doi: 10.3390/vaccines8030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang K, et al. Change of willingness to accept COVID-19 vaccine and reasons of vaccine hesitancy of working people at different waves of local epidemic in Hong Kong, China: repeated cross-sectional surveys. Vaccines. 2021;9:62. doi: 10.3390/vaccines9010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aikawa NE, et al. Immunogenicity and safety of two doses of the CoronaVac SARS-CoV-2 vaccine in SARS-CoV-2 seropositive and seronegative patients with autoimmune rheumatic diseases in Brazil: a subgroup analysis of a phase 4 prospective study. Lancet Rheumatol. 2022;4:e113–e124. doi: 10.1016/S2665-9913(21)00327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali, H. et al. Robust antibody levels in both diabetic and non-diabetic individuals after BNT162b2 mRNA COVID-19 vaccination. Front. Immunol. 12, 752233 (2021). [DOI] [PMC free article] [PubMed]
- 19.Notarte KI, et al. Effects of age, sex, serostatus, and underlying comorbidities on humoral response post-SARS-CoV-2 Pfizer-BioNTech mRNA vaccination: a systematic review. Crit. Rev. Clin. Lab. Sci. 2022;59:1–18. doi: 10.1080/10408363.2022.2038539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papadokostaki E, et al. Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in people with diabetes: a prospective observational study. Vaccines. 2022;10:382. doi: 10.3390/vaccines10030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou T, et al. A third dose of inactivated SARS-CoV-2 vaccine induces robust antibody responses in people with inadequate response to two-dose vaccination. Natl Sci. Rev. 2022;9:nwac066. doi: 10.1093/nsr/nwac066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finney Rutten LJ, et al. Evidence-based strategies for clinical organizations to address COVID-19 vaccine hesitancy. Mayo Clin. Proc. 2021;96:699–707. doi: 10.1016/j.mayocp.2020.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan RM, Milstein A. Influence of a COVID-19 vaccine’s effectiveness and safety profile on vaccination acceptance. Proc Natl Acad Sci USA. 2021;118:e2021726118. doi: 10.1073/pnas.2021726118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khoury DS, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nature Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 25.Grifoni A, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501.e1415. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jara A, et al. Effectiveness of homologous and heterologous booster doses for an inactivated SARS-CoV-2 vaccine: a large-scale prospective cohort study. Lancet Glob. Health. 2022;10:e798–e806. doi: 10.1016/S2214-109X(22)00112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi WS, Cheong HJ. COVID-19 vaccination for people with comorbidities. Infect. Chemother. 2021;53:155–158. doi: 10.3947/ic.2021.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.FDA. Vaccines and related biological products advisory committee meeting. December 10, 2020. FDA briefing document. Pfizer-BioNTech COVID-19 Vaccine. https://www.fda.gov/media/144245/download (2020).
- 29.FDA. Vaccines and related biological products advisory committee meeting. December 17, 2020. FDA briefing document. Moderna COVID-19 Vaccine. https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-december-17-2020-meeting-announcement#event-information (2020).
- 30.McMenamin ME, et al. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study. Lancet Infect. Dis. 2022;22:1435–1443. doi: 10.1016/S1473-3099(22)00345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng FWT, et al. The effectiveness and safety of mRNA (BNT162b2) and inactivated (CoronaVac) COVID-19 vaccines among individuals with chronic kidney diseases. Kidney Int. 2022;102:922–925. doi: 10.1016/j.kint.2022.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wan EYF, et al. Vaccine effectiveness of BNT162b2 and CoronaVac against SARS-CoV-2 Omicron BA.2 infection, hospitalisation, severe complications, cardiovascular disease and mortality in patients with diabetes mellitus: A case control study. J. Infect. 2022;85:e140–e144. doi: 10.1016/j.jinf.2022.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Netto LC, et al. Safety and immunogenicity of CoronaVac in people living with HIV: a prospective cohort study. Lancet HIV. 2022;9:e323–e331. doi: 10.1016/S2352-3018(22)00033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All data analyzed during this study are included in the Supplementary Data files 1–3. Supplementary Data 1 contains the source data for Fig. 2. Supplementary Data 2 contains the source data for Fig. 3. Supplementary Data 3 contains the source data for Fig. 4. Other data (such as flowcytometry data) are available from the corresponding author upon reasonable request.