Abstract
Overindulgence, excessive consumption, and a pattern of compulsive use of natural rewards, such as certain foods or drugs of abuse, may result in the development of obesity or substance use disorder, respectively. Natural rewards and drugs of abuse can trigger similar changes in the neurobiological substrates that drive food- and drug-seeking behaviors. This review examines the impact natural rewards and drugs of abuse have on perineuronal nets (PNNs). PNNs are specialized extracellular matrix structures that ensheathe certain neurons during development over the critical period to provide synaptic stabilization and a protective microenvironment for the cells they surround. This review also analyzes how natural rewards and drugs of abuse impact the density and maturation of PNNs within reward-associated circuitry of the brain, which may contribute to maladaptive food- and drug-seeking behaviors. Finally, we evaluate the relatively few studies that have degraded PNNs to perturb reward-seeking behaviors. Taken together, this review sheds light on the complex way PNNs are regulated by natural rewards and drugs and highlights a need for future studies to delineate the molecular mechanisms that underlie the modification and maintenance of PNNs following exposure to rewarding stimuli.
Subject terms: Motivation, Reward
Introduction
The extracellular matrix (ECM) surrounds neurons both loosely and also in highly-specialized structures called perineuronal nets (PNNs) [1] to direct changes in synaptic morphology that are critical for neuronal plasticity [2–5]. Activity-dependent changes in organization of the ECM alter synaptic architecture and physiology in a way that changes synaptic transmission [2, 6–9]. PNNs are densely organized ECM components generated by neurons and glia that ensheathe specific neuronal cell bodies and proximal dendrites with holes at the regions of synaptic contact [10, 11]. In adult rodents, PNNs are composed of chondroitin sulfate proteoglycans (CSPGs) including aggrecan, versican, brevican, and phosphacan, among others; tenascin-R; hyaluronan; and link proteins such as cartilage link protein 1 = Crtl1 (Hapln1) or brain link protein 2 (Bral2) [1, 12–14]. Recent excellent reviews provide detailed information about PNN structure and function in normal development, plasticity, and disease [15, 16]. PNNs are found mainly around fast-spiking, parvalbumin (PV)-containing GABAergic interneurons within many brain regions [17–19]. However, PNNs also surround glutamatergic neurons [20–24], which can be both PV positive or negative [20, 21], and other neurons involved in fast transmission, such as glycinergic output neurons in the medial nucleus of the trapezoid body (MNTB) at the calyx of Held synapse [25, 26] and excitatory neurons in the deep cerebellar nucleus (DCN) [27–29]. Relevant to reward-related brain regions, it remains unknown whether PNNs that surround PV neurons impart similar properties as those that surround other neuronal types. Future studies using genetic methods to target PNN degradation around specific neuronal subtypes to fully address this question are needed.
PNNs appear during critical periods of development in an experience-dependent manner [30–32] and restrict plasticity in adulthood. Wisteria floribunda agglutinin (WFA) is most often used to label PNNs [17] that surround the soma and dendrites of PV neurons. Hence, many of the studies reviewed below examined WFA staining as a proxy for PNN plasticity (e.g., changes in intensity, number, and colocalization with other markers), which we identify in Tables 1 and 2. Removal of PNNs is most commonly accomplished by the enzyme chondroitinase ABC (Ch-ABC) derived from the bacterium Proteus vulgaris that digests glycosaminoglycans [33]. Ch-ABC treatment restores ocular dominance plasticity in the visual cortex of adult animals [34], enhances reversal learning in the auditory cortex [35], promotes recovery of motor learning after spinal cord injury [36] or cortical ischemia [37], and influences extinction of fear conditioning [4]. Removal of PNNs also modifies plasticity by strong stimuli: PNN removal in the hippocampus or mPFC impairs reinstatement of fear conditioning [38]. Below we review the literature showing that plasticity induced by drugs of abuse is impaired after PNN removal.
Table 1.
Natural reward effects on PNNs.
| Environmental enrichment (EE) | Species/Sex | Age | Brain area | Treatment | Time after treatment | Behavior | PNN result | Citation |
|---|---|---|---|---|---|---|---|---|
| EE: Various enrichment paradigms before and after weaning | Mice/Male & Female | PND8 and PND10 | Striatum | (1) Preweaning = non-enriched, Postweaning = non-enriched (NN); (2) Pre = non-enriched, Post = enriched (NE); (3) Pre = enriched, Post = non-enriched (EN); 4) Pre = enriched, Post = enriched (EE) | PND8 and PND10 following treatment | Open field, Forced swim, and Morris water maze | Striatum-↑ in WFA density with EE at PND10 | Simonetti et al., 2009 |
| EE: 1 month exposure | Mice/Female | Adult | Deep cerebellar nuclei (DCN) | 1 month of EE | Following EE exposure | – | DCN: ↓ WFA positive cells | Foscarin et al., 2011 |
| EE: Acute (22 h) and extended (30d) exposure | Rat/Male | Adult | Prefrontal cortex (PFC): Prelimbic (PL), infralimbic (IL), orbitofrontal cortices (OFC) | 22 h or 30 days of EE | Following EE exposure | Coincided with incubation of sucrose craving studies (showed incubation) | PFC-PL: ↑ in WFA intensity with acute EE and extended + sucrose training, acute EE by itself ↓ WFA intensity; IL: ↑ in WFA intensity with acute EE + sucrose training; OFC: ↑ in WFA intensity with acute EE and extended + sucrose training, acute EE by itself ↑ WFA intensity | Slaker et al., 2016 |
| EE: Late-pregnant dams and offspring maintained in EE after weaning until euthanized (P15 or adults) | Mice/Male & Female | PND15 or Adult (12–14 wks) | Striatum: Medial and lateral | Late-pregnant dams and their offspring were maintained in either standard enrichment or EE after weaning coinciding with what what experienced by their respective dams | Following EE exposure | – | Striatum-Medial: ↑ in WFA density with EE between adult and PND15; Lateral: ↓ in WFA density with EE between adult and PND15, ↑ in WFA density with EE at PND15 | O’Connor et al., 2019 |
| Exercise | Species/Sex | Age | Brain area | Treatment | Time after treatment | Behavior | PNN result | Citation |
| Exercise: ad libitum access to a running wheel | Rat/Female | Adult | Cingulate cortex | 6 weeks ad libitum access to a running wheel | Following exercise exposure | Running Distance | Cingulate cortex: ↓ in PNN # and thickness | Smith et al., 2015 |
| Hippocampus: CA1, CA3, CA3, dentate gyrus (DG) | Hippocampus-CA1: ↓ in PNN # and thickness; CA3: ↓ in PNN thickness; DG: ↓ in PNN number and thickness | |||||||
| Lateral hypothalamus | Lateral hypothalamus: ↑ in PNN # | |||||||
| Striatum: Caudate/putamen | Striatum: ↓ in PNN # and thickness | |||||||
| Obesogenic diets | Species/Sex | Age | Brain area | Treatment | Time after treatment | Behavior | PNN result | Citation |
| Hight fat: 60.0% fat, 20.0% carbohydrates, 20.0% protein | Rat/Male | Adult (P60–80) | Prefrontal cortex (PFC): Prelimbic (PL), infralimbic (IL), orbitofrontal cortices (OFC) | Ad libitum or calorically-matched diet exposure for 21 days | Following diet exposure | Signficant weight gain in ad libitum 60% HF fed rats | PFC-PL: ↓ in WFA intensity with 60% HF exopsure regardless of weight gain; OFC: ↓ in WFA intensity and # with 60% HF exopsure regardless of weight gain | Dingess et al., 2018 |
| Hight fat: 60.0% fat, 20.0% carbohydrates, 20.0% protein | Rat/Sprague-Dawley (SD) and SD selectively bred obesity prone (OP) and obesity resistant (OR)/Male & Female | Adult (P60–80) | Prefrontal cortex (PFC): Prelimbic (PL), infralimbic (IL), orbitofrontal cortices (OFC) | Ad libitum diet exposure for 21 days | Following diet exposure | Signficant weight gain in ad libitum 60% HF fed male SD rats and female obesity prone rats | PFC-PL: ↓ in WFA intensity w/60% HF exposure in male SD and OP rats; IL: ↑ in WFA inensity w/60% HF exosure in female SD and OP rats, ↑ in colocalization of WFA/PV# in OP female rats w/60% HF exposure, ↓ in WFA intensity in OR female rats w/60% HF exposure; OFC: ↓ in WFA intensity and colocalization of WFA/PV# w/60% HF exopsure in SD and OP male rats, ↑ in WFA intensity in OR male rats w/60% HF exposure | Dingess et al., 2018 |
| Hight fat: 60.0% fat, 20.0% carbohydrates, 20.0% protein | Rat/Male | Adult (8 wks) | Hypothalamus: Medial arcuate (mARH), lateral arcuate (lARH) | Ad libitum diet for 2 weeks followed by streptozotocin injection to induce type II diabetic state and monitored for another 24 days | Following treatment | Significant weight gain and blood glucose levels in model of type II diabetes | Hypothalamus-mARH: ↓ WFA intensity in HF + streptozotocin injected rats, lARH: ↓ WFA intensity in HF + streptozotocin injected rats | Alonge et al., 2020 |
| Hight fat: 60.0% fat, 20.0% carbohydrates, 20.0% protein | Mice/Male & Female | Adult (15 wks) | Hypothalamus: Arcuate (ARH), terete (TE), paraventricular (PVH), lateral (LH), anterior (AH), ventromedial (VMH), dorsomedial (DMH), perifornical area of the anterior (PeFAH) | Ad libitum diet for 4 weeks ± sham/gonadectomy (GDX) ± fed or fasted state | Following diet exposure or overnight after fasting | Significant weight gain in HF fed mice amongst other metabolic parameters | Hypothalamus-ARH: ↓ WFA intensity in GDX mice regardless of sex and diet; TE: Female mice (sham) have an ↑ WFA intensity compared to intact males, High fat fed female mice have ↑ WFA intensity compared to chow fed female mice when not fasted | Zhang et al., 2021 |
| High fat and high sugar: 20% fat, 39.6% carbohydrates, 19.4% protein | Rat/Male | Adolescence (P28) | Prefrontal cortex (PFC): Prelimbic (PL), infralimbic (IL), anterior cingulate (ACC) | Intermitent diet exposure for 2 h/day for 28 days | Following diet exposure | Impaired social recognition memory | PFC-PL: ↑ in colocalization of PV/WFA; IL: ↓ in WFA# (layers II/III and V/VI) and an ↑ in PV/WFA colocalization in HFHS; ACC: ↑ in colocalization of PV/WFA in HFHS | Reichelt et al., 2019 |
| High fat and high sugar: 21.0% fat, 49.2% carbohydrates, 17.7% protein | Mice/Male | Adolescence (P26) & Adult (P68) | Hippocampus: CA1, CA2/3, Dentate gyrus (DG) | Ad libitum diet exposure for 5 week | Following diet exposure | Signficant weight gain in adolescent and adult mice | Hippocampus-CA1: ↓ in WFA# in adults fed HFHS and an ↑ in PV/WFA colocalization in adults regardless of diet; CA2/3: ↑ in PV/WFA colocalization in adults regardless of diet | Reichelt et al., 2021 |
| Prefrontal cortex (PFC): Prelimbic (PL), infralimbic (IL), orbitofrontal cortices (OFC) | PFC-PL: ↑ in WFA# in adults regardless of diet; IL: ↑ in WFA# and PV/WFA colocalization in adults regardless of diet | |||||||
| Sucrose | Species/Sex | Age | Brain area | Treatment | Time after treatment | Behavior | PNN result | Citation |
| Sucrose: 10% solution | Rat/Male | Adult | Prefrontal cortex (PFC): Prelimbic (PL), infralimbic (IL), orbitofrontal cortices (OFC) | 2 h/day X 10 days ± environmental enrichment | 1 or 30 days | Coincided with incubation of craving studies (showed incubation) | No significant sucrose-induced effects | Slaker et al., 2016 |
| Sucrose: pellets | Rat/Male | Adult | Prefrontal cortex (PFC): Prelimbic (PL), infralimbic (IL), orbitofrontal cortices (OFC) | 2 h/day X 10 days | 1 or 30 days | Coincided with incubation of craving studies (sucrose did not show incubation) | No significant sucrose-induced effects | Roura-Martinez et al., 2020 |
Table 2.
Drug reward effects on PNNs.
| Cocaine | Species/sex | Age | Brain area | Treatment | Time after treatment | Behavior | PNN result | Citation |
|---|---|---|---|---|---|---|---|---|
| Sensitization | ||||||||
| Cocaine sensitization (20 mg/kg), IP | Mice/Male | Adult (8 weeks) | Cerebellum | 1X/day x 6 days every 48 h | 1 month + acute cocaine challenge (10 mg/kg), IP | Sensitized locomotor activity and sniffing | Medial nucleus: ↓ WFA intensity | Vazquez-Sanroman et al., 2015a |
| Cocaine sensitization (20 mg/kg), IP | Mice/Male | Adult (PND 77) | Cerebellum | 1X/day x 6 days every 48 h | 7 days + acute cocaine challenge (10 mg/kg), IP | Sensitized locomotor activity and sniffing | Medial nucleus: ↑ WFA intensity | Vazquez-Sanroman et al., 2015b |
| Cocaine sensitization (15 mg/kg), IP | Rats/Male | Adult | Prefrontal cortex (PFC): Prelimbic (PL) and infralimbic (IL) | 1X or 5X daily | 2 or 24 h | Sensitized locomotor activity | 1 Day: PFC-PL and IL: ↓ WFA intensity 2 h later; IL: ↑ WFA intensity 24 h later | Slaker et al., 2018 |
| 5 Day: PFC-PL and IL: ↑ WFA intensity 2 h later | ||||||||
| Conditioned place preference (CPP) | Species/sex | Age | Brain area | Treatment | Time after treatment | Behavior | PNN result | Citation |
| Cocaine CPP (20 mg/kg), IP | Mice/Male | 7 weeks | Cerebellum | 1X/day alternating, 8 days total | 1 day after preference test (2 days after last cocaine) | Subset shows CPP = Preference (PREF) group | Golgi cells: ↑ WFA intensity in PREF group; DCN cells: ↓ WFA intensity in all cocaine groups independent of preference | Carbo-Gas et al., 2017 |
| Cocaine CPP (15 mg/kg), IP | Rats/Male | Adult | Cerebellum | 1X/day alternating, 8 days total | 90 min after preference test (2 days after last cocaine) | IL mPFC lidocaine infusion increased cocaine CPP | Golgi cells: ↑ WFA intensity in after IL mPFC lidocaine treatment during training days | Guarque-Chabrera et al., 2022 |
| Cocaine CPP (12 mg/kg), IP | Rats/Male | Adult | Prefrontal cortex (PFC): Prelimbic (PL) | 3X/day alternating | 30 min, 2 h, 24 h | CPP | No change in WFA intensity; Decreased PV intensity at all time points | Jorgensen et al., 2020 |
| Self-administration | Species/sex | Age | Brain area | Treatment | Time after treatment | Behavior | PNN result | Citation |
| Cocaine self-administration (0.3 mg/infusion), IV | Rats/Male | Adult | Cerebellar cortex: (vermis and hemispheres) | 2 h/day X 7 days; half given 1 h/day (ShA) or 6 h/day (LgA) X 20 days | 1, 7, or 28 days abstinence | 6 h vs 1 h access escalated cocaine intake | ↑ WFA intensity with abstinence in ShA and LgA rats 1 Day | Sanchez-Hernandez et al., 2021 |
| ↓ WFA intensity vs. naïve in ShA 28 days | ||||||||
| ↑ WFA intensity vs. naïve in LgA rats | ||||||||
| Cocaine self-administration (0.75 mg/kg/infusion), IV | Rats/Male | Adult | Prefrontal cortex (PFC): anterior cinglulate; ventral or dorsal prelimbic (v or dPL); ventral infralimbic (IL), insular, ventral orbitofrontal (vOFC), lateral orbitofrontal (lOFC) | 6 h/day X 10 days | 1 or 30 days abstinence | Self-administration | 1 Day: PFC-dPL, vPL, IL: ↑ number of WFA cells dependent on level of WFA intensity and hemisphere 30 Days: No changes | Roura-Martinez et al., 2020 |
| Ethanol | Species/sex | Age | Brain area | Treatment | Time after treatment | Behavior | PNN result | Citation |
| Ethanol (2.5 g/kg), SC | Mice/Male & Female | PND 7 | Barrel cortex | 2X injection, 2 h apart at PND 7 | 7 days or 83 days | Barrel cortex: PND 14: ↑ number of WFA cells in L4/5; PND 90: ↑ number of WFA cells not co-labeled with PV; ↓ number of PV/WFA cells; | Saito et al., 2019 | |
| Hippocampus: dentate gyrus (DG) | Hippocampus-DG: PND90: ↓ number of WFA cells | |||||||
| Ethanol (2.5 g/kg), SC | Mice/Male & Female | PND 7 | Hippocampus: CA1/CA3 | 2X injection, 2 h apart at PND 7 | 83 days | Decreased contextual fear conditioning; Increased fragmentation of slow-wave sleep; hyperactivity | Retrosplenial cortex: ↓ number of WFA cells | Lewin et al., 2018 |
| Retrosplenial cortex | ||||||||
| Ethanol, drinking-in-the dark procedure (20% ethanol vs. water) | Mice/Male | 8 weeks | Insular cortex and primary motor cortex | 4 h/day xX 4 days/week for 1 week or 6 weeks | 20 h | Alcohol self-administration | Insular cortex: ↑ WFA, aggrecan, brevican, and phosphacan intensity at 6 weeks | Chen et al., 2015 |
| Ethanol (5 g/kg, 25% ethanol) | Mice/Male | 28–37 days | Prefrontal cortex: Orbitofrontal cortex (OFC) | Ethanol (intragastic) 6 days between PND 28–37 | 73 days | Decreased reversal learning on Barnes maze; Decreased center time in open field | PFC-OFC: ↑ WFA, brevican, and neurocan intensity | Coleman et al., 2014 |
| Ethanol (25, 50, or 75 mM) or intragastric (5.25 g/kg) | Rats/Male | 21 days | Primary cortical astrocytes or hippocampus or intragastric treatment 2X at PND 4–9 | 1 day | 0 days | Cortical and hippocampal cultures: ↓ arylsulfatase B activity; ↑ C4S; ↑ cellular and medium neurocan mRNA and protein in cortical cultures; Hippocampus in vivo ethanol: ↑ neurocan | Zhang et al., 2014a | |
| Ketamine | Species/sex | Age | Brain area | Treatment | Time after treatment | Behavior | PNN result | Citation |
| Ketamine (30 mg/kg), IP | Mice/Male | Not specified | Hippocampus: CA1 | 7 daily | 1 day | Increased locomotion; decreased prepulse inhibition with low-intensity stimulus | Hippocampus-CA1 (so and sp), Basket cells: ↓Total PV + and Cat-315 + /PV + cells; Axoaxonic cells: ↓ total PV + and Cat-315-/PV + cells; O-LM/H-S* cells: ↓ total PV + , Cat-315 + /PV + , and Cat-315-/PV + , cells; Bistratified (so): ↓ PV + and Cat-315-/PV + cells | Fujikawa et al., 2021 |
| Ketamine (30 mg/kg), IP | Rats/Male | Adult (8 weeks) | Prefrontal cortex (PFC) | 5 daily/2 d off/5 daily/2 d/12 d isolation/2 d social interaction test | 2 days | Decreased non-aggressive social interaction | PFC: ↓ number of WFA cells; no change in number of PV cells or double-labeled WFA/PV cells | Matuszko et al., 2017 |
| Hippocampus: CA1 | Hippocampus-CA1: No change in WFA cell number or dendrite intensity of WFA | |||||||
| Ketamine (30 mg/kg), IP | Rats/Male | Adult (8 weeks) | Prefrontal cortex (PFC) | 5 daily/2 d off/5 daily/2 d/12 d isolation/2 d social interaction test | 2 days | PFC: PV + cells: ↓ WFA-labeled unit area; fine structure analysis: ↑ number but smaller, less circular WFA-labeled units | Kaushik et al., 2020 | |
| Ketamine (100 mg/kg)/xylazine (10 mg/kg)/acepromazine (3 mg/kg), IP (KXA) | Mice/Male & Female | Adult (8–12 weeks) | Somatosensory cortex: S1 | 1X, 2X, 3X, 6X; once every 72 h | 4 h | 3X KXA, every 2 days; Increased ocular dominance plasticity in layer 4 of V1 after brief (3 d) monocular deprivation | S1: ↓ percent of WFA-coated cells after 2–6 injections; | Venturino et al. 2021 |
| Nicotine | Species/sex | Age | Brain area | Treatment | Time after treatment | Behavior | PNN result | Citation |
| Nicotine self-administration (0.03 mg/kg/infusion), IV | Rats/Male | Adult | Prefrontal cortex: Orbitofrontal cortex (OFC) | 1 h/day X at least 21 days | 45 min or 3 days | Self-administration | PFC-OFC: ↓ number of PV cells surrounded by WFA & ↓ intensity of WFA at 45 min; | Vazquez-Sanroman et al., 2016 |
| Ventral tegmental area (VTA) | VTA: ↓ number of PV cells surrounded by WFA at 45 min and 3 days; ↓ intensity of WFA at 45 min | |||||||
| Opioids | Species/sex | Age | Brain area | Treatment | Time after treatment | Behavior | PNN result | Citation |
| Heroin self-administration (100 µg/kg/infusion), IV | Rats/Male | Adult | Dorsal striatum | 3 hr/day X 16 days; Extinction 1 h/day X 15 days | 21 days abstinence; Extinction; Reinstatement | Self-administraition | Van den Oever et al., 2010 | |
| Nucleus accumbens (Nac) | NAc: Extinction: ↓ tenascin-R (180 & 160 kDa); Cue reinstatement: partially restored tenascin-R | |||||||
| Prefrontal cortex (PFC) | PFC: 21 days abstinence: ↓ synaptosomal tenascin-R (160 kDa), ↓ brevican - Bcan; (145 kDa); Extinction: ↓ Bcan (145 kDa); Cue reinstatement: ↑ Bcan (145 kDa) to pre-extinction levels | |||||||
| Heroin self-administration (0.075 mg/kg/infusion), IV | Rats/Male | Adult | Prefrontal cortex (PFC): Infralimbic (IL) and orbiotofrontal cortex (OFC) | 6 h/day X 10 days | 1 or 30 days abstinence | Self-administration | PFC- IL and OFC: 1 Day: ↑ number of WFA cells | Roura-Martinez et al., 2020 |
| PFC- OFC: 30 Days: ↑ number of WFA cells | ||||||||
Natural rewards
Stimuli that are intrinsically rewarding (e.g., exercise, environmental enrichment, and food) dynamically alter the intensity and/or number of PNNs in numerous brain regions implicated in reward as well as in learning and memory processes (see Table 1). These regions include the prefrontal cortex (PFC) [39–43], striatum [44], cingulate cortex [44], hippocampus [42, 44], and hypothalamus [44–46]. Although natural rewards such as exercise can impact PNNs in areas outside those discussed above (i.e., areas of the central nervous system associated with motor control), discussion of these studies is outside the scope of this review, which is focused primarily on the interplay between PNNs and the circuitry involved in reward after exposure to reinforcing stimuli (see Table 1 for a summary of the findings).
Impact of food on PNNs
Growing evidence suggests that diet, particularly the Western diet (rich in saturated fats and refined sugars), influences the expression and intensity of PNNs [41, 42]. This diet may functionally impact the neurons they surround, altering the protective microenvironment PNNs provide [47–51] and impacting synaptic stabilization [49, 52]. Numerous reviews have also discussed the detrimental impact obesogenic diets have on neuroplasticity [53], neuroinflammation [54, 55], neuropsychiatric disorders [54], neurodegenerative and neurodevelopmental diseases [56], cognition [57], and the overall harmful effects obesity and obesogenic diets have on the brain [58]. Given the overlap between PNNs and the pathophysiological impact obesogenic diets have on the brain, understanding how diet influences the maturation and maintenance of PNNs may provide valuable insights into mechanisms that influence overconsumption of unhealthy foods that promote obesity.
Obesogenic diets
Foods that are high in saturated fat and refined sugars are prevalent and play a key role in overeating, weight gain, and obesity globally [59, 60]. The detrimental impact of these unhealthy diets on brain physiology and function is becoming evident [58]. Here we examine the impact obesogenic diets have on PNNs within the reward circuitry which may contribute to altered neuronal communication and promote maladaptive food-seeking behaviors.
Exposure to ad libitum 60% high fat diet in adult male rats for 3 weeks decreases PNN intensity in the prelimbic PFC and orbitofrontal cortex (OFC) regardless of weight gain [39]. Furthermore, when male rats selectively bred to be obesity prone or obesity resistant were exposed to the same dietary conditions, obesity prone rats showed decreases in PNN intensity similar to Sprague-Dawley outbred rats [40]. On the other hand, PNNs from obesity resistant rats had the opposite response to high fat diet, with an increase in PNN intensity in the OFC, indicating that genetic predisposition towards obesity may impact high fat-induced adaptations independent of weight gain. This is particularly intriguing as it suggests that other factors that distinguish obesity prone from obesity resistant rats, such as reward processing/perception, may be impacting PNNs to influence food-seeking behavior [61, 62].
In addition to examining the impact of diet on males, Dingess et al. [40] provided one of the few studies that conducted a comparative examination of diet on female rats in the PFC. The authors report that, unlike males, females had an increase in PNN intensity in the infralimbic region of the PFC, which was observed in both Sprague-Dawley and selectively bred obesity prone rats. In contrast, PNNs from obesity resistant females had decreased PNN intensity. However, unlike PNNs from males, we cannot conclude that the changes in PNNs are independent of weight gain, as the authors did not examine this in females [40].
Although the studies discussed above suggest that dietary high fat significantly alters PNN intensity, other studies suggest that the influence of diet is not so straightforward. Reichelt et al. [42] recently showed that male mice fed a diet high in fat (21%) and high in sugar (49%) for 5 weeks did not impact PNNs in the PFC. However, the same authors showed that intermittent exposure to the same diet for 28 days in male rats decreased the number of PNN-labeled cells in the infralimbic region of the PFC and increased colocalization of PV and PNN neurons in the prelimbic, infralimbic, and anterior cingulate regions of the PFC [41]. In addition, unpublished data by Dr. Brown’s lab showed a decrease in PNN intensity in males within the infralimbic region of the PFC after exposure to “junk-food” (mash of Ruffles potato chips (40 g), Chips Ahoy chocolate chip cookies (130 g), Jif smooth peanut butter (130 g), Nesquick powdered chocolate flavoring (130 g), powdered Lab Diet 5001 (220 g) [63]), which aligns with what has been found in the high-fat and high-sugar diets. Taken together, these findings suggest that there is a complex interplay between age, diet formulations, sex, genetic predisposition, and administration protocols that impacts PNNs within the PFC.
The role of PNNs in hippocampal plasticity has been well documented (for reviews refer to [16, 52]). However, few studies have examined the impact rewarding stimuli have on PNNs within the hippocampus. In addition to examining the PFC (discussed above) Reichelt et al. [42] showed that 5 weeks of exposure to a diet high in fat and sugar reduced the number of PNN positive cells in the CA1 subregion of the hippocampus in adult but not adolescent male mice, which correlated with adiposity [42]. The authors speculated that the CA1 subregion of the hippocampus may be more vulnerable to nutritional stress in adults, and that younger brains may have some resilience to diet-induced dysregulation, as PNNs are not fully mature.
The lateral hypothalamus plays a critical role in motivated behaviors and sends projections to the ventral tegmental area that are important for driving goal-oriented activities [64]. Rats exposed to dietary high fat in conjunction with type II diabetes have reduced PNN intensity in the arcuate nucleus of the hypothalamus [45]. However, Zhang et al. [46] showed that sex hormones impacted PNN intensity in the arcuate regardless of diet exposure in mice, suggesting an interplay between PNNs and metabolic dysfunction independent of diet. Additional evidence that metabolic dysfunction impacts PNNs has been observed in ob/ob mice, which show attenuated PNN immunoreactivity in the median eminence and failure to dynamically respond to food challenges [65]. Finally, ad libitum consumption of 60% high fat diet induces sex specific changes in PNN intensity (increase) in the terete nuclei of the hypothalamus in non-fasted female mice. Hence, numerous factors such as nutritional regulation, fasting state, metabolic dysfunction, and sex, can impact PNNs within the hypothalamus. Future studies will need to delineate the role PNNs play in hedonic vs. homeostatic feeding within the hypothalamus.
Sucrose
Data suggests that consumption of sugars and the development of obesity is a problem when sugar consumption results in excess caloric intake, and not because of sugar directly [66]. Sucrose consumption can modify neuronal characteristics similarly to those found after exposure to drugs of abuse within the reward circuit, distorting motivation towards an “addictive” phenotype [67]. Although dietary high fat and sucrose both alter dendritic spine morphology ([68–70] structural indicators of glutamatergic plasticity) in the PFC, there has been no evidence to date that sucrose causes adaptations to PNNs as does high fat.
Studies conducted by Slaker et al. [43] and Roura-Martinez et al. [71] found that 1 day or 30 days after sucrose consumption either 10% sucrose solution or sucrose pellets, respectively, resulted in no significant alterations in PNNs in the PFC. This is particularly intriguing given the evidence that high fat and combinations of high fat and high sugar can modify PNNs within the PFC. Hence, future studies need to determine the molecular changes that macronutrients may trigger to modify PNNs.
Exercise
Exercise has been shown to be rewarding, and inactivation of either the PFC or nucleus accumbens can diminish the rewarding aspects of exercise [72]. Smith et al. [44] showed that ad libitum access to a running wheel for 6 weeks influenced PNNs within numerous brain regions. Specifically, there was a decrease in PNN number and thickness in the striatum, cingulate cortex, and hippocampus, but an increase in PNN number in the lateral hypothalamus. Physical activity has been shown to be beneficial for health and alleviating a myriad of diseases, including drug addiction and obesity [73, 74]. Based on what little evidence we have for exercise-induced changes in PNNs and the beneficial consequences of exercise on physiological and pathophysiological processes that PNNs also respond to, it is surprising more research has not been carried out examining how PNNs in the brain respond to exercise, while several studies have examined the impact of exercise on PNNs in the spinal cord, typically after a spinal cord injury [44, 75–77].
Environmental enrichment
Environmental enrichment promotes behaviors that animals find rewarding, improves recovery from brain injury, and enhances learning and memory [78]. In addition, environmental enrichment diminishes the effects of chronic stress [79] and decreases the rewarding effects of drugs of abuse [80–88] and sucrose [89–91]. PNN plasticity is affected by environmental enrichment in several brain areas including, but not limited to, the striatum [92, 93], deep cerebellar nuclei [94], and PFC [43] (see Table 1). However, to date, only one study has examined the interaction between environmental enrichment and reward seeking on PNN changes. Slaker et al. [43] found that acute environmental enrichment (22 h) reduced PNN intensity in the prelimbic region of the PFC and increased PNN intensity in the OFC. There was no direct effect of extended (30 d) environmental enrichment on PNNs. However, when environmental enrichment was combined with sucrose self-administration training, there was a synergistic effect, as PNN intensity was increased within the prelimbic PFC in rats that were exposed to either acute or extended environmental enrichment combined with sucrose training. This effect was not seen in animals that were exposed to sucrose self-administration or environmental enrichment independently. Similar synergy was found in the infralimbic and OFC PFC regions (see Table 1). These data are intriguing considering the lack of evidence showing an effect of sucrose on PNNs. Further studies need to determine whether environmental enrichment triggers sucrose-induced PNN-associated plasticity, which is normally absent, or whether the experience of sucrose adds something to the augmented experience of environmental enrichment itself.
Drugs of abuse
Several studies have shown that drugs of abuse either decrease or increase the number and/or intensity of PNNs (see [95] for review). Different classes of drugs, including ethanol, nicotine, cocaine, ketamine, and heroin, alter the intensity or number of PNNs in various brain regions, including the mPFC [96–99], anterior cingulate cortex [100], OFC [101], barrel cortex [102, 103], insula [104], hypothalamus [105, 106], hippocampus [102, 107] ventral tegmental area [101], and cerebellum [24, 108–110] (see Table 2 for a summary of the findings).
Cocaine
Most studies examining drugs of abuse focused on how cocaine exposure altered PNNs, and most of these tested the effects of cocaine on PNNs in the mPFC and the cerebellum. Investigator-administered cocaine includes studies examining locomotor sensitization or conditioned place preference (CPP), while self-administration studies incorporate the commonly used 2 h or 6 h daily training sessions.
In the prelimbic PFC, investigator-administered cocaine decreased PNN intensity after an acute cocaine injection, but increased intensity after repeated cocaine [96], which induced locomotor sensitization. Cocaine-induced CPP studies have not shown changes in PNN intensity or number of PNNs upon retrieval of the cocaine-associated CPP memory [111]. The divergences may have been due to different timing of the cocaine injections, five daily injections for the sensitization studies and three cocaine injections (48 h apart) in the CPP study, as PNNs were assessed 2 h and 24 h after treatment in both studies. These findings are opposite to what was found in cocaine self-administering rats in which the number of PNNs was increased in the prelimbic PFC after 1 day but not after 30 days of abstinence from cocaine, although here the increase was dependent on the intensity and hemisphere in which PNNs were assessed [71]. Most of the studies discussed above compared sucrose to cocaine to delineate drug-induced changes from changes associated with natural rewards (Fig. 1).
Fig. 1. Comparison of the effects of sucrose and cocaine on PNNs and the role of PNNs in seeking behaviors.

Both sucrose and cocaine can facilitate motivated seeking behaviors. However, cocaine exposure and associated learning alters PNNs, and PNN degradation disrupts cocaine-seeking behaviors. To date, a role for PNN degradation in sucrose-seeking behaviors has not been observed.
In the cerebellum, repeated cocaine exposure increased the intensity of PNNs around neurons in the medial nucleus when examined after a 1-week abstinence period followed by an acute cocaine challenge [24], but the same cocaine challenge decreased the intensity around the same type of neurons when given after a 1-month abstinence period [108], suggesting that abstinence alone may induce further plasticity in cerebellar output neurons. Training for cocaine-induced conditioned place preference (CPP) increased PNN intensity in Golgi inhibitory interneurons in the cerebellum only in rats that showed place preference, while PNN intensity was decreased in medial deep cerebellar nucleus neurons independent of their place preference [110], together suggesting that Golgi neuron PNNs play a role in promoting cocaine-associated learning. The cerebellum has indirect functional connections to the PFC [97, 112], and a recent study showed that inactivation of the infralimbic but not prelimbic PFC, enhanced PNN expression around cerebellar Golgi interneurons [113]. For this latter study, animals were trained for CPP using odor cues paired with cocaine and a particular compartment but, on the preference test day, odors were presented in the opposite compartment in the absence of cocaine. Thus, it is likely that any preference exhibited was due to the conditioned odor cue rather than the contextual cue.
Cocaine self-administration studies show similar findings over abstinence time from short- (1 h) or long (6 h)- access to cocaine: in general, PNN intensity was decreased in Golgi interneurons in the cerebellar cortex at 1 day of abstinence in the short-access cocaine group, but was increased at 28 days of abstinence in the long-access cocaine group [109]. This increase in PNN intensity may therefore be associated with the “incubation” effect, which is the enhancement in drug-seeking behavior after long-term (2–4 weeks) of abstinence [114].
Ethanol
In general, ethanol exposure increases PNNs and PNN components (see [115] for review). Zhang et al. [116] showed that exposure of rat cortical or hippocampal cultures to ethanol increased sulfated forms of GAGs, neurocan, and C4S, the most prominent CS in PNNs during adulthood and a major CS associated with inhibition of axon growth [15]. These changes were accompanied by a decrease in arylsulfatase B, which removes sulfate groups from C4S, and by decreased neurite outgrowth during development. They showed similar changes in the hippocampus after in vivo exposure at PND 4–9. Another study that examined ethanol exposure early in development [117] used an adolescent binge model of ethanol exposure via intermittent intragastic administration for 6 days and reported an increase in the staining density of PNNs as well as their components, brevican and neurocan, in the OFC. These increases may be functionally related to the impairment of reversal learning, which is in part dependent on the OFC [118]. Using a different model of binge drinking (drinking in the dark procedure), Chen and Lasek [104] found similar increases in intensity staining of PNNs as well as in PNN components, including aggrecan, brevican, and phosphacan in the insular cortex 1 day after 6 weeks (but not after 1 week) of drinking bouts. These effects were not found in the primary motor cortex. In contrast to the increases found in PNNs or their components, one study showed that a double injection of ethanol to mice at PND 7 decreased the number of PNNs and PV cells in the retrosplenial cortex and CA3 region when examined in adulthood 3 months later [119]. The differences may be due to the long abstinence time. However, a follow-up study by this same group [102] found that ethanol administered at PND 7 increased the number of PNNs at PND 14 and PND 90 in the barrel cortex but decreased the number of PNNs in the dentate gyrus, suggesting region-specific effects by the same treatment and abstinence time. The number of PV cells surrounded by PNNs was decreased, suggesting a profound decrease in PV to non-detectable levels in PNN-surrounded cells or the potential loss of PV cells that were originally surrounded by PNNs.
Ketamine
Ketamine studies have largely focused on the impact of ketamine on PNNs or of removing PNNs in the context of alleviating depression or mimicking schizophrenia-like behaviors in animal models. Several studies examined the impact of ketamine in rodent models of schizophrenia. Matuskzko et al. [98] delivered daily ketamine (30 mg/kg, ip) to rats for 5 days, with a 2-day interval followed by another 5 daily injections. After 12 days of isolation housing, rats were given a social interaction test. In the mPFC, ketamine decreased the number of PNNs and PNN/PV-labeled cells, with no changes in the CA1. In a follow-up study, Kaushik et al. [99] developed a novel 2D and 3D analysis for PNN changes after repeated ketamine to assess the intensity, size, and shape of holes in mPFC PNNs. Ketamine altered several parameters in PNNs and their underlying PV neurons, including a decrease in PNN unit area and an increase in the number but smaller, less circular PNN units. Fujikawa et al. [107] identified four subtypes of PV neurons based on morphology and assessed in PV single or double-labeling using the Cat-315 antibody, which binds to aggrecan-containing PNNs with the human natural killer-1 (HNK-1) glycan [120]. In the CA1, daily ketamine decreased the number of some PV subtypes that was dependent on the presence of Cat-315 labeling, suggesting specificity of ketamine-induced plasticity within this brain region. An intriguing recent study by Venturino et al. [103] showed that as few as three anesthetic doses of ketamine given every few days profoundly depleted the number of PNNs in the barrel cortex and promoted ocular dominance plasticity after monocular deprivation. This PNN decrease was associated with activated microglia and was prevented by pharmacological depletion of microglia.
Nicotine
Only a single study has examined the impact of nicotine on PNNs. Vazquez-Sanroma et al. [101] reported decreased numbers of PV neurons surrounded by PNNs in the VTA just after the last self-administration session and at 3 days abstinence. Decreased PNN intensity was also found at the early time point, and the authors suggested that any accompanying changes in PV cell function might lead to disinhibition of burst firing of VTA dopamine neurons [121]. Nicotine self-administration also reduced PNN intensity and number of PV neurons surrounded by PNNs, but only immediately after discontinuing nicotine exposure. Unlike for cocaine and opioids, no changes in PNNs were found in the mPFC.
Opioids
Two studies have tested the impact of heroin self-administration on PNNs or PNN components. An earlier study showed that 21 days of abstinence from heroin self-administration in rats decreased synaptosomal tenascin-R (TnR) and brevican (Bcan) in the mPFC, (infralimbic and prelimbic PFC combined), while extinction over a 2-week period reduced Bcan, and remarkably, that a 30 min cue reinstatement increased Bcan back to pre-extinction levels. The latter finding suggests that rapid effects of environmental stimuli impact proteins such as Bcan that regulates localization of potassium channels and AMPAR trafficking on PV interneurons [122]. In another study, heroin self-administration increased the number of PNNs in the infralimbic PFC at 1 day of abstinence; this effect was absent by 30 days of abstinence [71]. These time-dependent effects were region-specific, since the same study demonstrated that heroin self-administration increased the number of PNNs in the ventral OFC on both 1 and 30 days of abstinence. Together, these studies are consistent with the idea that both abstinence and extinction can produce longer-term changes that may set the stage for drug- or drug-associated rapid changes in PNNs or their components.
Biological factors that may influence PNNs
The differential cocaine vs. sucrose findings are consistent with different neural ensembles mediating food or sucrose vs. cocaine seeking [123–125] and studies showing that treatments block reinstatement of cocaine seeking but not sucrose seeking [106, 126]. A key gap in determining why there is divergence in PNN regulation between non-drug and drug-rewarding stimuli is that little is known about what biological factors can trigger PNN plasticity. Although we speculate there is a wide range of factors, one such biological factor that may differentially trigger PNN plasticity is dopamine. For decades, the field has known that dopamine is central to the reinforcing properties of natural rewards and drugs; dopamine reinforces the consumption of drugs and moderates the salience of cues linked to the drug experience [127]. Dopamine increases the excitability of fast-spiking interneurons in the prefrontal cortex, which are primarily surrounded by PNNs and underlie the fidelity of gamma oscillations [128, 129]. In addition, dopamine promotes high-frequency cortical synchrony in anterior cingulate cortical slices, which can be enhanced by PNN degradation [100]. Furthermore, D1-type dopamine receptor stimulation induces proteolysis of brevican and aggrecan in the rat PFC, major constituents of PNNs [130]. However, it is unclear if there is a direct relationship between the rewarding effects of dopamine and PNNs. One could argue that if dopamine was playing a direct role on PNN plasticity, we would predict similar changes between drugs and natural rewards, which has not been the case. However, dopamine concentrations are greater in response to drug-rewarding stimuli vs. biological stimuli [131, 132] and thus complicate this prediction. Degradation of PNNs with Ch-ABC in the thalamic reticular nucleus [133] or in the ventral hippocampus [134] alters the control of dopamine activity in the ventral tegmental area. Future studies need to determine to what extent dopamine levels contribute to PNN changes. Changes in PNNs may be reflective of adaptations in combination with or independent from synaptic connectivity, and may reflect the protective role PNNs play against reactive oxygen species [47], which may be generated due to excess dopamine generation after exposure to rewarding stimuli [135, 136]. Hence, future studies will need to determine if dopamine is necessary for the changes in PNNs reviewed above and what the mechanistic trigger is for dopamine-induced PNN plasticity. The changes identified above may be entirely independent of dopamine and reflective of adaptations in glutamatergic signaling or other biological triggers seen during ‘critical periods’ of development [137–141].
Some natural and drug rewards alter the intensity of PNN-surrounded neurons, while other rewards alter the number of PNN-surrounded neurons, and some stimuli alter both parameters. Presumably, changes in intensity provide a more nuanced way to dynamically alter plasticity-related events, such as new synaptic inputs and/or intrinsic properties of the underlying neurons that change responsiveness to incoming stimuli. A recent study has shown that the extracellular matrix component tenascin-R and Crtl1 are endocytosed and subsequently recycled in an activity-dependent manner [142]. It is intriguing to speculate that dopamine or other neurotransmitters may generate changes in intensity or number of PNNs via enhanced recycling of PNN components. The significance of this recycling pathway is not known, but in addition to sparing the need for de novo synthesis of PNN components, the recycling of components may afford the rapid ability to change several properties of their underlying neurons, such as membrane capacitance [143], ionotropic receptors, or synaptic inputs [122], which collectively alter responses to incoming stimuli.
Impact of PNN degradation on behavior
Above we have characterized the impact of both natural rewards and drugs of abuse on PNN number, thickness, and intensity. To further understand the role PNNs play in motivated behaviors, a few studies have examined the impact of pharmacologically removing PNNs on reward-seeking behaviors (Table 3).
Table 3.
Impact of PNN attenuation on reward seeking.
| Natural reward | Species/Sex | Age | Brain area | Treatment | Time relative to treatment | Behavior | Citation |
|---|---|---|---|---|---|---|---|
| Food: consumption | Mice/Male | Adult | Hypothalamus: median eminence | Ch-ABC injection and then monitored food intake | Food intake monitored for 6 days after Ch-ABC injection | ↑ in cumulative food intake and weight gain | Kohnke et al., 2021 |
| Food: CPP | Rats/Male | Adult | Amygdala: basolateral, central | Ch-ABC after food CPP training; Prior to morphine CPP extinction | 12 days | No effect on acquired food CPP | Xue et al., 2014 |
| Sucrose: 2 bottle choice | Mice/Male | Adult | Insular cortex | Ch-ABC prior to 2-bottle choice (sucrose, water) | 3 days | No change | Chen and Lasek, 2020 |
| Sucrose: CPP | Rats/Male | Adult | Hypothalamus: anterior dorsal lateral hypothalamus | Ch-ABC prior to CPP training | 3 days before training for CPP | No effect on acquisition of CPP or self-administration | Blacktop et al. 2017 |
| Sucrose: self-administration | Rats/Male | Adult | Hypothalamus: anterior dorsal lateral hypothalamus | Prior to self-administration training | 1 day before training for self-administration | No effect on acquisition of CPP or self-administration | Blacktop et al. 2017 |
| Sucrose: self-administration | Rats/Male | Adult | Hypothalamus: anterior dorsal lateral hypothalamus | Ch-ABC prior to cue reinstatement | 16 h | Trend towards reduced cue reinstatement | Blacktop and Sorg, 2019 |
| Drug reward | Species/Sex | Age | Brain area | Treatment | Time after treatment | Behavior | Citation |
| Cocaine | |||||||
| Cocaine: CPP (12 mg/kg train, 10 mg/kg test), IP | Rats/Male | Adult | Prefrontal cortex (PFC): Prelimbic | Ch-ABC prior to CPP training; Prior to extinction of CPP; Prior to memory retrieval | 3 days before acquisition; 1 day before extinction; 3 days before memory retrieval | Acquisition: ↓ Reconsolidation: ↓ | Slaker et al., 2015 |
| Cocaine: CPP (5 mg/kg train) | Mice/Male | Adult | Prefrontal cortex (PFC): Prelimbic Hippocampus: Dorsal | Brevican KO heterozygous | 1 day: No change; 21 days: ↓ incubation of CPP; rescued by brevican expression in dorsal hippocampus but not mPFC | Lubbers et al., 2016 | |
| Cocaine: CPP (10 mg/kg train), IP | Rats/Male | Adult | Hypothalamus: Anterior dorsal lateral | Ch-ABC prior to CPP | 3 days | Acquisition: ↓ | Blacktop et al. 2017 |
| Cocaine: CPP (10 mg/kg train, 5 mg/kg test), IP | Rats/Male | Adult | Amygdala: Basolateral amygdala (BLA) and central amygdala (CeA) | Ch-ABC prior to extinction of CPP | 12 days | Cocaine-primed reinstatement: ↓ | Xue et al., 2014 |
| Cocaine: self-administration (0.75 mg/kg/infusion train) IV; (5 mg/kg reinstatement), IP | Rats/Male | Adult | Amygdala: Basolateral amygdala (BLA) and central amygdala (CeA) | Ch-ABC prior to extinction of CPP | 15 days | Cocaine-primed reinstatement: ↓ | Xue et al., 2014 |
| Cocaine: self-administration (0.125 mg/kg/infusion), IV | Rats/Male | Adult | Hypothalamus: Anterior dorsal lateral | Ch-ABC prior to training | 1 day | Acquisition: ↓ Cue-induced reinstatement: ↓ | Blacktop et al. 2017 |
| Cocaine: self-administration (0.5 mg/kg/infusion), IV | Rats/Male | Adult | Hypothalamus: Anterior dorsal lateral | Ch-ABC prior to cue reinstatement | 16 h | Cue-induced reinstatement: ↓ | Blacktop and Sorg, 2019 |
| Ethanol | Species/Sex | Age | Brain area | Treatment | Time after treatment | Behavior | Citation |
| Ethanol 2-bottle choice | Mice/Male | 8 weeks |
Insular cortex Motor cortex |
Ch-ABC prior to 2-bottle choice drinking over 24 h | 3 days | Insular cortex: ↑ sensitivity to quinine-induced aversion to EtOH | Chen and Lasek, 2020 |
| OPIOIDS | Species/Sex | Age | Brain area | Treatment | Time after treatment | Behavior | Citation |
| Morphine CPP (10 mg/kg train, 30 mg/kg test), SC | Rats/Male | Adult | Amygdala: Basolateral amygdala (BLA) and central amygdala (CeA) | Ch-ABC prior to extinction of CPP | 12 days (reinstatement); 10 days (retention); 4 days (reconsolidation) | Morphine-primed reinstatement of CPP: ↓ No effect on retrieval, reconsolidation, or retention | Xue et al., 2014 |
| Heroin self-administration (0.05 mg/kg/infusion train) IV; 0.25 mg/kg reinstatement) SC | Rats/Male | Adult | Amygdala: Basolateral amygdala (BLA) and central amygdala (CeA) | Ch-ABC prior to extinction of CPP | 13 days (reinstatement); 40 days (spontaneous recovery) | Heroin-primed reinstatement of self-administration: ↓ Spontaneous recovery: ↓ | Xue et al., 2014 |
Natural rewards
A half dozen studies to date have examined whether PNN degradation via Ch-ABC impacts the motivation to seek food, primarily sucrose [105, 106, 144, 145]. When 2-bottle choice (for ethanol), CPP, or self-administration was examined, there was no impact on sucrose-seeking behaviors. However, when ad libtum consumption of food was assessed after Ch-ABC infusion into the median eminence of the hypothalamus, there was an increase in cumulative food intake and weight gain [65]. Hence, although current evidence does not suggest a role for PNNs in motivated sucrose-seeking behaviors, it does suggest that there may be a role in consumption of some food types. This is not surprising, as there is a lack of evidence showing sucrose-induced effects on PNN plasticity, but future studies need to assess whether this lack of effect is specific to sucrose, motivated seeking for food in general, or a combination of both.
Drug rewards
Studies attempting to disrupt drug-seeking behaviors using Ch-ABC have generally found reduced responding to drugs or drug-associated cues. Removal of PNNs in the mPFC disrupted the acquisition and reconsolidation of cocaine CPP [146] and incubation of craving using a CPP paradigm [147]. PNN degradation in the hypothalamus prevented the acquisition of CPP [105] and blocked the acquisition and cue-induced reinstatement of cocaine self-administration [105, 106]. Finally, Ch-ABC infusion into the amygdala decreased cocaine-primed reinstatement in both CPP and self-administration models [145].
In addition to cocaine, Ch-ABC degradation of PNNs in the amygdala also reduced morphine-primed reinstatement of CPP and decreased heroin-primed reinstatement in a self-administration model [145]. Infusion of Ch-ABC into the insular cortex increased sensitivity to quinine-induced aversion to ethanol [144]. Therefore, disrupting PNN stability in numerous brain areas has been successful at reducing drug-seeking behaviors. Additional work is needed to determine whether this is specific to drugs, which may provide key insights into determining what physiological mechanisms trigger PNN plasticity.
Implications for PNNs on PV cell and circuit function
Most PNNs surround PV neurons (although there are several exceptions; see Introduction). As such, natural or drug reward-induced changes in PNNs can alter the firing properties of these neurons through several mechanisms that alter excitatory:inhibitory balance [148, 149]. These include the ability of PNNs to reduce membrane capacitance [143] and provide cation buffering capacity [150, 151]. PNNs or their component, brevican, also controls plasticity through glutamate receptors and specific potassium channels [122, 152]. The presence of PNNs or specific components also likely regulates synaptic inputs [122, 153], which in part may occur through the binding of PNNs to chemorepellents such as semaphorin 3 A [154].
In addition to changes in PNN-surrounded neurons themselves, reward-associated behaviors rely on the connectivity of several brain areas [155–159]. Therefore, it is important to consider how changes in PNNs could alter coordination of this circuitry. The mPFC and nucleus accumbens (NAc) are two major pathways in reward-seeking behavior [155, 158, 159]. In most, but not all brain regions, PNNs are found around PV neurons to allow for their fast-spiking nature [15, 26, 143, 160]. PV neurons are critical players in regulating the output of pyramidal neuron activity in the mPFC to the nucleus accumbens [161, 162] through their dense perisomatic connections to pyramidal neurons [163] and feedforward inhibition [164]. PV neurons are believed to communicate through precisely timed brain oscillations that synchronize neural activity. For example, PV neurons synchronize the output of pyramidal neurons into discrete groups of activated neurons thought to represent coding of separate memories [165, 166]; (see Fig. 2). Therefore, since PV neurons are essential for generating oscillations [167–170], the impact of PNNs on reward-associated behaviors is likely to be tightly linked to their influence on the PV neuron network. Gamma oscillations (30–120 Hz) are required for attention [171, 172], adapting new strategies [173, 174], and encoding reward outcomes [175] or expected outcomes [176]. PV neurons also contribute to theta oscillations (4–12 Hz) driven by cortical pyramidal neurons [170], and are thereby able to coordinate long-range communication within and across other brain regions through coupling of theta and gamma oscillations [177]. Removal of PNNs alters several properties of PV neurons [160] and increases the variability of spiking [149]. In support of the importance of normal PV function in excitatory:inhibitory balance, direct inhibition of PV neurons induces network instability [178, 179]. Thus, in the absence of PNNs or in instances where PNNs have been altered by palatable food or drugs of abuse, PV cells and pyramidal cells may not be well timed, or phase-locked, to theta or gamma rhythms [160], which could perturb behavioral output, including drug-associated memories that are thought to drive relapse. We speculate that dynamic changes in PNNs may optimize in a layer-specific manner low- and high-frequency inputs differently within distinct circuits to drive food- and drug-seeking behaviors. Altered PNN composition, intensity, or density, is expected to change PV neuronal function and in turn degrade the normally precise spatiotemporal firing patterns. This variability in firing patterns may produce overlapping neuronal ensembles, leading to less specificity of which ensembles represent a particular stimulus or memory [179, 180] (Fig. 2). Thus, on the one hand, by virtue of their ability to optimize and maintain the precise firing of PV neurons, PNNs appear to stabilize the network, and any food- or drug-induced alterations in PNNs within one brain region may manifest as impaired coupling of communication across many brain regions and disrupt desirable behaviors such as high-level cognition and decision making. On the other hand, too much rigid stabilization of a network in response to repeated exposure to drugs of abuse or highly palatable food may manifest as deeply entrenched and undesirable reward-related behaviors, and temporary degradation of PNNs might allow for the plasticity needed to render newer, healthy behaviors to replace drug-related behaviors.
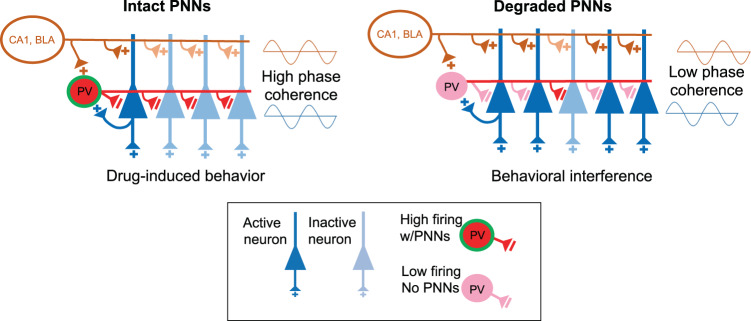
Fig. 2. Model for how PNN removal interferes with drug-induced behaviors.
PNN removal in the mPFC may alter activity of neural ensembles that form memories to drive relapse behavior. With intact PNNs, glutamatergic signaling from regions such as the BLA or CA1 increase activity of both pyramidal and PV neurons. PV neurons are activated slightly after pyramidal neurons to provide feedforward inhibition on pyramidal neurons to silence and reduce the competition from nearby pyramidal neurons. In this way, PV neurons synchronize output so that only discrete neural assemblies, groups of neurons activated and linked spatially and temporally to provide information, are activated [186]. PNN removal reduces PV cell firing and provides less inhibition to pyramidal neurons, causing overlap and thus interference with neurons representing the original drug memory [179, 180]. Thus, although excess pyramidal neuron activity may enhance plasticity, the consequence may be decreased coherence across brain regions, which interferes with drug-associated memories.
Limitations
Most of the studies we reviewed analyzed how WFA staining was modified after exposure to a rewarding stimulus, which can provide an indirect measure of PNN plasticity by measuring one of three parameters: (1) WFA intensity; (2) WFA number; and (3) WFA colocalization. WFA intensity is often used as a proxy for determining the maturity of the PNNs, with immature PNNs labeled with less WFA stain [181]. One problem comparing between studies with respect to intensity is the diverse way which intensity is quantified. That is, some studies use semi-quantitative measures and others average a set number of pixels within a given PNN. Work by Slaker et al. has decreased the variability in quantified WFA intensity measures using a “region of interest” strategy in conjunction with automated software, PIPSQUEAK [181]. The authors make a compelling argument for less biased data, which is reproducible and able to speed up analysis by 100-fold. Future studies need to use additional techniques, such as high- and super-resolution imaging to identify the fine structure of PNNs and changes after stimulation [99, 182] by natural and drug rewards to verify what is causing the alterations in WFA staining to better understand the specific modifications within PNNs.
Conclusions
Overall, the pattern of changes in PNNs after exposure to natural rewards and drugs of abuse is highly variable and dependent on brain area, drug dose and class, sex, genetic predisposition, and exposure duration. Some studies show that short-term abstinence from drugs of abuse reduces PNNs, whereas long-term abstinence increases PNNs or vice-versa, but one conclusion that can be drawn from these studies is that PNNs are dynamic and either or both exposure time and abstinence time change PNNs in opposing directions. Reward-induced changes in PNN numbers or intensity are expected to modulate the function of their underlying neurons. Removal of PNNs most consistently reduces the firing rate of fast-spiking PV neurons [160] by imposing changes in intrinsic and synaptic properties. These changes in firing pattern are likely to interfere with the exquisite timing of coordinated output needed to stabilize the network that may support drug-related behaviors.
Future research directions
Several fundamental questions remain regarding PNN regulation. Perhaps the greatest challenge facing the field currently is that numerous studies have characterized observational changes in PNNs, but little is known about the physiological mechanisms that trigger PNN plasticity and what adaptations within the PNNs account for the alterations in WFA staining. The field needs a better understanding of the relationship between PNNs and other molecules (e.g., PV and activity-dependent proteins), which may influence PNNs and vice versa. Once we have a better understanding of the physiological mechanisms triggering PNN plasticity, future studies will be able to address how physiological changes in PNNs by food/drugs of abuse impact local and downstream neural circuits to alter motivated behaviors. Another understudied area with respect to PNNs and reward circuity are sex differences. Several biological mechanisms (i.e., pharmacokinetics, neurotransmission, and hormones such as estrogen) differ between males and females and have been shown to influence drug sensitivity in almost all phases of substance use disorder (see [183, 184] for a comprehensive review on the influence of sex on drug abuse). Furthermore, sex differences have been reported for natural rewards [141, 185]. However, only two studies reported sex differences in PNNs and both reported region-specific differences [40, 46]. Hence, future studies need to consider sex differences when drawing conclusions about reward-triggered changes in PNNs. Finally, it is apparent that not all rewarding stimuli trigger PNN plasticity to the same degree and within the same brain regions. For instance, the studies reviewed above suggest sucrose by itself does not impact PNNs. However, sucrose in combination with other macronutrients (i.e., high fat) or environmental enrichment does seem to trigger PNNs plasticity. We need a better foundational understanding of what physiological mechanisms trigger PNN adaptations and the mechanisms by which PNNs maintain stability of the network. This level of understanding is expected to provide opportunities for innovative solutions to disrupt excessively stable drug-associated networks that are thought to underlie long-enduring drug behaviors.
Acknowledgements
The authors would like to acknowledge biorender.com for their services, which were used to generate Fig. 1.
Author contributions
TEB and BAS contributed equally to the writing of this review.
Funding
TEB was funded by startup funds from Washington State University and NIH grant DA040965. BAS was funded by the Good Samaritan Foundation of Legacy Health and NIH grants DA047121 and DA040965.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Deepa SS, Carulli D, Galtrey C, Rhodes K, Fukuda J, Mikami T, et al. Composition of perineuronal net extracellular matrix in rat brain: a different disaccharide composition for the net-associated proteoglycans. J Biol Chem. 2006;281:17789–800. doi: 10.1074/jbc.M600544200. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman KB, Martinez J, Lynch G. Proteolysis of cell adhesion molecules by serine proteases: a role in long term potentiation? Brain Res. 1998;811:29–33. doi: 10.1016/s0006-8993(98)00906-8. [DOI] [PubMed] [Google Scholar]
- 3.Letourneau PC, Condic ML, Snow DM. Interactions of developing neurons with the extracellular matrix. J Neurosci. 1994;14:915–28. doi: 10.1523/JNEUROSCI.14-03-00915.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gogolla N, Caroni P, Luthi A, Herry C. Perineuronal nets protect fear memories from erasure. Science. 2009;325:1258–61. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- 5.Gundelfinger ED, Frischknecht R, Choquet D, Heine M. Converting juvenile into adult plasticity: a role for the brain’s extracellular matrix. Eur J Neurosci. 2010;31:2156–65. doi: 10.1111/j.1460-9568.2010.07253.x. [DOI] [PubMed] [Google Scholar]
- 6.Lauri SE, Kaukinen S, Kinnunen T, Ylinen A, Imai S, Kaila K, et al. Reg1ulatory role and molecular interactions of a cell-surface heparan sulfate proteoglycan (N-syndecan) in hippocampal long-term potentiation. J Neurosci. 1999;19:1226–35. doi: 10.1523/JNEUROSCI.19-04-01226.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch G. Memory and the brain: unexpected chemistries and a new pharmacology. Neurobiol Learn Mem. 1998;70:82–100. doi: 10.1006/nlme.1998.3840. [DOI] [PubMed] [Google Scholar]
- 8.Rauvala H, Peng HB. HB-GAM (heparin-binding growth-associated molecule) and heparin-type glycans in the development and plasticity of neuron-target contacts. Prog Neurobiol. 1997;52:127–44. doi: 10.1016/s0301-0082(97)00007-5. [DOI] [PubMed] [Google Scholar]
- 9.Cremer H, Chazal G, Lledo PM, Rougon G, Montaron MF, Mayo W, et al. PSA-NCAM: an important regulator of hippocampal plasticity. Int J Dev Neurosci. 2000;18:213–20. doi: 10.1016/s0736-5748(99)00090-8. [DOI] [PubMed] [Google Scholar]
- 10.Hockfield S, McKay RD. A surface antigen expressed by a subset of neurons in the vertebrate central nervous system. Proc Natl Acad Sci USA. 1983;80:5758–61. doi: 10.1073/pnas.80.18.5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Celio MR, Blumcke I. Perineuronal nets-a specialized form of extracellular matrix in the adult nervous system. Brain Res Brain Res Rev. 1994;19:128–45. doi: 10.1016/0165-0173(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 12.Carulli D, Rhodes KE, Fawcett JW. Upregulation of aggrecan, link protein 1, and hyaluronan synthases during formation of perineuronal nets in the rat cerebellum. J Comp Neurol. 2007;501:83–94. doi: 10.1002/cne.21231. [DOI] [PubMed] [Google Scholar]
- 13.Carulli D, Pizzorusso T, Kwok JC, Putignano E, Poli A, Forostyak S, et al. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain. 2010;133:2331–47. doi: 10.1093/brain/awq145. [DOI] [PubMed] [Google Scholar]
- 14.Galtrey CM, Fawcett JW. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res Rev. 2007;54:1–18. doi: 10.1016/j.brainresrev.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Fawcett JW, Oohashi T, Pizzorusso T. The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat Rev Neurosci. 2019;20:451–65. doi: 10.1038/s41583-019-0196-3. [DOI] [PubMed] [Google Scholar]
- 16.Carulli D, Verhaagen J. An Extracellular Perspective on CNS Maturation: Perineuronal Nets and the Control of Plasticity. Int J Mol Sci. 2021;22:1-26. [DOI] [PMC free article] [PubMed]
- 17.Hartig W, Brauer K, Bruckner G. Wisteria floribunda agglutinin-labelled nets surround parvalbumin-containing neurons. Neuroreport. 1992;3:869–72. doi: 10.1097/00001756-199210000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Schuppel K, Brauer K, Hartig W, Grosche J, Earley B, Leonard BE, et al. Perineuronal nets of extracellular matrix around hippocampal interneurons resist destruction by activated microglia in trimethyltin-treated rats. Brain Res. 2002;958:448–53. doi: 10.1016/s0006-8993(02)03569-2. [DOI] [PubMed] [Google Scholar]
- 19.Dityatev A, Bruckner G, Dityateva G, Grosche J, Kleene R, Schachner M. Activity-dependent formation and functions of chondroitin sulfate-rich extracellular matrix of perineuronal nets. Dev Neurobiol. 2007;67:570–88. doi: 10.1002/dneu.20361. [DOI] [PubMed] [Google Scholar]
- 20.Horii-Hayashi N, Sasagawa T, Hashimoto T, Kaneko T, Takeuchi K, Nishi M. A newly identified mouse hypothalamic area having bidirectional neural connections with the lateral septum: the perifornical area of the anterior hypothalamus rich in chondroitin sulfate proteoglycans. Eur J Neurosci. 2015;42:2322–34. doi: 10.1111/ejn.13024. [DOI] [PubMed] [Google Scholar]
- 21.Meszar Z, Girard F, Saper CB, Celio MR. The lateral hypothalamic parvalbumin-immunoreactive (PV1) nucleus in rodents. J Comp Neurol. 2012;520:798–815. doi: 10.1002/cne.22789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada J, Ohgomori T, Jinno S. Perineuronal nets affect parvalbumin expression in GABAergic neurons of the mouse hippocampus. Eur J Neurosci. 2015;41:368–78. doi: 10.1111/ejn.12792. [DOI] [PubMed] [Google Scholar]
- 23.Wegner F, Hartig W, Bringmann A, Grosche J, Wohlfarth K, Zuschratter W, et al. Diffuse perineuronal nets and modified pyramidal cells immunoreactive for glutamate and the GABA(A) receptor alpha1 subunit form a unique entity in rat cerebral cortex. Exp Neurol. 2003;184:705–14. doi: 10.1016/S0014-4886(03)00313-3. [DOI] [PubMed] [Google Scholar]
- 24.Vazquez-Sanroman D, Leto K, Cerezo-Garcia M, Carbo-Gas M, Sanchis-Segura C, Carulli D, et al. The cerebellum on cocaine: plasticity and metaplasticity. Addict Biol. 2015;20:941–55. doi: 10.1111/adb.12223. [DOI] [PubMed] [Google Scholar]
- 25.Blosa M, Sonntag M, Jager C, Weigel S, Seeger J, Frischknecht R, et al. The extracellular matrix molecule brevican is an integral component of the machinery mediating fast synaptic transmission at the calyx of Held. J Physiol. 2015;593:4341–60. doi: 10.1113/JP270849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balmer TS. Perineuronal nets enhance the excitability of fast-spiking neurons. eNeuro. 2016;3:1–13. [DOI] [PMC free article] [PubMed]
- 27.Carulli D, Broersen R, de Winter F, Muir EM, Meskovic M, de Waal M, et al. Cerebellar plasticity and associative memories are controlled by perineuronal nets. Proc Natl Acad Sci USA. 2020;117:6855–65. doi: 10.1073/pnas.1916163117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edamatsu M, Miyano R, Fujikawa A, Fujii F, Hori T, Sakaba T, et al. Hapln4/Bral2 is a selective regulator for formation and transmission of GABAergic synapses between Purkinje and deep cerebellar nuclei neurons. J Neurochem. 2018;147:748–63. doi: 10.1111/jnc.14571. [DOI] [PubMed] [Google Scholar]
- 29.Hirono M, Watanabe S, Karube F, Fujiyama F, Kawahara S, Nagao S, et al. Perineuronal Nets in the Deep Cerebellar Nuclei Regulate GABAergic Transmission and Delay Eyeblink Conditioning. J Neurosci. 2018;38:6130–44. doi: 10.1523/JNEUROSCI.3238-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balmer TS, Carels VM, Frisch JL, Nick TA. Modulation of perineuronal nets and parvalbumin with developmental song learning. J Neurosci. 2009;29:12878–85. doi: 10.1523/JNEUROSCI.2974-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carulli D, Pizzorusso T, Kwok JC, Putignano E, Poli A, Forostyak S, et al. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain. 2010;133:2331–47. doi: 10.1093/brain/awq145. [DOI] [PubMed] [Google Scholar]
- 32.Dityatev A, Bruckner G, Dityateva G, Grosche J, Kleene R, Schachner M. Activity dependent formation and functions of chondroitin sulfate rich extracellular matrix of perineuronal nets. Dev Neurobiol. 2007;67:570–88. doi: 10.1002/dneu.20361. [DOI] [PubMed] [Google Scholar]
- 33.Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–40. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 34.Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–51. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 35.Happel MF, Niekisch H, Castiblanco Rivera LL, Ohl FW, Deliano M, Frischknecht R. Enhanced cognitive flexibility in reversal learning induced by removal of the extracellular matrix in auditory cortex. Proc Natl Acad Sci USA. 2014;111:2800–5. doi: 10.1073/pnas.1310272111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao RR, Fawcett JW. Combination treatment with chondroitinase ABC in spinal cord injury-breaking the barrier. Neurosci Bull. 2013;29:477–83. doi: 10.1007/s12264-013-1359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gherardini L, Gennaro M, Pizzorusso T. Perilesional Treatment with Chondroitinase ABC and Motor Training Promote Functional Recovery After Stroke in Rats. Cereb Cortex. 2013;25:202–12. [DOI] [PubMed]
- 38.Hylin MJ, Orsi SA, Moore AN, Dash PK. Disruption of the perineuronal net in the hippocampus or medial prefrontal cortex impairs fear conditioning. Learn Mem. 2013;20:267–73. doi: 10.1101/lm.030197.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dingess P, Harkness J, Slaker M, Zhang Z, Wulff S, Sorg B, et al. Consumption of a high-fat diet alters perineuronal nets in the prefrontal cortex. Neural plasticity. 2018;2018:1–8. [DOI] [PMC free article] [PubMed]
- 40.Dingess PM, Zhang Z, Sorg BA, Ferrario CR, Brown TE. Sex and region-specific effects of high fat diet on PNNs in obesity susceptible rats. Physiol Behav. 2020;222:112963. doi: 10.1016/j.physbeh.2020.112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reichelt AC, Gibson GD, Abbott KN, Hare DJ. A high-fat high-sugar diet in adolescent rats impairs social memory and alters chemical markers characteristic of atypical neuroplasticity and parvalbumin interneuron depletion in the medial prefrontal cortex. Food Funct. 2019;10:1985–98. doi: 10.1039/c8fo02118j. [DOI] [PubMed] [Google Scholar]
- 42.Reichelt AC, Lemieux CA, Princz-Lebel O, Singh A, Bussey TJ, Saksida LM. Age-dependent and region-specific alteration of parvalbumin neurons, perineuronal nets and microglia in the mouse prefrontal cortex and hippocampus following obesogenic diet consumption. Sci Rep. 2021;11:5593. doi: 10.1038/s41598-021-85092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slaker M, Barnes J, Sorg BA, Grimm JW. Impact of Environmental Enrichment on Perineuronal Nets in the Prefrontal Cortex following Early and Late Abstinence from Sucrose Self-Administration in Rats. PLoS One. 2016;11:e0168256. doi: 10.1371/journal.pone.0168256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith CC, Mauricio R, Nobre L, Marsh B, Wust RC, Rossiter HB, et al. Differential regulation of perineuronal nets in the brain and spinal cord with exercise training. Brain Res Bull. 2015;111:20–6. doi: 10.1016/j.brainresbull.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Alonge KM, Mirzadeh Z, Scarlett JM, Logsdon AF, Brown JM, Cabrales E, et al. Hypothalamic perineuronal net assembly is required for sustained diabetes remission induced by fibroblast growth factor 1 in rats. Nat Metab. 2020;2:1025–33. doi: 10.1038/s42255-020-00275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang N, Yan Z, Liu H, Yu M, He Y, Liu H, et al. Hypothalamic Perineuronal Nets Are Regulated by Sex and Dietary Interventions. Front Physiol. 2021;12:714104. doi: 10.3389/fphys.2021.714104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cabungcal JH, Steullet P, Morishita H, Kraftsik R, Cuenod M, Hensch TK, et al. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci USA. 2013;110:9130–5. doi: 10.1073/pnas.1300454110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morawski M, Bruckner MK, Riederer P, Bruckner G, Arendt T. Perineuronal nets potentially protect against oxidative stress. Exp Neurol. 2004;188:309–15. doi: 10.1016/j.expneurol.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 49.Reichelt AC, Hare DJ, Bussey TJ, Saksida LM. Perineuronal Nets: Plasticity, Protection, and Therapeutic Potential. Trends Neurosci. 2019;42:458–70. doi: 10.1016/j.tins.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 50.Suttkus A, Rohn S, Jager C, Arendt T, Morawski M. Neuroprotection against iron-induced cell death by perineuronal nets - an in vivo analysis of oxidative stress. Am J Neurodegener Dis. 2012;1:122–9. [PMC free article] [PubMed] [Google Scholar]
- 51.Suttkus A, Rohn S, Weigel S, Glockner P, Arendt T. Morawski M. Aggrecan, link protein and tenascin-R are essential components of the perineuronal net to protect neurons against iron-induced oxidative stress. Cell Death Dis. 2014;5:e1119. doi: 10.1038/cddis.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sorg BA, Berretta S, Blacktop JM, Fawcett JW, Kitagawa H, Kwok JC, et al. Casting a Wide Net: Role of Perineuronal Nets in Neural Plasticity. J Neurosci. 2016;36:11459–68. doi: 10.1523/JNEUROSCI.2351-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacques A, Chaaya N, Beecher K, Ali SA, Belmer A, Bartlett S. The impact of sugar consumption on stress driven, emotional and addictive behaviors. Neurosci Biobehav Rev. 2019;103:178–99. doi: 10.1016/j.neubiorev.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 54.Melo HM, Santos LE, Ferreira ST. Diet-Derived Fatty Acids, Brain Inflammation, and Mental Health. Front Neurosci. 2019;13:265. doi: 10.3389/fnins.2019.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valencia AP, Nagaraj N, Osman DH, Rabinovitch PS, Marcinek DJ. Are fat and sugar just as detrimental in old age? Geroscience. 2021;43:1615–25. doi: 10.1007/s11357-021-00390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flores-Dorantes MT, Diaz-Lopez YE, Gutierrez-Aguilar R. Environment and Gene Association With Obesity and Their Impact on Neurodegenerative and Neurodevelopmental Diseases. Front Neurosci. 2020;14:863. doi: 10.3389/fnins.2020.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lowe CJ, Reichelt AC, Hall PA. The Prefrontal Cortex and Obesity: A Health Neuroscience Perspective. Trends Cogn Sci. 2019;23:349–61. doi: 10.1016/j.tics.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Morris MJ, Beilharz JE, Maniam J, Reichelt AC, Westbrook RF. Why is obesity such a problem in the 21st century? The intersection of palatable food, cues and reward pathways, stress, and cognition. Neurosci Biobehav Rev. 2015;58:36–45. doi: 10.1016/j.neubiorev.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 59.Giskes K, van Lenthe F, Avendano-Pabon M, Brug J. A systematic review of environmental factors and obesogenic dietary intakes among adults: are we getting closer to understanding obesogenic environments? Obes Rev. 2011;12:e95–e106. doi: 10.1111/j.1467-789X.2010.00769.x. [DOI] [PubMed] [Google Scholar]
- 60.Monteiro CA, Moubarac JC, Cannon G, Ng SW, Popkin B. Ultra-processed products are becoming dominant in the global food system. Obes Rev. 2013;14:21–8. doi: 10.1111/obr.12107. [DOI] [PubMed] [Google Scholar]
- 61.Derman RC, Ferrario CR. Enhanced incentive motivation in obesity-prone rats is mediated by NAc core CP-AMPARs. Neuropharmacology. 2018;131:326–36. doi: 10.1016/j.neuropharm.2017.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vollbrecht PJ, Nobile CW, Chadderdon AM, Jutkiewicz EM, Ferrario CR. Pre-existing differences in motivation for food and sensitivity to cocaine-induced locomotion in obesity-prone rats. Physiol Behav. 2015;152:151–60. doi: 10.1016/j.physbeh.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oginsky MF, Goforth PB, Nobile CW, Lopez-Santiago LF, Ferrario CR. Eating ‘Junk-Food’ Produces Rapid and Long-Lasting Increases in NAc CP-AMPA Receptors: Implications for Enhanced Cue-Induced Motivation and Food Addiction. Neuropsychopharmacology. 2016;41:2977–86. doi: 10.1038/npp.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tyree SM, de Lecea L. Lateral Hypothalamic Control of the Ventral Tegmental Area: Reward Evaluation and the Driving of Motivated Behavior. Front Syst Neurosci. 2017;11:50. doi: 10.3389/fnsys.2017.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kohnke S, Buller S, Nuzzaci D, Ridley K, Lam B, Pivonkova H, et al. Nutritional regulation of oligodendrocyte differentiation regulates perineuronal net remodeling in the median eminence. Cell Rep. 2021;36:109362. doi: 10.1016/j.celrep.2021.109362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prinz P. The role of dietary sugars in health: molecular composition or just calories? Eur J Clin Nutr. 2019;73:1216–23. doi: 10.1038/s41430-019-0407-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.DiNicolantonio JJ, O’Keefe JH, Wilson WL. Sugar addiction: is it real? A narrative review. Br J Sports Med. 2018;52:910–13. doi: 10.1136/bjsports-2017-097971. [DOI] [PubMed] [Google Scholar]
- 68.Crombag HS, Gorny G, Li Y, Kolb B, Robinson TE. Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. Cereb Cortex. 2005;15:341–8. doi: 10.1093/cercor/bhh136. [DOI] [PubMed] [Google Scholar]
- 69.Dingess PM, Darling RA, Derman RC, Wulff SS, Hunter ML, Ferrario CR, et al. Structural and functional plasticity within the nucleus accumbens and prefrontal cortex associated with time-dependent increases in food cue-seeking behavior. Neuropsychopharmacology. 2017;42:2354–64. doi: 10.1038/npp.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dingess PM, Darling RA, Dolence EK, Culver BW, Brown TE. Exposure to a diet high in fat attenuates dendritic spine density in the medial prefrontal cortex. Brain Struct Funct. 2017;222:1077–85. doi: 10.1007/s00429-016-1208-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roura-Martinez D, Diaz-Bejarano P, Ucha M, Paiva RR, Ambrosio E, Higuera-Matas A. Comparative analysis of the modulation of perineuronal nets in the prefrontal cortex of rats during protracted withdrawal from cocaine, heroin and sucrose self-administration. Neuropharmacology. 2020;180:108290. doi: 10.1016/j.neuropharm.2020.108290. [DOI] [PubMed] [Google Scholar]
- 72.Basso JC, Morrell JI. The medial prefrontal cortex and nucleus accumbens mediate the motivation for voluntary wheel running in the rat. Behav Neurosci. 2015;129:457–72. doi: 10.1037/bne0000070. [DOI] [PubMed] [Google Scholar]
- 73.Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA. Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis. Neurosci Biobehav Rev. 2013;37:1622–44. doi: 10.1016/j.neubiorev.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vina J, Sanchis-Gomar F, Martinez-Bello V, Gomez-Cabrera MC. Exercise acts as a drug; the pharmacological benefits of exercise. Br J Pharmacol. 2012;167:1–12. doi: 10.1111/j.1476-5381.2012.01970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Orlando C, Raineteau O. Integrity of cortical perineuronal nets influences corticospinal tract plasticity after spinal cord injury. Brain Struct Funct. 2015;220:1077–91. doi: 10.1007/s00429-013-0701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sanchez-Ventura J, Gimenez-Llort L, Penas C, Udina E. Voluntary wheel running preserves lumbar perineuronal nets, enhances motor functions and prevents hyperreflexia after spinal cord injury. Exp Neurol. 2021;336:113533. doi: 10.1016/j.expneurol.2020.113533. [DOI] [PubMed] [Google Scholar]
- 77.Takeda A, Shuto M, Funakoshi K. Chondroitin Sulfate Expression in Perineuronal Nets After Goldfish Spinal Cord Lesion. Front Cell Neurosci. 2018;12:63. doi: 10.3389/fncel.2018.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McDonald MW, Hayward KS, Rosbergen ICM, Jeffers MS, Corbett D. Is Environmental Enrichment Ready for Clinical Application in Human Post-stroke Rehabilitation? Front Behav Neurosci. 2018;12:135. doi: 10.3389/fnbeh.2018.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zanca RM, Braren SH, Maloney B, Schrott LM, Luine VN, Serrano PA. Environmental Enrichment Increases Glucocorticoid Receptors and Decreases GluA2 and Protein Kinase M Zeta (PKMzeta) Trafficking During Chronic Stress: A Protective Mechanism? Front Behav Neurosci. 2015;9:303. doi: 10.3389/fnbeh.2015.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology. 2001;155:278–84. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- 81.Bowling SL, Rowlett JK, Bardo MT. The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology. 1993;32:885–93. doi: 10.1016/0028-3908(93)90144-r. [DOI] [PubMed] [Google Scholar]
- 82.Chauvet C, Goldberg SR, Jaber M, Solinas M. Effects of environmental enrichment on the incubation of cocaine craving. Neuropharmacology. 2012;63:635–41. doi: 10.1016/j.neuropharm.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chauvet C, Lardeux V, Goldberg SR, Jaber M, Solinas M. Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology. 2009;34:2767–78. doi: 10.1038/npp.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Green TA, Cain ME, Thompson M, Bardo MT. Environmental enrichment decreases nicotine-induced hyperactivity in rats. Psychopharmacology. 2003;170:235–41. doi: 10.1007/s00213-003-1538-3. [DOI] [PubMed] [Google Scholar]
- 85.Green TA, Gehrke BJ, Bardo MT. Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacology. 2002;162:373–8. doi: 10.1007/s00213-002-1134-y. [DOI] [PubMed] [Google Scholar]
- 86.Nicolas C, Hofford RS, Dugast E, Lardeux V, Belujon P, Solinas M, et al. Prevention of relapse to methamphetamine self-administration by environmental enrichment: involvement of glucocorticoid receptors. Psychopharmacology. 2021;239:1009–18. [DOI] [PubMed]
- 87.Sikora M, Nicolas C, Istin M, Jaafari N, Thiriet N, Solinas M. Generalization of effects of environmental enrichment on seeking for different classes of drugs of abuse. Behav Brain Res. 2018;341:109–13. doi: 10.1016/j.bbr.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 88.Thiriet N, Gennequin B, Lardeux V, Chauvet C, Decressac M, Janet T, et al. Environmental enrichment does not reduce the rewarding and neurotoxic effects of methamphetamine. Neurotox Res. 2011;19:172–82. doi: 10.1007/s12640-010-9158-2. [DOI] [PubMed] [Google Scholar]
- 89.Grimm JW, Hyde J, Glueck E, North K, Ginder D, Jiganti K, et al. Examining persistence of acute environmental enrichment-induced anti-sucrose craving effects in rats. Appetite. 2019;139:50–58. doi: 10.1016/j.appet.2019.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grimm JW, Osincup D, Wells B, Manaois M, Fyall A, Buse C, et al. Environmental enrichment attenuates cue-induced reinstatement of sucrose seeking in rats. Behav Pharmacol. 2008;19:777–85. doi: 10.1097/FBP.0b013e32831c3b18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rodriguez-Ortega E, Alcaraz-Iborra M, de la Fuente L, Cubero I. Protective and therapeutic benefits of environmental enrichment on binge-like sucrose intake in C57BL/6 J mice. Appetite. 2019;138:184–89. doi: 10.1016/j.appet.2019.03.033. [DOI] [PubMed] [Google Scholar]
- 92.O’Connor AM, Burton TJ, Mansuri H, Hand GR, Leamey CA, Sawatari A. Environmental Enrichment From Birth Impacts Parvalbumin Expressing Cells and Wisteria Floribunda Agglutinin Labelled Peri-Neuronal Nets Within the Developing Murine Striatum. Front Neuroanat. 2019;13:90. doi: 10.3389/fnana.2019.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Simonetti T, Lee H, Bourke M, Leamey CA, Sawatari A. Enrichment from birth accelerates the functional and cellular development of a motor control area in the mouse. PLoS One. 2009;4:e6780. doi: 10.1371/journal.pone.0006780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Foscarin S, Ponchione D, Pajaj E, Leto K, Gawlak M, Wilczynski GM, et al. Experience-dependent plasticity and modulation of growth regulatory molecules at central synapses. PLoS One. 2011;6:e16666. doi: 10.1371/journal.pone.0016666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lasek AW, Chen H, Chen WY. Releasing Addiction Memories Trapped in Perineuronal Nets. Trends Genet. 2018;34:197–208. doi: 10.1016/j.tig.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Slaker ML, Jorgensen ET, Hegarty DM, Liu X, Kong Y, Zhang F, et al. Cocaine Exposure Modulates Perineuronal Nets and Synaptic Excitability of Fast-Spiking Interneurons in the Medial Prefrontal Cortex. eNeuro. 2018;5:1–17. [DOI] [PMC free article] [PubMed]
- 97.Gil-Miravet I, Guarque-Chabrera J, Carbo-Gas M, Olucha-Bordonau F, Miquel M. The role of the cerebellum in drug-cue associative memory: functional interactions with the medial prefrontal cortex. Eur J Neurosci. 2019;50:2613–22. doi: 10.1111/ejn.14187. [DOI] [PubMed] [Google Scholar]
- 98.Matuszko G, Curreli S, Kaushik R, Becker A, Dityatev A. Extracellular matrix alterations in the ketamine model of schizophrenia. Neuroscience. 2017;350:13–22. doi: 10.1016/j.neuroscience.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 99.Kaushik R, Lipachev N, Matuszko G, Kochneva A, Dvoeglazova A, Becker A, et al. Fine structure analysis of perineuronal nets in the ketamine model of schizophrenia. Eur J Neurosci. 2021;53:3988–4004. doi: 10.1111/ejn.14853. [DOI] [PubMed] [Google Scholar]
- 100.Steullet P, Cabungcal JH, Cuenod M, Do KQ. Fast oscillatory activity in the anterior cingulate cortex: dopaminergic modulation and effect of perineuronal net loss. Front Cell Neurosci. 2014;8:244. doi: 10.3389/fncel.2014.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vazquez-Sanroman DB, Monje RD, Bardo MT. Nicotine self-administration remodels perineuronal nets in ventral tegmental area and orbitofrontal cortex in adult male rats. Addict Biol. 2017;22:1743–55. doi: 10.1111/adb.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saito M, Smiley JF, Hui M, Masiello K, Betz J, Ilina M, et al. Neonatal Ethanol Disturbs the Normal Maturation of Parvalbumin Interneurons Surrounded by Subsets of Perineuronal Nets in the Cerebral Cortex: Partial Reversal by Lithium. Cereb Cortex. 2019;29:1383–97. doi: 10.1093/cercor/bhy034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Venturino A, Schulz R, De Jesus-Cortes H, Maes ME, Nagy B, Reilly-Andujar F, et al. Microglia enable mature perineuronal nets disassembly upon anesthetic ketamine exposure or 60-Hz light entrainment in the healthy brain. Cell Rep. 2021;36:109313. doi: 10.1016/j.celrep.2021.109313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen H, He D, Lasek AW. Repeated Binge Drinking Increases Perineuronal Nets in the Insular Cortex. Alcohol Clin Exp Res. 2015;39:1930–8. doi: 10.1111/acer.12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Blacktop JM, Todd RP, Sorg BA. Role of perineuronal nets in the anterior dorsal lateral hypothalamic area in the acquisition of cocaine-induced conditioned place preference and self-administration. Neuropharmacology. 2017;118:124–36. doi: 10.1016/j.neuropharm.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Blacktop JM, Sorg BA. Perineuronal nets in the lateral hypothalamus area regulate cue-induced reinstatement of cocaine-seeking behavior. Neuropsychopharmacology. 2019;44:850–58. doi: 10.1038/s41386-018-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fujikawa R, Yamada J, Jinno S. Subclass imbalance of parvalbumin-expressing GABAergic neurons in the hippocampus of a mouse ketamine model for schizophrenia, with reference to perineuronal nets. Schizophrenia Res. 2021;229:80–93. doi: 10.1016/j.schres.2020.11.016. [DOI] [PubMed] [Google Scholar]
- 108.Vazquez-Sanroman D, Carbo-Gas M, Leto K, Cerezo-Garcia M, Gil-Miravet I, Sanchis-Segura C, et al. Cocaine-induced plasticity in the cerebellum of sensitised mice. Psychopharmacology. 2015;232:4455–67. doi: 10.1007/s00213-015-4072-1. [DOI] [PubMed] [Google Scholar]
- 109.Sanchez-Hernandez A, Nicolas C, Gil-Miravet I, Guarque-Chabrera J, Solinas M, Miquel M. Time-dependent regulation of perineuronal nets in the cerebellar cortex during abstinence of cocaine-self administration. Psychopharmacology. 2021;238:1059–68. doi: 10.1007/s00213-020-05752-0. [DOI] [PubMed] [Google Scholar]
- 110.Carbo-Gas M, Moreno-Rius J, Guarque-Chabrera J, Vazquez-Sanroman D, Gil-Miravet I, Carulli D, et al. Cerebellar perineuronal nets in cocaine-induced pavlovian memory: Site matters. Neuropharmacology. 2017;125:166–80. doi: 10.1016/j.neuropharm.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 111.Jorgensen ET, Gonzalez AE, Harkness JH, Hegarty DM, Thakar A, Burchi DJ, et al. Cocaine memory reactivation induces functional adaptations within parvalbumin interneurons in the rat medial prefrontal cortex. Addict Biol. 2020;26:e12947. [DOI] [PMC free article] [PubMed]
- 112.Miquel M, Gil-Miravet I, Guarque-Chabrera J. The Cerebellum on Cocaine. Front Syst Neurosci. 2020;14:586574. doi: 10.3389/fnsys.2020.586574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Guarque-Chabrera J, Gil-Miravet I, Olucha-Bordonau F, Melchor-Eixea I, Miquel M. When the front fails, the rear wins. Cerebellar correlates of prefrontal dysfunction in cocaine-induced memory in male rats. Prog Neuropsychopharmacol Biol Psychiatry. 2022;112:110429. doi: 10.1016/j.pnpbp.2021.110429. [DOI] [PubMed] [Google Scholar]
- 114.Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–2. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lasek AW. Effects of Ethanol on Brain Extracellular Matrix: Implications for Alcohol Use Disorder. Alcohol Clin Exp Res. 2016;40:2030–42. doi: 10.1111/acer.13200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang X, Bhattacharyya S, Kusumo H, Goodlett CR, Tobacman JK, Guizzetti M. Arylsulfatase B modulates neurite outgrowth via astrocyte chondroitin-4-sulfate: dysregulation by ethanol. Glia. 2014;62:259–71. doi: 10.1002/glia.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Coleman LG, Jr, Liu W, Oguz I, Styner M, Crews FT. Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility. Pharm Biochem Behav. 2014;116:142–51. doi: 10.1016/j.pbb.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Riceberg JS, Shapiro ML. Reward stability determines the contribution of orbitofrontal cortex to adaptive behavior. J Neurosci. 2012;32:16402–9. doi: 10.1523/JNEUROSCI.0776-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lewin M, Ilina M, Betz J, Masiello K, Hui M, Wilson DA, et al. Developmental Ethanol-Induced Sleep Fragmentation, Behavioral Hyperactivity, Cognitive Impairment and Parvalbumin Cell Loss are Prevented by Lithium Co-treatment. Neuroscience. 2018;369:269–77. doi: 10.1016/j.neuroscience.2017.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Matthews RT, Kelly GM, Zerillo CA, Gray G, Tiemeyer M, Hockfield S. Aggrecan glycoforms contribute to the molecular heterogeneity of perineuronal nets. J Neurosci. 2002;22:7536–47. doi: 10.1523/JNEUROSCI.22-17-07536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tolu S, Eddine R, Marti F, David V, Graupner M, Pons S, et al. Co-activation of VTA DA and GABA neurons mediates nicotine reinforcement. Mol Psychiatry. 2013;18:382–93. doi: 10.1038/mp.2012.83. [DOI] [PubMed] [Google Scholar]
- 122.Favuzzi E, Marques-Smith A, Deogracias R, Winterflood CM, Sanchez-Aguilera A, Mantoan L, et al. Activity-Dependent Gating of Parvalbumin Interneuron Function by the Perineuronal Net Protein Brevican. Neuron. 2017;95:639–55 e10. doi: 10.1016/j.neuron.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 123.Bobadilla AC, Dereschewitz E, Vaccaro L, Heinsbroek JA, Scofield MD, Kalivas PW. Cocaine and sucrose rewards recruit different seeking ensembles in the nucleus accumbens core. Mol Psychiatry. 2020;25:3150–63. doi: 10.1038/s41380-020-00888-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kane L, Venniro M, Quintana-Feliciano R, Madangopal R, Rubio FJ, Bossert JM, et al. Fos-expressing neuronal ensemble in rat ventromedial prefrontal cortex encodes cocaine seeking but not food seeking in rats. Addict Biol. 2021;26:e12943. doi: 10.1111/adb.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Guillem K, Ahmed SH. Reorganization of theta phase-locking in the orbitofrontal cortex drives cocaine choice under the influence. Sci Rep. 2020;10:8041. doi: 10.1038/s41598-020-64962-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guercio LA, Hofmann ME, Swinford-Jackson SE, Sigman JS, Wimmer ME, Dell’Acqua ML, et al. A-Kinase Anchoring Protein 150 (AKAP150) Promotes Cocaine Reinstatement by Increasing AMPA Receptor Transmission in the Accumbens Shell. Neuropsychopharmacology. 2018;43:1395–404. doi: 10.1038/npp.2017.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–94. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 128.Gorelova N, Seamans JK, Yang CR. Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. J Neurophysiol. 2002;88:3150–66. doi: 10.1152/jn.00335.2002. [DOI] [PubMed] [Google Scholar]
- 129.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mitlohner J, Kaushik R, Niekisch H, Blondiaux A, Gee CE, Happel MFK, et al. Dopamine Receptor Activation Modulates the Integrity of the Perisynaptic Extracellular Matrix at Excitatory Synapses. Cells. 2020;9:1–21. [DOI] [PMC free article] [PubMed]
- 131.Baik JH. Dopamine signaling in reward-related behaviors. Front Neural Circuits. 2013;7:152. doi: 10.3389/fncir.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–80. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- 133.Zhu X, Cabungcal JH, Cuenod M, Uliana DL, Do KQ, Grace AA. Thalamic reticular nucleus impairments and abnormal prefrontal control of dopamine system in a developmental model of schizophrenia: prevention by N-acetylcysteine. Mol Psychiatry. 2021;26:7679–89. doi: 10.1038/s41380-021-01198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shah A, Lodge DJ. A loss of hippocampal perineuronal nets produces deficits in dopamine system function: relevance to the positive symptoms of schizophrenia. Transl Psychiatry. 2013;3:e215. doi: 10.1038/tp.2012.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cadet JL, Brannock C. Free radicals and the pathobiology of brain dopamine systems. Neurochem Int. 1998;32:117–31. doi: 10.1016/s0197-0186(97)00031-4. [DOI] [PubMed] [Google Scholar]
- 136.Rabinovic AD, Hastings TG. Role of endogenous glutathione in the oxidation of dopamine. J Neurochem. 1998;71:2071–8. doi: 10.1046/j.1471-4159.1998.71052071.x. [DOI] [PubMed] [Google Scholar]
- 137.Huang YH, Lin Y, Mu P, Lee BR, Brown TE, Wayman G, et al. In vivo cocaine experience generates silent synapses. Neuron. 2009;63:40–7. doi: 10.1016/j.neuron.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Huang YH, Schluter OM, Dong Y. Silent Synapses Speak Up: Updates of the Neural Rejuvenation Hypothesis of Drug Addiction. Neuroscientist. 2015;21:451–9. doi: 10.1177/1073858415579405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci. 2011;31:5737–43. doi: 10.1523/JNEUROSCI.0350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wolf ME, Tseng KY. Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Front Mol Neurosci. 2012;5:72. doi: 10.3389/fnmol.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Alonso-Caraballo Y, Fetterly TL, Jorgensen ET, Nieto AM, Brown TE, Ferrario CR. Sex specific effects of “junk-food” diet on calcium permeable AMPA receptors and silent synapses in the nucleus accumbens core. Neuropsychopharmacology. 2021;46:569–78. doi: 10.1038/s41386-020-0781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dankovich TM, Kaushik R, Olsthoorn LHM, Petersen GC, Giro PE, Kluever V, et al. Extracellular matrix remodeling through endocytosis and resurfacing of Tenascin-R. Nat Commun. 2021;12:7129. doi: 10.1038/s41467-021-27462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tewari BP, Chaunsali L, Campbell SL, Patel DC, Goode AE, Sontheimer H. Perineuronal nets decrease membrane capacitance of peritumoral fast spiking interneurons in a model of epilepsy. Nat Commun. 2018;9:4724. doi: 10.1038/s41467-018-07113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen H, Lasek AW. Perineuronal nets in the insula regulate aversion-resistant alcohol drinking. Addict Biol. 2019;25:e12821. [DOI] [PMC free article] [PubMed]
- 145.Xue YX, Xue LF, Liu JF, He J, Deng JH, Sun SC, et al. Depletion of perineuronal nets in the amygdala to enhance the erasure of drug memories. J Neurosci. 2014;34:6647–58. doi: 10.1523/JNEUROSCI.5390-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Slaker M, Churchill L, Todd RP, Blacktop JM, Zuloaga DG, Raber J, et al. Removal of perineuronal nets in the medial prefrontal cortex impairs the acquisition and reconsolidation of a cocaine-induced conditioned place preference memory. J Neurosci. 2015;35:4190–202. doi: 10.1523/JNEUROSCI.3592-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lubbers BR, Matos MR, Horn A, Visser E, Van der Loo RC, Gouwenberg Y, et al. The Extracellular Matrix Protein Brevican Limits Time-Dependent Enhancement of Cocaine Conditioned Place Preference. Neuropsychopharmacology. 2016;41:1907–16. doi: 10.1038/npp.2015.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bozzelli PL, Alaiyed S, Kim E, Villapol S, Conant K. Proteolytic Remodeling of Perineuronal Nets: Effects on Synaptic Plasticity and Neuronal Population Dynamics. Neural Plast. 2018;2018:5735789. doi: 10.1155/2018/5735789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lensjo KK, Lepperod ME, Dick G, Hafting T, Fyhn M. Removal of Perineuronal Nets Unlocks Juvenile Plasticity Through Network Mechanisms of Decreased Inhibition and Increased Gamma Activity. J Neurosci. 2017;37:1269–83. doi: 10.1523/JNEUROSCI.2504-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bruckner G, Brauer K, Hartig W, Wolff JR, Rickmann MJ, Derouiche A, et al. Perineuronal nets provide a polyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain. Glia. 1993;8:183–200. doi: 10.1002/glia.440080306. [DOI] [PubMed] [Google Scholar]
- 151.Hartig W, Derouiche A, Welt K, Brauer K, Grosche J, Mader M, et al. Cortical neurons immunoreactive for the potassium channel Kv3.1b subunit are predominantly surrounded by perineuronal nets presumed as a buffering system for cations. Brain Res. 1999;842:15–29. doi: 10.1016/s0006-8993(99)01784-9. [DOI] [PubMed] [Google Scholar]
- 152.Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D, Gundelfinger ED. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci. 2009;12:897–904. doi: 10.1038/nn.2338. [DOI] [PubMed] [Google Scholar]
- 153.Carceller H, Guirado R, Ripolles-Campos E, Teruel-Marti V, Nacher J. Perineuronal Nets Regulate the Inhibitory Perisomatic Input onto Parvalbumin Interneurons and gamma Activity in the Prefrontal Cortex. J Neurosci. 2020;40:5008–18. doi: 10.1523/JNEUROSCI.0291-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.de Winter F, Kwok JC, Fawcett JW, Vo TT, Carulli D, Verhaagen J. The Chemorepulsive Protein Semaphorin 3 A and Perineuronal Net-Mediated Plasticity. Neural Plast. 2016;2016:3679545. doi: 10.1155/2016/3679545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Feltenstein MW, See RE, Fuchs RA. Neural Substrates and Circuits of Drug Addiction. Cold Spring Harb Perspect Med. 2021;11:1–33. [DOI] [PMC free article] [PubMed]
- 156.Lovinger DM, Gremel CM. A Circuit-Based Information Approach to Substance Abuse Research. Trends Neurosci. 2021;44:122–35. doi: 10.1016/j.tins.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–73. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nall RW, Heinsbroek JA, Nentwig TB, Kalivas PW, Bobadilla AC. Circuit selectivity in drug versus natural reward seeking behaviors. J Neurochem. 2021;157:1450–72. doi: 10.1111/jnc.15297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dong Y, Taylor JR, Wolf ME, Shaham Y. Circuit and Synaptic Plasticity Mechanisms of Drug Relapse. J Neurosci. 2017;37:10867–76. doi: 10.1523/JNEUROSCI.1821-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wingert JC, Sorg BA. Impact of Perineuronal Nets on Electrophysiology of Parvalbumin Interneurons, Principal Neurons, and Brain Oscillations: A Review. Front Synaptic Neurosci. 2021;13:673210. doi: 10.3389/fnsyn.2021.673210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 162.Sparta DR, Hovelso N, Mason AO, Kantak PA, Ung RL, Decot HK, et al. Activation of prefrontal cortical parvalbumin interneurons facilitates extinction of reward-seeking behavior. J Neurosci. 2014;34:3699–705. doi: 10.1523/JNEUROSCI.0235-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Packer AM, Yuste R. Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? J Neurosci. 2011;31:13260–71. doi: 10.1523/JNEUROSCI.3131-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Faini G, Aguirre A, Landi S, Lamers D, Pizzorusso T, Ratto GM, et al. Perineuronal nets control visual input via thalamic recruitment of cortical PV interneurons. Elife. 2018;7:1–21. [DOI] [PMC free article] [PubMed]
- 165.Ye L, Allen WE, Thompson KR, Tian Q, Hsueh B, Ramakrishnan C, et al. Wiring and Molecular Features of Prefrontal Ensembles Representing Distinct Experiences. Cell. 2016;165:1776–88. doi: 10.1016/j.cell.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tonegawa S, Pignatelli M, Roy DS, Ryan TJ. Memory engram storage and retrieval. Curr Opin Neurobiol. 2015;35:101–9. doi: 10.1016/j.conb.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 167.Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–16. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- 168.Uhlhaas PJ, Roux F, Rodriguez E, Rotarska-Jagiela A, Singer W. Neural synchrony and the development of cortical networks. Trends Cogn Sci. 2010;14:72–80. doi: 10.1016/j.tics.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 169.Buzsaki G, Wang XJ. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–25. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Stark E, Eichler R, Roux L, Fujisawa S, Rotstein HG, Buzsaki G. Inhibition-induced theta resonance in cortical circuits. Neuron. 2013;80:1263–76. doi: 10.1016/j.neuron.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kim H, Ahrlund-Richter S, Wang X, Deisseroth K, Carlen M. Prefrontal Parvalbumin Neurons in Control of Attention. Cell. 2016;164:208–18. doi: 10.1016/j.cell.2015.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bueno-Junior LS, Simon NW, Wegener MA, Moghaddam B. Repeated Nicotine Strengthens Gamma Oscillations in the Prefrontal Cortex and Improves Visual Attention. Neuropsychopharmacology. 2017;42:1590–98. doi: 10.1038/npp.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Guise KG, Shapiro ML. Medial Prefrontal Cortex Reduces Memory Interference by Modifying Hippocampal Encoding. Neuron. 2017;94:183–92 e8. doi: 10.1016/j.neuron.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cho KKA, Davidson TJ, Bouvier G, Marshall JD, Schnitzer MJ, Sohal VS. Cross-hemispheric gamma synchrony between prefrontal parvalbumin interneurons supports behavioral adaptation during rule shift learning. Nat Neurosci. 2020;23:892–902. doi: 10.1038/s41593-020-0647-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Donnelly NA, Holtzman T, Rich PD, Nevado-Holgado AJ, Fernando AB, Van Dijck G, et al. Oscillatory activity in the medial prefrontal cortex and nucleus accumbens correlates with impulsivity and reward outcome. PLoS One. 2014;9:e111300. doi: 10.1371/journal.pone.0111300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Del Arco A, Park J, Wood J, Kim Y, Moghaddam B. Adaptive Encoding of Outcome Prediction by Prefrontal Cortex Ensembles Supports Behavioral Flexibility. J Neurosci. 2017;37:8363–73. doi: 10.1523/JNEUROSCI.0450-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–9. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 178.Miao C, Cao Q, Moser MB, Moser EI. Parvalbumin and Somatostatin Interneurons Control Different Space-Coding Networks in the Medial Entorhinal Cortex. Cell. 2017;171:507–21 e17. doi: 10.1016/j.cell.2017.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Christensen AC, Lensjo KK, Lepperod ME, Dragly SA, Sutterud H, Blackstad JS, et al. Perineuronal nets stabilize the grid cell network. Nat Commun. 2021;12:253. doi: 10.1038/s41467-020-20241-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Agetsuma M, Hamm JP, Tao K, Fujisawa S, Yuste R. Parvalbumin-Positive Interneurons Regulate Neuronal Ensembles in Visual Cortex. Cereb Cortex. 2018;28:1831–45. doi: 10.1093/cercor/bhx169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Slaker ML, Harkness JH, Sorg BA. A standardized and automated method of perineuronal net analysis using Wisteria floribunda agglutinin staining intensity. IBRO Rep. 2016;1:54–60. doi: 10.1016/j.ibror.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sigal YM, Bae H, Bogart LJ, Hensch TK, Zhuang X. Structural maturation of cortical perineuronal nets and their perforating synapses revealed by superresolution imaging. Proc Natl Acad Sci USA. 2019;116:7071–76. doi: 10.1073/pnas.1817222116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharm Sci. 2004;25:273–9. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 184.Cornish JL, Prasad AA. Sex Differences in Substance Use Disorders: A Neurobiological Perspective. Front Glob. Women’s Health. 2021;2:778514. doi: 10.3389/fgwh.2021.778514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Alonso-Caraballo Y, Jorgensen ET, Brown TE, Ferrario CR. Functional and structural plasticity contributing to obesity: roles for sex, diet, and individual susceptibility. Curr Opin Behav Sci. 2018;23:160–70. doi: 10.1016/j.cobeha.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Badin AS, Fermani F, Greenfield SA. The Features and Functions of Neuronal Assemblies: Possible Dependency on Mechanisms beyond Synaptic Transmission. Front Neural Circuits. 2016;10:114. doi: 10.3389/fncir.2016.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]