Abstract
Dopamine (DA) is one of the chief neurotransmitters present in the central nervous system of mammals. Therefore detection of DA in presence of various analytes like paracetamol has great importance. In the current work, we are proposing that Triton X-100 (TX-100) pretreated carbon paste electrode (CPE) can be useful to detect the DA selectively in presence of PA. After the pretreatment CPE can detect DA in presence of PA effectively. Cyclic voltammetry was employed to observe the amplified electron transfer reaction between the modified CPE and DA. To understand electron transfer regioselectivity at the TX-100 pretreated CPE, a dual descriptor was used. The prepared electrode showed satisfactory stability when kept under ambient conditions. The proposed approach also showed excellent analytical applicability to identify DA and PA in commercial formulations. The scope of the work is limited to detecting DA in presence of PA. We will consider the other interferes for future works.
Subject terms: Biochemistry, Chemistry, Engineering
Introduction
Now-day researchers are more concentrating on carbon paste electrodes (CPEs) to utilize them as working electrodes in electrochemical sensors applications. CPEs offer several appealing properties, including surface repeatability, stability, and renewability, making them appealing substances for working electrodes1–3. Due to their inexpensive cost relative to other materials, CPEs are becoming more widely used in areas such as pharmaceutical, biological, and environmental analysis4,5.
Physical or chemical modifications can improve CPE's sensing properties like selectivity, adsorption capacity, and sensitivity6,7. The goal of modifying a CPE matrix is to create an efficient electrode with sensing properties that will be useful for users. In general, after modifications, electrocatalytic activities at the CPE interface will improve, which may be due to the increased surface area which in turn gives better current sensitivity for electron transfer reactions. CPE can be chemically modified using conductive substrates modified with electroactive thin films, monolayers, or thick coatings8,9.
The electrochemical modifications may include the use of different types of surfactants to modify the CPE. Clay minerals treated with surfactants have excellent sorption capabilities for both organic and inorganic substances in aqueous solutions10,11. In terms of electrode modification, T-octylphenoxypolyethoxyethanol (Triton X-100 (TX100)) is one of the best nonionic surfactants12–16. The properties of hybrid materials (carbon paste + TX-100) are highly promising. TX-100 modified carbon hybrid materials are previously reported to use as electrodes. TX-100 adsorbed electrode alters the electrode–electrolyte interface, making it easier to create poly (m-toluidine) films with higher current densities16. TX-100 modified CPE was prepared by surface immobilization technique by B.N. Chandrashekar et al. and this method exhibit good sensitivity, but TX-100 pretreatment is more applicable than this method17. According to the literature, modification of electrodes using TX-100 has several works but the pre-treatment method good one for analysis of biomolecules proved by the present work18,19.
The modifications of electrodes can be done by methods like mechanical grinding, self-assembled layers, covalently bonded electrolyzers, surfactant immobilization, etc. The electrochemical pre-treatment process increases the electron transfer compared to others. To use carbon surface effectively one must take care of the degree of activation20. The modification of CPEs is achieved simply by pre-treatment of TX-100 modifier with the carbon paste which is enhancing the sensitivity and selectivity of the electrochemical analysis technique.
Because of its simplicity, sensitivity, efficiency, and low cost, electrochemical pretreatment was chosen for this study. Pretreatment is considered environmentally benign since it uses less dangerous chemicals than other time-consuming and sophisticated modification materials. The electrochemical pretreatment procedure results in transducers that produce signals that are undistorted, well-defined, and repeatable. By creating charge-carrying functional groups, the pretreatment of the CPE enhances the selectivity and sensitivity of the sensors for detecting selected electroactive components. In the present work, we study the effect of TX-100 pretreatment on CPE, and it is used in applications for DA and PA sensors with performing the detection of DA in the presence of PA because the usage of PA protects dopaminergic neurons against oxidative stress damage produced by acute exposure to increased amounts of DA, according to in vitro studies21 and it shows outstanding stability, sensitivity, and selectivity for biomedical applications.
Experimental section
Reagents and chemicals
Paracetamol (PA) was obtained from Merck, sodium hydroxide (NaOH), and dopamine hydrochlorides (DA) from nice chemicals. The graphite powder (LobaChemie), Silicone oil and TX-100 are obtained from Himedia.
Apparatus
The TX-100 atomic model has created with Sinapsis software (Version: Sinapsis.XIII Weblink: http://sinapsis.sourceforge.net Acess date: accessed July 20, 2020)22. The experiments were done using the potentiostat model CHI-660c (CH Instrument-660 electrochemical workstation) and the generated data was transferred to origin 6.1. Bare CPE (BCPE), NaOH pretreated modified CPE (NaOH-PT-MCPE), and TX-100 pretreated modified CPE (TX-100PT-MCPE) are used as working electrodes, saturated calomel as the reference electrode, and a platinum electrode as a counter electrode.
Preparation of BCPE
The BCPE was prepared by hand mixing 70% graphite powder with 30% silicon oil in an agate mortar for approximately 30 min to produce a homogenous carbon paste. The paste was packed into the homemade cavity of a Teflon tube and polished on a paper napkin to obtain a uniform surface.
Pretreatment of CPE with TX-100
The pretreatment process was carried out using cyclic voltammetry (CV), potential cycles were between − 0.4 and 0.9 V at a scan rate (ν) of 1 V/s for 100 cycles as depicted in Fig. 1A. With an increase in the cycle number voltammogram, currents decreased gradually which indicates that during the pretreatment process TX-100 is adsorbing on the electrode surface. To eliminate contaminants electrode was dipped in deionized water. The electrochemical response of TX-100PT-MCPE with different cycles is measured by using potassium ferrocyanide, K4(Fe (CN)6) at a sweep rate of 50 mV/s is depicted in Fig. 1B. TX-100PT-MCPE prepared by 100 cycles showed better sensing capability hence it is considered for further analysis.
Figure 1.

(A) CVs for electrodeposition of TX-100 on a BCPE in 0.1 M NaOH by 100 cycles. Sweep rate: 1 Vs−1. (B)The graph of anodic peak current versus several cycles for potassium ferrocyanide at a sweep rate of 50 mV/s.
Computational analysis
The TX-100 atomic model was created with Sinapsis software22 as shown in Fig. 2 and geometry optimization was done with deMon2k software23 using PBE exchange - correlation functions24,25 and TZVP basis sets26, employing density functional theory (DFT) as described in our previous articles. Sinapsis software was used to plot the dual descriptor results.
Figure 2.
Atomistic model of Triton X-100 (H = white, C = grey, and O = red).
Results and discussion
The PTTMCPE electrochemical response towards potassium ferrocyanide
The cyclic voltammetric (CV) response of K4(Fe(CN)6 at TX-100-PTCPE is shown in Fig. 3A. The BCPEs had poor peak current and high redox peak potential differences, but the MCPEs had much-improved redox peak current signals and smaller redox peak potential discrepancies. Randle’s Sevick's equation27 was used to calculate the total active surface area. The active surface area for electrodes (BCPE, NaOH-PT-MCPE, and TX-100PT-MCPE) is shown in Table 1.
Figure 3.

(A) CVs of 1 mM potassium ferrocyanide in 1 M KCl solution at a speed rate of 50 mV/s. (B) Dual descriptor result of TX-100 [ISO = 0.07, grid = 0.3].
Table 1.
The electrochemical performance of the electrodes.
| Electrodes | Ipa (A) | ∆E (V) [at ν = 0.05 V] | Area (cm2) |
|---|---|---|---|
| BCPE | 2.12 × 10–05 | 0.110 | 0.0321 |
| Pretreated NaOH MCPE | 2.70 × 10–05 | 0.103 | 0.0372 |
| PTTMCPE | 3.26 × 10–05 | 0.050 | 0.0412 |
The heterogeneous rate constant (k0) values for K4(Fe (CN)6 are calculated from the ΔEp values (> 10 mV) using Eq. (1). The k0 values are listed in Table 2.
| 1 |
Table 2.
The rate constant values for potassium ferrocyanide at different electrodes.
| υ/mV/s | ΔEp/mV | k°(s−1) | ||||
|---|---|---|---|---|---|---|
| BCPE | Pretreated NaOH MCPE | PTT MCPE | BCPE | Pretreated NaOH MCPE | PTT MCPE | |
| 50 | 110 | 103 | 50 | 0.895798 | 0.488696 | 0.451108 |
| 100 | 131 | 130 | 82 | 0.709635 | 0.717796 | 1.242637 |
| 150 | 141 | 140 | 87 | 0.949449 | 0.960367 | 1.760387 |
| 200 | 153 | 150 | 97 | 1.103633 | 1.142144 | 2.093592 |
| 250 | 159 | 158 | 104 | 1.288076 | 1.302888 | 2.415703 |
TX-100 redox sites are found using ADPT-based dual descriptor calculations. The dual descriptors or dual Fukui functions are obtained using Fukui functions. Fukui functions can be defined according to Eq. (2)26. Previously we have used the Fukui functions to analyze surfactants28,29.
| 2 |
where N is the number of electrons in the system, ρ(r) is the electron density, + and − signs correspond to the addition or remotion of electrons, respectively. The dual descriptor () is plotted using Eq. (3).
| 3 |
The dual descriptor charts for TX-100 are shown in Fig. 3B22. The blue colour regions in our dual descriptor maps indicate TX-100 oxidation sites, whereas the red colour regions represent TX-100 reduction sites. TX-100 is oxidized via the tail due to the presence of oxygen atoms with lone pair electrons, and it is reduced via the head phenyl ring.
The electrochemical response for DA at PTTMCPE
The voltammetric response of DA (10 µM) at the BCPE, NaOH-PTMCPE, and TX-100PT-MCPE was displayed in Fig. 4. The peak potential difference (ΔEp) was 58 mV at BCPE. However, ΔEp values are 28 mV and 25 mV for NaOH-PT-MCPE and TX-100-PTMCPE respectively. This indicates that pretreated electrodes are good for sensing applications.
Figure 4.

CVs for 10 µM DA in 0.2 M PBS solution of pH 7.4 at pretreated NaOH and TX-100-PT-MCPE at the sweep rate of 100 mV/s. Inset: compares the BCPE and NaOH pretreatment.
Electrochemical investigation of DA with the different parameters at TX-100-PT-MCPE
As shown in Fig. 5A, the effect of change in the ν for 10 µM DA in 0.2 M PBS was studied by CV at TX-100PT-MCPE. The TX-100PT-MCPE demonstrates that as the ν increases the redox peak current steadily increases. The plot of anodic peak current (Ipa) vs ν verifies the type of electrode process (Inset of Fig. 5B). The correlation coefficient was originally set at R2 = 0.9997, however, Fig. 5C depicts a plot of log Ipa vs log ν with 0.8362 slopes. Therefore, the mass transfer process is adsorption controlled30.
Figure 5.

(A) CVs of 10 µM DA in 0.2 M PBS solution of pH 7.4 at TX-100-PT-MCPE at different sweep rates. (a–j; 20–200 mV/s). (B) Graph of peak current versus sweep rate. (C) Graph of the log of peak current versus the log of sweep rate.
Figure 6A displays the CV response of DA at TX-100-PT-MCPE at different concentrations of 10–60 μM using 0.2 M PBS (pH 7.4) with a ν 100 mV/s. With a rise in concentration, the corresponding anodic peak current (Ipa) of DA enhances. Figure 6B shows the curve of Ipa vs DA concentration. It has high linearity with regression equations are Ipa(μA) = 42 (μM) + 0.105 (R2 = 0.9981) and the limit of detection (LOD) and limit of quantification (LOQ) are calculated according to Eqs. (4, 5)30. Where S is the standard deviation and M is the slope obtained from the calibration plot:
| 4 |
| 5 |
Figure 6.
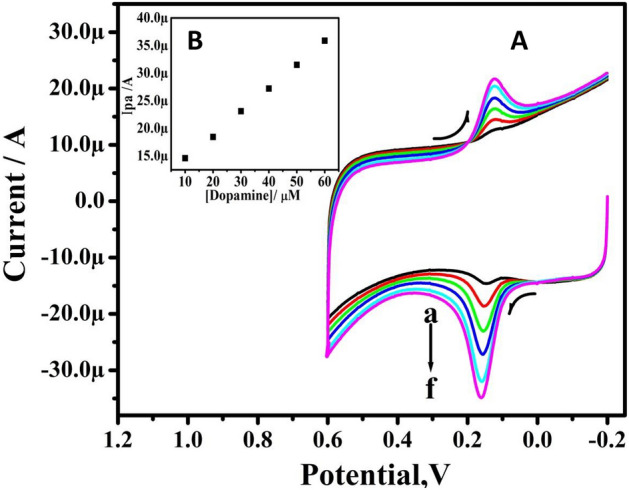
(A) CVs of 10 µM DA in 0.2 M PBS solution of pH 7.4 at TX-100-PT-MCPE at a sweep rate of 50 mV/s with different concentrations (a–f: 10–60 μM). (B) Graph of anodic peak current versus concentration of DA.
The comparative analytical performance electrode for DA was presented in Table 331–47 and LOQ was found to be 4.08 μM.
Table 3.
Comparative analytical performance electrode for DA.
| Sl. no. | Electrodes | Linear range (µM) | LOD (µM) | Method | References |
|---|---|---|---|---|---|
| 01 | Au/Gr-Au | 0.03–30 | 30 | SW | 31 |
| 02 | Pt–Au hybrid | 103–165 | 24 | CV | 32 |
| 03 | CTAB/CPE | 0–130 | 11.0 | DPV | 33 |
| 04 | Fc-MCPE | 120–11,000 | 9.4 | CV | 34 |
| 05 | Poly (Sudan III)/MCPE | 10–60 | 9.3 | CV | 35 |
| 06 | SWCNT/GCE | – | 7.0 | DPV | 36 |
| 07 | Metallothioneins self-assembled gold electrode | 0.08–0.2 | 6.0 | CV | 37 |
| 08 | LDH/CILE | 10–1100 | 5.0 | DPV | 38 |
| 09 | Ag-reduced GO/GCE | 10–800 | 5.4 | LSV | 39 |
| 10 | Poly-VA/MWCNT/GCE | 5–120 | 4.5 | CV | 40 |
| 11 | Ag/Ag2S-CNT-Nafion | 250–2000 | 4.7 | DPV | 41 |
| 12 | BPVCM-e/MWCNT/GCE | 5–5000 | 2.25 | CV | 42 |
| 13 | Poly (amido black) MCPE | 10–60 | 2.03 | CV | 43 |
| 14 | CTAB-GO/MWNT | 5.0–500 | 1.5 | DPV | 44 |
| 15 | TX-100-PT-MCPE | 10–60 | 1.22 | CV | Present work |
| 16 | SiTi/AuNP/CPE | 20–180 | 0.57 | CV | 45 |
| 17 | Ni-Zr/MSN/GCE | 0.3–100 | 0.13 | CV | 46 |
| 18 | PSi NPs modified GCE | 0.5–333.3 | 0.032 | CV | 47 |
The CVs for DA obtained by the TX-100-PT-MCPE at ν of 100 mV/s in PBS of various pH from 6.2 to 7.8 for DA are shown in Fig. 7A. The Ipa of DA changed to a less positive potential as the pH increased. A graph of Epa vs different pH levels was used to create the potential diagram (Fig. 7B). With a slope of 55 mV/pH, the obtained graphs exhibit acceptable linearity with regression equations are EpDA (mV) = − 55 pH + 56 (R2 = 0.9989). The calculated slope is extremely near to the Nernstian value of 59 mV, as shown in the graphs. Therefore, an equal number of protons and electrons are transferred in the reactions (Fig. 7C).
Figure 7.

(A) CVs of 10 µM DA for different pH (6.2–7.8 pH) at TX-100-PT-MCPE. (B) Graph of Epa versus different pH. (C) Redox mechanism dopamine.
Electrochemical investigation of PA with different parameters at TX-100PT-MCPE
A CV profile of 10 μM PA at BCPE and TX-100PT-MCPE in 0.2 M PBS is shown in Fig. 8 (pH 7.4). The TX-100PT-MCPE shows an increase in redox Ipa and a small shift in potential, but the BCPE, PA shows less current and more widespread peaks. Our findings show that following the change, the electrode performs well and lowers ΔE while increasing Ipa. These findings show that the proposed sensor has a catalytic effect on PA analysis.
Figure 8.

CVs of 10 µM PA in 0.2 M PBS solution of pH 7.4 at BCPE and TX-100-PT-MCPE at the sweep rate of 100 mV/s.
At TX-100-PT-MCPE, the effect of ν on the CV performance of the PA was investigated in PBS (pH 7.4). Ipa increased with a slight positive shift in the peak potential displayed in Fig. 9A increased in the region of 20–200 mV/s. In Fig. 9B,C, the current intensity changes in terms of sweep rates are shown in a graph with good linearity. The findings show that the oxidation–reduction process of PA is influenced by adsorption.
Figure 9.

(A) Cyclic voltammograms of 10 µM PA in 0.2 M PBS solution of pH 7.4 at TX-100-PT-MCPE at different sweep rates. (a–j; 20–200 mV/s). (B) Graph of peak current versus sweep rate. (C) Graph of the log of peak current versus the log of sweep rate.
PA CVs at TX-100-PT-MCPE at various concentrations are depicted in Fig. 10A. PA's Ipa hikes with an increase in concentration shown in Fig. 10B. The linearity of the Ipa vs. PA concentration was found high linearity with regression equations are Ipa(μA) = 01 (μM) + 0.15 (R2 = 0.99979) and the limit of detection (LOD) and limit of quantification (LOQ) are calculated according to Eqs. (4, 5) and are found to be 5.031 μM and 16.77 μM respectively.
Figure 10.

(A) CVs of 10 µM PA in 0.2 M PBS solution of pH 7.4 at TX-100-PT-MCPE at a sweep rate of 50 mV/s with different concentrations (a–f: 10–60 μM). (B) Graph of anodic peak current versus concentration of PA.
The CVs profile for PA, at TX-100-PT-MCPE in 0.2 M PBS range 6.2–7.8 is portrayed in Fig. 11A. The Epa was moved negative with an increase in pH values. Figure 11B portrayed the linear establishment between Epa and pH and provide a slope of 57 mV for PA with regression equations are EpDA (mV) = − 57 pH + 58 (R2 = 0.9997). The resulting slope is close to the Nernstian value. Therefore, an equal number of protons and electrons are transferred in the reactions (Fig. 11C).
Figure 11.

(A) Cyclic voltammograms of 10 µM PA for different pH (from a–e; 6.2–7.8 pH) at pretreated TX-100 modified CPE. (B) Graph of Epa versus different pH. (C) Redox mechanism paracetamol.
Selectivity and reproducibility study for TX-100-PT-MCPE
Figure 12A displays the CV responses of 10 µM of DA and 10 µM PA in 0.2 M PBS of both BCPE and TX-100PT-MCPE. The BCPE showed short current signals with poor selectivity indicating that electrode is not selective for simultaneous detection of DA and PA. But after TX-100 pretreatment current signals are enhanced and selectivity was improved (oxidation potential of DA and PA are separated). Therefore, TX-100PT-MCPE can effectively be used to detect DA in presence of PA. The fabrication reproducibility was checked by successively scanning 50 cycle’s depicted in Fig. 12B. The Ip signals retained approximately 90.6% of the original value. The experimental results indicated that the TX-100PT-MCPE possesses a long service life.
Figure 12.
(A) CVs for simultaneous determination for 10 µM DA and 10 µM PA at BCPE (dashed line) and TX-100-PT-MCPE (solid line) at a sweep rate of 100 mV/s. (B) CVs of 1 mM potassium ferrocyanide in 1 M KCl solution at a speed rate of 50 mV/s for stability analysis for TX-100-PT-MCPE at 50 cycles.
Interference study
Figure 13A displays the DPVs of DA at 5–40 µM concentration in presence of 5 µM PA. As a result, the Ipa of DA increases with an increase in DA concentration, and the Ipa of PA remains constant. The obtained results are good as shown in the graph of Ipa versus the concentration of DA (Fig. 13B). This indicates that TX-100-PT-MCPE can selectively sense DA in presence of PA.
Figure 13.
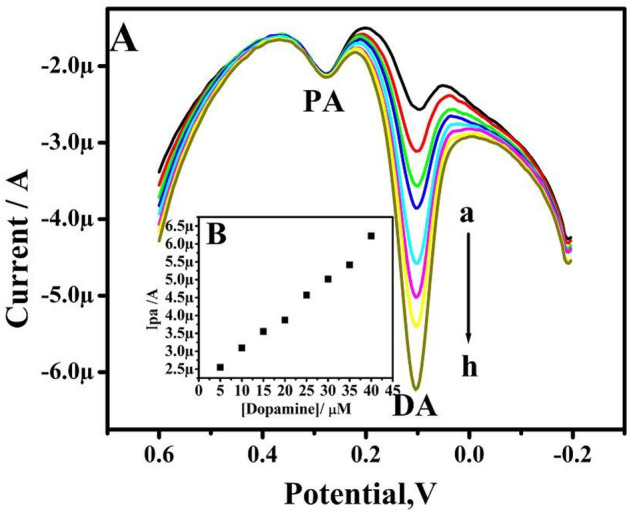
(A) Differential pulse voltammograms were obtained for variation of concentration 5–40 µM (a–h) DA, in PBS (pH 7.4), in the presence of 5 µM PA at TX-100-PT-MCPE. (B) Graph of anodic peak current versus concentration of DA.
Figure 14A demonstrates raising the concentration of PA from 5 to 40 µM while maintaining the DA concentration constant (5 µM). The curve of Ipa vs concentration of PA is depicted in Fig. 14B and it is linear. The result confirms that changing the concentration of one analyte does not affect the peak current and peak potential of another analyte; it also demonstrates the modified carbon paste electrode's outstanding selectivity and sensitivity towards DA and PA.
Figure 14.
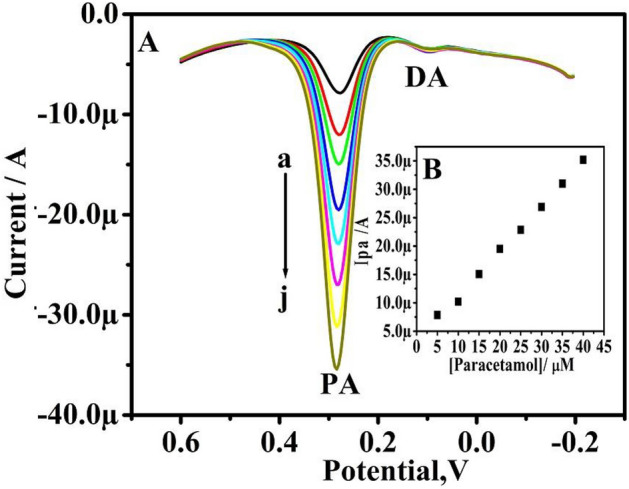
(A) Differential pulse voltammograms obtained for variation of concentration 5–40 µM (a–h) PA, in PBS (pH 7.4), in the presence of 5 µM DA at pretreated TX-100 modified CPE. (B) Graph of anodic peak current versus concentration of PA.
Real sample analysis
For the examination of DA-containing injectable samples and PA tablet samples by standard addition method using TX-100PT-MCPE. The injection samples and DA injection were utilized after a sufficient dilution with a 0.2 M phosphate buffer. Dolo-500, Micro Labs Ltd provided the PA tablet sample, which had a PA 500 mg concentration. The obtained results are tabulated in Table 4. The recovery was acceptable, indicating that the proposed methods may be utilized to detect DA in injections with a recovery rate of 98.20–99.10 percent and a recovery rate of 98.5–99.45 percent for the PA tablet sample.
Table 4.
Detection of DA and PA in the real sample (n = 3).
| Sample | DA added (µM) | Found (µM) | Recovery (%) |
|---|---|---|---|
| DA injections | 10 | 9.84 | 98.4 ± 0.976 |
| 20 | 19.46 | 98.20 ± 0.876 | |
| 30 | 29.73 | 99.10 ± 0.135 | |
| PA tablet | 10 | 9.85 | 98.5 ± 0.911 |
| 20 | 19.69 | 98.45 ± 0.992 | |
| 30 | 29.28 | 97.6 ± 0.997 |
Conclusion
The pretreatment of the CPE with TX-100 in 0.1 M NaOH solution results in a very stable DA sensor with outstanding sensitivity and selectivity. The TX-100PT-MCPE reduced the overpotential and enhanced the current responsiveness. The pH study reveals that the redox mechanism involves an equal quantity of protons and electrons being transferred. This demonstrated a promising biological use. The prepared electrode showed satisfactory stability when kept under ambient conditions. The proposed approach is also used to identify DA in injectable samples and PA in commercial tablet formulations.
Author contributions
J.K.S.K: electrochemical sensors experiments, formal analysis, writing—original draft. B.E.K.S.: conceptualization, supervision, writing—review and editing. G.K.J.: theoretical work, writing—review. S.C.S.: writing—review and editing. R.F.-M.: theoretical work, writing—review. K.M.: writing—review. S.A.H.: writing—review and editing.
Funding
This article was funded by Jain University.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
B. E. Kumara Swamy, Email: bek@kuvempu.ac.in.
S. C. Sharma, Email: scsharma.bangalore@gmail.com
References
- 1.Abdel Rahim AM, Abdel Gaber AA. Fabrication and characterization of extrinsic electrochemically modified graphite reinforcement carbon paste electrode for selective determination of Cu(II) in trace levels. Appl. Surf. Sci. Adv. 2020;2:100031. doi: 10.1016/j.apsadv.2020.100031. [DOI] [Google Scholar]
- 2.Cariati LSS, Buoro RM. Evaluation of ionic natural deep eutectic solvents (NADES) modified binders towards the chemical properties of carbon paste electrodes. Electrochem. Commun. 2019;109:106605. doi: 10.1016/j.elecom.2019.106605. [DOI] [Google Scholar]
- 3.Walid B, et al. Elaboration of carbon paste electrode containing pentadentate Nickel-(II) Schiff base complex: Application to electrochemical oxidation of thiosulfate in alkaline medium. Arab. J. Chem. 2020;13(7):6072–6083. doi: 10.1016/j.arabjc.2020.05.007. [DOI] [Google Scholar]
- 4.Olga V, et al. Use of carbon paste electrode and modified by gold nanoparticles for selected macrolide antibiotics determination as standard and in pharmaceutical preparations. J. Electroanal. Chem. 2020;873:114324. doi: 10.1016/j.jelechem.2020.114324. [DOI] [Google Scholar]
- 5.Mehdi R-N, et al. A modified sensitive carbon paste electrode for 5-fluorouracil based using a composite of praseodymium erbium tungstate. Microchem. J. 2020;154:104654. doi: 10.1016/j.microc.2020.104654. [DOI] [Google Scholar]
- 6.Piovesan JV, Santana ER, Almir S. A carbon paste electrode improved with poly (ethylene glycol) for tannic acid surveillance in beer samples. Food Chem. 2020;326:127055. doi: 10.1016/j.foodchem.2020.127055. [DOI] [PubMed] [Google Scholar]
- 7.Saleh AT, Khaled MM, Abdur R. Electrochemical sensor for the determination of ketoconazole based on gold nanoparticles modified carbon paste electrode. J. Mol. Liq. 2018;256:39–48. doi: 10.1016/j.molliq.2018.02.006. [DOI] [Google Scholar]
- 8.Janata J, Josowicz M. Conducting polymers in electronic chemical sensors. Nat. Mater. 2003;2:19–24. doi: 10.1038/nmat768. [DOI] [PubMed] [Google Scholar]
- 9.Cristina S, et al. Sensitive voltammetry determination of riboflavin in pharmaceutical and biological samples using FSN-Zonyl-Nafion modified carbon paste electrode. Microchem. J. 2020;155:104729. doi: 10.1016/j.microc.2020.104729. [DOI] [Google Scholar]
- 10.Lili Z, Yuhua C, Guangqun C. Electrochemical sensor based on magnetic molecularly imprinted nanoparticles at surfactant modified magnetic electrode for determination of bisphenol A. Biosens. Bioelectron. 2014;54:258–261. doi: 10.1016/j.bios.2013.10.072. [DOI] [PubMed] [Google Scholar]
- 11.Thomas T, et al. Pristine multi-walled carbon nanotubes/SDS modified carbon paste electrode as an amperometric sensor for epinephrine. Talanta. 2014;125:352–360. doi: 10.1016/j.talanta.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 12.Lokhande PE, Pawar K, Chavan US. Chemically deposited ultrathin α-Ni(OH)2 nanosheet using surfactant on Ni foam for high-performance supercapacitor application. Mater. Sci. Energy Technol. 2018;1:166–170. [Google Scholar]
- 13.Biaselle CJ, Millar DB. Studies on triton X-100 detergent micelles. Biophys. Chem. 1975;3:355. doi: 10.1016/0301-4622(75)80029-9. [DOI] [PubMed] [Google Scholar]
- 14.Jayaprakash GK, Flores-Moreno R. Quantum chemical study of TX-100 modified graphene surface. Electrochim. Acta. 2017;248:225–231. doi: 10.1016/j.electacta.2017.07.109. [DOI] [Google Scholar]
- 15.Marzieh M, Shahla E. Colorimetric detection of insulin in human serum using GO/AuNPs/TX-100 nanocomposite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020;240:118617. doi: 10.1016/j.saa.2020.118617. [DOI] [PubMed] [Google Scholar]
- 16.Jayaprakash GK, Kumara Swamy BE, Casillas N, Flores-Moreno R. Analytical Fukui and cyclic voltammetric studies on ferrocene modified carbon electrodes and effect of TX-100 on it. Electrochim. Acta. 2017;258:1025–1034. doi: 10.1016/j.electacta.2017.11.154. [DOI] [Google Scholar]
- 17.Chandrashekara BN, Kumara Swamy BE. Simultaneous cyclic voltammetric determination of norepinephrine, ascorbic acid and uric acid using TX-100 modified carbon paste electrode: A cyclic voltammetric study. Anal. Methods. 2012;4:849. doi: 10.1039/c2ay05632a. [DOI] [Google Scholar]
- 18.Fang J, Xie Z, Wallace G, Wang X. Co-deposition of carbon dots and reduced graphene oxide nanosheets on carbon-fiber microelectrode surface for selective detection of dopamine. Appl. Surf. Sci. 2017;412:131–137. doi: 10.1016/j.apsusc.2017.03.257. [DOI] [Google Scholar]
- 19.Andrea P, Rojas S, Cortes M, Hurtado J. Electrochemical synthesis of poly (3,4-ethylene dioxythiophene) doped with a new bis(pyrazolyl)methane disulfonate and its behaviour towards dopamine detection. J. Electroanal. Chem. 2019;837:200–207. doi: 10.1016/j.jelechem.2019.02.041. [DOI] [Google Scholar]
- 20.Rana A, Baig N, Saleh TA. Electrochemically pretreated carbon electrodes and their electroanalytical applications—a review. J. Electroanal. Chem. 2019;833:313–332. doi: 10.1016/j.jelechem.2018.12.019. [DOI] [Google Scholar]
- 21.Sukanya Swamy BEK, Shashikumara JK, Sharma SC, Hariprasad SA. Poly (Orange CD) sensor for paracetamol in presence of folic acid and dopamine. Sci. Rep. 2021;11:22332. doi: 10.1038/s41598-021-01311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flores-Moreno, R., Urbina, K. P. & Sandoval, Z. G. Sinapsis,Version XII-V. Sinapsis developers, 2012. (Version: Sinapsis. XIII Weblink: http://sinapsis.sourceforge.net. Accessed 20 July 2020.
- 23.Gerald G, et al. demon2k. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012;2:548–555. doi: 10.1002/wcms.98. [DOI] [Google Scholar]
- 24.Perdew JP, et al. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996;77:3865–3868. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 25.Perdew JP, et al. Generalized gradient approximation made simple. Phys. Rev. Lett. 1997;78:1396–1396. doi: 10.1103/PhysRevLett.78.1396. [DOI] [PubMed] [Google Scholar]
- 26.Godbout N, et al. Optimization of Gaussian-type basis sets for local spin density functional calculations. Part I. Boron through neon, optimization technique and validation. Can. J. Chem. 1992;70:560–571. doi: 10.1139/v92-079. [DOI] [Google Scholar]
- 27.Parr RG, Yang W. Density functional approach to the frontier–electron theory of chemical reactivity. J. Am. Chem. Soc. 1984;106:4049–4050. doi: 10.1021/ja00326a036. [DOI] [Google Scholar]
- 28.Jayaprakash GK, et al. Dual descriptor analysis of cetylpyridinium modified carbon paste electrodes for ascorbic acid sensing applications. J. Mol. Liquids. 2021;334:116348/1-17. doi: 10.1016/j.molliq.2021.116348. [DOI] [Google Scholar]
- 29.Jayaprakash GK, Swamy BEK, Sánchez JPM, Li X, Sharma SC, Lee S-L. Electrochemical and quantum chemical studies of cetylpyridinium bromide modified carbon electrode interface for sensor applications. J. Mol. Liq. 2020;315(113719):1–10. [Google Scholar]
- 30.Shashikumara JK, Kalaburgi B, Swamy BEK, Nagabhushana H, Sharma SC, Lalitha P. Effect of RGO-Y2O3 and RGO-Y2O3:Cr3+ nanocomposite sensor for dopamine. Sci. Rep. 2021;11:9372/1–11. doi: 10.1038/s41598-021-87749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pruneanu S, Biris AR, Pogacean F, Socaci C, Coros M, Rosu MC, Watanabe F, Biris AS. The influence of uric and ascorbic acid on the electrochemical detection of dopamine using graphene-modified electrodes. Electrochim. Acta. 2015;154:197. doi: 10.1016/j.electacta.2014.12.046. [DOI] [Google Scholar]
- 32.Thiagarajan S, Chen SM. Preparation and characterization of Pt Au hybrid film modified electrodes and their use in the simultaneous determination of dopamine, ascorbic acid and uric acid. Talanta. 2007;74(2):212–222. doi: 10.1016/j.talanta.2007.05.049. [DOI] [PubMed] [Google Scholar]
- 33.Corona-Avendano S, Ramirez-Silva MT, Palomar-Pardave M, Hernandez-Martinez L, Romero-Romo M, Alarcon-Angeles G. Influence of CTAB on the electrochemical behaviour of dopamine and its analytic determination in the presence of ascorbic acid. J. Appl. Electrochem. 2010;40:463. doi: 10.1007/s10800-009-0017-x. [DOI] [Google Scholar]
- 34.Ali Kamyabi M, Aghajanloo F. Electrocatalytic response of dopamine at a CPE modified with ferrocene. Roat Che Act. 2009;82:599–606. [Google Scholar]
- 35.Sunil Kumar Naik TS, Muthui Martin M, Kumara Swamy BE. Fabrication of poly (Sudan III) modified carbon paste electrode sensor for dopamine: A voltammetric study. J. Electroanal. Chem. 2018;834:73. [Google Scholar]
- 36.Goyal RN, Singh SP. Simultaneous voltammetric determination of dopamine and adenosine using a single-walled carbon nanotube—MCPE. Carbon. 2008;46:1556–1562. doi: 10.1016/j.carbon.2008.06.051. [DOI] [Google Scholar]
- 37.Wang Q, Li N, Wang W. Electrocatalytic response of dopamine at a metallothioneins self-assembled gold electrode. Anal. Sci. 2002;18:635. doi: 10.2116/analsci.18.635. [DOI] [PubMed] [Google Scholar]
- 38.Zhu Z, Lining Qu, Guo Y, Zeng Y, Sun W, Xintang H. Electrochemical detection of dopamine on a Ni/Al layered double hydroxide modified carbon ionic liquid electrode. Sens. Actuators B. 2010;151:146–152. doi: 10.1016/j.snb.2010.09.032. [DOI] [Google Scholar]
- 39.Kaur B, Pandiyan T, Satpati B, Srivastava R. Simultaneous and sensitive determination of ascorbic acid, dopamine, uric acid, and tryptophan with silver nanoparticles-decorated reduced graphene oxide modified electrode. Colloids Surf. B. 2013;111:97–106. doi: 10.1016/j.colsurfb.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 40.Silva LVD, Silva FAS, Kubota LT, Lopes CB, Lima PR, Costa EO, Junior WP, Goulart MOF. Amperometric sensor based on carbon nanotubes and electropolymerized vanillic acid for simultaneous determination of ascorbic acid, dopamine, and uric acid. J. Solid State Electrochem. 2016;20:2389–2393. doi: 10.1007/s10008-016-3129-3. [DOI] [Google Scholar]
- 41.Chih YK, Yang MC. Simultaneous detection of dopamine and ascorbic acid using silver. Taiwan Inst. Chem. Eng. 2014;45:833–839. doi: 10.1016/j.jtice.2013.09.005. [DOI] [Google Scholar]
- 42.Liu R, Zeng XB, Liu JC, Luo J, Zheng YY, Liu XY. A glassy carbon electrode modified with an amphiphilic, electroactive and photosensitive polymer and with multi-walled carbon nanotubes for simultaneous determination of dopamine and paracetamol. Microchim. Acta. 2016;183:1543–1551. doi: 10.1007/s00604-016-1763-1. [DOI] [Google Scholar]
- 43.Shashikumara JK, KumaraSwamy BE, Madhuchandra HD. Poly (amido black) modified carbon paste electrode sensor for dopamine in the presence of uric acid. Mater. Sci. Energy Technol. 2020;3:390–396. [Google Scholar]
- 44.Yang YJ, Li WK. CTAB functionalized graphene oxide/multiwalled carbon nanotube composite modified electrode for the simultaneous determination of ascorbic acid, dopamine, uric acid and nitrite. Sens. Actuators B Chem. 2014;56:300–306. doi: 10.1016/j.bios.2014.01.037. [DOI] [PubMed] [Google Scholar]
- 45.de Matos Morawski F, Xavier BB, Virgili AH, dos Santos Caetano K, de Menezes EW, Benvenutti EV, Arenas LT. A novel electrochemical platform based on mesoporous silica/titania and gold nanoparticles for simultaneous determination of norepinephrine and dopamine. Mater. Sci. Eng. C. 2021;120:111646. doi: 10.1016/j.msec.2020.111646. [DOI] [PubMed] [Google Scholar]
- 46.Roduan MRAM, Saidin MI, Sidik SM, Abdullah J, Isa IM, Hashim N, Bahari AA. New modified mesoporous silica nanoparticles with bimetallic Ni-Zr for electroanalytical detection of dopamine. J. Electrochem. Sci. Eng. 2022;12:463–474. [Google Scholar]
- 47.Ahmed J, Faisal M, Harraz FA, Jalalah M, Alsareii SA. Development of an amperometric biosensor for dopamine using novel mesoporous silicon nanoparticles fabricated via a facile stain etching approach. Phys. E. 2022;135:114952. doi: 10.1016/j.physe.2021.114952. [DOI] [Google Scholar]




