Abstract
Endocannabinoids (eCBs) are lipid neuromodulators that suppress neurotransmitter release, reduce postsynaptic excitability, activate astrocyte signaling, and control cellular respiration. Here, we describe canonical and emerging eCB signaling modes and aim to link adaptations in these signaling systems to pathological states. Adaptations in eCB signaling systems have been identified in a variety of biobehavioral and physiological process relevant to neuropsychiatric disease states including stress-related disorders, epilepsy, developmental disorders, obesity, and substance use disorders. These insights have enhanced our understanding of the pathophysiology of neurological and psychiatric disorders and are contributing to the ongoing development of eCB-targeting therapeutics. We suggest future studies aimed at illuminating how adaptations in canonical as well as emerging cellular and synaptic modes of eCB signaling contribute to disease pathophysiology or resilience could further advance these novel treatment approaches.
Subject terms: Neuroscience, Cellular neuroscience
Introduction
Interest in all things “cannabinoid” has grown dramatically over the past two decades. Understanding potential therapeutic and adverse effects of cannabis and elucidating long-term consequences of in utero exposure, for example, are subjects of intense and growing interest within the scientific community and public health sectors. Paralleling these important investigations, a rapidly advancing field of study aimed at elucidating the physiology and therapeutic potential of endogenous cannabinoid (eCB) signaling systems as emerged: beginning with the discovery of cannabinoid receptors, eCB ligands, and the subsequent elucidation of canonical eCB retrograde synaptic signaling and arriving at early clinical trials of eCB modulating compounds for a variety of psychiatric and neurological disorders. Here, we aim to link advances in our understanding of eCB signaling at the cellular and synaptic level with adaptations in these signaling systems under a variety of conditions relevant to neuropsychiatric illnesses. Elucidating environmental, genetic, and experience-dependent adaptations in eCB signaling could provide insights into the physiology of stress reactivity, feeding, and motivation, and could reveal pathophysiological mechanisms contributing to, and potential treatment opportunities for, autism spectrum disorders, substance use disorders, and epilepsy, amongst others. Dysregulation in eCB function have been implicated in numerous additional behavioral domains including sleep [1] and pain [2], for example, and have been reviewed thoroughly elsewhere. Here, we begin by describing canonical synaptic eCB signaling and emerging modes of signaling being discovered at the cellular level. We then describe how eCB signaling and adaptations in this system relate to a series of fundamental disease-relevant biobehavioral processes. When applicable, we highlight how understanding adaptations in eCB signaling can provide insight into the pathophysiology of disease or reveal new opportunities for therapeutic intervention.
Biochemistry and pharmacology of eCB signaling
Molecular and biochemical roots of endocannabinoid signaling
Extensive reviews of the history of cannabinoid receptors, as well as their endogenous and exogenous ligands, have been published [3, 4]. Briefly, the eCB system is comprised of the two primary cannabinoid receptors (CB1R and CB2R) and endogenous ligands 2-arachidonylglycerol (2-AG) and anandamide (AEA) [5]. The CB1R and CB2R are generally Gi/o-coupled receptors expressed throughout the CNS and periphery. While CB1Rs are enriched within the nervous system, CB2Rs are primarily expressed within immune cells and to a lesser degree within some CNS cell types. Both receptors are membrane bound and subject to internalization and desensitization via C-terminal phosphorylation [6]. Lipid rafts also play an important role in the localization of CB1Rs [7, 8], in particular in their endocytosis, as cholesterol depletion has been shown to improve ligand binding and increase CB1R localization at the plasma membrane [9, 10].
Several eCB ligands have been discovered that bind and activate these cannabinoid receptors including 2-AG and AEA. 2-AG is synthesized from diacylglycerol (DAG) via sn1-specific DAG lipase-α (DAGL α) and lipase-ß (DAGL ß) [11]. Synthesis of AEA is mediated via the formation of N-arachidonoyl phosphatidylethanolamine (NAPE) from phosphatidylethanolamine and phosphatidylcholine via a calcium-dependent transacylase enzyme followed by phospholipase-D-mediated conversion to AEA. However, additional redundant pathways of AEA synthesis have been suggested [12]. Unlike classical neurotransmitters, the majority of eCB production is de novo or “on-demand,” and release occurs via non-vesicular mechanisms, though in some cases eCBs may be excreted from microglial cells via exosomes [13]. Following their release, 2-AG and AEA are primarily metabolized to arachidonic acid via monoacylglycerol lipase (MAGL) and fatty acid amide hydrolase (FAAH), respectively [14, 15]. Additional eCB-metabolizing enzymes include α,ß-hydrolase domain-containing 6 (ABHD6) and ABHD12 for 2-AG, as well as cytochrome p450 enzymes, lipoxygenases, and cyclooxygenase 2 for both. Degradation of AEA and 2-AG occurs at post- and presynaptic compartments in the brain, respectively [16]. However, some 2-AG degrading enzymes such as ABHD6 and ABHD12 are postsynaptically localized at intracellular and extracellular sites, respectively [17], supporting their potential role in limiting cellular/synaptic 2-AG signaling [18].
In addition to the CB1R and CB2R, the eCBs act as endogenous ligands for several other receptors. Most notably, AEA acts as a ligand for the transient receptor potential vanilloid 1 receptor (TRPV1) [19–21]. The de-orphaned receptor, GPR55, may also be a target for AEA, albeit through a non-GTPγS binding mechanism with significant heterogeneity in activity in various biological systems [22]. Finally, some data have highlighted the potential role of 2-AG as a ligand for the GABAA receptor acting via allosteric potentiation [23]. Elucidation of the molecular constituents of eCB signaling systems has opened the door to the development of numerous pharmacological tools to investigate the physiological roles of eCB signaling and validating eCB-related molecular targets for therapeutic intervention.
Pharmacological modulation of eCB signaling
∆9-tetrahydrocannabinol (∆9-THC) is the most well-known exogenous cannabinoid and is a partial agonist at the CB1R and CB2R [4]. Structure-activity relationship research later led to the identification of many highly potent ligands for the CB1R and CB2R such as CP55940 and Win55212-2 [24]. The first CB1R antagonist (inverse agonist), SR141716A [25], was developed by the French pharmaceutical company, Sanofi Recherche, while several years later the CB2R-selective antagonists, AM630 and SR144528, were identified [26]. Since then, positive and negative allosteric modulators (PAMs/NAMs) [27–30], as well as biased ligands, which preferentially engage specific downstream signaling pathways, have also been developed for cannabinoid receptors. In fact, both orthosteric [31, 32] and allosteric [33–35] ligands have been found to act via distinct intracellular second messenger systems.
Aside from direct agonist/antagonists, manipulation of eCB synthetic and degrading enzymes has offered further insights into the functions of eCB signaling in several physiological processes [36]. The modulation of eCB synthesis and degradation has been employed for both in vitro and in vivo investigations. Indeed, potent, selective, and brain penetrant or peripherally restricted inhibitors of the AEA degrading enzyme, FAAH [37], have been extensively investigated for almost two decades [38]. More recently, inhibitors of MAGL have provided substantial insights into the therapeutic potential of 2-AG augmentation for a variety of neuropsychiatric conditions [39]. Importantly, manipulation of eCB synthesis or degradation pathways impact additional non-eCB signaling molecules. For instance, inhibition of MAGL to elevate 2-AG (and other monoacylglycerols) results in decreased free arachidonic acid [40], thus impacting the synthesis of prostaglandins that modulate neuroinflammation and immune function in a non-eCB-dependent manner. Most recently, inhibitors of 2-AG and AEA synthesis have been developed [15] and provided critical experimental data on the importance of distinct eCB ligands in the regulation of appetite, stress responsivity, and motivation. For example, DO34 is a covenant DAGL inhibitor that causes rapid and profound reductions in 2-AG (and other monoacylglycerol) levels in the brain, impairs synaptic 2-AG signaling, and has provided important insights into the roles of 2-AG in regulation of reward, stress reactivity and food intake [41]. While a single pathway for AEA synthesis has not been considered dogmatic, the discovery of the NAPE-PLD inhibitor LEI-401, which reduces N-acylethanolamine levels (including AEA) in vitro and in vivo and recapitulates effects of a CB1R antagonist in vivo, indicated that NAPE-PLD inhibition may represent a promising means of manipulating AEA synthesis [42]. Despite these important advances, issues related to potency and selectivity remain caveats of currently available tools. Medicinal chemistry efforts to develop eCB-modulating compounds have been, and will undoubtedly continue to be, critical to the advancement of eCB-based therapeutics, which are at various stages of development for several CNS indications [4].
Endocannabinoid signaling at the synapse and beyond
Synaptic eCB signaling
eCBs are well known to act as retrograde synaptic messengers capable of inducing both short- and long-term forms of synaptic suppression (Fig. 1). The role of eCBs as mediators of short-term plasticity was first identified in the scheme of depolarization-induced suppression of inhibition (DSI) wherein postsynaptic depolarization produces a transient (10–60 s) presynaptic inhibition of GABA release [43, 44]. An analogous form of short-term plasticity, depolarization-induced suppression of excitation (DSE), occurs at excitatory synapses [45, 46]. Mechanistically, postsynaptic depolarization increases intracellular Ca2+ via voltage-gated Ca2+ channels, thereby inducing the rapid synthesis and release of 2-AG followed by presynaptic activation of inhibitory CB1Rs. This phenomenon has been identified in a wide range of brain regions including hippocampus, amygdala, neocortex, cerebellum, and ventral tegmental area (VTA), amongst others [47]. Furthermore, activation of several GPCRs, including group-1 metabotropic glutamate receptors (mGlu1 and mGlu5) and muscarinic acetylcholine receptors (mAChR M1/3), can trigger 2-AG release in a calcium-independent manner and augment DSI/DSE [48–52]. Finally, a variety of ligand-gated ion channels allowing the passage of Ca2+ into the postsynaptic neuron are known to participate in short-term plasticity of this nature, including NMDA, kainate, Ca2+-permeable AMPA, and TRPV1 receptors [53–57].
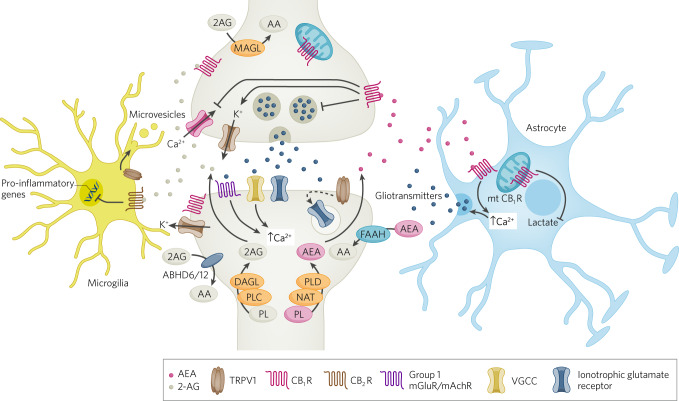
Fig. 1. Cellular aspects of eCB signaling.
Schematic diagram of eCB signaling elements within synapses, astrocytes and microglia. At the synapse, eCBs are synthesized postsynaptically, through a variety of mechanisms that result in increases in intracellular calcium, and activate presynaptic CB1 receptors to mediate retrograde suppression of neurotransmitter release. AEA can also activate TRPV1 receptors to initiate postsynaptic LTD via glutamate receptor internalization. CB1 receptors expressed on astrocytes can lead to increases in calcium release and release of gliotransmitters, which can indirectly affect synaptic transmission. CB2 receptors on microglia can regulate cytokine release. CB1 receptors expressed on mitochondria can also regulate a variety of intracellular processes including cellular energetics.
In addition to short-term plasticity such as DSI/DSE, eCBs participate in several forms of long-term synaptic plasticity, including long-term potentiation (LTP) and long-term depression (LTD) [58–60], metaplasticity [61–64], and synaptic scaling [65, 66]. Like DSI/DSE, eCB-LTD/LTP are primarily postsynaptically induced and presynaptically expressed. eCB-LTP is often a heterosynaptic form of plasticity, characterized by the long-lasting disinhibition secondary to eCB-mediated LTD of inhibitory GABAergic inputs (eg. [67–69], though may also be homosynaptic, mediated via TRPV1 receptor or CB1R activation [70–72]). Similarly, eCB-LTD may be either homo- or heterosynaptic and occurs at both glutamatergic and GABAergic synapses [67, 73–76]. These long-lasting forms of plasticity may be induced via forms of afferent-only protocols such as low-frequency, high-frequency, or theta-burst stimulation [67, 77, 78], spike-timing dependent [79, 80], or input-timing dependent stimuli [81], as well as through the activation of some Gq-coupled GPCRs [82–85].
In addition to these well-established and ubiquitous forms of eCB plasticity mediated via post-synaptic synthesis and release of eCBs (i.e., retrograde transmission to modify presynaptic release), several non-canonical forms of eCB plasticity have subsequently been identified. For example, a form of slow self-inhibition has been identified and attributed to activation of postsynaptic CB1Rs following autocrine release of 2-AG in neocortical GABA neurons [86, 87] and potentially in glutamatergic excitatory neurons [88]. Additionally, a form of LTD mediated by postsynaptic TRPV1 was identified at excitatory synapses onto hippocampal neurons [89] and further defined to require endogenous AEA release [90]. Similar TRPV1-mediated LTD induced by AEA release has been identified at somatic, but not dendritic inhibitory synapses of the dentate gyrus [91].
Given the on-demand nature of eCB synthesis and release, initial conceptualizations focused on the phasic nature of eCB signaling that enables short-term synaptic suppression and triggers eCB-LTD. However, more recent studies have identified sustained, or “tonic,” actions of eCBs at synapses. In contrast to the phasic action of eCBs, tonic CB1R-mediated suppression of neurotransmitter release can occur two ways: either by constitutive eCB synthesis and release or by constitutively active intracellular CB1R signaling cascades in the absence of agonist binding. Importantly, both AEA and 2-AG can be released on a tonic manner in some cases, and tonic signaling can co-exist with phasic signaling at the same synapses [92]. While most of the physiological aspects of eCB signaling have generally been attributed to synaptic eCB signaling, other forms of signaling including astrocytic and mitochondrial eCB signaling have begun to be implicated in some physiological and behavioral effects of cannabinoids and are discussed below.
Synaptic transport of eCBs
While the mechanisms of eCB signaling, synthesis, and degradation have been well defined, intracellular and intercellular transport of eCBs remains poorly understood [93]. Two prevailing models of eCB transport across the lipid bilayer of the postsynaptic membrane posit either a simple [94, 95] or facilitated diffusion [96, 97]. The uncharged nature of eCBs, permitting diffusion across protein-free bilayers [98–100], as well as the non-tissue-dependent and non-saturable characteristics of transmembrane diffusion [95, 101, 102] support a passive or simple model of diffusion. Conversely, the use of structural eCB analogs which target proteins associated with both transport and metabolism [96, 97, 103–106] and their action primarily at the plasma membrane [107, 108] have provided support for a facilitated transport model. Small molecule inhibitors of the putative eCB membrane transporter may shed further light on this discrepancy in future studies [106, 109, 110].
Once released, eCBs rapidly traverse the synaptic cleft to activate presynaptic CBRs. The synaptic cleft presents a hydrophilic/lipophobic barrier, suggesting some form of active intercellular transport could facilitate retrograde eCB synaptic signaling. Recent studies have found transmembrane secretion of fatty-acid biding proteins (FABPs) [111–113], and specifically FABP5 released by astrocytes [114], as potential candidates. Indeed, disruption of FABP5 inhibits synaptic 2-AG signaling [114]. An alternative or complementary mechanism for the synaptic transport of eCBs is the employment of extracellular vesicles. Indeed, isolated extracellular vesicles from activated microglia have been found to be enriched in AEA and, when added to a primary culture of GABAergic neurons, inhibit synaptic transmission through CB1R-dependent mechanisms [13]. Further data have identified a G-protein-dependent mechanism associated with the Sigma-1 receptor that regulates extracellular vesicle trafficking [115]. Disruption of this mechanism was found to suppress retrograde 2-AG transmission in GABAergic neurons without altering tonic eCB release, indicating potentially divergent synaptic transport mechanisms for tonic and phasic synaptic eCB signaling. Identification of proteins and processes involved in eCB transport could represent additional molecular targets for drug development.
Astrocytic eCB signaling
Astrocytes represent a large population of glial cells that plays an active role in synaptic transmission and plasticity (reviewed in [116]). Several lines of investigation have elucidated a wide spectrum of molecular signaling candidates that enable communication between neurons and astrocytes [116]. Ultrastructural analyses indicate regional variability in the abundance of astrocytic CB1Rs but also demonstrate considerable spatial overlap with neuronal CB1Rs in the synaptic and perisynaptic space across different brain areas [117, 118]. Activation of astrocytic CB1R modulates synaptic function through a myriad of signaling strategies (reviewed in [119]). In addition to the expression of CB1R, astrocytes also synthesize eCBs [120, 121]. Accumulating evidence highlights the sufficiency, and circuit specificity, of astrocytic CB1R downstream signaling in modulating synaptic function, circuit excitability and behavioral outputs such as sleep [122], learning and memory [118, 123, 124].
Unlike the widespread downstream effects of neuronal CB1R activation that lead to dampening of presynaptic neurotransmitter release, CB1R activation in astrocytes leads to an increase in intracellular Ca2+ which eventually culminates in the release of various neuroactive compounds termed gliotransmitters (e.g., glutamate, serine, and adenosine) [124, 125] (Fig. 1). Indeed, an intriguing aspect of astrocytic CB1R is the downstream signaling pathway: unlike the neuronal CB1R (associated with Gαi/o proteins) [60], astrocytic CB1Rs are associated with Gq-protein activation, which leads to an increase in intracellular calcium in an IP3R-dependent manner [126]. Depending on the target synapses and neuronal activity pattern, these gliotransmitters can lead to potentiation or depression of the synaptic transmission. For instance, studies by Navarrete and Araque showed that CB1R-mediated release of glutamate from astrocytes in the hippocampus leads to potentiation of excitatory synaptic transmission through presynaptic mGlu1 activation [126, 127]. Subsequent investigations have highlighted that synaptic plasticity mediated via astrocytic CB1R activation can significantly affect hippocampal-dependent learning and memory function. Selective deletion of CB1Rs from the astrocytes results in impairment of spatial working memory and hippocampal LTD [118]. Activation of astrocytic CB1Rs leads to the release of D-serine onto CA1 synapses, which supports NMDA receptor activation and LTP induction. Selective CB1R deletion from astrocytes impairs memory in object recognition task and LTP induction in CA1 [123]. Conversely, adenosine released by astrocytes in response to the activation of the CB1R acts on type-1 Adenosine receptor (A1R) eventually reducing presynaptic neurotransmitter release [128]. In addition, a study by Martin-Fernandez and colleagues showed that astrocytic CB1R activation in centro-medial amygdala (CeM) increases intracellular calcium signaling and release of adenosine. On the one hand, this enhances inhibitory transmission in the centro-lateral amygdala to CeM pathway through adenosine receptor type-2A activation and, on the other hand, depressed excitatory synapses in the BLA to CeM pathway through A1R [124]. This pathway-specific synaptic modulation led to a reduction in learned fear expression in rodents [124]. Therefore, alongside the neuronal CB1Rs, astrocytic CB1Rs form another key facet of the eCB signaling pathway that can influence circuit dynamics, shape network output, and modulate behavior.
Mitochondrial CB1 receptor signaling and function
The functional impact of CB1Rs on synaptic transmission has been largely focused around its presence on the plasma membrane. However, CB1Rs in sub-cellular compartments, such as mitochondria, has been increasingly investigated (Fig. 1). A study by Bartova and Birmigham in 1976 is one of the earliest reports elucidating the effects of Δ9-THC on mitochondrial NADH-oxidase activity in several brain areas [129]. Since then, a growing number of studies have reported the presence of CB1Rs in the mitochondria of several non-neuronal tissues such as cardio-myocytes, hepatic cells and adipocytes. [130]. It is only in the last decade that studies have demonstrated the presence of CB1Rs in brain mitochondria in both neurons and astrocytes and underscored its role in modulating neuronal activity and synaptic transmission [131–133]. Approximately 10–15% of the total CB1R labeling in hippocampus is on mitochondrial membranes [132]. Mitochondrial CB1Rs (mtCB1Rs) are Gαi-protein coupled receptors, the activation of which leads to a reduction in cellular respiration through inhibition of the electron transfer system in an adenylyl cyclase-protein kinase A-dependent manner [131]. An in vitro assessment using purified rodent hippocampal mitochondria has demonstrated that CB1R agonists reduced the activity of mitochondrial respiratory complex 1 [131]. Concomitantly, activation of intracellular mtCB1Rs in hippocampal CA1 pyramidal neurons may be necessary for regulating presynaptic GABA release through DSI [131]. In addition to the presence of CB1Rs, the same study found MAGL in mitochondria, which plays an important role in regulating levels of 2-AG [131]. The activation of mtCB1Rs leads to inhibition of field excitatory post-synaptic currents in the hippocampal CA3-CA1 pathway and is sufficient to induce cannabinoid-induced amnesia as observed through an impairment in novel object recognition [134]. The modulation of energetics and metabolism by exogenous cannabinoids and eCBs through their action at mtCB1Rs is not restricted to neurons. Activation of mtCB1Rs in astrocytes reduces glycolytic capacity and lactate production which eventually causes metabolic stress in nearby neurons, thereby affecting network output leading to social behavioral deficits [135]. However, only approximately 11–13% of astrocytic mitochondria were found to be positive for CB1R [132]. Since astrocytes are the primary source of lactate available to the neurons for their metabolic demands [136], these findings highlight the potential impact that astrocytic mtCB1Rs might have in influencing neuronal activity in health and disease.
Functional implications of eCB signaling in neuropsychiatric disorders
Network effects and neural oscillations
Neural oscillations, particularly in the gamma range, have been associated with sensory perception, attention, and object recognition, amongst other functions. Gamma oscillations interact with theta oscillations, particularly in the hippocampus and neocortex, and play an essential role in spatial, episodic and working memory, both in animal models and humans [137, 138]. Neural oscillations are primarily orchestrated by GABAergic interneurons, and CB1Rs, being highly expressed on a subset of large cholecystokinin-expressing GABAergic interneurons, are well placed to regulate neural oscillations and thereby influence behavioral and cognitive manifestations of brain oscillatory activity. Consistent with this anatomical distribution, the CB1R agonist CP55490 can reduce 40 Hz power in kainate-induced synchronous gamma oscillation in vitro in the hippocampal slices [139]. Additionally, in vivo THC and CP55940 administration in rodents reduces neuronal synchrony, reduces power of local field potentials in various frequency bands in a dose dependent manner, and does so without substantial changes in average firing rates of neurons [140]. Recurrent exposure to cannabinoids in adolescence, but not during adulthood, has been shown to reduce pharmacologically-induced theta and gamma oscillations in rodents [141]. A separate study found that repeated stimulation of CB1Rs during adolescence leads to a state of disinhibition in the prefrontal cortex, elicited by downregulation of the GABAergic inhibitory network [142]. This could explain the increased vulnerability towards development of impairments in neural oscillatory activity and behavioral deficits observed with cannabinoid overuse during development. Acute cannabis use disrupts working and episodic memory in human subjects with a concomitant reduction in theta power and stimulus-locked event-related potentials (ERPs), which are a proxy of neuronal activity [143]. Chronic cannabis consumption alters broadband neural oscillations in humans, particularly in gamma range [144]. EEG recordings in humans have shown that intravenous administration of THC, at concentrations that induce psychotomimetic effects, disrupts gamma range synchronization, which is inversely related to positive psychotic symptom severity [145]. Studies showing gamma band abnormalities in schizophrenia patients raise the possibility that THC-induced psychotomimetic effects may be related to gamma alterations [137]. Similarly, intravenous THC can reduce theta power and theta coherence between bi-frontal brain regions, which strongly correlates with positive psychotic symptoms [146]. Thus, CB1R agonists can cause disruptions in network oscillations relevant to the subjective effects of cannabis intoxication. The degree to which specific CB1R populations and synaptic mechanisms contribute to these alterations is not well understood and represents an important area for continued investigation.
Seizures and epilepsy
The eCB system is heavily implicated in the pathophysiology of epileptic seizures (reviewed in [147–149]). CB1Rs are known to reduce glutamatergic transmission at hippocampal synapses [150, 151] and thus have been examined for their role in the regulation of epileptiform activity in a variety of model systems. For example, in a pilocarpine model of temporal lobe epilepsy, CB1R expression is increased in the dentate gyrus of mice and CB1R activation reduces the increased spontaneous glutamatergic currents and excessive recurrent excitatory transmission induced by glutamate uncaging in dentate gyrus granule neurons [152]. Similarly, epileptiform activity in the hippocampus has been shown to be reduced by CB1R activation, while CB1R blockade increased epileptiform activity in vivo [153] and in vitro [154, 155]. Importantly, mice lacking DAGLα show increased susceptibility to kainate-induced seizures while 2-AG augmentation suppressed excitatory input to the hippocampus granule cells, decreasing excitability and reducing seizure severity [156]. Other studies have demonstrated that increasing 2-AG levels slows the progression of epileptogenesis [157]. Consistent with these data, mice lacking CB1Rs on glutamatergic axon terminals exhibit more severe behavioral consequences in a kainate model of epilepsy [158]. Combined with data demonstrating that seizure activity is associated with protective increases in 2-AG levels [153], these data suggest that eCB, and specifically 2-AG augmentation, could represent a viable approach to the treatment of seizure disorders.
While the above cited data support the notion that 2-AG- and CB1R-mediated suppression of glutamatergic transmission may improve physiological and behavioral consequences of seizures, CB1Rs are also heavily expressed by subsets of GABAergic neurons. Indeed, eCB-mediated suppression of GABA release has been demonstrated in human tissue derived from resected localized epileptogenic lesions [159]. However, lack of control samples in this study precludes conclusions related to effects of epilepsy on eCB synaptic signaling. In an animal model of developmental seizures induced by hyperthermia, DSI is enhanced in hippocampal CA1 neurons [160, 161]. In this model, CB1Rs were also upregulated, and blockade of CB1 receptors during hyperthermic seizure induction prevented later enhancement of DSI, CB1R upregulation, and increases in network excitability. Thus, pathological upregulation of CB1R and eCB signaling at GABAergic synapses could contribute to the pathogenesis of seizure disorders.
The recent development of optical sensors for endocannabinoids (GRABeCB 2.0), enabling quantification of activity induced release of eCBs with high temporal and spatial resolution [162], has highlighted a potentially more complex role for 2-AG in the regulation of seizure-related consequences. Specifically, an otherwise tight spatiotemporal regulation of 2-AG in the hippocampus is lost during seizures [163] and dysregulated increase in 2-AG during seizures leads to prostaglandin-dependent post-ictal hypoxia that is associated with post seizure behavioral deficits [163]. Therefore, temporal precision of therapeutic 2-AG modulation could be a key factor in designing interventions to counteract seizures.
Additionally, recent investigations utilizing non-human primate models of epilepsy have been instrumental in dissecting the temporal profile of eCB dynamics during epileptogenesis. A recent study utilizing PET imaging during electrical kindling in the amygdala of rhesus monkeys found regional increases in CB1R binding during seizures, consistent with rodent literature cited above [164]. However, these data contrast with a clinical report that showed selectively downregulated CB1R in glutamatergic axon terminals in the dentate gyrus [165]. These authors also showed that DAGLα was reduced in sclerotic tissue from patients with epilepsy and suggest that the protective eCB system is impaired in the hippocampus of patients with temporal lobe epilepsy. Thus, there may be significant differences between model systems of epilepsy and patients with severe long-term seizure disorders.
Patients with mesial temporal lobe epilepsy (MTLE) exhibit alterations in levels of 2-AG and AEA and their synthetic enzymes in brain tissue collected during the postictal phase of epileptic seizures [156, 166]. Although several studies have reported a reduction in 2-AG levels and DAGL mRNA from MTLE patients compared to controls, there is significant variability in the reported changes in AEA levels. While the study by Rocha and colleagues shows a significant increase in the temporal lobe AEA of MTLE patients [167], a contrasting report demonstrates a reduction in circulating AEA in the CSF collected from MTLE patients [168].
These data support the notion that eCBs acutely suppress epileptiform activity and likely protects against the epileptogenesis via suppression of glutamatergic transmission within hippocampal circuits. While the role of eCB signaling at GABAergic synapses remains less clear, it may counteract this protective effect. Importantly, while CB1R upregulation is a consistent feature of rodent and non-human primate model systems, the degree to which this extends to clinical populations is unclear, and work demonstrating reduced CB1R expression in patients with sclerotic disease suggests impairment in eCB signaling systems may in fact contribute to disease etiology or progression. Future studies will be required to test this hypothesis. To summarize, the eCB system undergoes multifaceted alterations depending on the brain-region, cell type, seizure phase under investigation and the kind of model used. To complicate matters further, these alterations can, in principle, achieve different end points and hence appear counterproductive. Understanding the spatiotemporal specificity of eCB signaling is crucial, as this would allow investigators to make and test reasonable predictions on the net effect of a particular intervention, and aid in development of targeted, effective and safe therapeutic intervention.
Stress
Stress is a major risk factor for the development and exacerbation of major psychiatric disorders including major depression, anxiety disorders, psychotic disorders, and substance use disorders. Understanding how environmental stressors affect brain structure and function to impact biobehavioral processes relevant to psychiatric illness could provide new insight into the pathophysiology of stress-exacerbated disorders and reveal potential novel treatment approaches. Indeed, several lines of evidence support widespread adaptations in central eCB signaling in response to stress and have revealed potential therapeutic opportunities to mitigate the adverse consequences of stress and promote resilience. Here we focus on two key brain regions critically involved in stress responsivity and affect regulation, the paraventricular hypothalamus (PVN) and amygdala, and highlight experience-dependent changes in synaptic eCB signaling that could provide insight into how stress exposure is translated into affective and physiological pathology.
The hypothalamic-pituitary-adrenal (HPA)-axis is a core component of the central stress response, and dysregulation of HPA-axis function is associated with a variety of psychiatric disorders [169]. Indeed, prolonged pathological increases in corticosterone/cortisol, as the product of HPA-axis activation, can have broad-ranging deleterious somatic and CNS effects. PVN neurons releasing corticotrophin releasing factor represent the initial entryway to the HPA-axis, and eCBs are known to regulate glutamatergic and GABAergic synaptic transmission onto PVN neurons. Initial studies demonstrating a role for synaptic eCB signaling in the regulation of HPA-axis function came from the seminal work of Tasker and co-workers who showed that tonic eCB signaling, triggered by non-genomic effects of membrane-bound glucocorticoid receptors, suppresses glutamate release onto putative PVN CRF neurons, thus highlighting a potential role for eCB signaling in negative feedback inhibition of HPA-axis function [170]. This notion is supported by in vivo studies demonstrating that intra-PVN infusions of the CB1 antagonist AM251 block fast feedback of the HPA-axis triggered by dexamethasone, and that stress increases 2-AG levels in the PVN [171]. Subsequent studies revealed phasic eCB signaling at PVN synapses and demonstrated the existence of both DSI and DSE at synapses onto PVN neurons [172, 173]. Interestingly, DSI is limited by presynaptic mGlu5 via heterologous desensitization of CB1Rs, suggesting during times of high glutamatergic transmission, glutamate spillover can limit DSI, and further promoting PVN neuron firing by limiting the temporal window of eCB signaling efficacy at GABA synapses [173].
Initial studies examining stress adaptations in eCB signaling in the PVN revealed that repeated restraint stress substantially blunted DSI via a mechanism involving CB1R desensitization/downregulation [172]. This impairment in DSI was reversible after stress removal and blocked by pre-stress administration of the glucocorticoid antagonist, RU-486. Similar effects were observed when examining DSE, suggesting a broad ranging desensitization of CB1Rs within the PVN after repeated stress exposure. Indeed, this phenomenon is also observed in the amygdala and striatum [174, 175], but not hippocampus [172]. Initial explanations of these data focused on the possibility of reduced expression of CB1Rs, mediated by prolonged elevations in corticosterone levels [176]. However, more recent studies showed that, at least in the PVN, deficient CB1R signaling after repeated homotypic stress can be rapidly overcome by either novel stress exposure or increased afferent neuronal activity. These data suggest a rapidly reversible phenomenon by which repeated homotypic stress functionally inhibits synaptic eCB signaling at both glutamatergic and GABAergic synapses in the PVN.
GABAergic afferents to the PVN suppress the firing rate of PVN neurons [173, 177], an effect counteracted by DSI. Given that repeated homotypic stressors lead to habituation of the HPA-axis, it is possible that stress-related impairment in CB1R function at GABA synapses could contribute to this habituation. The rapidly reversible nature of this signaling impairment is not well understood but could include receptor internalization or transient functional G-protein uncoupling. Fast HPA-axis feedback inhibition is also known to be lost after chronic stress [178], and CB1Rs on glutamatergic afferents likely involved in fast feedback inhibition also show functional impairment after chronic homotypic stress, suggesting this adaptation in eCB signaling at PVN glutamatergic synapses could contribute to this phenomenon. Studies examining selective deletion of CB1R from GABAergic and glutamatergic terminals innervating PVN CRF neurons could be used to experimentally test this hypothesis. Mechanisms underlying divergence of long-term effects of chronic stress, leading to HPA-axis hyperactivity (which is undesirable) compared to those responsible for HPA-axis habituation (which is desirable) are not clear. Additionally, how concomitant adaptations in eCB signaling at glutamatergic and GABAergic synapses interact to sculpt HPA-axis function after stress exposure remains an open question.
In addition to the PVN, the amygdala is a key limbic brain region regulating stress responses, emotional learning, and valence coding, which is highly regulated by synaptic eCB signaling [179–181]. The amygdala is divided into two components: (1) the basolateral complex, which is a cortical-like structure that projects to other cortical regions and subcortical structures including the ventral striatum and central amygdala and (2) the central amygdala, which projects to hypothalamic, midbrain, and brainstem strictures to control behavioral and autonomic responses to stress and other physiological challenges. CB1Rs depress both glutamatergic and GABAergic transmission in the basolateral amygdala (BLA) and central amygdala [182–184]. Furthermore, DSE and DSI are expressed by BLA neurons and DSE is also present at excitatory synapses in the central amygdala [184, 185]. In addition, eCB-LTD of GABAergic and glutamatergic transmission have been demonstrated in the BLA [83, 186]. Importantly, LTD of GABAergic transmission promotes intra-amygdala LTP via a disinhibition mechanism. Lastly, M1 muscarinic receptors can trigger tonic eCB release at central amygdala glutamatergic synapses [184]. Thus, the amygdala is highly enriched in functional eCB signaling and adaptations in this system have been examined in multiple studies (Fig. 2).
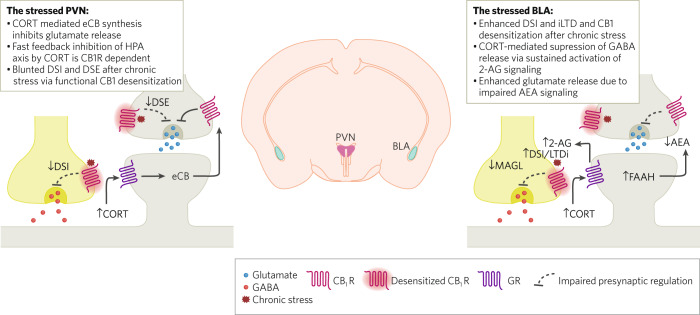
Fig. 2. Stress-induced adaptations in eCB signaling.
Synaptic activity in the paraventricular hypothalamus and the amygdala is heavily modulated by stress. Excitatory neurotransmission in the neurosecretory cells of PVN is acutely suppressed by corticosterone mediated eCB release. Sustained high levels of corticosterone during repeated stress exposure leads to impairment in DSI and DSE through the reversible downregulation of CB1Rs in PVN. In the amygdala, however, DSI and LTD of GABAergic synapses are enhanced, likely by increased 2-AG signaling, despite impaired CB1R function. Conversely, stress leads to enhanced glutamate release, likely as a result of increased FAAH activity and reduction in AEA-mediated suppression of glutamate release.
Initial investigations into stress effects on eCB signaling revealed impaired CB1R function at GABAergic synapses after repeated restraint stress exposure [175], like that seen in the PVN and striatum (above). Interestingly, DSI and LTD of GABAergic transmission were both enhanced despite this CB1R signaling impairment [175, 187]. Gating of LTD of GABAergic transmission by repeated stress was likely mediated by 2-AG signaling, as membrane bound MAGL expression was reduced by repeated stress and MAGL inhibition mimicked the gating of LTD at GABAergic synapses seen after repeated stress exposure. These data are also consistent with those demonstrating increased 2-AG levels in the amygdala after stress [187–189]. Subsequent studies revealed an additional role for glucocorticoid-stimulated tonic 2-AG release in the inhibition of GABA release in the BLA [190]. This study also found that in vivo acute stress exposure triggers corticosterone-mediated tonic 2-AG signaling to suppress GABAergic transmission in the BLA, and that this may contribute to stress-induced anxiogenesis. Interestingly, acute restraint stress and foot-shock increase glutamatergic transmission in the BLA, which are reversed by MAGL inhibition [189, 191]. In the case of foot-shock exposure, this is only true for mice showing behavioral susceptibility to stress exposure in the form of increased avoidance behavior, and in the case of acute restraint stress, the ability of MAGL inhibition to reverse the increase in glutamatergic transmission was correlated with the anxiolytic effects in vivo. In the foot-shock model, mice resilient to the behavioral consequences of stress exposure exhibited a larger dynamic range of eCB-mediated suppression of glutamate release in the BLA relative to stress-susceptible mice. These data suggest that stress can increase 2-AG signaling at both GABAergic and glutamatergic synapses and that stress-enhanced 2-AG signaling at GABAergic synapses could promote anxiety while enhanced 2-AG signaling at glutamate synapses could act to counter adverse effects of stress. This hypothesis is consistent with data demonstrating that augmentation of 2-AG signaling can reduce adverse effects of stress (see [192]) but may also worsen some aspects of stress responsivity under some conditions [193–195].
Stress also reduces AEA levels in the amygdala, an effect mediated by CRF signaling which acts to increase FAAH activity and contributes to immediate and long-lasting changes in basal glutamate release and structural remodeling promoting amygdala hyperactivity [196–198]. Specifically, acute stress increases glutamate release in the BLA, which is prevented by inhibition of FAAH activity prior to stress exposure [198], an effect blocked by in vivo administration of a CB1R antagonist during stress exposure. Similarly, FAAH inhibition prevents long term increases in glutamate release probability and spine density observed 10 days after a single restraint stress exposure in rats [198]. These data are also consistent with preclinical studies showing that FAAH inhibition can reduce the adverse behavioral and physiological consequences of stress [199].
Human data have also demonstrated stress related changes in eCB levels. Briefly, major depressive disorder patients have reduced serum 2-AG levels, which inversely correlated with depressive episode durations, while AEA showed a strong negative correlation with anxiety indices [200, 201]. Interestingly, 2-AG levels correlated with increasing differential fear ratings in a fear conditioning paradigm. In this same study, plasma AEA was increased during fear acquisition and extinction [202]. Indeed, pharmacological FAAH inhibition potentiated fear extinction recall and attenuated autonomic stress reactivity in a recent human study [203]. Based on these preclinical studies, FAAH and MAGL inhibitors are currently under investigation for a variety of stress-related disorders.
Obesity, diet, and energy state
eCB signaling participates in several aspects of feeding behavior, energy homeostasis, and dietary regulation [204]. Blockade of CB1Rs or inhibition of eCB synthesis can reduce food intake and body weight [177, 205]. Moreover, changes in eCB synaptic signaling have been observed after manipulations of diet and energy state in animal models. Indeed, significantly altered levels of both 2-AG and AEA have been observed in the hippocampus of adult mice fed a high-fat diet over the course of 12 weeks [206]. This increase was associated with elevated DAGLα and increased CB1R density without alteration to agonist-stimulated GTPγS binding. Consistent with these data, DSI and LTD at inhibitory synapses were both enhanced in the CA1 region of the hippocampus, an effect which was absent in mice genetically deficient in CB1Rs. These data suggest the possibility that increases in body weight associated with high fat diet can upregulate eCB-mediated synaptic signaling in the brain.
Deficiency in n-3 polyunsaturated fatty acids (PUFA) may affect brain structure and has been implicated as a modifiable risk factor for a variety of neurodevelopmental and psychiatric disorders [207]. PUFA deficiency has also been shown to disrupt synaptic signaling, owing to their role as crucial precursors for eCB synthesis. For example, eCB-LTD in the hippocampus was impaired, particularly at inhibitory synapses of the CA1 region, in the offspring of mice fed a PUFA-deficient diet throughout the gestational and weaning period [208]. In this condition, NMDAR-dependent LTP was similarly disrupted in the offspring of n-3 PUFA-deficient dams. Later in development, n-3 PUFA dietary deficiency similarly impacts eCB-LTD in the medial prefrontal cortex (mPFC) and nucleus accumbens (NAc) during adulthood [209]. As in the hippocampus of n-3 PUFA-deficient mice [208], these rats also exhibited abolished NMDAR-dependent LTP in the mPFC. Together, these data highlight the synaptic consequences of deficiencies in crucial precursors to eCB synthesis caused by dietary fat manipulation.
In the hypothalamus, a biphasic impact of a chronic high-fat diet has been observed in juvenile rats [210]. Following short exposure to this diet, excitatory synapses onto orexin neurons of the hypothalamus acquired a form of presynaptic mGlu5-dependent LTD requiring retrograde eCB signaling, which was no longer present after 4 weeks of high-fat diet. These authors suggest the possibility that a high-fat diet increases eCB synaptic signaling via increases in triglycerides or dynamic changes in diet-sensitive hormone levels. Interestingly, DSI is also induced at GABAergic synapses onto hypothalamic orexin neurons in obese leptin deficient ob/ob mice, but not wild-type mice, supporting previous data that obesity is associated with enhanced synaptic eCB signaling [211]. Furthermore, these authors showed a reorganization of CB1Rs shifting from expression on excitatory terminals to expression predominantly on GABAergic terminals in ob/ob mice. These data suggest that the upregulation of eCB signaling at GABAergic synapses disinhibits orexin neurons and results in increased orexin release in terminal fields and could thus contribute to the metabolic phenotypes observed in ob/ob mice, consistent with the notion that reducing CB1R signaling can reduce body weight and food intake [205, 212].
More recently, analysis of eCB signaling within melanocortin-4 receptor-expressing neurons within the PVN (MC4R-PVN) has revealed a key role for 2-AG signaling in the energy-state dependent activity of these cells (Fig. 3). MC4R neurons in the PVN have been heavily implicated in the regulation of feeding behavior and energy homeostasis [213, 214]. During fasting, the action potential frequency of lateral parabrachial nucleus (LPBN)-projecting MC4R-PVN neurons increases relative to the fed state, and this increase is accompanied by a reduction in GABAergic inhibitory transmission onto these cells [177]. Indeed, GABAergic transmission onto MC4R-PVN neurons is an important regulator of action potential firing in these cells. This reduction in GABAergic transmission is mediated by increased tonic 2-AG-mediated suppression of GABA release. This relatively enhanced 2-AG signaling normalizes in the fed state, resulting in increased GABA transmission onto MC4R-PVN neurons and a concomitant reduction in action potential firing. Consistent with these data, inhibition of 2-AG synthesis or deletion of DAGLα from MC4R neurons reduced food intake and body weight in this study. These data indicate that energy state-dependent dynamic changes in 2-AG signaling regulate the activity of MC4R-PVN neurons and play an important role in the regulation of feeding behavior via modulation of PVN hypothalamic neuron output.
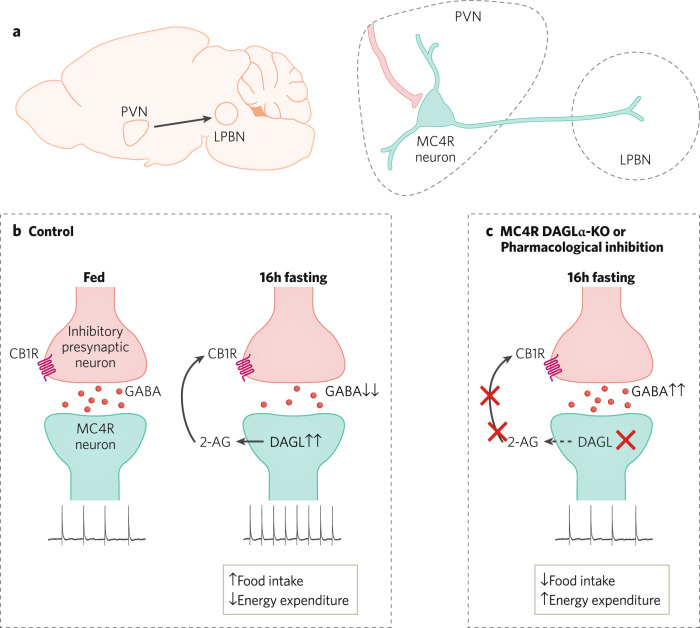
Fig. 3. eCB regulation of hypothalamic feeding circuits.
a LPBN-projecting MC4R neurons withing PVN play a key role in feeding behavior and energy homeostasis and are regulated by GABAergic inputs from a variety of sources including the arcuate nucleus. b Increased food intake and reduced energy expenditure observed after 16 h fasting is associated with enhanced 2-AG mediated suppression of GABA release from its inhibitory presynaptic afferents and increased action potential frequency of MC4R PVN neurons compared to the fed mice. c Pharmacological inhibition of DAGL causes impairment in 2-AG-mediated suppression of GABA release and blocks fasting-induced increase in action potential firing of MC4R PVN neurons. Concomitantly, MC4R-selective DAGLα-KO mice demonstrate lower food intake post-fasting and increased energy expenditure.
Together, these data suggest complex and multifaceted adaptations in eCB signaling in response to diet, body weight, and acute energy state, supporting the notion that changes in dietary constituents can influence central synaptic eCB signaling, and that in some cases adaptations in eCB signaling could contribute to metabolic phenotypes observed in genetic models of obesity. Lastly, emerging data provide insight into how acute energy-states rapidly modulate synaptic eCB signaling to gate the activity of neural circuits regulating food intake. A deeper understanding of these processes could result in more refined and specific eCB-based therapeutics for the treatment of obesity and metabolic disorders.
Autism Spectrum/Neurodevelopmental disorders
Autism spectrum disorder (ASD) is characterized by varied levels of deficits in communication, social function, and the development of repetitive behaviors, along with a host of other co-morbid symptoms [215]. Some cases of ASD can be linked to single-gene mutations, among which fragile X syndrome (FXS), caused by defective FMRP gene, is the largest monogenetic contributor [216]. In this context, several studies have examined adaptations in synaptic eCB signaling using models of FXS with implications for novel eCB-based treatment modalities (Fig. 4).
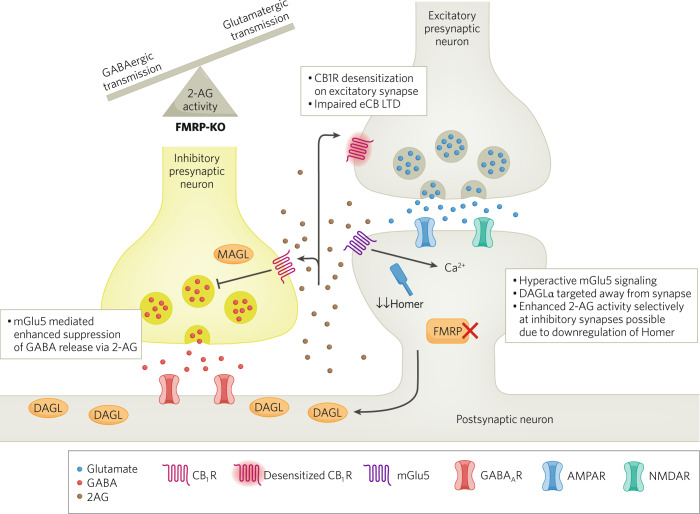
Fig. 4. Altered eCS in a FXS model.
Hyperactive mGlu5 signalling in FMRP-KO mice leads to increased 2-AG-mediated suppression of GABA release in neurons within the striatum and the hippocampus. Desensitized CB1R receptor function is observed at glutamatergic synapses possibly due to excess 2-AG release and receptor overactivation. Additionally, mGlu5-mediated eCB-LTD is impaired in prefrontal cortex and ventral striatum of FMRP-KO mice. These impairments are associated with targeting of DAGLα away from the postsynaptic density, caused by reduction in the scaffold protein Homer, selectively enhancing 2-AG-mediated heterosynaptic suppression of GABA release. Ultimately, these eCB signaling adaptations shift excitation/inhibition balance toward excess synaptic excitation.
Early studies examining FXS-eCB interactions were spurred by the well-known role of mGlu5 in the pathogenesis of FXS and the important coupling between group-1 metabotropic glutamate receptors, including mGlu5, and eCB signaling. Using the FMPR-KO mice, Maccarrone et al showed that striatal activity of DAGL and MAGL were both increased, and dual mGlu1/5-stimulated 2-AG-mediated suppression of GABA release was enhanced, without concomitant changes in CB1R function [217]. These data suggested that synaptic 2-AG signaling at GABAergic synapses is negatively regulated by FMRP and that there may be higher flux of 2-AG signaling within the striatum of FMRP-KO mice. In the hippocampus, dual mGlu1/5-stimulated eCB suppression of GABA release, but not calcium-stimulated eCB release in the form of DSI, was found to be enhanced in FMRP-KO mice [218]. In contrast, DSE was found to be enhanced in autaptic hippocampal preparations from FMRP-KO mice, wherein dual mGlu1/5-stimulated eCB suppression of glutamatergic transmission was not altered. These studies also revealed that CB1Rs are desensitized in cultures from FMRP-KO mice, likely due to chronically elevated 2-AG levels [219]. Somewhat surprisingly, studies using mouse forebrain have demonstrated that DAGLα mRNA binds to FMRP and that genetic disruption of FMRP leads to impaired targeting of DAGLα away from the postsynaptic density, an effect which is associated with impaired dual mGlu1/5-stimulated eCB-LTD of glutamatergic transmission in the PFC and NAc [220]. Interestingly, M1 mAChR-stimulated eCB-LTD of glutamatergic transmission is also impaired within the NAc and hippocampus of FMRP-KO mice, suggesting broad impairments in Gq-receptor-stimulated 2-AG signaling in this FXS model [221, 222]. While in apparent contradiction to the above-cited studies, these latter authors suggest aberrant localization of DAGLα away from dendritic spine heads could result in enhanced heterosynaptic eCB signaling at GABAergic synapses. Deeper mechanistic investigations have revealed that reductions in the postsynaptic density scaffold protein, Homer, leads to enhanced dual mGlu1/5-stimulated eCB signaling at GABAergic synapses, but impaired signaling at glutamatergic synapses, suggesting that known reductions in Homer expression in FMRP-KO mice could explain divergent effects of eCB signaling at GABAergic and glutamatergic synapses [223]. Thus, FMRP-KO mice exhibit altered DAGLα targeting away from excitatory synapses, possibly secondary to reduced Homer expression, which impairs eCB-LTD at glutamatergic synapses and could result in aberrantly enhanced heterosynaptic dual mGlu1/5-stimulated suppression of GABA transmission (Fig. 4).
These synaptic and biochemical studies reveal generally impaired 2-AG-mediated synaptic signaling at glutamatergic synapses, in addition to enhanced signaling at GABAergic synapses. In line with these data, both inhibition and augmentation of eCB signaling have been shown to alleviate various behavioral phenotypes observed in FMRP-KO mice. For example, CB1R blockade normalized cognitive impairment, nociceptive desensitization, and audiogenic seizures, while blockade of CB2Rs normalized anxiolytic-like behavior in FRMP-KO mice [224]. Surprisingly, 2-AG augmentation via inhibition of MAGL also reduced anxiolytic behavior [220] and rescued impaired cognitive function [222] in FMRP-KO mice. In both studies, 2-AG augmentation also reversed impaired eCB-synaptic transmission deficits. Interestingly, increasing brain AEA levels via inhibition of FAAH reversed social impairments in FMRP-KO mice [225]. Overall, the preponderance of available data suggests that correction of deficient 2-AG synaptic signaling, which is primarily observed at excitatory forebrain synapses, could represent a novel approach to the treatment of FXS and possibly other autism spectrum disorders.
Substance use disorders
eCB signaling mechanisms are known to be engaged during the development and maintenance of various phases of substance use disorders modeled in rodents. Briefly, rewarding drugs, including opioids, psychomotor stimulants, alcohol, and nicotine, enhance the activity of the mesolimbic dopamine (DA) system, increasing DA release in crucial regions of the brain’s reward circuitry including the NAc and VTA [226]. Repeated administration of these substances induces adaptive changes in DA reward circuitry leading to attenuated release in both rodent and human models [227, 228], and simultaneously induces adaptive changes in the amygdala, leading to enhanced stress-reactivity and negative affect which promote negative reinforcement-driven drug taking [229]. Further neuroplastic and morphological changes in the prefrontal cortex (PFC), which regulates executive functions, behavioral control, and flexibility, are associated with the loss of control and impairments in decision making in substance use disorders [230, 231].
The necessary participation of eCB signaling in the regulation of drug reward has been demonstrated by a lack of DA increases in response to nicotine, heroin, morphine, ethanol, or THC in CB1R-KO mice [232–235] and the enhancement of conditioned place preference for nicotine and ethanol in rodents concomitantly administered CB1R agonists [236–238]. Similarly, pharmacological or genetic inhibition of CB1Rs decreases operant self-administration of several drugs including morphine [239], heroin [240], ethanol [241], and nicotine [235]. Due to a more direct modulation of monoamine release, often via transport proteins at DA terminals [242], the role of CB1Rs described above does not apply to many psychomotor stimulants such as cocaine, methamphetamine, or MDMA. Indeed, CB1R-KO mice maintain conditioned place preference for MDMA and cocaine, though self-administration is attenuated in some [234, 243] but not all cases.
Given the critical participation of the eCB system in positive reinforcement driven drug taking behavior, substantial data have implicated the VTA as a site of eCB action to modulate drug reward. Activity within the mesolimbic DA system is regulated by a balance of inhibitory GABAergic and excitatory glutamatergic synapses. Acutely, retrograde eCB transmission at glutamatergic synapses limits DA release while CB1R activation at GABAergic synapses induces disinhibition of VTA DA neurons [244, 245]. CB1Rs depress glutamatergic transmission onto VTA DA neurons, and VTA DA neurons express eCB-mediated DSE and LTD, as well as tonic eCB signaling that suppresses afferent glutamate transmission [246, 247]. Similarly, eCBs mediate LTD of GABAergic transmission onto VTA DA neurons [248, 249], and tonic inhibition of GABA release onto VTA DA neurons [250], likely via release of 2-AG. More recently, eCBs have been shown to mediate LTD of glutamatergic transmission onto local VTA GABAergic neurons, which could further disinhibit VTA DA neuron activity [251]. Thus, eCB signaling is well positioned to affect reward-related behavioral processes via synaptic modulation of excitation-inhibition balance onto VTA DA neurons, with the predominant effect likely resulting in disinhibition of VTA DA neuron activity.
Adaptations in mesolimbic DA system eCB signaling have been detected in a variety of models of substance use and withdrawal. For example, cocaine-self administration reduces eCB-LTD of glutamatergic transmission onto VTA DA neurons via a mechanism involving reductions in CB1R function [252]. Prenatal alcohol exposure also reduces eCB-mediated LTD at VTA glutamatergic synapses via impaired CB1R function [253]. Protracted alcohol withdrawal is associated with enhanced tonic eCB signaling at GABAergic synapses onto VTA neurons, which opposes CRF-mediated enhancement of GABA release [254]. In vivo chronic nicotine exposure increases tonic 2-AG mediated suppression of GABA release onto VTA DA neurons [250]. In addition to adaptations in eCB signaling caused by in vivo drug exposure, ex vivo cocaine can enhance eCB-LTD at GABAergic synapses of the VTA DA neurons, an effect mediated via D2 dopamine receptors [248, 255]. Acute ex vivo ethanol also suppresses GABAergic transmission onto VTA DA neurons through tonic release of 2-AG [256]. Interestingly, glutamatergic transmission in crucial regions of the brain’s reward circuitry, such as the NAc, undergoes plasticity during protracted abstinence from cocaine or methamphetamine self-administration, during which time there is a progressive increase in craving termed incubation of craving [257, 258]. mGlu5-driven eCB LTD in the NAc is disrupted during cocaine incubation, an effect not mediated via loss of CB1R function [259]. Instead, disruption of this LTD during incubation may be associated with functional uncoupling of mGlu5 signaling from eCB production [257]. Whether pharmacological normalization of mGlu5-eCB synthesis coupling could affect incubation at the behavioral level remains to be determined. Such studies could help clarify whether this adaptation contributes to incubation or serves as resiliency mechanism, which would in turn be critical for guiding potential eCB-based therapeutic strategies for stimulant use disorders. Furthermore, both acute and chronic exposure to alcohol diminishes eCB-LTD at inhibitory striatal synapses [260], while cocaine similarly inhibits or abolishes eCB-LTD of excitatory transmission in the NAc [261] and BNST [262]. Disruption of balanced signaling in regions of the extended amygdala, such as the BNST, may enhance maladaptive stress-reward interactions, contributing to relapse or reinstatement. Indeed, cue-induced reinstatement of nicotine-seeking behavior requires CB1R-LTP in the BNST, further underscoring the role of eCB synaptic plasticity and its involvement in maladaptive stress responding in substance use-related behavior development, maintenance, and retrieval [263].
Postmortem studies have revealed several abnormalities in eCB signaling of individuals with substance use disorders. In one study, the cerebellum, hippocampus, and caudate nucleus of subjects with alcohol use disorder were studied in comparison to those of control subjects with respect to CB1R expression [264]. Using a GTPγS binding assay, subjects with alcohol use disorder were found to exhibit hyperfunctional CB1Rs in the caudate nucleus, with no change in CB1R expression and no distinct changes found in the other two brain regions. An earlier study identified decreased MAGL activity in alcohol use disorder subjects, along with decreased levels of activated ERK and CREB [264]. Interestingly, a separate study identified hypofunctional CB1Rs with decreased density in the ventral striatum in subjects with alcohol use disorder as compared to healthy controls [265]. In this latter study, activity of FAAH was also lower in subjects with alcohol use disorder as compared to control. These data are in line with those found in postmortem studies of cocaine, but not mixed cocaine/opiate or opiate users, wherein CB1R density was found to be significantly lower in the prefrontal cortex as compared to healthy controls [266]. Combined with the finding that CB1Rs in the prefrontal cortex of patients with cocaine use disorder were redistributed (i.e., increased cytosolic expression over membrane expression) rather than fully reduced, as well as an observed reduction in GRK2/3/5, these data indicate significant receptor desensitization after chronic cocaine use.
In sum, available preclinical data support the notion that eCBs regulate afferent synaptic input onto VTA DA and GABA neurons, and that disinhibition of DA neuron activity may be the net result of eCB signaling under some circumstances. Current literature strongly suggests some psychoactive drugs with dependence liability may engage eCB signaling to ultimately facilitate DA release and contribute to positive reinforcement driven drug taking. Consistent with this notion, CB1 receptor blockade reduces positive reinforcement driven self-administration in preclinical models and clinical trials. Initial studies also suggest blockade of 2-AG synthesis may exert similar effects on alcohol self-administration [256], which is also associated with impaired ethanol-mediated suppression of GABA release onto VTA DA neurons, presumably preventing ethanol-induced disinhibition of DA release. Future studies examining the effects of AEA and 2-AG synthesis inhibition in preclinical models of substance use disorders should be a high priority and could reveal novel treatment approaches.
Outlook and future directions
Despite decades of investigation into the molecular mechanisms regulating eCB signaling, our understanding of the cellular and synaptic effects of this system are continuing to evolve at a rapid pace. How the multifaceted components of this system beyond retrograde synaptic suppression (indirect astrocytic signaling, autocrine signaling, and eCB control of cellular respiration) adapt in response to environmental pressures and disease states represents a major open question in the field. Similarly, the ability of eCBs to regulate both excitatory and inhibitory neurotransmission onto the same neurons makes interpretation of net effects on neuronal excitability, and ultimately functional output, difficult to predict. Indeed, we have reviewed data demonstrating that eCB signaling at GABA vs. glutamate synapses could have opposing behavioral consequences. Utilization of emerging genetic and molecular biology approaches to selectively examine and target eCB signaling at distinct cell and synapse types and within intracellular compartments will be critical to elucidate differential functional consequences of distinct eCB signaling mechanisms. Moreover, elucidation of differential molecular signaling cascades regulating eCB production and effector systems downstream of CB1Rs could ultimately reveal pharmacological approaches to target eCB signaling at distinct types of synapses for therapeutic purposes. In this context, development of biased CB1R and CB2R ligands may present a unique opportunity to differentially target eCB signaling at subsets of synapses or engage subsets of effector pathways for therapeutic interventions. These issues notwithstanding, the future of eCB-based therapeutics development appears promising with several clinical trials of eCB degradation inhibitors currently underway for a variety of neurological and psychiatric indications. For example, small molecule inhibitors of FAAH have been examined for pain associated with osteoarthritis (albeit with negative results [267]) and are currently being evaluated for anxiety and other affective disorders. More recently, irreversible small molecule inhibitors of MAGL have entered early phase clinical trials for neurological and psychiatric disorders [268, 269]. Interestingly, the potential anti-inflammatory effects of MAGL inhibitors in the CNS, mediated via reductions in prostaglandin synthesis, have expanded the potential therapeutic potential of this mechanism [270]. Additionally, ongoing development of allosteric modulators, transport inhibitors, and eCB synthesis inhibitors, will further expand the repertories of potential therapeutic approaches for stress-related disorders, obesity, ASD, and substance use disorders [3].
Author contributions
SP, AFS, FY and SN conceived of content, wrote, and edited the manuscript.
Funding
This work was supported by NIH grants MH107452, MH119817, and AA026186 (SP).
Competing interests
SP is a scientific consultant for Janssen, Psy Therapeutics, and Jazz Pharmaceuticals. The remaining authors have nothing to disclose.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kesner AJ, Lovinger DM. Cannabinoids, Endocannabinoids and Sleep. Front Mol Neurosci. 2020;13:125. doi: 10.3389/fnmol.2020.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zieglgansberger W, Brenneisen R, Berthele A, Wotjak CT, Bandelow B, Tolle TR, et al. Chronic Pain and the Endocannabinoid System: Smart Lipids - A Novel Therapeutic Option? Med Cannabis Cannabinoids. 2022;5:61–75. doi: 10.1159/000522432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ligresti A, De Petrocellis L, Di Marzo V. From Phytocannabinoids to Cannabinoid Receptors and Endocannabinoids: Pleiotropic Physiological and Pathological Roles Through Complex Pharmacology. Physiol Rev. 2016;96:1593–659. doi: 10.1152/physrev.00002.2016. [DOI] [PubMed] [Google Scholar]
- 4.Mechoulam R, Hanus LO, Pertwee R, Howlett AC. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat Rev Neurosci. 2014;15:757–64. doi: 10.1038/nrn3811. [DOI] [PubMed] [Google Scholar]
- 5.Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br J Pharmacol. 2006;147:S163–71. doi: 10.1038/sj.bjp.0706406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nie J, Lewis L. The Proximal and DIstal C-Terminal Tail Domains of the CB1 Cannabinoid Receptor Mediate G Protein Coupling. Neuroscience. 2001;107:161–7. doi: 10.1016/s0306-4522(01)00335-9. [DOI] [PubMed] [Google Scholar]
- 7.Sarnataro D, Grimaldi C, Pisanti S, Gazzerro P, Laezza C, Zurzolo C, et al. Plasma membrane and lysosomal localization of CB1 cannabinoid receptor are dependent on lipid rafts and regulated by anandamide in human breast cancer cells. FEBS Lett. 2005;579:6343–9. doi: 10.1016/j.febslet.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Maccarrone M, De Chiara V, Gasperi V, Viscomi MT, Rossi S, Oddi S, et al. Lipid rafts regulate 2-arachidonoylglycerol metabolism and physiological activity in the striatum. J Neurochem. 2009;109:371–81. doi: 10.1111/j.1471-4159.2009.05948.x. [DOI] [PubMed] [Google Scholar]
- 9.Bari M, Battista N, Fezza F, Finazzi-Agro A, Maccarrone M. Lipid rafts control signaling of type-1 cannabinoid receptors in neuronal cells. Implications for anandamide-induced apoptosis. J Biol Chem. 2005;280:12212–20. doi: 10.1074/jbc.M411642200. [DOI] [PubMed] [Google Scholar]
- 10.Bari M, Oddi S, De Simone C, Spagnolo P, Gasperi V, Battista N, et al. Type-1 cannabinoid receptors colocalize with caveolin-1 in neuronal cells. Neuropharmacology. 2008;54:45–50. doi: 10.1016/j.neuropharm.2007.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–8. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung D, Saghatelian A, Simon GM, Cravatt BF. Inactivation of N-Acyl Phosphatidylethanolamine Phospholipase D Revealse Multiple Mechanisms for the Biosynthesis of Endocannabinoids. Biochemistry. 2006;45:4720–6. doi: 10.1021/bi060163l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabrielli M, Battista N, Riganti L, Prada I, Antonucci F, Cantone L, et al. Active endocannabinoids are secreted on extracellular membrane vesicles. EMBO Rep. 2015;16:213–20. doi: 10.15252/embr.201439668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blankman JL, Cravatt BF. Chemical probes of endocannabinoid metabolism. Pharmacol Rev. 2013;65:849–71. doi: 10.1124/pr.112.006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pertwee RG. Elevating endocannabinoid levels: pharmacological strategies and potential therapeutic applications. Proc Nutr Soc. 2014;73:96–105. doi: 10.1017/S0029665113003649. [DOI] [PubMed] [Google Scholar]
- 16.Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, et al. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci. 2004;20:441–58. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- 17.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–24. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schurman LD, Lu D, Kendall DA, Howlett AC, Lichtman AH. Molecular Mechanism and Cannabinoid Pharmacology. Handb Exp Pharmacol. 2020;258:323–53. doi: 10.1007/164_2019_298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross RA. Anandamide and vanilloid TRPV1 receptors. Br J Pharmacol. 2003;140:790–801. doi: 10.1038/sj.bjp.0705467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Petrocellis L, Bisogno T, Maccarrone M, Davis JB, Finazzi-Agro A, Di, Marzo V. The activity of anandamide at vanilloid VR1 receptors requires facilitated transport across the cell membrane and is limited by intracellular metabolism. J Biol Chem. 2001;276:12856–63. doi: 10.1074/jbc.M008555200. [DOI] [PubMed] [Google Scholar]
- 21.Di Marzo V, Bisogno T, De Petrocellis L. Anandamide: Some Like it Hot. Trends in Pharmacological Sciences. 2001;22:346–9. doi: 10.1016/s0165-6147(00)01712-0. [DOI] [PubMed] [Google Scholar]
- 22.Sharir H, Console-Bram L, Mundy C, Popoff SN, Kapur A, Abood ME. The endocannabinoids anandamide and virodhamine modulate the activity of the candidate cannabinoid receptor GPR55. J Neuroimmune Pharmacol. 2012;7:856–65. doi: 10.1007/s11481-012-9351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baur R, Kielar M, Richter L, Ernst M, Ecker GF, Sigel E. Molecular analysis of the site for 2-arachidonylglycerol (2-AG) on the beta(2) subunit of GABA(A) receptors. J Neurochem. 2013;126:29–36. doi: 10.1111/jnc.12270. [DOI] [PubMed] [Google Scholar]
- 24.Howlett AC, Champion TM, Wilken GH, Mechoulam R. Stereochemical effects of 11-OH-∆8-Tetrahydrocannabinol-dimethylheptyl to inhibit adenylate cyclase and bind to the cannabinoid receptor. Neuropharmacology. 1990;29:161–5. doi: 10.1016/0028-3908(90)90056-w. [DOI] [PubMed] [Google Scholar]
- 25.Rinaldi-Carmona M, Pialot F, Congy C, Redon E, Barth F, Bachy A, et al. Characterization and distribution of binding sites for [3H]-SR 141716A, a selective brain (CB1) cannabinoid receptor antagonist, in rodent brain. Life Sciences. 1996;58:1239–47. doi: 10.1016/0024-3205(96)00085-9. [DOI] [PubMed] [Google Scholar]
- 26.Pertwee R, Griffin G, Fernando S, Li C, Hill A, Makriyannis A. AM630. A COMPETITIVE CANNABINOID RECEPTOR ANTAGONIST. Life Sciences. 1995;56:1949–55. doi: 10.1016/0024-3205(95)00175-6. [DOI] [PubMed] [Google Scholar]
- 27.Gamage TF, Ignatowska-Jankowska BM, Wiley JL, Abdelrahman M, Trembleau L, Greig IR, et al. In-vivo pharmacological evaluation of the CB1-receptor allosteric modulator Org-27569. Behav Pharmacol. 2014;25:182–5. doi: 10.1097/FBP.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ignatowska-Jankowska BM, Baillie GL, Kinsey S, Crowe M, Ghosh S, Owens RA, et al. A Cannabinoid CB1 Receptor-Positive Allosteric Modulator Reduces Neuropathic Pain in the Mouse with No Psychoactive Effects. Neuropsychopharmacology. 2015;40:2948–59. doi: 10.1038/npp.2015.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slivicki RA, Xu Z, Kulkarni PM, Pertwee RG, Mackie K, Thakur GA, et al. Positive Allosteric Modulation of Cannabinoid Receptor Type 1 Suppresses Pathological Pain Without Producing Tolerance or Dependence. Biol Psychiatry. 2018;84:722–33. doi: 10.1016/j.biopsych.2017.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cairns EA, Szczesniak AM, Straiker AJ, Kulkarni PM, Pertwee RG, Thakur GA, et al. The In Vivo Effects of the CB1-Positive Allosteric Modulator GAT229 on Intraocular Pressure in Ocular Normotensive and Hypertensive Mice. J Ocul Pharmacol Ther. 2017;33:582–90. doi: 10.1089/jop.2017.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lauckner JE, Hille B, Mackie K. The cannabinoid agonist WIN55,212-2 increases intracellular calcium via CB1 receptor coupling to Gq/11 G proteins. Proc Natl Acad Sci U S A. 2005;102:19144–9. doi: 10.1073/pnas.0509588102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukhopadhyay S, Howlett AC. Chemically distinct ligands promote differential CB1 cannabinoid receptor-Gi protein interactions. Mol Pharmacol. 2005;67:2016–24. doi: 10.1124/mol.104.003558. [DOI] [PubMed] [Google Scholar]
- 33.Khurana L, Mackie K, Piomelli D, Kendall DA. Modulation of CB1 cannabinoid receptor by allosteric ligands: Pharmacology and therapeutic opportunities. Neuropharmacology. 2017;124:3–12. doi: 10.1016/j.neuropharm.2017.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahn KH, Mahmoud MM, Shim JY, Kendall DA. Distinct roles of beta-arrestin 1 and beta-arrestin 2 in ORG27569-induced biased signaling and internalization of the cannabinoid receptor 1 (CB1) J Biol Chem. 2013;288:9790–800. doi: 10.1074/jbc.M112.438804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jagla CAD, Scott CE, Tang Y, Qiao C, Mateo-Semidey GE, Yudowski GA, et al. Pyrimidinyl Biphenylureas Act as Allosteric Modulators to Activate Cannabinoid Receptor 1 and Initiate beta-Arrestin-Dependent Responses. Mol Pharmacol. 2019;95:1–10. doi: 10.1124/mol.118.112854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Egmond N, Straub VM, van der Stelt M. Targeting Endocannabinoid Signaling: FAAH and MAG Lipase Inhibitors. Annu Rev Pharmacol Toxicol. 2021;61:441–63. doi: 10.1146/annurev-pharmtox-030220-112741. [DOI] [PubMed] [Google Scholar]
- 37.Slivicki RA, Xu Z, Mali SS, Hohmann AG. Brain permeant and impermeant inhibitors of fatty-acid amide hydrolase suppress the development and maintenance of paclitaxel-induced neuropathic pain without producing tolerance or physical dependence in vivo and synergize with paclitaxel to reduce tumor cell line viability in vitro. Pharmacol Res. 2019;142:267–82. doi: 10.1016/j.phrs.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 39.Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MC, et al. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011;334:809–13. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogasawara D, Deng H, Viader A, Baggelaar MP, Breman A, den Dulk H, et al. Rapid and profound rewiring of brain lipid signaling networks by acute diacylglycerol lipase inhibition. Proc Natl Acad Sci U S A. 2016;113:26–33. doi: 10.1073/pnas.1522364112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mock ED, Mustafa M, Gunduz-Cinar O, Cinar R, Petrie GN, Kantae V, et al. Discovery of a NAPE-PLD inhibitor that modulates emotional behavior in mice. Nat Chem Biol. 2020;16:667–75. doi: 10.1038/s41589-020-0528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohno-Shosaku T, Maejima T. Endogenous Cannabinoids Mediate Retrograde Signals from Depolarized Postsynaptic Neurons to Presynaptic Terminals. Neuron. 2001;29:729–38. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 44.Wilson RI, Nicoll RA. Endogenous Cannabinoids Mediate Retrograde Signalling at Hippocampal Synapses. Nature. 2001;410:588–92. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 45.Diana MA, Marty A. Endocannabinoid-mediated short-term synaptic plasticity: depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE) Br J Pharmacol. 2004;142:9–19. doi: 10.1038/sj.bjp.0705726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kreitzer AC, Regehr WG. Retrograde Inhibition of Presynaptic Calcium Influx by Endogenous Cannabinoids at Excitatory Synapses onto Purkinje Cells. Neuron. 2001;29:717–27. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 47.Winters BL, Vaughan CW. Mechanisms of endocannabinoid control of synaptic plasticity. Neuropharmacology. 2021;197:108736. doi: 10.1016/j.neuropharm.2021.108736. [DOI] [PubMed] [Google Scholar]
- 48.Hoffman AF, Riegel AC, Lupica CR. Functional localization of cannabinoid receptors and endogenous cannabinoid production in distinct neuron populations of the hippocampus. Eur J Neurosci. 2003;18:524–34. doi: 10.1046/j.1460-9568.2003.02773.x. [DOI] [PubMed] [Google Scholar]
- 49.Kim J, Isokawa M, Ledent C, Alger BE. Activation of Muscarinic Acetylcholine Receptors Enhances the Release of Endogenous Cannabinoids in the Hippocampus. The Journal of Neuroscience. 2002;22:10182–91. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohno-Shosaku T, Shosaku J, Tsubokawa H, Kano M. Cooperative endocannabinoid production by neuronal depolarization and group I metabotropic glutamate receptor activation. European Journal of Neuroscience. 2002;15:953–61. doi: 10.1046/j.1460-9568.2002.01929.x. [DOI] [PubMed] [Google Scholar]
- 51.Ohno-Shosaku T, Matsui M, Fukudome Y, Shosaku J, Tsubokawa H, Taketo MM, et al. Postsynaptic M1 and M3 receptors are responsible for the muscarinic enhancement of retrograde endocannabinoid signalling in the hippocampus. Eur J Neurosci. 2003;18:109–16. doi: 10.1046/j.1460-9568.2003.02732.x. [DOI] [PubMed] [Google Scholar]
- 52.Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic Glutamate Receptors Drive the Endocannabinoid System in the Hippocampus. The Journal of Neuroscience. 2001;21:1–5. doi: 10.1523/JNEUROSCI.21-24-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lourenco J, Matias I, Marsicano G, Mulle C. Pharmacological activation of kainate receptors drives endocannabinoid mobilization. J Neurosci. 2011;31:3243–8. doi: 10.1523/JNEUROSCI.3512-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manz KM, Ghose D, Turner BD, Taylor A, Becker J, Grueter CA, et al. Calcium-Permeable AMPA Receptors Promote Endocannabinoid Signaling at Parvalbumin Interneuron Synapses in the Nucleus Accumbens Core. Cell Rep. 2020;32:107971. doi: 10.1016/j.celrep.2020.107971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marshall JJ, Xu J, Contractor A. Kainate Receptors Inhibit Glutamate Release Via Mobilization of Endocannabinoids in Striatal Direct Pathway Spiny Projection Neurons. J Neurosci. 2018;38:3901–10. doi: 10.1523/JNEUROSCI.1788-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohno-Shosaku T, Hashimotodani Y, Ano M, Takeda S, Tsubokawa H, Kano M. Endocannabinoid signalling triggered by NMDA receptor-mediated calcium entry into rat hippocampal neurons. J Physiol. 2007;584:407–18. doi: 10.1113/jphysiol.2007.137505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liao HT, Lee HJ, Ho YC, Chiou LC. Capsaicin in the periaqueductal gray induces analgesia via metabotropic glutamate receptor-mediated endocannabinoid retrograde disinhibition. Br J Pharmacol. 2011;163:330–45. doi: 10.1111/j.1476-5381.2011.01214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Piette C, Cui Y, Gervasi N, Venance L. Lights on Endocannabinoid-Mediated Synaptic Potentiation. Front Mol Neurosci. 2020;13:132. doi: 10.3389/fnmol.2020.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Araque A, Castillo PE, Manzoni OJ, Tonini R. Synaptic functions of endocannabinoid signaling in health and disease. Neuropharmacology. 2017;124:13–24. doi: 10.1016/j.neuropharm.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Castillo PE, Younts TJ, Chavez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012;76:70–81. doi: 10.1016/j.neuron.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- 62.Jensen KR, Berthoux C, Nasrallah K, Castillo PE. Multiple cannabinoid signaling cascades powerfully suppress recurrent excitation in the hippocampus. Proc Natl Acad Sci U S A. 2021;118. [DOI] [PMC free article] [PubMed]
- 63.Melis M, Greco B, Tonini R. Interplay between synaptic endocannabinoid signaling and metaplasticity in neuronal circuit function and dysfunction. Eur J Neurosci. 2014;39:1189–201. doi: 10.1111/ejn.12501. [DOI] [PubMed] [Google Scholar]
- 64.Xu J, Antion MD, Nomura T, Kraniotis S, Zhu Y, Contractor A. Hippocampal metaplasticity is required for the formation of temporal associative memories. J Neurosci. 2014;34:16762–73. doi: 10.1523/JNEUROSCI.2869-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim J, Alger BE. Reduction in endocannabinoid tone is a homeostatic mechanism for specific inhibitory synapses. Nat Neurosci. 2010;13:592–600. doi: 10.1038/nn.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang SY, Xu M, Miao QL, Poo MM, Zhang XH. Endocannabinoid-dependent homeostatic regulation of inhibitory synapses by miniature excitatory synaptic activities. J Neurosci. 2009;29:13222–31. doi: 10.1523/JNEUROSCI.1710-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chevaleyre V, Castillo PE, Heterosynaptic LTD. of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–72. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- 68.Chevaleyre V, Castillo PE. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron. 2004;43:871–81. doi: 10.1016/j.neuron.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 69.Zhu PJ, Lovinger DM. Persistent synaptic activity produces long-lasting enhancement of endocannabinoid modulation and alters long-term synaptic plasticity. J Neurophysiol. 2007;97:4386–9. doi: 10.1152/jn.01228.2006. [DOI] [PubMed] [Google Scholar]
- 70.Cui Y, Paille V, Xu H, Genet S, Delord B, Fino E, et al. Endocannabinoids mediate bidirectional striatal spike-timing-dependent plasticity. J Physiol. 2015;593:2833–49. doi: 10.1113/JP270324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cui Y, Perez S, Venance L. Endocannabinoid-LTP Mediated by CB1 and TRPV1 Receptors Encodes for Limited Occurrences of Coincident Activity in Neocortex. Front Cell Neurosci. 2018;12:182. doi: 10.3389/fncel.2018.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang W, Trieu BH, Palmer LC, Jia Y, Pham DT, Jung KM, et al. A Primary Cortical Input to Hippocampus Expresses a Pathway-Specific and Endocannabinoid-Dependent Form of Long-Term Potentiation. eNeuro. 2016;3:ENEURO.0160-16.2016. [DOI] [PMC free article] [PubMed]
- 73.Adermark L, Talani G, Lovinger DM. Endocannabinoid-dependent plasticity at GABAergic and glutamatergic synapses in the striatum is regulated by synaptic activity. Eur J Neurosci. 2009;29:32–41. doi: 10.1111/j.1460-9568.2008.06551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arami MK, Sohya K, Sarihi A, Jiang B, Yanagawa Y, Tsumoto T. Reciprocal Homosynaptic and heterosynaptic long-term plasticity of corticogeniculate projection neurons in layer VI of the mouse visual cortex. J Neurosci. 2013;33:7787–98. doi: 10.1523/JNEUROSCI.5350-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Penasco S, Rico-Barrio I, Puente N, Gomez-Urquijo SM, Fontaine CJ, Egana-Huguet J, et al. Endocannabinoid long-term depression revealed at medial perforant path excitatory synapses in the dentate gyrus. Neuropharmacology. 2019;153:32–40. doi: 10.1016/j.neuropharm.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 76.Peterfi Z, Urban GM, Papp OI, Nemeth B, Monyer H, Szabo G, et al. Endocannabinoid-mediated long-term depression of afferent excitatory synapses in hippocampal pyramidal cells and GABAergic interneurons. J Neurosci. 2012;32:14448–63. doi: 10.1523/JNEUROSCI.1676-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–51. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- 78.Park H, Rhee J, Lee S, Chung C. Selectively Impaired Endocannabinoid-Dependent Long-Term Depression in the Lateral Habenula in an Animal Model of Depression. Cell Rep. 2017;20:289–96. doi: 10.1016/j.celrep.2017.06.049. [DOI] [PubMed] [Google Scholar]
- 79.Andrade-Talavera Y, Duque-Feria P, Paulsen O, Rodriguez-Moreno A. Presynaptic Spike Timing-Dependent Long-Term Depression in the Mouse Hippocampus. Cereb Cortex. 2016;26:3637–54. doi: 10.1093/cercor/bhw172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sjöström PJ, Turrigiano GG, Nelson SB, Neocortical LTD. via Coincident Activation of Presynaptic NMDA and Cannabinoid Receptors. Neuron. 2003;39:641–54. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- 81.Basu J, Srinivas KV, Cheung SK, Taniguchi H, Huang ZJ, Siegelbaum SA. A cortico-hippocampal learning rule shapes inhibitory microcircuit activity to enhance hippocampal information flow. Neuron. 2013;79:1208–21. doi: 10.1016/j.neuron.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Adermark L, Lovinger DM. Combined activation of L-type Ca2+ channels and synaptic transmission is sufficient to induce striatal long-term depression. J Neurosci. 2007;27:6781–7. doi: 10.1523/JNEUROSCI.0280-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Azad SC, Monory K, Marsicano G, Cravatt BF, Lutz B, Zieglgansberger W, et al. Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. J Neurosci. 2004;24:9953–61. doi: 10.1523/JNEUROSCI.2134-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haj-Dahmane S, Shen RY. Chronic stress impairs alpha1-adrenoceptor-induced endocannabinoid-dependent synaptic plasticity in the dorsal raphe nucleus. J Neurosci. 2014;34:14560–70. doi: 10.1523/JNEUROSCI.1310-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Izumi Y, Zorumski CF. Direct cortical inputs erase long-term potentiation at Schaffer collateral synapses. J Neurosci. 2008;28:9557–63. doi: 10.1523/JNEUROSCI.3346-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–6. doi: 10.1038/nature02913. [DOI] [PubMed] [Google Scholar]
- 87.Marinelli S, Pacioni S, Bisogno T, Di Marzo V, Prince DA, Huguenard JR, et al. The endocannabinoid 2-arachidonoylglycerol is responsible for the slow self-inhibition in neocortical interneurons. J Neurosci. 2008;28:13532–41. doi: 10.1523/JNEUROSCI.0847-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marinelli S, Pacioni S, Cannich A, Marsicano G, Bacci A. Self-modulation of neocortical pyramidal neurons by endocannabinoids. Nat Neurosci. 2009;12:1488–90. doi: 10.1038/nn.2430. [DOI] [PubMed] [Google Scholar]
- 89.Gibson HE, Edwards JG, Page RS, Van Hook MJ, Kauer JA. TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron. 2008;57:746–59. doi: 10.1016/j.neuron.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chavez AE, Chiu CQ, Castillo PE. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat Neurosci. 2010;13:1511–8. doi: 10.1038/nn.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chavez AE, Hernandez VM, Rodenas-Ruano A, Chan CS, Castillo PE. Compartment-specific modulation of GABAergic synaptic transmission by TRPV1 channels in the dentate gyrus. J Neurosci. 2014;34:16621–9. doi: 10.1523/JNEUROSCI.3635-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Newsom RJ, Garcia RJ, Stafford J, Osterlund C, O'Neill CE, Day HEW, et al. Remote CB1 receptor antagonist administration reveals multiple sites of tonic and phasic endocannabinoid neuroendocrine regulation. Psychoneuroendocrinology. 2020;113:104549. doi: 10.1016/j.psyneuen.2019.104549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaczocha M, Haj-Dahmane S Mechanisms of endocannabinoid transport in the brain. Br J Pharmacol. 2021. [DOI] [PMC free article] [PubMed]
- 94.Fasia L, Karava V, Siafaka-Kapadai A. Uptake and metabolism of [3H]anandamide by rabbit platelets. Lack of transporter? Eur J Biochem. 2003;270:3498–506. doi: 10.1046/j.1432-1033.2003.03724.x. [DOI] [PubMed] [Google Scholar]
- 95.Glaser ST, Abumrad NA, Fatade F, Kaczocha M, Studholme KM, Deutsch DG. Evidence against the presence of an anandamide transporter. Proc Natl Acad Sci U S A. 2003;100:4269–74. doi: 10.1073/pnas.0730816100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D. Functional Role of High-Affinity Anandamide Transport, as Revealed by Selective Inhibition. Science. 1997;277:1094–7. doi: 10.1126/science.277.5329.1094. [DOI] [PubMed] [Google Scholar]
- 97.Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, et al. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–91. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- 98.Kaczocha M, Lin Q, Nelson LD, McKinney MK, Cravatt BF, London E, et al. Anandamide externally added to lipid vesicles containing trapped fatty acid amide hydrolase (FAAH) is readily hydrolyzed in a sterol-modulated fashion. ACS Chem Neurosci. 2012;3:364–8. doi: 10.1021/cn300001w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tian X, Guo J, Yao F, Yang DP, Makriyannis A. The conformation, location, and dynamic properties of the endocannabinoid ligand anandamide in a membrane bilayer. J Biol Chem. 2005;280:29788–95. doi: 10.1074/jbc.M502925200. [DOI] [PubMed] [Google Scholar]
- 100.Lynch DL, Reggio PH. Cannabinoid CB1 receptor recognition of endocannabinoids via the lipid bilayer: molecular dynamics simulations of CB1 transmembrane helix 6 and anandamide in a phospholipid bilayer. J Comput Aided Mol Des. 2006;20:495–509. doi: 10.1007/s10822-006-9068-9. [DOI] [PubMed] [Google Scholar]
- 101.Fowler CJ. Transport of endocannabinoids across the plasma membrane and within the cell. FEBS J. 2013;280:1895–904. doi: 10.1111/febs.12212. [DOI] [PubMed] [Google Scholar]
- 102.Kaczocha M, Hermann A, Glaser ST, Bojesen IN, Deutsch DG. Anandamide uptake is consistent with rate-limited diffusion and is regulated by the degree of its hydrolysis by fatty acid amide hydrolase. J Biol Chem. 2006;281:9066–75. doi: 10.1074/jbc.M509721200. [DOI] [PubMed] [Google Scholar]
- 103.Piomelli D, Beltramo M, Glasnapp S, Lin SY, Goutopoulos A, Xie X-Q, et al. Structural determinants for recognition and translocation by the anandamide transporter. Proc Natl Acad Sci. 1999;96:5802–7. doi: 10.1073/pnas.96.10.5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moore SA, Nomikos GG, Dickason-Chesterfield AK, Schober DA, Schaus JM, Ying BP, et al. Identification of a high-affinity binding site involved in the transport of endocannabinoids. Proc Natl Acad Sci U S A. 2005;102:17852–7. doi: 10.1073/pnas.0507470102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fegley D, Kathuria S, Mercier R, Li C, Goutopoulos A, Makriyannis A, et al. Anandamide transport is independent of fatty-acid amide hydrolase activity and is blocked by the hydrolysis-resistant inhibitor AM1172. Proc Natl Acad Sci U S A. 2004;101:8756–61. doi: 10.1073/pnas.0400997101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chicca A, Marazzi J, Nicolussi S, Gertsch J. Evidence for bidirectional endocannabinoid transport across cell membranes. J Biol Chem. 2012;287:34660–82. doi: 10.1074/jbc.M112.373241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ligresti A, De Petrocellis L, Hernan Perez de la Ossa D, Aberturas R, Cristino L, Moriello AS, et al. Exploiting nanotechnologies and TRPV1 channels to investigate the putative anandamide membrane transporter. PLoS ONE. 2010;5:e10239. doi: 10.1371/journal.pone.0010239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oddi S, Bari M, Battista N, Barsacchi D, Cozzani I, Maccarrone M. Confocal microscopy and biochemical analysis reveal spatial and functional separation between anandamide uptake and hydrolysis in human keratinocytes. Cell Mol Life Sci. 2005;62:386–95. doi: 10.1007/s00018-004-4446-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nicolussi S, Chicca A, Rau M, Rihs S, Soeberdt M, Abels C, et al. Correlating FAAH and anandamide cellular uptake inhibition using N-alkylcarbamate inhibitors: from ultrapotent to hyperpotent. Biochem Pharmacol. 2014;92:669–89. doi: 10.1016/j.bcp.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 110.Nicolussi S, Viveros-Paredes JM, Gachet MS, Rau M, Flores-Soto ME, Blunder M, et al. Guineensine is a novel inhibitor of endocannabinoid uptake showing cannabimimetic behavioral effects in BALB/c mice. Pharmacol Res. 2014;80:52–65. doi: 10.1016/j.phrs.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 111.Ertunc ME, Sikkeland J, Fenaroli F, Griffiths G, Daniels MP, Cao H, et al. Secretion of fatty acid binding protein aP2 from adipocytes through a nonclassical pathway in response to adipocyte lipase activity. J Lipid Res. 2015;56:423–34. doi: 10.1194/jlr.M055798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Josephrajan A, Hertzel AV, Bohm EK, McBurney MW, Imai SI, Mashek DG, et al. Unconventional Secretion of Adipocyte Fatty Acid Binding Protein 4 Is Mediated By Autophagic Proteins in a Sirtuin-1-Dependent Manner. Diabetes. 2019;68:1767–77. doi: 10.2337/db18-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Villeneuve J, Bassaganyas L, Lepreux S, Chiritoiu M, Costet P, Ripoche J, et al. Unconventional secretion of FABP4 by endosomes and secretory lysosomes. J Cell Biol. 2018;217:649–65. doi: 10.1083/jcb.201705047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Haj-Dahmane S, Shen RY, Elmes MW, Studholme K, Kanjiya MP, Bogdan D, et al. Fatty-acid-binding protein 5 controls retrograde endocannabinoid signaling at central glutamate synapses. Proc Natl Acad Sci U S A. 2018;115:3482–7. doi: 10.1073/pnas.1721339115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nakamura Y, Dryanovski DI, Kimura Y, Jackson SN, Woods AS, Yasui Y, et al. Cocaine-induced endocannabinoid signaling mediated by sigma-1 receptors and extracellular vesicle secretion. Elife. 2019;8. [DOI] [PMC free article] [PubMed]
- 116.Lyon KA, Allen NJ. From Synapses to Circuits, Astrocytes Regulate Behavior. Front Neural Circuits. 2021;15:786293. doi: 10.3389/fncir.2021.786293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rodríguez JJ, Mackie K, Pickel VM. Ultrastructural Localization of the CB1 Cannabinoid Receptor in μ-Opioid Receptor Patches of the Rat Caudate Putamen Nucleus. The Journal of Neuroscience. 2001;21:823–33. doi: 10.1523/JNEUROSCI.21-03-00823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Han J, Kesner P, Metna-Laurent M, Duan TT, Xu L, Georges F, et al. Acute Cannabinoids Impair Working Memory through Astroglial CB1 Receptor Modulation of Hippocampal LTD. Cell. 2012;148:1039–50. doi: 10.1016/j.cell.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 119.Covelo A, Eraso-Pichot A, Fernández-Moncada I, Serrat R, Marsicano G. CB1R-dependent regulation of astrocyte physiology and astrocyte-neuron interactions. Neuropharmacology. 2021;195:108678. doi: 10.1016/j.neuropharm.2021.108678. [DOI] [PubMed] [Google Scholar]
- 120.Walter L, Stella N. Endothelin-1 increases 2-arachidonoyl glycerol (2-AG) production in astrocytes. Glia. 2003;44:85–90. doi: 10.1002/glia.10270. [DOI] [PubMed] [Google Scholar]
- 121.Walter L, Dinh T, Stella N. ATP induces a rapid and pronounced increase in 2-arachidonoylglycerol production by astrocytes, a response limited by monoacylglycerol lipase. J Neurosci. 2004;24:8068–74. doi: 10.1523/JNEUROSCI.2419-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hablitz LM, Gunesch AN, Cravetchi O, Moldavan M, Allen CN. Cannabinoid Signaling Recruits Astrocytes to Modulate Presynaptic Function in the Suprachiasmatic Nucleus. eNeuro. 2020;7:ENEURO.0081-19.2020. [DOI] [PMC free article] [PubMed]
- 123.Robin LM, Oliveira da Cruz JF, Langlais VC, Martin-Fernandez M, Metna-Laurent M, Busquets-Garcia A, et al. Astroglial CB1 Receptors Determine Synaptic D-Serine Availability to Enable Recognition Memory. Neuron. 2018;98:935–44 e5. doi: 10.1016/j.neuron.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 124.Martin-Fernandez M, Jamison S, Robin LM, Zhao Z, Martin ED, Aguilar J, et al. Synapse-specific astrocyte gating of amygdala-related behavior. Nat Neurosci. 2017;20:1540–8. doi: 10.1038/nn.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Min R, Nevian T. Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nature Neuroscience. 2012;15:746–53. doi: 10.1038/nn.3075. [DOI] [PubMed] [Google Scholar]
- 126.Navarrete M, Araque A. Endocannabinoids mediate neuron-astrocyte communication. Neuron. 2008;57:883–93. doi: 10.1016/j.neuron.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 127.Navarrete M, Araque A. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron. 2010;68:113–26. doi: 10.1016/j.neuron.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 128.Covelo A, Araque A Neuronal activity determines distinct gliotransmitter release from a single astrocyte. Elife. 2018;7. [DOI] [PMC free article] [PubMed]
- 129.Bartova A, Birmingham MK. Effect of delta9-tetrahydrocannabinol on mitochondrial NADH-oxidase activity. Journal of Biological Chemistry. 1976;251:5002–6. [PubMed] [Google Scholar]
- 130.Lipina C, Irving AJ, Hundal HS. Mitochondria: a possible nexus for the regulation of energy homeostasis by the endocannabinoid system? Am J Physiol Endocrinol Metab. 2014;307:E1–13. doi: 10.1152/ajpendo.00100.2014. [DOI] [PubMed] [Google Scholar]
- 131.Benard G, Massa F, Puente N, Lourenco J, Bellocchio L, Soria-Gomez E, et al. Mitochondrial CB(1) receptors regulate neuronal energy metabolism. Nat Neurosci. 2012;15:558–64. doi: 10.1038/nn.3053. [DOI] [PubMed] [Google Scholar]
- 132.Gutierrez-Rodriguez A, Bonilla-Del Rio I, Puente N, Gomez-Urquijo SM, Fontaine CJ, Egana-Huguet J, et al. Localization of the cannabinoid type-1 receptor in subcellular astrocyte compartments of mutant mouse hippocampus. Glia. 2018;66:1417–31. doi: 10.1002/glia.23314. [DOI] [PubMed] [Google Scholar]
- 133.Hebert-Chatelain E, Reguero L, Puente N, Lutz B, Chaouloff F, Rossignol R, et al. Cannabinoid control of brain bioenergetics: Exploring the subcellular localization of the CB1 receptor. Mol Metab. 2014;3:495–504. doi: 10.1016/j.molmet.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hebert-Chatelain E, Desprez T, Serrat R, Bellocchio L, Soria-Gomez E, Busquets-Garcia A, et al. A cannabinoid link between mitochondria and memory. Nature. 2016;539:555–9. doi: 10.1038/nature20127. [DOI] [PubMed] [Google Scholar]
- 135.Jimenez-Blasco D, Busquets-Garcia A, Hebert-Chatelain E, Serrat R, Vicente-Gutierrez C, Ioannidou C, et al. Glucose metabolism links astroglial mitochondria to cannabinoid effects. Nature. 2020;583:603–8. doi: 10.1038/s41586-020-2470-y. [DOI] [PubMed] [Google Scholar]
- 136.Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–23. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Skosnik PD, Cortes-Briones JA, Hajós M. It’s all in the rhythm: the role of cannabinoids in neural oscillations and psychosis. Biological psychiatry. 2016;79:568–77. doi: 10.1016/j.biopsych.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 138.Lisman J, Buzsáki G. A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophrenia bulletin. 2008;34:974–80. doi: 10.1093/schbul/sbn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hájos N, Katona I, Naiem S, Mackie K, Ledent C, Mody I, et al. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. European Journal of Neuroscience. 2000;12:3239–49. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- 140.Robbe D, Montgomery SM, Thome A, Rueda-Orozco PE, McNaughton BL, Buzsaki G. Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nature neuroscience. 2006;9:1526–33. doi: 10.1038/nn1801. [DOI] [PubMed] [Google Scholar]
- 141.Raver SM, Haughwout SP, Keller A. Adolescent cannabinoid exposure permanently suppresses cortical oscillations in adult mice. Neuropsychopharmacology. 2013;38:2338–47. doi: 10.1038/npp.2013.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cass DK, Flores-Barrera E, Thomases DR, Vital WF, Caballero A, Tseng KY. CB1 cannabinoid receptor stimulation during adolescence impairs the maturation of GABA function in the adult rat prefrontal cortex. Molecular psychiatry. 2014;19:536–43. doi: 10.1038/mp.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ilan AB, Smith ME, Gevins A. Effects of marijuana on neurophysiological signals of working and episodic memory. Psychopharmacology. 2004;176:214–22. doi: 10.1007/s00213-004-1868-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Skosnik PD, D'souza DC, Steinmetz AB, Edwards CR, Vollmer JM, Hetrick WP, et al. The effect of chronic cannabinoids on broadband EEG neural oscillations in humans. Neuropsychopharmacology. 2012;37:2184–93. doi: 10.1038/npp.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cortes-Briones J, Skosnik PD, Mathalon D, Cahill J, Pittman B, Williams A, et al. Δ9-THC disrupts gamma (γ)-band neural oscillations in humans. Neuropsychopharmacology. 2015;40:2124–34. doi: 10.1038/npp.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Morrison PD, Nottage J, Stone JM, Bhattacharyya S, Tunstall N, Brenneisen R, et al. Disruption of frontal theta coherence by Δ9-tetrahydrocannabinol is associated with positive psychotic symptoms. Neuropsychopharmacology. 2011;36:827–36. doi: 10.1038/npp.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cheung KAK, Peiris H, Wallace G, Holland OJ, Mitchell MD. The Interplay between the Endocannabinoid System, Epilepsy and Cannabinoids. Int J Mol Sci. 2019;20:6079. [DOI] [PMC free article] [PubMed]
- 148.Soltesz I, Alger BE, Kano M, Lee SH, Lovinger DM, Ohno-Shosaku T, et al. Weeding out bad waves: towards selective cannabinoid circuit control in epilepsy. Nat Rev Neurosci. 2015;16:264–77. doi: 10.1038/nrn3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vega-Garcia A, Feria-Romero I, Garcia-Juarez A, Munguia-Madera AC, Montes-Aparicio AV, Zequeida-Munoz E, et al. Cannabinoids: A New Perspective on Epileptogenesis and Seizure Treatment in Early Life in Basic and Clinical Studies. Front Behav Neurosci. 2020;14:610484. doi: 10.3389/fnbeh.2020.610484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Guggenhuber S, Romo-Parra H, Bindila L, Leschik J, Lomazzo E, Remmers F, et al. Impaired 2-AG Signaling in Hippocampal Glutamatergic Neurons: Aggravation of Anxiety-Like Behavior and Unaltered Seizure Susceptibility. Int J Neuropsychopharmacol. 2015;19:pyv091. [DOI] [PMC free article] [PubMed]
- 151.Ohno-Shosaku T, Tsubokawa H, Mizushima I, Yoneda N, Zimmer A, Kano M. Presynaptic Cannabinoid Sensitivity Is a Major Determinant of Depolarization-Induced Retrograde Suppression at Hippocampal Synapses. The Journal of Neuroscience. 2002;22:3864–72. doi: 10.1523/JNEUROSCI.22-10-03864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bhaskaran MD, Smith BN. Cannabinoid-mediated inhibition of recurrent excitatory circuitry in the dentate gyrus in a mouse model of temporal lobe epilepsy. PLoS ONE. 2010;5:e10683. doi: 10.1371/journal.pone.0010683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wallace MJ, Blair RE, Falenski KW, Martin BR, DeLorenzo RJ. The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J Pharmacol Exp Ther. 2003;307:129–37. doi: 10.1124/jpet.103.051920. [DOI] [PubMed] [Google Scholar]
- 154.Deshpande LS, Sombati S, Blair R, Carter D, Martin BR, DeLorenzo RJ. Cannabinoid CB1 receptor antagonists cause status epilepticuslike activity in the hippocampal neuronal culture model of acquired epilepsy. Neurosci Lett. 2007;411:11–4. doi: 10.1016/j.neulet.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Deshpande LS, Blair RE, Ziobro JM, Sombati S, Martin BR, DeLorenzo RJ. Endocannabinoids block status epilepticus in cultured hippocampal neurons. Eur J Pharmacol. 2007;558:52–9. doi: 10.1016/j.ejphar.2006.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sugaya Y, Yamazaki M, Uchigashima M, Kobayashi K, Watanabe M, Sakimura K, et al. Crucial Roles of the Endocannabinoid 2-Arachidonoylglycerol in the Suppression of Epileptic Seizures. Cell Rep. 2016;16:1405–15. doi: 10.1016/j.celrep.2016.06.083. [DOI] [PubMed] [Google Scholar]
- 157.Griebel G, Pichat P, Beeske S, Leroy T, Redon N, Jacquet A, et al. Selective blockade of the hydrolysis of the endocannabinoid 2-arachidonoylglycerol impairs learning and memory performance while producing antinociceptive activity in rodents. Sci Rep. 2015;5:7642. doi: 10.1038/srep07642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Monory K, Massa F, Egertova M, Eder M, Heike B, Westenbroek R, et al. The Endocannabinoid System Controls Key Epileptogenic Circuits in the Hippocampus. Neuron. 2006;51:455–66. doi: 10.1016/j.neuron.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kovacs FE, Knop T, Urbanski MJ, Freiman I, Freiman TM, Feuerstein TJ, et al. Exogenous and endogenous cannabinoids suppress inhibitory neurotransmission in the human neocortex. Neuropsychopharmacology. 2012;37:1104–14. doi: 10.1038/npp.2011.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen K, Neu A, Howard AL, Foldy C, Echegoyen J, Hilgenberg L, et al. Prevention of plasticity of endocannabinoid signaling inhibits persistent limbic hyperexcitability caused by developmental seizures. J Neurosci. 2007;27:46–58. doi: 10.1523/JNEUROSCI.3966-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen K, Ratzliff A, Hilgenberg L, Gulyás A, Freund TF, Smith M, et al. Long-Term Plasticity of Endocannabinoid Signaling Induced by Developmental Febrile Seizures. Neuron. 2003;39:599–611. doi: 10.1016/s0896-6273(03)00499-9. [DOI] [PubMed] [Google Scholar]
- 162.Dong A, He K, Dudok B, Farrell JS, Guan W, Liput DJ, et al. A fluorescent sensor for spatiotemporally resolved imaging of endocannabinoid dynamics in vivo. Nat Biotechnol. 2022;40:787–98. [DOI] [PMC free article] [PubMed]
- 163.Farrell JS, Colangeli R, Dong A, George AG, Addo-Osafo K, Kingsley PJ, et al. In vivo endocannabinoid dynamics at the timescale of physiological and pathological neural activity. Neuron. 2021;109:2398–+. doi: 10.1016/j.neuron.2021.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cleeren E, Casteels C, Goffin K, Koole M, Van Laere K, Janssen P, et al. Positron emission tomography imaging of cerebral glucose metabolism and type 1 cannabinoid receptor availability during temporal lobe epileptogenesis in the amygdala kindling model in rhesus monkeys. Epilepsia. 2018;59:959–70. doi: 10.1111/epi.14059. [DOI] [PubMed] [Google Scholar]
- 165.Ludanyi A, Eross L, Czirjak S, Vajda J, Halasz P, Watanabe M, et al. Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. J Neurosci. 2008;28:2976–90. doi: 10.1523/JNEUROSCI.4465-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sugaya Y, Kano M. Endocannabinoid-Mediated Control of Neural Circuit Excitability and Epileptic Seizures. Front Neural Circuits. 2021;15:781113. doi: 10.3389/fncir.2021.781113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rocha L, Cinar R, Guevara-Guzman R, Alonso-Vanegas M, San-Juan D, Martinez-Juarez I, et al. Endocannabinoid System and Cannabinoid 1 Receptors in Patients With Pharmacoresistant Temporal Lobe Epilepsy and Comorbid Mood Disorders. Front Behav Neurosci. 2020;14:52. doi: 10.3389/fnbeh.2020.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Romigi A, Bari M, Placidi F, Marciani MG, Malaponti M, Torelli F, et al. Cerebrospinal fluid levels of the endocannabinoid anandamide are reduced in patients with untreated newly diagnosed temporal lobe epilepsy. Epilepsia. 2010;51:768–72. doi: 10.1111/j.1528-1167.2009.02334.x. [DOI] [PubMed] [Google Scholar]
- 169.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature reviews neuroscience. 2009;10:434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 170.Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic Glucocorticoid Inhibition via Endocannabinoid Release in the Hypothalamus: A Fast Feedback Mechanism. The Journal of Neuroscience. 2003;23:4850–7. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Evanson NK, Tasker JG, Hill MN, Hillard CJ, Herman JP. Fast Feedback Inhibition of the HPA Axis by Glucocorticoids Is Mediated by Endocannabinoid Signaling. Endocrinology. 2010;151:4811–9. doi: 10.1210/en.2010-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wamsteeker JI, Kuzmiski JB, Bains JS. Repeated stress impairs endocannabinoid signaling in the paraventricular nucleus of the hypothalamus. J Neurosci. 2010;30:11188–96. doi: 10.1523/JNEUROSCI.1046-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Colmers PLW, Bains JS. Presynaptic mGluRs Control the Duration of Endocannabinoid-Mediated DSI. J Neurosci. 2018;38:10444–53. doi: 10.1523/JNEUROSCI.1097-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rossi S, De Chiara V, Musella A, Kusayanagi H, Mataluni G, Bernardi G, et al. Chronic psychoemotional stress impairs cannabinoid-receptor-mediated control of GABA transmission in the striatum. J Neurosci. 2008;28:7284–92. doi: 10.1523/JNEUROSCI.5346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Patel S, Kingsley PJ, Mackie K, Marnett LJ, Winder DG. Repeated homotypic stress elevates 2-arachidonoylglycerol levels and enhances short-term endocannabinoid signaling at inhibitory synapses in basolateral amygdala. Neuropsychopharmacology. 2009;34:2699–709. doi: 10.1038/npp.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mailleux P, Vanderhaeghen J-J. Glucocorticoid regulation of cannabinoid receptor messenger RNA levels in the rat caudate-putamen. An in situ hybridization study. Neuroscience Letters. 1993;156:51–3. [DOI] [PubMed]
- 177.Yong Y, Cakir I, Lining Pan P, Biddinger JE, Bluett RJ, Mackie K, et al. Endogenous cannabinoids are required for MC4R-mediated control of energy homeostasis. Proc Natl Acad Sci U S A. 2021;118:e2015990118. [DOI] [PMC free article] [PubMed]
- 178.Young EA, Akana S, Dallman MF. Decreased Sensitivity to Glucocorticoid Fast Feedback in Chronically Stressed Rats. Neuroendocrinology. 1990;51:536–42. doi: 10.1159/000125388. [DOI] [PubMed] [Google Scholar]
- 179.Ramikie TS, Patel S. Endocannabinoid signaling in the amygdala: anatomy, synaptic signaling, behavior, and adaptations to stress. Neuroscience. 2012;204:38–52. doi: 10.1016/j.neuroscience.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sears RM, Schiff HC, LeDoux JE. Molecular mechanisms of threat learning in the lateral nucleus of the amygdala. Prog Mol Biol Transl Sci. 2014;122:263–304. doi: 10.1016/B978-0-12-420170-5.00010-6. [DOI] [PubMed] [Google Scholar]
- 181.Tye KM. Neural Circuit Motifs in Valence Processing. Neuron. 2018;100:436–52. doi: 10.1016/j.neuron.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Azad SC, Eder M, Marsicano G, Lutz B, Zieglgansberger W, Rammes G. Activation of the cannabinoid receptor type 1 decreases glutamatergic and GABAergic synaptic transmission in the lateral amygdala of the mouse. Learn Mem. 2003;10:116–28. doi: 10.1101/lm.53303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, et al. Distribution of CB1 Cannabinoid Receptors in the Amygdala and their Role in the Control of GABAergic Transmission. The Journal of Neuroscience. 2001;21:9506–18. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ramikie TS, Nyilas R, Bluett RJ, Gamble-George JC, Hartley ND, Mackie K, et al. Multiple mechanistically distinct modes of endocannabinoid mobilization at central amygdala glutamatergic synapses. Neuron. 2014;81:1111–25. doi: 10.1016/j.neuron.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yoshida T, Uchigashima M, Yamasaki M, Katona I, Yamazaki M, Sakimura K, et al. Unique inhibitory synapse with particularly rich endocannabinoid signaling machinery on pyramidal neurons in basal amygdaloid nucleus. Proc Natl Acad Sci U S A. 2011;108:3059–64. doi: 10.1073/pnas.1012875108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Huang Y-C, Wang S-J, Chiou L-C, Gean P-W. Mediation of Amphetamine-Induced Long-Term Depression of Synaptic Transmission by CB1 Cannabinoid Receptors in the Rat Amygdala. The Journal of Neuroscience. 2003;23:10311–20. doi: 10.1523/JNEUROSCI.23-32-10311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sumislawski JJ, Ramikie TS, Patel S. Reversible gating of endocannabinoid plasticity in the amygdala by chronic stress: a potential role for monoacylglycerol lipase inhibition in the prevention of stress-induced behavioral adaptation. Neuropsychopharmacology. 2011;36:2750–61. doi: 10.1038/npp.2011.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Patel S, Roelke CT, Rademacher DJ, Hillard CJ. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci. 2005;21:1057–69. doi: 10.1111/j.1460-9568.2005.03916.x. [DOI] [PubMed] [Google Scholar]
- 189.Bedse G, Hartley ND, Neale E, Gaulden AD, Patrick TA, Kingsley PJ, et al. Functional Redundancy Between Canonical Endocannabinoid Signaling Systems in the Modulation of Anxiety. Biol Psychiatry. 2017;82:488–99. doi: 10.1016/j.biopsych.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Di S, Itoga CA, Fisher MO, Solomonow J, Roltsch EA, Gilpin NW, et al. Acute Stress Suppresses Synaptic Inhibition and Increases Anxiety via Endocannabinoid Release in the Basolateral Amygdala. J Neurosci. 2016;36:8461–70. doi: 10.1523/JNEUROSCI.2279-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bluett RJ, Baldi R, Haymer A, Gaulden AD, Hartley ND, Parrish WP, et al. Endocannabinoid signalling modulates susceptibility to traumatic stress exposure. Nat Commun. 2017;8:14782. doi: 10.1038/ncomms14782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bedse G, Hill MN, Patel S. 2-Arachidonoylglycerol Modulation of Anxiety and Stress Adaptation: From Grass Roots to Novel Therapeutics. Biol Psychiatry. 2020;88:520–30. doi: 10.1016/j.biopsych.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hartley ND, Gunduz-Cinar O, Halladay L, Bukalo O, Holmes A, Patel S. 2-arachidonoylglycerol signaling impairs short-term fear extinction. Transl Psychiatry. 2016;6:e749. doi: 10.1038/tp.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Llorente-Berzal A, Terzian AL, di Marzo V, Micale V, Viveros MP, Wotjak CT. 2-AG promotes the expression of conditioned fear via cannabinoid receptor type 1 on GABAergic neurons. Psychopharmacology (Berl). 2015;232:2811–25. doi: 10.1007/s00213-015-3917-y. [DOI] [PubMed] [Google Scholar]
- 195.Johansson M, Stomrud E, Johansson PM, Svenningsson A, Palmqvist S, Janelidze S, et al. Development of Apathy, Anxiety, and Depression in Cognitively Unimpaired Older Adults: Effects of Alzheimer's Disease Pathology and Cognitive Decline. Biol Psychiatry. 2022;92:34–43. [DOI] [PubMed]
- 196.Hill MN, Kumar SA, Filipski SB, Iverson M, Stuhr KL, Keith JM, et al. Disruption of fatty acid amide hydrolase activity prevents the effects of chronic stress on anxiety and amygdalar microstructure. Mol Psychiatry. 2013;18:1125–35. doi: 10.1038/mp.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gray JM, Vecchiarelli HA, Morena M, Lee TT, Hermanson DJ, Kim AB, et al. Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J Neurosci. 2015;35:3879–92. doi: 10.1523/JNEUROSCI.2737-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yasmin F, Colangeli R, Morena M, Filipski S, van der Stelt M, Pittman QJ, et al. Stress-induced modulation of endocannabinoid signaling leads to delayed strengthening of synaptic connectivity in the amygdala. Proc Natl Acad Sci U S A. 2020;117:650–5. doi: 10.1073/pnas.1910322116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hill MN, Campolongo P, Yehuda R, Patel S. Integrating Endocannabinoid Signaling and Cannabinoids into the Biology and Treatment of Posttraumatic Stress Disorder. Neuropsychopharmacology. 2018;43:80–102. doi: 10.1038/npp.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hill MN, Miller GE, Ho WS, Gorzalka BB, Hillard CJ. Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry. 2008;41:48–53. doi: 10.1055/s-2007-993211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hill MN, Bierer LM, Makotkine I, Golier JA, Galea S, McEwen BS, et al. Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the World Trade Center attacks. Psychoneuroendocrinology. 2013;38:2952–61. doi: 10.1016/j.psyneuen.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Weisser S, Mueller M, Rauh J, Esser R, Fuss J, Lutz B, et al. Acquisition of threat responses are associated with elevated plasma concentration of endocannabinoids in male humans. Neuropsychopharmacology. 2022 10.1038/s41386-022-01320-6. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 203.Mayo LM, Asratian A, Linde J, Morena M, Haataja R, Hammar V, et al. Elevated Anandamide, Enhanced Recall of Fear Extinction, and Attenuated Stress Responses Following Inhibition of Fatty Acid Amide Hydrolase: A Randomized, Controlled Experimental Medicine Trial. Biol Psychiatry. 2020;87:538–47. doi: 10.1016/j.biopsych.2019.07.034. [DOI] [PubMed] [Google Scholar]
- 204.Cristino L, Palomba L, Di Marzo V. New horizons on the role of cannabinoid CB1 receptors in palatable food intake, obesity and related dysmetabolism. Int J Obes Suppl. 2014;4:S26–30. doi: 10.1038/ijosup.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life sciences. 1998;63:PL113–PL7. doi: 10.1016/s0024-3205(98)00322-1. [DOI] [PubMed] [Google Scholar]
- 206.Massa F, Mancini G, Schmidt H, Steindel F, Mackie K, Angioni C, et al. Alterations in the hippocampal endocannabinoid system in diet-induced obese mice. J Neurosci. 2010;30:6273–81. doi: 10.1523/JNEUROSCI.2648-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.McNamara RK, Almeida DM. Omega-3 Polyunsaturated Fatty Acid Deficiency and Progressive Neuropathology in Psychiatric Disorders: A Review of Translational Evidence and Candidate Mechanisms. Harv Rev Psychiatry. 2019;27:94–107. doi: 10.1097/HRP.0000000000000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Thomazeau A, Bosch-Bouju C, Manzoni O, Laye S. Nutritional n-3 PUFA Deficiency Abolishes Endocannabinoid Gating of Hippocampal Long-Term Potentiation. Cereb Cortex. 2017;27:2571–9. doi: 10.1093/cercor/bhw052. [DOI] [PubMed] [Google Scholar]
- 209.Manduca A, Bara A, Larrieu T, Lassalle O, Joffre C, Layé S, et al. Amplification of mGlu5-endocannabinoid signaling rescues behavioral and synaptic deficits in a mouse model of adolescent and adult dietary polyunsaturated fatty acid imbalance. Journal of Neuroscience. 2017;37:6851–68. doi: 10.1523/JNEUROSCI.3516-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Linehan V, Fang LZ, Hirasawa M. Short-term high-fat diet primes excitatory synapses for long-term depression in orexin neurons. J Physiol. 2018;596:305–16. doi: 10.1113/JP275177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Cristino L, Busetto G, Imperatore R, Ferrandino I, Palomba L, Silvestri C, et al. Obesity-driven synaptic remodeling affects endocannabinoid control of orexinergic neurons. Proc Natl Acad Sci U S A. 2013;110:E2229–38. doi: 10.1073/pnas.1219485110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Cota D, Marsicano G, Tschöp M, Grübler Y, Flachskamm C, Schubert M, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. The Journal of clinical investigation. 2003;112:423–31. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Garfield AS, Li C, Madara JC, Shah BP, Webber E, Steger JS, et al. A neural basis for melanocortin-4 receptor–regulated appetite. Nature neuroscience. 2015;18:863–71. doi: 10.1038/nn.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 215.Lord C, Brugha TS, Charman T, Cusack J, Dumas G, Frazier T, et al. Autism spectrum disorder. Nat Rev Dis Primers. 2020;6:5. doi: 10.1038/s41572-019-0138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Crawford DC, Acuna JM, Sherman SL. FMR1 and the fragile X syndrome: human genome epidemiology review. Genet Med. 2001;3:359–71. doi: 10.1097/00125817-200109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Maccarrone M, Rossi S, Bari M, De Chiara V, Rapino C, Musella A, et al. Abnormal mGlu 5 receptor/endocannabinoid coupling in mice lacking FMRP and BC1 RNA. Neuropsychopharmacology. 2010;35:1500–9. doi: 10.1038/npp.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhang L, Alger BE. Enhanced endocannabinoid signaling elevates neuronal excitability in fragile X syndrome. J Neurosci. 2010;30:5724–9. doi: 10.1523/JNEUROSCI.0795-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Straiker A, Min KT, Mackie K. Fmr1 deletion enhances and ultimately desensitizes CB(1) signaling in autaptic hippocampal neurons. Neurobiol Dis. 2013;56:1–5. doi: 10.1016/j.nbd.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Jung KM, Sepers M, Henstridge CM, Lassalle O, Neuhofer D, Martin H, et al. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nat Commun. 2012;3:1080. doi: 10.1038/ncomms2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Neuhofer D, Lassalle O, Manzoni OJ. Muscarinic M1 Receptor Modulation of Synaptic Plasticity in Nucleus Accumbens of Wild-Type and Fragile X Mice. ACS Chem Neurosci. 2018;9:2233–40. doi: 10.1021/acschemneuro.7b00398. [DOI] [PubMed] [Google Scholar]
- 222.Wang W, Cox BM, Jia Y, Le AA, Cox CD, Jung KM, et al. Treating a novel plasticity defect rescues episodic memory in Fragile X model mice. Mol Psychiatry. 2018;23:1798–806. doi: 10.1038/mp.2017.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tang AH, Alger BE. Homer protein-metabotropic glutamate receptor binding regulates endocannabinoid signaling and affects hyperexcitability in a mouse model of fragile X syndrome. J Neurosci. 2015;35:3938–45. doi: 10.1523/JNEUROSCI.4499-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Busquets-Garcia A, Gomis-Gonzalez M, Guegan T, Agustin-Pavon C, Pastor A, Mato S, et al. Targeting the endocannabinoid system in the treatment of fragile X syndrome. Nat Med. 2013;19:603–7. doi: 10.1038/nm.3127. [DOI] [PubMed] [Google Scholar]
- 225.Wei D, Dinh D, Lee D, Li D, Anguren A, Moreno-Sanz G, et al. Enhancement of Anandamide-Mediated Endocannabinoid Signaling Corrects Autism-Related Social Impairment. Cannabis Cannabinoid Res. 2016;1:81–9. doi: 10.1089/can.2015.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, et al. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47:227–41. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 227.Volkow ND, Koob GF, McLellan AT. Neurobiologic Advances from the Brain Disease Model of Addiction. N Engl J Med. 2016;374:363–71. doi: 10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zhang Y, Schlussman SD, Rabkin J, Butelman ER, Ho A, Kreek MJ. Chronic escalating cocaine exposure, abstinence/withdrawal, and chronic re-exposure: effects on striatal dopamine and opioid systems in C57BL/6J mice. Neuropharmacology. 2013;67:259–66. doi: 10.1016/j.neuropharm.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, et al. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–8. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Belin D, Belin-Rauscent A, Murray JE, Everitt BJ. Addiction: failure of control over maladaptive incentive habits. Curr Opin Neurobiol. 2013;23:564–72. doi: 10.1016/j.conb.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 231.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–69. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cohen C, Perrault G, Voltz C, Steinberg R, Soubrié P. SR141716, a central cannabinoid (CB1) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behavioural Pharmacology. 2002;13:451–63. doi: 10.1097/00008877-200209000-00018. [DOI] [PubMed] [Google Scholar]
- 233.Hungund BL, Szakall A, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid CB1 receptor knockout mice exhibit markedly reducted voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- 234.Soria G, Mendizabal V, Tourino C, Robledo P, Ledent C, Parmentier M, et al. Lack of CB1 cannabinoid receptor impairs cocaine self-administration. Neuropsychopharmacology. 2005;30:1670–80. doi: 10.1038/sj.npp.1300707. [DOI] [PubMed] [Google Scholar]
- 235.Tanda G. Cannabinoid and Heroin Activation of Mesolimbic Dopamine Transmission by a Common µ1 Opioid Receptor Mechanism. Science. 1997;276:2048–50. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- 236.Colombo G, Serra S, Brunetti G, Gomez R, Melis S, Vacca G, et al. Stimulation of voluntary ethanol intake by cannabinoid receptor agonists in ethanol-preferring sP rats. Psychopharmacology (Berl). 2002;159:181–7. doi: 10.1007/s002130100887. [DOI] [PubMed] [Google Scholar]
- 237.Gallate JE, Saharov T, Mallet PE, McGregor IS. Increased motivation for beer in rats following administration of a cannabinoid CB1 receptor agonist. European Journal of Pharmacology. 1999;370:233–40. doi: 10.1016/s0014-2999(99)00170-3. [DOI] [PubMed] [Google Scholar]
- 238.Valjent E, Mitchell JM, Besson M, Caboche J, Maldonado R. Behavioural and biochemical evidence for interactions between ∆9-tetrahydrocannabinol and nicotine. British Journal of Pharmacology. 2002;135:564–78. doi: 10.1038/sj.bjp.0704479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Navarro M, Carrera MR, Del Arco I, Trigo JM, Koob GF, Rodriguez de Fonseca F. Cannabinoid receptor antagonist reduces heroin self-administration only in dependent rats. Eur J Pharmacol. 2004;501:235–7. doi: 10.1016/j.ejphar.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 240.Navarro M, Carrera MRA, Fratta W, Valverde O, Cossu G, Fattore L, et al. Functional Interaction between Opioid and Cannabinoid Receptors in Drug Self-Administration. The Journal of Neuroscience. 2001;21:5344–50. doi: 10.1523/JNEUROSCI.21-14-05344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Arnone M, Maruani J, Chaperon F, Thiébot MH, Poncelet M, Soubrié P, et al. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology. 1997;132:104–6. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- 242.Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur J Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 243.Tourino C, Ledent C, Maldonado R, Valverde O. CB1 cannabinoid receptor modulates 3,4-methylenedioxymethamphetamine acute responses and reinforcement. Biol Psychiatry. 2008;63:1030–8. doi: 10.1016/j.biopsych.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 244.Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29:225–32. doi: 10.1016/j.tins.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 245.Parsons LH, Hurd YL. Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci. 2015;16:579–94. doi: 10.1038/nrn4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Melis M, Pistis M, Perra S, Muntoni AL, Pillolla G, Gessa GL. Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J Neurosci. 2004;24:53–62. doi: 10.1523/JNEUROSCI.4503-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Haj-Dahmane S, Shen RY. Regulation of plasticity of glutamate synapses by endocannabinoids and the cyclic-AMP/protein kinase A pathway in midbrain dopamine neurons. J Physiol. 2010;588:2589–604. doi: 10.1113/jphysiol.2010.190066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Pan B, Hillard CJ, Liu QS. Endocannabinoid signaling mediates cocaine-induced inhibitory synaptic plasticity in midbrain dopamine neurons. J Neurosci. 2008;28:1385–97. doi: 10.1523/JNEUROSCI.4033-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Pan B, Hillard CJ, Liu QS. D2 dopamine receptor activation facilitates endocannabinoid-mediated long-term synaptic depression of GABAergic synaptic transmission in midbrain dopamine neurons via cAMP-protein kinase A signaling. J Neurosci. 2008;28:14018–30. doi: 10.1523/JNEUROSCI.4035-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Buczynski MW, Herman MA, Hsu KL, Natividad LA, Irimia C, Polis IY, et al. Diacylglycerol lipase disinhibits VTA dopamine neurons during chronic nicotine exposure. Proc Natl Acad Sci U S A. 2016;113:1086–91. doi: 10.1073/pnas.1522672113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Friend L, Weed J, Sandoval P, Nufer T, Ostlund I, Edwards JG. CB1-Dependent Long-Term Depression in Ventral Tegmental Area GABA Neurons: A Novel Target for Marijuana. J Neurosci. 2017;37:10943–54. doi: 10.1523/JNEUROSCI.0190-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wang R, Hausknecht KA, Gancarz-Kausch AM, Oubraim S, Shen R-Y, Haj-Dahmane S. Cocaine self-administration abolishes endocannabinoid-mediated long-term depression of glutamatergic synapses in the ventral tegmental area. Eur J Neurosci. 2020;52:4517–24. doi: 10.1111/ejn.14980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Hausknecht K, Shen YL, Wang RX, Haj-Dahmane S, Shen RY. Prenatal Ethanol Exposure Persistently Alters Endocannabinoid Signaling and Endocannabinoid-Mediated Excitatory Synaptic Plasticity in Ventral Tegmental Area Dopamine Neurons. J Neurosci. 2017;37:5798–808. doi: 10.1523/JNEUROSCI.3894-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Harlan BA, Becker HC, Woodward JJ, Riegel AC. Opposing actions of CRF-R1 and CB1 receptors on VTA-GABAergic plasticity following chronic exposure to ethanol. Neuropsychopharmacology. 2018;43:2064–74. doi: 10.1038/s41386-018-0106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Liu Q, Lu P, Poo M. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–31. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Winters ND, Bedse G, Astafyev AA, Patrick TA, Altemus M, Morgan AJ, et al. Targeting diacylglycerol lipase reduces alcohol consumption in preclinical models. J Clin Invest. 2021;131:e146861. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 257.Loweth JA, Scheyer AF, Milovanovic M, LaCrosse AL, Flores-Barrera E, Werner CT, et al. Synaptic depression via mGluR1 positive allosteric modulation suppresses cue-induced cocaine craving. Nat Neurosci. 2014;17:73–80. doi: 10.1038/nn.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Scheyer AF, Loweth JA, Christian DT, Uejima J, Rabei R, Le T, et al. AMPA Receptor Plasticity in Accumbens Core Contributes to Incubation of Methamphetamine Craving. Biol Psychiatry. 2016;80:661–70. doi: 10.1016/j.biopsych.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.McCutcheon JE, Loweth JA, Ford KA, Marinelli M, Wolf ME, Tseng KY, Group I. mGluR activation reverses cocaine-induced accumulation of calcium-permeable AMPA receptors in nucleus accumbens synapses via a protein kinase C-dependent mechanism. J Neurosci. 2011;31:14536–41. doi: 10.1523/JNEUROSCI.3625-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Clarke RB, Adermark L. Acute ethanol treatment prevents endocannabinoid-mediated long-lasting disinhibition of striatal output. Neuropharmacology. 2010;58:799–805. doi: 10.1016/j.neuropharm.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 261.Fourgeaud L, Mato S, Bouchet D, Hemar A, Worley PF, Manzoni OJ. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J Neurosci. 2004;24:6939–45. doi: 10.1523/JNEUROSCI.0671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Grueter BA, Gosnell HB, Olsen CM, Schramm-Sapyta NL, Nekrasova T, Landreth GE, et al. Extracellular-signal regulated kinase 1-dependent metabotropic glutamate receptor 5-induced long-term depression in the bed nucleus of the stria terminalis is disrupted by cocaine administration. J Neurosci. 2006;26:3210–9. doi: 10.1523/JNEUROSCI.0170-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Reisiger AR, Kaufling J, Manzoni O, Cador M, Georges F, Caille S. Nicotine self-administration induces CB1-dependent LTP in the bed nucleus of the stria terminalis. J Neurosci. 2014;34:4285–92. doi: 10.1523/JNEUROSCI.3149-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Erdozain AM, Rubio M, Valdizan EM, Pazos A, Meana JJ, Fernandez-Ruiz J, et al. The endocannabinoid system is altered in the post-mortem prefrontal cortex of alcoholic subjects. Addict Biol. 2015;20:773–83. doi: 10.1111/adb.12160. [DOI] [PubMed] [Google Scholar]
- 265.Vinod KY, Kassir SA, Hungund BL, Cooper TB, Mann JJ, Arango V. Selective alterations of the CB1 receptors and the fatty acid amide hydrolase in the ventral striatum of alcoholics and suicides. J Psychiatr Res. 2010;44:591–7. doi: 10.1016/j.jpsychires.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Alváro-Bartolomé M, Garcià-Sevilla JA. Dysregulation of cannabinoid CB1 receptor and associated signaling networks in brains of cocaine addicts and cocaine-treated rodents. Neuroscience. 2013;247:294–308. doi: 10.1016/j.neuroscience.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 267.Huggins JP, Smart TS, Langman S, Taylor L, Young T. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain. 2012;153:1837–46. doi: 10.1016/j.pain.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 268.Jiang M, van der Stelt M. Activity-Based Protein Profiling Delivers Selective Drug Candidate ABX-1431, a Monoacylglycerol Lipase Inhibitor, To Control Lipid Metabolism in Neurological Disorders. J Med Chem. 2018;61:9059–61. doi: 10.1021/acs.jmedchem.8b01405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Muller-Vahl KR, Fremer C, Beals C, Ivkovic J, Loft H, Schindler C. Endocannabinoid Modulation Using Monoacylglycerol Lipase Inhibition in Tourette Syndrome: A Phase 1 Randomized, Placebo-Controlled Study. Pharmacopsychiatry. 2022;55:148–56. doi: 10.1055/a-1675-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Papa A, Pasquini S, Contri C, Gemma S, Campiani G, Butini S, et al. Polypharmacological Approaches for CNS Diseases: Focus on Endocannabinoid Degradation Inhibition. Cells. 2022;11:471. [DOI] [PMC free article] [PubMed]