Abstract
Background
Fragile X syndrome (FXS) is associated with dysregulated endocannabinoid signaling and may therefore respond to cannabidiol therapy.
Design
CONNECT-FX was a double-blind, randomized phase 3 trial assessing efficacy and safety of ZYN002, transdermal cannabidiol gel, for the treatment of behavioral symptoms in children and adolescents with FXS.
Methods
Patients were randomized to 12 weeks of ZYN002 (250 mg or 500 mg daily [weight-based]) or placebo, as add-on to standard of care. The primary endpoint assessed change in social avoidance (SA) measured by the Aberrant Behavior Checklist–Community Edition FXS (ABC-CFXS) SA subscale in a full cohort of patients with a FXS full mutation, regardless of the FMR1 methylation status. Ad hoc analyses assessed efficacy in patients with ≥ 90% and 100% methylation of the promoter region of the FMR1 gene, in whom FMR1 gene silencing is most likely.
Results
A total of 212 patients, mean age 9.7 years, 75% males, were enrolled. A total of 169 (79.7%) patients presented with ≥ 90% methylation of the FMR1 promoter and full mutation of FMR1. Although statistical significance for the primary endpoint was not achieved in the full cohort, significant improvement was demonstrated in patients with ≥ 90% methylation of FMR1 (nominal P = 0.020). This group also achieved statistically significant improvements in Caregiver Global Impression‐Change in SA and isolation, irritable and disruptive behaviors, and social interactions (nominal P-values: P = 0.038, P = 0.028, and P = 0.002). Similar results were seen in patients with 100% methylation of FMR1. ZYN002 was safe and well tolerated. All treatment-emergent adverse events (TEAEs) were mild or moderate. The most common treatment-related TEAE was application site pain (ZYN002: 6.4%; placebo: 1.0%).
Conclusions
In CONNECT-FX, ZYN002 was well tolerated in patients with FXS and demonstrated evidence of efficacy with a favorable benefit risk relationship in patients with ≥ 90% methylation of the FMR1 gene, in whom gene silencing is most likely, and the impact of FXS is typically most severe.
Trial registration
The CONNECT-FX trial is registered on Clinicaltrials.gov (NCT03614663).
Supplementary Information
The online version contains supplementary material available at 10.1186/s11689-022-09466-6.
Keywords: Fragile X syndrome, Clinical trial, Endocannabinoid system, Cannabidiol
Introduction
Fragile X syndrome
Fragile X syndrome (FXS) is a rare X-linked genetic disorder that has a prevalence of approximately 1 in 4000 males and 1 in 6000 females [1]. FXS is the most common inherited cause of intellectual disability and monogenetic cause of autism spectrum disorder (ASD) and is also associated with anxiety and a variety of problem behaviors such as aggression, irritability, temper tantrums, hyperactivity, attention deficits, shyness, and preference for solitary activities [2, 3]. Despite decades of preclinical research and interventional clinical trials, there are no regulatory approved treatments for FXS.
FXS pathophysiology
FXS is typically caused by a trinucleotide repeat expansion containing more than 200 cytosine, guanine, and guanine (CGG) repeats in the 5′ untranslated region of the FMR1 gene on the X chromosome (full mutation, [FM]). This generally leads to methylation of the promoter region of FMR1, producing subsequent gene silencing and absent or reduced levels of the protein product, FMRP [4–9]. Thus, FXS is caused by the deficiency or absence of FMRP [10].
FMRP is an RNA-binding protein that is important for normal synaptic function, synaptic plasticity, and the development of neuronal connections over time during brain maturation [11]. In general, the FXS cognitive and emotional phenotype depends on the amount of FMRP that is produced, which is determined in part by the degree of methylation of FMR1 [9, 12]. Males with a fully methylated FM generally do not produce FMRP, while FMRP can range from near normal to significantly reduced in females with a fully methylated FM of FMR1, depending on the pattern of X-inactivation in the affected female [13, 14]. In general, patients with a higher degree of methylation have a more severe phenotype, including lower IQ and more severe symptoms of ASD, although there is wide variability for any given level of methylation [14]. Some individuals with FXS are mosaics and can present with a high degree of mosaicism due to the presence of a percentage of cells with the FMR1 premutation (i.e., 55 to 200 CGG repeats) in addition to cells with a full mutation. They can also present with incomplete methylation and can produce elevated FMR1 mRNA, which can cause toxicity to the cells of the central nervous system (CNS). Those with > 90% methylation produce lower levels of FMR1 mRNA and little or no FMRP. Therefore, they present the classical and most severe phenotype of FXS that is recapitulated by the knockout mouse model of FXS.
Endocannabinoid system is dysregulated in FXS
The endocannabinoid (EC) system includes 2 types of G-protein–coupled receptors, termed CB1 and CB2 [15, 16]. Cannabinoid receptors are found in a variety of diverse organisms [17], indicating that the EC system is highly evolutionarily conserved and may play central roles in human physiology and pathophysiology [18]. CB1 receptors are among the most abundant G-protein–coupled receptors in the brain and are present at lower concentrations in a variety of peripheral tissues and cells [19]. CB2 receptors are expressed primarily in the immune and hematopoietic systems, as well as in the brain, pancreas, and bone.
The primary endogenous ligands for CB1 and CB2 receptors are called ECs and include anandamide (AEA) and 2-arachidonoylglycerol (2-AG). The ECs modulate synaptic transmission throughout the CNS, yielding widespread influence on cognition and behavior [20, 21]. The ECs are synthesized and released “on demand” from post-synaptic membrane-bound phospholipids in response to neuronal signaling and act as retrograde signaling molecules across the synaptic cleft to stimulate CB1 receptors on the presynaptic terminal [15, 22] and attenuate further activity through an inhibitory feedback loop. Enzymes that function in synthesizing 2-AG include phospholipase C and diacylglycerol lipase (DAGL) [15, 23]. At developed synapses, 2-AG released from postsynaptic terminals binds to presynaptic CB1 receptors to inhibit the secretion of both excitatory and inhibitory neurotransmitters; this DAGL-dependent synaptic plasticity operates throughout the nervous system [24].
The functional consequences of reduced FMRP in FXS likely reflect changes in both developmental and dynamic regulation of multiple intracellular processes. Among these, loss of FMRP function is thought to alter DAGL function and retrograde EC signaling in neuronal synapses, thereby affecting excitatory and inhibitory neurotransmitter release [24]. Downstream dysregulation of EC signaling in the CNS is one proposed mechanism that may contribute to the clinical abnormalities seen in FXS [24, 25].
Cannabidiol, the main non-euphoric component of the Cannabis plant, has a variety of effects on the EC system that may improve the behavioral symptoms of FXS. These include, (A) attenuating the loss of endogenous, on-demand EC signaling by increasing 2-AG availability and serving as retrograde signaling molecules in the regulation of synaptic transmission through negative allosteric modulation of the CB1 receptor [24, 26, 27]; (B) preventing internalization of CB1 receptors and restoring membrane expression of receptors [28–32]; and (C) increasing the availability of AEA by reducing its access to the catabolic pathway [26, 31, 33–36].
Cannabidiol may also have other effects related to FXS. Cannabidiol binds to the 5HT1A receptor with moderate affinity and possesses agonist efficacy in 5HT1A signal transduction studies [37]. Cannabidiol has also been shown to act as a positive allosteric modulator at GABAA receptors [38]. Cannabidiol’s ability to enhance EC levels and facilitate GABAergic transmission may serve to improve the balance in inhibitory and excitatory transmission and help restore neuronal function and synaptic plasticity in patients with FXS. Cannabidiol is also a dopamine partial agonist [39].
Rationale for the CONNECT-FX trial
ZYN002 is a permeation-enhanced transdermal gel, consisting of a hydro-alcoholic gel containing cannabidiol, which is manufactured at a 4.2% (w/w) cannabidiol concentration. An exploratory phase 2 open-label clinical trial (ZYN2-CL-009) found that 12-week treatment with ZYN002 resulted in clinically meaningful reductions in anxiety and behavioral symptoms in 20 children and adolescents with FXS and full FMR1 mutation [40]. In light of these findings, the placebo-controlled, phase 3, CONNECT-FX trial was conducted to assess the efficacy and safety of ZYN002 for the treatment of behavioral symptoms in children and adolescents with FXS.
Methods
Study design
CONNECT-FX (Study ZYN2-CL-016) was a randomized, double-blind, placebo-controlled efficacy and safety study in pediatric and adolescent patients with FXS aged 3 to < 18 years. The study design for the CONNECT-FX trial is shown in Fig. 1. Patients with FXS were treated for 12 weeks with a 2-week, single-blind placebo run-in preceding the 12-week double-blind treatment period. Following the placebo run-in period, patients who met the following criteria were randomized 1:1 to receive ZYN002 or placebo: ABC-CFXS Social Avoidance (SA) score ≥ 4 prior to randomization, with no more than a 30% improvement during the placebo run-in, or ABC-CFXS SA score of 2 or 3 with an ABC-CFXS Irritability (Irr) score ≥ 18 prior to randomization, with no more than a 30% improvement in ABC-CFXS SA score during the placebo run-in. Randomization was stratified by gender, weight category, and region.
Fig. 1.
Study design. a indicates the following: following a 2-week single-blind placebo run-in period
Dose selection for this trial was based upon findings from the initial open-label trial in patients with FXS in which the majority of individuals were titrated up to 250 mg/day [40]. The open-label data demonstrated a potential efficacy signal with good tolerability. These findings led to the selection of doses of 250 mg/day or 500 mg/day for individuals ≤ 35 kg or > 35 kg respectively in CONNECT-FX, supported by population pharmacokinetic modeling to predicted dosing to provide similar steady-state concentrations found in adults at a dose of 500 mg/day which had been demonstrated to be safe and well tolerated. ZYN002 was provided in sealed individual dose packets containing 125 mg cannabidiol per individual packet and placebo packets matched the study drug dosing packets in appearance and gel contents (without cannabidiol). In a blinded fashion, ZYN002-treated patients who weighed ≤ 35 kg received 125 mg cannabidiol Q12H (1 individual dose packet every 12 h) (± 2 h), for a total daily dose of 250 mg cannabidiol. Patients who weighed > 35 kg received 250 mg cannabidiol (2 dose packets) Q12H (± 2 h), for a total daily dose of 500 mg cannabidiol. All patients remained on their assigned dose during the 12 weeks of the treatment phase of the study. Study visits occurred at week 4, week 8, and week 12 post randomization. Patients who successfully completed the 12 weeks of the double-blind study and were at least 90% compliant with the trial drug and visits had the option to enroll in an open-label extension study.
Study population
The trial was conducted at 21 investigative centers in the USA (17 sites), Australia (3 sites), and New Zealand (1 site). Children and adolescents aged 3 to < 18 years with a body mass index of 12–30 kg/m2 and a diagnosis of FXS through molecular documentation of the full FMR1 mutation were enrolled. At screening, all patients were required to have an ABC-CFXS SA subscale score ≥ 4 or an ABC CFXS SA subscale score of 2 or 3 with an ABC-CFXS Irr subscale score ≥ 18. All patients were also required to have a Clinical Global Impression-Severity (CGI-S) score ≥ 3. Patients who were receiving psychotropic medication(s) were eligible provided they were taking ≤ 2 medications for ≥ 4 weeks prior to enrollment. Patients with a history of seizure disorders who were receiving ≤ 2 antiseizure medications or who were seizure-free for ≥ 1 year prior to the study were eligible. Key exclusion criteria were as follows: use of cannabis or any tetrahydrocannabinol (THC) or cannabidiol-containing product within 3 months of study entry or during the study; alanine aminotransferase, aspartate aminotransferase, or total bilirubin levels ≥ 2 × upper limit of normal (ULN) or alkaline phosphatase levels ≥ 3 × ULN; positive drug screen (ethanol, cocaine, THC, barbiturates, amphetamines [unless prescribed], benzodiazepines [except midazolam], and opiates); use of the following antiepileptic drugs: clobazam, phenobarbital, ethosuximide, felbamate, or vigabatrin; and use of a strong inhibitor/inducer of cytochrome P450 3A4.
Patients were randomly assigned to treatment according to a computer-generated randomization scheme. Randomization was coordinated centrally through an interactive response system (IRT). Patients who met all eligibility requirements and randomization criteria were randomly allocated to active or placebo treatment groups using a 1:1 allocation ratio. Randomization was stratified by sex (male vs female), weight (≤ 35 kg vs > 35 kg), and region (North America vs non-North America).
CGG repeat sizing and methylation status
Genomic DNA was isolated from peripheral blood leukocytes using Gentra Puregene Blood Kit (Qiagen, Valencia, CA). Molecular DNA testing for FXS, to establish CGG repeat length, percentage of CpG methylated FMR1 alleles, and, in females, activation ratio was carried out by PCR and Southern blot analysis as previously described [41–43].
Outcome measures
The primary efficacy end point was the change from baseline (day 1 of randomized treatment) to week 12 in the ABC-CFXS SA subscale score. Key secondary end points included change from baseline to week 12 in ABC-CFXS Irr subscale score, change from baseline to week 12 in ABC-CFXS Social Unresponsiveness/Lethargy (SU/L) subscale score, and Clinical Global Impression, Improvement (CGI-I) at week 12. Exploratory end points to support meaningful change analyses included domain specific (SA, Irr, and SU/L) and overall behavior Caregiver Global Impression of Severity and Change items (CaGI-S and CaGI-C).
The ABC-C is an established observer-reported outcome measure of inappropriate and maladaptive behavior in children, adolescents, and adults with autism spectrum disorder and intellectual disability [44]. Caregivers rate how problematic a particular behavior has been over the past week on a 4-point rating scale ranging from “not at all a problem” to “the problem is severe in degree.” The ABC-CFXS specific scoring algorithm developed by Sansone and colleagues [45] assesses behavior across 6 domains including SA, Irr, and SU/L.
The CaGI-S asks caregivers to rate the overall severity of their child’s problems with social avoidance and isolation (nervousness, shyness, avoidance of other people), social interactions (communicating verbally and with body language), and irritable/disruptive behavior (temper tantrums, crying, whining) as well as overall behavior over the past week on a 4-point rating scale ranging from “no problems” to “severe problems.” The CaGI-C asks caregivers to rate the amount of change in their child’s problems compared to the beginning of the study with these behaviors on a 7-point rating scale ranging from “much better” to “much worse.”
Safety assessments
Safety assessments included physical and neurological exams, Tanner Stage assessment, examination of skin at application sites for irritation, vital signs, 12-lead electrocardiograms, the Columbia Suicide Severity Rating Scale (children’s version), the 15-item Marijuana Withdrawal Checklist–Short Form, the Penn Physician Withdrawal Checklist, safety laboratory tests, urinalysis, seizure assessment, and adverse event (AE) monitoring.
Caregivers used a daily diary to record the presence of any skin irritation at the gel application sites, indicating whether there was redness of varying intensities. Additionally, a skin examination was conducted by the investigator at each study visit. The following Skin Irritation Check Scale was used by caregivers and investigators: 0, no erythema; 1, minimal erythema; 2, moderate erythema with sharply defined borders; 3, intense erythema with or without edema; and 4, intense erythema with edema and blistering/erosion.
Statistical analysis
The study was designed to have 90% power to detect a − 1.3-point difference between treatments in change from baseline to week 12 ABC-CFXS SA subscale score, assuming a standard deviation of 2.8 and a two-sided test at the 5% significance level. This required 102 patients per group.
The primary efficacy analysis was performed on the full analysis set (FAS), which included all patients who received ≥ 1 dose of study medication and with baseline and ≥ 1 post-baseline ABC-CFXS assessments. Continuous measures including the primary endpoint were analyzed with a mixed model for repeated measures (MMRM) using an unstructured covariance matrix to estimate within-subject error, under missing at random assumption for missing data. The model included stratification variables gender and region (North America/non-North America), treatment, repeated measures for week (4, 8, 12), treatment-by-week interaction, and baseline score with baseline score-by-week interaction. Categorical response measures were analyzed with a logistic MMRM model including the same terms for stratification, treatment, week, and treatment-by-week interaction as the linear model. The hypothesis tests for the primary end point and key secondary end points were included in the strategy for strong control of the type I error probability.
Ad hoc analyses were conducted to evaluate the efficacy of ZYN002 vs placebo in patients with ≥ 90% methylation of the promoter region of the FMR1 gene and in patients with 100% methylation of the FMR1 gene. For analyses of outcomes in the subgroups defined by ≥ 90% methylation and 100% methylation, the MMRM statistical models described above also included the appropriate interaction terms to obtain treatment-by-subgroup interaction and inference regarding the treatment comparisons within methylation subgroups at the week 12 end point reported herein. The hypothesis tests for these treatment comparisons were exploratory in nature; therefore, nominal P-values are presented without adjustment for multiplicity of tests.
To aid in the interpretation of the point change on the ABC-CFXS subscale scores, separate pre-planned analyses were conducted to establish meaningful change thresholds (MCTs) over 12 weeks of treatment using anchor-based methods according to the US FDA recommendation [46]. The CaGI-S and CaGI-C items served as the anchors. Change categories from baseline to week 12 were created for the CaGI-S to represent whether the severity of child’s domain-specific and overall behavior worsened or improved. Change from baseline to week 12 for each ABC-CFXS subscale score was calculated for each CaGI-S change category from baseline to week 12 and for each value of the CaGI-C at week 12. Empirical cumulative distribution functions (eCDFs) of the ABC-CFXS subscale scores by CaGI-S change category (Supplemental Fig. S1) and CaGI-C value (Supplemental Fig. S2) at week 12 were plotted.
MCTs were established for the ABC-CFXS subscale scores by triangulation of the anchor-based analyses, eCDFs, and findings regarding meaningful change from a qualitative study in caregivers of children and adolescents with FXS [47].
Safety analyses were conducted on the safety analysis population, which included all randomized patients who received ≥ 1 dose of study medication.
All statistical analyses were performed using SAS software version 9.4 (SAS Institute).
The CONNECT-FX trial is registered on Clinicaltrials.gov (NCT03614663). This article presents analyses included in the final statistical analysis plan.
Results
Patients
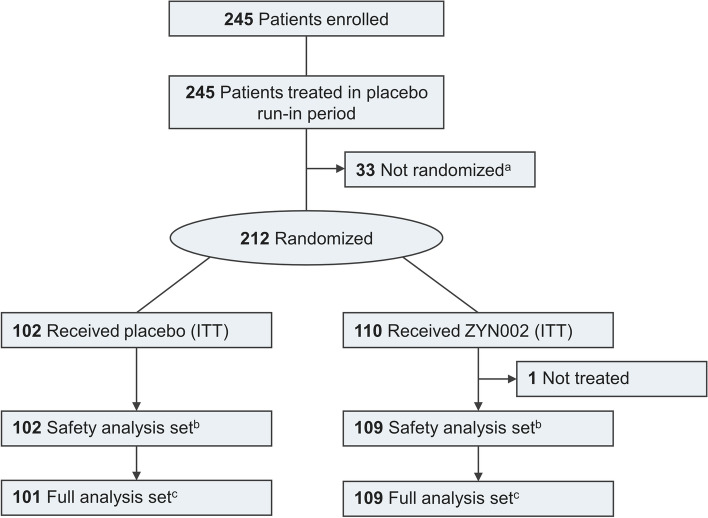
A total of 212 patients were randomized at 21 clinical sites in the USA, Australia, and New Zealand. The first patient was randomized on August 8, 2018, and the final patient visit occurred on May 15, 2020. Patient disposition is shown in Fig. 2. Of the 212 patients randomized (ZYN002, 110; placebo, 102), one did not receive study treatment. Thus, 211 patients were included in the safety analysis set. One treated patient did not have a post-baseline efficacy measure, resulting in 210 patients in the full analysis set (FAS).
Fig. 2.
Flow diagram of participants in the CONNECT-FX trial. a indicates the following: failure to meet randomization criteria. b indicates the following: received at least one dose of double-blind treatment. c indicates the following: all patients in the safety analysis set with both a baseline and at least one post-baseline efficacy measurement
Baseline demographics and disease characteristics
The patient populations were well matched in terms of demographic and disease characteristics (Table 1). Most patients were white (78.3%) and male (75%).
Table 1.
Baseline demographics
| ITT analysis set (all randomized) | ≥ 90% methylation group | < 90% methylation group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo (n = 102) | ZYN002 (n = 110) | Total (N = 212) | Placebo (n = 77) | ZYN002 (n = 92) | Total (n = 169) | Placebo (n = 25) | ZYN002 (n = 17) |
Total (n = 42) |
|
| Age (years) | |||||||||
| Mean | 9.8 | 9.6 | 9.7 | 9.6 | 9.2 | 9.4 | 10.4 | 12.1 | 11.1 |
| Median (min, max) | 10.0 (3, 17) | 9.0 (3, 17) | 10.0 (3, 17) | 9.0 (3, 17) | 9.0 (3, 17) | 9.0 (3, 17) | 11.0 (5, 16) | 12.0 (5, 17) | 11.0 (5, 17) |
| Sex—males, n (%) | 78 (76.5) | 81 (73.6) | 159 (75.0) | 54 (70.1) | 65 (70.7) | 119 (70.4) | 24 (96.0) | 15 (88.2) | 39 (92.9) |
| Weight (kg) | |||||||||
| Median (min, max) |
34.3 (15.6, 104.7) |
36.8 (14.6, 87.0) |
35.7 (14.6, 104.7) |
33.9 (15.6, 104.7) |
35.7 (14.6, 87.0) |
35.0 (14.6, 104.7) |
40.3 (21.9, 79.3) |
50.0 (21.4, 66.4) |
41.5 (21.4, 79.3) |
| Weight category | |||||||||
| > 35 kg, n (%) | 49 (48.0) | 61 (55.5) | 110 (51.9) | 35 (45.5) | 49 (53.3) | 84 (49.7) | 14 (56.0) | 12 (70.6) | 26 (61.9) |
| ADOS®-2 | |||||||||
| Comparison scorea | |||||||||
| Median (min, max) | 7.0 (1, 10) | 7.0 (1, 10) | 7.0 (1, 10) | 8.0 (1, 10) | 7.0 (1, 10) | 7.0 (1, 10) | 6.0 (1, 10) | 7.0 (3, 10) | 7.0 (1, 10) |
| Comparison score | |||||||||
| Categories,a n (%) | |||||||||
| Minimal/no evidence | 9 (9.1) | 9 (8.4) | 18 (8.7) | 6 (7.9) | 9 (10.0) | 15 (9.0) | 3 (13.0) | 0 | 3 (7.7) |
| Mild | 11 (11.1) | 11 (10.3) | 22 (10.7) | 9 (11.8) | 10 (11.1) | 19 (11.4) | 2 (8.7) | 1 (6.3) | 3 (7.7) |
| Moderate | 29 (29.3) | 37 (34.6) | 66 (32.0) | 19 (25.0) | 30 (33.3) | 49 (29.5) | 10 (43.5) | 6 (37.5) | 16 (41.0) |
| Severe | 45 (45.5) | 47 (43.9) | 92 (44.7) | 37 (48.7) | 41 (45.6) | 78 (47.0) | 8 (34.8) | 6 (37.5) | 14 (35.9) |
| Not applicable | 5 (5.1) | 3 (2.8) | 8 (3.9) | 5 (6.6) | 0 | 5 (3.0) | 0 | 3 (18.8) | 3 (7.7) |
| Missing | 3 | 3 | 6 | 1 | 2 | 3 | 2 | 1 | 3 |
| VABS-3 | |||||||||
| Overall—adaptive behavior composite scores | |||||||||
| n | 100 | 102 | 202 | 76 | 84 | 160 | 24 | 17 | 41 |
| Median (min, max) | 53.5 (25, 93) | 51.1 (23, 119) | 52.0 (23, 119) | 51.5 (24, 119) | 51.5 (24, 119) | 51.5 (24, 119) | 60.0 (27, 84) | 47.0 (23, 74) | 57.0 (23, 84) |
ADOS®-2 Autism Diagnostic Observation Schedule®-2, VABS-3 Vineland Adaptive Behavior Scales™, 3rd Edition
aComparison score categories: minimal/no evidence = 1 or 2, mild = 3 or 4, moderate = 5 to 7, severe = 8 to 10
As described in the “Methods” section, ad hoc analyses were conducted to evaluate the effect of ZYN002 vs placebo in patients with ≥ 90% methylation of the promoter region of the FMR1 gene and in patients with 100% methylation of the FMR1 gene. The ≥ 90% methylation group represented 79.7% of the total study population. The ≥ 90% methylation patients were similar to the overall intention-to-treat (ITT) population with respect to baseline demographic and disease characteristics (Table 1). The 100% methylation group (64.8% of total study population) also had similar baseline characteristics to the ITT population and the cohort of patients with ≥ 90% methylation.
Consistent with the published literature [14, 48], patients with ≥ 90% methylation generally presented with a more severe phenotype than those who were < 90% methylated (Table 1). Baseline demographics and disease characteristics were well matched between placebo and ZYN002 in the ≥ 90% methylation group of patients (Table 1).
Results for the primary analyses (FAS)
ABC-CFXS subscale scores
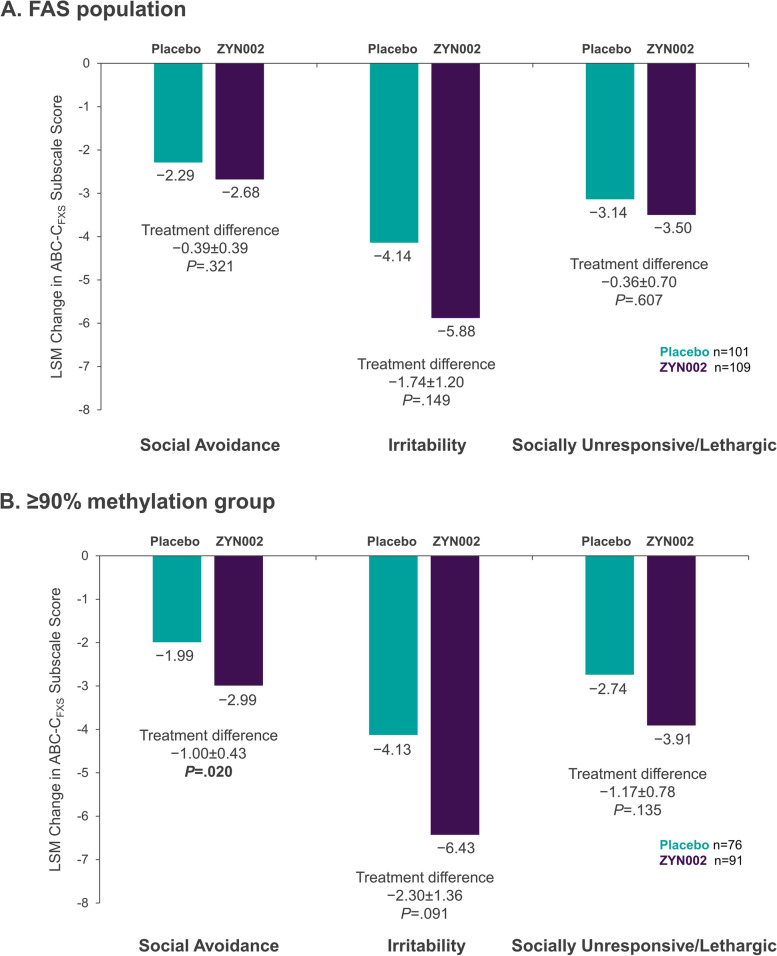
The primary efficacy end point was the change from baseline to week 12 in social avoidance (SA) as measured by the Aberrant Behavior Checklist-Community FXS specific (ABC-CFXS) SA subscale score [45, 49, 50]. Week 12 changes from baseline in ABC-CFXS subscale scores measuring primary and secondary end points in the FAS population are shown in Fig. 3A. Although improvements in SA, irritability (Irr), and social unresponsiveness/lethargy (SU/L) (indicated by decreases in score) were greater in the ZYN002 group than in the placebo group, the differences were not statistically significant.
Fig. 3.
Changes from baseline in ABC-CFXS subscale scores. Least square mean (LSM) changes from baseline in ABC-CFXS subscale scores are shown for each treatment group along with the LSM treatment differences. A Results from the full analysis set. B Results from the patients who had ≥ 90% methylation of the promoter region of the FMR1 gene. FAS, full analysis set
Meaningful change threshold
Mean change scores on the ABC-CFXS subscales by CaGI-S baseline to week 12 change category are shown in Supplemental Table S1. For the change categories indicating improvement (i.e., 1 or 2 category change), mean change scores on the ABC-CFXS SA, Irr, and SU/L subscales ranged from − 3.0 (2.96) to − 5.6 (3.06), − 8.9 (9.43) to − 13.8 (11.73), and − 3.9 (5.69) to − 7.2 (5.48) respectively for the CaGI-S domain specific and − 3.3 (4.85) to overall behavior items, respectively. A one category improvement (e.g., severe to moderate problem) on the CaGI-S were reported as a meaningful improvement in SA, Irr, and SU/L and overall behavior in the qualitative interviews [47].
Mean change scores on the ABC-CFXS subscales by CaGI-C value at week 12 are presented in Supplemental Table S2. For the values indicating improvement (i.e., a little better, moderately better, and much better), mean change scores on the ABC-CFXS SA, Irr, and SU/L subscales ranged from − 2.6 (3.47) to − 4.7 (3.44), − 8.6 (9.71) to − 17.4 (13.12), and − 5.1 (6.00) to − 7.8 (5.60). Any positive change on the CaGI-C was reported as meaningful or important in the caregiver cognitive interviews. As with the CaGI-S, meaningful change on the CaGI-C was considered by caregivers to imply improvement [51].
The eCDFs show clear separation between the CaGI-S change categories and the CaGI-C values representing improvement and deterioration (Supplemental Figs. 1 and 2).
Triangulating the results from the anchor-based analyses, the visual plots, and the levels of meaningful change reported by caregivers in the qualitative study, MCTs for changes from baseline to week 12 were determined to be 3 or more points on the ABC-CFXS SA subscale, 9 or more points on the ABC-CFXS Irr subscale, and 5 or more points on the ABC-CFXS SU/L.
Responder analysis—ABC-CFXS
Responder analyses using the MCT estimates did not find a statistically significant difference between placebo and ZYN002 for SA (nominal P = 0.254) and SU/L (nominal P = 0.190) subscale scores; however, there was a trend toward statistical significance in favor of ZYN002 for Irr (nominal P = 0.057). The model-based estimates of percent improved at week 12 were higher for the ZYN002 group than the placebo group for all 3 subscales: 54% vs 46% for SA, 37% vs 24% for Irr, and 41% vs 32% for SU/L.
Clinical Global Impression, Improvement (CGI-I)
For the key secondary end point of CGI-I, the percentage of patients with improvement at week 12 was higher in the ZYN002 group compared with the placebo group, but the difference was not statistically significant. Ratings of “Very much improved,” “Much improved,” or “Minimally improved” were reported for 40.5% of the placebo group vs 46.8% of the ZYN002 group (P = 0.376).
Caregiver Global Impression-Change (CaGI-C)
The percentage of patients whose parents/caregivers indicated that their child was “a little better,” “moderately better,” or “much better” on the CaGI-C was higher for patients receiving ZYN002 compared with those receiving placebo for SA/isolation (least squares [LS] means 57.1% vs 47.6%, nominal P = 0.184), social interactions (61.5% vs 44.9%, nominal P = 0.021), irritable/disruptive behavior (48.6% vs 38.7%, nominal P = 0.185), and overall behavior (55.9% vs 44.9%, nominal P = 0.145).
Ad hoc analyses: results in the ≥ 90% methylation group
ABC-CFXS subscale scores
In this study, there was clear evidence of a threshold effect at 90% methylation based on a statistically significant treatment-by-subgroup interaction in change from baseline to week 12 ABC-CFXS SA, (nominal P = 0.002); the 2 subgroups were qualitatively different, i.e., the treatment effect was reversed in the < 90% methylation group. There was no statistical evidence of a similar or greater treatment difference between subgroups below 90% methylation. The treatment effect observed in patients with ≥ 90% methylation was more than double the treatment effect observed in the overall sample.
Analysis of week 12 changes from baseline in ABC-CFXS subscale scores in the ≥ 90% methylation group demonstrated statistically significant improvement in the ZYN002 patients vs placebo patients for the primary end point of SA as measured by the ABC-CFXS SA subscale (treatment difference of − 1.00, nominal P = 0.020) (Fig. 3B). The median percent of improvement from baseline was 40.0% in the ZYN002 group vs 21.1% in the placebo group. Although statistical significance was not observed for the ABC-CFXS subscales of Irr (nominal P = 0.091) and SU/L (nominal P = 0.135) in the ≥ 90% methylation group, the mean changes in score were greater for the ZYN002 group than for the placebo group for both subscales (Fig. 3B).
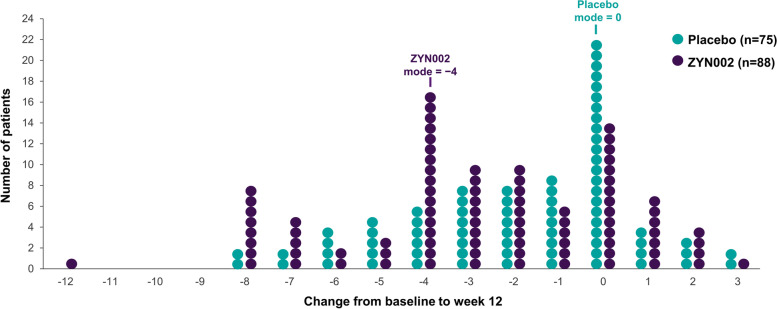
The distribution of responses to ZYN002 (change from baseline in SA subscale scores) in the ≥ 90% methylation group illustrated the response to treatment, as shown in Fig. 4. In the ≥ 90% methylation group, the mode for change from baseline among patients receiving ZYN002 was − 4 vs 0 for patients receiving placebo. In the < 90% methylation group, the mode was 0 for both placebo and ZYN002; a similar number of patients in the ZYN002 treatment group improved (5/17) or worsened (6/17).
Fig. 4.
Individual patient changes from baseline to week 12 in ABC-CFXS Social Avoidance subscale scores: ≥ 90% methylation group. Distribution of changes in ABC-CFXS SA subscale scores at 12 weeks in the patients who had ≥ 90% methylation of the promoter region of the FMR1 gene. Each circle represents one patient in the study. The mode for the distribution of each group is shown in the figure
Responder analysis—ABC-CFXS
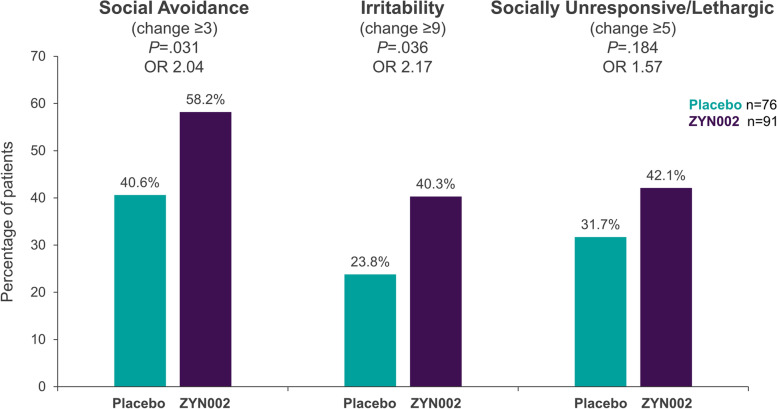
In the responder analysis for clinically meaningful within-patient change for the ≥ 90% methylation group, significant differences between placebo and ZYN002 were observed for the percent of patients improved at week 12 in SA (odds ratio 2.04, nominal P = 0.031) and Irr (odds ratio 2.17, nominal P = 0.036) (Fig. 5). The model-based estimate of percent improved in the ZYN002 group was 58.2% for SA and 40.3% for Irr. The improvement at Week 12 in SA corresponded to a number needed to treat (NNT) of 5.7.
Fig. 5.
Meaningful change in ABC-CFXS subscales in the ≥ 90% methylation group. Percentage of patients who experienced meaningful changes from baseline in ABC-CFXS subscale scores, defined as a change of ≥ 3 for SA, ≥ 9 for Irr, or ≥ 5 for SU/L. The data represent results from the patients who had ≥ 90% methylation of the promoter region of the FMR1 gene
Clinical Global Impression, Improvement (CGI-I)
In the ≥ 90% methylation group, ratings of “Very much improved,” “Much improved,” or “Minimally improved” were reported in 37.7% of the placebo population vs 51.1% in the ZYN002 group (nominal P = 0.056).
Caregiver Global Impression-Change (CaGI-C)
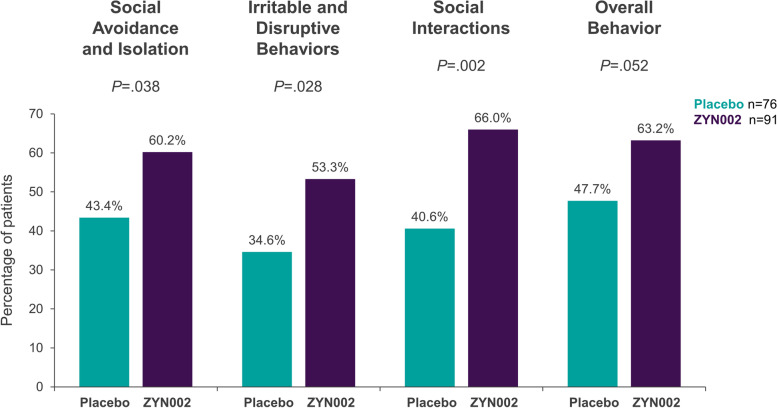
In the ≥ 90% methylation group, the percentage of patients whose parents/caregivers indicated that their child was “a little better,” “moderately better,” or “much better” was statistically significantly higher for patients receiving ZYN002 compared with those receiving placebo for all 3 items in CaGI-C, SA/isolation, social interactions, and irritable/disruptive behavior (all nominal P < 0.05) and neared significance for overall behavior (nominal P = 0.052) (Fig. 6).
Fig. 6.
Caregiver Global Impression-Change, any improvement: ≥ 90% methylation group. Percentage of patients who recorded improvements in Caregiver Global Impression-Change. The data represent results from the patients who had ≥ 90% methylation of the promoter region of the FMR1 gene
Ad hoc analysis: results in the 100% methylation group
An ad hoc analysis of patients with 100% methylation of the promoter region of the FMR1 gene was conducted to further explore the impact of complete methylation on response to ZYN002. This analysis revealed a further increase in treatment effect. The effect sizes were − 0.14 for the primary analysis, − 0.36 for the ≥ 90% methylation group, and − 0.39 for 100% methylation group. In patients with 100% methylation, ZYN002 was associated with 40% median improvement in the ABC-CFXS SA (treatment difference of − 1.08, nominal P = 0.027). Statistically significant effects were also observed for responder analyses for clinically meaningful change in ABC-CFXS SA (≥ 3 points; 56% for ZYN002 vs 37% for placebo [nominal P = 0.030]) and in the CaGI-C for any improvement in social interaction (63% for ZYN002 vs 37% for placebo [nominal P = 0.005]) and irritable/disruptive behaviors (54% for ZYN002 vs 33% for placebo [nominal P = 0.027]).
Safety
Approximately half (54.0%) of the 211 patients included in the safety analysis population experienced at least one treatment-emergent adverse event (TEAE). The frequency of TEAEs was similar for the placebo and ZYN002 treatment groups (50.0% and 57.8%, respectively) (Table 2). In the ≥ 90% methylation group, 55.4% of patients in the safety analysis population (n = 168) experienced at least one TEAE (placebo: 51.9%; ZYN002: 58.2%). In the < 90% methylation group, 47.6% experienced at least one TEAE (placebo: 44.0%; ZYN002: 52.9%). All TEAEs were mild or moderate in severity.
Table 2.
Treatment-emergent adverse events occurring in > 1% of patients (safety analysis set)
| Adverse event, n (%) | Placebo (n = 102) | ZYN002 (n = 109) |
|---|---|---|
| Patients with at least 1 TEAE | 51 (50) | 63 (57.8) |
| Upper respiratory tract infection | 7 (6.9) | 15 (13.8) |
| Nasopharyngitis | 9 (8.8) | 10 (9.2) |
| Vomiting | 6 (5.9) | 8 (7.3) |
| Pyrexia | 7 (6.9) | 5 (4.6) |
| Application site pain | 1 (1.0) | 7 (6.4) |
| Diarrhea | 0 (0) | 5 (4.6) |
| Gastroenteritis | 1 (1.0) | 7 (6.4) |
| Pharyngitis streptococcal | 2 (2.0) | 3 (2.8) |
| Rhinorrhea | 2 (2.0) | 2 (1.8) |
| Cough | 0 (0) | 3 (2.8) |
| Rash | 1 (1.0) | 2 (1.8) |
| Skin abrasion | 1 (1.0) | 2 (1.8) |
| Viral upper respiratory tract infection | 2 (2.0) | 1 (0.9) |
There were no serious adverse events (SAEs) or severe TEAEs reported during the study. One patient in the placebo group discontinued study treatment due to a TEAE (stereotypy). The most frequently reported TEAEs in either treatment group were upper respiratory tract infections. Psychiatric disorder TEAEs, primarily symptoms of FXS, were reported for 5 (4.9%) patients in the placebo group (anxiety, impulsive behavior, irritability, staring, stereotypy: 1 patient each) and 2 (1.8%) patients in the ZYN002 group (aggression, bruxism: 1 patient each).
Treatment-related TEAEs were reported for 4 (3.9%) patients in the placebo group (total of 6 events) and 11 (10.1%) patients in the ZYN002 group (total of 14 events). In the ≥ 90% methylation group, 8.3% had at least one treatment-related TEAE (placebo: 5.2%; ZYN002: 11.0%). In the < 90% methylation group, 2.4% experienced at least one treatment-related TEAE. The most common treatment-related TEAE was application site pain, reported in 1 (1.0%) placebo-treated patient and 7 (6.4%) ZYN002-treated patients. Application site pain was mild except for 2 events of moderate severity in the ZYN002 group. All other application site TEAEs reported in the ZYN002 group (application site dryness and application site pruritus in 1 patient, application site rash in 1 patient, and application site reaction in 1 patient) were of mild severity. Application site urticaria of moderate severity was reported for 1 patient in the placebo group.
Regarding skin assessments, 97% and 89% of individual daily diary skin irritation scores assessed by caregivers were “0,” no erythema, for placebo- and ZYN002-treated patients, respectively. The percentage of patients with a score of “2,” moderate erythema with sharply defined borders, recorded by caregivers during months 1, 2, and 3 of treatment, respectively, were 2.9%, 2.0%, and 2.0% for the placebo group and 17%, 5.6%, and 1.9% for the ZYN002 group. The only scores of “3,” intense erythema with or without edema, were recorded during month 1 of randomized treatment for 1 patient (1%) in the placebo group and 3 patients (2.8%) in the ZYN002 group. There were no scores of “4,” intense erythema with edema and blistering/erosion, reported by caregivers for any patients.
The highest skin irritation score assigned by investigators was a score of “2” recorded for 2 of 104 (1.9%) patients in the ZYN002 group at week 4, 1 of 98 (1.0%) at week 8, and for 1 of 98 (1.0%) patients in the placebo group at weeks 8 and 12.
Changes from baseline in laboratory values for chemistry and hematology were comparable between the placebo and ZYN002 treatment groups, and there were no clinically relevant abnormalities in either group. There were no clinically significant changes in liver function tests reported in any patient. In both treatment groups, overall changes from baseline in vital signs and ECG parameters were minimal and not clinically significant.
Discussion
Overview
CONNECT-FX was the single largest double-blind, randomized, placebo-controlled trial performed in FXS. The number of children and adolescents included in the ≥ 90% methylation group (169 patients), alone, was as large as study populations in most other clinical trials of FXS.
In the ≥ 90% methylation group, ZYN002 was superior to placebo in multiple analyses. In addition, an analysis of responder thresholds for meaningful within-patient behavioral change on the ABC-CFXS revealed specific thresholds for the ABC-CFXS subscales, indicating that the ABC-CFXS is well suited for assessing meaningful changes in these behaviors [47]. In the ZYN002 group, the LS mean change from baseline at week 12 in the ABC-CFXS SA subscale (− 2.99) met the threshold for meaningful within-patient change [47]. The proportions of patients attaining a threshold of meaningful within patient change in SA and Irr were significantly greater with ZYN002 vs placebo. There was a statistically significantly higher percentage of patients reported as improved based on caregiver reported global impression of change for SA, social interaction, and irritable behaviors with ZYN002 vs placebo. Parents/caregivers consider any improvement in this measure to be important, highlighting the potential benefit of ZYN002 in this patient population. These results demonstrate the consistency of the effect of ZYN002 in the treatment of behavioral symptoms associated with FXS in patients with ≥ 90% methylation of the FMR1 gene. A confirmatory phase 3, randomized, controlled trial (ZYN2-CL-033, RECONNECT; NCT04977986) is being conducted in children and adolescent patients with FXS.
ZYN002 was well tolerated. There were no SAEs reported during the study. All TEAEs (any event, whether unrelated or related to study drug) were mild or moderate. There were fewer psychiatric disorder TEAEs reported in the ZYN002 patients compared with the placebo patients, suggesting that ZYN002 treatment may have reduced the periodic exacerbations of symptoms that are typically associated with FXS (i.e., anxiety, impulsive behavior, irritability, staring, and stereotypy). There were no apparent differences in safety or tolerability based on the methylation status of patients. No clinically significant changes in liver function tests were reported in this trial. Liver enzyme elevations have been documented in previous clinical trials with oral formulations of cannabidiol [52, 53]. The lack of liver function elevations in the CONNECT-FX trial may be due to the transdermal route of administration of cannabidiol in ZYN002.
Biologically identifiable population
As described above, improvements in ABC-CFXS SA subscale scores were greater in the ZYN002 group than in the placebo group. Although the differences were not statistically significant in the FAS, the differences were statistically significant in the patients with ≥ 90% methylation of the promoter region of the FMR1 gene, with the greatest treatment effect seen in those patients with 100% methylation of the FMR1 gene promoter region. Thus, CONNECT-FX appears to provide evidence that identifies a biologically identifiable and clinically responsive population of patients affected by FXS who are defined by both full mutation and ≥ 90% methylation of the FMR1 gene. CONNECT-FX may also support the hypothesis that targeted intervention with cannabidiol, intended to modulate EC system dysfunction in FXS, can produce clinically relevant improvement in behavioral symptoms of FXS.
Patients with ≥ 90% methylation of the promoter region of the FMR1 gene represented 80% of the patients in the CONNECT-FX trial and are estimated to represent approximately 70% of patients with FXS. Previous studies demonstrated that persons with FXS and a FM may not have complete silencing of the FMR1 gene and as such may still be producing FMRP [9, 54]. The patients in CONNECT-FX who had FXS with an FM with ≥ 90% methylation may, therefore, represent a population with almost complete or complete silencing of the FMR1 gene with little to no FMRP production and, further, may represent a population that is most responsive to ZYN002. This may help explain why those with < 90% methylation did not respond as well as those with ≥ 90% methylation. We could hypothesize that those who are mosaic with unmethylated alleles, whether in the premutation or in the full mutation range, produce mRNA and FMRP that could potentially interfere with the putative positive effects of ZYN002.
While girls with ≥ 90% methylation likely produce more FMRP than boys due to the presence of a normal allele, girls with greater methylation of the affected allele and/or a low activation ratio are more phenotypically similar to boys; for example, like boys, significant cognitive impairment occurs in girls with significant methylation [55]. Withdrawn and anxious behaviors have also been reported to be greater in girls with FXS [56] and in girls in the general population [57]. The underlying physiology of the EC system in girls may also lead to increased responsiveness in girls [58]. Taken together, these findings may explain why the girls with ≥ 90 methylation responded to ZYN002 despite the likely presence of more FMRP than boys.
Study limitations
Because the study was limited to children and adolescents with FXS, the study results may not necessarily be generalizable to adult patients with FXS. Moreover, the effects of ZYN002 on the outcomes measures was seen predominantly in the patients with ≥ 90% methylation in FMR1 with less pronounced effects in patients with < 90% methylation in FMR1. While the results of this trial suggest a lower response for patients with < 90% methylation in FMR1, the sample size of that population was small and as such a definitive conclusion cannot be drawn in regard to the effect of ZYN002 in that population. As such, the study results may therefore not be generalizable to patients with < 90% methylation in FMR1. The primary endpoint, the SA subscale of the ABC-CFXS, includes only 4 items and may therefore not measure all aspects of anxiety and social anxiety in patients with FXS that may be affected by ZYN002. A small number of patients (n = 13) were enrolled who had ABC-CFXS SA subscale scores of 2 to 3 at baseline (plus Irr score ≥ 18; as described in the “Methods” section), and it is possible the inclusion of these patients may have limited the ability to detect improvement in SA in those patients. In addition, the use of fixed, weight-based dosing for ZYN002 led to various drug doses on a mg/kg basis, especially in higher weight individuals. A broader dose range, up to 750 mg/day in patients weighing more than 50 kg, has been incorporated in a follow-up study to reconfirm the results seen in CONNECT-FX.
Conclusions
In this trial, ZYN002 was well tolerated in patients with FXS and demonstrated evidence of efficacy with a favorable benefit risk relationship in patients with ≥ 90% methylation of the promoter region of the FMR1 gene, in whom gene silencing is most likely, with little or no FMRP production, and the impact of FXS is typically most severe.
Supplementary Information
Additional file 1: Supplemental Table S1. Change in ABC-CFXS Social Avoidance, Irritability, and Socially Unresponsive/Lethargic Subscale Scores by CaGI-S Change Categories. Supplemental Table S2. Change in ABC-CFXS Social Avoidance, Irritability, and Socially Unresponsive/Lethargic Subscale Scores by CaGI-C Week 12 Values. Supplemental Figure S1. Empirical cumulative distribution function curves of change in the ABC-CFXS SA, Irritability, and SU/L subscale scores by change in the CaGI-S domain-specific and overall behavior scores. Supplemental Figure S2. Empirical cumulative distribution function curves of change in the ABC-CFXS SA, Irritability, and SU/L subscale scores by change in the CaGI-C domain-specific and overall behavior scores.
Acknowledgements
The CONNECT-FX trial was funded by Zynerba Pharmaceuticals Inc. Devon, PA, USA. We would like to thank p-value communications for providing support for technical writing, editing, and publication assistance; this support was funded by Zynerba Pharmaceuticals Inc. Devon, PA, USA. EB-K, RA, CE, and NTartaglia are members of the Scientific Advisory Board for Zynerba Pharmaceuticals Inc. We thank the clinical site principal investigators who participated in the CONNECT-FX trial: Darius Adams, Jacobs Levy Personalized Genomic Medicine and Research Program, Morristown, NJ, USA; Elizabeth Berry-Kravis, Rush University Medical Center, Department of Pediatrics, Neurological Sciences and Biochemistry, Chicago, IL, USA; Caroline Buchanan, Greenwood Genetic Center, Greenville Clinic, Greenville, SC, USA; Dejan Budimirovic, Department of Psychiatry & Behavioral Sciences-Child Psychiatry, Fragile X Clinic and Clinical Trials Unit, Kennedy Krieger Institute/the Johns Hopkins Medical Institutions, Baltimore, MD, USA; Diana Cejas, University of North Carolina & Translational Research Center, Chapel Hill, NC, USA; Jonathan Cohen, Genetic Clinics Australia, Melbourne, Victoria, Australia; Craig Erickson, Cincinnati Children's Hospital Medical Center, Department of Psychiatry, Cincinnati, OH, USA; Richard Frye, Phoenix Children's Hospital, Phoenix, AZ, USA; Randi Hagerman, UC Davis Health System, MIND Institute, Department of Pediatrics, Sacramento, CA, USA; Shivkumar Hatti, Suburban Research Associates, Media, PA, USA; Helen Heussler, Queensland Childrens’ Hospital, South Brisbane, Queensland, Australia; Soo-Jeong Kim, Bernier Lab, University of Washington, Seattle, WA, USA; Sarah Land, Central States Research, Tulsa, OK, USA; Reymundo Lozano, Seaver Autism Center for Research and Treatment, Departments of Genetics and Genomic Sciences, Psychiatry and Pediatrics at Icahn School of Medicine at Mount Sinai, New York, NY, USA; Andrew Marshall, Wellington Hospital, Wellington, New Zealand; Nora McNamara, University Hospitals Cleveland Medical Center, Cleveland, OH, USA; Raun Melmed, Southwest Autism Research and Resource Center, Department of Research, Phoenix, AZ, USA; Lisa Prock, Boston Children's Hospital, Boston, MA, USA; Natalie Silove, Westmead Children’s Hospital, Sydney, New South Wales, Australia; Amy Talboy, Emory University School of Medicine, Atlanta, GA, USA; Nicole Tartaglia, Children's Clinical Research Organization, Research Institute, Children’s Hospital Colorado, University of Colorado School of Medicine, Aurora, CO, USA.
Abbreviations
- 2-AG
2-Arachidonoylglycerol
- ABC-CFXS
Aberrant Behavior Checklist–Community Edition FXS
- ADOS®-2
Autism Diagnostic Observation Schedule®-2
- AE
Adverse event
- AEA
Anandamide
- ASD
Autism spectrum disorder
- CaGI-C
Caregiver Global Impression-Change
- CGG
Cytosine, guanine, and guanine
- CGI-I
Clinical Global Impression, Improvement
- CGI-S
Clinical Global Impression-Severity
- CNS
Central nervous system
- DAGL
Diacylglycerol lipase
- EC
Endocannabinoid
- FAS
Full analysis set
- FM
Full mutation
- FXS
Fragile X syndrome
- Irr
Irritability
- IRT
Interactive response system
- ITT
Intention-to-treat
- LS
Least squares
- MMRM
Mixed model for repeated measures
- NNT
Number needed to treat
- SA
Social avoidance
- SAEs
Serious adverse events
- SU/L
Social Unresponsiveness/Lethargy
- TEAE
Treatment-emergent adverse event
- THC
Tetrahydrocannabinol
- ULN
Upper limit of normal
- VABS-3
Vineland Adaptive Behavior Scales™, 3rd Edition
Authors’ contributions
Conceptualization: EB-K, RH, EM, TS, NTich; methodology: EBK, RH, NTartaglia, FT, TD, EM, TS, NTich; software: TD; validation: TS, NTich, SO’Q; formal analysis: TD; investigation: EB-K, RH, DB, CE, HH, NTartaglia, JC, FT; resources: EB-K, RH, DB, CE, HH, NTartaglia, JC, TS, NTich; data curation: EB-K, RH, DB, CE, HH, NTartaglia, JC, FT, TD, TS, NTich, SO’Q; writing—original draft: JMP, SO’Q. Writing—review and editing: EB-K, RH, DB, CE, HH, NTartaglia, JC, FT TD, EM, TS, NTich; visualization: JMP, SO’Q; supervision: EB-K, RH, DB, CE, HH, NTartaglia, JC, TS, NTich, JMP; project administration: EB-K, RH, DB, CE, HH, NTartaglia, JC, TS, NTich, SO’Q; funding acquisition: TS. The authors read and approved the final manuscript.
Funding
The funding for this clinical trial was provided by Zynerba Pharmaceuticals, Inc.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
The protocol for the study was approved by the individual institution Investigational Review Boards (IRB) and Ethic Committees or by the central IRB, WCG IRB.
Consent for publication
Not applicable.
Competing interests
EB-K, RH, CE, and NTartaglia have received funding from Zynerba Pharmaceuticals for the conduct of the study as investigators and are on scientific advisory board for fragile X syndrome for Zynerba Pharmaceuticals. DB, HH, JC, and FT have received funding from Zynerba Pharmaceuticals for the conduct of the study as investigators. TD and EM are paid consultants for Zynerba Pharmaceuticals. TS, NTich, and SO’Q are employees of Zynerba Pharmaceuticals. JMP was an employee of Zynerba Pharmaceuticals at the time of the study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coffee B, Keith K, Albizua I, Malone T, Mowrey J, Sherman SL, et al. Incidence of fragile X syndrome by newborn screening for methylated FMR1 DNA. Am J Hum Genet. 2009;85(4):503–514. doi: 10.1016/j.ajhg.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagerman RJ. Fragile X syndrome. Molecular and clinical insights and treatment issues. West J Med. 1997;166(2):129–37. [PMC free article] [PubMed] [Google Scholar]
- 3.Tranfaglia MR. The psychiatric presentation of fragile X: evolution of the diagnosis and treatment of the psychiatric comorbidities of fragile X syndrome. Dev Neurosci. 2011;33(5):337–348. doi: 10.1159/000329421. [DOI] [PubMed] [Google Scholar]
- 4.Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65(5):905–914. doi: 10.1016/0092-8674(91)90397-H. [DOI] [PubMed] [Google Scholar]
- 5.Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, et al. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66(4):817–822. doi: 10.1016/0092-8674(91)90125-I. [DOI] [PubMed] [Google Scholar]
- 6.Sutcliffe JS, Nelson DL, Zhang F, Pieretti M, Caskey CT, Saxe D, et al. DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum Mol Genet. 1992;1(6):397–400. doi: 10.1093/hmg/1.6.397. [DOI] [PubMed] [Google Scholar]
- 7.Zafarullah M, Tassone F. Molecular biomarkers in fragile X syndrome. Brain Sci. 2019;9(5):96. doi: 10.3390/brainsci9050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darnell JC, Klann E. The translation of translational control by FMRP: therapeutic targets for FXS. Nat Neurosci. 2013;16(11):1530–1536. doi: 10.1038/nn.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Budimirovic DB, Schlageter A, Filipovic-Sadic S, Protic DD, Bram E, Mahone EM, et al. A genotype-phenotype study of high-resolution FMR1 nucleic acid and protein analyses in fragile X patients with neurobehavioral assessments. Brain Sci. 2020;10(10):694. doi: 10.3390/brainsci10100694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagerman RJ, Berry-Kravis E, Hazlett HC, Bailey DB, Jr, Moine H, Kooy RF, et al. Fragile X syndrome. Nat Rev Dis Primers. 2017;3:17065. doi: 10.1038/nrdp.2017.65. [DOI] [PubMed] [Google Scholar]
- 11.Sidorov MS, Auerbach BD, Bear MF. Fragile X mental retardation protein and synaptic plasticity. Mol Brain. 2013;6:15. doi: 10.1186/1756-6606-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Usdin K, Hayward BE, Kumari D, Lokanga RA, Sciascia N, Zhao XN. Repeat-mediated genetic and epigenetic changes at the FMR1 locus in the Fragile X-related disorders. Front Genet. 2014;5:226. doi: 10.3389/fgene.2014.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiraanont P, Kumar M, Tang HT, Espinal G, Hagerman PJ, Hagerman RJ, et al. Size and methylation mosaicism in males with Fragile X syndrome. Expert Rev Mol Diagn. 2017;17(11):1023–1032. doi: 10.1080/14737159.2017.1377612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pretto D, Yrigollen CM, Tang HT, Williamson J, Espinal G, Iwahashi CK, et al. Clinical and molecular implications of mosaicism in FMR1 full mutations. Front Genet. 2014;5:318. doi: 10.3389/fgene.2014.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mouslech Z, Valla V. Endocannabinoid system: an overview of its potential in current medical practice. Neuro Endocrinol Lett. 2009;30(2):153–179. [PubMed] [Google Scholar]
- 16.Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58(3):389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stefano GB, Liu Y, Goligorsky MS. Cannabinoid receptors are coupled to nitric oxide release in invertebrate immunocytes, microglia, and human monocytes. J Biol Chem. 1996;271(32):19238–19242. doi: 10.1074/jbc.271.32.19238. [DOI] [PubMed] [Google Scholar]
- 18.Elphick MR. The evolution and comparative neurobiology of endocannabinoid signalling. Philos Trans R Soc Lond B Biol Sci. 2012;367(1607):3201–3215. doi: 10.1098/rstb.2011.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackie K. Mechanisms of CB1 receptor signaling: endocannabinoid modulation of synaptic strength. Int J Obes (Lond) 2006;30(Suppl 1):S19–23. doi: 10.1038/sj.ijo.0803273. [DOI] [PubMed] [Google Scholar]
- 20.Castillo PE, Younts TJ, Chavez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012;76(1):70–81. doi: 10.1016/j.neuron.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohno-Shosaku T, Kano M. Endocannabinoid-mediated retrograde modulation of synaptic transmission. Curr Opin Neurobiol. 2014;29:1–8. doi: 10.1016/j.conb.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Vaughan CW, Christie MJ. Retrograde signalling by endocannabinoids. Handb Exp Pharmacol. 2005;168:367–383. doi: 10.1007/3-540-26573-2_12. [DOI] [PubMed] [Google Scholar]
- 23.Reisenberg M, Singh PK, Williams G, Doherty P. The diacylglycerol lipases: structure, regulation and roles in and beyond endocannabinoid signalling. Philos Trans R Soc Lond B Biol Sci. 2012;367(1607):3264–3275. doi: 10.1098/rstb.2011.0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung KM, Sepers M, Henstridge CM, Lassalle O, Neuhofer D, Martin H, et al. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nat Commun. 2012;3:1080. doi: 10.1038/ncomms2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Busquets-Garcia A, Gomis-Gonzalez M, Guegan T, Agustin-Pavon C, Pastor A, Mato S, et al. Targeting the endocannabinoid system in the treatment of fragile X syndrome. Nat Med. 2013;19(5):603–607. doi: 10.1038/nm.3127. [DOI] [PubMed] [Google Scholar]
- 26.Elmes MW, Kaczocha M, Berger WT, Leung K, Ralph BP, Wang L, et al. Fatty acid-binding proteins (FABPs) are intracellular carriers for Delta9-tetrahydrocannabinol (THC) and cannabidiol (CBD) J Biol Chem. 2015;290(14):8711–8721. doi: 10.1074/jbc.M114.618447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Marzo V, Maccarrone M. FAAH and anandamide: is 2-AG really the odd one out? Trends Pharmacol Sci. 2008;29(5):229–233. doi: 10.1016/j.tips.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Pertwee RG, Ross RA, Craib SJ, Thomas A. (-)-Cannabidiol antagonizes cannabinoid receptor agonists and noradrenaline in the mouse vas deferens. Eur J Pharmacol. 2002;456(1–3):99–106. doi: 10.1016/S0014-2999(02)02624-9. [DOI] [PubMed] [Google Scholar]
- 29.Ryan D, Drysdale AJ, Pertwee RG, Platt B. Interactions of cannabidiol with endocannabinoid signalling in hippocampal tissue. Eur J Neurosci. 2007;25(7):2093–2102. doi: 10.1111/j.1460-9568.2007.05448.x. [DOI] [PubMed] [Google Scholar]
- 30.Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150(5):613–623. doi: 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McPartland JM, Duncan M, Di Marzo V, Pertwee RG. Are cannabidiol and Delta(9) -tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol. 2015;172(3):737–53. [DOI] [PMC free article] [PubMed]
- 32.Laprairie RB, Bagher AM, Kelly ME, Dupre DJ, Denovan-Wright EM. Type 1 cannabinoid receptor ligands display functional selectivity in a cell culture model of striatal medium spiny projection neurons. J Biol Chem. 2014;289(36):24845–24862. doi: 10.1074/jbc.M114.557025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreutz S, Koch M, Bottger C, Ghadban C, Korf HW, Dehghani F. 2-Arachidonoylglycerol elicits neuroprotective effects on excitotoxically lesioned dentate gyrus granule cells via abnormal-cannabidiol-sensitive receptors on microglial cells. Glia. 2009;57(3):286–294. doi: 10.1002/glia.20756. [DOI] [PubMed] [Google Scholar]
- 34.Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134(4):845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153(2):199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30(8):1037–1043. doi: 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- 38.Bakas T, van Nieuwenhuijzen PS, Devenish SO, McGregor IS, Arnold JC, Chebib M. The direct actions of cannabidiol and 2-arachidonoyl glycerol at GABAA receptors. Pharmacol Res. 2017;119:358–370. doi: 10.1016/j.phrs.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 39.McGuire P, Robson P, Cubala WJ, Vasile D, Morrison PD, Barron R, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225–231. doi: 10.1176/appi.ajp.2017.17030325. [DOI] [PubMed] [Google Scholar]
- 40.Heussler H, Cohen J, Silove N, Tich N, Bonn-Miller MO, Du W, et al. A phase 1/2, open-label assessment of the safety, tolerability, and efficacy of transdermal cannabidiol (ZYN002) for the treatment of pediatric fragile X syndrome. J Neurodev Disord. 2019;11(1):16. doi: 10.1186/s11689-019-9277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tassone F, Pan R, Amiri K, Taylor AK, Hagerman PJ. A rapid polymerase chain reaction-based screening method for identification of all expanded alleles of the fragile X (FMR1) gene in newborn and high-risk populations. J Mol Diagn. 2008;10(1):43–49. doi: 10.2353/jmoldx.2008.070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Filipovic-Sadic S, Sah S, Chen L, Krosting J, Sekinger E, Zhang W, et al. A novel FMR1 PCR method for the routine detection of low abundance expanded alleles and full mutations in fragile X syndrome. Clin Chem. 2010;56(3):399–408. doi: 10.1373/clinchem.2009.136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tassone F, Hagerman RJ, Ikle DN, Dyer PN, Lampe M, Willemsen R, et al. FMRP expression as a potential prognostic indicator in fragile X syndrome. Am J Med Genet. 1999;84(3):250–261. doi: 10.1002/(SICI)1096-8628(19990528)84:3<250::AID-AJMG17>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 44.Aman MG, Singh NN. Aberrant Behavior Checklist Manual. 2. East Aurora, New York: Slosson Educational Publications, Inc; 2017. [Google Scholar]
- 45.Sansone SM, Widaman KF, Hall SS, Reiss AL, Lightbody A, Kaufmann WE, et al. Psychometric study of the Aberrant Behavior Checklist in Fragile X Syndrome and implications for targeted treatment. J Autism Dev Disord. 2012;42(7):1377–1392. doi: 10.1007/s10803-011-1370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.United States Food and Drug Administration. Incorporating clinical outcome assessments into endpoints for regulatory decision-making 2019. Available from: https://www.fda.gov/media/132505/download.
- 47.Merikle E, Patel V, Dobbins T, O'Quinn S, Tich N, Palumbo J. Cannabidiol in fragile X syndrome (FXS): proposed mechanism of action (MOA) translates into meaningful clinical benefits [CONNECT-FX (ZYN2-CL-016)]. Biol Psychiatry. 2021;89(9, Supplement):S143.
- 48.Jacquemont S, Curie A, des Portes V, Torrioli MG, Berry-Kravis E, Hagerman RJ, et al. Epigenetic modification of the FMR1 gene in fragile X syndrome is associated with differential response to the mGluR5 antagonist AFQ056. Sci Transl Med. 2011;3(64):64ral. doi: 10.1126/scitranslmed.3001708. [DOI] [PubMed] [Google Scholar]
- 49.Budimirovic DB, Bukelis I, Cox C, Gray RM, Tierney E, Kaufmann WE. Autism spectrum disorder in Fragile X syndrome: differential contribution of adaptive socialization and social withdrawal. Am J Med Genet A. 2006;140A(17):1814–1826. doi: 10.1002/ajmg.a.31405. [DOI] [PubMed] [Google Scholar]
- 50.Budimirovic DB, Berry-Kravis E, Erickson CA, Hall SS, Hessl D, Reiss AL, et al. Updated report on tools to measure outcomes of clinical trials in fragile X syndrome. J Neurodev Disord. 2017;9:14. doi: 10.1186/s11689-017-9193-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Merikle E, Patel VP, Tich N, Sebree T, Hagerman RJ, Palumbo JM. Caregiver-perceived behavioral challenges in fragile x syndrome (FXS) and their measurement using the Aberrant Behavior Checklist-Community FXS specific (ABC-CFXS) Value Health. 2021;24:S241–S242. doi: 10.1016/j.jval.2021.04.1215. [DOI] [Google Scholar]
- 52.Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376(21):2011–2020. doi: 10.1056/NEJMoa1611618. [DOI] [PubMed] [Google Scholar]
- 53.Devinsky O, Patel AD, Cross JH, Villanueva V, Wirrell EC, Privitera M, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018;378(20):1888–1897. doi: 10.1056/NEJMoa1714631. [DOI] [PubMed] [Google Scholar]
- 54.Schneider A, Winarni TI, Cabal-Herrera AM, Bacalman S, Gane L, Hagerman P, et al. Elevated FMR1-mRNA and lowered FMRP - a double-hit mechanism for psychiatric features in men with FMR1 premutations. Transl Psychiatry. 2020;10(1):205. doi: 10.1038/s41398-020-00863-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim K, Hessl D, Randol JL, Espinal GM, Schneider A, Protic D, et al. Association between IQ and FMR1 protein (FMRP) across the spectrum of CGG repeat expansions. PLoS ONE. 2019;14(12):e0226811. doi: 10.1371/journal.pone.0226811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hessl D, Dyer-Friedman J, Glaser B, Wisbeck J, Barajas RG, Taylor A, et al. The influence of environmental and genetic factors on behavior problems and autistic symptoms in boys and girls with fragile X syndrome. Pediatrics. 2001;108(5):E88. doi: 10.1542/peds.108.5.e88. [DOI] [PubMed] [Google Scholar]
- 57.Rubinow DR, Schmidt PJ. Sex differences and the neurobiology of affective disorders. Neuropsychopharmacol. 2019;44(1):111–128. doi: 10.1038/s41386-018-0148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cooper ZD, Craft RM. Sex-dependent effects of cannabis and cannabinoids: a translational perspective. Neuropsychopharmacol. 2018;43(1):34–51. doi: 10.1038/npp.2017.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplemental Table S1. Change in ABC-CFXS Social Avoidance, Irritability, and Socially Unresponsive/Lethargic Subscale Scores by CaGI-S Change Categories. Supplemental Table S2. Change in ABC-CFXS Social Avoidance, Irritability, and Socially Unresponsive/Lethargic Subscale Scores by CaGI-C Week 12 Values. Supplemental Figure S1. Empirical cumulative distribution function curves of change in the ABC-CFXS SA, Irritability, and SU/L subscale scores by change in the CaGI-S domain-specific and overall behavior scores. Supplemental Figure S2. Empirical cumulative distribution function curves of change in the ABC-CFXS SA, Irritability, and SU/L subscale scores by change in the CaGI-C domain-specific and overall behavior scores.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.