Abstract
Porphyromonas gingivalis fimbriae elicit many responses in eukaryotic cells, including mitogenicity, cytokine production, epithelial cell invasion, and cellular immune response. Specific domains of the major fimbrial protein (FimA) have been shown to be important in triggering some of these functions. The goal of the present study was to identify the domain(s) of P. gingivalis FimA responsible for specific interaction with human mucosal epithelial cells. Fimbriated P. gingivalis strains have been shown to bind to buccal epithelial cells, whereas nonfimbriated strains bind at low levels or not at all. This and other studies provide evidence that FimA mediates the adherence of P. gingivalis to oral epithelial cells. To determine the specific region(s) of P. gingivalis FimA involved in epithelial cell binding, specific antipeptide antibodies were used to inhibit the binding of iodinated purified fimbriae as well as the binding of P. gingivalis cells to epithelial cells. Antibodies directed against peptides 49 to 68 (VVMANTAGAMELVGKTLAEVK) and 69 to 90 (ALTTELTAENQEAAGLIMTAEP) were found to highly inhibit both the binding of fimbriae and the binding of P. gingivalis cells to epithelial cells. The antibody against FimA peptides 69 to 90 also reacted with P. gingivalis fimbriae in immunogold labeling and immunoblot analysis, thereby indicating that this peptide domain is exposed on the surface of fimbriae. Our results suggest that the amino-terminal domain corresponding to amino acid residues 49 to 90 of the fimbrillin protein is a major epithelial cell binding domain of P. gingivalis fimbriae.
Porphyromonas gingivalis plays an important role in the initiation and progression of periodontal disease. P. gingivalis has been shown to attach to and invade oral epithelial cells in vitro (22, 26). Intracellular invasion of human epithelial cells is a key pathogenic property for a number of bacterial species (4–6). For these events, bacteria bind to epithelial cell membranes and induce a series of biochemical changes (27) that involve the induction of protein kinase activity (24, 25). A variety of cell surface structures have been postulated to play roles in P. gingivalis interaction with host cells (2), and the initial binding of P. gingivalis to target cells appears to be fimbriae mediated. Recent reports from several laboratories have shown that fimbriae bind to saliva-coated hydroxyapatite (13), human erythrocytes (17, 18), monocytes and macrophages (19), epithelial cells (7, 11, 16, 30), and gingival fibroblasts (8). The afimbriated mutants of P. gingivalis have been shown to possess diminished capacity for adherence to oral epithelial cells in vitro (7, 16, 30). In addition, antifimbrial monoclonal antibodies have been shown to block the adhesion of P. gingivalis to human buccal epithelial cells in an in vitro assay (11). The above studies strongly implicate the major fimbrial protein FimA in adherence to these mammalian cells. Recent studies from Ogawa et al. (21) have shown that synthetic peptides of fimbrillin corresponding to its binding domains (amino acid residues 1 to 20, 69 to 80, and 171 to 181) inhibited the binding of P. gingivalis fimbriae to gingival fibroblasts. Fimbriae have also been reported to induce the expression of inflammatory cytokines in human gingival fibroblasts and mouse peritoneal macrophages, strongly suggesting that fimbriae are crucial in bacterial interactions with the host gingival tissue (9).
P. gingivalis fimbriae can induce protein kinase-mediated phosphorylation of a signaling protein in mouse peritoneal macrophages and in human monocytes (15, 20). Since P. gingivalis FimA appears to be key in the binding to and possible invasion of epithelial cells, we attempted to elucidate the important region(s) of fimbrillin that are involved in the attachment process. To this end, we have mapped the surface regions of the fimbrillin molecule by utilizing antipeptide antibodies against synthetic peptides corresponding to the FimA sequence (3). The selection of peptides was based on surface predictions incorporating three different parameters: hydrophilicity, accessibility, and mobility (23). Antipeptide antibodies that reacted with native fimbriae, thereby suggesting that the corresponding peptides are surface exposed, were used as inhibitors of P. gingivalis binding to epithelial cells.
The peptides synthesized are listed in Table 1. Peptide synthesis and preparation of peptide conjugates were carried out by a method described earlier (14). The composition and sequence of peptides were confirmed by amino acid analysis on a Beckman model 121MB sequencer, and the amino acid sequence was confirmed by an automated stepwise procedure on an Applied Biosystems model 477A sequencer.
TABLE 1.
Sequence of synthetic peptides corresponding to the segments of P. gingivalis fimbrillina
| Peptide position | Amino acid sequence |
|---|---|
| 42–61 | SAGQRTLVVMANTGAMELVG |
| 49–68 | VVMANTGAMELVGKTLAEVK |
| 69–90 | ALTTELTAENQEAAGLIMTAEP |
| 81–98 | AAGLIMTAEPKTIVLKAG |
| 99–110 | KNYIGYSGTGEG |
| 226–245 | IHPTILCVYGKLQKNGADLA |
| 287–306 | NKIGRNHKYDIKLTITGPGT |
Numbering amino acids did not take into account that the first amino acids appeared as the leader sequence in the fimbrillin sequence by Dickinson et al. (3).
Immunization.
For the production of polyclonal antibodies to synthetic peptides, each peptide was conjugated to thyroglobulin using m-maleinimido-benzoyl-N-hydroxy-succinimide ester (MBS) as a cross-linker by a method described by Schmidt et al. (28). Briefly, 10 mg of thyroglobulin, dissolved in 3 ml of phosphate buffered saline (PBS), pH 7.4, was activated by mixing with 5 mg of MBS in 1 ml of N-N-dimethylformamide. The solution was stirred for 2 h at room temperature, and unreacted MBS was removed by gel filtration on Sephadex G-25 in 0.1 M PBS, pH 6.0. Synthetic peptides (5 mg each) were reduced with sodium borohydride, and excess borohydride was destroyed with acetic acid. The neutralized and reduced peptide was combined with MBS-activated thyroglobulin and was stirred overnight at room temperature. The resulting peptide-carrying conjugate was subsequently isolated by gel filtration on Sephadex G-25 in 0.1 M ammonium bicarbonate buffer, pH 8.0.
New Zealand White rabbits (2 to 3 kg) were injected subcutaneously at multiple sites with 500 μg (total dosage) of the appropriate conjugated peptide carrier in complete Freund's adjuvant. Beginning 2 weeks after the first injection, the rabbits were boosted weekly with immunogens in incomplete adjuvant. Six weeks after the first injection, each rabbit was bled, and the antibodies were tested against the corresponding antigen by enzyme-linked immunoabsorbent assay and immunoblot analysis. After appropriate antibody titer was obtained, rabbits were bled by heart puncture, and the sera were collected and stored at −70°C.
Bacterial culture conditions.
P. gingivalis 2561 was grown in one-half-strength (18 mg/ml) brain heart infusion broth, supplemented with 5 mg of yeast extract per ml and buffered at a pH of 7.4. Cells were then incubated for 2 days in an anaerobic chamber (85% N2, 10% H2, 5% CO2).
Fimbrial preparation and iodination.
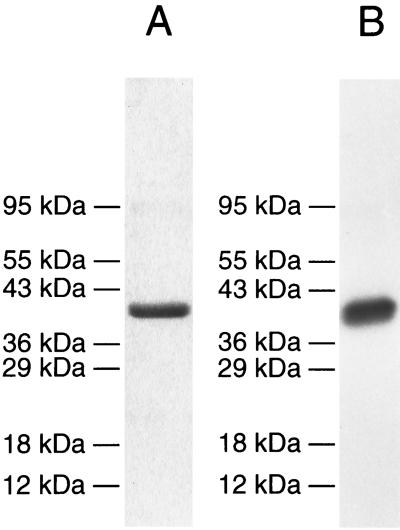
P. gingivalis fimbriae were purified by the procedure of Sojar et al. (29), and purity was confirmed by a sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (12) (Fig. 1A). Purified fimbriae were iodinated using the chloramine T method (10) with the following modification. Ten micrograms of purified fimbriae was labeled with 0.5 mCi of sodium iodine-125 (Amersham Pharmacia Biotech Inc., N.J.) in 0.5 M PBS, pH 7.2, in the presence of 10 μl of chloramine T (1 mg/ml) for 60 s. Adding 20 μl of sodium metabisulfite (2 mg/ml) terminated iodination. After termination, 100 μl of PBS containing 10% sucrose and 10% potassium iodide was added, and the mixture was loaded on a Sephadex G-75 column (1 by 30 cm) saturated with 1% bovine serum albumin (BSA) to prevent nonspecific binding. The column was extensively washed after saturation with BSA. The iodinated fimbrial peak was collected without a carrier protein, and the integrity of labeled fimbriae was confirmed by autoradiography (Fig. 1B).
FIG. 1.
SDS-polyacrylamide gel electrophoresis of purified fimbriae and autoradiograph of iodinated fimbriae. (A) SDS-polyacrylamide gel stained with Coomassie blue. (B) Autoradiograph of iodinated fimbriae. Molecular mass is indicated.
Dot blot assay.
Five micrograms of purified fimbriae was spotted on nitrocellulose membranes. Unoccupied sites were blocked with 1% BSA, and membranes were incubated with respective antipeptide antibodies. Following washing with Tris-buffered saline (20 mM Tris-Cl, 0.5M NaCl [pH 7.5]), membranes were incubated with goat anti-rabbit immunoglobulin G horseradish peroxidase conjugate for 1 h at room temperature. The blots were washed as above, and bound antibodies were visualized by adding 4-chloro-1-naphthol color-developing reagent. Prebleed rabbit serum and a blot without primary antibodies were used as negative controls. The results showed that the antibodies against FimA peptides 42 to 61, 49 to 68, 69 to 90, 81 to 98, 99 to 110, 226 to 245, and 287 to 306 reacted positively with purified fimbriae (data not shown).
Inhibition of binding of purified P. gingivalis fimbriae to KB cells by antipeptide antibodies.
Inhibition of fimbrial binding to oral epithelial KB cell line ATCC CCL17 (American Type Culture Collection, Rockville, Md.) was carried out. The culture was maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum. The ability of fimbrial peptide antibodies to inhibit fimbrial binding to KB cells was examined by preincubating purified iodinated fimbriae with antipeptide antibodies at room temperature for 1 h. Normal rabbit serum was used as a negative control. Epithelial cells were grown to confluent monolayers, the medium was removed, and cells were washed with the same medium without serum. Iodinated fimbriae (100,000 cpm) with and without antipeptide antibodies were added in equal volumes of medium without fetal calf serum and were incubated at 37°C for 1 h. The unbound iodinated fimbriae were aspirated, and cells were washed with medium without fetal bovine serum. The washed cells were solubilized in 0.5 M NaOH plus 1% SDS at 37°C for a few minutes, and the radioactivity was counted in a Beckman gamma counter. When antipeptide antibodies were incubated with iodinated fimbriae prior to their addition to KB cells, it was found that, in the presence of the antibodies against FimA peptides 49 to 68 and peptides 69 to 90, binding of fimbriae to Kb cells was reduced to 30 and 26%, respectively (Table 2).
TABLE 2.
Effects of antipeptide serum on binding of iodinated purified fimbriae
| Inhibitor | Percent binding |
|---|---|
| None | 94.61 ± 9.02 |
| Normal rabbit serum | 94.98 ± 9.08 |
| Antifimbria antibodies | 29.10 ± 0.79 |
| Antibody against peptides 49–68 | 30.27 ± 3.01 |
| Antibody against peptides 69–90 | 26.02 ± 2.89 |
| Antibody against peptides 226–245 | 101.6 ± 6.60 |
| Antibody against peptides 287–306 | 94.42 ± 2.64 |
Inhibition of adherence of P. gingivalis cells to KB cells by antipeptide antibodies.
Adherence of P. gingivalis and its inhibition in the presence of antipeptide antibodies were assessed in an oral epithelial cell invasion model described earlier (16). The oral epithelial KB cell line ATCC CCL17 was maintained in DMEM supplemented with 10% fetal bovine serum and gentamicin. Cells were seeded into 24-well plates 24 h earlier and were allowed to grow to a density of 5 × 104 cells/well. Bacterial cultures grown to late log phase were centrifuged, were washed in PBS, and were resuspended in DMEM containing 1 mM MgCl2 and 0.2 mM CaCl2 at a final concentration of 106 cells per ml. The bacterial suspensions (1.0 ml) were added to confluent epithelial monolayers and were incubated at 37°C in 5% CO2 for 2 h. After incubation, unattached bacteria were removed following the washing of the monolayers twice in PBS. KB cells were lysed in 1 ml of sterile distilled water per well and were incubated for 10 min. Lysates were serially diluted, were plated on abscisic acid plates, and were incubated anaerobically at 37°C for 7 days. All assays were performed in triplicate.
The ability of antipeptide antibodies to inhibit the adherence of P. gingivalis to epithelial cells was examined by preincubating P. gingivalis with antipeptide antibodies which were found to be inhibitory in a purified fimbria binding inhibition assay. Normal rabbit serum, as well as carboxy-terminal antibodies, were used as negative controls. Freshly grown P. gingivalis cells were resuspended in 0.05 M PBS (pH 7.2) at a concentration of 109 cells/ml and were incubated anaerobically at 37°C for 60 min in 1:500 dilution of sera raised against fimbrial peptide. Normal rabbit serum was used as a negative control. After 100 μl of each P. gingivalis culture was plated on brain heart infusion blood agar plates to confirm viability, cultures were used to infect KB monolayers as described above. Preincubation of P. gingivalis strains with normal rabbit sera did not inhibit the binding of P. gingivalis to KB cells. Antipeptide antibodies that inhibited the binding of purified fimbriae to epithelial cells were also found to inhibit the binding of P. gingivalis to KB cells. In the presence of antibodies against FimA peptides 49 to 68 and 69 to 90, P. gingivalis attachment to epithelial cells was reduced to 0.52 and 0.056%, respectively, compared to 5.06% attachment levels without inhibitor (Table 3). However, the antibody to FimA peptides 226 to 245 failed to block fimbrial binding, yet inhibited P. gingivalis binding by 60%. This might suggest that antipeptide antibodies react with another protein on the P. gingivalis cell surface.
TABLE 3.
Effects of antipeptide serum on attachment of P. gingivalis to KB cells
| Inhibitor | % Total inoculum attachment | % Binding | % Inhibition |
|---|---|---|---|
| None | 5.06 ± 0.66 | 100 | 0 |
| Antibody against peptides 49–68 | 0.52 ± 0.16 | 10.3 | 89.7 |
| Antibody against peptides 69–90 | 0.06 ± 0.02 | 1.2 | 98.8 |
| Antibody against peptides 226–245 | 2.04 ± 0.55 | 40.3 | 59.7 |
| Antibody against peptides 287–306 | 4.35 ± 0.18 | 86.0 | 14.0 |
Immunogold labeling.
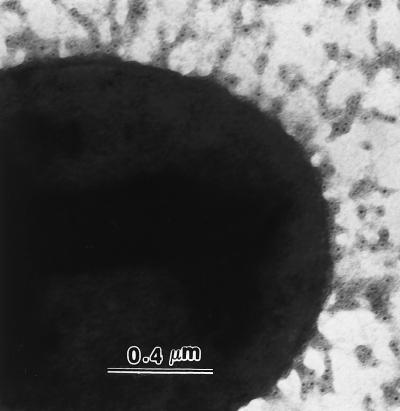
P. gingivalis 2561 was grown as described above. Cells were washed with PBS and transferred to nickel grids coated with Formvar film to air dry. The cells were then incubated with 10 μl of rabbit polyclonal antibodies raised against peptides (1:500 dilution of antisera in PBS containing 1% BSA) at 37°C for 1 h. Following washing five times with PBS, cells were incubated for 30 min with sheep anti-rabbit immunoglobulin G conjugated with 5-nm gold particles (1:20 AuroProbe EM; Amersham) at 37°C for 30 min. The cells were rinsed twice with PBS and negatively stained with 2% (wt/vol) uranyl acetate for 1 min. The fixed and stained cells were examined and photographed with a Hitachi H-600 electron microscope operating at 75 kV. The results showed that the antibody against FimA peptides 69 to 90 recognized the native fimbriae on the surface of whole cells (Fig. 2). No labeling was observed when normal rabbit sera or only secondary antibodies were used as controls (data not shown).
FIG. 2.
Localization of fimbriae on a whole cell visualized by immunogold electron microscopy. Cells were incubated with antibody against FimA peptides 69 to 90 followed by gold-labeled goat anti-rabbit antibody. Samples were prepared by negative staining with 2% uranyl acetate.
Many researchers have suggested that major P. gingivalis fimbriae play an important role in P. gingivalis adherence and invasion of oral epithelial cells (16, 30). Njoroge et al. (16) found that incubation of P. gingivalis with antisera raised to major fimbriae prior to infection of epithelial cells resulted in complete inhibition of adherence to these cells. In addition, adherence to and invasion of oral epithelial cells were not detected with the fimA-knockout strains DPG3 and MPG1. The interaction of P. gingivalis with epithelial cells appears to involve at least a two-stage process of initial and intimate attachment. The initial attachment of P. gingivalis with epithelial cells appears to be mediated by the major fimbriae. In this study, we have elucidated the region(s) of fimbrillin that is involved in the initial attachment process.
To elucidate the regions of P. gingivalis fimbrillin that may be involved in epithelial binding, we first identified the surface-exposed regions of fimbrillin by utilizing antibodies raised against synthetic peptides of fimbrillin. We found that a majority of the antibodies against the amino-terminal region recognized the native fimbriae. Additionally, secondary structure and hydrophilicity prediction of fimbrillin protein according to Chou and Fasman algorithms (1) showed that the amino-terminal region is surface exposed. Taken together, these studies showed that the amino-terminal region of fimbrillin is surface exposed and may be available for binding. It should be noted here that the failure of antibodies directed against other domains of fimbrillin to recognize native fimbriae does not suggest that such domains are not surface exposed. It is probable that these synthetic peptide antibodies do not recognize the native peptide conformation in fimbriae. By utilizing antipeptide antibodies to inhibit P. gingivalis binding to epithelial cells, we found that antibodies against the FimA amino-terminal region (from peptides 49 to 90) were most effective in inhibiting P. gingivalis attachment to epithelial cells; the antibodies against FimA peptides 49 to 68 and 69 to 90 inhibited the binding to 89.7 and 98.9%, respectively.
In conclusion, our results suggest that the amino-terminal region of fimbrillin (from amino acid residues 49 to 90) is the potential epithelial binding domain of P. gingivalis fimbriae.
Acknowledgments
This study was supported in part by U.S. Public Health Service grant no. DE08240, DE04898, DE12320-01, and AI09268.
We thank Darlene Badgett for her excellent technical assistance and Sara Saldi for editing the manuscript.
REFERENCES
- 1.Chou P Y, Fasman G D. Confirmational parameters for amino acids in helical, β sheet, and random coil regions calculated from proteins. Biochemistry. 1974;13:211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- 2.Cutler C W, Kalmar J, Genco C A. Pathogenic strategies of the oral anaerobe Porphyromonas gingivalis. Trends Microbiol. 1995;3:45–51. doi: 10.1016/s0966-842x(00)88874-5. [DOI] [PubMed] [Google Scholar]
- 3.Dickinson D P, Kubiniec M A, Yoshimura F, Genco R J. Molecular cloning and sequencing of the gene encoding the fimbrial subunit protein of Bacteroides gingivalis. J Bacteriol. 1988;170:1658–1665. doi: 10.1128/jb.170.4.1658-1665.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falkaw S. Bacterial entry into eukaryotic cells. Cell. 1991;65:1099–1102. doi: 10.1016/0092-8674(91)90003-h. [DOI] [PubMed] [Google Scholar]
- 5.Finlay B B, Starnbach M N, Francis C L, Stocker B A, Chatfield S, Dougan G, Falkow S. Identification and characterization of TnphoA mutants of Salmonella that are unable to pass through a polarized MDCK epithelial cell monolayer. Mol Microbiol. 1988;2:757–766. doi: 10.1111/j.1365-2958.1988.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 6.Finlay B B, Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989;53:210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamada N, Watanabe K, Sasakawa C, Yoshikaa M, Yoshimura F, Umemoto T. Construction and characterization of a fimA mutant of Porphyromonas gingivalis. Infect Immun. 1994;62:1696–1704. doi: 10.1128/iai.62.5.1696-1704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanazawa S, Hirose K, Ohmori Y, Amano S, Kitano S. Bacteroides gingivalis fimbriae stimulate production of thymocyte-activating factor by human gingival fibroblasts. Infect Immun. 1988;56:272–274. doi: 10.1128/iai.56.1.272-274.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanazawa S, Mrakami Y, Hirose K, Amano S, Ohmori Y, Higuchi H, Kitano S. Bacteroides (Porphyromonas) gingivalis fimbriae activate mouse peritoneal macrophages and induce gene expression and production of interleukin-1. Infect Immun. 1991;59:1972–1977. doi: 10.1128/iai.59.6.1972-1977.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter W M, Greenwood F C. Preparation of 131iodine labeled growth hormone of high specific activity. Nature (London) 1962;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- 11.Isogai H, Isogai E, Yoshimura F, Suzuki T, Kagota W, Takano K. Specific inhibition of adherence of an oral strain of Bacteroides gingivalis 381 to epithelial cells by monoclonal antibodies against the bacterial fimbriae. Arch Oral Biol. 1988;33:479–485. doi: 10.1016/0003-9969(88)90028-3. [DOI] [PubMed] [Google Scholar]
- 12.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 13.Lee J Y, Sojar H T, Bedi G S, Genco R J. Porphyromonas (Bacteroides) gingivalis fimbrillin: size, amino-terminal sequence, and antigenic heterogeneity. Infect Immun. 1991;59:383–389. doi: 10.1128/iai.59.1.383-389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J Y, Sojar H T, Bedi G S, Genco R J. Synthetic peptides analogous to the fimbrillin sequence inhibit adherence of Porphyromonas gingivalis. Infect Immun. 1992;60:1662–1670. doi: 10.1128/iai.60.4.1662-1670.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murakami Y, Hanazawa S, Watanbe A, Naganuma K, Iwasaki H, Kawakami K, Kitano S. Porphyromonas gingivalis fimbriae induce a 68-kilodalton phosphorylated protein in macrophages. Infect Immun. 1994;62:5242–5246. doi: 10.1128/iai.62.12.5242-5246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Njoroge T, Genco R J, Sojar H T, Hamada N, Genco C A. A role for fimbriae in Porphyromonas gingivalis invasion of oral epithelial cells. Infect Immun. 1997;65:1980–1984. doi: 10.1128/iai.65.5.1980-1984.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogawa T, Hamada S. Hemagglutinating and chemotactic properties of synthetic peptide segments of fimbrial protein from Porphyromonas gingivalis. Infect Immun. 1994;62:3305–3310. doi: 10.1128/iai.62.8.3305-3310.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogawa T, Kusumoto Y, Uchida H, Nagashima S, Ogo H, Hamada S. Immunological activities of synthetic peptide segment of fimbrial protein from Porphyromonas gingivalis. Biochem Biophys Res Commun. 1991;180:1335–1341. doi: 10.1016/s0006-291x(05)81342-7. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa T, Ogo H, Hamada S. Chemotaxis of human monocytes by synthetic peptides that mimic segments of Porphyromonas gingivalis fimbrial protein. Oral Microbiol Immunol. 1994;9:257–261. doi: 10.1111/j.1399-302x.1994.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 20.Ogawa T, Uchida H, Hamada S. Porphyromonas gingivalis fimbriae and their synthetic peptides induce proinflammatory cytokines in human peripheral blood monocyte cultures. FEMS Microbiol Lett. 1994;116:237–242. doi: 10.1111/j.1574-6968.1994.tb06707.x. [DOI] [PubMed] [Google Scholar]
- 21.Ogawa T, Ogo H, Akohiro Kinoshita Antagonistic effect of synthetic peptides corresponding to the binding regions within fimbrial subunit protein from Porphyromonas gingivalis to human gingival fibroblasts. Vaccine. 1997;15:230–236. doi: 10.1016/s0264-410x(96)00127-2. [DOI] [PubMed] [Google Scholar]
- 22.Pappanou P N, Sandros J, Lindberg K, Duncan M J, Niederman R, Nannmark U. Porphyromonas gingivalis may multiply and advance within stratified human functional epithelium in vitro. J Periodontal Res. 1994;29:374–375. doi: 10.1111/j.1600-0765.1994.tb01237.x. [DOI] [PubMed] [Google Scholar]
- 23.Parker J M R, Guo D C, Hodges R S. New hydrophilicity scale derived from high performance liquid chromatography peptide retention data: correlation of predicted surface residues with antigenicity and X-ray derived accessible sites. Biochemistry. 1986;25:5425–5432. doi: 10.1021/bi00367a013. [DOI] [PubMed] [Google Scholar]
- 24.Rosenshine I, Duronio V, Finlay B B. Tyrosine protein kinase inhibitors block invasion-promoted bacterial uptake by epithelial cells. Infect Immun. 1992;60:2211–2217. doi: 10.1128/iai.60.6.2211-2217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenshine I, Finlay B B. Exploitation of host signal transduction pathways and cytoskeletal functions by invasive bacteria. Bioessays. 1993;15:17–24. doi: 10.1002/bies.950150104. [DOI] [PubMed] [Google Scholar]
- 26.Sandros J, Papapanou P N, Nannmark U, Dahlen G. Porphyromonas gingivalis invades pocket epithelium in vitro. J Periodontal Res. 1994;29:62–69. doi: 10.1111/j.1600-0765.1994.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 27.Sansonetti P J. Bacterial pathogens, from adherence to invasion: comparative strategies. Med Microbiol Immunol. 1993;182:223–232. doi: 10.1007/BF00579621. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt A, Hanley P O, Schoolnik G K. Gal-Gal pyelonephritis Escherichia coli pili linear immunogenic and antigenic epitopes. J Exp Med. 1984;161:705–717. doi: 10.1084/jem.161.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sojar H T, Lee J Y, Bedi G S, Cho M I, Genco R J. Purification, characterization and immunolocalization of fimbrial protein from Porphyromonas (Bacteroides) gingivalis. Biochem Biophys Res Commun. 1991;175:713–719. doi: 10.1016/0006-291x(91)91624-l. [DOI] [PubMed] [Google Scholar]
- 30.Weinberg A, Belton C M, Park Y, Lamont R J. Role of Porphyromonas gingivalis fimbriae in invasion of gingival epithelial cells. Infect Immun. 1997;65:313–316. doi: 10.1128/iai.65.1.313-316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]