Abstract
Background:
Previous studies have observed an increased incidence of Cetuximab-induced hypersensitivity infusion reactions (CI-IRs) in the southeastern states of the USA. Tick’s bites were suspected of generating cross-reactions between cetuximab and alpha-gal. This study aims was to describe the incidence and associated risk factors of CI-IRs, in the French areas chosen according to their Lyme disease incidence.
Patients and methods:
A retrospective chart review was conducted on patients that received cetuximab infusion from January 2010 to June 2019 in 4 French areas with different Lyme disease incidence rates.
Results:
Of 1392 patients, 117 (8.4%) experienced a CI-IR, including 68 severe (grade 3 or 4) reactions (4.9%). This CI-IR incidence was significantly higher in the Lyme disease high-risk area than in the other areas (13.2% versus 7.1%, 8.1% and 6.4%; P = 0.016). Sex (P = 0.53), premedication (P = 0.91), primary cancer location (P = 0.46) and chemotherapy regimen type (P = 0.78) had no impact on CI-IR incidence in the overall population. In the head and neck squamous cell carcinoma (HNSCC) patient subgroup, CI-IRs were significantly more frequent in the high-risk area (16.4% versus 6.7%, 7.1% and 7.0%; P = 0.0015).
Conclusion:
This study suggests that patients treated in the French area with the highest incidence of Lyme disease are at a higher risk of CI-IRs.
Keywords: Cetuximab, Infusion reaction, Lyme disease, Hypersensitivity, Risk factors, Alpha-gal, Head, And neck neoplasms
Highlights
In a large series of 1392 patients, the 266 patients treated in the French area with the highest incidence of Lyme disease were at a higher risk of cetuximab-induced hypersensitivity infusion reactions compared to those from other areas (16.4% versus 6.7%, 7.1% and 7.0% in medium-, low- and very-low-risk areas; P = 0.0015).
The risk of cetuximab-induced hypersensitivity infusion reactions in head and neck squamous cell carcinoma patients was higher only in the area with the highest incidence of Lyme disease.
Age, sex, premedication, primary cancer location and chemotherapy regimen type had no impact on the incidence of cetuximab-induced infusion reactions in the overall population.
Introduction
Cetuximab is a recombinant human-murine chimeric immunoglobulin G1 monoclonal antibody targeting the human epidermal growth factor receptor (EGFr) by competitively inhibiting the binding of EGF and other ligands on non-tumoral and tumoral cells [1]. Cetuximab has been approved for the treatment of metastatic colorectal cancer in association with anticancer chemotherapy regimen (only for wild type Rat Sarcoma Virus (RAS) status) and advanced head and neck squamous cell carcinoma (HNSCC), in combination with radiotherapy or platinum-based anticancer chemotherapy regimen [1–8].
The most common adverse events include cutaneous eruptions and hypersensitivity infusion reactions [2, 9–11]. Cetuximab-induced hypersensitivity infusion reactions (CI-IRs) occurred in 8.4% of 1373 patients who received cetuximab across clinical trials, mostly on first infusion [1]. Among them, severe CI-IRs were reported in 2.2% with one fatal outcome [1]. An anaphylactic mechanism mediated by immunoglobulin E seems to be a predominant pathway in those hypersensitivity reactions [12–16]. CI-IR incidence could be reduced using combined corticosteroid and histamine H-1 antagonist (H1A) premedication in metastatic colorectal cancer [2, 17–20]. A systematic double premedication is now recommended in Summary Product Characteristics (SPC) of cetuximab. The SPC also specifies an infusion flow rate of 5 and 10 mg/min for first and subsequent courses respectively to prevent CI-IRs [2].
Geography, allergy history, cancer type (HNSCC), ethnic group, premedication, tobacco and alcohol consumption have been reported as potential risk factors for CI-IRs, but these factors remain controversial [21–29].
In southeastern states of the United State of America (USA), an increased incidence of CI-IRs was observed in real life during post-marketing surveillance [21–23, 26, 28, 30–33]. High rates of severe CI-IRs have been reported in North Carolina (14.4%), Tennessee (14%), Missouri (24.6%), Oklahoma (12.4%), Florida (27%) and Arkansas (14.5%) [21, 23, 26, 28, 30, 33]. Hypotheses have been put forward that a history of lone star tick (Amblyomma americanum) bites prior to cetuximab infusion could generate crossed reactions between cetuximab and alpha-gal, an oligosaccharide also observed in cetuximab heavy chain, through IgE [12, 13, 15, 16, 31, 34–39, 40, 41].
The European data related to CI-IR incidence and geographic area as a risk factor are poor [27, 29]. In Europe, the Ixodes ricinus tick is responsible for transmitting the Borellia burgdorferi bacterium causing Lyme disease [42]. This European tick is also associated with IgE responses to alpha-gal and red meat allergy [39, 43–46]. Variability in incidence rates of Lyme disease could be explained by differences in geographical and climate characteristics, in types of exposure and the presence of competent reservoir hosts [47].
The aims of the present CETUXIR study were to describe the incidence of CI-IRs, their management and associated risk factors in four French areas with different Lyme disease incidence rates.
Patients and methods
Study design and patients
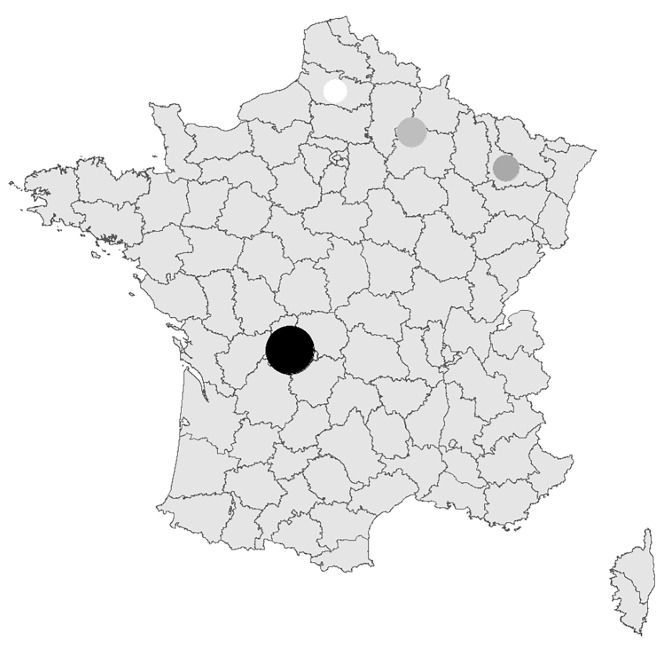
The CETUXIR study was a retrospective multicentric cohort study of all consecutive cancer patients treated with cetuximab conducted at 6 tertiary centres, in four French areas (defined by administrative regions): Amiens University Hospital, Limoges University Hospital, the University Hospital and Lorraine Cancer Institute in Nancy, the University Hospital and Godinot Cancer Institute in Reims. Areas were chosen and ranked in distinct categories according to their known Lyme disease incidence, using reference work from epidemiological surveillance by the Sentinelles network between 2005 and 2016: high risk (> 150/100,000 inhabitants) for the Limoges area, medium risk (50–100/100,000 inhabitants) for the Nancy area, low risk (20–50/100,000 inhabitants) for the Reims area and very low risk (5–20/100,000 inhabitants) for the Amiens area [47] (Fig. 1).
Fig. 1.
French map with estimated mean annual regional incidence rates of Lyme disease (per 100 000 inhabitants) and incidence of cetuximab-induced hypersensitivity infusion reactions (CI-IRs) in each of the four French areas
CI-IRs, cetuximab-induced hypersensitivity infusion reactions; N, number of patients
Inclusion criteria included patients over 18 years old undergoing the administration of a first infusion of cetuximab from 1st January, 2010 to 30th June, 2019. Patients were excluded if they were opposed to the study.
Data collection
Data from eligible patients were extracted and collected from the computerized order entry (CPOE) software for anticancer chemotherapy (CHIMIO v5.7, Computer Engineering, Paris, France). The following clinical, pathological and therapeutic variables were collected: age, sex, primary cancer location, cetuximab-based regimen type (monotherapy or chemotherapy-based combination, concomitant radiotherapy) and use of premedication with corticosteroids and/or H1A. Lyme disease history of the included patients were not collected. Finally, the occurrence and symptoms of CI-IRs (any grades) on the first infusion of cetuximab and outcome (potential cetuximab rechallenge, desensitization procedure, and switch to panitumumab) were recorded. CI-IRs were graded retrospectively based on National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTC-AE) version 5.0 using medical records or via symptom review [48]. Severe CI-IRs were defined as grade 3- and 4- reactions.
Outcomes
The primary objective was to describe the incidence of CI-IRs in different French areas according to Lyme disease incidence. The secondary objectives were to describe therapeutic management after CI-IRs and to identify CI-IR-associated risk factors.
Ethical considerations
Patients’ records were anonymized prior to analysis. A database was created in accordance with the reference methodology MR004 of the National Commission of Liberties and Informatics (n°2,206,749, 13/09/2018). A non-opposition form was sent to each living patient included in the study. As per French regulations, no additional ethical committee review was required.
Statistical analysis
Quantitative data were described with their median and inter-quartile ranges (Q1-Q3) and compared with the non-parametric Kruskall-Wallis test. Qualitative data were described by frequencies and percentages and compared with the Chi-square test or Fisher’s exact test when appropriate. Subgroup analyses of CI-IR risk factors by area were carried out. Multivariate analyses were conditioned to a minimal number of events at the statistician’s discretion. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Statistical significance was defined as a P-value < 0.05 for all tests.
Results
Population characteristics
A total of 1392 consecutive patients were included. The main characteristics of patients treated by cetuximab, overall and per area, are presented in Table 1. Most patients were male (78.4%) and received cetuximab in combination with polychemotherapy (65.5%). Most primary cancer locations were advanced HNSCC (69%) or metastatic colorectal cancer (24%). Almost all patients (97.8%) received double premedication (corticosteroids and H1A). Mean age was 63 + 10 years, but patients were younger in the medium-risk area (61.6 + 10; P = 0.006). Frequencies of HNSCC and colorectal cancer were significantly different in the 4 areas (for HNSCC: 59.8% in the high-risk area, 70.7% in the medium-risk area, 69.5% in the low-risk area and 73.8% in the very-low-risk area; P = 0.0003; and for colorectal cancer: 37.2% in the high-risk area, 24.4% in the medium-risk area, 19.4% in the low-risk area, and 21.8% in the very-low-risk area; P < 0.0001. Double premedication frequency was also significantly different in the 4 areas (99.6% in the high-risk area, 98.9% in the medium-risk area, 94.3% in the low-risk area and 99.2% in the very-low-risk area, P < 0.0001) (Table 1).
Table 1.
Population characteristics and univariate analysis on the association between population characteristics and 4 areas of treatment
| Characteristics | Total (N = 1392) | High-risk areaa Limoges (N = 266) | Medium-risk areab Nancy (N = 467) |
Low-risk areac Reims (N = 407) | Very-low-risk aread Amiens (N = 252) |
P-value | ||
|---|---|---|---|---|---|---|---|---|
| Age (years), median (inter-quartile) | 63.0 (56–70) | 64.5 (57–72) | 62.0 (55–68) | 63.0 (56–71) | 63.0 (58–70) | 0.0064 | ||
| Sex | ||||||||
| Male, N (%) | 1091 (78.4) | 199 (74.8) | 368 (78.8) | 329 (80.8) | 195 (77.4) | NS | ||
| Female, N (%) | 301 (21.6) | 67 (25.2) | 99 (21.2) | 78 (19.2) | 57 (22.6) | NS | ||
| Primary cancer locations | ||||||||
| HNSCC, N (%) | 958 (68.8) | 159 (59.8) | 330 (70.7) | 283 (69.5) | 186 (73.8) | 0,003 | ||
| Colorectal, N (%) | 347 (24.9) | 99 (37.2) | 114 (24.4) | 79 (19.4) | 55 (21.8) | < 10− 4 | ||
| CSCC, N (%) | 65 (4.7) | 7 (2.6) | 8 (1.7) | 44 (10.8) | 6 (2.4) | NS | ||
| Cervical, N (%) | 2 (0.1) | 0 | 0 | 1 (0.3) | 1 (0.4) | NS | ||
| Other, N (%) | 20 (1.4) | 1 (0.4) | 15 (3.2) | 0 | 4 (1.6) | NS | ||
| Premedications | ||||||||
| Corticosteroids alone, N (%) | 19 (1.4) | 1 (0.4) | 1 (0.2) | 15 (3.7) | 2 (0.8) | NS | ||
| H1A alone, N (%) | 11 (0.8) | 0 | 4 (0.9) | 7 (1.7) | 0 | NS | ||
| Corticosteroids + H1A, N (%) | 1361 (97.8) | 265 (99.6) | 462 (98.9) | 384 (94.6) | 250 (99.2) | < 10− 4 | ||
| Types of chemotherapy | ||||||||
| Monotherapy, N (%) | 49 (3.5) | 11 (4.1) | 5 (1.1) | 32 (7.9) | 1 (0.4) | NS | ||
| Polychemotherapy, N (%) | 911 (65.5) | 205 (77.1) | 335 (71.7) | 198 (48.6) | 173 (68.6) | NS | ||
| Concomittant radiotherapy, N (%) | 432 (31) | 50 (18.8) | 127 (27.2) | 177 (43.5) | 78 (31) | NS | ||
N, number of patients; HNSCC, head and neck squamous-cell carcinoma; CSCC, cutaneous squamous-cell carcinoma; H1A, histamine-1 receptor antagonist; NS, not significant. a, b, c, d defined according to estimated mean annual regional incidence rates of Lyme disease from Septfons A, et al. Eurosurveillance. 2019;24(11). doi:10.2807/1560-7917.ES.2019.24.11.1800134 [41] : a > 150 / 100,000 inhabitants; b 50–100 / 100,000 inhabitants; c 20–50 / 100,000 inhabitants; d 5–20 / 100,000 inhabitants
Incidence of CI-IRs
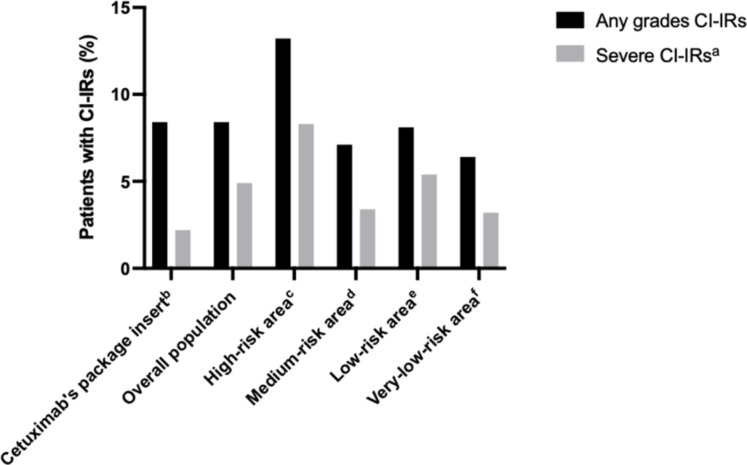
Of the 1392 patients, 117 (8.4%) experienced one CI-IR including 68 (4.8%) severe infusion reactions. No fatal outcome occurred. CI-IR incidences that occurred among patients in each area according to their grade are presented in Fig. 2; Table 2. Incidence of CI-IRs of any grade was significantly higher in the Limoges high-risk area of Lyme disease (13.2% versus 7.1%, 8.1% and 6.4%; P = 0.016). Severe CI-IRs were also more frequent in the high-risk area (8.3% versus 3.4%, 5.4% and 3.2%; P = 0.04) (Fig. 2).
Fig. 2.
Distribution of any grades and severe grades of cetuximab-induced hypersensitivity infusion reactions (CI-IRs) in each area with different Lyme disease risks (N = 117)
CI-IRs, cetuximab-induced hypersensitivity infusion reactions. a defined as grade 3 or grade 4 infusion reactions. b from ImClone Systems Incorporated. U.S. Food and Drug Administration. Erbitux (cetuximab) prescribing information. 2019 [1]. c, d, e, f defined according to estimated mean annual regional incidence rates of Lyme disease from Septfons A, et al. Eurosurveillance. 2019;24(11). doi:10.2807/1560-7917.ES.2019.24.11.1800134 [41] : c > 150 / 100,000 inhabitants; d 50–100 / 100,000 inhabitants; e 20–50 / 100,000 inhabitants; f 5–20 / 100,000 inhabitants
Table 2.
Incidences and grades of cetuximab-induced hypersensitivity infusion reactions (CI-IRs) in each area with different Lyme disease risks
| Areas | Estimated mean annual regional incidence rates of Lyme diseasea(per 100 000 inhabitants) | Incidences of CI-IRs | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade 1, N (%) | Grade 2, N (%) | Grade 3, N (%) | Grade 4, N (%) | Grade 5, N (%) | Low gradeb, N (%) |
Severe gradec, N (%) |
Any grades, N (%) | ||
|
High-risk area Limoges (N = 266) |
> 150 | 3 (1.1) | 10 (3.8) | 14 (5.3) | 8 (3.0) | 0 | 13 (4.9) | 22 (8.3) | 35 (13.2) |
|
Medium-risk area Nancy (N = 467) |
50–100 | 7 (1.5) | 10 (2.1) | 14 (3.0) | 2 (0.4) | 0 | 17 (3.6) | 16 (3.4) | 33 (7.1) |
|
Low-risk area Reims (N = 407) |
20–50 | 4 (1.0) | 7 (1.7) | 14 (3.4) | 8 (2.0) | 0 | 11 (2.7) | 22 (5.4) | 33 (8.1) |
|
Very-low-risk area Amiens (N = 252) |
5–20 | 4 (1.6) | 4 (1.6) | 5 (2.0) | 3 (1.2) | 0 | 8 (3.2) | 8 (3.2) | 16 (6.4) |
| Overall study population (N = 1392) | 18 (1.3) | 31 (2.2) | 47 (3.4) | 21 (1.5) | 0 | 49 (3.5) | 68 (4.9) | 117 (8.4) | |
CI-IRs, cetuximab induced hypersensitivity infusion reactions; N, number of patients
a from Septfons A, et al. Epidemiology of Lyme borreliosis through two surveillance systems: the national Sentinelles GP network and the national hospital discharge database, France, 2005 to 2016. Eurosurveillance. 2019;24(11). doi:10.2807/1560-7917.ES.2019.24.11.1800134 [41]. b defined as grade 1 or grade 2 infusion reactions. c defined as grade 3 or grade 4 infusion reactions
Therapeutic management after CI- IRs
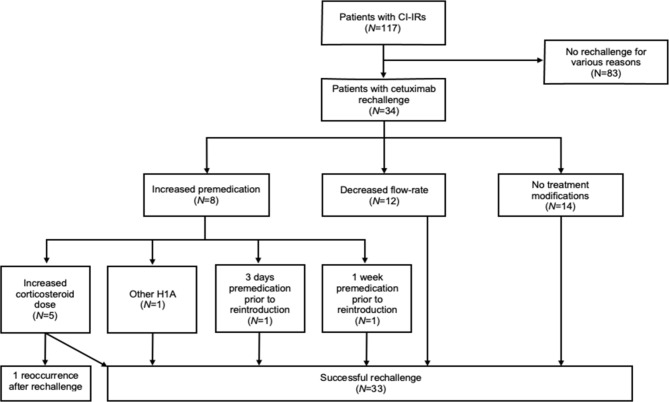
The flow chart of cetuximab rechallenge after cetuximab-induced infusion reaction (CI-IRs) is presented in Fig. 3. Thirty-four patients among 117 (29%) who had a CI-IR were rechallenged. Among them, 8 patients were rechallenged with increased premedication and 12 patients received cetuximab with a decreased flow-rate infusion. A total of 14 patients were rechallenged without treatment modification. Only one patient experienced a subsequent reaction despite premedication reinforcement. In this study, no patient was desensitized. Among the 30 patients treated for metastatic colorectal cancer, 13 (43%) were switched to panitumumab without infusion reaction.
Fig. 3.
Flow chart of cetuximab rechallenge after cetuximab-induced infusion reaction (CI-IRs).
CI-IRs, cetuximab-induced infusion reactions; N, number of patients; H1A, histamine-1 receptor antagonist
Associated variables with CI-IRs
In the overall population, age, sex, primary cancer location, premedication type and cetuximab-based regimen were not significantly associated with CI-IRs (Table 3).
Table 3.
Univariate analysis on the risk factors of cetuximab-induced hypersensitivity infusion reactions (CI-IRs)
| Characteristics | N | Patients with CI-IRs (N = 117) |
Patients without CI-IRs (N = 1275) | P-value | ||
|---|---|---|---|---|---|---|
| Age (years), mean ± SD | 1392 | 64.0 ± 9.7 | 62.9 ± 10.6 | 0.28 | ||
| Sex | 0.53 | |||||
| Male, N (%) | 1091 | 89 (8.2) | 1002 (91.8) | |||
| Female, N (%) | 301 | 28 (9.3) | 273 (90.7) | |||
| Primary cancer locations | 0.91 | |||||
| Head and Neck, N (%) | 958 | 81 (8.5) | 877 (91.5) | |||
| Colorectal, N (%) | 347 | 30 (8.6) | 317 (91.3) | |||
| Premedications | 0.46 | |||||
| Corticosteroids alone, N (%) | 19 | 0 | 19 (100) | |||
| H1A alone, N (%) | 11 | 0 | 11 (100) | |||
| Corticosteroids + H1A, N (%) | 1361 | 117 (8.6) | 1244 (91.4) | |||
| Types of chemotherapy regimen | 0.78 | |||||
| Monotherapy, N (%) | 49 | 4 (8.2) | 45 (91.8) | |||
| Polychemotherapy, N (%) | 911 | 80 (8.8) | 831 (91.2) | |||
| Concomittant radiotherapy, N (%) | 432 | 33 (7.6) | 399 (92.4) | |||
| Lyme disease risk areasa | 0.016 | |||||
| High-risk areab, N (%) | 266 | 35 (13.2) | 231 (86.8) | |||
| Medium-risk areac, N (%) | 467 | 33 (7.1) | 434 (92.9) | |||
| Low-risk aread, N (%) | 407 | 33 (8.1) | 374 (91.9) | |||
| Very-low-risk areae, N (%) | 252 | 16 (6.4) | 236 (93.6) | |||
CI-IRs, cetuximab induced hypersensitivity infusion reactions; SD, standard deviation; H1A, histamine-1 receptor antagonist
a defined according to estimated mean annual regional incidence rates of Lyme disease from Septfons A, et al. Epidemiology of Lyme borreliosis through two surveillance systems: the national Sentinelles GP network and the national hospital discharge database, France, 2005 to 2016 Eurosurveillance. 2019;24(11). doi:10.2807/1560-7917.ES.2019.24.11.1800134 [41]. : b > 150 / 100 000 inhabitants; c 50–100 / 100 000 inhabitants; d 20–50 / 100 000 inhabitants; e 5–20 / 100 000 inhabitants
All 117 patients who experienced a CI-IR received the recommended double premedication.
In the HNSCC patient subgroup, CI-IRs were more frequent in the high-risk area of Lyme disease (16.4%) than in the 3 other areas (6.7% in the medium-risk area, 7.1% in the low-risk area and 7.0% in the very-low-risk area; P = 0.0015). There was no significant difference in CI-IR incidence concerning the colorectal patient subgroup in the four areas (8.1% in the high-risk area, 9.7% in the medium-risk area, 11.4% in the low-risk area and 3.7% in the very-low-risk area), nor concerning sex, cetuximab-based regimen and pre-medication subgroups (P not evaluable (futile); data not shown).
Discussion
The CETUXIR study is the first one to investigate CI-IR distribution in a large cohort of 1392 consecutive patients treated in real life in four different French areas. Our study shows a higher incidence of CI-IRs in the area with the highest risk of Lyme disease in a European country, in line with previous non-European findings.
The present study found an overall incidence of 8.4% of any grades CI-IRs, consistent with previous data provided by the cetuximab SCP [1] (Fig. 2). CI-IR rates were significantly higher in the high-risk area of Lyme disease compared with others (13.2% versus 7.1%, 8.1%, 6.4%; P = 0.016) and compared with the literature [1, 2, 4, 5, 7, 49–51]. Severe CI-IR rates also seemed to be higher in the high-risk area (8.3% versus 3.4%, 5.4% and 3.2%). However, no statistical test was performed for severe CI-IRs due to small samples per group despite the large number of patients in the study. A previous French monocentric study found a 5.4% rate of CI-IRs in 428 patients treated for advanced HNSCC at Gustave Roussy cancer centre in Paris, Île de France (urban low-risk area) [29]. In another French small cohort of 229 patients living in the Normandy very-low-risk area, the CI-IRs rates were 10.5% (any grades) and 4.8% (grades 3–4) respectively [52]. In this study, no clinical variables predicted CI-IR risk but anti-cetuximab IgE was found in 13 of 17 patients (76.5%) when experiencing CI-IRs compared with 17 of 91 control patients (18.7%).
Our results suggest the role of Lyme disease and tick bites in the occurrence of CI-IRs. An increased rate of CI-IRs has also been largely studied in the southeastern states of the USA in which the distribution of the Amblyomma americanum tick (lone star tick) overlaps with the region of both cetuximab sensitivity and red meat allergy [21, 22, 26, 28, 30, 33, 34]. The Ixodes ricinus tick is the main European vector of Lyme disease (a borreliosis) and is also associated with alpha-gal sensitization. In Sweden, Hamsten and al. screened 207 patients with Lyme disease as a confirmed recently tick-bitten population and found 22% to have positive IgE levels to alpha-gal [46]. In a second study, Hamsten and al. demonstrated that the alpha-gal epitope was present in the gastrointestinal tract of Ixodes ricinus [43].
Despite the identification of different individual risk factors for CI-IRs, these associated factors are not consistent in retrospective studies [22, 24, 25, 28, 33]. Among the 374 CI-IRs reported during a 15-year period in The French Pharmacovigilance database, the indication of cetuximab was more likely HNSCC than colorectal cancer (P < 0.001) [27]. In a French monocentric study, combined tobacco and alcohol history (P = 0.009) and prior allergy history (P = 0.003) were associated with CI-IRs in 428 patients treated for HNSCC [29]. The pathophysiological mechanisms of the relation between CI-IR risk and HNSCC with alcohol and tobacco history remain unclear. Tobacco and alcohol exposure could mediate local chronic inflammation and favour an IgE-mediated reaction [29]. In the present study, tobacco-alcohol consumption was not collected and the risk of CI-IRs in HNSCC patients was higher only in the Limoges high-risk area. This result suggests the existence of a still unknown confusion bias between HNSCC and Lyme disease.
The ability of double premedication to reduce the incidence and severity of Ci-IRs was not clear according to current published data [17–19, 21, 22, 24, 28, 52]. Although the administration of H1A before infusion of cetuximab may limit the occurrence of anaphylaxis, the addition of corticosteroids does not seem to be effective. Interestingly, in our study, all the patients who experienced a CI-IR received a double premedication with corticosteroid and H1A as recommended by SPC and the latest international guidelines [1]. Thus, the high incidence of CI-IRs observed in the Limoges high-risk area was not explained by the use of a non-optimal premedication.
Management of CI-IRs is well described in the cetuximab SPC whatever the grade [1]. However, the present study is in line with previous ones that reported heterogeneous therapeutic management after low-grade (grade 1 and 2) infusion reactions [9, 26, 52, 53]. This might result from the difficulty to detect and input subtle symptoms that occur during low-grade CI-IRs. Some studies have found that the practice of cetuximab rechallenges in low-grade CI-IRs was feasible and safe [24, 53]. Indeed, among the 34 patients who were rechallenged in our study, all experienced low-grade infusion reactions and only one re-challenged patient developed a subsequent reaction. However, it is preferable to avoid cetuximab rechallenge in patients who experienced a severe CI-IR. When performed, it should be justified by suboptimal carcinological outcomes, after drug desensitization. In previous studies, several desensitization protocols were developed to safely carry on using cetuximab [54–60]. Despite this fact, no desensitization procedure was performed in our study and definitive discontinuation of cetuximab was decided for all patients that experienced severe CI-IRs. For patients with metastatic colorectal cancer who experienced CI-IRs, switching to panitumumab seems to be a safe therapeutic alternative, especially in cases of severe CI-IRs [61]. Panitumumab is a fully recombinant IgG2 human monoclonal antibody targeting EGFR with restricted approval to metastatic colorectal cancer treatment alone [62]. Clinical trials have shown that, unlike cetuximab, panitumumab is rarely associated with infusion reactions (3–4%) even without premedication [50, 63–66].
This study has several limitations. First, the rates of CI-IRs might be underestimated due to the retrospective collection of data, as some low-grade IRs (grade 1 or 2) might not have been reported in the health record. Moreover, history of tick bites and anti-cetuximab IgE assay, which could have strengthened this association with chronological and biological arguments were not collected. We were, therefore, unable to compare the incidence of anti-bodies directed against Lyme disease based on the geographical area of the patient. Besides, we considered only variables that could be extracted from clinical databases in our analysis. Additional unmeasured confounding factors (tobacco, alcohol, atopy history, surgery, chemotherapy regimen, radiotherapy doses) contributing to CI-IRs may exist. Furthermore, patients may live or have lived in areas where Lyme disease incidences are different from the area where they were treated. An exhaustive data collection of patients’ current and past locations may be needed.
This study suggests that patients previously infected with Lyme disease are at a higher risk of CI-IRs. HNSCC seems to be a predictive risk factor of CI-IRs only in the Lyme disease high-risk area. This result suggests a possible association between HNSCC and Lyme disease. Further studies are needed to investigate these associations. A geographic and ecological study could be performed to investigate the influence of Lyme disease on the appearance of CI-IRs at the scale of groups of individuals. This retrospective study could be carried out prior to analytical epidemiological studies (cohort or case-control studies carried out at the individual level) to establish a strong association between Lyme disease and CI-IRs. An anti-cetuximab IgE assay could be used prior to cetuximab treatment to identify patients at higher risk of CI-IRs, especially in the area with the highest incidence of Lyme disease.
Acknowledgements
The authors would like to thank Ms. Daniela Pellot of the SERRA at Reims Faculty of Medicine for assistance with English language editing.
Funding
No external funding was received for this study or for the preparation of this manuscript.
Data availability
All data are availlable by asking the corresponding author at claire.carlier@chu-reims.fr.
Declarations
Ethical approval and consent to participate
A database was created in accordance with the reference methodology MR004 of the National Commission of Liberties and Informatics (n°2206749, 13/09/2018). A non-opposition form was sent to each living patient included in the study. As per French regulations, no additional ethical committee review was required.
Decree No. 2016 − 1537 of November 16, 2016 relating to research involving the human person. This decree is taken for the application of the modified law n° 2012 − 300 of March 12, 2012 relating to research involving the human person known as “Loi Jardé”.
Non-interventional research (Category 3): This is research that does not involve any risk or constraint, does not modify the care of the participants and in which all the acts are performed and the products used in the usual way. They are also referred to as “observational research”. For example, they may relate to treatment compliance, monitoring of a drug after it has been marketed, the practices of one healthcare center compared to those of another, etc.
Category 3 searches also require receiving:
• an authorization from the Commission Nationale Informatique et Liberté (CNIL) or respecting a reference methodology (MR004) concerning the processing of the personal data of the persons involved.
• informing the ANSM of the opinion issued by the Committee for the Protection of Persons, without its authorization being required.
• the information of the people involved in the research and the collection of their non-objection to the use of the data collected.
The Jardé law considers that it is not a study on human beings but a study on medical records (and therefore that there is no need for an opinion from a committee for the protection of people) The CNIl reference methodology MR004 imposes patient information (non-objection form) with an exemption from information for deceased subjects (if the subject did not express his opposition to the use of its data for research).
No administrative permissions were required to access the raw data used in ourstudy.
The data used in this study were anonymised before its use.
Consent for publication
NA.
Competing interests
C-GR reports honoraria as a speaker and/or in an advisory role from Takeda, Gilead, Ferring, and Tillots, unrelated to this work. DB reports honoraria as a speaker and/or in an advisory role from Amgen, Accord Healthcare, Sanofi, Servier, Roche and Pierre Fabre, unrelated to this work.MB reports honoraria as a speaker and/or in an advisory role from Astra-Zeneca, Amgen and Bayer, unrelated to this work. VH reports honoraria as a speaker and/or in an advisory role from Merck KGaA and Amgen, unrelated to this work. AL reports research funding from Roche, and honoraria as a speaker and/or in an advisory role from Amgen, and receiving lecture fees from Vifor Pharma, Bayer, Merck, Sanofi, and Ipsen, unrelated to this work. FS reports honoraria as a speaker and/or in an advisory role from Gilead, and Astra-Zeneca, unrelated to this work. OB reports honoraria as a speaker and/or in an advisory role from Merck KGaA, Roche Genentech, Bayer, MSD, Amgen, Sanofi, Servier, and Pierre Fabre, unrelated to this work. All remaining authors have declared no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
F Slimano and M Brugel contributed equally to this study
References
- 1.ImClone Systems Incorporated. U.S. Food and Drug Administration. Erbitux (cetuximab) prescribing information. 2019.
- 2.European Medicines Agency. European public assessment report for Erbitux (cetuximab). 2020.
- 3.Phelip JM, Tougeron D, Léonard D, et al. Metastatic colorectal cancer (mCRC): French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR) Dig Liver Dis. 2019;51(10):1357–63. doi: 10.1016/j.dld.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 4.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus Cetuximab for Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2006;354(6):567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 5.Vermorken JB, Mesia R, Rivera F, et al. Platinum-Based Chemotherapy plus Cetuximab in Head and Neck Cancer. N Engl J Med. 2008;359(11):1116–27. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 6.Machiels J-P, René Leemans C, Golusinski W, et al. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS–ESMO–ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(11):1462–75. doi: 10.1016/j.annonc.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the Treatment of Colorectal Cancer. N Engl J Med. 2007;357(20):2040–8. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham D, Humblet Y, Siena S, et al. Cetuximab Monotherapy and Cetuximab plus Irinotecan in Irinotecan-Refractory Metastatic Colorectal Cancer. N Engl J Med. 2004;351(4):337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 9.Foley KA, Wang PF, Barber BL, et al. Clinical and economic impact of infusion reactions in patients with colorectal cancer treated with cetuximab. Ann Oncol. 2010;21(7):1455–61. doi: 10.1093/annonc/mdp535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaguchi K, Watanabe T, Satoh T, et al. Severe Infusion Reactions to Cetuximab Occur within 1 h in Patients with Metastatic Colorectal Cancer: Results of a Nationwide, Multicenter, Prospective Registry Study of 2126 Patients in Japan. Jpn J Clin Oncol. 2014;44(6):541–6. doi: 10.1093/jjco/hyu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song X, Long R, Barber S, et al. Systematic Review on Infusion Reactions Associated with Chemotherapies and Monoclonal Antibodies for Metastatic Colorectal Cancer. Curr Clin Pharmacol. 2012;7(1):56–65. doi: 10.2174/157488412799218806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung CH, Mirakhur B, Chan E, et al. Cetuximab-Induced Anaphylaxis and IgE Specific for Galactose-α-1,3-Galactose. N Engl J Med. 2008;358(11):1109–17. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Platts-Mills TAE, Li RC, Keshavarz B, et al. Diagnosis and Management of Patients with the alpha-Gal Syndrome. J Allergy Clin Immunol Pract. 2020;8(1):15–23.e1. doi: 10.1016/j.jaip.2019.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chinuki Y, Morita E. Alpha-Gal-containing biologics and anaphylaxis. Allergol Int. 2019;68(3):296–300. doi: 10.1016/j.alit.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Commins SP. Diagnosis & management of alpha-gal syndrome: lessons from 2,500 patients. Expert Rev Clinical Immunol. 2020;16(7):667–77. doi: 10.1080/1744666X.2020.1782745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de la Fuente J, Pacheco I, Villar M, et al. The alpha-Gal syndrome: new insights into the tick-host conflict and cooperation. Parasit Vectors. 2019;12(1):154. doi: 10.1186/s13071-019-3413-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung CH. Managing Premedications and the Risk for Reactions to Infusional Monoclonal Antibody Therapy. Oncologist. 2008;13(6):725–32. doi: 10.1634/theoncologist.2008-0012. [DOI] [PubMed] [Google Scholar]
- 18.Ikegawa K, Suzuki S, Nomura H, et al. Retrospective analysis of premedication, glucocorticosteroids, and H 1 -antihistamines for preventing infusion reactions associated with cetuximab treatment of patients with head and neck cancer. J Int Med Res. 2017;45(4):1378–85. doi: 10.1177/0300060517713531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siena S, Glynne-Jones R, Adenis A, et al. Reduced incidence of infusion-related reactions in metastatic colorectal cancer during treatment with cetuximab plus irinotecan with combined corticosteroid and antihistamine premedication. Cancer. 2010;116(7):1827–37. doi: 10.1002/cncr.24945. [DOI] [PubMed] [Google Scholar]
- 20.Durham CG, Thotakura D, Sager L, et al. Cetirizine versus diphenhydramine in the prevention of chemotherapy-related hypersensitivity reactions. J Oncol Pharm Pract. 2019;25(6):1396–401. doi: 10.1177/1078155218811505. [DOI] [PubMed] [Google Scholar]
- 21.O’Neil BH, Allen R, Spigel DR, et al. High Incidence of Cetuximab-Related Infusion Reactions in Tennessee and North Carolina and the Association With Atopic History. J Clin Oncol. 2007;25(24):3644–8. doi: 10.1200/JCO.2007.11.7812. [DOI] [PubMed] [Google Scholar]
- 22.Hansen NL, Chandiramani DV, Morse MA, et al. Incidence and predictors of cetuximab hypersensitivity reactions in a North Carolina academic medical center. J Oncol Pharm Pract. 2011;17(2):125–30. doi: 10.1177/1078155209360853. [DOI] [PubMed] [Google Scholar]
- 23.Atwal D, Safar AM, Govindarajan R, Makhoul I. Severe first infusion reaction related to cetuximab in cancer patients in Arkansas. J Oncol Pharm Pract. 2019;25(5):1130–4. doi: 10.1177/1078155218780514. [DOI] [PubMed] [Google Scholar]
- 24.Touma W, Koro SS, Ley J, et al. Risk factors for and pre-medications to prevent cetuximab-induced infusion reactions in patients with squamous cell carcinoma of the head and neck. Oral Oncol. 2014;50(9):895–900. doi: 10.1016/j.oraloncology.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waqar SN, Tan BR, Zubal B, et al. Race and albuterol premedication are risk factors for hypersensitivity reactions to cetuximab. J Clin Oncol. 2008;26(15_suppl):20503. doi: 10.1200/jco.2008.26.15_suppl.20503. [DOI] [Google Scholar]
- 26.George TJ, Laplant KD, Walden EO, et al. Managing cetuximab hypersensitivity-infusion reactions: incidence, risk factors, prevention, and retreatment. J Support Oncol. 2010;8(2):72–7. [PubMed] [Google Scholar]
- 27.Grandvuillemin A, Disson-Dautriche A, Miremont-Salamé G, et al. Cetuximab infusion reactions: French pharmacovigilance database analysis. J Oncol Pharm Pract. 2013;19(2):130–7. doi: 10.1177/1078155212457965. [DOI] [PubMed] [Google Scholar]
- 28.Hopps S, Medina P, Pant S, et al. Cetuximab hypersensitivity infusion reactions: Incidence and risk factors. J Oncol Pharm Pract. 2013;19(3):222–7. doi: 10.1177/1078155212462440. [DOI] [PubMed] [Google Scholar]
- 29.Palomar Coloma V, Bravo P, Lezghed N, et al. High incidence of cetuximab-related infusion reactions in head and neck patients. ESMO Open. 2018;3(5):e000346. doi: 10.1136/esmoopen-2018-000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keating K, Walko C, Stephenson B, et al. Incidence of cetuximab-related infusion reactions in oncology patients treated at the University of North Carolina Cancer Hospital. J Oncol Pharm Pract. 2014;20(6):409–16. doi: 10.1177/1078155213510542. [DOI] [PubMed] [Google Scholar]
- 31.Adams CB, Street DS, Crass M, Bossaer JB. Low rate of cetuximab hypersensitivity reactions in Northeast Tennessee: An Appalachian effect? J Oncol Pharm Pract. 2016;22(6):784–9. doi: 10.1177/1078155215618771. [DOI] [PubMed] [Google Scholar]
- 32.Baxley AA, Doyin-Lipede OA, Razaq MA. Hypersensitivity to Cetuximab After Geographic Relocation. Am J Ther. 2018;25(6):e699–700. doi: 10.1097/MJT.0000000000000709. [DOI] [PubMed] [Google Scholar]
- 33.Owera R, Gill A, Haddadin S, et al. High incidence of hypersensitivity reactions to cetuximab infusions in mid-Missouri: Association with prior history of atopy. J Clin Oncol. 2008;26(15_suppl):20747. doi: 10.1200/jco.2008.26.15_suppl.20747. [DOI] [Google Scholar]
- 34.Commins SP, James HR, Kelly LA, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-α-1,3-galactose. J Allergy Clin Immunol. 2011;127(5):1286–93.e6. doi: 10.1016/j.jaci.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Commins SP, Platts-Mills TAE. Allergenicity of Carbohydrates and Their Role in Anaphylactic Events. Curr Allergy Asthma Rep. 2010;10(1):29–33. doi: 10.1007/s11882-009-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michel S, Scherer K, Heijnen IAFM, Bircher AJ. Skin prick test and basophil reactivity to cetuximab in patients with IgE to alpha-gal and allergy to red meat. Allergy. 2014;69(3):403–5. doi: 10.1111/all.12344. [DOI] [PubMed] [Google Scholar]
- 37.Platts-Mills TAE, Schuyler AJ, Tripathi A, Commins SP. Anaphylaxis to the Carbohydrate Side Chain Alpha-gal. Immunol Allergy Clin North Am. 2015;35(2):247–60. doi: 10.1016/j.iac.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JH, Kim JH, Kim TH, Kim S-C. Delayed mammalian meat-induced anaphylaxis confirmed by skin test to cetuximab. J Dermatol. 2013;40(7):577–8. doi: 10.1111/1346-8138.12140. [DOI] [PubMed] [Google Scholar]
- 39.Steinke JW, Platts-Mills TAE, Commins SP. The alpha-gal story: Lessons learned from connecting the dots. J Allergy Clin Immunol. 2015;135(3):589–96. doi: 10.1016/j.jaci.2014.12.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young I, Prematunge C, Pussegoda K, et al. Tick exposures and alpha-gal syndrome: A systematic review of the evidence. Ticks Tick Borne Dis. 2021;12(3):101674. doi: 10.1016/j.ttbdis.2021.101674. [DOI] [PubMed] [Google Scholar]
- 41.Sharma SR, Karim S. Tick Saliva and the Alpha-Gal Syndrome: Finding a Needle in a Haystack. Front Cell Infect Microbiol. 2021;11:680264. doi: 10.3389/fcimb.2021.680264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. The Lancet. 2012;379(9814):461–73. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- 43.Hamsten C, Starkhammar M, Tran TAT, et al. Identification of galactose-α-1,3-galactose in the gastrointestinal tract of the tick Ixodes ricinus; possible relationship with red meat allergy. Allergy. 2013;68(4):549–52. doi: 10.1111/all.12128. [DOI] [PubMed] [Google Scholar]
- 44.Beaudouin E, Kanny G, Guerin B, et al. Unusual Manifestations of Hypersensitivity After a Tick Bite: Report of Two Cases. Ann Allergy Asthma Immunol. 1997;79(1):43–6. doi: 10.1016/S1081-1206(10)63082-7. [DOI] [PubMed] [Google Scholar]
- 45.Fernández-Soto P, Dávila I, Laffond E, et al. Tick-bite-induced anaphylaxis in Spain. Ann Trop Med Parasitol. 2001;95(1):97–103. doi: 10.1080/00034983.2001.11813619. [DOI] [PubMed] [Google Scholar]
- 46.Hamsten C, Tran TAT, Starkhammar M, et al. Red meat allergy in Sweden: Association with tick sensitization and B-negative blood groups. J Allergy Clin Immunol. 2013;132(6):1431–4.e6. doi: 10.1016/j.jaci.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Septfons A, Goronflot T, Jaulhac B, et al. Epidemiology of Lyme borreliosis through two surveillance systems: the national Sentinelles GP network and the national hospital discharge database, France, 2005 to 2016. Euro Surveill. 2019;24(11):1800134. doi: 10.2807/1560-7917.ES.2019.24.11.1800134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.National Cancer Institute (U.S. Department of Health and Human Services). Common Terminology Criteria for Adverse Events (CTCAE) v5.0. 2017.
- 49.Vermorken JB, Trigo J, Hitt R, et al. Open-Label, Uncontrolled, Multicenter Phase II Study to Evaluate the Efficacy and Toxicity of Cetuximab As a Single Agent in Patients With Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck Who Failed to Respond to Platinum-Based Therapy. J Clin Oncol. 2007;25(16):2171–7. doi: 10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 50.Van Cutsem E, Peeters M, Siena S, et al. Open-Label Phase III Trial of Panitumumab Plus Best Supportive Care Compared With Best Supportive Care Alone in Patients With Chemotherapy-Refractory Metastatic Colorectal Cancer. J Clin Oncol. 2007;25(13):1658–64. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 51.Van Cutsem E, Nowacki M, Lang I, et al. Randomized phase III study of irinotecan and 5-FU/FA with or without cetuximab in the first-line treatment of patients with metastatic colorectal cancer (mCRC): The CRYSTAL trial. J. Clin. Oncol. 2007; 25(18_suppl):4000.
- 52.Dupont B, Mariotte D, Clarisse B, et al. Risk factors associated with hypersensitivity reactions to cetuximab: anti-cetuximab IgE detection as screening test. Future Oncol. 2014;10(14):2133–40. doi: 10.2217/fon.14.153. [DOI] [PubMed] [Google Scholar]
- 53.Burke E, Rockey M, Grauer D, et al. Assessment of cetuximab-induced infusion reactions and administration rechallenge at an academic medical center. Med Oncol. 2017;34(4):51. doi: 10.1007/s12032-017-0902-9. [DOI] [PubMed] [Google Scholar]
- 54.Bavbek S, Kendirlinan R, Çerçi P, et al. Rapid Drug Desensitization with Biologics: A Single-Center Experience with Four Biologics. Int Arch Allergy Immunol. 2016;171(3–4):227–33. doi: 10.1159/000454808. [DOI] [PubMed] [Google Scholar]
- 55.García-Menaya JM, Cordobés-Durán C, Gómez-Ulla J, et al. Successful Desensitization to Cetuximab in a Patient With a Positive Skin Test to Cetuximab and Specific IgE to Alpha-gal. J Investig Allergol Clin Immunol. 2016;26(2):132–4. doi: 10.18176/jiaci.0031. [DOI] [PubMed] [Google Scholar]
- 56.Hong DI, Bankova L, Cahill KN, et al. Allergy to monoclonal antibodies: cutting-edge desensitization methods for cutting-edge therapies. Expert Rev Clin Immunol. 2012;8(1):43–54. doi: 10.1586/eci.11.75. [DOI] [PubMed] [Google Scholar]
- 57.Jerath MR, Kwan M, Kannarkat M, et al. A desensitization protocol for the mAb cetuximab. J Allergy Clin Immunol. 2009;123(1):260–2. doi: 10.1016/j.jaci.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 58.Pérez-Rodríguez E, Martínez-Tadeo JA, Pérez-Rodríguez N, et al. Outcome of 490 Desensitizations to Chemotherapy Drugs with a Rapid One-Solution Protocol. J Allergy Clin Immunol Pract. 2018;6(5):1621–7.e6. doi: 10.1016/j.jaip.2017.11.033. [DOI] [PubMed] [Google Scholar]
- 59.Saif MW, Syrigos KI, Hotchkiss S, et al. Successful desensitization with cetuximab after an infusion reaction to panitumumab in patients with metastatic colorectal cancer. Cancer Chemother Pharmacol. 2009;65(1):107–12. doi: 10.1007/s00280-009-1009-6. [DOI] [PubMed] [Google Scholar]
- 60.Solduzian M, Anvari S, Taghvaye Masoumi H, et al. Successful desensitization of a patient with cetuximab hypersensitivity: A case report. J Oncol Pharm Pract. 2019;25(7):1726–30. doi: 10.1177/1078155218793505. [DOI] [PubMed] [Google Scholar]
- 61.Heun J, Holen K. Treatment with Panitumumab After a Severe Infusion Reaction to Cetuximab in a Patient with Metastatic Colorectal Cancer: A Case Report. Clin Colorectal Cancer. 2007;6(7):529–31. doi: 10.3816/CCC.2007.n.019. [DOI] [PubMed] [Google Scholar]
- 62.Amgen, Incorporated. U.S. Food and Drug Administration. Vectibix (panitumumab) prescribing information. 2017.
- 63.Berlin J, Neubauer M, Swanson P, et al. Panitumumab antitumor activity in patients (pts) with metastatic colorectal cancer (mCRC) expressing ≥ 10% epidermal growth factor receptor (EGFr) J Clin Oncol. 2006;24(18_suppl):3548. doi: 10.1200/jco.2006.24.18_suppl.3548. [DOI] [Google Scholar]
- 64.Cohenuram M, Saif MW. Panitumumab the first fully human monoclonal antibody: from the bench to the clinic. Anticancer Drugs. 2007;18(1):7–15. doi: 10.1097/CAD.0b013e32800feecb. [DOI] [PubMed] [Google Scholar]
- 65.Gibson TB, Ranganathan A, Grothey A, Randomized Phase III. Trial Results of Panitumumab, a Fully Human Anti—Epidermal Growth Factor Receptor Monoclonal Antibody, in Metastatic Colorectal Cancer. Clin Colorectal Cancer. 2006;6(1):29–31. doi: 10.3816/CCC.2006.n.01. [DOI] [PubMed] [Google Scholar]
- 66.Hecht J, Mitchell E, Baranda J, et al. Panitumumab antitumor activity in patients (pts) with metastatic colorectal cancer (mCRC) expressing low (1–9%) or negative (< 1%) levels of epidermal growth factor receptor (EGFr) J Clin Oncol. 2006;24:3547. doi: 10.1200/jco.2006.24.18_suppl.3547. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are availlable by asking the corresponding author at claire.carlier@chu-reims.fr.