Abstract
BACKGROUND
Experimental studies and small clinical trials have suggested that treatment with intranasal oxytocin may reduce social impairment in persons with autism spectrum disorder. Oxytocin has been administered in clinical practice to many children with autism spectrum disorder.
METHODS
We conducted a 24-week, placebo-controlled phase 2 trial of intranasal oxytocin therapy in children and adolescents 3 to 17 years of age with autism spectrum disorder. Participants were randomly assigned in a 1:1 ratio, with stratification according to age and verbal fluency, to receive oxytocin or placebo, administered intranasally, with a total target dose of 48 international units daily. The primary outcome was the least-squares mean change from baseline on the Aberrant Behavior Checklist modified Social Withdrawal subscale (ABC-mSW), which includes 13 items (scores range from 0 to 39, with higher scores indicating less social interaction). Secondary outcomes included two additional measures of social function and an abbreviated measure of IQ.
RESULTS
Of the 355 children and adolescents who underwent screening, 290 were enrolled. A total of 146 participants were assigned to the oxytocin group and 144 to the placebo group; 139 and 138 participants, respectively, completed both the baseline and at least one postbaseline ABC-mSW assessments and were included in the modified intention-to-treat analyses. The least-squares mean change from baseline in the ABC-mSW score (primary outcome) was −3.7 in the oxytocin group and −3.5 in the placebo group (least-squares mean difference, −0.2; 95% confidence interval, −1.5 to 1.0; P = 0.61). Secondary outcomes generally did not differ between the trial groups. The incidence and severity of adverse events were similar in the two groups.
CONCLUSIONS
This placebo-controlled trial of intranasal oxytocin therapy in children and adolescents with autism spectrum disorder showed no significant between-group differences in the least-squares mean change from baseline on measures of social or cognitive functioning over a period of 24 weeks. (Funded by the National Institute of Child Health and Human Development; SOARS-B ClinicalTrials.gov number, NCT01944046.)
The neuropeptide oxytocin has been used as a potential therapy to reduce social impairment in autism spectrum disorder. In animals, oxytocin increases social approach and social memory,1 both of which are impaired in persons with autism. In persons without known developmental or psychiatric disorders, the use of intranasal oxytocin increases social affiliation, social memory, and empathy.2–5 Some, but not all, studies have shown reduced plasma oxytocin levels in children with autism spectrum disorder.6 A meta-analysis has tentatively supported an association between autism spectrum disorder and polymorphisms in the oxytocin receptor gene, OXTR, but not at the level of genomewide significance.7 Elevated promoter methylation in OXTR has also been reported in persons with autism spectrum disorder, as compared with controls.8 Decreased oxytocin-receptor density has been found in the ventral pallidum of postmortem brain tissue obtained from a few persons with autism spectrum disorder.9
These findings have led to many clinical investigations of oxytocin therapy in persons with autism spectrum disorder, most of which have had inconclusive findings. Several trials have shown that a single dose of intranasal oxytocin enhanced performance on measures of social cognition10,11 or motivation,12–14 as compared with placebo, in persons with autism spectrum disorder. These results have been supported by functional neuroimaging studies involving persons with autism spectrum disorder, which have shown differences in regional brain activation in response to social stimuli after the administration of intranasal oxytocin, as compared with placebo.11,15–17 Small, randomized, controlled trials of intranasal oxytocin administered for 4 to 24 weeks in persons with autism spectrum disorder have had equivocal results with regard to oxytocin-associated improvements in social functioning, social cognition, or social attention.18–21 The inconsistent results among these various investigations may have been the result of limited power or differences in participant age, oxytocin formulation or dose, treatment duration, outcome measures, or analytic methods.22–24 We performed a randomized, placebo-controlled phase 2 trial (Autism Centers of Excellence Network Study of Oxytocin in Autism to Improve Reciprocal Social Behaviors [SOARS-B]) to evaluate the efficacy of 24 weeks of intranasal oxytocin treatment to enhance social function in children and adolescents with autism spectrum disorder.
METHODS
TRIAL DESIGN AND OVERSIGHT
We conducted an investigator-initiated, randomized, double-blind, parallel-group, placebo-controlled trial of daily, flexible-dose intranasal oxytocin treatment in children and adolescents with autism spectrum disorder. A detailed rationale for the trial, as well as its design and methods, has been published previously.25 The trial protocol (available with the full text of this article at NEJM.org) was approved by the institutional review board at each trial site. Participants were recruited from seven academic sites with the use of research registries and community outreach (see the Supplementary Appendix, available at NEJM.org). Written informed consent for trial participation was obtained from the parent or guardian of each participant; assent from participants was obtained when appropriate. The trial was conducted in accordance with the amended Declaration of Helsinki guidelines. There was no commercial involvement in the trial. The roles of the authors in designing the trial, gathering and analyzing the data, and writing the manuscript are listed in the Supplementary Appendix.
Intranasal oxytocin in concentrations of 8 IU per 0.1 ml and 24 IU per 0.1 ml and matched placebo were manufactured by Tergus Pharma and were labeled and distributed by Patwell Pharmaceutical Solutions in compliance with current Good Manufacturing Practice regulations of the Food and Drug Administration. The active product contained synthetic oxytocin peptide.25 The placebo contained no oxytocin but was otherwise identical to the intranasal oxytocin product with respect to the other ingredients, volume, labeling, container system, and other features.
PARTICIPANTS
Children and adolescents 3 to 17 years of age were assessed by site investigators and trained staff with the use of clinical interviews, physical and neurologic examinations, cognitive profiles using either the Stanford–Binet Intelligence Scales, fifth edition (SB5), or the Mullen Scales of Early Learning, and diagnostic testing using the Autism Diagnostic Observation Schedule, second edition (ADOS-2). Participants met the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5), criteria for autism spectrum disorder. Parents and guardians were required to speak English. Participants could not have received a diagnosis of the Rett syndrome or childhood disintegrative disorder, deafness or blindness, active cardiovascular or renal disease, or uncontrolled epilepsy or be pregnant, lactating, or sexually active without contraception. Previous daily treatment with intranasal oxytocin for more than 30 days was an exclusion criterion. Changes in neuropsychiatric medications were not allowed within 1 month before randomization; changes in nonmedication therapies for autism spectrum disorder were not allowed within 2 months before randomization. Use of antipsychotic agents, anticonvulsants, and stimulants was allowed during the trial.
Participants were randomly assigned in a 1:1 ratio, by means of a centralized randomization table, to receive intranasal oxytocin or matching intranasal placebo, with stratification according to verbal fluency (minimally verbal or fluently verbal, with the ability to perform ADOS-2 module 3 or 4 as an indication of fluent speech) and age group (3 to 6 years, 7 to 11 years, and 12 to 17 years). Study visits were scheduled at baseline and at weeks 4, 8, 12, 16, 20, and 24. At each visit, the trial physician, who was unaware of the participant’s trial-group assignment, completed a physical examination, systematically elicited a history of adverse events, verified concomitant treatments, and, at visits after the baseline visit, assessed current symptoms of autism spectrum disorder using the Clinical Global Impressions Scale of Improvement.
Parents or guardians completed the Aberrant Behavior Checklist (ABC) and the Pervasive Developmental Disorders Behavior Inventory–Screening Version (PDDBI-SV) at each visit; the Vineland Adaptive Behavior Scales, second edition (VABS-II), at baseline and week 24; and the Social Responsiveness Scale, second edition (SRS-2), at baseline and weeks 12 and 24. When feasible, the same caregiver responded to all questionnaires. Participants who were able to describe basic emotions completed the Reading the Mind in the Eyes test at baseline and at weeks 8 and 24.
OUTCOMES
The primary outcome was the least-squares mean change from baseline to 24 weeks in the score on the ABC modified Social Withdrawal subscale (ABC-mSW), which consists of 13 items; scores range from 0 to 39, with higher scores indicating less social interaction. The ABC instrument, from which the ABC-mSW is extracted, is a 58-item caregiver-rated questionnaire assessing problem behaviors across five subscales: irritability, lethargy and social withdrawal, stereotypic behavior, hyperactivity and noncompliance, and inappropriate speech. Use of a total score for the ABC is not advised. The ABC-mSW that was used to determine the primary outcome was a modification of the ABC Lethargy–Social Withdrawal subscale that omitted three questions (3, 32, and 53) pertaining to reduced physical movement in order to increase specificity for social function.
The three secondary outcomes were the least-squares mean changes from baseline in the T-score for the SRS-2 Social Motivation subscale (SRS-2-SM) and in the scores for the Sociability Factor and SB5 Abbreviated IQ. On the SRS-2-SM, sex-adjusted T-scores range from 42 to 90, with T-scores greater than 59 indicating less social motivation. The Sociability Factor consists of 31 items that combine the ABC-mSW and PDDBI-SV; total scores range from 0 to 93, with higher scores indicating poorer social function. Scores on the SB5 Abbreviated IQ range from 47 to 153, with higher scores indicating greater cognitive abilities. Exploratory outcomes and their measurement characteristics are listed in Table S1 in the Supplementary Appendix. At the end of the trial, the trial physician, primary caregiver, and participant (if appropriate) were asked to guess whether the participant had been receiving intranasal oxytocin or placebo.
TRIAL REGIMENS
The dose of oxytocin was flexible and was not dependent on participant age or weight. The dose of oxytocin (or matched placebo) began at 8 international units (IU) administered each morning, with a target total daily dose of 48 IU, typically begun at week 8 and administered as 24 IU twice daily. Once the target dose was maintained for 7 weeks, the dose could be escalated further by 16 IU every 4 weeks to reach a maximal total daily dose of 80 IU (Table S2). The dose could be reduced by 8 to 16 IU or maintained at the same dose, rather than increased as suggested by the dose-adjustment schedule, at any time if requested by the trial physician, caregiver, or participant. Dose increases before the suggested time point in the schedule were not permitted. If assessment of the participant indicated moderate or severe worsening symptoms, or if the participant had two consecutive Clinical Global Impressions Improvement scores that indicated poorer functioning than the preceding two scores, the protocol required dose reduction.
SAFETY MONITORING
At each visit, the participant’s pulse, blood pressure, temperature, height, and weight were measured. At screening and week 24, electrocardiograms, urinalyses, pregnancy status, blood chemical levels, liver enzyme levels, and prolactin levels were obtained. At each visit, trial physicians who were unaware of the assigned trial groups reviewed previously reported medical conditions and adverse events and systematically elicited information regarding potential new adverse events, including specifically asking the parent or guardian, as well as the participant only if the clinician determined that the participant could understand the concepts of “on purpose” death and suicide, about the participant’s suicidal thoughts and statements and self-injurious behaviors.
Safety and adherence to the protocol were monitored by means of weekly telephone calls with all the investigators to discuss all reported adverse events of moderate or greater severity, concerns raised by participants, and ongoing trial conduct. Regularly scheduled site-monitoring visits were conducted. The data and safety monitoring board reviewed unblinded safety data twice yearly. The medical monitor, data and safety monitoring board, and the institutional review board at each site were notified of all serious adverse events that were considered to be related to blinded oxytocin or placebo, deaths, and unexpected problems within 14 days. The medical monitor was notified of all serious adverse events that were considered by the investigators to be unrelated to the trial; the medical monitor also had access to unblinded data on request.
STATISTICAL ANALYSIS
Power calculations were based on a two-group Student’s t-test of changes in scores because some aspects of the mixed-effect model, such as covariance structure, were unknown. We assumed that a between-group difference of 5 points in the least-squares mean change from baseline in the ABC-mSW scores (primary outcome) was clinically meaningful on the basis of data from published trials of risperidone therapy in persons with autism spectrum disorder.26 We estimated a 9-point standard deviation for the change in score on the basis of previous trials of treatment for autism spectrum disorder.27–32 We estimated that the enrollment of 71 participants in each group would provide the trial with 90% power to distinguish between the two groups at an alpha level of 0.05. To allow for a sensitivity analysis in the minimally verbal and fluently verbal subgroups, we aimed to enroll 284 participants in a modified intention-to-treat analysis.
All the efficacy analyses were performed with the use of a modified intention-to-treat approach that included all the participants who had undergone randomization, received oxytocin or placebo for at least 1 day, and had both a baseline and at least one postbaseline ABC-mSW score. This population was also used for the secondary, exploratory, and sensitivity analyses. Only non-missing data from the modified intention-to-treat population were included in the analyses.
Efficacy analyses used a mixed-effect model with repeated measures that considered the least-squares mean change from baseline to each postbaseline time point through 24 weeks as the response variable; we included the baseline value of the response variable, verbal fluency subgroup, continuous time, treatment, and treatment-by-time interaction as fixed effects and the intercept and time slope as random effects. Age group and trial site were considered as additional fixed effects but were removed from models owing to lack of significance. There was no prespecified plan for adjustment of confidence intervals to account for multiple comparisons for secondary, exploratory, or sensitivity outcomes. Results of these analyses are therefore reported as point estimates with 95% confidence intervals and cannot be used to infer definitive treatment effects.
Sensitivity analyses of the primary outcome included the least-squares mean changes from baseline in the original ABC Lethargy–Social Withdrawal score in the modified intention-to-treat population and the least-squares mean changes from baseline in the ABC-mSW scores separately in the subgroups of participants who were minimally verbal or fluently verbal, the subgroups of participants with a baseline ABC-mSW score at or above the sample median or with a baseline ABC-mSW score below the sample median, and the per-protocol population (defined as all the patients who met the target dose by week 8 and had no subsequent reduction in the dose, took at least 80% of the prescribed doses, and had ABC-mSW scores at all scheduled visits). The statistical analysis plan is available with the trial protocol. Safety analyses were descriptive and included all the participants who received at least one dose of oxytocin or placebo.
RESULTS
PARTICIPANTS
The trial was conducted from August 2014 through June 2017. Of the 355 children and adolescents who underwent screening, 290 met the eligibility criteria, were randomly assigned to a trial group, received at least one dose of oxytocin or placebo, and were included in the safety analyses. The efficacy analyses excluded 11 participants who had no ABC-mSW assessments after randomization and 2 who did not have a baseline ABC-mSW score, which yielded 277 participants for the modified intention-to-treat analysis. In the modified intention-to-treat population, 14 participants in the oxytocin group and 13 in the placebo group withdrew before week 24; thus, 90% of the participants in the modified intention-to-treat population (125 in each group) completed the trial (Fig. 1). The per-protocol population included 114 participants in the oxytocin group and 107 in the placebo group.
Figure 1. Randomization and Follow-up of the Participants.
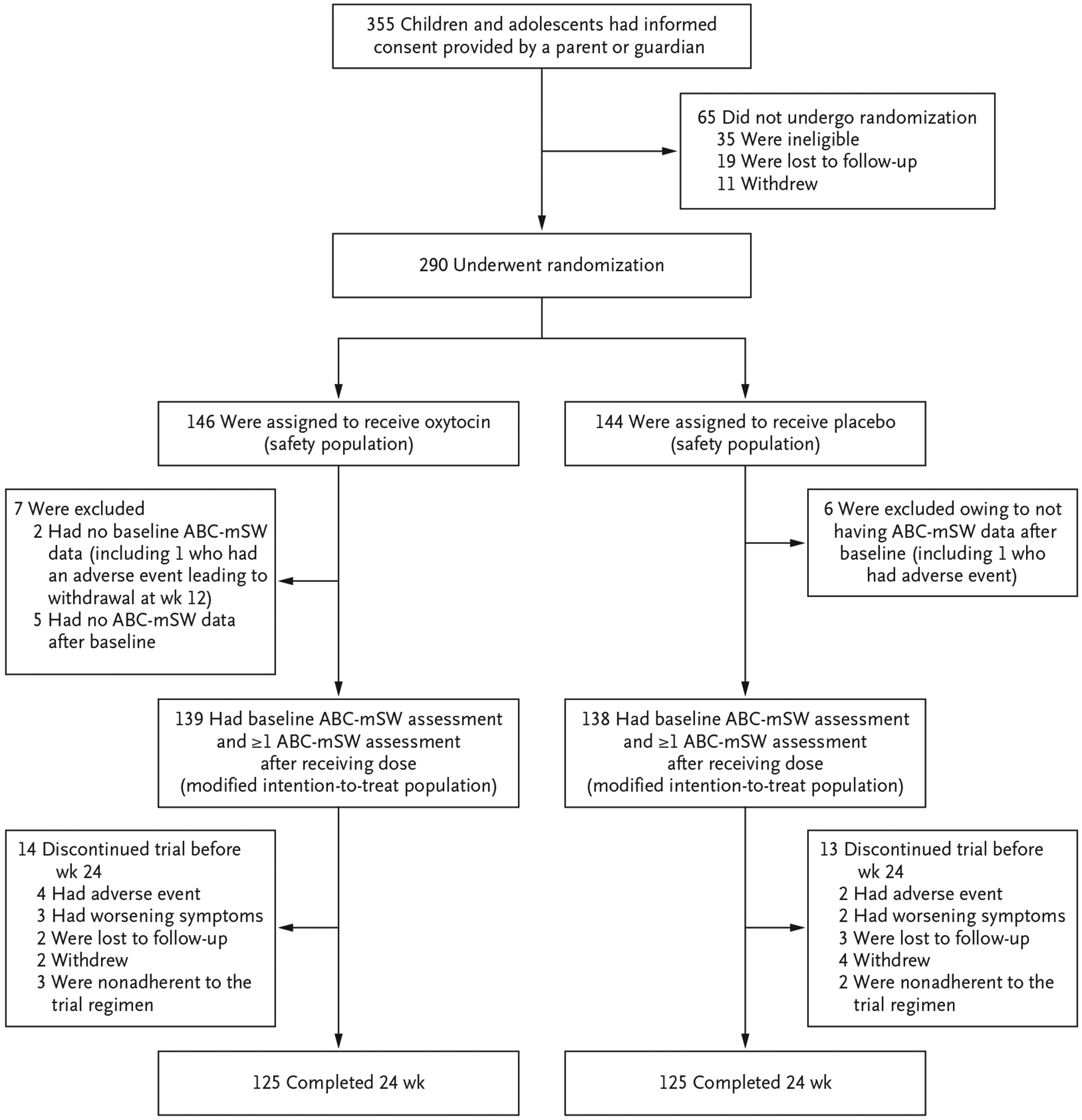
The primary outcome was the least-squares mean change in the score on the Aberrant Behavior Checklist modified Social Withdrawal subscale (ABC-mSW).
Among all the participants who were included in the efficacy analysis, 48% had minimal verbal fluency and 52% had fluent verbal speech. The distribution across age groups was as follows: 25% of the participants were 3 to 6 years of age, 39% were 7 to 11 years of age, and 36% were 12 to 17 years of age. Most of the participants (87%) were male. The participants’ demographic characteristics, symptom severity, and use of concomitant medications were similar at baseline in the two trial groups (Tables 1 and S3). The median ABC-mSW score at baseline was 11 points. Of the 1939 ABC-mSW assessments that were expected if every participant in the modified intention-to-treat population completed all scheduled assessments, 128 (6.6%) were missing; the data were considered to be missing at random (Table S4).
Table 1.
Demographic Characteristics of the Participants at Baseline, According to Verbal Fluency Subgroups and Overall (Full Analysis Set).*
| Characteristic | Minimally Verbal Subgroup (N = 132) |
Fluently Verbal Subgroup (N = 145) |
All Participants (N = 277) |
|||
|---|---|---|---|---|---|---|
| Oxytocin (N = 65) |
Placebo (N = 67) |
Oxytocin (N = 74) |
Placebo (N = 71) |
Oxytocin (N = 139) |
Placebo (N = 138) |
|
| Age | ||||||
| Mean — yr | 9.7±4.0 | 9.7±3.7 | 11.0±4.1 | 11.1±4.2 | 10.4±4.1 | 10.4±4.0 |
| Distribution — no. (%) | ||||||
| 3–6 yr | 20 (31) | 20 (30) | 14 (19) | 15 (21) | 34 (24) | 35 (25) |
| 7–11 yr | 25 (38) | 26 (39) | 29 (39) | 27 (38) | 54 (39) | 53 (38) |
| 12–17 yr | 20 (31) | 21(31) | 31 (42) | 29 (41) | 51 (37) | 50 (36) |
| Sex — no. (%) | ||||||
| Male | 56 (86) | 56 (84) | 66 (89) | 64 (90) | 122 (88) | 120 (87) |
| Female | 9 (14) | 11 (16) | 8 (11) | 7 (10) | 17 (12) | 18 (13) |
| Race — no./total no. (%)† | ||||||
| White | 48/65 (74) | 44/65 (68) | 56/73 (77) | 56/70 (80) | 104/138 (75) | 100/135 (74) |
| Black | 4/65 (6) | 10/65 (15) | 5/73 (7) | 4/70 (6) | 9/138 (7) | 14/135 (10) |
| Asian | 7/65 (11) | 7/65 (11) | 4/73 (5) | 5/70 (7) | 11/138 (8) | 12/135 (9) |
| Multiracial | 6/65 (9) | 4/65 (6) | 8/73 (11) | 5/70 (7) | 14/138 (10) | 9/135 (7) |
| Hispanic ethnic group — no./total no. (%)† | 8/65 (12) | 8/65 (12) | 5/74 (7) | 7/71 (10) | 13/139 (9) | 15/136 (11) |
| ABC-mSW score‡ | 11.9±8.0 | 13.7±7.6 | 10.2±6.7 | 9.6±7.4 | 11.0±6.9 | 11.6±7.8 |
| Original ABC Lethargy-Social Withdrawal score§ | 12.5±7.5 | 14.9±8.3 | 11.4±7.7 | 10.6±8.3 | 11.9±7.6 | 12.7±8.5 |
| Sociability Factor score¶ | 39.0±15.6 | 42.9±14.0 | 33.1±13.2 | 31.1±13.9 | 35.8±14.6 | 36.8±15.1 |
| SRS-2‖ | ||||||
| No. of participants with data | 65 | 66 | 74 | 71 | 139 | 137 |
| Total T-score | 78.2±10.1 | 80.6±9.3 | 76.1±9.5 | 74.7±9.7 | 77.1±9.8 | 77.5±10.0 |
| SRS-2-SM T-score | 70.6±11.3 | 72.7±11.3 | 67.6±10.8 | 66.7±11.8 | 69.0±11.1 | 69.6±11.9 |
| ADOS-2 comparison score — no. (%)** | ||||||
| Minimal | 0 | 0 | 0 | 1 (1) | 0 | 1 (1) |
| Low severity | 1 (2) | 0 | 5 (7) | 0 | 6 (4) | 0 |
| Moderate | 20 (31) | 19 (28) | 24 (32) | 24 (34) | 44 (32) | 43 (31) |
| High severity | 44 (68) | 48 (72) | 45 (61) | 46 (65) | 89 (64) | 94 (68) |
| VABS-II Socialization Standard score†† | ||||||
| No. of participants with data | 42 | 45 | 60 | 57 | 102 | 102 |
| Mean | 58.9±15.4 | 52.9±10.4 | 76.3±17.5 | 73.4±13.9 | 69.1±18.7 | 64.4±16.1 |
| SB5 Abbreviated IQ‡‡ | ||||||
| No. of participants with data | 37 | 38 | 73 | 71 | 110 | 109 |
| Mean | 66.8±18.8 | 61.7±13.7 | 94.8±20.1 | 92.6±19.1 | 85.4±23.7 | 81.8±22.8 |
Plus–minus values are means ±SD. Percentages may not total 100 because of rounding. CI denotes confidence interval.
Race was reported by the participant’s parent or guardian.
The Aberrant Behavior Checklist (ABC) modified Social Withdrawal subscale (ABC-mSW) omits questions 3, 32, and 53 from the original ABC Lethargy–Social Withdrawal subscale in order to eliminate confounding by questions that reflect lack of physical movement rather than lack of social interaction. Scores range from 0 to 39, with higher scores indicating less social interaction.
Scores on the original ABC Lethargy–Social Withdrawal subscale range from 0 to 48, with higher scores indicating reduced physical movements and less social interaction.
The Sociability Factor score is the sum of the scores on the ABC-mSW and the Pervasive Developmental Disorders Behavior Index–Screening Version. Scores range from 0 to 93, with higher scores indicating poorer social function.
Total T-scores on the Social Responsiveness Scale, second edition (SRS-2), which indicate the sum of all items on this scale, range from 0 to 192, with higher scores indicating fewer or more impaired social behaviors and more frequent or intense repetitive behaviors. Raw scores are converted to T-scores with sex-based cutoffs for clinical significance. Sex-adjusted T-scores on the SRS-2 Social Motivation subscale (SRS-2-SM) range from 42 to 90, with T-scores greater than 59 indicating less social motivation.
The Autism Diagnostic Observation Schedule, second edition (ADOS-2), comparison score allows for the comparison of younger and older persons who completed the same module of the ADOS-2 as well as for the comparison of persons who completed different modules with different scoring paradigms. Scores range from 1 to 10, with higher scores indicating more severe autistic behaviors. A score of 1 or 2 indicates minimal autistic behaviors, a score of 3 or 4 low-severity autistic behaviors, a score of 5 to 7 moderate autistic behaviors, and a score of 8 to 10 high-severity autistic behaviors.
The Vineland Adaptive Behavior Scale, second edition (VABS-II), Caregiver Report Form subscale and total composite standard scores cannot be calculated if more than two items have missing or “I don’t know” responses. The Socialization Standard score ranges from 20 to 159, with higher scores indicating better social functioning; a score of 85 to 115 indicates average social functioning.
The Abbreviated IQ score was assessed by means of the Stanford–Binet Intelligence Scales, fifth edition (SB5), Abbreviated IQ. Scores range from 47 to 153, with higher scores indicating greater cognitive abilities. The Abbreviated IQ assessment was not completed in participants with a mental age of younger than 18 months or in those who could not complete the verbal or nonverbal routing subtests.
Most participants (126 in the oxytocin group and 130 in the placebo group) continued the 48 IU total daily dose of oxytocin or matching placebo for at least 7 weeks, a duration that was prespecified in the protocol. The mean (±SD) maximal total daily dose was 67.6±16.9 IU in the oxytocin group, with a volume equivalent of 69.5±16.1 IU in the placebo group. A total of 52% of the trial physicians, 49% of the primary caregivers, and 22 of the 44 participants who answered (50%) guessed the trial-group assignment correctly at the end of the trial.
EFFICACY
Across the 24 weeks of the trial, the least-squares mean change from baseline in the ABC-mSW score (primary outcome) was −3.7 in the oxytocin group and −3.5 in the placebo group (difference, −0.2 points; 95% confidence interval [CI], −1.5 to 1.0; P = 0.61). Figure 2 shows raw mean ABC-mSW scores across the 24-week trial. Figure S1 shows the least-squares mean changes from baseline to each time point as assessed by a model based on the least-squares mean change with adjustment for baseline values of the ABC-mSW score for each participant at each time point. The values from the model correspond to the primary-outcome results in Table 2. Among participants with fluent verbal speech, the between-group difference in the least-squares mean change from baseline was 0.3 (95% CI, −1.4 to 2.1); among participants with minimal verbal fluency, the between-group difference in the least-squares mean change from baseline was −0.9 (95% CI, −2.7 to 1.0). Sensitivity analyses that used least-squares mean changes from baseline in scores from the original ABC Lethargy–Social Withdrawal subscale showed results that did not differ appreciably between the trial groups (Table 2).
Figure 2. Scores on the Aberrant Behavior Checklist Modified Social Withdrawal Subscale (ABC-mSW) over 24 Weeks.
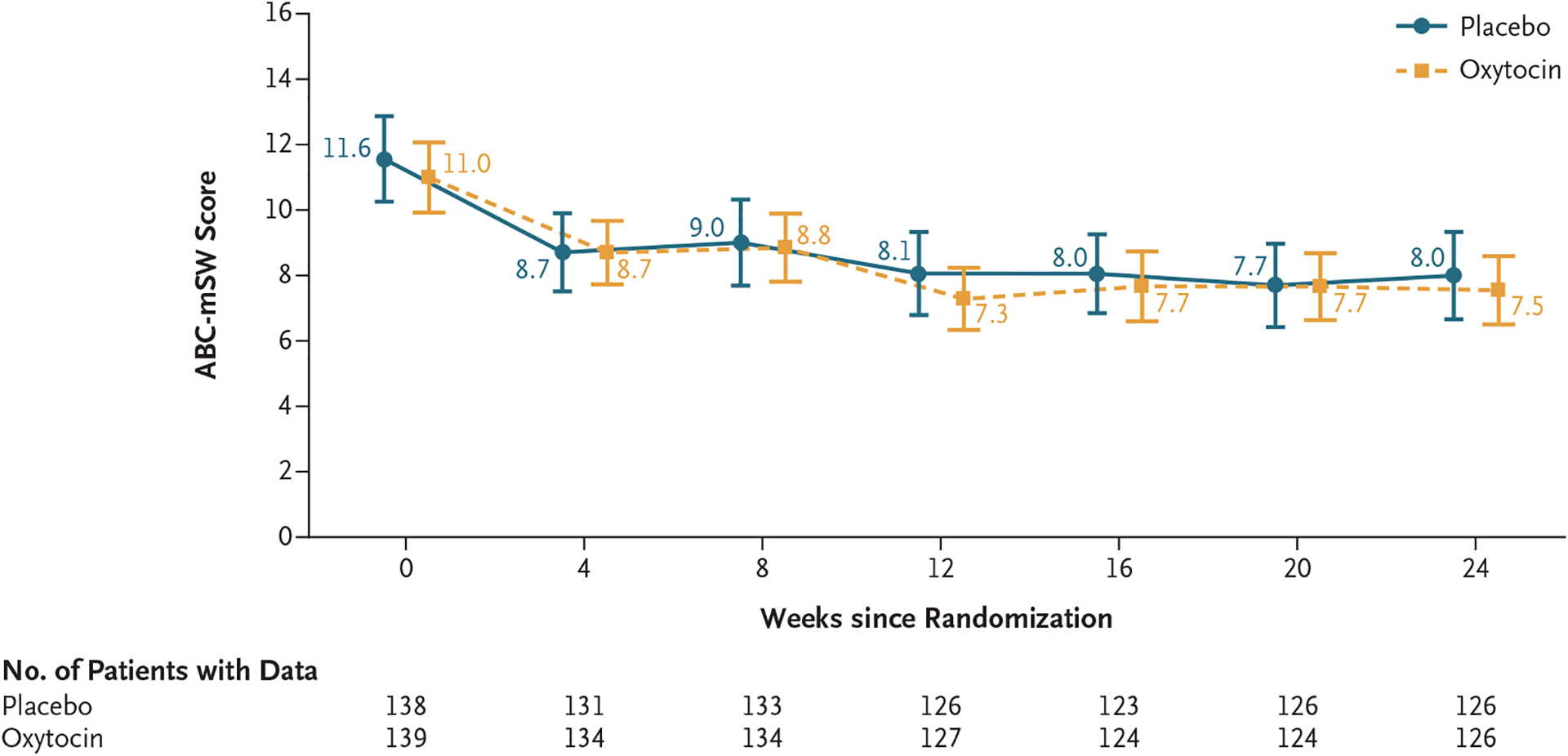
Shown are raw mean scores on the ABC-mSW subscale across the 24-week trial of intranasal oxytocin as compared with placebo. Scores on the ABC-mSW range from 0 to 39, with higher scores indicating less social interaction. I bars indicate 95% confidence intervals. Values are offset from each other at each time point for readability. A graph showing least-squares mean changes from baseline in the ABC-mSW subscale scores (primary outcome) is provided in Figure S1. The changes between baseline and each time point that are based on the mean raw values differ from the least-squares mean change from baseline values at each time point because the least-squares mean values are adjusted for the baseline value of the ABC-mSW for each participant at each time point.
Table 2.
Least-Squares Mean Changes from Baseline to Week 24 in the Primary and Secondary Outcomes and in Key Sensitivity Analyses.*
| Variable | Oxytocin Group | Placebo Group | Difference (95% CI) | P Value | ||
|---|---|---|---|---|---|---|
| No. of Participants | Least-Squares Mean Change (95% CI) | No. of Participants | Least-Squares Mean Change (95% CI) | |||
| Primary outcome: ABC-mSW score change from baseline† | 139 | −3.7 (−4.6 to −2.8) | 138 | −3.5 (−4.4 to −2.6) | −0.2 (−1.5 to 1.0) | 0.61 |
| Secondary outcomes | ||||||
| SRS-2-SM score | 139 | −4.5 (−5.9 to −3.1) | 137 | −5.4 (−6.8 to −4.0) | 0.9 (−1.0 to 2.9) | |
| Sociability Factor score | 139 | −7.7 (−9.5 to −5.9) | 138 | −8.3 (−10.1 to −6.5) | 0.6 (−1.8 to 3.1) | |
| SB5 Abbreviated IQ | 110 | 0.9 (−1.3 to 3.0) | 109 | 0.8 (−1.3 to 2.9) | 0.1 (−2.9 to 3.0) | |
| Sensitivity analyses | ||||||
| Original ABC Lethargy-Social Withdrawal score | 139 | −4.2 (−5.2 to −3.2) | 138 | −3.9 (−4.9 to −2.9) | −0.4 (−1.8 to 1.1) | |
| ABC-mSW score according to verbal fluency | ||||||
| Fluently verbal subgroup | 74 | −3.3 (−4.5 to −2.1) | 71 | −3.7 (−4.9 to −2.4) | 0.3 (−1.4 to 2.1) | |
| Minimally verbal subgroup | 65 | −4.1 (−5.4 to −2.8) | 67 | −3.3 (−4.5 to −2.0) | −0.9 (−2.7 to 1.0) | |
| ABC-mSW score in per-protocol population‡ | 114 | −3.6 (−4.5 to −2.7) | 107 | −3.5 (−4.5 to −2.5) | −0.1 (−1.4 to 1.2) | |
| ABC-mSW score according to baseline-score subgroup§ | ||||||
| <11 points | 70 | −1.3 (−2.2 to −0.4) | 63 | −1.6 (−2.5 to −0.7) | 0.4 (−0.9 to 1.7) | |
| ≥11 points | 69 | −6.0 (−7.5 to −4.4) | 75 | −5.2 (−6.7 to −3.7) | −0.8 (−2.9 to 1.4) | |
Least-squares means are from the statistical model described in the statistical analysis plan; this model included the baseline score as a covariate. Higher scores indicate more impairment for all outcomes, except for the SB5 Abbreviated IQ, on which higher scores indicate greater cognitive abilities. Because the statistical analysis plan did not include a provision for correcting confidence intervals for between-group differences for multiplicity in tests for secondary or other outcomes, results are reported as point estimates with 95% confidence intervals and cannot be used to infer treatment effects.
Graphed results for the primary analysis are shown in Figure S1.
The per-protocol population included all the participants who met the target dose by week 8 and had no subsequent reduction in dose, took at least 80% of the prescribed doses, and had ABC-mSW scores at all scheduled visits.
The results of the sensitivity analyses examining the least-squares mean changes from baseline among participants with a baseline ABC-mSW scores at or above the median score of 11 and among those with a baseline score lower than the median are shown in Figure S2A and S2B.
The three secondary outcomes essentially affirmed the absence of a difference between the trial groups. The point estimate of the least-squares mean change from baseline in the SRS-2-SM T-score was −4.5 in the oxytocin group and −5.4 in the placebo group (difference, 0.9 points; estimated 95% CI, −1.0 to 2.9). The point estimate of the least-squares mean change from baseline in the Sociability Factor score was −7.7 in the oxytocin group and −8.3 in the placebo group (difference, 0.6 points; 95% CI, −1.8 to 3.1). The point estimate of the least-squares mean change from baseline in the SB5 Abbreviated IQ was 0.9 in the oxytocin group and 0.8 in the placebo group (difference, 0.1 point; 95% CI, −2.9 to 3.0). These results were not appreciably altered by the addition of the baseline plasma oxytocin level to the model (Table S5). Exploratory outcomes, including scores on the Clinical Global Impressions Scale of Improvement indicating “improved” or “very much improved,” are shown in Table S6.
SAFETY
Three serious adverse events occurred during the trial. One serious adverse event was considered by the investigators to be related to oxytocin: sedation while driving that led to a motor vehicle accident while the participant was taking a total daily dose of 48 IU. In the safety population, four participants in the oxytocin group and three in the placebo group discontinued the trial regimen owing to adverse events. Most of the discontinuations in the oxytocin group were related to irritability or aggression; only one of the discontinuations in the placebo group was due to behavioral causes (increased libido with impulsivity).
Adverse events occurred in 82% of the participants in the oxytocin group and in 83% of those in the placebo group (Table 3). The oxytocin group had higher incidences of increased appetite (16%, vs. 10% in the placebo group), increased energy (10% vs. 3%), restlessness (8% vs. 2%), subjective weight loss (7% vs. 3%), increased thirst (6% vs. 3%), inattention (6% vs. 3%), and myalgia (3% vs. 1%) (Table S7). The mean weight gain was 1.6±2.2 kg in the oxytocin group and 2.3±2.8 kg in the placebo group. Specific adverse events in each trial group, classified according to terms from the Medical Dictionary for Regulatory Activities, version 22.0, are shown in Table S5. There were no other clinically meaningful changes in vital signs, height, clinical laboratory assessments, or electrocardiographic findings in either group.
Table 3.
Adverse Events (Safety Population).*
| Event | Oxytocin (N = 146) | Placebo (N = 144) |
|---|---|---|
| Any adverse event — no. of participants (%) | 120 (82) | 120 (83) |
| Maximum intensity of any adverse event in each participant — no. of participants (%) | ||
| Death | 0 | 0 |
| Life-threatening | 0 | 1 (1) |
| Severe | 7 (5) | 10 (7) |
| Moderate | 67 (46) | 52 (36) |
| Mild | 46 (32) | 57 (40) |
| Adverse event considered to be related to oxytocin or placebo, according to intensity category — no. of events/total no. (%) | ||
| Severe | 5/13 (38) | 13/25 (52) |
| Moderate | 76/171 (44) | 68/172 (40) |
| Mild | 154/301 (51) | 158/313 (50) |
| Adverse event leading to withdrawal from trial — no. of participants (%)† | 4 (3) | 3 (2) |
| Serious adverse event — no. of participants (%)‡ | 2 (1) | 1 (1) |
The safety population included all the participants who received at least one dose of oxytocin or placebo. A list of specific adverse events is provided in Table S5.
In the oxytocin group, irritability occurred in two patients and aggression and sedation occurred in one patient each. In the placebo group, viral infection, gastrointestinal discomfort, and increased libido with impulsivity occurred in one patient each.
In the oxytocin group, appendicitis and sedation leading to a motor vehicle accident occurred in one patient each. In the placebo group, a single patient had dysphoria and irritability, which were considered to be separate serious adverse events.
DISCUSSION
Many children with autism spectrum disorder are thought to have tried intranasal oxytocin therapy33 on the basis of putatively promising data. This treatment approach has been driven by trials of a single dose of oxytocin or by small clinical trials involving the administration of various doses of oxytocin over multiple days,10–21 but the limited power and differences among the trials in the participants’ ages and symptom profiles, outcome measures, oxytocin dose and duration, and trial designs make comparisons of these trials difficult.18–24 In the absence of an objective diagnostic test for autism spectrum disorder, we used the DSM-5 clinical diagnostic criteria to determine eligibility in the current trial, and we attempted to be inclusive with respect to participants’ age, verbal ability, and intellectual ability.
In contrast to some previous trials, our randomized, controlled trial showed no significant difference between oxytocin and placebo, each administered daily for 24 weeks, in the least-squares mean change from baseline in the score on the ABC-mSW scale, which assesses social interaction in persons with autism spectrum disorder. The absence of between-group differences in outcomes was similar among participants with fluent verbal communication and among those with minimal verbal communication, and the results for the secondary outcomes were generally similar to that for the primary outcome. One previous trial showed a significant benefit of intranasal oxytocin therapy on the SRS-2 total score only when the baseline plasma oxytocin level was incorporated into the analysis.18 However, our sensitivity analyses, which incorporated the baseline plasma oxytocin level as a covariate, did not show a benefit of intranasal oxytocin therapy over placebo with regard to the least-squares mean change from baseline on either the ABC-mSW score or the SRS-2 total T-score. In the absence of trials showing a replicable benefit of any intervention for social functioning in persons with autism spectrum disorder, it is difficult to know which outcome measure is most appropriate to assess potential social improvement in future trials of an intervention targeting core social deficits in autism spectrum disorder.
We used the least-squares mean change from baseline in the ABC-mSW score as the primary outcome because a consensus panel found that the original ABC Lethargy–Social Withdrawal subscale had evidence supporting its use to assess social behavior in clinical trials involving persons with autism spectrum disorder34 and because the ABC-mSW increases specificity for social interaction. A sensitivity analysis of the least-squares mean changes from baseline in scores on the original ABC Lethargy–Social Withdrawal subscale provided results similar to that for the primary outcome, as did analyses for other outcomes relevant to social functioning. The least-squares mean changes from baseline in the ABC Lethargy–Social Withdrawal scores that were observed in both the oxytocin and placebo groups in this trial were similar to that observed in the placebo group of the licensing trial of risperidone for irritable behaviors in autism spectrum disorder but were considerably smaller than the change in score that was observed with risperidone in that trial.26
This trial has limitations. First, our primary outcome was based on the use of the ABC-mSW, which has not been validated. A minimum score on this scale was not required for enrollment, which potentially limited our ability to detect improvements in children who had low ABC-mSW scores at baseline. However, a benefit with oxytocin therapy was not observed when the analysis was limited to the subgroup of participants with a baseline ABC-mSW score of at least 11.
Another limitation is that the flexible dose strategy for oxytocin in this trial differed from strategies used in previous clinical trials. Previous trials used 12 IU of oxytocin in children younger than 13 years of age and 24 IU once or twice daily in participants 13 years of age or older. In contrast, the total daily doses of oxytocin in our trial ranged from 8 to 80 IU, depending on adverse effects in the participants rather than on participant age or weight. This flexible-dose strategy allowed the exploration of safety across a range of oxytocin doses.
There is uncertainty regarding absorption, brain penetration, and time course of effects with intranasal oxytocin35,36 that may be amplified by differences among specific preparations of intranasal oxytocin. The formulation that was used in our trial differed from the Novartis Syntocinon product that has been used in some, but not all, previous clinical trials. The concentration of the Novartis product is 4 IU of oxytocin per 0.1 ml of solution; a dose of 24 IU requires that 0.6 ml of solution be delivered at one time, which may influence absorption. The highest concentration of the formulation used in our trial was 24 IU per 0.1 ml. Finally, it is possible that the treatment period of 24 weeks that was used in this trial might attenuate an initial early response to oxytocin.
In this trial involving children and adolescents with autism spectrum disorder, we found that 24 weeks of daily intranasal oxytocin treatment, as compared with placebo, did not improve social interaction or other measures of social function related to autism spectrum disorder.
Supplementary Material
Acknowledgments
We thank the participants in this trial and their families; the numerous research support staff members at each site, who facilitated subcontracting, ethical oversight, and implementation of the trial; Evdokia Anagnostou for serving as the medical monitor; Robert M. Hamer (deceased), Latha Soorya, Alice Kau, Joseph P. Horrigan, William Rencher, and Cheryl Walker; Scott Compton, Gordon Keeler, Katarina Tsilou, and Geraldine Dawson; and the staff of the Psychiatry Division of the Food and Drug Administration, Alera Labs, Tergus Pharma, and ACTA Laboratories.
Supported by a grant (U01HD073984) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development through the Autism Centers of Excellence Program and the Department of Psychiatry and Behavioral Sciences at Duke University. The data and safety monitoring board was funded by a grant (UL1TR002489) from the National Center for Advancing Translational Sciences.
Footnotes
The content of this article is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
REFERENCES
- 1.Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron 2010; 65: 768–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature 2005; 435: 673–6. [DOI] [PubMed] [Google Scholar]
- 3.Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry 2007; 61: 731–3. [DOI] [PubMed] [Google Scholar]
- 4.De Dreu CKW, Greer LL, Handgraaf MJJ, et al. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science 2010; 328: 1408–11. [DOI] [PubMed] [Google Scholar]
- 5.Bartz JA, Zaki J, Bolger N, et al. Oxytocin selectively improves empathic accuracy. Psychol Sci 2010; 21: 1426–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rutigliano G, Rocchetti M, Paloyelis Y, et al. Peripheral oxytocin and vasopressin: biomarkers of psychiatric disorders? A comprehensive systematic review and preliminary meta-analysis. Psychiatry Res 2016; 241: 207–20. [DOI] [PubMed] [Google Scholar]
- 7.LoParo D, Waldman ID. The oxytocin receptor gene (OXTR) is associated with autism spectrum disorder: a meta-analysis. Mol Psychiatry 2015; 20: 640–6. [DOI] [PubMed] [Google Scholar]
- 8.Gregory SG, Connelly JJ, Towers AJ, et al. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med 2009; 7: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman SM, Palumbo MC, Lawrence RH, Smith AL, Goodman MM, Bales KL. Effect of age and autism spectrum disorder on oxytocin receptor density in the human basal forebrain and midbrain. Transl Psychiatry 2018; 8: 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guastella AJ, Einfeld SL, Gray KM, et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry 2010; 67: 692–4. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe T, Abe O, Kuwabara H, et al. Mitigation of sociocommunicational deficits of autism through oxytocin-induced recovery of medial prefrontal activity: a randomized trial. JAMA Psychiatry 2014; 71: 166–75. [DOI] [PubMed] [Google Scholar]
- 12.Kanat M, Spenthof I, Riedel A, van Elst LT, Heinrichs M, Domes G. Restoring effects of oxytocin on the attentional preference for faces in autism. Transl Psychiatry 2017; 7(4): e1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andari E, Duhamel J-R, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci U S A 2010; 107: 4389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Althaus M, Groen Y, Wijers AA, Noltes H, Tucha O, Hoekstra PJ. Oxytocin enhances orienting to social information in a selective group of high-functioning male adults with autism spectrum disorder. Neuropsychologia 2015; 79: Pt A: 53–69. [DOI] [PubMed] [Google Scholar]
- 15.Gordon I, Vander Wyk BC, Bennett RH, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci U S A 2013; 110: 20953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kruppa JA, Gossen A, Oberwelland Weiß E, et al. Neural modulation of social reinforcement learning by intranasal oxytocin in male adults with high-functioning autism spectrum disorder: a randomized trial. Neuropsychopharmacology 2019; 44: 749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greene RK, Spanos M, Alderman C, et al. The effects of intranasal oxytocin on reward circuitry responses in children with autism spectrum disorder. J Neurodev Disord 2018; 10: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker KJ, Oztan O, Libove RA, et al. Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proc Natl Acad Sci U S A 2017; 114: 8119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe T, Kuroda M, Kuwabara H, et al. Clinical and neural effects of six-week administration of oxytocin on core symptoms of autism. Brain 2015; 138: 3400–12. [DOI] [PubMed] [Google Scholar]
- 20.Yamasue H, Okada T, Munesue T, et al. Effect of intranasal oxytocin on the core social symptoms of autism spectrum disorder: a randomized clinical trial. Mol Psychiatry 2020; 25: 1849–58. [DOI] [PubMed] [Google Scholar]
- 21.Anagnostou E, Soorya L, Chaplin W, et al. Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: a randomized controlled trial. Mol Autism 2012; 3: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Wang M-J, Rong Y, He H-Z, Yang C-J. Oxytocin therapy for core symptoms in autism spectrum disorder: an updated meta-analysis of randomized controlled trials. Res Autism Spectr Disord 2019; 64: 63–75. [Google Scholar]
- 23.Ooi YP, Weng S-J, Kossowsky J, Gerger H, Sung M. Oxytocin and autism spectrum disorders: a systematic review and meta-analysis of randomized controlled trials. Pharmacopsychiatry 2017; 50: 5–13. [DOI] [PubMed] [Google Scholar]
- 24.Keech B, Crowe S, Hocking DR. Intranasal oxytocin, social cognition and neuro-developmental disorders: a meta-analysis. Psychoneuroendocrinology 2018; 87: 9–19. [DOI] [PubMed] [Google Scholar]
- 25.Spanos M, Chandrasekhar T, Kim SJ, et al. Rationale, design, and methods of the autism centers of excellence (ACE) network study of oxytocin in autism to improve reciprocal social behaviors (SOARS-B). Contemp Clin Trials 2020; 98: 106103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scahill L, Hallett V, Aman MG, et al. Brief report: social disability in autism spectrum disorder: results from Research Units on Pediatric Psychopharmacology (RUPP) Autism Network trials. J Autism Dev Disord 2013; 43: 739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shea S, Turgay A, Carroll A, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics 2004; 114(5): e634–e641. [DOI] [PubMed] [Google Scholar]
- 28.McCracken JT, McGough J, Shah B, et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002; 347: 314–21. [DOI] [PubMed] [Google Scholar]
- 29.Owen R, Sikich L, Marcus RN, et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics 2009; 124: 1533–40. [DOI] [PubMed] [Google Scholar]
- 30.Marcus RN, Owen R, Kamen L, et al. A placebo-controlled, fixed-dose study of aripiprazole in children and adolescents with irritability associated with autistic disorder. J Am Acad Child Adolesc Psychiatry 2009; 48: 1110–9. [DOI] [PubMed] [Google Scholar]
- 31.King BH, Hollander E, Sikich L, et al. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Arch Gen Psychiatry 2009; 66: 583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aman MG, McDougle CJ, Scahill L, et al. Medication and parent training in children with pervasive developmental disorders and serious behavior problems: results from a randomized clinical trial. J Am Acad Child Adolesc Psychiatry 2009; 48: 1143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hopf KP, Madren E, Santianni KA. Use and perceived effectiveness of complementary and alternative medicine to treat and manage the symptoms of autism in children: a survey of parents in a community population. J Altern Complement Med 2016; 22: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anagnostou E, Jones N, Huerta M, et al. Measuring social communication behaviors as a treatment endpoint in individuals with autism spectrum disorder. Autism 2015; 19: 622–36. [DOI] [PubMed] [Google Scholar]
- 35.Lee MR, Shnitko TA, Blue SW, et al. Labeled oxytocin administered via the intranasal route reaches the brain in rhesus macaques. Nat Commun 2020; 11: 2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benner S, Aoki Y, Watanabe T, et al. Neurochemical evidence for differential effects of acute and repeated oxytocin administration. Mol Psychiatry 2021; 26: 710–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.


