Abstract
Medical management of heart failure (HF) has evolved achieving significant survival benefits, resulting in highly complex medication regimens. Complex medication regimens create challenges for older adults, including nonadherence and increased adverse drug events, especially associated with cognitive impairment, physical limitations, or lack of social support. However, the association between medication complexity and patients’ health outcomes among older adults with HF is unclear. The purpose of this review is to address how the complexity of HF medications has been assessed in the literature and what clinical outcomes are associated with medication regimen complexity in HF. Further, we aimed to explore how older adults were represented in those studies. The Medication Regimen Complexity Index was the most commonly used tool for assessment of medication regimen complexity. Rehospitalization was most frequently assessed as the clinical outcome, and other studies used medication adherence, quality of life, healthcare utilization, healthcare cost, or side effect. However, the studies showed inconsistent results in the association between the medication regimen complexity and clinical outcomes. We also identified an extremely small number of studies that focused on older adults. Notably, current medication regimen complexity tools did not consider a complicated clinical condition of an older adult with multimorbidity, therapeutic competition, drug interactions, or altered tolerance to the usual dose strength of the medications. Furthermore, the outcomes that studies assessed were rarely comprehensive or patient-centered. More studies are required to fill the knowledge gap identifying more comprehensive and accurate medication regimen complexity tools and more patient-centered outcome assessment.
Keywords: heart failure, aged, polypharmacy, pharmacotherapy
1. INTRODUCTION
Heart failure (HF) is a significant public health issue in the United States. Almost 6 million Americans have HF, and the prevalence is projected to increase, to 8 million in 2030. Subsequently, the economic burden of HF management is substantial. In 2014, it was reported that almost $40 billion per year were spent on HF care, projected to cost $70 billion by 2030.[1,2] A more recent systematic review estimated that the median cost for HF care could reach $24,383 per patient.[3] HF is also a disease of older adults. The incidence and prevalence of HF increase with age, with more than half of patients with HF in the United States are 75 years or older. [4,5] Furthermore, HF is one of the leading causes of hospitalizations among older adults posing a substantial burden on patients and society.[6,7] HF-related hospitalization among older adults was 3,527 per 100,000 person-years and accounted 38% of adult HF-related hospitalizations.[8]
In the last few decades, guideline-directed medical therapy for HF has evolved by adding medications with survival benefits one by one to the long list of medications.[9–11]Such combinations of medications have shown survival benefit among patients with HF and are recommended by the guideline. However, older adults with HF are also more likely to have other medical conditions, making to take other medications besides the recommended ones for HF. Although the medications that they are recommended are shown to be beneficial to distinct medications, taking multiple medications puts the older adults at a higher risk of medication-related issues including medication errors, drug-drug or drug-disease interactions, and adverse drug effects. Such medication-related problems could be associated with adverse clinical outcomes and compromise each medication’s expected clinical benefit among older adults with HF.[12–15]
Besides the high number of medications, another issue is the increased complexity of the regimen of the medications. Per the 2022 AHA/ACC/HFSA Guideline for the Management of HF, depending on the severity of the HF and other characteristics, a patient with HF with reduced ejection fraction (HFrEF) is recommended to take up to seven or more different medications that have shown benefits in the management of HF (including angiotensin receptor-neprilysin inhibitor/angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, beta-blocker, aldosterone antagonist, sodium-glucose cotransporter-2 inhibitor, diuretic agent, hydralazine+isosorbide dinitrate, and ivabradine).[11] Some medications are indicated to be taken once a day, others twice or three times a day, making the overall medication regimen highly complex. Furthermore, an older adult with HF typically has multiple comorbidities requiring additional medications. They include but are not limited to other cardiologic diseases such as atrial fibrillation or coronary artery disease, diabetes, chronic pulmonary disease, depression, anemia, or chronic kidney disease.[16–19] Subsequently, patients with HF take multiple medications with complex dosing schedules. A study showed that a patient with HF takes an average of 6.8 prescribed medications per day with 10.1 doses per day.[20]
Such a complex medication regimen may create challenges for older adults, including nonadherence and increased adverse drug events, especially when they also have cognitive impairment, physical limitations, or lack of social support. Patients with HF are already known to have poor medication adherence as low as 40–50%.[16] And, they are at higher risks of developing (x4 times) cognitive impairment than those without HF, which can further compromise the ability to comply with such complex medication regimens adequately.[21,22] When cognitive impairment or executive dysfunction prevents patients from taking therapeutic medications as prescribed, such as missing or doubling the doses, it may cause emergency room visits, readmission to the hospital, poor quality of life due to pill burden, and overall poor health outcome. Indeed, a growing number of studies have shown that medication complexity is associated with medication nonadherence, poor quality of life, and increased healthcare resource utilization in the general population.[23–25]
Optimization of the medical management for HF based on the guideline, using multiple medications, is still critical to improving the survival of HF. Older adults with HF need proper medications to benefit from the pharmacotherapy. However, what is not thoroughly studied is what would be the most effective tool to address the complexity of the medication regimen among older adults with HF and the association between the medication complexity and patients’ health outcome. The purpose of this review is to address how the complexity of HF medications has been assessed in the literature and how its related outcomes were associated with medication regimen complexity in HF management. Furthermore, we also aimed to explore how older adults in the studies were represented in the previous studies, and how their unique characteristics could be better represented for complexity measurement in the future.
2. LITERATURE SEARCH METHODS
We conducted the initial literature review in Ovid MEDLINE on March 2nd, 2021. The search terms that we used were “heart failure/ or ((cardiac or heart or myocardial) adj2 (decompensation* or de-compensation* or dysfunction* or failure*)).mp. or cardiomyopath*.mp.” AND “((drug* or medic* or pharmac*) adj2 complex*).mp.” This search yielded 269 articles. We only included studies assessing the medication complexity among patients with HF and association with any health outcomes. Since we aimed to explore how older adults were included in the studies, we did not limit the search using any term related to older adults. The first screening process included a review of the title and abstract resulting in 19 studies for in-depth review. Then we conducted the second screening, reviewing the full-text to identify studies that assessed medication complexity among patients with HF, which resulted 8 original research articles. Then, one original research article was supplemented from the reference of the included articles. Finally, we included 9 articles to inform the current review. We conducted the second round of literature review in Ovid MEDLINE on September 9th, 2022 to include more recent studies using the same search terms. However, it did not change the final selection of the studies. Table 1 shows the author, the year of the study, the population of the study, the tool that they used to assess medication regimen complexity, outcomes that the study assessed, and brief results. We also acknowledge that the medications could differ between inpatient or outpatient settings. Therefore, we specified if they included inpatient medications or outpatient medications in the “population” and “complexity assessment” sections of the table.
Table 1.
Studies that assessed the medication complexity and clinical or economic outcomes
| Author, year | Study design | Population | Complexity assessment | Outcome | Results |
|---|---|---|---|---|---|
| Masoudi, 2005[20] | Retrospective | Hospitalized patients with HF and 65 years or older (n=62,376) | The mean number of long term medications, the mean daily number of doses | The estimated annual cost of medications | The mean number of prescribed medications increased from 7.4 to 8.3 between 1998–1999 and 2000–2001, and the estimated annual cost per drug prescribed also increased from $US498 to $US545. |
| Udelson, 2009[40] | RCT | Multicenter study 18–85 years with stable mild-to-severe HF in an outpatient setting (n=405) | CR carvedilol OD vs. IR carvedilol BID | Compliance, QoL, side effect, adverse effect, and healthcare utilization | No difference in compliance, QoL measured with KCCQ, PHQ-8 Depressive Symptoms Questionnaire, TSQM, side effect, adverse effect, or healthcare utilization. |
| Nieuwenhuis, 2012[35] | A substudy of an RCT | Hospitalized patients with HF, 18 years and older (n=37) | Dosage frequency (>1 times a day) | Adherence | A higher percentage of frequent dosage medications among the nonadherent population than adherent population (78% vs. 21%, p<0.01) |
| Yam, 2016[29] | Retrospective | Hospitalized veterans with HF and followed up at the Veterans Affairs outpatient clinics (n=174) | MRCI | 90-day readmission and/or ER visit | No significant association between MRCI and 90-day readmission and/or ER visit |
| Abou-Karam, 2016[30] | Retrospective | Hospitalized patients with HF 18 years and older, AMI, pneumonia, and COPDa (n=756) | MRCI | 30-day readmission or revisit | No significant association with MRCI and 30-day readmission or revisit |
| Goldstein, 2016[31] | A subanalysis of a longitudinal observational study | In both outpatient or inpatient settings, patients age between 50 and 85 with HF (n=299) | MRCI | Interaction of depression and MRCI toward adherence | For individuals with higher levels of depressive symptoms, more MRCI was associated with lower adherence (with low or average levels of depressive symptoms, MRCI had no effect on medication adherence) |
| Colavecchia, 2017[39] | Retrospective | Hospitalized patients age 18 and older with HF | MRCI | 30-day readmission | MRCI ≥15was associated with 30-day readmission (odds ratio 1.62 95% confidence interval 1.01 – 2.59) |
| Cobretti, 2017[37] | Retrospective | The outpatient setting, patients with HF age between 60 and 89 (n=145) | pMRCIb, number of medications | Comparison pMRCI between young-old group (60–74 years) and old-old group (75–89 years), comparison pMRCI between ischemic cardiomyopathy group and nonischemic cardiomyopathy group | No difference in pMRCI or number of medications between the young-old and old-old groups. Higher pMRCI scores (34.5 ± 15.2 vs. 28.8 ± 12.7 p=0.009), and number of medications (14.1 ± 4.9 vs. 12.2 ± 4.5, p=0.008) in ischemic cardiomyopathy group than nonischemic cardiomyopathy group |
| Wilkening, 2020[32] | Retrospective | Outpatient setting with regular visits with HF (n=72) | MRCI | Association between MRCI and QoL | No correlation between baseline MRCI and quality of life was measured with MLHFQ. A moderate, negative correlation (r = −0.47; p = 0.009) existed between change in MRCI and MLHFQ from baseline to follow-up improved QoL despite increasing MRCI during follow-up. |
Also included other diseases.
Compared with MRCI, pMRCI counts all of the medications that the patient is taking, including over-the-counter medications.
AMI acute myocardial infarction, BID twice daily, COPD chronic obstructive pulmonary disease, CR controlled-release, HF heart failure, ER emergency room, IR immediate-release, KCCQ Kansas City Cardiomyopathy Questionnaire, MLHFQ Minnesota Living with Heart Failure Questionnaire, MRCI Medication Regimen Complexity Index, OD once daily, PHQ-8 Personal Health Questionnaire Depression Scale, QoL quality of life, RCT randomized controlled trial, TSQM Treatment Satisfaction Questionnaire with Medication
3. ASSESSMENT OF MEDICATION REGIMEN COMPLEXITY
Medication regimen complexity describes multiple characteristics of a patient’s medication regimen beyond the number of the prescribed medications. It considers the number of doses per day, number of units per dose, dosage form, and additional instructions.[26] There have been several tools that assess the medication regimen complexity and calculate its degree as a score.
The most-studied instruments to assess the complexity of the medication regimen are the Medication Complexity Index (MCI) and the Medication Regimen Complexity Index (MRCI).
3.1. Medication Complexity Index (MCI)
The Medication Complexity Index (MCI) was first introduced by Kelley in 1988[27] and became the foundation of other medication complexity tools such as the Medication Regimen Complexity Index (MRCI). It assessed the number and frequency of medications and considered the type of actions that the individual is required to manage the regimens. It measures the number of medications in the regimen, the number of doses per day, additional directions that the individual must follow. The total MCI score is the sum of the points awarded for each action and decision required for each medication.[28]
3.2. Medication Regimen Complexity Index (MRCI)
The MRCI is a commonly used tool to assess the complexity of medication regimens. This tool was developed by George et al. with the concept that when it comes to the accurate assessment of the complexity of medication regimen, we should not only take into account the number of medications, but also consider the doses per day, number of units per dose, dosage forms, and any additional instructions.[26] The MRCI has been validated to measure the complexity of a medication list, and it is known to have good inter-rater and test-retest reliability, providing a weighted score indicating complexity. MRCI is calculated based on dosage form (section A), dosing frequency (section B), and additional directions for administration (section C). The more difficult or complex dosage form to administer the medication gets higher weights in section A. More frequent medication administration or more strict intervals received higher weights in section B. In section C, if there is additional instruction in administering the medication, it adds more weight to the total score. The higher total score indicates a more complex medication regimen.[26] The MRCI was widely validated and demonstrated good reliability, and it also has been most commonly used to assess medication regimen complexity among patients with HF.[29–32]
After the MRCI was developed, Libby et al. expanded it and proposed the patient-level Medication Regimen Complexity Index (pMRCI), including both prescribed and over-the-counter medications. The rationale of such expansion of the concept is to reflect the patient’s real-world settings by accepting that over-the-counter medication administration will contribute greatly to medication complexity.[33]
3.3. Other complexity assessment tools
Several studies used different tools to assess the medication complexity, other than MCI or MRCI. For example, Vik et al. created a complexity index for each medication by multiplying the frequency of administration by the amount administered. The total complexity index is then calculated by adding the individual drug complexity indices for each subject’s total prescribed medications.[34] In other studies focused on patients with HF, only frequency was used to assess the complexity of the medication regimen, such as comparing once-daily or more than one time a day.[35,36]
4. OUTCOME ASSESSMENT IN RELATION TO MEDICATION REGIMEN COMPLEXITY
4.1. Medication regimen complexity index among older adults with heart failure
Cobretti et al. conducted a retrospective study assessing pMRCI as the outcome and compared it between the young-old group (60–74 years) and the old-old group (75–89 years). They also compared the pMRCI between ischemic and nonischemic cardiomyopathy groups. Although there was no difference in pMRCI or the number of medications between the young-old group and old-old group, they reported that the ischemic cardiomyopathy group had higher pMRCI scores (34.5 ± 15.2 vs. 28.8 ± 12.7 p=0.009) and a higher number of medications (14.1 ± 4.9 vs. 12.2 ± 4.5, p=0.008) than the nonischemic cardiomyopathy group (Table 1).[37]
4.2. The association with medication regimen complexity and rehospitalization
As adverse drug events are a leading cause of hospitalization, understandably, a complex medication regimen is known to be related to unplanned hospitalization. In a study from Sweden, higher MRCI score or number of medications was shown to be related to higher chance of unplanned hospitalization.[38] Through our review for studies among patients with HF, rehospitalization was also one of the most commonly assessed clinical outcomes in relation to MRCI. Several studies used MRCI to assess the medication complexity and clinical outcomes in patients with HF, but the results were heterogeneous and inconsistent (Table 1). Colavecchia et al. conducted a retrospective study among adult hospitalized patients with a diagnosis of HF (n=1,452) and found that MRCI ≥ 15 was independently associated with a higher 30-day rehospitalization rate (odds ratio (OR) 1.62; 95% confidence interval (CI): 1.01 – 2.59).[39] However, a retrospective cohort study by Yam et al. that studied 174 veterans who were admitted to the hospital due to HF (mean age 71.2 ± 12 years) found no significant association between MRCI and 90-day readmission or E.R. visits, although they found that the mean MRCI score at discharge (40.2 ± 18.2) was significantly higher than the MRCI score at admission (35.5 ± 19.4) (p<0.0001).[29] Abou-Karam and colleagues conducted a retrospective study with a parallel-group case-control design among hospitalized adult patients with HF, acute myocardial infarct, pneumonia, and chronic obstructive pulmonary disease, comparing patients with 30-day all-cause readmission and without readmission, and they also did not find any significant association with MRCI and 30-day readmission or revisit after discharge.[30]
4.3. The association with medication regimen complexity and medication adherence
One of the other clinical outcomes assessed with medication complexity among patients with HF was medication adherence (Table 1). Most of the studies that evaluated adherence as the outcome assessed the complexity using the frequency of the medication use per day. For example, Udelson et al. assessed if the adherence differed between once-daily controlled-release carvedilol and twice-daily immediate-release carvedilol and found no significant difference.[40] In a substudy of a randomized controlled study (COACH – Coordinating study evaluating Outcomes of Advising and Counselling in Heart failure patients), Nieuwenhuis et al. compared the two groups of HF patients, adherent group and nonadherent group. They found out that a higher percentage (78%) of the nonadherent group were taking medications more than once a day than those in the adherent group (21%).[35] Another study used MRCI as the complexity assessment to assess the association with adherence. Goldstein et al. assessed the interaction of depression and MRCI toward the patient’s adherence and reported that for individuals with higher depressive symptoms, more MRCI was associated with lower adherence, but MRCI was not related to adherence for those with little or no depressive symptoms.[31]
4.4. The association with medication regimen complexity and quality of life
Patients with HF often have poor quality of life due to symptoms and related healthcare utilization such as frequent hospitalization.[32] Additionally, the complex medication regimen itself can reduce quality of life, mediated by increased drug interactions, inappropriate dosing, therapeutic failure, nonadherence, and functional decline.[33,41,42] However, few studies have assessed quality of life in relation to medication complexity in patients with HF (Table 1). Udelson et al. compared quality of life using Kansas City Cardiomyopathy Questionnaire (KCCQ), PHQ-8 Depressive Symptoms Questionnaire, and Treatment Satisfaction Questionnaire with Medication between the once-daily controlled-release carvedilol and twice-daily immediate-release carvedilol groups but did not find any differences.[40] Notably, this study was the only one that assessed the overall quality of life of the patients (using PHQ-8 and Treatment Satisfaction Questionnaire with Medication) using non-HF specific quality of life assessment tool.[40] Wilkening et al. assessed the correlation between the medication complexity and the quality of life using MRCI and the Minnesota Living with Heart Failure Questionnaire (MLHFQ). In this retrospective study, they found no significant association between baseline MRCI and MLHFQ, but found that improvement of MLHFQ score despite an increase MRCI during the follow-up.[32] The authors explained that the increased complexity was more likely to be from complex instruction for dosing titration and indicated that they could not assess if the improvement of quality of life preceded the increase of complexity or not due to the nature of retrospective study.
4.5. The association with medication regimen complexity and economic outcomes
One important outcome in estimating the impact of medication complexity on the patient’s health is associated cost (Table 1). The authors could not find studies assessing the direct cost associated with medication complexity using MRCI or other validated complexity assessment tools. We found only one study evaluated the association between the number of prescribed medications and the estimated total annual healthcare cost. From a retrospective study using national data, Masoudi et al. reported that the mean number of prescribed medications increased from 7.4 to 8.3 between 1998–1999 and 2000–2001, and the estimated annual cost per drug prescribed also increased from $498 to $545 among patients who were hospitalized with HF.[20] They also reported an overall increase of total annual healthcare costs from $3,649 to $4,526 within the same study period.
5. STUDIES FOCUSING ON OLDER ADULTS
While HF is a disease of older adults, studies assessing the medication complexity focusing on the geriatric population were few. Among the nine studies we reviewed in-depth, [20,29–32,35,37,39,40], only three studies[20,31,37] focused on older adults. However, among the three, two studies excluded very old patients or patients with cognitive impairment. Cobretti et al. included somewhat “older” adults, only including patients 60 years and older, but they excluded patients 90 years or older.[37] Goldstein et al. conducted a subanalysis including patients between 50 to 85, but excluded patients with any cognitive impairment.[31] Patients with HF and cognitive impairment have poor medication adherence and poor medication self-management skills (i.e., inability to read pill bottle labels, inability to open pill bottle safety cap, more errors of omission, and more knowledge-based mistakes).[22,43] Excluding older adults who have the most difficulties managing complex medication regimens will be less likely to produce reliable results reflecting real-world practice.
6. TACKLING MEDICATION REGIMEN COMPLEXITY
6.1. Knowledge gap and heterogeneity in medication regimen complexity assessment exist
We found a knowledge gap evidenced by a wide variety and heterogeneity in assessing medication regimen complexity and evaluating its associated outcomes and lack of inclusion of older adults with geriatric syndromes. The most commonly used assessment tool was MRCI, and the most frequently assessed outcomes were readmission to the hospital and adherence. The results of the studies consistently showed that the patients with HF experience high complexity regimens. However, the relationship between medication complexity and clinical outcomes is not clear. Results from studies assessing the association between MRCI and readmission rates have been inconsistent. Some studies show that high MRCI is associated with a higher readmission rate[39], but other studies show no associations[29,30]. Furthermore, in regards to adherence, studies that assessed the relationship between the dosing frequencies of drugs and adherence showed no significant association. Still, a study that evaluated the interaction of depression and MRCI toward adherence found that MRCI was associated with lower adherence among patients with higher depressive symptoms.
Through literature review, we could only identify nine studies assessing medication complexity and outcomes among patients with HF. Furthermore, the number of studies focusing on older adults and reflecting the real-world situation is extremely small. It was also noticeable that no study focused on HF with preserved ejection fraction, which disproportionally affects older adults. Considering that among older adults with HF, their poor clinical outcomes are mediated by medication nonadherence, poor quality of life, or cognitive impairment,[32,41,44] it would also be important to investigate if medication regimen complexity also is a mediator associated with negative health outcomes.
6.2. More practical medication complexity regimen tools are needed for older adults
Furthermore, medication regimen for HF patient is continuously evolving as more studies identify medications with survival benefits.[45] Although the current review mainly focused on the complexity of the medication regimen that patients experience in their daily lives, the importance of adherence of appropriate medications for optimal management of HF should not be discredited, and older adults with HF still need proper medications based on the guideline as tolerated. What should be done in the future is to capture the accurate complexity focused on older adults’ unique characteristics to avoid any adverse consequences from the complexity of medication regimens. As a patient’s condition changes, including aging, frailty, multimorbidity, and HF severity, the patient’s tolerability for each medication can change.
Current medication complexity assessment tools do not fully capture related issues such as therapeutic competition, polypharmacy, multimorbidity, or drug interactions with geriatric syndromes. In a recent study, Brinker et al. reported a high prevalence of polypharmacy (74%) and 100% prevalence of prescription for potentially inappropriate medications based on the 2016 American Heart Association Scientific Statement on drugs that pose a major risk of causing or exacerbating HF, the 2018 Beers Criteria, or medications associated with geriatric syndromes[18,46,47]. Nearly half (49%) of the study cohort were taking a medication having therapeutic competition, a condition in which a medication for one condition may directly worsen a coexisting medical condition or decrease medication efficacy for a coexisting medical condition.[48,49] Further studies to identify a more appropriate tool to assess the medication regimen complexity that includes not only the aspects of the drug itself (dose strength, frequency, and complex instructions) but potential therapeutic competitions due to multimorbidity or drug interactions (drug-drug interaction, drug-age interaction, and drug-geriatric syndrome interaction) to address the real challenge that patients with HF face, and assess the real “burden” of the complex medication regimen. Again, it is noteworthy that older adults with HF often have lower physical or cognitive function, such as frailty or dementia, which will require further attention in assessing complexity. Such drug-geriatric syndrome interactions should be counted as one of the therapeutic competition components. A comprehensive assessment to capture this complexity, competing conditions, and burden could show the true impact of complex medication utilization on patients’ health outcomes. A practical but comprehensive complexity assessment tool could include the previous complexity measures, such as frequency or instructions, but it also needs to incorporate older adults’ unique age-related characteristics, such as therapeutic competition due to multimorbidity or drug-age interactions (Figure 1). For instance, adding a weighted score to the overall complexity measurement score could be an exemplar, so that it can be calculated easily in daily practice. However, again, future studies are warranted to develop a practical and comprehensive tool and assess validity and feasibility.
Figure 1.
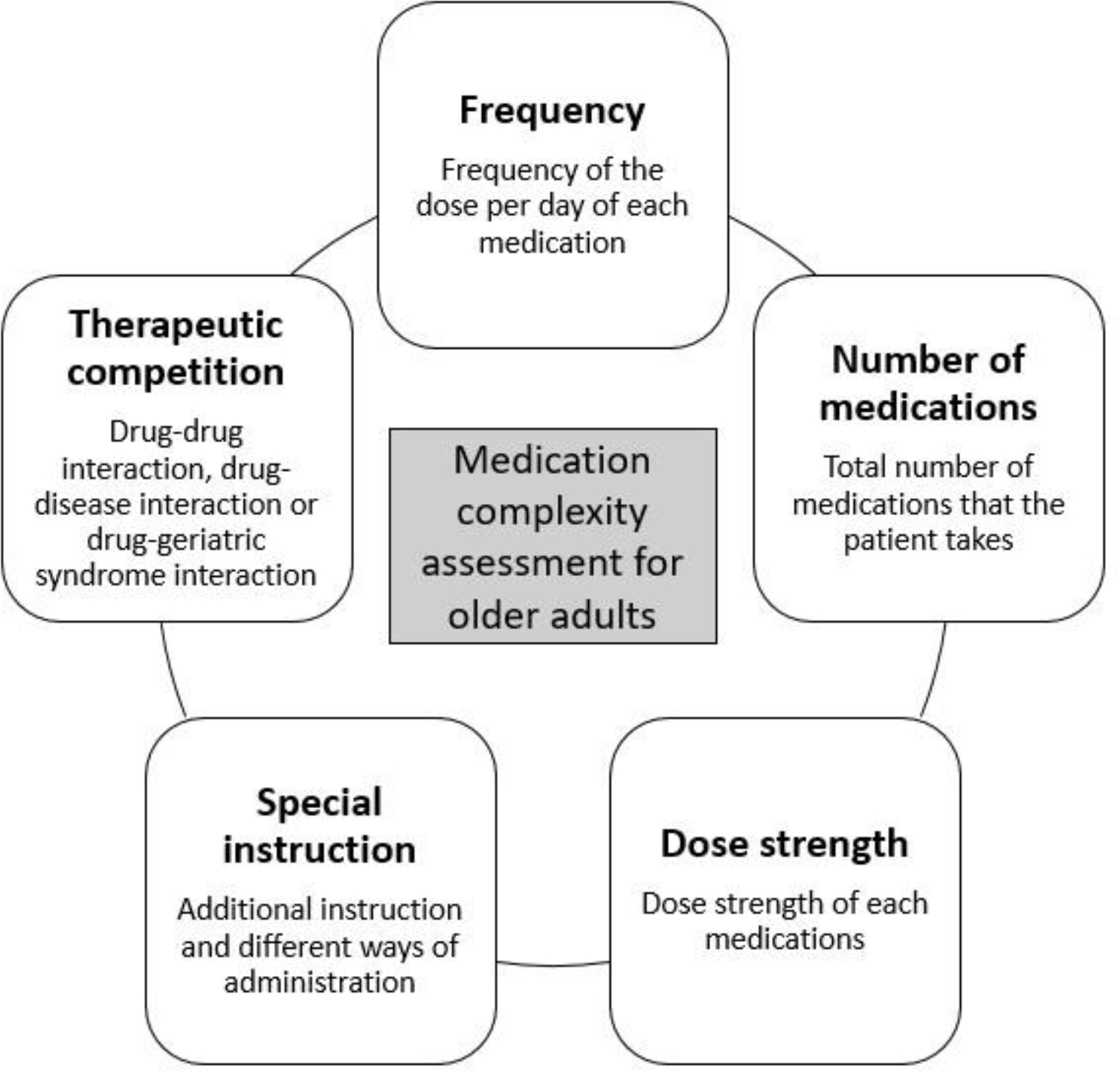
Factors to consider for medication complexity assessment for older adults with heart failure
6.3. Future studies are needed to assess the impact of medication regimen complexity and patient-centered outcomes
Furthermore, we also need more studies identifying the appropriate patient-centered outcomes associated with medication regimen complexity. We noticed that the most common clinical outcomes that they assessed were rehospitalization or admission to the hospital. Preventing hospitalization among older adults with HF is an important clinical outcome, but outcomes such as functional or cognitive decline are greatly important for older adults. Therefore, more patient-centered outcomes should be assessed to evaluate the real impact of medication regimen complexity on overall health. Such outcomes could include quality of life related to pharmacotherapy, functional status changes, home time, or caregiver burdens.
7. CONCLUSION
Complex medication regimens are significant burden to older adults with HF. Although medication regimens are becoming more complex for management for HF and aging continues to affect older adults’ cognitive and executive function, there have been very few studies to evaluate medication complexity among older adults with HF. Furthermore, several studies excluded very old individuals or those with cognitive impairment. Studies are still heterogeneous in both assessment tools and association with outcomes. More studies focusing on older adults, assessing the medication regimen complexity and its clinical outcomes in a more comprehensive fashion considering their unique physiologic and psychologic conditions such as frailty or cognitive impairment are needed.
KEY POINTS.
Medical management of heart failure has become complex and older adults with limited physical or cognitive function experience difficulties managing such complex medication regimens.
The Medication Regimen Complexity Index was the most commonly used tool for assessment of medication regimen complexity, but studies showed inconsistent results in the association between the medication regimen complexity and clinical outcomes.
We suggest that future studies focusing on older adults, assessing the medication regimen complexity and its clinical outcomes in a more comprehensive fashion considering their unique physiologic and psychologic conditions such as frailty or cognitive impairment are needed.
Funding
This work was supported by the National Institutes on Aging R24AG064025. The funding agency did not have any involvement in study design, data collection, analysis or interpretation of the data, writing of the manuscript or decision to submit the article for publication.
Footnotes
Conflict of Interest
Dr. Kwak receives funding from National Institute on Aging 1R24AG064025 and receives consult fee from the Endocrine & Diabetes Plus Clinic of Houston and Institute for Healthcare Improvement. Dr. Goyal is supported by American Heart Association grant 20CDA35310455, National Institute on Aging grant K76AG064428, and Loan Repayment Program award L30AG060521; Dr. Goyal receives personal fees for medicolegal consulting related to heart failure; and has received honoraria from Akcea inc and Bionest inc. Dr. Hummel is the PI or co-I for studies funded by Pfizer, Novartis, Corvia and Axon, and has funding from National Institute on Aging, National Heart, Lung, and Blood Institute, and Veterans Affairs Clinical Science Research and Development. Dr. Hummel also had previously received research funding from PurFoods, LLC. Dr. Kim receives personal fees from Alosa Health and research funding from the National Institute of Health for projects unrelated to the current work. Dr. Holly Holmes received research funding from Healthcare Services Corporation, which is a foundation of Blue Cross Blue Shield, for a study of deprescribing. The funding is unrelated to the manuscript under consideration. Dr. Dhoble is a consultant and proctor for Abbott Vascular. Dr. Aparasu receives research funding from Astellas Inc., Incyte Corp., Gilead, and Novartis Inc. for projects unrelated to the current work.
Ethics approval: Not applicable
Consent to participate: Not applicable
Consent for publication: Not applicable
Code availability: Not applicable
Availability of data and materials:
Not applicable
REFERENCE
- [1].Voigt J, Sasha John M, Taylor A, Krucoff M, Reynolds MR, Michael Gibson C. A reevaluation of the costs of heart failure and its implications for allocation of health resources in the United States. Clin Cardiol 2014; 37:312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013; 6:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Urbich M, Globe G, Pantiri K, et al. A Systematic Review of Medical Costs Associated with Heart Failure in the USA (2014–2020). Pharmacoeconomics 2020; 38:1219–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tsao CW, Aday AW, Almarzooq ZI, et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022; 145:e153–e639. [DOI] [PubMed] [Google Scholar]
- [5].Rich MW. Management of heart failure in the elderly. Heart Fail Rev 2002; 7:89–97. [DOI] [PubMed] [Google Scholar]
- [6].Azad N, Lemay G. Management of chronic heart failure in the older population. Journal of Geriatric Cardiology 2014; 11:329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Freidman B (AHRQ), Jiang HJ (AHRQ) and Russo CA (Thomson Reuters). Medicare Hospital Stays: Comparisons between the Fee-for-Service Plan and Alternative Plans, 2006. 2009. [PubMed]
- [8].Minhas A,Khan Mannan, Ijaz SH, Jamal S, et al. Trends in Characteristics and Outcomes in Primary Heart Failure Hospitalizations Among Older Population in the United States, 2004 to 2018. Circulation: Heart Failure 2022; 15:e008943. [DOI] [PubMed] [Google Scholar]
- [9].Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62:e147–e239. [DOI] [PubMed] [Google Scholar]
- [10].Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136:e137–e161. [DOI] [PubMed] [Google Scholar]
- [11].Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022; 145:e895–e1032. [DOI] [PubMed] [Google Scholar]
- [12].Gastelurrutia P, Benrimoj SI, Espejo J, Tuneu L, Mangues MA, Bayes-Genis A. Negative clinical outcomes associated with drug-related problems in heart failure (HF) outpatients: impact of a pharmacist in a multidisciplinary HF clinic. J Card Fail 2011; 17:217–223. [DOI] [PubMed] [Google Scholar]
- [13].Volpe M, Chin D, Paneni F. The challenge of polypharmacy in cardiovascular medicine. Fundam Clin Pharmacol 2010; 24:9–17. [DOI] [PubMed] [Google Scholar]
- [14].Marcum ZA, Amuan ME, Hanlon JT, et al. Prevalence of unplanned hospitalizations caused by adverse drug reactions in older veterans. J Am Geriatr Soc 2012; 60:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wong CY, Chaudhry SI, Desai MM, Krumholz HM. Trends in comorbidity, disability, and polypharmacy in heart failure. Am J Med 2011; 124:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wu J, Moser DK, Lennie TA, Burkhart PV. Medication Adherence in Patients Who Have Heart Failure: a Review of the Literature. Nurs Clin North Am 2008; 43:133–153. [DOI] [PubMed] [Google Scholar]
- [17].Braunstein JB, Anderson GF, Gerstenblith G, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol 2003; 42:1226–1233. [DOI] [PubMed] [Google Scholar]
- [18].Page RL2, O’Bryant CL, Cheng D, et al. Drugs That May Cause or Exacerbate Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2016; 134:e32–69. [DOI] [PubMed] [Google Scholar]
- [19].Lazzarini V, Mentz RJ, Fiuzat M, Metra M, O’Connor CM. Heart failure in elderly patients: distinctive features and unresolved issues. Eur J Heart Fail 2013; 15:717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Masoudi FA, Baillie CA, Wang Y, et al. The complexity and cost of drug regimens of older patients hospitalized with heart failure in the United States, 1998–2001. Arch Intern Med 2005; 165:2069–2076. [DOI] [PubMed] [Google Scholar]
- [21].Sauvé MJ, Lewis WR, Blankenbiller M, Rickabaugh B, Pressler SJ. Cognitive Impairments in Chronic Heart Failure: A Case Controlled Study. J Card Fail 2009; 15:1–10. [DOI] [PubMed] [Google Scholar]
- [22].Hawkins LA, Kilian S, Firek A, Kashner TM, Firek CJ, Silvet H. Cognitive impairment and medication adherence in outpatients with heart failure. Heart & Lung 2012; 41:572–582. [DOI] [PubMed] [Google Scholar]
- [23].Paquin AM, Zimmerman KM, Kostas TR, et al. Complexity perplexity: a systematic review to describe the measurement of medication regimen complexity. Expert Opin Drug Saf 2013; 12:829–840. [DOI] [PubMed] [Google Scholar]
- [24].Mansur N, Weiss A, Beloosesky Y. Looking beyond polypharmacy: quantification of medication regimen complexity in the elderly. Am J Geriatr Pharmacother 2012; 10:223–229. [DOI] [PubMed] [Google Scholar]
- [25].Willson MN, Greer CL, Weeks DL. Medication regimen complexity and hospital readmission for an adverse drug event. Ann Pharmacother 2014; 48:26–32. [DOI] [PubMed] [Google Scholar]
- [26].George J, Phun YT, Bailey MJ, Kong DC, Stewart K. Development and validation of the medication regimen complexity index. Ann Pharmacother 2004; 38:1369–1376. [DOI] [PubMed] [Google Scholar]
- [27].Kelley S Measurement of Complexity of Medication Regimens of the Elderly. 1988.
- [28].Conn VS, Taylor SG, Kelley S. Medication regimen complexity and adherence among older adults. Image J Nurs Sch 1991; 23:231–235. [DOI] [PubMed] [Google Scholar]
- [29].Yam FK, Lew T, Eraly SA, Lin H, Hirsch JD, Devor M. Changes in medication regimen complexity and the risk for 90-day hospital readmission and/or emergency department visits in U.S. Veterans with heart failure. Research In Social & Administrative Pharmacy 2016; 12:713–721. [DOI] [PubMed] [Google Scholar]
- [30].Abou-Karam N, Bradford C, Lor KB, Barnett M, Ha M, Rizos A. Medication regimen complexity and readmissions after hospitalization for heart failure, acute myocardial infarction, pneumonia, and chronic obstructive pulmonary disease. SAGE Open Medicine 2016; 4:2050312116632426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Goldstein CM, Gathright EC, Gunstad J, et al. Depressive symptoms moderate the relationship between medication regimen complexity and objectively measured medication adherence in adults with heart failure. J Behav Med 2017; 40:602–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wilkening GL, Brune S, Saenz PF, Vega LM, Kalich BA. Correlation between medication regimen complexity and quality of life in patients with heart failure. Research In Social & Administrative Pharmacy 2020; 16:1498–1501. [DOI] [PubMed] [Google Scholar]
- [33].Libby AM, Fish DN, Hosokawa PW, et al. Patient-level medication regimen complexity across populations with chronic disease. Clin Ther 2013; 35:385–398.e1. [DOI] [PubMed] [Google Scholar]
- [34].Vik SA, Hogan DB, Patten SB, Johnson JA, Romonko-Slack L, Maxwell CJ. Medication nonadherence and subsequent risk of hospitalisation and mortality among older adults. Drugs Aging 2006; 23:345–356. [DOI] [PubMed] [Google Scholar]
- [35].Nieuwenhuis MMW, Jaarsma T, van Veldhuisen DJ, van der Wal MHL Self-reported versus ‘true’ adherence in heart failure patients: a study using the Medication Event Monitoring System. Netherlands Heart Journal 2012; 20:313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hauptman PJ, Pressler SJ, Sackner-Bernstein J, Ordronneau P, Udelson JE, Investigators C. Rationale and design of CASPER: compliance and quality of life study comparing once-daily carvedilol CR and twice-daily carvedilol IR in patients with heart failure. Am J Cardiol 2006; 98:6066. [DOI] [PubMed] [Google Scholar]
- [37].Cobretti MR, Page RL 2nd, Linnebur SA, et al. Medication regimen complexity in ambulatory older adults with heart failure. Clinical Interventions In Aging 2017; 12:679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wimmer BC, Bell JS, Fastbom J, Wiese MD, Johnell K. Medication Regimen Complexity and Polypharmacy as Factors Associated With All-Cause Mortality in Older People: A Population-Based Cohort Study. Ann Pharmacother 2016; 50:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Colavecchia AC, Putney DR, Johnson ML, Aparasu RR. Discharge medication complexity and 30-day heart failure readmissions. Research In Social & Administrative Pharmacy 2017; 13:857–863. [DOI] [PubMed] [Google Scholar]
- [40].Udelson JE, Pressler SJ, Sackner-Bernstein J, et al. Adherence with once daily versus twice daily carvedilol in patients with heart failure: the Compliance And Quality of Life Study Comparing Once-Daily Controlled-Release Carvedilol CR and Twice-Daily Immediate-Release Carvedilol IR in Patients with Heart Failure (CASPER) Trial. J Card Fail 2009; 15:385–393. [DOI] [PubMed] [Google Scholar]
- [41].Peron EP, Gray SL, Hanlon JT. Medication Use and Functional Status Decline in Older Adults: A Narrative Review. The American Journal of Geriatric Pharmacotherapy 2011; 9:378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Brysch EG, Cauthon KAB, Kalich BA, Sarbacker GB. Medication Regimen Complexity Index in the Elderly in an Outpatient Setting: A Literature Review. Consult Pharm 2018; 33:484–496. [DOI] [PubMed] [Google Scholar]
- [43].Howell EH, Senapati A, Hsich E, Gorodeski EZ. Medication self-management skills and cognitive impairment in older adults hospitalized for heart failure: A cross-sectional study. SAGE Open Med 2017; 5:2050312117700301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fröhlich SE, Zaccolo AV, da Silva SL, Mengue SS. Association between drug prescribing and quality of life in primary care. Pharm World Sci 2010; 32:744–751. [DOI] [PubMed] [Google Scholar]
- [45].Maddox Thomas M, Januzzi James L, Allen Larry A, et al. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction. J Am Coll Cardiol 2021; 77:772–810. [DOI] [PubMed] [Google Scholar]
- [46].American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc 2019; 67:674–694. [DOI] [PubMed] [Google Scholar]
- [47].Saraf AA, Petersen AW, Simmons SF, et al. Medications associated with geriatric syndromes and their prevalence in older hospitalized adults discharged to skilled nursing facilities. J Hosp Med 2016; 11:694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Brinker LM, Konerman MC, Navid P, et al. Complex and Potentially Harmful Medication Patterns in Heart Failure with Preserved Ejection Fraction. Am J Med 2021; 134:374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lorgunpai SJ, Grammas M, Lee DS, McAvay G, Charpentier P, Tinetti ME. Potential therapeutic competition in community-living older adults in the US: use of medications that may adversely affect a coexisting condition. PloS one 2014; 9:e89447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable


