Abstract
Glycoinositolphospholipids (GIPLs) are some of the major glycolipids of the Trypanosoma cruzi surface that were previously shown to activate B cells. In the present study, we investigated whether (i) T. cruzi GIPLs could induce immunoglobulin secretion from B cells in the absence of T cells and NK cells and whether (ii) NK cells are also stimulated by the GIPLs. B cells purified from mice deficient in both T and NK cells (CD3ɛ transgenic mice) secreted immunoglobulin in response to the GIPL. This response was increased by coculture with a murine NK cell line. The T. cruzi GIPL also increased the NK cell (interleukin-2 induced) proliferative response. Our data indicate that the T. cruzi GIPL has a direct stimulatory effect on NK cells and induces immunoglobulin secretion in the absence of T lymphocytes and NK cells. These findings suggest that this T. cruzi-derived molecule may be one of the stimulators that lead to NK cell activation during T. cruzi infection.
Glycoinositolphospholipids (GIPLs) are some of the major glycoconjugates present on the cellular surface of Leishmania (17) and different strains of Trypanosoma cruzi (6). T. cruzi GIPL was shown to contain a glycan moiety linked through a non-N-acetylated glucosamine residue to an inositolphosphoryl-N-lignoceroyldihydrosphingosine (19). The main chain of the glycan moiety has a tetramannose structure in which the nonreducing mannose is substituted for by β-galactofuranose residues. The third mannose distal to the inositolphosphate component can be replaced by nonreducing galactofuranosyl end units (or by ethanolamine phosphate), and the glucosamine contains a 2-aminoethylphosphonate substituent (6). GIPL purified from Leishmania major inhibits the leishmanicidal activity of murine macrophages (21), and the T. cruzi GIPL induces macrophage apoptosis in the presence of gamma interferon (IFN-γ), a process that is associated with increased parasite release (10). T. cruzi GIPL blocks T-cell responses induced by different polyclonal activators (11), while it activates murine B cells in vitro (2). In the absence of added costimuli, the T. cruzi G strain GIPL stimulates detectable immunoglobulin M (IgM) production by both low- and high-density B cells and potentiates the response induced by either surface Ig ligation or cytokines. The B-cell stimulatory effect of the GIPL is mediated mainly by its oligosaccharide moiety (2).
Infection with T. cruzi is associated with a polyclonal B-cell activation, and increased circulating Ig levels are detected both early during infection and throughout the chronic phase (8, 27). A transient increase in NK cell cytotoxic activity is also observed during infection (13), and NK cells have been described as being necessary for resistance to T. cruzi infection, probably due to the secretion of IFN-γ (5). Besides their role in the resistance against different infections due to their cytotoxic activity, NK cells have an important role in regulating B-cell activation. Thus, in vitro polysaccharide antigen-induced B-cell response requires the presence of NK cells (25). This accessory role is mediated both by the secretion of cytokines by the NK cells and by B-lymphocyte–NK cell contact (12, 25).
A previous study (2) showed that the inositol (PIns)-oligosaccharide derived from the T. cruzi G strain GIPL has a stimulatory effect on B cells. However, the requirement for accessory cells in the induction of B-cell stimulation had not been addressed yet. In the present study, we investigated the effect of the GIPL on B cells purified from mice deficient in both T lymphocytes and NK cells (30). The role of NK cells in the GIPL-induced B-cell response was assessed by using an NK cell line recently described to enhance Ig secretion in an in vitro model of T-cell-independent type 2 (polysaccharide) antigen (29).
CD3ɛ transgenic (tg) mice [B6,CBA-TgN(CD3E)26Cpt] were obtained from the Jackson Laboratories (Bar Harbor, Maine); the strain used has abnormal differentiation of both T lymphocytes and NK cells, lacking mature peripheral NK and T cells (29). Mice were used at 8 to 12 weeks of age. The experiments were conducted according to the principles set forth in the Guide for the Care and Use of Laboratory Animals (18a). The dextran-conjugated anti-IgD antibody (anti-delta-dextran) was prepared by the conjugation of the AF3 anti-IgD monoclonal antibody (28) to high-molecular-weight dextran, as previously described (4). Murine recombinant interleukin-2 (IL-2) (specific activity, 1.6 × 106 U/mg) and murine recombinant IL-12 (heterodimeric form) were obtained from Genzyme Corporation (Cambridge, Mass.). Splenic B cells were obtained by discontinuous Percoll gradient fractionation (7, 22). Gradients consisting of 70, 60, and 50% Percoll (with densities of 1.086, 1.074, and 1.062 g/ml, respectively) were used. The cells were collected from the 70 to 60% interface after centrifugation (1,900 × g for 15 min). The B-cell preparation obtained after Percoll fractionation was composed of 95% B cells and 5% non-B cells (data not shown). The B cells were cultured for 7 days in RPMI 1640 supplemented with 10% fetal calf serum (GIBCO, Grand Island, N.Y.), l-glutamine (2 mM), 2-mercaptoethanol (50 μM), nonessential amino acids (100 μM), sodium pyruvate (1 mM), and gentamicin (50 μg/ml) (complete RPMI medium), in a final volume of 200 μl in flat-bottom 96-well trays (Costar, Cambridge, Mass.). Quantification of IgM was performed by a modification of a previously described capture Ig enzyme-linked immunosorbent assay (ELISA) (24). The PKO cell line was obtained by stimulation, with both IL-2 and poly(I-C), of spleen cells not expressing either major histocompatibility complex class II or Thy1 obtained from p53 knockout mice (29). Flow cytometry analysis showed that the PKO cell line expressed IL-2 receptor α and β chains and expressed low levels of B220. The cell line had a uniform Ly-49-positive phenotype and did not express the NK 1.1 antigen. No CD3, CD8, CD4, and secretory Ig expression was detected (29). The PKO cell line lacked T-cell-receptor rearrangement, showed cytotoxic activity, and expressed mRNA for perforin and granzymes (29). The PKO cell line was weekly split after detachment with trypsin-EDTA solution. Split cells were cultured in the presence of IL-2 at 50 U/ml in complete RPMI medium. For measurement of NK cell proliferation, the PKO cells were cultured for 48 h as described above. Proliferation was measured by tritiated thymidine incorporation and analyzed by liquid scintillation spectrometry; the results are expressed as the arithmetic means of triplicate cultures. T. cruzi (G strain) epimastigote forms were grown in brain heart infusion medium (Difco Laboratories, Inc., Detroit, Mich.) (6), and the harvested cells (approximately 1011) were extracted at 80°C with 45% aqueous phenol. The aqueous layer was dialyzed, freeze dried, and applied to a column (2 cm by 100 cm) of Bio-Gel P-60. The excluded material was lyophilized, and the GIPL was recovered by extraction with chloroform-methanol-water (10:10:3). The extract was evaporated to dryness, dissolved in water and lyophilized (20). The dry material was diluted in water and added to the cultures after gamma irradiation. The intact GIPL appeared on sodium dodecyl sulfate-polyacrylamide gel electrophoresis as a fast-moving single-molecule species of 2.08 kDa. Approximately 3 μg of GIPL (1.4 nmol) was obtained from 4.5 × 107 T. cruzi cells (6). The structure of T. cruzi G strain GIPL was resolved by nuclear magnetic resonance (NMR) spectroscopy and fast atom bombardment mass spectrometry analysis. A mixture at a 7:3 molar ratio of two closely related ceramide-containing structures, (i) Galf β1-3 Manp α1-2 (EtNP) Manp α1-2 Manp α1-6 Man α1-4(2-AEP) GlcN α1-6 InsPO4 and (ii) Manp α1-2 (EtNP) Manp α1-2 Manp α1-6 Man α1-4(2-AEP) GlcN α1-6 InsPO4, was found in the purified GIPL (6). The major GIPL (i) contains a nonreducing β-galactofuranose residue, and the third mannose in the tetramannose chain is replaced by ethanolaminephosphate. The PIns-oligosaccharides are linked to a ceramide (N-lignoceroyldihydrosphingosine).
The virtual absence of contaminating peptide material was confirmed by NMR analysis of PIns-oligosaccharides. Analysis of the hydrolysis product of the GIPL (see below) revealed no contamination with bacterial lipopolysaccharide (LPS) (6). The presence of 3-deoxyoctulosonic acid (KDO), a characteristic product of the hydrolysis of LPS, was not detected in material analyzed by high-pH anion-exchange chromatography with an electrochemical detector. Also, LPS per-O-trimethylsilylated derivative was not detected by gas-liquid chromatography. The sensitivity of pulse amperometric detection is at least 0.05 nmol, and that of gas-liquid chromatography is around 2 pmol. The PIns-oligosaccharides were isolated from the intact GIPL by alkaline hydrolysis (6) and have a molecular size of 1.46 kDa.
Statistical analysis was performed with Student's t test.
It has previously been observed that the PIns-oligosaccharide moiety of the T. cruzi GIPL stimulates Ig secretion by a B-cell preparation obtained from normal mice (2). In order to test if either contaminating NK or T cells would interfere in this response, B lymphocytes were obtained from tg mice expressing a high copy number of the CD3ɛ gene. Those mice develop neither T lymphocytes nor NK cells (30). B cells obtained from these mice were stimulated with either the GIPL-derived PIns-oligosaccharide (Table 1, experiment 1) or the whole GIPL (Table 1, experiment 2). As can be seen in Table 1, B cells purified from CD3ɛ tg mice were induced to secrete Ig in the absence of T cells or NK cells when stimulated with either the GIPL or the GIPL-derived PIns-oligosaccharide. The observed effect of the glycoconjugate was not detected at either higher or lower doses of the GIPL (data not shown). Addition of IL-2 led to an additional enhancement in IgM secretion. To test whether GIPL synergized with a multivalent membrane Ig cross-linking signal, we cultured anti-Ig-dextran together with GIPL in the presence or absence of IL-2. Addition of GIPL-derived oligosaccharide to the combination of anti-Ig-dextran and IL-2 led to a threefold increase in the amount of secreted IgM compared to that of the control (16.2 versus 5.7 μg/ml). Also, in an independent experiment, we observed an even higher increase in IgM secretion when the whole GIPL was added to the B cells in the presence of anti-Ig-dextran and IL-2 (Table 1). These data indicate that B cells from mice deficient in both T lymphocytes and NK cells can be stimulated to secrete IgM by GIPL and GIPL-derived PIns-oligosaccharide. Furthermore, the data demonstrate that this glycoconjugate can significantly enhance IgM secretion stimulated by IL-2 and anti-Ig-dextran.
TABLE 1.
Ig secretion by B cells purified from the CD3ɛ tg mice stimulated with T. cruzi GIPL
| Stimulusa | IgM secretion (ng/ml)b
|
|||
|---|---|---|---|---|
| Expt 1
|
Expt 2
|
|||
| No oligosaccharide | Plus oligosaccharide (6.5 μM) | No GIPL | Plus GIPL (1.5 μM) | |
| Medium | 160 | 600 | <50 | <50 |
| IL-2 | 180 | 1,500 | <50 | 4,000 |
| Anti-delta-dextran | 4,750 | 5,000 | 350 | 2,700 |
| Anti-delta-dextran + IL-2 | 5,750 | 16,250 | 350 | 18,500 |
IL-2 was added at 50 U/ml, dextran-conjugated anti-delta antibody (AF3-dextran) was used at 20 ng/ml, and splenic B cells from CD3ɛ tg mice were added at 105/ml.
Culture supernatants were obtained after 7 days of triplicate cultures, and IgM levels were determined by ELISA. The results shown are representative of two independent experiments.
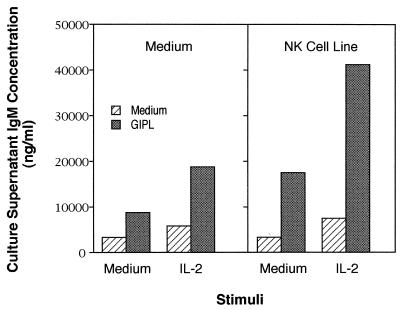
T lymphocytes and NK cells play an important role in the enhancement of Ig secretion by B cells, and depletion of these populations decreases B-cell response to polysaccharide (type 2) antigens (18). To test if GIPL-induced Ig secretion would be increased in the presence of activated NK cells, we added an NK cell line previously reported to support B-cell Ig secretion (29) to cultures containing GIPL. As can be seen in Fig. 1, addition of NK cells to cultures containing medium and GIPL led to an increase in Ig secretion that was enhanced even further when IL-2 was added. These data suggest that the induction of an optimally stimulatory response may require the activation of both B and NK cells by IL-2.
FIG. 1.
Enhancement of the GIPL-stimulated IgM secretion in the presence of an NK cell line. Splenic B cells were purified from CD3ɛ tg mice and used at 105/ml. Some cultures were stimulated with T. cruzi GIPL (added at 1.5 μM) and IL-2 (50 U/ml) either alone or in combination. The PKO NK cell line (400 cells/ml) was added where indicated. The dextran-conjugated anti-delta antibody (AF3-dextran) was added at 20 ng/ml. Culture supernatants were obtained from triplicate cultures after 7 days, and IgM levels were determined by ELISA. The results are representative of three independent experiments.
The B-cell preparation used in the present work is depleted of both T lymphocytes and NK cells due to the blockage in the development of these cell populations in the CD3ɛ tg mice. Contaminating macrophages may probably be present in our cell preparation and could have interfered with the response we studied, since it is well known that macrophage-derived cytokines can modify B-cell response (18). However, we do not believe macrophages interfered in either the B-cell or the NK cell response described here, since it was shown that in vitro T. cruzi GIPL treatment has a major inhibitory effect on macrophage activity and can even lead to apoptosis in those cells (10).
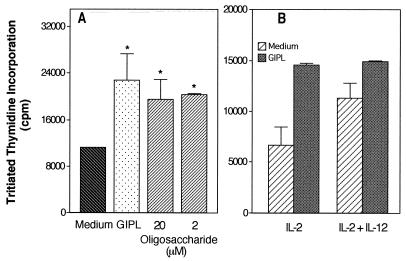
As shown above (Fig. 1), the increase in Ig secretion following anti-delta-dextran-induced stimulation of B cells obtained from CD3ɛ transgenic mice was best detected if both the GIPL and IL-2 were added in combination with the NK line. This finding suggests either that the GIPL affects the B cells only (and in conjunction with IL-2-induced NK cell activation there is a significant increase in Ig secretion) or, possibly, that the GIPL affects both the B-cell and NK cell populations. To test if the GIPL would have any stimulatory effect on the NK cell line, we investigated its effect on NK cell proliferation. The IL-2-induced NK line proliferation was significantly increased in the presence of either the GIPL or the GIPL-derived oligosaccharide (Fig. 2A). We also tested if the IL-2-induced response could be increased by IL-12, a cytokine that increases NK cell cytotoxic activity (23), and we did not observe any potentiating effect (Fig. 2B). This finding is consistent with the report that even though IL-12 can potentiate IFN-γ secretion, it may have an inhibitory effect on NK cell proliferation (23). Also, IL-12 did not have any stimulatory effect on the NK line when added either alone or in the presence of T. cruzi GIPL (data not shown). We also tested the induction of NK cell IFN-γ secretion by the GIPL, and no effect was detected (data not shown). This absence of IFN-γ secretion, despite the observation of NK-mediated B-cell costimulatory effect, was not unexpected, since the different NK effector functions were described to be independently regulated (15).
FIG. 2.
Proliferation of the PKO NK cell line in the presence of cytokines and T. cruzi-derived glycoconjugates. Cells of the PKO NK line (1.25 × 105/ml) were stimulated with the indicated glycoconjugates. GIPL purified from T. cruzi was used at 15 μM. This was observed to be the most effective dose in titration studies (data not shown). The indicated doses (micromolar) of the PIns-oligosaccharide were also added. IL-2 was added at 50 U/ml, and IL-12 was added at 0.2 ng/ml. Tritiated thymidine incorporation was measured after a 48-h culture. Cultures performed in the absence of IL-2 showed thymidine incorporation below 300 cpm irrespective of the stimulus used. Asterisks indicate statistically significant differences at a P value of ≤0.005.
NK cells have receptors for oligosaccharides, and incubation of these cells with some mannosylated oligosaccharides stimulates cytotoxic activity (3). Most NK cell surface receptors recognize glycosylated molecules (16). Although we did not characterize the mechanism of binding of the GIPL to NK cells, it is possible that this binding occurs on mannose receptors present on NK cells, since the T. cruzi GIPL has a tetramannose core (6). Previous data published by our group have indicated that a mixture at a 7:3 molar ratio of two closely related ceramide-containing structures is found in the purified T. cruzi G strain GIPL (6). The major GIPL contained a terminal nonreducing β-galactofuranose residue. However, the minor fraction had a terminal mannose. The lower content of the structure containing free terminal mannose may be one of the reasons why the use of high doses of GIPL may be necessary in vitro.
Recent studies (reviewed in reference 26) suggested that several bacterially derived products would generate accessory cell signals necessary for B-cell activation. A well-characterized activator is the bacterial CpG oligodeoxynucleotide, which was previously shown to activate both B lymphocytes and NK cells (1, 14) and to have adjuvant effects on Th1 cell activation (31). The response induced by this molecule resembles the one induced by the GIPL, and it is possible that the GIPL may be one of the protozoan-derived molecules modulating the host B cells' response during infection.
Infection with T. cruzi is associated with altered function of different lymphoid cells. One important abnormality is a polyclonal B-cell activation that is associated with increased circulating Ig levels (8, 27). Our finding that multivalent Ig cross-linking and GIPL synergize to induce enhanced Ig secretion may be a model that reflects the Ig secretion induced by T. cruzi that displays repetitive antigen epitopes on its surface in conjunction with the GIPL. During T. cruzi infection, a suppression of T-cell function (9) and a transient increase in NK cell cytotoxic activity (13) also occur. It is noteworthy that the effect of GIPL on B and T lymphocytes and on NK cells (references 2 and 11 and the present study) parallels the alterations in cell function observed during T. cruzi infection. Taken together, these data suggest that the T. cruzi GIPL could be one of the molecules involved in the induction of abnormal lymphoid cell function detected during infection.
Acknowledgments
We are indebted to Sidney Gomes da Costa and Nelson Martins Ferreira for technical assistance. We thank George A. dosReis for critically reviewing the manuscript.
This work was supported by Nacional de Desenvolvimento Científico e Tecnológico (CNPq; CNPq/RHAE), Financiadora de Estudos e Projetos (FINEP), Ministério da Ciência e Tecnologia (PRONEX-MCT), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Third World Academy of Sciences, and National Institutes of Health grant AI36588-02A1. L. Mendonça-Previato was supported by a Howard Hughes International Research Scholarship. L.B.D.H is a fellow from FAPERJ.
REFERENCES
- 1.Ballas Z D, Rasmussen W L, Krieg A M. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J Immunol. 1996;157:1840–1845. [PubMed] [Google Scholar]
- 2.Bento C A M, Melo M B, Previato J O, Mendonça-Previato L, Peçanha L M T. Glycoinositolphospholipids purified from Trypanosoma cruzi stimulate immunoglobulin production in vitro. J Immunol. 1996;157:4996–5001. [PubMed] [Google Scholar]
- 3.Bezouska K, Yuen C-T, O'Brien J, Childs R A, Chai W, Lawson A M, Drbal K, Fiserova A, Pospisl M, Feizi T. Oligosaccharide ligands for NKR-P1 protein activate NK cells and cytotoxicity. Nature. 1994;372:150–156. doi: 10.1038/372150a0. [DOI] [PubMed] [Google Scholar]
- 4.Brunswick M, Finkelman F D, Highet P, Inman J K, Dintzis H M, Mond J J. Picogram quantities of anti-Ig antibodies coupled to dextran induce B cell proliferation. J Immunol. 1988;140:3364–3372. [PubMed] [Google Scholar]
- 5.Cardillo F, Voltarelli J C, Reed S G, Silva J S. Regulation of Trypanosoma cruzi infection in mice by gamma interferon and interleukin 10: role of NK cells. Infect Immun. 1996;64:128–134. doi: 10.1128/iai.64.1.128-134.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carreira J, Jones C, Wait R, Previato J O, Mendonça-Previato L. Structural variation in the glycoinositolphospholipids of different strains of Trypanosoma cruzi. Glycoconj J. 1996;13:955–966. doi: 10.1007/BF01053191. [DOI] [PubMed] [Google Scholar]
- 7.De Franco A L, Raveche E S, Asofsky R, Paul W E. Frequency of B lymphocytes responsive to anti-immunoglobulin. J Exp Med. 1982;155:1523–1534. doi: 10.1084/jem.155.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Imperio Lima M, Eisen H, Minoprio P, Joskowicz M, Coutinho A. Persistence of polyclonal B cell activation with undetectable parasitemia in late stages of experimental Chagas disease. J Immunol. 1986;137:353–356. [PubMed] [Google Scholar]
- 9.DosReis G A. Cell mediated immunity in experimental Trypanosoma cruzi infection. Parasitol Today. 1997;13:335–342. doi: 10.1016/s0169-4758(97)01073-9. [DOI] [PubMed] [Google Scholar]
- 10.Freire de Lima C G, Nunes M P, Corte-Real S, Soares M P, Previato J O, Mendonça-Previato L, dosReis G A. Pro-apoptotic activity of a Trypanosoma cruzi ceramide-containing glycolipid turned on in host macrophages by interferon-γ. J Immunol. 1998;161:4909–4916. [PubMed] [Google Scholar]
- 11.Gomes N A, Previato J O, Zingales B, Mendonça-Previato L, dosReis G A. Down-regulation of T lymphocyte activation in vitro and in vivo induced by glycoinositolphospholipids from Trypanosoma cruzi. Assignment of the T cell-suppressive determinant to the ceramide domain. J Immunol. 1996;156:628–635. [PubMed] [Google Scholar]
- 12.Gray J D, Horwitz D A. Activated human NK cells can stimulate resting B cells to secrete immunoglobulin. J Immunol. 1995;154:5656–5662. [PubMed] [Google Scholar]
- 13.Hatcher F M, Kuhn R E, Cerrone M C, Burton R C. Increased natural killer cell activity in experimental American trypanosomiasis. J Immunol. 1981;127:1126–1130. [PubMed] [Google Scholar]
- 14.Kreig A M, Yi A K, Matson S, Waldschmidt T J, Bishop G A, Teasdale R, Koretsky G A, Klinman D M. CpG motifs in bacterial DNA trigger direct B cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 15.Kurago Z B, Lutz C T, Smith K D, Colonna M. NK cell natural cytotoxicity and IFN-γ production are not always coordinately regulated: engagement of DX9 KIR+ NK cells by HLA-B7 variants and target cells. J Immunol. 1998;160:1573–1580. [PubMed] [Google Scholar]
- 16.Lanier L L. NK cell receptors. Annu Rev Immunol. 1998;16:359–393. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 17.McConville M J, Ferguson M A J. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochemistry. 1993;294:61–80. doi: 10.1042/bj2940305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mond J J, Lees A, Snapper C M. T cell independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 18a.National Institutes of Health. Guide for the care and use of laboratory animals. Department of Health, Education, and Welfare publication 78-23. Bethesda, Md: National Institutes of Health; 1985. [Google Scholar]
- 19.Previato J O, Gorin P A J, Mazurek M, Xavier M T, Fournet B, Wieruszesk J M, Mendonça-Previato L. Primary structure of the oligosaccharide chain of lipopeptidophosphoglycan of epimastigote forms of Trypanosoma cruzi. J Biol Chem. 1990;265:2518–2526. [PubMed] [Google Scholar]
- 20.Previato J O, Mendonça-Previato L, Jones C, Wait R, Fournet B. Structural characterization of a novel class of glycophosphosphingolipids from the protozoan Leptomonas samueli. J Biol Chem. 1992;267:24279–24286. [PubMed] [Google Scholar]
- 21.Proudfoot L, O'Donnell C A, Liew F Y. Glycoinositolphospholipids of Leishmania major inhibit nitric oxide synthesis and reduce leishmanicidal activity in murine macrophages. Eur J Immunol. 1995;25:745–750. doi: 10.1002/eji.1830250318. [DOI] [PubMed] [Google Scholar]
- 22.Rabin E M, Ohara J, Paul W E. B-cell stimulatory factor 1 activates resting B cells. Proc Natl Acad Sci USA. 1985;82:2935–2939. doi: 10.1073/pnas.82.9.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robertson M J, Soiffer R J, Wolf S F, Manley T J, Donohue C, Young D, Herrmann S H, Ritz J. Response of human natural killer (NK) cells to NK cell stimulatory factor (NKSF): cytolytic activity and proliferation of NK cells are differently regulated by NKSF. J Exp Med. 1992;175:779–788. doi: 10.1084/jem.175.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snapper C M, Paul W E. B cell stimulatory factor 1 (interleukin 4) prepares resting murine B cells to secrete IgG1 upon subsequent stimulation with bacterial lipopolysaccharide. J Immunol. 1987;139:10–17. [PubMed] [Google Scholar]
- 25.Snapper C M, Yamaguchi H, Moorman M A, Mond J J. An in vitro model for T cell-independent induction of humoral immunity: a requirement for NK cells. J Immunol. 1994;152:4884–4992. [PubMed] [Google Scholar]
- 26.Snapper C M, Mond J J. A model for induction of T cell independent humoral immunity in response to polysaccharide antigens. J Immunol. 1996;157:2229–2232. [PubMed] [Google Scholar]
- 27.Spinella S, Liegeard P, Hontebeyrie-Joskowicz M. Trypanosoma cruzi: predominance of IgG2a in nonspecific humoral response during experimental Chagas' disease. Exp Parasitol. 1992;74:46–56. doi: 10.1016/0014-4894(92)90138-z. [DOI] [PubMed] [Google Scholar]
- 28.Stall A, Loken M. Allotypic specificities of murine IgD and IgM recognized by monoclonal antibodies. J Immunol. 1984;132:787–795. [PubMed] [Google Scholar]
- 29.Vos Q, Ortaldo J R, Conan-Cibotti M, Vos M D, Young H A, Anderson S K, Witherspoon K, Prager I, Snapper C M, Mond J J. Phenotypic and functional characterization of a panel of cytotoxic murine NK cell clones that are heterogeneous in their enhancement of Ig secretion in vitro. Int Immunol. 1998;10:1093–1101. doi: 10.1093/intimm/10.8.1093. [DOI] [PubMed] [Google Scholar]
- 30.Wang B, Biron C, She J, Higgins K, Sunshine M-J, Lacy E, Lonberg N, Terhorst C. A block in both early T lymphocyte and natural killer cell development in transgenic mice with high copy number of the human CD3ɛ gene. Proc Natl Acad Sci USA. 1994;91:9402–9406. doi: 10.1073/pnas.91.20.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zimmermann S, Egeter O, Hausmann S, Lipford G B, Rockën M, Wagner H, Heeg K. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J Immunol. 1998;160:3627–3630. [PubMed] [Google Scholar]