Abstract
Rationale
This report investigated the role of endocannabinoids in the encoding of task-relevant information by ensembles of hippocampal neurons under conditions in which the CB1 receptor antagonist, rimonabant, was administered during performance of a short-term memory delayed non-match to sample (DNMS) task in rats.
Objective
The influence of endocannabinoids on the encoding of task relevant information was determined via examination of the firing patterns of ensembles of CA1/CA3 hippocampal neurons during individual trials while rats performed a DNMS task.
Materials and methods
Multivariate discriminant analysis of the firing patterns of ensembles of hippocampal neurons was used to extract trial-specific codes for task-relevant information under different types of trial sequences.
Results
It was discovered that rimonabant blocked an inherent hippocampal memory encoding bias used by all animals. This bias was characterized as the preferential encoding of sample information on individual trials based on the similarity (i.e., same or different) and duration of the delay in the preceding trial.
Conclusions
The results indicate that endocannabinoids are a major influence on the strategic encoding biases of hippocampal ensembles and that pharmacological blockade of CB1 receptors facilitated performance by eliminating such influences.
Keywords: Endocannabinoids, Trial sequence, Hippocampus, Neural ensemble codes, CB1 antagonist, Linear discriminant analyses
Introduction
Understanding the neurobiological basis of learning and memory has incurred interest with respect to the role of endogenous cannabinoids (i.e., endocannabinoids) as determined via blockade of cannabinoid receptors or through manipulations of other junctures in the cannabinoid signaling pathway (Safo et al. 2006; Gipson and Yeckel 2007; Zangen et al. 2006; Hashimotodani et al. 2007). The fact that CB1 cannabinoid receptors appear to be strategically poised to regulate transmission at both inhibitory and excitatory synapses in several key brain structures (Wilson and Nicoll 2001; Edwards et al. 2006; Chevaleyre and Castillo 2003; Beierlein and Regehr 2006) suggests that an understanding of the circumstances that provoke verifiable endocannabinoid action can provide insight into how behavioral and cognitive processes are differentially modulated within different contextual frameworks. Recent reports suggest that endocannabinoids may be more influential during the extinction of previously learned behaviors and associations than during acquisition; however, this influence appears be specific to the type of behavioral task employed (Holter et al. 2005; Cannich et al. 2004; Marsicano and Lutz 2006). If it is true that endocannabinoids have selective influences on mechanisms of learning and memory, but only during particular behavioral circumstances, such selectivity may reside in the contextual control of endocannabinoid actions. Demarcation of those particular instances requires the means to isolate and identify when performance varies as a function of endocannabinoid action (Marsicano et al. 2002; Cravatt and Lichtman 2004; Marsicano and Lutz 2006; Laviolette and Grace 2006).
In previous work from this laboratory, the manner in which exogenously administered cannabinoids disrupt performance of a short-term memory task in rats was identified in relation to the action of CB1 receptors in altering the ensemble processing of task-relevant information (Heyser et al. 1993; Hampson and Deadwyler 2000; Hampson et al. 2003a; Deadwyler and Hampson 2004). In a recent report (Deadwyler et al. 2007), we described how performance of a delayed non-match to sample (DNMS) short-term memory task was positively modulated on a trial-to-trial basis by administration of rimonabant (Sanofi Reserche), the well-known CB1 cannabinoid receptor antagonist. In this paper, we describe “how” such a trial-by-trial influence of endocannabinoids alters encoding of task relevant information by hippocampal ensembles during DNMS performance. These new results provide unique insight into the role of endocannabinoids in regulating hippocampal neuronal processes requisite for the encoding of short-term memory and indicate functional endpoints for the recently discovered capacity for CB1 receptors to modify synaptic processes on hippocampal neurons (Wilson and Nicoll 2001; Alger 2002; Katona et al. 2006).
Materials and methods
Subjects
Male Long–Evans rats (n=13) ranging in age from 200 to 250 days were used as subjects. Animals were deprived of water for 15–20 h to facilitate performance of the DNMS task for water reward, but allowed free access to food. Total fluid intake was adjusted daily for maintenance at 85% of normal body weight. All animal care and experimental procedures including water deprivation and surgery conformed to National Institutes of Health (NIH) and Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) regulations.
Apparatus and behavioral training
Complete details of apparatus design and behavioral training in the DNMS task have been reported elsewhere (Deadwyler and Hampson 2004). The apparatus consisted of a behavioral testing chamber with two retractable levers mounted on one wall, positioned to either side of a water trough, and a nosepoke device with cuelight mounted in the center of the opposite wall. The DNMS task was identical to that described previously (Deadwyler et al. 1996) and consisted of three main phases: sample, delay, and non-match. In the sample phase, either the left or right lever was selected at random and extended and when pressed (sample response, SmR), was immediately retracted and the 1–30 s delay phase of the task initiated thereafter. The animal was required to nose poke into a photobeam on the opposite wall until the delay interval timed out. The last nose poke in the delay turned off the nose poke cue light and extended both levers signaling onset of the non-match phase of the task. A response on the lever opposite the SmR, constituting the “nonmatch response” (NR), was rewarded with a drop of water. A response on the same lever as the SmR caused the houselights to be turned off for 5 s, with both levers retracted signaling an error with no reward. A 10-s intertrial interval was employed. “Probe trials” with delays longer than 30 s (40–60 s) were presented randomly to test for improved performance in drug sessions.
Surgery
All surgical procedures conformed to NIH and AAALAC guidelines and were performed in a rodent surgical facility approved by the Wake Forest University IACUC. Animals were anesthetized with ketamine (100 mg/kg, I.P.) and xylazine (10 mg/kg, I.P.). Multineuron recording arrays, each consisting of sixteen 25-μm wire electrodes (Neurolinc, New York, NY, USA) were implanted in the CA1 and CA3 subfields bilaterally at coordinates: 3.8 mm posterior to bregma, 3.0 mm left or right of midline following the longitudinal axis of the hippocampus (Hampson et al. 1999). Single neuron firing was monitored from the array during surgery to ensure placement in appropriate hippocampal cell layers. After positioning of the array, the cranium was sealed with bone wax and dental cement and the animal allowed to recover with appropriate infection preventive treatment for 1 week before resumption of behavioral testing.
Drug preparation and administration
Rimonabant (SR141716A, Sanofi Reserche, provided by Research Triangle Institute, Cary, NC, USA) was prepared daily from a 20 mg/ml stock in ethanol. Rimonabant stock (0.5 ml) was added to 2.0 ml of Pluronic F68 detergent (Sigma) in ethanol solution (20 mg/ml) in which 2.0 ml of saline (0.9%) was slowly added. The solution was stirred rapidly and placed under a steady stream of nitrogen gas to evaporate the ethanol (approximately 10 min) resulting in a detergent/drug suspension of rimonabant (5.0 mg/ml) which was then sonicated and diluted with 3.0 ml of saline to a final injection concentration of 2.0 mg/ml. Vehicle solutions were prepared in a similar manner without rimonabant. Animals were injected I.P. with the rimonabant/pluronic solution (1.0 ml/kg) or vehicle approximately 10 min before the start of the behavioral session. One day of vehicle testing was imposed between each drug testing session.
Analyses of behavioral data
The primary measures of DNMS performance were mean percent of correct trials during the session and mean percent of correct trials at each delay interval (assessed in 5.0 s increments). Multifactor analyses of variance (ANOVA) were employed, and main effects were examined further via adjusted pairwise linear contrasts for individual comparisons of drug effects at specific delays.
Multineuron recording technique
Extracellular action potentials and behavioral events within each DNMS trial were digitized and time-stamped for computer processing (Deadwyler and Hampson 2004). Single neurons were isolated and selected for analysis from each of the 32 (16 per hemisphere) different hippocampal recording electrodes. Action potential waveforms were digitized at 40 kHz and isolated in real-time by derivation of individual waveform characteristics via a Plexon multineuron acquisition processor (MAP, Plexon, Dallas, TX, USA). A limit of two separately identified neurons recorded from the same electrode location was employed for analyses of ensemble activity to limit spatial bias of sampled activity (Hampson et al. 1999).
Hippocampal ensembles and extraction of neural codes
Analysis of hippocampal ensemble firing has been described in detail in several reports (Hampson et al. 2005;Simeral et al. 2005; Deadwyler et al. 2007). Recordings of 15–25 isolated CA1 and CA3 neurons in each animal were obtained over at least five successive 100–150 trial sessions. Behavioral correlates were determined for each neuron via perievent histograms computed ±1.5 s before and after the occurrence of the SmR and NR in the respective phases of the DNMS task. A canonical discriminant analysis (CDA) utilizing multivariate procedures (Stevens 2002) assessed ensemble neural firing from the SmR and NR perievent histograms in terms of multiple sources of variance or discriminant functions (DFs) from a time × neuron matrix of ensemble firing rates in each animal. DFs were computed by canonical correlation and eigenvector decomposition (Rao 2002) of ensemble firing rate matrices compiled from data collected over at least five consecutive daily DNMS sessions (≥500 total trials). Each extracted significant source of variance (DF) represented a proportion of the total variance associated with the SmR or NR. Details of this type of analysis have been presented elsewhere (Simeral et al. 2005). Single trial DF scores for NRs and SmRs were computed by multiplying the single trial ensemble firing rate matrices by the respective eigenvector DF coefficients and summing the resultant products. The source of variance associated with the NR (DF4) showed significant representation of lever position [F(1,7437)=11.83, p<0.001], revealed by differential mean (±SEM) scores of 1.88±0.10 for left NRs and −1.62±0.08 for right NRs. Although DF4 consistently discriminated NR position (left or right) on a given trial in all animals, it did not significantly indicate trial outcome (i.e., correct vs error trials) as described previously (Hampson et al. 2001, 2005). SmRs on each trial were associated with a DF5 scores that (1) reflected lever position by magnitude and sign and (2) trial outcome as analyzed across sessions by multi-factor ANOVA, thus, validating the fact that DF5 scores not only discriminated left from right SmRs but also the degree to which that representation was successful on a given trial (Deadwyler et al. 2007).
Results
Rimonabant improves neural encoding of DNMS task-relevant information
Figure 1a illustrates the relationship between SmR ensemble firing indicative of both strong (correct) and weak (error) codes (DF5 scores) related to DNMS performance on single trials (Deadwyler and Hampson 2004). Figure 1b shows DNMS performance plotted as a function of duration of delay interval for vehicle and rimonabant sessions across all trials including “probes” with delays that extended to 80 s. Trials with weak SmR code strengths in vehicle sessions were plotted separately (weak codes) to indicate the significant decrease in performance on trials with delays >10 s. In contrast, in the same vehicle sessions on trials with medium and strong SmR codes, performance was significantly higher and decreased in a delay-dependent manner between 20 and 40 s. In rimonabant sessions, DNMS performance showed a similar delay dependence (Fig. 1b), but was superior to vehicle sessions on trials with delays 20–60 s [pairwise linear contrasts F(1,2514)>13.69, p<0.001]. Figure 1c shows a comparison of the frequency distributions of DF5 scores, indicating SmR code strengths, for vehicle vs rimonabant sessions on individual probe trials (delays 40–80 s) summed across all animals. As shown previously (Deadwyler et al. 2007), the distribution of DF5 scores for rimonabant was markedly shifted toward higher values (Wilcoxon rank test, Z=13.15, p<0.001), indicating more trials with increased SmR code strength (DF5 scores, 2.0–3.0) and fewer with low SmR code strength (DF5 scores, 0–1.0).
Fig. 1.
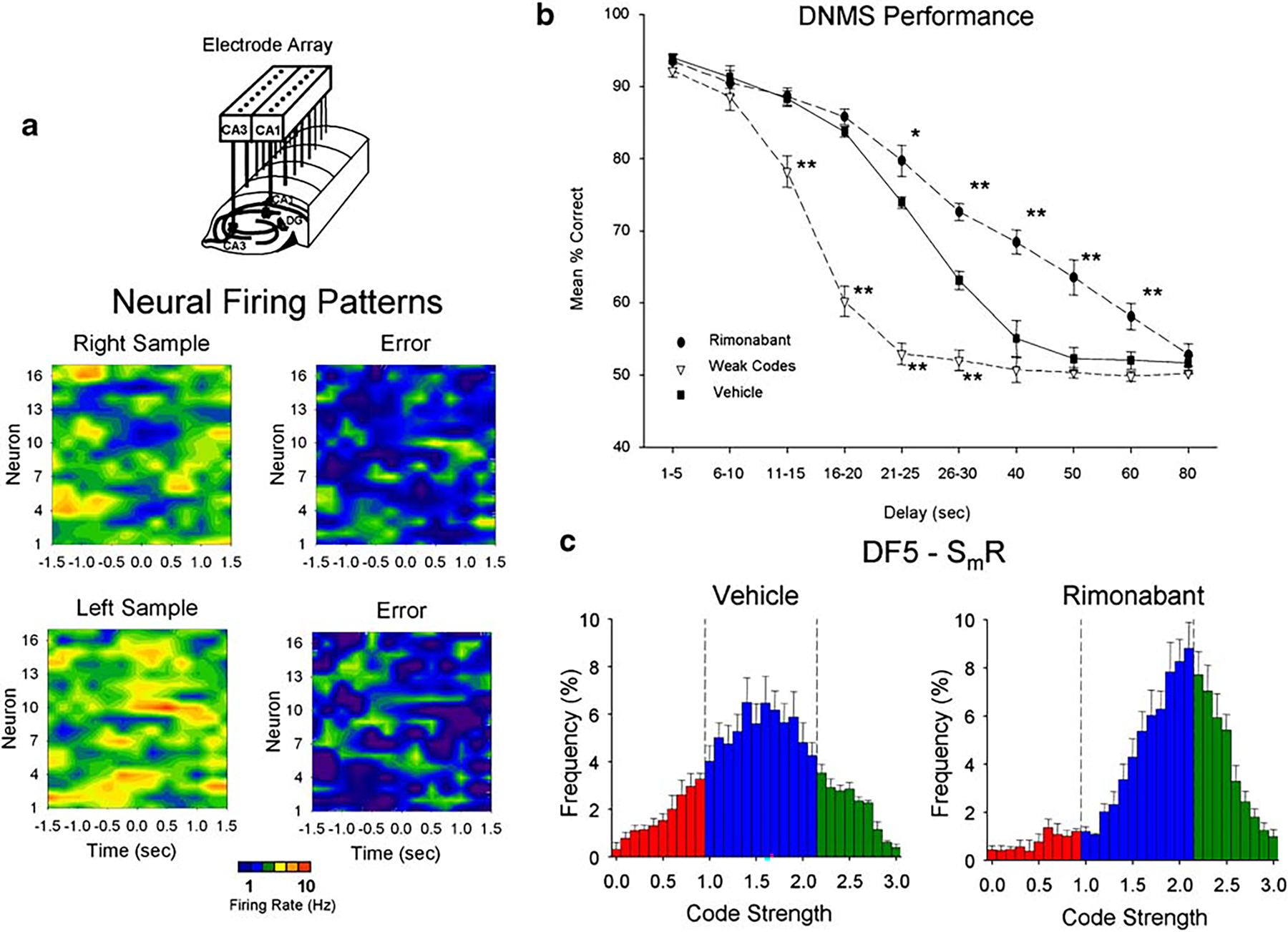
Hippocampal neural activity and performance of the delayed-non-match-to-sample (DNMS) task. a Ensemble firing patterns on single DNMS trials recorded from electrode arrays (illustrated at top) were analyzed by multivariate CDA. The discriminant function (DF5) that contributed to the sample response (SmR) yielded differential firing for correct and error trials plotted as perievent histograms (±1.5 s), represented here as color contours of firing rates across all neurons recorded in a single animal. Each of the four contour plots represents the firing pattern of the same ensemble on a different individual DNMS trial. Patterns in the left column illustrate “strong” encoding of right and left SmRs respectively, while patterns in the right column reflect “weak” encoding of SmRs resulting in behavioral errors. Individual neurons are listed (1–16) on vertical axis and time on the horizontal axis with “0.0 s” demarcating the occurrence of the SmR. The color scale depicts firing rate for contour plots: blue ≤1 Hz to red ≥10 Hz. b DNMS behavioral performance curves depicting mean (±SEM) percent correct responses on trials with delays of 1–80 s. Animals were trained with delays of 1–30 s and probe trials with delays of 40, 50, 60, or 80 s interspersed randomly to test strength of encoding. Mean correct performance in vehicle sessions (solid line), rimonabant sessions (dashed line and filled circles). Plot of weak code (DF5s < 0.90: performance) trials in vehicle sessions (dashed line and unfilled circles). *F(1,2514)>7.39, p<0.01; **F(1,2514)>13.69, p<0.001. c Frequency distribution of SmR code strengths (DF5 scores) for 40–80 s trials shown in B computed for all animals (n=13) across five to ten sessions ≥ 100 trials. Mean (±SEM) frequency (% of total trials) of occurrence of each of code strength (DF5 score bin=0.1, range 0–3.0) classified as weak (DF5=0–1.0, red), normal (1.0–2.0, blue), and strong (2.0–3.0, green) for vehicle (left) and rimonabant (right) sessions
Effects of trial sequence on SmR encoding
The sequence of DNMS behavioral trials has been shown previously to affect both behavioral performance and the relative firing rates of hippocampal and subicular neuron ensembles (Deadwyler and Hampson 2004). Therefore, we examined trial-to-trial influences on SmR code strength to determine the processes underlying generation of weak vs strong SmR codes for the same types of trials. Figure 2a reveals the effect of trial sequence on the strength of SmR encoding in which mean (±SEM) DF5 scores are shown across a sequence of left (L) and right (R) trials with short and long delays. Negative and positive score values correspond to right vs left current trial mean (±SEM) DF5 scores, respectively. The scores are plotted according to the classification scheme shown on the x-axis for prior trial occurrence as: (1) the duration of the delay on the prior trial and (2) whether the prior trial was the same or different from the current trial. This sequential dependency is also summarized in Fig. 2b, which shows the overall mean (±SEM) absolute values of DF5 scores for each of the categories (A–D) in Fig. 2a. It is clear that a strong bias existed for trials that were different from the prior trial especially if both the prior and current trials had long (>15 s) delays (category A, Fig. 2b). The analysis (ANOVA) of code strengths over all possible trial sequences [F(7,1474)=17.22, p<0.001] revealed significant main effects of prior trial type [F(1,1474)=11.55, p<0.001] and prior trial delay [F(1,1474)=7.09, p<0.01] but no significant interaction. Thus, although DF5 reflected the current trial SmR code appropriately for each lever position (positive DF5 scores-left or negative scores-right), the “strength” of DF5 scores on those trials was influenced significantly by prior trial factors as illustrated by the diagram in Fig. 2c.
Fig. 2.
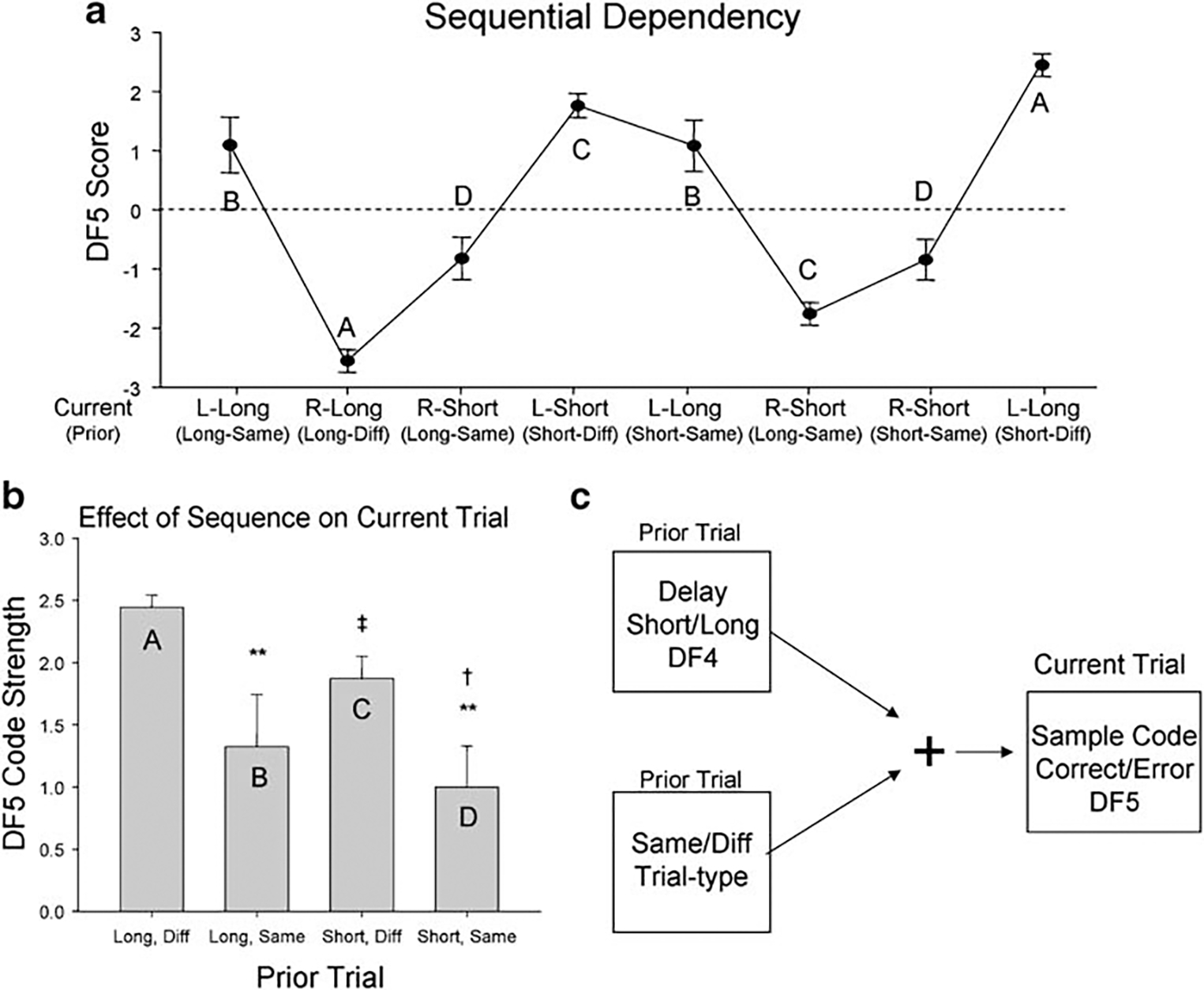
Influence of trial sequence on SmR code strength (DF5) during vehicle sessions. a Sequence of left (L) or right (R) trial types comprised of either long (>15 s) or short (<15 s) delay durations constructed to show effects of prior trial on current trial SmR code strength. Prior trial type and delay are listed below (in parentheses) the current trial type. Filled circles depict mean (±SEM) DF5 discriminant scores (n≥50 trials for each point) from current trials with the indicated prior trial sequence. Letters correspond to SmR code strengths graphed in B. b Mean (±SEM) DF5 code strength (irrespective of sign) on current trial sorted by delay (long/short) and similarity (same vs different) of prior trial corresponding to the same letter symbols in a. *F(1,1474)>6.89, p<0.01, **F(1,1474)>12.16, p<0.001 comparison of prior trial same vs different; †F(1,1474)>7.08, p<0.01, ‡F(1,1474)>11.91, p<0.001 comparison of prior trial long vs short. c Schematic indicating strength of current trial SmR code (DF5) in vehicle sessions as a function of similarity of prior and current trial (same/different) and duration of prior trial delay as encoded by strength of the prior trial NR (DF4)
The basis for the above trial sequence influence on current trial SmR code strength was examined further using another measure of ensemble encoding in the DNMS task, the code strength of the non-match response (NR), indicated by scores for ensemble variance characterized by DF4. Figure 3 displays single trial results from all animals in both vehicle and rimonabant sessions as correlations between: (a) the strength of NR code (DF4 score) and duration of delay on the current trial, (b) strength of NR on the prior trial and SmR strength (DF5) on the current trial, and (c) current trial NRs and SmRs. Figure 3 indicates three important changes in the relationship of DF4 to the above factors on individual trials in vehicle vs rimonabant sessions: (1) elimination in rimonabant sessions (Fig. 3a, right) of the significant correlation in vehicle sessions between strength of NR and the duration of the delay interval on the current trial [r2=0.41, regression F(1,398)=13.61, p<0.001]; (2) a shift from the lack of a significant correlation between the SmR and NR in vehicle sessions to a significant correlation [r2=0.42, F(1,287)=9.53, p<0.01] in rimonabant sessions (Fig. 3b); and most importantly, (3) elimination in rimonabant sessions of the marked correlation (Fig. 3c) between NR code strength on the prior trial and SmR code strength on the current trial [r2=0.52, F(1,344)=17.48, p<0.001] in vehicle sessions. Figure 3d shows the frequency distributions of DF4 scores for vehicle and rimonabant sessions in which the shift toward higher NR code strengths (DF4 scores) in rimonabant sessions (Wilcoxon rank test, Z=25.17, p<0.001) was similar to that shown in Fig. 1c for SmR.
Fig. 3.
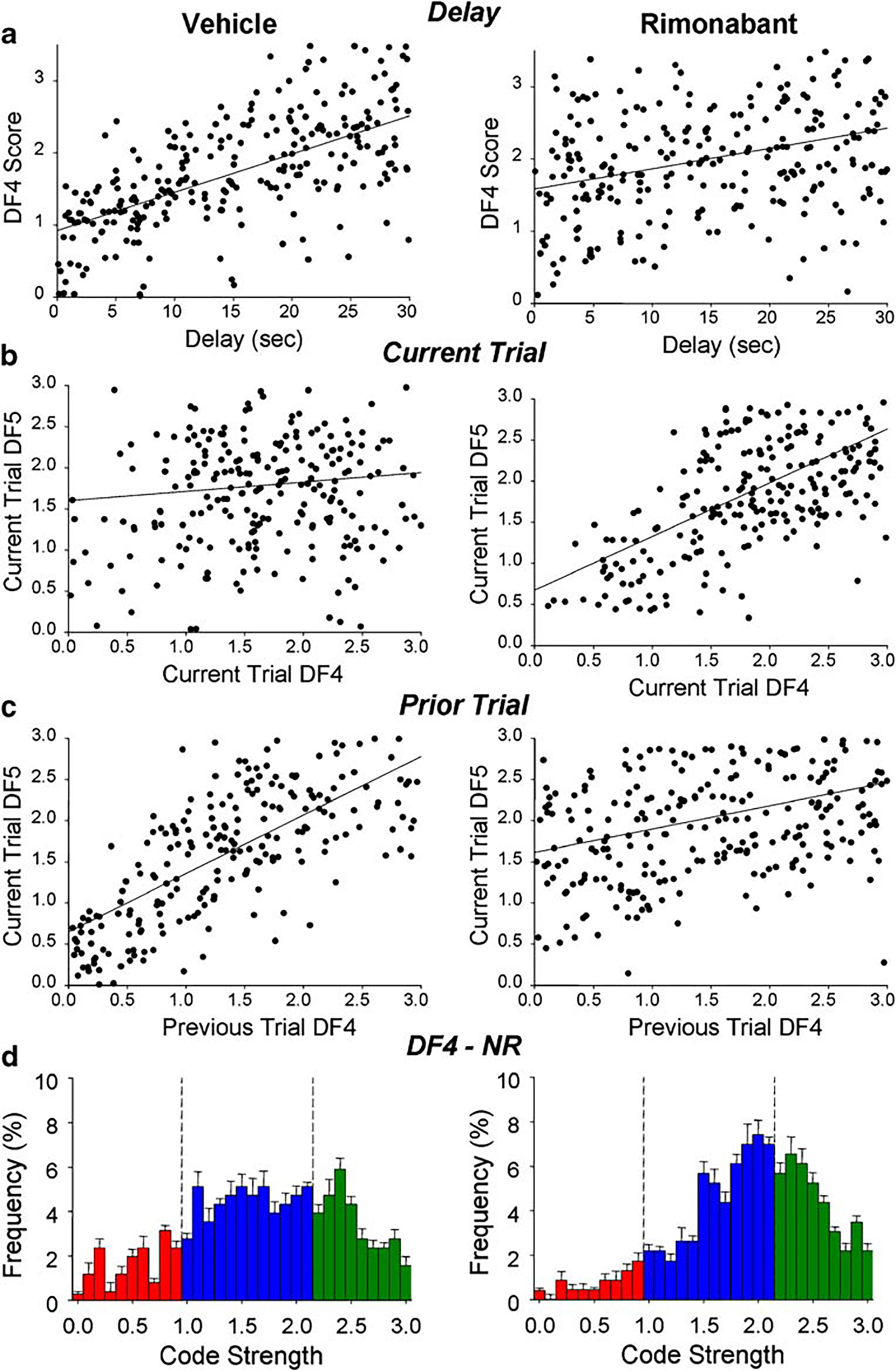
Change in correlations of single trial SmR (DF5) and NR (DF4) scores both within and between trials for vehicle and rimonabant sessions. a Scatter plots of NR (DF4) scores at different durations of delay (each dot) show that significant correlation between both factors on the same (current) trial in vehicle sessions was decreased for in rimonabant sessions (vehicle: slope=0.53, r2=0.41, p<0.001; rimonabant: slope=0.28, r2=0.11, N.S.). b Scatter plot of SmR (DF5) vs NR (DF4) scores on the same (current) trial. Correlation was not significant in vehicle sessions, but became significant for rimonabant sessions (slope=0.65, r2=0.42, p<0.01). c Scatter plot of current trial SmR (DF5) vs prior trial NR (DF4) scores shows that DF5 scores were highly correlated with prior trial DF4 scores for vehicle sessions (slope=0.71, r2=0.52, p<0.001) and that the correlation was decreased significantly in rimonabant sessions (slope=0.28, r2=0.27, p<0.01). In the scatter plots, each dot indicates single trial scores for the indicated conditions and each graph display = 500 dots. d Frequency distribution of NR (DF4) code strengths for vehicle (left) and rimonabant (right) sessions. Colored bars indicate classification of code strength based on same scale as DF5 codes shown in Fig. 1c
Figure 4 shows the consequences of eliminating the above biasing influence of the prior trial NR (Figs. 2 and 3) on SmR encoding on the current trial during sessions in which rimonabant was administered. The same trial sequence plot of DF5 scores shown for vehicle sessions in Fig. 2a is shown in Fig. 4a for rimonabant sessions. It is clear that the mean overall left and right trial scores were significantly increased when compared with vehicle session scores shown in Fig. 2a [left trials: F(1,1474)=14.22, p<0.001; right trials: F(1,1474)=16.43, p<0.001]. More specifically, the major change is the lack of a reduced SmR encoding when the current trial remains the same as the previous trial. Figure 4b shows that DF5 scores were not significantly different for any combination of prior trial irrespective of delay or trial type and that SmR code strength was symmetric and only reflected the parameters of the current trial. Moreover, the prior trial influence of NR was transferred to the current trial (Fig. 3b current trial rimonabant sessions). Thus, the influence of the prior trial on SmR code strength was no longer present when animals were tested after injection of rimonabant, which provided a basis for the marked improvement in overall performance in rimonabant sessions with longer delays (Fig. 1c).
Fig. 4.
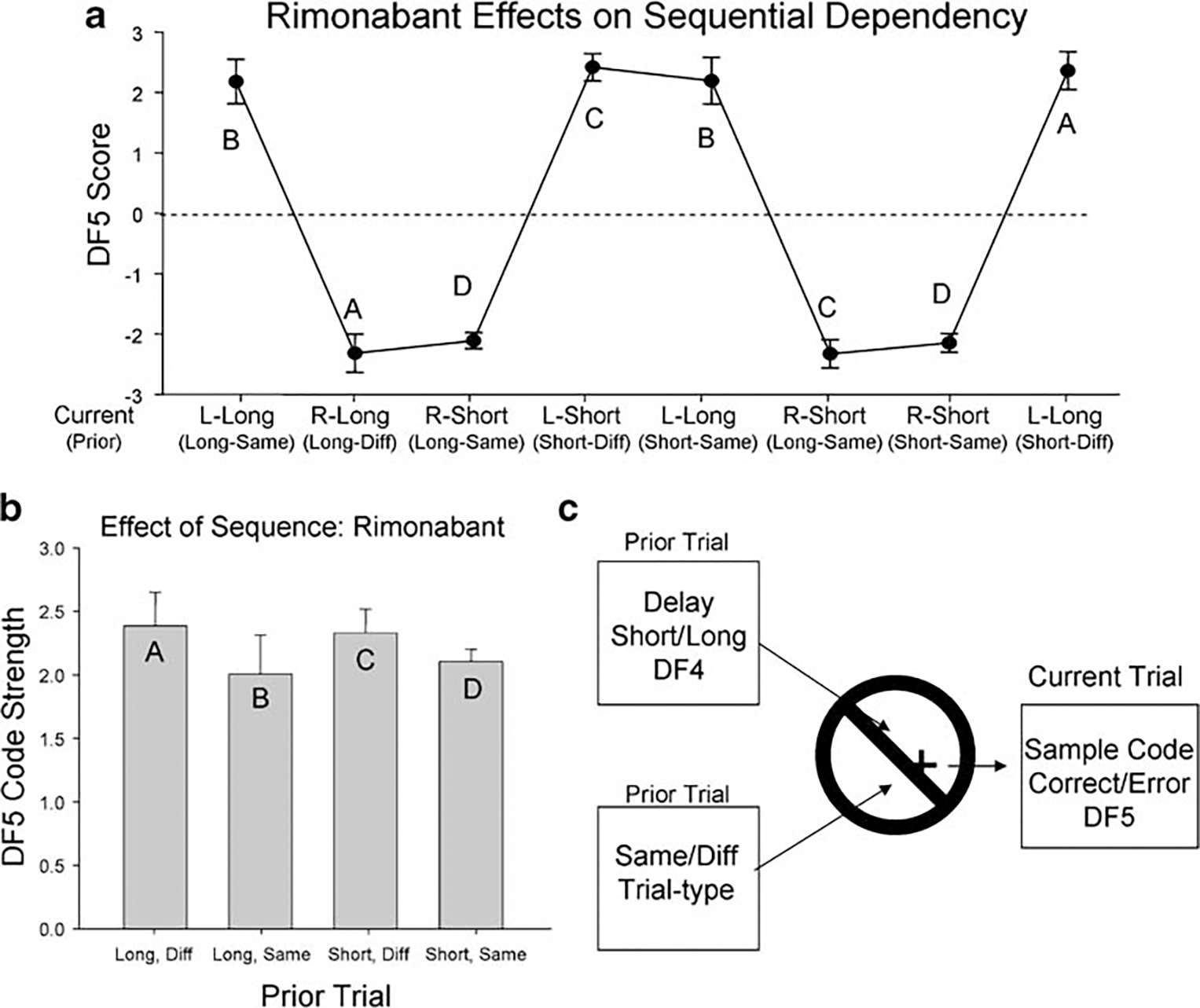
Rimonabant alters the influence of trial sequence on SmR (DF5) code strength shown in Fig. 2. a Mean (±SEM) DF5 scores for rimonabant sessions plotted for the same trial sequence shown in Fig. 2a. b Absolute values of current trial DF5 scores (mean±SEM) sorted by prior trial type and delay in rimonabant sessions did not differ significantly as a function of prior trial sequence in rimonabant sessions as shown in Fig. 2b for vehicle sessions. c Schematic of trial sequence dependence shows that current trial SmR (DF5) code strength was not influenced by either prior trial type nor prior trial delay (DF4), corroborating the changes in correlation between DF4 and DF5 shown in Fig. 3b and c for rimonabant sessions
Rimonabant eliminates between trial influences on hippocampal ensemble firing
Figure 5 illustrates how rimonabant changed the influence of trial sequence on weak vs strong SmR codes in terms of hippocampal neuron ensemble firing patterns. The color contour plots in Fig. 5 depict typical robust right and left trial ensemble firing patterns corresponding to strong SmR codes as shown in Fig. 1a. Immediately below the two strong code patterns are corresponding right and left trial SmR firing patterns obtained in vehicle sessions on trials preceded by either: (1) long delay duration trial of a different type (A) or a short delay trial of the same type (B). Note that the right and left trial firing patterns for the long/different trial sequences (vehicle A) are distinct and robust strong SmR codes, while the patterns for the short/same trial sequence (vehicle B) are clearly reduced with respect to firing intensity and specificity across neurons, characteristic of weak SmR codes (Figs. 1 and 2). After administration of rimonabant, SmR ensemble firing patterns were shifted to more intense neuronal firing patterns, indicating stronger SmR encoding for the same conditions (C and D) and were no longer subject to the prior trial influence that decreased ensemble firing in vehicle sessions (Fig. 5; rimonabant). It is clear that the same trial sequences that resulted in reduced firing in vehicle sessions (A and B) did not influence ensemble firing for the same sequences during rimonabant sessions (C and D).
Fig. 5.
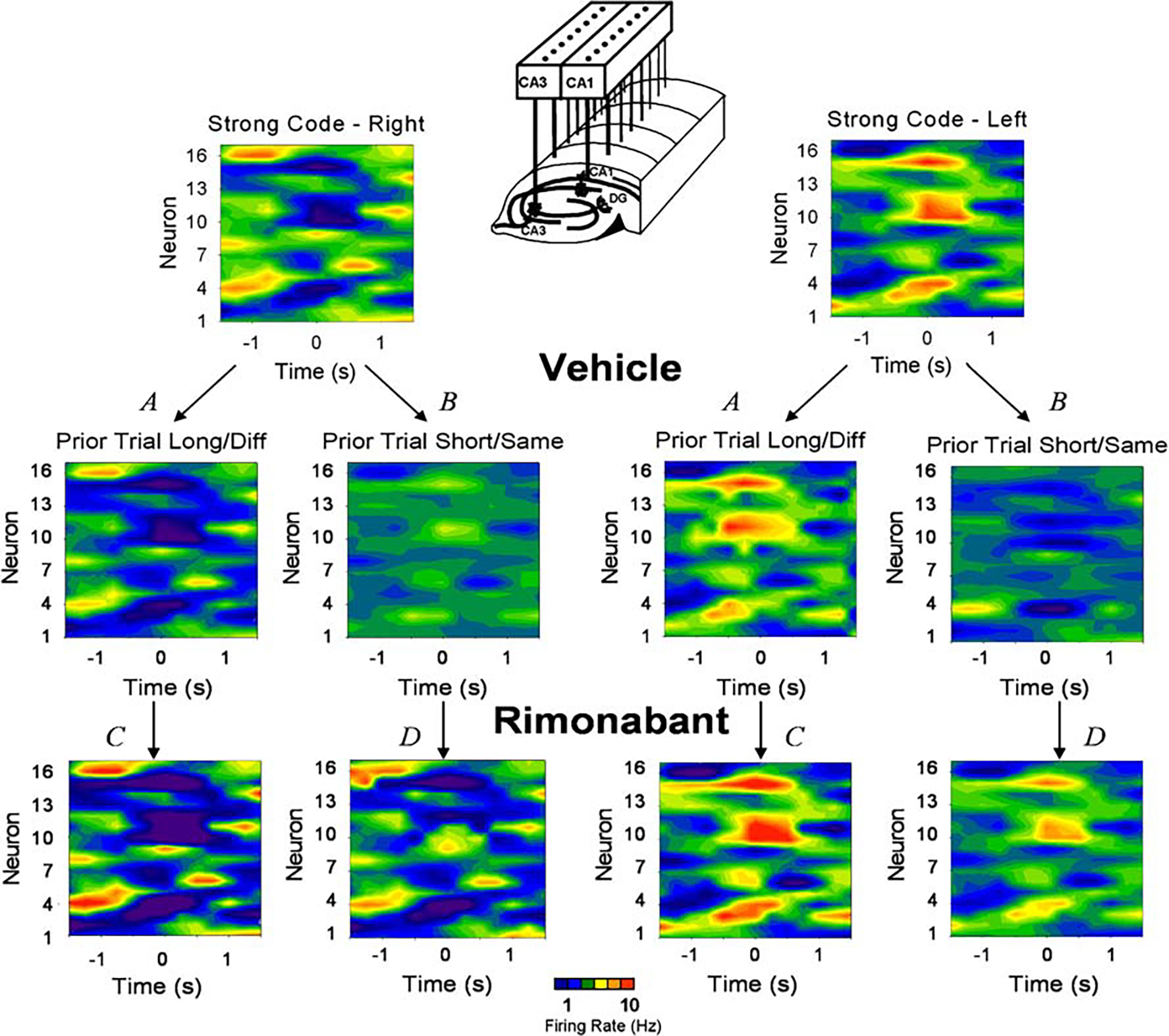
Rimonabant eliminates prior trial effects on hippocampal ensemble firing and associated SmR code strength. Color contour firing maps (perievent histograms) of ensemble firing patterns for a single animal similar to those shown in Fig. 1a. The influence of prior trial type (same vs diff) and prior trial delay (short vs long) on ensemble firing in vehicle sessions (vehicle A and B) was eliminated in rimonabant sessions (rimonabant C and D). On the vertical axis of the contour maps are listed the individual neurons in the ensemble; the horizontal axis shows time (±1.5 s) relative to occurrence of the SmR (0.0 s). Firing rate is shown in 250 ms bins and reflected by the color scale: blue ≤1 Hz to red ≥10 Hz
Discussion
The above results provide important new evidence that endocannabinoids are involved in the trial-to-trial encoding of task-relevant information in rats trained to perform a DNMS short-term memory task. These findings demonstrate that when the actions of spontaneously released endocannabinoids were blocked by rimonabant, the carryover influences from the prior trial no longer impacted the encoding strength of the SmR on the current trial (Figs. 2, 3 4, 5). Under normal conditions, this interaction was associated with a marked increase in the tendency to make errors (Fig. 2b) primarily because encoding of the SmR on the current trial was biased by an inherent tendency to encode the next trial as opposite (i.e., different) from the prior trial especially if the duration of the delay on the prior trial was long (Figs. 2a,b and 5). Figure 3c shows that this biasing tendency in vehicle sessions was associated directly with the strength of code for the NR (DF4) on the prior trial which was highly correlated with SmR code strength on the current trial. This linkage was the primary factor that determined performance when the trial sequence conformed to either similar long or short trials in vehicle sessions, and the actions of endocannabinoids were not blocked by rimonabant (Figs. 2a,b).
In a recent report, it was demonstrated that rimonabant significantly improved DNMS performance by increasing SmR code strength on the current trial relative to vehicle sessions (Deadwyler et al. 2007). However, in that report, the basis as to how rimonabant achieved this change in ensemble firing was not determined. The current results show that the increase in SmR code strength produced by rimonabant was a consequence of (1) increasing the correlation of code strengths between the NR (DF4) and SmR (DF5) on the same trial (Fig. 3 current trial), and perhaps more importantly, (2) elimination of the influence of the NR from the prior trial (Fig. 3, prior trial). This is also consistent with the previous report (Deadwyler et al. 2007) showing that the actions of endocannabinoids appeared to impair performance by increasing the number of weak SmR codes within the session (Fig. 1b,c), an effect mimicked by administration of exogenous cannabinoids (Hampson and Deadwyler 2000; Deadwyler et al. 2007).
The basis for rimonabant’s improvement of performance more than likely reflects elimination of the above explained trial sequence influence or bias on encoding of the SmR on the current trial (Figs. 2 and 4). The relationship of SmR code strength to DNMS trial delay reflects the firing pattern of what have been previously classified as “trial-type” cells in the hippocampal ensemble that fire only during a specific type of DNMS trial to satisfy the respective non-match contingencies (Deadwyler and Hampson 2004; Hampson et al. 2005; Simeral et al. 2005). Trial-type cell firing has also been shown to be influenced by trial sequence as indicated in Fig. 5. Previous reports showed that if the trial sequence did not conform to this biased or “predetermined” firing pattern of the hippocampal ensemble (i.e., “long-diff” in Fig. 2), performance was “at risk” for error (Deadwyler and Hampson 2004). The fact that rimonabant dissociated this relationship between the current and prior trial influence suggests that endocannabinoids may be responsible, at least in part, for this trial-dependent firing bias and would explain further why exogenous cannabinoids produce a similar delay-dependent deficit in performance (Heyser et al. 1993; Hampson and Deadwyler 2000; Hampson et al. 2003a).
The strategic location of CB1 cannabinoid receptors on presynaptic terminals of both excitatory and inhibitory neurons in hippocampus (Freund et al. 2003; Katona et al. 2006) provides a basis for the endocannabinoid modulation of task-specific firing of hippocampal ensembles shown here. The retrograde manner in which cannabinoids have been shown to alter presynaptic processes (Wilson and Nicoll 2001) and the relatively long duration of those synaptic changes demonstrated in vitro (Alger 2002), have not, to date, been identified in behaving animals. However, the trial-to-trial nature of this action as demonstrated here, strongly supports an interpretation in which patterned ensemble discharge on particular trial sequences provokes endocannabinoid synaptic modification of firing in key hippocampal neurons (i.e., trial-type cells) on subsequent trials (Diana and Marty 2004; Alger 2002; Wilson and Nicoll 2001; Hampson et al. 2003b; Zhuang et al. 2005).
It is possible that some of the effects of rimonabant stem from its well-described inverse agonist properties (Vasquez and Lewis 1999; Meschler et al. 2000; Vasquez et al. 2003; Pertwee 2005). However, such a scenario is inconsistent with prior investigations showing that exogenous cannabinoids produce effects opposite to rimonabant on DNMS performance and hippocampal ensemble encoding (Heyser et al. 1993; Deadwyler et al. 2007) and that rimonabant effectively blocks the impairments produced by exogenous cannabinoids (Hampson et al. 2003a). The trial-specific nature of the influence of rimonabant shown here is supported by recent investigations in which endocannabinoids increased the rate of extinction of previously trained behaviors without altering other behavioral parameters (Holter et al. 2005; Marsicano et al. 2002). Moreover, as demonstrated previously, the shift in DF5 code strength was opposite that produced by exogenous cannabinoids (Hampson and Deadwyler 2000; Hampson et al. 2003a) and is consistent with the effects of pharmacological blockade of endocannabinoid action shown in other behavioral tests (Kreitzer and Regehr 2002; Cravatt and Lichtman 2004; Marsicano and Lutz 2006). Such findings suggest that the endocannabinoid system may have a specific role in behavioral modification and training which may only be expressed in certain phases or within contextually defined task events (Varvel et al. 2005). In conclusion, the results shown here and elsewhere clearly indicate that the role of endogenous cannabinoids in hippocampal information processing is complex and can be considered as a part of a routine influence in establishing strategies to resolve complex task demands such as those imposed by the DNMS paradigm. The presence in hippocampus of such endocannabinoid-sensitive encoding strategies suggests that repetition of similar trial events within a session may provoke encoding mechanisms based on past reinforcement history rather than the actual events present on a given trial. Blockade of endocannabinoid action by rimonabant provided a basis for trial-specific encoding unhindered by such prior trial influences.
Acknowledgments
The authors wish to thank the following for assistance with the project: David B. King, Wilson Davis, Nicholas Jones (University of Bath, UK), Lin Lin Ginzberg (University of Bath), Anushka V. Goonawardena, D. Lynn Johnson, Jason Locke, Joanne Konstantopoulos.
This work was supported by NIH grants MH613972 and DA08549 to R.E.H., and DA00119, DA07525 to S.A.D.
References
- Alger BE (2002) Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol 68:247–286 [DOI] [PubMed] [Google Scholar]
- Beierlein M, Regehr WG (2006) Local interneurons regulate synaptic strength by retrograde release of endocannabinoids. J. Neurosci 26:9935–9943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannich A, Wotjak CT, Kamprath K, Hermann H, Lutz B, Marsicano G (2004) CB1 cannabinoid receptors modulate kinase and phosphatase activity during extinction of conditioned fear in mice. Learn Mem 11:625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE (2003) Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron 38:461–472 [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Lichtman AH (2004) The endogenous cannabinoid system and its role in nociceptive behavior. J Neurobiol 61:149–160 [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Bunn T, Hampson RE (1996) Hippocampal ensemble activity during spatial delayed-nonmatch-to-sample performance in rats. J Neurosci 16:354–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deadwyler SA, Hampson RE (2004) Differential but complementary mnemonic functions of the hippocampus and subiculum. Neuron 42:465–476 [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Goonawardena AV, Hampson RE (2007) Short-term memory is modulated by the spontaneous release of endocannabinoids: evidence from hippocampal population codes. Behav Pharm 18:571–580 [DOI] [PubMed] [Google Scholar]
- Diana MA, Marty A (2004) Endocannabinoid-mediated short-term synaptic plasticity: depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE). Br J Pharmacol 142:9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DA, Kim J, Alger BE (2006) Multiple mechanisms of endocannabinoid response inhibition in hippocampus. J Neurophysiol 95:67–75 [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D (2003) Role of endogenous cannabinoids in synaptic signaling. Physiol Rev 83:1017–1066 [DOI] [PubMed] [Google Scholar]
- Gipson KE, Yeckel MF (2007) Coincident glutamatergic and cholinergic inputs transiently depress glutamate release at rat Schaffer collateral synapses. J Neurophysiol 97:4108–4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA (2000) Cannabinoids reveal the necessity of hippocampal neural encoding for short-term memory in rats. J Neurosci 20:8932–8942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson RE, Simeral JD, Deadwyler SA (1999) Distribution of spatial and nonspatial information in dorsal hippocampus. Nature 402:610–614 [DOI] [PubMed] [Google Scholar]
- Hampson RE, Simeral JD, Deadwyler SA (2001) What ensemble recordings reveal about functional hippocampal cell encoding. Prog Brain Res 130:345–357 [DOI] [PubMed] [Google Scholar]
- Hampson RE, Simeral JD, Kelly EJ, Deadwyler SA (2003a) Tolerance to the memory disruptive effects of cannabinoids involves adaptation by hippocampal neurons. Hippocampus 13:543–556 [DOI] [PubMed] [Google Scholar]
- Hampson RE, Zhuang S-Y, Weiner JL, Deadwyler SA (2003b) Functional significance of cannabinoid-mediated, depolarization-induced suppression of inhibition (DSI) in the hippocampus. J Neurophysiol 90:55–64 [DOI] [PubMed] [Google Scholar]
- Hampson RE, Simeral J, Deadwyler SA (2005) Cognitive processes in replacement brain parts: a code for all reasons. In: Berger TW, Glanzman DL (eds) Toward replacement parts for the brain. MIT Press, Cambridge, pp 111–128 [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M (2007) Presynaptic monoacylglycerol lipase activity determines basal endocannabinoid tone and terminates retrograde endocannabinoid signaling in the hippocampus. J Neurosci 27:1211–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser CJ, Hampson RE, Deadwyler SA (1993) The effects of delta-9-THC on delayed match to sample performance in rats: alterations in short-term memory produced by changes in task specific firing of hippocampal neurons. J Pharmacol Exp Ther 264:294–307 [PubMed] [Google Scholar]
- Holter SM, Kallnik M, Wurst W, Marsicano G, Lutz B, Wotjak CT (2005) Cannabinoid CB1 receptor is dispensable for memory extinction in an appetitively-motivated learning task. Eur J Pharmacol 510:69–74 [DOI] [PubMed] [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung K, Piomelli D, Mackie K, Freund TF (2006) Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci 26:5628–5637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG (2002) Retrograde signaling by endocannabinoids. Curr Opin Neurobiol 12:324–330 [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA (2006) Cannabinoids potentiate emotional learning plasticity in neurons of the medial prefrontal cortex through basolateral amygdala inputs. J Neurosci 26:6458–6468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Lutz B (2006) Neuromodulatory functions of the endocannabinoid system. J Endocrinol Invest 29:27–46 [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di MV, Lutz B (2002) The endogenous cannabinoid system controls extinction of aversive memories. Nature 418:530–534 [DOI] [PubMed] [Google Scholar]
- Meschler JP, Kraichely DM, Wilken GH, Howlett AC (2000) Inverse agonist properties of N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2, 4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide HCl (SR141716A) and 1-(2-chlorophenyl)-4-cyano-5-(4-methoxyphenyl)-1H-pyrazole-3-carboxyl ic acid phenylamide (CP-272871) for the CB(1) cannabinoid receptor. Biochem Pharmacol 60:1315–1323 [DOI] [PubMed] [Google Scholar]
- Pertwee RG (2005) Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci 76:1307–1324 [DOI] [PubMed] [Google Scholar]
- Rao CR (2002) Linear statistical inference and its applications. Wiley, New York, NY [Google Scholar]
- Safo PK, Cravatt BF, Regehr WG (2006) Retrograde endocannabinoid signaling in the cerebellar cortex. Cerebellum 5:134–145 [DOI] [PubMed] [Google Scholar]
- Simeral JD, Hampson RE, Deadwyler SA (2005) Behaviorally relevant neural codes in hippocampal ensembles: Detection on single trials. In: Baudry M, Bi X, Schreiber S (eds) Synaptic plasticity: from basic mechanisms to clinical applications. MIT Press, Cambridge, MA, pp 459–476 [Google Scholar]
- Stevens J (2002) Applied multivariate statistics for the social sciences. Erlbaum, Hillsdale [Google Scholar]
- Varvel SA, Anum EA, Lichtman AH (2005) Disruption of CB(1) receptor signaling impairs extinction of spatial memory in mice. Psychopharmacology (Berl) 179:863–872 [DOI] [PubMed] [Google Scholar]
- Vasquez C, Lewis DL (1999) The CB1 cannabinoid receptor can sequester G-proteins, making them unavailable to couple to other receptors. J Neurosci 19(21):9271–9280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez C, Navarro-Polanco RA, Huerta M, Trujillo X, Andrade F, Trujillo-Hernandez B, Hernandez L (2003) Effects of cannabinoids on endogenous K+ and Ca2+ currents in HEK293 cells. Can J Physiol Pharmacol 81(5):436–442 [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA (2001) Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410:588–592 [DOI] [PubMed] [Google Scholar]
- Zangen A, Solinas M, Ikemoto S, Goldberg SR, Wise RA (2006) Two brain sites for cannabinoid reward. J Neurosci 26:4901–4907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang S, Hampson RE, Deadwyler SA (2005) Behaviorally relevant endocannabinoid action in hippocampus: dependence on temporal summation of multiple inputs. Behav Pharmacol 16:463–471 [DOI] [PubMed] [Google Scholar]


