Abstract
BACKGROUND
The role of adjuvant chemotherapy in stage II colon cancer continues to be debated. The presence of circulating tumor DNA (ctDNA) after surgery predicts very poor recurrence-free survival, whereas its absence predicts a low risk of recurrence. The benefit of adjuvant chemotherapy for ctDNA-positive patients is not well understood.
METHODS
We conducted a trial to assess whether a ctDNA-guided approach could reduce the use of adjuvant chemotherapy without compromising recurrence risk. Patients with stage II colon cancer were randomly assigned in a 2:1 ratio to have treatment decisions guided by either ctDNA results or standard clinicopathological features. For ctDNA-guided management, a ctDNA-positive result at 4 or 7 weeks after surgery prompted oxaliplatin-based or fluoropyrimidine chemotherapy. Patients who were ctDNA-negative were not treated. The primary efficacy end point was recurrence-free survival at 2 years. A key secondary end point was adjuvant chemotherapy use.
RESULTS
Of the 455 patients who underwent randomization, 302 were assigned to ctDNA-guided management and 153 to standard management. The median follow-up was 37 months. A lower percentage of patients in the ctDNA-guided group than in the standard-management group received adjuvant chemotherapy (15% vs. 28%; relative risk, 1.82; 95% confidence interval [CI], 1.25 to 2.65). In the evaluation of 2-year recurrence-free survival, ctDNA-guided management was noninferior to standard management (93.5% and 92.4%, respectively; absolute difference, 1.1 percentage points; 95% CI, −4.1 to 6.2 [noninferiority margin, −8.5 percentage points]). Three-year recurrence-free survival was 86.4% among ctDNA-positive patients who received adjuvant chemotherapy and 92.5% among ctDNA-negative patients who did not.
CONCLUSIONS
A ctDNA-guided approach to the treatment of stage II colon cancer reduced adjuvant chemotherapy use without compromising recurrence-free survival. (Supported by the Australian National Health and Medical Research Council and others; DYNAMIC Australian New Zealand Clinical Trials Registry number, ACTRN12615000381583.)
Colorectal cancer remains common worldwide.1 The current standard care for nonmetastatic colon cancer is surgery, with histopathological staging informing the use of up to 6 months of adjuvant chemotherapy. Although the benefit of adjuvant chemotherapy has been unequivocally established for patients with stage III colon cancer, its usefulness for patients with stage II disease continues to be debated.2 Surgery alone can cure more than 80% of patients with stage II colon cancer, and no clear overall survival benefit has been observed in trials of adjuvant therapy.3–5 Therefore, guidelines currently recommend that adjuvant chemotherapy be considered for patients who have stage II colon cancer with high-risk clinicopathological features, who may be more likely to benefit from adjuvant treatment.6–8 However, the current definitions of “high risk” are inadequate, since many patients who have cancer with high-risk features do not have disease recurrence, whereas some with disease that is deemed low-risk do. Furthermore, the survival benefit conferred by adjuvant chemotherapy remains modest (<5%) even when patients with high-risk disease are selectively treated, and therefore many patients are exposed to unnecessary chemotherapy.4,9,10
More precise prediction of recurrence risk after surgery for stage II colon cancer could address this clinical dilemma, limiting treatment to the group of patients who have disease with well-defined high-risk features and are most likely to derive a survival benefit. This approach would also allow patients who are at low risk for recurrence to be spared the physical and financial cost of unnecessary treatment. To date, efforts to refine recurrence risk for nonmetastatic colon cancer have focused on examinations of the resected tumor with various biomarkers. Although such tissue-based biomarkers have been reported to be associated with recurrence risk, the hazard ratios are typically modest, and their clinical application is still contentious.11–14
Circulating tumor DNA (ctDNA) analysis is a promising alternative strategy in which peripheral blood (a “liquid biopsy”) is directly evaluated for evidence of minimal residual disease that could ultimately be the source of a later clinical recurrence. Several observational studies involving patients with solid tumors, including those with stage II colon cancer, have confirmed a very high risk of recurrence (>80%) when ctDNA is detected after curative-intent therapy without further adjuvant treatment.15–17 Nevertheless, uncertainty remains as to whether adjuvant treatment is beneficial for these ctDNA-positive patients who are at high risk for recurrence.
The Circulating Tumour DNA Analysis Informing Adjuvant Chemotherapy in Stage II Colon Cancer (DYNAMIC) trial was a randomized trial designed to investigate whether a ctDNA-guided approach as compared with a standard approach in stage II colon cancer could reduce the use of adjuvant treatment without compromising the risk of recurrence. We further examined outcomes among ctDNA-positive patients who received adjuvant chemotherapy, to assess the benefit of treating this high-risk group of patients, as well as outcomes among ctDNA-negative patients whose disease was managed by surveillance alone.
METHODS
PATIENTS
We enrolled patients with resected histologically confirmed stage II (T3 or T4, N0, M0)18 colon or rectal adenocarcinoma with negative resection margins. To be eligible for enrollment, patients needed to have an Eastern Cooperative Oncology Group (ECOG) performance-status score of 0 to 2 (scores range from 0 to 5, with higher numbers reflecting greater disability) and had to be medically able to receive adjuvant oxaliplatinbased or single-agent fluoropyrimidine chemotherapy. Patients with evidence of macroscopic metastatic disease on computed tomography (CT) of the chest, abdomen, and pelvis performed within 8 weeks before enrollment were excluded. Other exclusion criteria were a history of another primary cancer within the previous 3 years, the presence of synchronous primary colorectal cancer, or treatment with neoadjuvant chemoradiotherapy. Patients were enrolled within 3 weeks after surgery, and an adequate specimen from the resected tumor needed to be provided for mutation analysis by 4 weeks after surgery.
TRIAL DESIGN AND INTERVENTIONS
This trial was a phase 2, multicenter, randomized, controlled trial of biomarker-driven adjuvant therapy. Patients were randomly assigned in a 2:1 ratio to have their disease managed according to ctDNA results (ctDNA-guided management) or managed by the treating clinician according to standard clinicopathological criteria (standard management). Individual patients were assigned to trial groups with the use of block randomization stratified according to the participating center location (regional or metropolitan) and tumor stage (T3 or T4).
Plasma specimens were obtained for ctDNA analysis from all patients at week 4 and week 7 after surgery. Patients underwent randomization after confirmation of adequate tumor tissue by central pathological review and confirmation of an adequate week 4 blood specimen. For patients assigned to ctDNA-guided management, week 4 and week 7 specimens were analyzed concurrently, and ctDNA results were made available to the treating clinician 8 to 10 weeks after surgery. Patients with a positive ctDNA result at either week 4 or week 7 received adjuvant singleagent fluoropyrimidine or oxaliplatin-based chemotherapy, with the treatment regimen chosen at the clinician’s discretion. Patients with negative ctDNA results at both week 4 and week 7 were not treated with adjuvant chemotherapy.
In the standard-management group, all treatment decisions were based on conventional clinicopathological criteria. Acceptable chemotherapy regimens for patients in either group are listed in Table S1 of the Supplementary Appendix, available with the full text of this article at NEJM.org. Dose modifications to chemotherapy were made in accordance with local standards.
END POINTS AND ASSESSMENTS
The primary efficacy end point was recurrence-free survival at 2 years. The recurrence-free survival time was calculated from the date of randomization to the date of confirmation of disease recurrence or death from any cause (whichever occurred earlier) or the last date at which the patient was known to be free of disease (censoring time). Recurrence was defined as local, regional, or distant relapse. A key secondary end point was treatment with adjuvant chemotherapy. Other secondary end points included recurrence-free survival among ctDNA-positive and ctDNA-negative patients in the ctDNA-guided group, time to recurrence, and overall survival. Exploratory end points included the ctDNA clearance rate in ctDNA-positive patients treated with adjuvant chemotherapy, levels of fear of recurrence among the patients, and cost-effectiveness. Overall survival data and outcomes for exploratory end points are not reported here.
All patients were to be followed for 5 years, with carcinoembryonic antigen measured every 3 months for 24 months and then every 6 months for 36 months. Contrast-enhanced CT of the chest, abdomen, and pelvis was performed every 6 months for 24 months and then at 36 months. Because only standard treatments were used in this trial, adverse events were not assessed. Dose intensity and dose adjustments for administered chemotherapy were recorded.
TRIAL OVERSIGHT
The trial was initiated by investigators based at the Walter and Eliza Hall Institute of Medical Research (WEHI), which was responsible for overseeing the conduct of the trial. All tumor and plasma specimens were analyzed by academic collaborators in a central research laboratory (Ludwig Center at Johns Hopkins) using Safe-Sequencing System tumor-informed personalized ctDNA assays.15,19 Further details are provided in the Supplementary Appendix. The protocol, available at NEJM.org, was approved by the institutional review board or ethics committee at the WEHI, Johns Hopkins Medicine, and each participating site. All the participants provided written informed consent in accordance with the principles of the Declaration of Helsinki. A statistical analysis plan was written and made publicly accessible before the database lock, and the final analysis was conducted accordingly.20 Trial data were collected and managed with the use of REDCap electronic datacapture tools hosted at the WEHI.21,22 The authors vouch for the accuracy and completeness of the data and for the adherence of the trial to the protocol. No one who is not an author contributed to writing the article.
STATISTICAL ANALYSIS
The overall sample size was chosen to ensure a minimum of 30 patients with a ctDNA-positive result in the ctDNA-guided group and an acceptable noninferiority margin of 8.5 percentage points for the analysis of 2-year recurrence-free survival, to exclude the largest absolute benefit that could be derived from adjuvant oxaliplatin-based chemotherapy for patients with stage II disease.3,23 We calculated that a total sample of 450 patients would provide 80% power with a type I error of 5% to show noninferiority of ctDNA-guided management to standard management, under the assumption of a 2-year recurrence-free survival of 84% with standard management and of 85% with ctDNA-guided management and allowing for a 10% dropout rate. The trial was powered to detect a noninferiority margin of 5 percentage points for the percentage of patients with recurrence within 2 years in a time-to-event analysis, as well as a 20-percentage-point difference between the standard-management group and the ctDNA-guided group in the percentage of patients treated with adjuvant chemotherapy, under the assumption that 30% of the patients in the standard-management group and 10% of those in the ctDNA-guided group would receive treatment.
The primary efficacy end point was assessed in the intention-to-treat population, which included all eligible patients who underwent randomization and had both week 4 and week 7 postsurgical blood specimens. A sensitivity analysis was performed in the per-protocol population, which included patients who had undergone 24-month surveillance imaging (unless recurrence or death had already occurred) and, for ctDNA-guided management, ctDNA-positive patients who received at least 12 weeks of chemotherapy or ctDNA-negative patients who received no more than 4 weeks of chemotherapy. Noninferiority of ctDNA-guided management to standard management was to be accepted if the lower bound of the 95% confidence interval around the estimated difference in the 2-year recurrence-free survival was above −8.5 percentage points. In addition, recurrence-free survival and percentages of patients with recurrence within 1, 2, and 3 years in a time-to-event analysis were computed from the Kaplan–Meier survival curves along with the associated 95% confidence intervals. Hazard ratios and associated 95% confidence intervals were also reported after evaluation of the proportional hazards assumption with the use of the Schoenfeld residuals test. The between-group difference in the use of adjuvant chemotherapy was assessed as percentages of patients in each group and as relative risk. No prespecified plan to control for multiplicity of testing was made, and therefore the 95% confidence intervals cannot be used to infer effects. This analysis was conducted when the last patient reached a minimum follow-up of 2 years. Statistical analyses were performed with R software, version 3.6.1 (R Project for Statistical Computing), and SAS software, version 9.4 (SAS Institute).
RESULTS
PATIENT CHARACTERISTICS AND FOLLOW-UP
A total of 459 patients were enrolled from 23 Australian centers between August 10, 2015, and August 2, 2019, of whom 455 underwent randomization. Of the 302 patients assigned to ctDNA-guided management, 8 (3%) were excluded from the intention-to-treat population, and of the 153 patients assigned to standard management, 6 (4%) were excluded (Fig. S1). A successful ctDNA analysis was performed for 291 of 294 patients (99%) in the ctDNA-guided group. Of these patients, only 2 did not receive ctDNA-guided management of their disease. Of the 45 ctDNA-positive patients in the ctDNA-guided group, 1 did not receive adjuvant chemotherapy, and 1 ctDNA-negative patient received chemotherapy. The median follow-up from randomization to database lock for analysis (October 15, 2021) was 37 months (37 months in the ctDNA-guided group and 38 months in the standard-management group).
The baseline characteristics of the patients in the main analysis population were generally balanced between the two groups, with the exception of a higher percentage of patients in the ctDNA-guided group than in the standard-management group having tumors on the right side (Tables 1 and S3). The median age of the patients was 64 years, and the majority of patients (99%) had an ECOG performance-status score of 0 or 1. T4 disease was present in 15% of the patients, and 5% had a lymph node yield of less than 12. Clinical high-risk disease, defined as one or more clinicopathological risk features (T4, poor tumor differentiation, lymph node yield <12, lymphovascular invasion, tumor perforation, or bowel obstruction) in association with a proficient mismatch-repair tumor, was present in 176 patients (40%). The baseline characteristics of the patients in the per-protocol population are shown in Table S2.
Table 1.
Characteristics of the Patients at Baseline in the Intention-to-Treat Population.*
| Characteristic | Standard Management (N = 147) | ctDNA-Guided Management (N = 294) | Overall (N = 441) |
|---|---|---|---|
| Male sex — no. (%) | 81 (55) | 154 (52) | 235 (53) |
| Median age (range) — yr | 62 (28–84) | 65 (30–94) | 64 (28–94) |
| Age group — no. (%) | |||
| ≤70 yr | 113 (77) | 207 (70) | 320 (73) |
| >70 yr | 34 (23) | 87 (30) | 121 (27) |
| ECOG performance‑status score — no./total no. (%)† | |||
| 0 | 124/147 (84) | 226/293 (77) | 350/440 (80) |
| 1 | 20/147 (14) | 65/293 (22) | 85/440 (19) |
| 2 | 3/147 (2) | 2/293 (1) | 5/440 (1) |
| Type of center — no. (%) | |||
| Metropolitan | 121 (82) | 240 (82) | 361 (82) |
| Regional | 26 (18) | 54 (18) | 80 (18) |
| Primary tumor site — no. (%)‡ | |||
| Left side | 78 (53) | 126 (43) | 204 (46) |
| Right side | 69 (47) | 168 (57) | 237 (54) |
| Tumor stage — no. (%) | |||
| T3 | 127 (86) | 250 (85) | 377 (85) |
| T4 | 20 (14) | 44 (15) | 64 (15) |
| Poor tumor differentiation — no. (%) | 17 (12) | 43 (15) | 60 (14) |
| Lymph node yield <12 — no. (%) | 7 (5) | 13 (4) | 20 (5) |
| Tumor perforation — no. (%) | 7 (5) | 7 (2) | 14 (3) |
| Bowel obstruction — no./total no. (%)† | 18/147 (12) | 26/291 (9) | 44/438 (10) |
| Lymphovascular invasion — no. (%) | 38 (26) | 82 (28) | 120 (27) |
| Deficient mismatch repair — no. (%) | 27 (18) | 59 (20) | 86 (20) |
| Clinical risk group — no./total no. (%)§ | |||
| High | 60/147 (41) | 116/293 (40) | 176/440 (40) |
| Low | 87/147 (59) | 177/293 (60) | 264/440 (60) |
| Median time from surgery to randomization (IQR) — days | 33 (28–41) | 32 (28–39) | 32 (28–39.5) |
The abbreviation ctDNA denotes circulating tumor DNA, and IQR interquartile range.
Eastern Cooperative Oncology Group (ECOG) performance-status scores range from 0 to 5, with higher numbers reflecting greater disability.
A tumor on the left side was defined as a tumor arising in the area from the splenic flexure to the rectum; a tumor on the right side was defined as a tumor arising in the area from the cecum to the transverse colon.
Clinical high risk was defined as the presence of tumors with proficient mismatch repair along with any clinicopathological risk feature, including T4 extension, poor tumor differentiation, a lymph node yield of less than 12, lymphovascular invasion, tumor perforation, or bowel obstruction. Clinical low risk was defined as the presence of a tumor with deficient mismatch repair or a tumor with proficient mismatch repair and none of the above risk features. One case could not be classified because of missing information on bowel obstruction.
TREATMENT DELIVERED
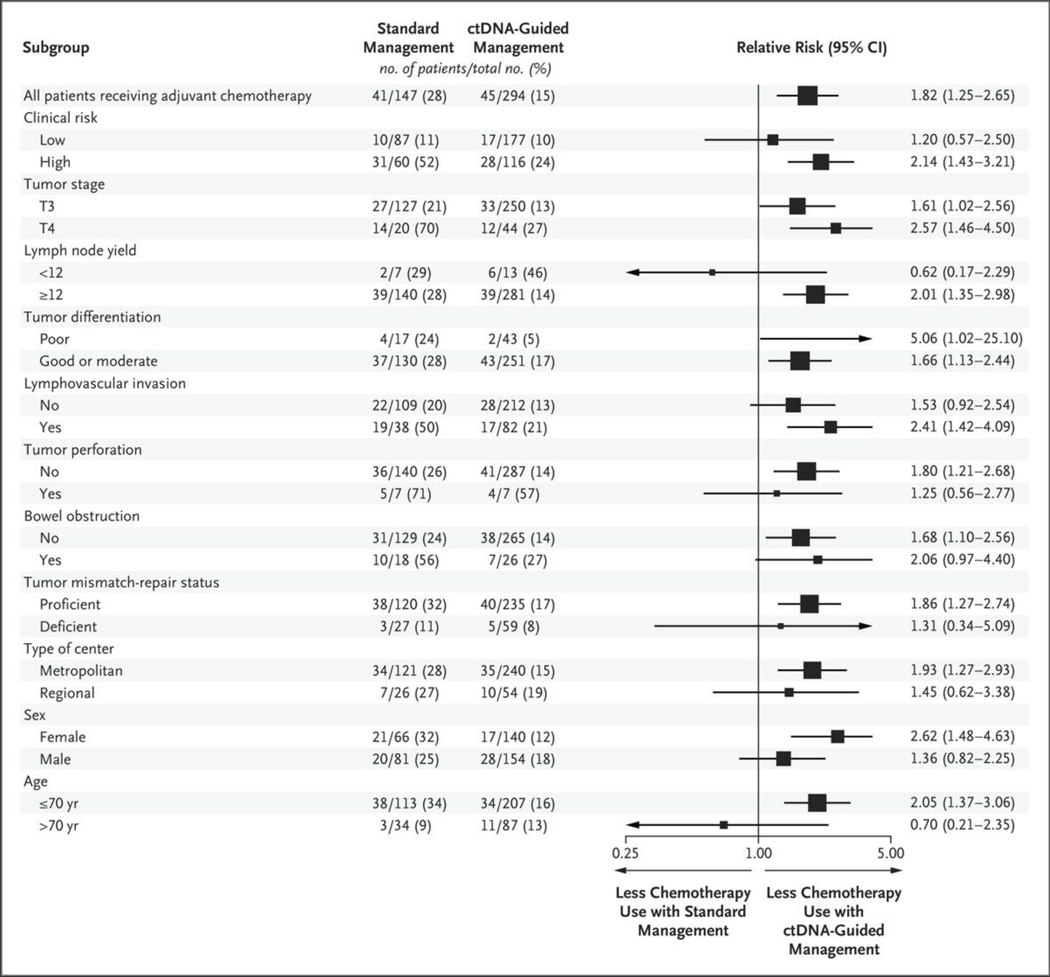
A summary of the treatment delivered and adherence in both trial groups is provided in Table 2. A lower percentage of patients in the ctDNA-guided group than in the standard-management group received adjuvant chemotherapy (15% vs. 28%; relative risk, 1.82; 95% confidence interval [CI], 1.25 to 2.65). This difference was observed across almost all patient subgroups, with the exception of patients with a lymph node yield of less than 12 and patients older than 70 years of age (Fig. 1); the most notable difference was seen among patients with T4 or poorly differentiated tumors (relative risk, 2.57 and 5.06, respectively). For patients with high-risk clinicopathological features, the likelihood of receiving adjuvant chemotherapy was 2.14 times as high in the standard-management group as in the ctDNA-guided group.
Table 2.
Treatment Delivery and Adherence.*
| Treatment Characteristic | Standard Management (N = 147) | ctDNA-Guided Management (N = 294) | Relative Risk (95% CI) |
|---|---|---|---|
| Adjuvant chemotherapy received — no. (%) | |||
| No | 106 (72) | 249 (85) | |
| Yes | 41 (28) | 45 (15) | 1.82 (1.25–2.65) |
| Chemotherapy regimen received — no./total no. (%) | |||
| Oxaliplatin-based doublet | 4/41 (10) | 28/45 (62) | |
| Single-agent fluoropyrimidine | 37/41 (90) | 17/45 (38) | 2.39 (1.62–3.52) |
| Median time from surgery to start of chemotherapy (IQR) — days | 53 (49–61) | 83 (76–89) | |
| Median treatment duration (IQR) — wk | 24 (21–24) | 24 (19–24) | |
| Reason for stopping chemotherapy — no./total no. (%) | |||
| Completion of planned treatment | 32/41 (78) | 38/45 (84) | |
| Disease relapse | 1/41 (2) | 0/45 (0) | |
| Patient request | 1/41 (2) | 1/45 (2) | |
| Toxic effects | 7/41 (17) | 6/45 (13) | |
| Percentage of full dose delivered | |||
| Mean | 77±26 | 74±24 | |
| Median (IQR) | 84 (64–100) | 78 (56–100) |
Plus–minus values are means ±SD. CI denotes confidence interval.
Figure 1. Receipt of Adjuvant Chemotherapy in the Intention-to-Treat Population According to Subgroup.
The relative risk and 95% confidence intervals for the receipt of adjuvant chemotherapy in the standard-management group as compared with the circulating tumor DNA (ctDNA)–guided group are shown. The intention-to-treat population included all eligible patients who underwent randomization and had both week 4 and week 7 postsurgical blood specimens. The size of each square corresponds to the size of the subgroup. For the subgroup with poorly differentiated tumors, the relative risk lies beyond the upper limit of the horizontal axis and is not shown.
Among those who received adjuvant chemotherapy, an oxaliplatin-based doublet was administered to a higher percentage of patients in the ctDNA-guided group than in the standardmanagement group (62% vs. 10%). In total, 8 of 86 patients (9%) with deficient mismatch-repair tumors received adjuvant chemotherapy, 6 (75%) of whom (including 4 patients in the ctDNA-guided group) were treated with oxaliplatin-based combination chemotherapy. The median time to the start of treatment after surgery was longer in the ctDNA-guided group than in the standard-management group (83 days vs. 53 days); this difference was driven by the wait time for the ctDNA result. No patient had disease recurrence during this waiting period.
EFFICACY ACCORDING TO TREATMENT GROUP
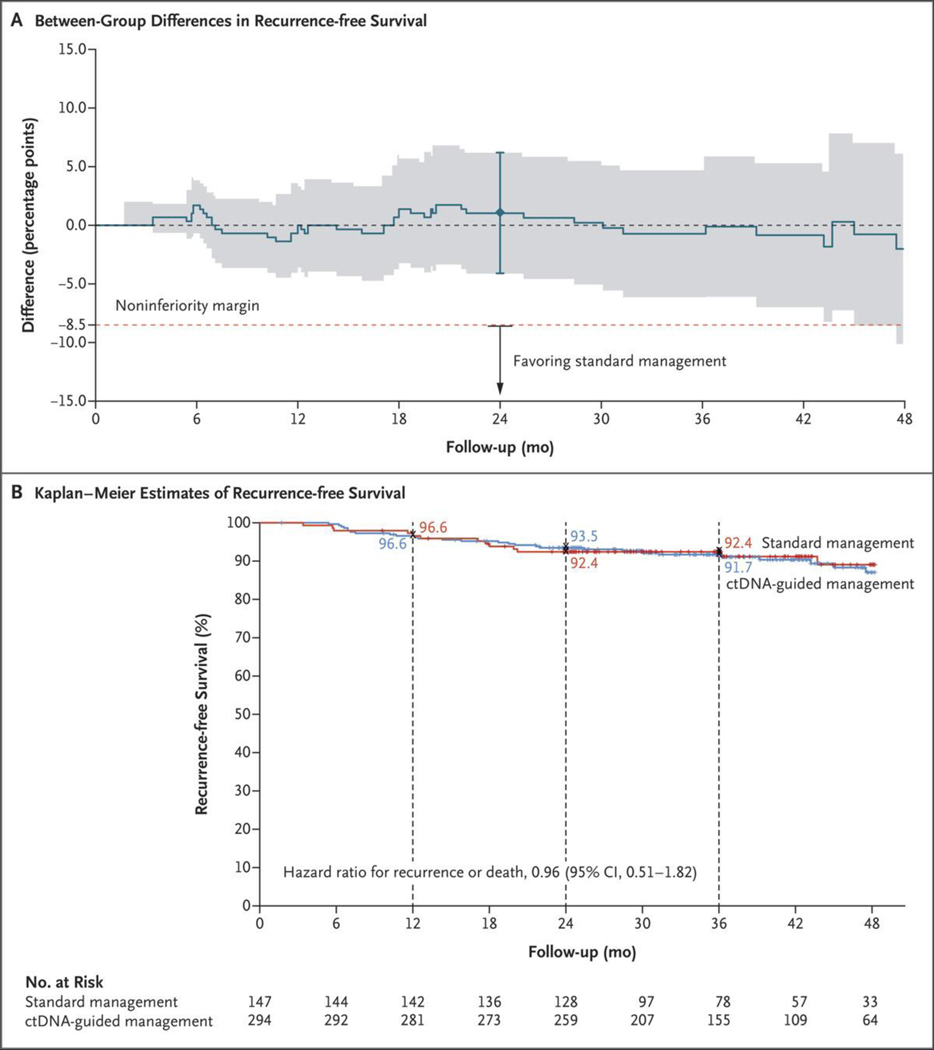
At the time of database lock, 43 events of disease recurrence or death had occurred. Noninferiority of ctDNA-guided management to standard management was confirmed in the intention-to-treat population for both 2-year recurrence-free survival (absolute difference, 1.1 percentage points; 95% CI, −4.1 to 6.2) and the percentage of patients with recurrence within 2 years in the time-to-event analysis (absolute difference, 0.7 percentage points; 95% CI, −4.3 to 5.7) (Figs. 2A, S2, S3, and S4). The percentages of patients surviving without disease recurrence at 2 years and at 3 years were similar in the ctDNA-guided group and the standard-management group (2-year recurrence-free survival, 93.5% and 92.4%, respectively; 3-year recurrence-free survival, 91.7% and 92.4%, respectively; hazard ratio, 0.96; 95% CI, 0.51 to 1.82) (Fig. 2B). The analysis involving the per-protocol population provided similar results (Figs. S6 and S7). Results were also generally similar in prespecified subgroup analyses (Fig. S5).
Figure 2. Outcomes with ctDNA-Guided as Compared with Standard Management in the Intention-to-Treat Population.
Panel A shows the absolute difference in recurrence-free survival over time between the ctDNA-guided and standard-management groups; shading indicates the 95% confidence interval. The noninferiority margin of −8.5 percentage points for the primary end point of recurrence-free survival at 2 years is indicated by the dashed red line; the 𝙸 bar indicates the 95% confidence interval at 2 years, the lower bound of which (−4.1 percentage points) lies above −8.5 percentage points, which confirms noninferiority of ctDNA-guided management to standard management. Kaplan–Meier estimates of recurrence-free survival according to the assigned management group are shown in Panel B. Tick marks indicate censored data. At 3 years, 91.7% of the patients in the ctDNA-guided group and 92.4% of those in the standard-management group were alive without disease recurrence.
OUTCOMES ACCORDING TO CTDNA STATUS IN THE CTDNA-GUIDED GROUP
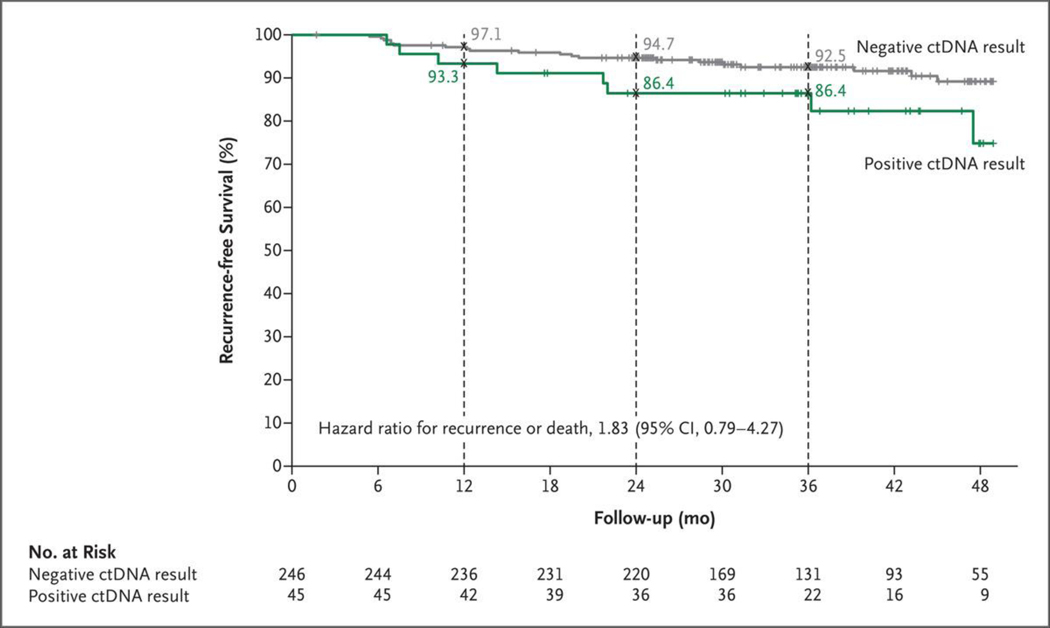
In the ctDNA-guided group, recurrence or death occurred in 15 of 246 ctDNA-negative patients (6%) and 8 of 45 ctDNA-positive patients (18%). The estimated 3-year recurrence-free survival was 92.5% among ctDNA-negative patients and 86.4% among ctDNA-positive patients (hazard ratio, 1.83; 95% CI, 0.79 to 4.27) (Fig. 3), and the percentage of patients who had had a recurrence at 3 years was 7% among ctDNA-negative patients, as compared with 14% among ctDNA-positive patients (hazard ratio, 2.45; 95% CI, 1.00 to 5.99) (Fig. S8). Among the ctDNA-positive patients treated with adjuvant chemotherapy, 3-year recurrence-free survival was 92.6% among those who received oxaliplatin-based chemotherapy and 76.0% among those who received single-agent fluoropyrimidine chemotherapy.
Figure 3. Recurrence-free Survival in the ctDNA-Guided Group According to ctDNA Status.
Kaplan–Meier estimates of recurrence-free survival according to ctDNA result (positive or negative) are shown. The 3-year recurrence-free survival was 92.5% among ctDNA-negative patients who did not receive adjuvant chemotherapy and 86.4% among ctDNA-positive patients who received adjuvant chemotherapy. Tick marks indicate censored data.
In accordance with current guidelines, clinicians routinely base treatment recommendations on clinical risk, with a T4 tumor being the strongest risk factor.6–8 In a post hoc exploratory analysis, we examined the effect of ctDNA-negative status on recurrence-free survival among patients with low-risk or high-risk disease and T3 or T4 tumors. Among ctDNA-negative patients, 3-year recurrence-free survival was higher among patients with clinical low-risk cancers than among those with high-risk cancers (96.7% vs. 85.1%; hazard ratio, 3.04; 95% CI, 1.26 to 7.34) (Fig. S9). Similarly, 3-year recurrence-free survival was higher among patients with T3 tumors than among those with T4 tumors (94.2% vs. 81.3%; hazard ratio, 2.60; 95% CI, 1.01 to 6.71) (Fig. S10). We did not investigate the effect of ctDNA-positive status according to clinical risk because of the small total number of patients with a ctDNA-positive result.
DISCUSSION
The risk of cancer recurrence after curative-intent surgery for solid tumors has traditionally been estimated on the basis of formal histologic assessment of the resected specimen. This type of analysis defines the tumor stage and determines the presence of any adverse features, which inform the use of adjuvant chemotherapy. Efforts to improve treatment and outcomes in stage II colon cancer have explored the effect of various adjuvant therapy combinations or have been aimed at defining a subgroup of patients who are most likely to derive benefit from treatment. To date, such approaches have led to limited progress. In this trial, we found that a ctDNA-guided approach reduced the number of patients who received adjuvant therapy and did not alter the risk of recurrence. Furthermore, ctDNA-positive patients appeared to derive considerable benefit from adjuvant treatment, given the low percentage of patients with recurrence in this trial as compared with previously reported high recurrence rates in this subgroup of patients when no adjuvant chemotherapy was administered.15,24 We confirm the very low risk of recurrence in untreated ctDNA-negative patients.
Across various cohorts of patients with non-metastatic colon cancer and resected colorectal liver metastases, the percentage of patients with disease recurrence among those who had detectable ctDNA and did not receive adjuvant therapy has consistently been in excess of 80%.15,24–28 The time to recurrence in these studies was also short; all untreated ctDNA-positive patients in our previous study of stage II colon cancer had disease recurrence within 2 years.15 In this context, the percentage of patients with recurrence within 3 years among the treated ctDNA-positive patients in the current trial (14%) is encouraging, notwithstanding the longer median time to chemotherapy commencement in the ctDNA-guided group of 11.9 weeks, as compared with the guidelines-recommended time of 8 weeks or less after surgery.8 However, more mature data are needed to rule out the possibility that the treatment of ctDNA-positive patients with chemotherapy may have delayed rather than prevented recurrence in some instances. It is conceivable that earlier initiation of chemotherapy for ctDNA-positive patients could lead to even more favorable outcomes. Because the turnaround time from the time a blood specimen is obtained to the time a ctDNA result is available is approximately 2 weeks, it would be useful for future studies to consider analyzing blood specimens at week 4 and week 7 after surgery (or later) sequentially instead of concurrently, with a positive week 4 ctDNA result triggering the start of adjuvant chemotherapy within the time frame recommended in guidelines. In addition, serial ctDNA analysis for patients who are ctDNA-negative after surgery may reduce the risk of undertreatment because of an initially false negative ctDNA result.
At the clinician’s discretion, the majority of ctDNA-positive patients in the ctDNA-guided group received oxaliplatin-based therapy rather than fluoropyrimidine alone. This approach was likely to have been driven by the known prognostic significance of ctDNA positivity and previous data suggesting a benefit for oxaliplatin-based therapy in patients with high-risk stage II colon cancer.23 Given the fact that our trial design predates the International Duration Evaluation of Adjuvant Therapy (IDEA) meta-analysis,29 the majority of patients were scheduled for 24 weeks of treatment, with 84% of the patients in the ctDNA-guided group and 78% of those in the standard-management group completing the planned treatment. Although we observed numerically better recurrence-free survival among ctDNA-positive patients treated with oxaliplatin-based treatment than among those treated with single-agent fluoropyrimidine, this finding should be considered hypothesis-generating only. Further studies with much larger sample sizes will be required in order to define the relative effect of fluoropyrimidine alone as compared with an oxaliplatin-based combination regimen, as well as to define appropriate treatment duration in this subgroup of patients.
Along with defining a subgroup of patients with stage II colon cancer who benefit from adjuvant therapy, defining a subgroup in whom treatment can be avoided with minimal risk of recurrence is also an important goal. To this end, our results indicated an overall very low risk of recurrence in untreated patients who were ctDNA-negative, with 3-year recurrence-free survival of 92.5%. Given the current focus of using clinicopathological risk to select patients with stage II colon cancer for adjuvant therapy,6–8,23 we explored outcomes among patients with high-risk or low-risk disease. Most notable was the 3-year recurrence-free survival of 96.7% among patients with low-risk disease, indicating that adjuvant therapy should not be considered for ctDNA-negative patients who are at clinicopathological low risk. This is an important observation, because in routine clinical practice adjuvant chemotherapy is still administered to some patients at low risk (11% in our standard-management group), particularly younger patients.
The strength of our trial is the random assignment of patients to receive ctDNA-guided or standard treatment. However, there are several limitations. The trial was adequately powered to address the primary end point, but a larger trial might have provided more definitive findings for specific patient subgroups. We did not examine the effect of a ctDNA-guided approach beyond the initial decision for adjuvant chemotherapy, because this would have compromised the trial end points. We did not randomly assign the ctDNA-positive and ctDNA-negative patients to receive treatment or no treatment, a trial design that would have provided more definitive evidence of the effect of treatment or lack thereof in each subgroup. Multiple other groups are exploring additional ways in which ctDNA analysis could inform adjuvant therapy for nonmetastatic colon cancer, including therapeutic approaches in patients who remain ctDNA-positive after completing standard adjuvant therapy (e.g., ClinicalTrials.gov numbers, NCT03803553 and NCT03832569, among other studies30–36). Data from these studies are eagerly awaited.
The results of this trial suggest that a survival benefit from adjuvant chemotherapy may be obtained in a well-defined subgroup of patients with stage II colon cancer — namely, those with detectable ctDNA after surgery. Treating only the patients who had detectable ctDNA reduced the percentage of patients who received adjuvant therapy as compared with standard management and did not compromise recurrence-free survival.
Supplementary Material
Acknowledgments
Supported by a grant (APP1085531) from the Australian National Health and Medical Research Council, a grant (APP1194970) from the Medical Research Future Fund, the Marcus Foundation, the Virginia and D.K. Ludwig Fund for Cancer Research, the Lustgarten Foundation, the Conrad R. Hilton Foundation, the Sol Goldman Charitable Trust, John Templeton Foundation, grants (CA62924, CA009071, GM136577, and CA06973) from the National Institutes of Health, and a Linda Williams Memorial Grant from the Eastern Health Research Foundation.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
We thank the patients and their caregivers and the study coordinators who participated in this trial; Michael Christie for providing central pathological review of tumor tissue; Matthew Chapman for providing assistance with project management; Siavash Foroughi for developing the electronic database used in the trial; and Cherie Blair for sample management.
Footnotes
A list of the principal investigators in the DYNAMIC trial is provided in the Supplementary Appendix, available at NEJM.org.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Rebuzzi SE, Pesola G, Martelli V, Sobrero AF. Adjuvant chemotherapy for stage II colon cancer. Cancers (Basel) 2020; 12: 2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Figueredo A, Coombes ME, Mukherjee S. Adjuvant therapy for completely resected stage II colon cancer. Cochrane Database Syst Rev 2008; 3: CD005390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.André T, de Gramont A, Vernerey D, et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J Clin Oncol 2015; 33: 4176–87. [DOI] [PubMed] [Google Scholar]
- 5.Böckelman C, Engelmann BE, Kaprio T, Hansen TF, Glimelius B. Risk of recurrence in patients with colon cancer stage II and III: a systematic review and meta-analysis of recent literature. Acta Oncol 2015; 54:5–16. [DOI] [PubMed] [Google Scholar]
- 6.Costas-Chavarri A, Nandakumar G, Temin S, et al. Treatment of patients with early-stage colorectal cancer: ASCO resource-stratified guideline. J Glob Oncol 2019; 5:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. Colon cancer: NCCN guidelines with evidence blocks (version 1.2022) (https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1428).
- 8.Argilés G, Tabernero J, Labianca R, et al. Localised colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020;31: 1291–305. [DOI] [PubMed] [Google Scholar]
- 9.O’Connor ES, Greenblatt DY, LoConte NK, et al. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol 2011; 29: 3381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verhoeff SR, van Erning FN, Lemmens VE, de Wilt JH, Pruijt JF. Adjuvant chemotherapy is not associated with improved survival for all high-risk factors in stage II colon cancer. Int J Cancer 2016; 139: 187–93. [DOI] [PubMed] [Google Scholar]
- 11.Yamanaka T, Oki E, Yamazaki K, et al. 12-Gene Recurrence Score assay stratifies the recurrence risk in stage II/III colon cancer with surgery alone: the SUNRISE study. J Clin Oncol 2016; 34: 2906–13. [DOI] [PubMed] [Google Scholar]
- 12.Venook AP, Niedzwiecki D, Lopatin M, et al. Biologic determinants of tumor recurrence in stage II colon cancer: validation study of the 12-gene recurrence score in cancer and leukemia group B (CALGB) 9581. J Clin Oncol 2013; 31: 1775–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kopetz S, Tabernero J, Rosenberg R, et al. Genomic classifier ColoPrint predicts recurrence in stage II colorectal cancer patients more accurately than clinical factors. Oncologist 2015; 20: 127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pagès F, Mlecnik B, Marliot F, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 2018; 391:2 128–39. [DOI] [PubMed] [Google Scholar]
- 15.Tie J, Wang Y, Tomasetti C, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med 2016; 8: 346ra92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christensen E, Birkenkamp-Demtröder K, Sethi H, et al. Early detection of metastatic relapse and monitoring of therapeutic efficacy by ultra-deep sequencing of plasma cell-free DNA in patients with urothelial bladder carcinoma. J Clin Oncol 2019; 37: 1547–57. [DOI] [PubMed] [Google Scholar]
- 17.Moding EJ, Liu Y, Nabet BY, et al. Circulating tumor DNA dynamics predict benefit from consolidation immunotherapy in locally advanced non-small cell lung cancer. Nat Cancer 2020; 1: 176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17:1 471–4. [DOI] [PubMed] [Google Scholar]
- 19.Kinde I, Wu J, Papadopoulos N, Kinzler KW, Vogelstein B. Detection and quantification of rare mutations with massively parallel sequencing. Proc Natl Acad Sci U S A 2011; 108: 9530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tie J, Lo SN, Cohen JD, et al. Circulating tumour DNA analysis informing adjuvant chemotherapy in stage II colon cancer (DYNAMIC trial): statistical analysis plan. September 6, 2021 ( 10.1101/2021.09.02.21262816v1). preprint. [DOI]
- 21.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) — a meta-data-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019; 95: 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tournigand C, André T, Bonnetain F, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol 2012; 30: 3353–60. [DOI] [PubMed] [Google Scholar]
- 24.Tie J, Cohen JD, Wang Y, et al. Serial circulating tumour DNA analysis during multimodality treatment of locally advanced rectal cancer: a prospective biomarker study. Gut 2019; 68:6 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parikh AR, Van Seventer EE, Siravegna G, et al. Minimal residual disease detection using a plasma-only circulating tumor DNA assay in patients with colorectal cancer. Clin Cancer Res 2021; 27: 5586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tie J, Wang Y, Cohen J, et al. Circulating tumor DNA dynamics and recurrence risk in patients undergoing curative intent resection of colorectal cancer liver metastases: a prospective cohort study. PLoS Med 2021; 18(5): e1003620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loupakis F, Sharma S, Derouazi M, et al. Detection of molecular residual disease using personalized circulating tumor DNA assay in patients with colorectal cancer undergoing resection of metastases. JCO Precis Oncol 2021; 5: 1166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diehl F, Schmidt K, Choti MA, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med 2008; 14: 985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grothey A, Sobrero AF, Shields AF, et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med 2018; 378: 1177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taïeb J, Benhaim L, Laurent Puig P, et al. Decision for adjuvant treatment in stage II colon cancer based on circulating tumor DNA: the CIRCULATE-PRODIGE 70 trial. Dig Liver Dis 2020; 52: 730–3. [DOI] [PubMed] [Google Scholar]
- 31.Taniguchi H, Nakamura Y, Kotani D, et al. CIRCULATE-Japan: circulating tumor DNA-guided adaptive platform trials to refine adjuvant therapy for colorectal cancer. Cancer Sci 2021; 112: 2915–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schraa SJ, van Rooijen KL, van der Kruijssen DEW, et al. Circulating tumor DNA guided adjuvant chemotherapy in stage II colon cancer (MEDOCC-CrEATE): study protocol for a trial within a cohort study. BMC Cancer 2020; 20: 790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anandappa G, Starling N, Peckitt C, et al. TRACC: tracking mutations in cell-free DNA to predict relapse in early colorectal cancer — a randomized study of circulating tumour DNA (ctDNA) guided adjuvant chemotherapy versus standard of care chemotherapy after curative surgery in patients with high risk stage II or stage III colorectal cancer (CRC). J Clin Oncol 2020;3 8: Suppl 15: TPS4120. [Google Scholar]
- 34.Morris VK, Yothers G, Kopetz S, et al. Phase II/III study of circulating tumor DNA as a predictive biomarker in adjuvant chemotherapy in patients with stage II colon cancer: NRG-GI005 (COBRA). J Clin Oncol 2020; 38: Suppl 15: TPS4121. [Google Scholar]
- 35.Folprecht G, Reinacher-Schick A, Tannapfel A, et al. Circulating tumor DNA-based decision for adjuvant treatment in colon cancer stage II evaluation: (CIRCULATE-trial) AIO-KRK-0217. J Clin Oncol 2020; 38: Suppl 4: TPS273. [DOI] [PubMed] [Google Scholar]
- 36.Nors J, Henriksen TV, Gotschalck KA, et al. IMPROVE-IT2: implementing non-invasive circulating tumor DNA analysis to optimize the operative and postoperative treatment for patients with colorectal cancer — intervention trial 2. Study protocol. Acta Oncol 2020; 59: 336–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.